Abstract
The use of a variety of neuroanatomical techniques has led to a greater understanding of the adverse effects of stress on psychiatric health. One recent advance that has been particularly valuable is the development of resting state functional connectivity (RSFC) in clinical studies. The current study investigates changes in RSFC in F1 adult female rats exposed to the early life chronic social stress (ECSS) of the daily introduction of a novel male intruder to the cage of their F0 mothers while the F1 pups are in the cage. This ECSS for the F1 animals consists of depressed maternal care from their F0 mothers and exposure to conflict between their F0 mothers and intruder males. Analyses of the functional connectivity data in ECSS exposed adult females versus control females reveal broad changes in the limbic and reward systems, the salience and introspective socioaffective networks, and several additional stress and social behavior associated nuclei. Substantial changes in connectivity were found in the prefrontal cortex, nucleus accumbens, hippocampus, and somatosensory cortex. The current rodent RSFC data support the hypothesis that the exposure to early life social stress has long term effects on neural connectivity in numerous social behavior, stress, and depression relevant brain nuclei. Future conscious rodent RSFC studies can build on the wealth of data generated from previous neuroanatomical studies of early life stress and enhance translational connectivity between animal and human fMRI studies in the development of novel preventative measures and treatments.
Keywords: Social stress, fMRI, Resting state functional connectivity, Prefrontal cortex, Hippocampus, Nucleus accumbens
1. Introduction
The use of a variety of neuroanatomical techniques has led to a greater understanding of the adverse effects of stress on psychiatric health. One recent advance that has been particularly valuable is the development of resting state functional connectivity (RSFC) in clinical studies. This technique measures intrinsic neural connectivity through the measurement of spontaneous fluctuations in BOLD activity in different brain regions [1,2]. RSFC analysis allows for the simultaneous assessment of long term changes in multiple neural circuits involved in psychiatric etiology. This method has recently been adapted to imaging in conscious rodents [3,4], and is a valuable tool to enhance the translational value of behavioral neuroscience studies of rodent models of psychiatric illness. Comparisons of clinical and animal model RSFC data will enhance our understanding of susceptibility and resilience, pathological etiology, and treatment response. When compared to other methods of assessing neural activity and connectivity (immunohistochemistry, various tract tracing techniques, pcr for neural activity), a single RFSC study can add a temporal dimension, even allowing the longitudinal collection of several months or years’ worth of data, a scale typically not possible with other time course approaches such as electrophysiological methods. Given similar financial resources, collecting similar amounts of data using other techniques may be impossible. In addition, RSFC in conscious rodents presents tremendous potential for enhanced etiological relevance through the longitudinal assessment of the effects of stress on multiple neural networks at several life history stages.
We have developed an ethologically relevant transgenerational model of the effects of chronic social stress (CSS) in postpartum depression and anxiety [5–9] (Fig. 1). Exposure of F0 dams to the chronic social stress of a daily exposure to a novel male intruder depresses maternal care, impairs lactation in both the F0 dams as well as their female F1 offspring, where CSS is an early life CSS (ECSS) Neuroendocrine studies of the F1 offspring of stressed F0 dams have revealed several behaviorally relevant changes in gene expression in a select set of nuclei involved in both the control of social behavior and the stress response that parallel similar findings in human studies of depression, anxiety and autism [5]. However, given that tissues were sampled at the end of lactation, it is unclear when the neural changes in gene expression occurred; for example, if they were present prior to gestation.
Fig. 1.
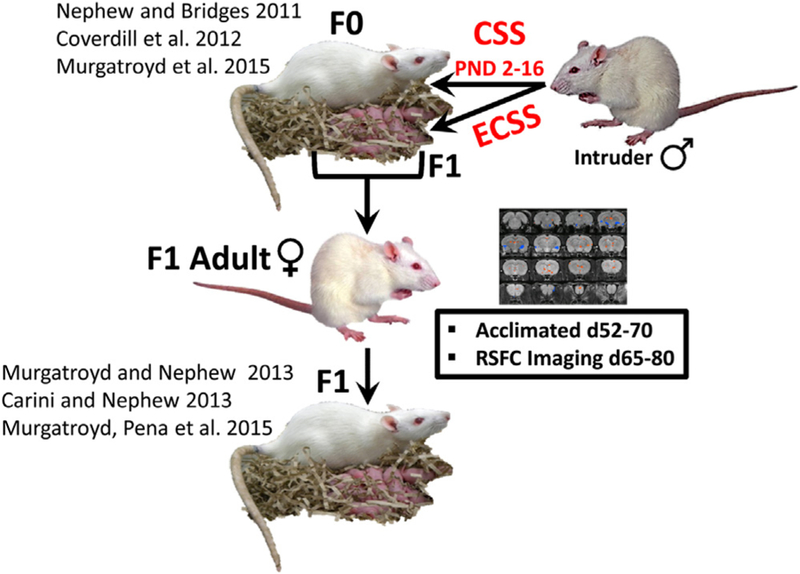
The chronic social stress (CSS) model of postpartum depression and anxiety. F0 dams and their F1 pups are exposed to novel male intruder stress for one hour/day during postnatal days 2–15. This social stress is an early life chronic social stressor (ECSS) for the F1 generation. Both the F0 and F1 dams exhibit depressed maternal care and increased maternal anxiety. The current study focused on female F1 adults, who were imaged for resting state functional connectivity (RSFC).
Recent study of the effects of a single traumatic stress exposure in rats indicated that it can cause long-term changes in the RSFC between the amygdala and mPFC, providing additional, clinically relevant support for the face and construct validity of this animal model of PTSD [10]. The development of RSFC in related animal models of psychiatric illness is postulated to enhance the identification of susceptibility indicators and the identification of effective preventative measures and treatments. The current study sought to build on this finding and apply RSFC to the study of the long term effects of early life stress, a mechanism relevant to a vast array of psychiatric disorders. It was hypothesized that animals exposed to ECSS would exhibit substantial changes in connectivity between several nuclei implicated in the early life stress associated disorders in both humans and animal models, including components of the Default Mode Network (DMN) and limbic network.
2. Methods
2.1. Animals
Sprague Dawley rats (Charles River, Wilmington, MA) in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts University and University of Massachusetts Institutional Animal Care and Use Committees. For an overview of the CSS paradigm, see Fig. 1. “CSS dams” refers to the adult females exposed to CSS during lactation (F0), and “ECSS females” refers to the adult female offspring of the CSS dams (F1); the focus of the present study. The F0 CSS stage of the study was conducted at Tufts University and the F1 pups were transported 10 miles at 30–40 days of age to the University of Massachusetts Center for Comparative Neuroimaging. The sample size for F1 control and ECSS female groups was 14.
2.2. ECSS model: creation of F0 dams and F1 females
F0 Dams (Charles River, Wilmington, MA) mated at Tufts University were subjected to the CSS protocol at the Cummings School (previously described) [7,9] consisting of placing a similarly sized (220–300 g) novel male intruder into a lactating female’s home cage for 1 h from days 2–16 of lactation. Control dams were not exposed to the CSS protocol, and were only tested for maternal care and maternal aggression between 0800 and 1200 on days 2, 9, and 16 of lactation (both control and CSS dams were tested for maternal care and maternal aggression on these days). The F1 pups were left in the cage during the intruder presentation and the CSS F1 pups were exposed to depressed maternal care from their F0 mothers and the daily conflict between the mother and the male intruder (Early life CSS, ECSS) [7,9]. The F1 control and ECSS females of the current study were the offspring of the F0 control and CSS dams; the differences between the treatments of the control and ECSS F1 females were limited to the exposure of the ECSS F1 females to depressed maternal care and daily conflict between their F0 mothers and the male intruders during age 2–16 days. The F1 control and ECSS animals were treated identically after the age of 16 days. All F1 (CSS and control) females were transported to the UMass CCNI at 30–40 days of age (10 miles from the Cummings School), quarantined for 21 days, and then acclimated to the imaging procedures for 8 days.
2.3. Acclimation to imaging procedures
A total of 27 F1 adult females (65–90 days old) were exposed to the imaging protocol (13 CSS and 14 control). Before the imaging experiment, these rats were acclimated to the environment and imaging acoustic noise produced by the MR scanner using the procedure previously described [4]. Briefly, rats were anesthetized with isoflurane (2%) and secured in a head holder using plastic bite bar and ear bars. EMLA cream (Lidocaine 2.5% and Prilocaine 2.5% cream, Hi-Tech Pharmacal Co., Inc.) was topically applied to relieve any pain associated with the head holder. Animals were then placed into a black opaque tube (mock scanner) with tape-recorded scanner noise played. Animals were acclimated for eight days, one session per day. The time of acclimation was 15 min on day 1 with an increment of 15 min per day up until day 4. A maximum of 60 min was used on days 4–8, and all animals completed the acclimation procedures successfully.
2.4. Imaging
Animals were imaged at 65–90 days of age to assess the long term effects of early life stress on adult RSFC. All MR images were acquired on a 4.7T/40 cm horizontal magnet (Oxford, UK) interfaced with a Biospec Bruker console (Bruker, Germany) and equipped with a 20G/cm magnetic field gradient. A custom built 1H radiofrequency (RF) volume coil was used. Anatomical images were acquired using a multi-slice fast spin-echo sequence (RARE) with the parameters: repetition time (TR) = 3000 ms; RARE factor = 8; effective echo time (TE) = 50 ms; matrix size = 256 × 256 × 20; in-plane field of view (FOV) = 3.2cm 3.2 cm; slice thickness = 1 mm; n of averages = 4. Functional images were acquired using echo-planar imaging (EPI) with the parameters: TR = 1089 ms; Flip Angle = 60°; TE = 30 ms; matrix size = 64 × 64 × 20; in-plane FOV = 3.2 cm × 3.2 cm; slice thickness = 1 mm; voxel size 0.5 mm × 0.5 mm × 1 mm. Each EPI scan had 600 repetitions, lasting about 10 min.
2.5. Resting state functional connectivity (RSFC) data analysis
All fMRI data were processed and analyzed using Medical Image Visualization and Analysis (MIVA, http://ccni.wpi.edu), Statistical Parametric Mapping (SPM8) software (Wellcome Department of Cognitive Neurology, London, UK) and Matlab (The Mathworks Inc., Natick, MA, USA), following a similar processing pipeline addressed in our previous publications [3,4,10,11]. Anatomical and fMRI images were registered from multiple subjects to a fully segmented rat brain atlas (Swanson). Seed regions were selected from the neuroendocrine targets described in experiment based on previous imaging and gene expression studies [5,12–14], including the prefrontal area (PFC) – prelimbic (PL) and infralimbic (IL) areas, nucleus accumbens (NAc), hippocampus (HP), insula, bed nucleus stria terminalis (BST), amygdala, lateral septum (LS), periaqueductal gray (PAG), primary somatosensory area (SSp), secondary somatosensory area (SSs) and thalamus. After registration, fMRI data went through the following pre-processing steps: (a) motion correction (threshold of 0.5 mm), (b) spatial smoothing using a 3D Gaussian filter (1-mm FWHM) to account for small variations in signal due to movement and vascular effects, (c) regressions of motion parameters and white matter/ventricle signal, and (d) band-pass filtering (0.002–0.1 Hz) to adjust for scanner drift between runs and help remove other sources of noise. Functional connectivity was evaluated using a region-of-interest (ROI) seed-based correlational analysis. For each seed ROI, a time course was created by averaging time courses of all voxels inside it. This time course was then used as the reference to calculate functional connectivity to each voxel of the rest of the brain. Pearson correlation coefficient (CC) between reference time course and the time course of each individual voxel was calculated. This correlation analysis was carried out for each run. CC’s were transformed using Fisher’s z transformation and then averaged across runs and animals. Subsequently, the averaged z values were transformed back to r values, yielding a mean correlation map for each seed.
Based on the individual CC maps of significant group differences (Fig. 2), two sample t-tests were carried out to generate group differences of RSFC for each seed region, using a threshold of p < 0.05 after false discovery rate (FDR) correction.
Fig. 2.
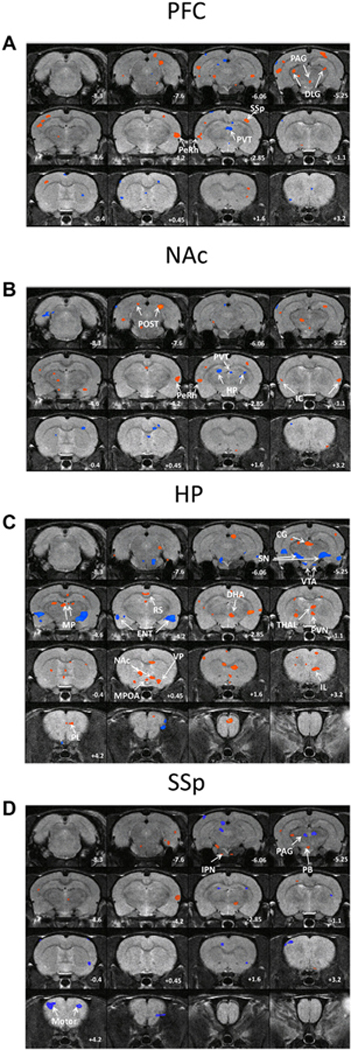
A–D Composite maps of significant changes in resting state functional connectivity in ECSS animals compared to controls using the prefrontal cortex (A), nucleus accumbens (B), hippocampus (C), and primary somatosensory cortex (D) as seeds. Red denotes increased connectivity between the seed and denoted region in ECSS animals, blue denotes decreased connectivity. Numerical values are coronal position relative to bregma. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Results
Table 1 lists the brain regions discussed in the current study. Table 2 summarizes all the significant differences in RSFC between the F1 controls and the ECSS adult females for the 13 seed regions. Fig. 2A–D display the difference maps for four specific ROIs where there were significant differences (or multiple trends) in RSFC: PFC, NAc, HP and SSp. Fig. 3A–D are graphs of the differences in these nuclei, with asterisks denoting significance at p < 0.05.
Table 1.
Definitions of brain region abbreviations.
| ACC | Anterior cingulate cortex |
|---|---|
| AMG | Amygdala |
| BLA | Basolateral amygdala |
| BNST | Bed nucleus of the stria terminalis |
| CeA | Central amygdala |
| CG | Central geniculate |
| DHA | Dorsal hypothalamus |
| DLG | Dorsolateral geniculate |
| ENT | Entorhinal |
| HP | Hippocampus |
| IC | Insular cortex |
| IL | Infralimbic cortex |
| IPN | Interpeduncular |
| LS | Lateral septum |
| MC | Motor cortex |
| MeA | Medial amygdala |
| MP | Magnocellular precommissural |
| MPOA | Medial preoptic area |
| NAc | Nucleus accumbens |
| OC | Olfactory cortex |
| PAG | Periaqueductal gray |
| PaS | Parasubiculum |
| PB | Parabrachial |
| PeRh | Perirhinal cortex |
| PFC | Prefrontal Cortex |
| PL | Prelimbic cortex |
| POST | Posterior subiculum |
| PVN | Paraventricular nucleus |
| PVT | Paraventricular thalamus |
| RS | Retrosplenial |
| SN | Substantia nigra |
| SSp | Primary somatosensory cortex |
| SSs | Secondary somatosensory cortex |
| TC | Temporal cortex |
| VAL | Ventral anterior lateral thalamus |
| VMH | Ventromedial hypothalamus |
| VP | Ventral Pallidum |
| VTA | Ventral tegmental area |
Table 2.
Summary of changes in resting state functional connectivity (RSFC) between control and ECSS exposed F1 adult females.
| Seed region | Resting State Functional Connectivity, ECSS relative to Control | |
|---|---|---|
| INCREASED RSFC | DECREASED RSFC | |
| PFC | PVT | |
| NAc | PeRH | HP |
| HP | DHA, NAc, IL, PL | SN, VTA, MPOA, |
| IC | VAL | SSp |
| BNST | ENT | Cg, SSp |
| BLA | Cg | HP, DLG |
| CeA | IC | |
| MeA | PAG | SSp |
| AMG | PAG | |
| LS | PaS | SSp |
| PAG | IC, Cg | OC |
| SSp | IPN, PB | MC |
| SSs | TC, VMH, LS | |
Fig. 3.
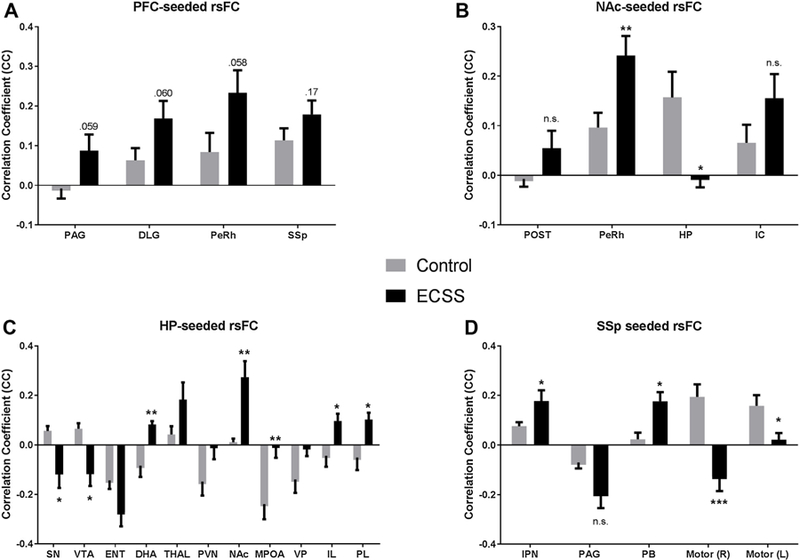
A–D Graphical representation of significant changes in resting state functional connectivity in ECSS animals compared to controls using the prefrontal cortex (A), nucleus accumbens (B), hippocampus (C), and primary somatosensory cortex (D) as seeds. Bars are mean correlation coefficient ±SEM. * denotes a significant difference (p < 0.05).
Using the PFC as a seed, there are several trends for increases in connectivity with multiple other regions in ECSS animals versus controls, specifically the periaqueductal gray (PAG), dorsal lateral geniculate (DLG), and perirhinal cortex (PeRh). In contrast, RSFC between the PFC and periventricular thalamus (PVT) decreased (Table 2). NAc seeded RSFC increased in the PeRh and decreased in the hippocampus (HP) (fig. 2B, 3B). With the HP as seed, the RSFC changed from a positive to a negative correlation in the substantia nigra (SN) and ventral tegmental area (VTA), and changed from negative to positive in the dorsal hypothalamic area (DHA), infralimbic area (IL) and prelimbic cortex (PL). Connectivity between the HP and NAc increased, and there was a loss of connectivity in the MPOA (Figs. 2C, 3C). The seemingly contradictory patterns in HP-NAc connectivity when using the NAc or HP as seed indicates that there are likely subregion-specific changes in RSFC in one or both regions. This is not surprising given the size of the HP seed and the diverse subregions of both brain regions. SSp seeded RSFC showed an increase in the interpeduncular nucleus (IPN) and parabrachial nucleus (PB), and decreases in the left motor area. Instead of the positive correlation between the SSp and the motor areas found in control animals, the connectivity between these regions switched to a negative correlation for the right hemisphere motor cortex in the ECSS females (Figs. 2D, 3D).
4. Discussion
Previous behavioral and neuroendocrine gene expression analyses of ECSS exposed animals have described depressed maternal care, decreased aggression, increased anxiety, and numerous behaviorally relevant changes in gene expression in stress and depression associated nuclei [5,6,15]. The present approach identified functional connectivity in the range of our previous rat studies [10,11,16] and those of other groups [17,18], and was sensitive to group differences even at modest connectivity levels. These rodent RSFC data support the hypotheses that the ECSS procedure has long term effects on neural connectivity in numerous social behavior, stress, and depression relevant brain nuclei and that socially focused pathological animal models involve robust changes in resting connectivity in multiple clinically relevant networks, including the limbic system, the reward system, and the introspective socioaffective and salience networks. Data are discussed in terms of individual nuclei to avoid making assumptions on functional network wide changes that would be best addressed with additional stimulus-based fMRI data and/or behavioral data (reward tasks, social behavior, fear-related behavior) as well as allow for more flexibility in comparing with prior research. The results establish a broad neural foundation for further studies focused on additional life history stages (including longitudinal studies), behavioral stimulus dependent imaging, morphological, immunohistochemical, and genetic analyses of specific nuclei and networks, and manipulative studies aimed at treating the adverse effects of early life stress.
4.1. Prefrontal cortex (PFC)
The PFC (including changes between HP and PL, IL) connectivity data fit with reported depression associated changes in clinical studies [19]. While depressive behavior data are not available for the F1 adult females used in the present study, ECSS exposure is associated with saccharin based anhedonia and depressed maternal care in F1 dams [6], and it is hypothesized that these behavioral effects are mediated by the long-term effects of social stress on the F1 animals as pups and would be expected to be present at the adult and dam stages. RSFC of depressed individuals revealed hyperconnectivity in the extended social affective default network, which includes the HP, cingulate, amygdala, and PFC [19,20]. While we did not see the exact same changes, we did observe trends for increased connectivity between the PFC and several regions, significant increased connectivity between the HP and the PL and IL (rat homologs of the dorsal anterior cingulate and subgenual vmPFC, respectively [21,22]), as well as several other changes in HP connectivity.
The anterior cingulate cortex has been identified as an area that displays increased activity in depressed subjects [23] and it is also a target for deep brain stimulation for the treatment of depression which induces changes in multiple cortical and limbic regions [24–26]. Length of depressive episodes are positively correlated with general RSFC with the ACC [27]. Connectivity between the amygdala and ACC is disrupted in postpartum depressed women compared to non-depressed women [28], although this involves a decrease in connectivity in contrast to the increased HP and PL connectivity reported here. However, a, women with PTSD related to early life stress exhibit a general increase in ACC activity [29], which is a more relevant comparison. It is hypothesized that the reported increases in connectivity between the HP and PL may mediate accentuated responses to stressful stimuli in ECSS females, but further research is needed on this topic.
Interestingly, the current PFC connectivity data fit more closely with RSFC studies of depression than some studies of early life stress which involved individuals without a psychiatric illness that may have selected for a certain degree of resilience or used younger subjects [30,31]. Other studies suggest that early life stress induces region specific changes in the direction of PFC RSFC, with increased connectivity in some regions and decreases in others [32]. Changes in the PFC also suggest that cognition may be altered in the stressed F1 females, and it is suggested that disrupted maternal care in ECSS dams represents maternal specific cognitive impairment.
One specific behavioral role of these changes in PFC activity may be to increase retrieval latencies in the ECSS dams, as the PFC is involved in the development of maternal care [33] and is a primary mediator of pup retrieval [34]. The increased connectivity in the PFC of ECSS females may interfere with typical processing of pup related stimuli and motor output necessary for the efficient retrieval of pups to the nest, possibly involving the changes in the SSp and MC as well. Since subsequent maternal care behaviors require that the litter be retrieved to the nest, this disruption in the PFC could impact pup grooming and nursing. Differences in PFC RSFC in early life stress exposed and depressed humans and animals may be relevant to the identification of social specific mechanisms of susceptibility and resilience.
4.2. Periventricular thalamus (PVT)
The PVT is connected to several nuclei that mediate mood, critically involved in the response to chronic stress, displays abnormal functioning in anxiety and depression, and contains orexin positive neurons [35]. Increased orexinergic activity has been found in the dorsal hypothalamus of rodent models of depression [36] and orexin is a potential treatment target [37,38]. The Fos response to restraint in PVT orexin neurons is dampened in female rats exposed to early life stress [39], and disruption of PVT functioning in ECSS adult females is indicated by the decreased RSFC between the PFC and PVT. In the SON of ECSS dams, gene expression of orexin and orexin receptors are decreased, and these changes are correlated with maladaptive changes in both maternal care and maternal anxiety [15]. Similar changes in PVT orexin may mediate early life social stress related behavioral changes [40] and the reported change in PFC-PVT RSFC. When taken together, these recent studies support a role for orexin activity in the PVT in early life stress induced changes in maternal behavior and associated disorders such as depression, anxiety, and PTSD.
4.3. Nucleus accumbens (NAc)
The NAc is key factor in the cognitive processing of rewarding stimuli and has been associated with the adverse effects of early life stress, depression and anxiety, and the control of maternal behaviors. Previous conscious rodent fMRI study revealed increased BOLD in the NAc and IC in response to the presentation of novel male intruder rat [13]. Maternal separation in rodents disrupts the dendritic morphology of neurons in the NAc and HP [41], and similar changes may mediate the decrease in NAc-HP connectivity in the ECSS females that are exposed to depressed maternal care, as maternal care and maternal anxiety associated ERK levels, a factor in the BDNF pathway, are decreased in the NAc of ECSS dams (Murgatroyd 15). These neuronal effects may be caused by corticosterone exposure which induces morphological changes in the NAc and HP of rats [42]. Studies in prairie voles has implicated OXT signaling in the NAc facilitation of alloparental care [43], and variations in NAc DA mediates individual differences in rat maternal care [44]. The NAc response to affective stimuli was identified as a factor mediating depression susceptibility in humans [45], and the NAc is a target for deep brain stimulation treatment for severe depression and anxiety [46,47]. Future longitudinal studies of OXT, DA, and neuronal morphology of the ECSS model may generate valuable insight on the role of early life stress on depression etiology in the NAc and guide novel intervention strategies, especially for maternal depression and/or deficient maternal care.
4.4. Hippocampus (HP)
Similar to studies of childhood emotional maltreatment, we report substantial changes in limbic and salience network RSFC, most notably in the HP, thalamus, cingulate, SN, and VTA [48]. Behaviorally, ECSS exposed dams are less maternally responsive towards pups and less aggressive towards a novel male intruder, indicating attenuated salience of these stimuli which are both critical components of adequate maternal behavior. The changes in HP RSFC in ECSS F1 adults are extensive, and include increased connectivity in the thalamus and PL similar to studies of depression in humans [27]. The CA1 region of the HP, ACC, ENT, and the thalamus have been implicated in the neural responses of lactating dams in previous fMRI studies [13]. In addition, central vasopressin V1a receptor blockade alters BOLD responses in the SN, MPOA, and IL to novel male intruders [12], areas where connectivity with the HP is also altered in ECSS females. The reversal in connectivity between the HP and SN and VTA provide additional evidence that the reward system of the ECSS females is affected, and this is supported by behavioral assessment of depressed maternal care in social stress exposed F1 dams [6] due to the importance of the reward system in mediating maternal care [49,50]. Early social stress exposure in male Guinea pigs decreases androgen receptors in MPOA and alters social behavior [51], and a related neural mechanism may mediate the decrease in HP-MPOA RSFC in ECSS females. The broad effects on HP RSFC may be the result of decreases in BDNF activity and associated morphological changes, as early maternal deprivation decreases the expression of BDNF receptor subunits in the rat HP [52]. In support of this hypothesis, early maternal separation produces a delayed effect on HP structure by decreasing rates of synaptic development [53].
Similar to the PVT data, the current imaging results suggest that orexin activity in the DHA is involved in ECSS induced changes in connectivity. Preweaning maternal deprivation and postweaning social isolation in Octodon degus increases synaptic densities in the IL [54], and this mechanism may explain the increased HP-IL RSRC in ECSS females. In Cushing’s disease patients who often suffer from comorbid depression, previous exposure to high levels of cortisol is related to increased connectivity between the limbic network and the cingulate [55], and ECSS associated exposure to corticosterone may be involved in increased connectivity between the HP and PL in the present study. The broad and robust changes in HP connectivity suggest that the effects of ECSS in the limbic and salience networks are mediated by alterations in HP neuroplasticity.
4.5. Primary somatosensory cortex (SSp)
In addition to the increased negative correlation between the SSp and PAG, ECSS also disrupted connectivity between the SSp and motor cortex. The SSp has been implicated in the role of AVP in the BOLD responses of maternal rats [12]. This impact of ECSS on basal neural functioning may indicate a disruption in the processing of sensory input and the translation of sensory input to motor output, and this hypothesis is supported by the display of disorganized maternal care in ECSS dams. Specifically, changes in the SSp-motor cortex connectivity mediate the adverse effects of ECSS on maternal care by disrupting the translation of pup stimuli perception into rewarding maternal care behaviors. Other connections with the SSp which were affected by ECSS are with the IPN and PB nuclei.
4.6. Interpeduncular nucleus (IPN)
The IPN has been implicated in a wide range of behaviors, including nociception, learning and memory, motor activity, sexual and maternal behavior, stress, depression, and anxiety behavior, and reward mediated behavior [56]. Activity in the IPN is generally associated with the inhibition of dopamine release. DA release in several brain regions mediates maternal care, including the neighboring ventral mesencephalic tegmentum (VMT) [57], so this is another change in RSFC which may mediate depressed ECSS maternal care through decreased DA activity in maternally relevant nuclei such as the VMT. The increase in SSp-IPN RSFC in ECSS females may represent SSp mediated inhibition of DA release in the IPN. Lesions of the peripenduncular decrease aggression towards a novel male intruder in lactating dams [58]. It is postulated that input from the SSp has inhibitory actions on the IPN which decrease aggressive responses of ECSS dams towards a novel male intruder [6]. The IPN has been associated with both maternal aggression and lactation in other lesion studies, and these effects are likely to be mediated by OXT [59]. Although OXT activity in the IPN of ECSS dams has not been measured, OXT activity in several other nuclei, maternal aggression, and lactation are all decreased in ECSS dams [5]. In social defeat models of social stress, Fos activity in the IPN is associated with defeat [60], and adverse life experiences and adult social stress have been hypothesized to mediate stress related neuropsychiatric disorders through dysregulated serotonergic activity in the IPN [61]. 5HT, DA, and OXT targeted investigations of the IPN in the CSS model and similar paradigms may generate more specific insights on the role of the IPN in the adverse effects of early life stress.
4.7. Parabrachial nucleus (PB)
The PB is involved in integrating ascending and descending inputs and coordinating experience induced neuroplasticity [62]. It is associated with opioid mediated conditioned place preference and taste preference as part of the reward pathway [63,64] (Simon, 11, 13), and it has been postulated to exert inhibitory effects on the reward pathway in obese rats [65]. Furthermore, maternally deprived male rats exhibit an 86% increase in PB CRH concentrations, and CRH infusions into the PB induce both depression and anxiety [66], behavioral changes also found in ECSS dams [6]. The increased RSFC in between the SSp and PB of ECSS females may represent CRF mediated basal hypersensitivity to sensory stimuli, resulting in an impaired reward pathway and associated decreases in maternal care and maternal aggression.
4.8. Comparisons with other rodent RSFC studies: changes in the DMN and limbic networks
Two other recent studies have investigated RSFC changes in rodent models of depression/anxiety [67,68]. These studies differed from the current study in that the rats were anesthetized during imaging (isoflurane and mechanical ventilation [68] or medetomadine [67]). Compared to data from anesthetized rats, awake imaging appears to identify a greater number of significant changes in RFSC, although it is possible this could be due to differences in rodent paradigm, with ECSS vs. chronic adult restraint or a congenital learned helplessness rat strain. These differences, as well as differences in depression models, make it difficult to compare results, but there are general similarities between the studies.
The most consistent finding among the three paradigms was changes in the somatosensory cortex, with increased RSFC [68], decreased [67], or changes in both directions in the present study. The current changes in somatosensory cortex RSFC are indirectly related to previous behavioral rodent fMRI work from the Febo lab, where manipulation of the vasopressin system, which is implicated in chronic stress induced behavioral changes as well as maternal care [69], affects BOLD activity in the SSp [12]. One hypothesis is that chronic stress related depression etiology involves changes in SSp RSFC which mediates adverse responses to subsequent stressors; the induction of a hypervigilant state where sensory processing is accentuated. Further behavioral studies, in conjunction with comprehensive stimulus based conscious rodent fMRI would generate valuable insight concerning this hypothesis.
Henckens et al. reported an increase in the DMN, and while we did not assess connectivity in the DMN as a whole, there were general increases in connectivity in the PL, IL, HP, and RS in ECSS females compared to controls. While increased DMN connectivity has been hypothesized to be a significant component in stress related depressive etiology in humans [70], the exact role of this network in rodents is unclear and direct comparisons between clinical and animal work would require substantial anthropomorphism. ECSS exposure also resulted in several RSFC changes in the limbic system (both increases and decreases in connectivity), which is also a common observation in clinical studies [71]. These limbic changes may mediate previously documented effects of ECSS on social behaviors such as maternal care [5,6].
5. Conclusions
The simultaneous evaluation of numerous brain regions in conscious rodent fMRI studies facilitates the integration of findings from many previous neuroanatomical studies to generate robust, broad conclusions which may span several neural networks and enhance translational connectivity between clinical and animal investigations. While data on stress induced changes in RSFC in rodents has recently been published [68], comparisons with this study are difficult given the major differences in awake vs. anesthetized and mechanically ventilated animal imaging, notably anesthesia associated confounds in cerebral hemodynamics and neuronal activity [72]. The current rodent RSFC data support the hypotheses that the ECSS procedure has long term effects on neural connectivity in numerous social behavior, stress, and depression relevant brain nuclei and that socially focused pathological animal models involve robust changes in resting connectivity in multiple clinically relevant networks, including the limbic system, the reward system, and the introspective socioaffective and salience networks. Functional connectivity data specifically support the importance of the PFC, NAc, HP, and SSp nuclei in long term early life social stress induced changes in neuroplasticity. Future conscious rodent RSFC studies possess substantial potential to build on the wealth of basic data generated from past neuroanatomical studies of social behavior and integrate conclusions from recent pathological studies on single nuclei or small groups of nuclei. As with past lesion studies, one key to the successful application of conscious rodent RSFC is to start with uncomplicated experiments to build a solid foundation of dependable fMRI data to support later, more complex investigations. All imaging studies generate an abundance of data, and it is suggested that simple studies that allow for more complete discussion of the results will provide the greatest benefit to subsequent investigations. Longitudinal conscious rodent fMRI investigations will generate valuable findings on the etiology of several psychiatric disorders associated with early life stress and/or long term changes in neuroplasticity, such as depression, anxiety, schizophrenia, PTSD, addiction, and eating disorders.
HIGHLIGHTS.
Early life social stress induces changes in resting state functional connectivity.
The prefrontal cortex and hippocampus exhibited particularly robust changes.
Stress affects connectivity in social and depression relevant brain regions.
Acknowledgements
The authors would like to thank Dr. Jessica Babb for assistance with the CSS protocol at the Cummings School and Kelly Tam for experimental logistics at the CCNI. The laboratory animal medicine service staff at the Cummings School provided exceptional care of our rodents. This work was supported by a National Institutes of Health award (NICHD R00 HD056643) and a Brain and Behavior Research Foundation NARSAD Young Investigator Award to BCN and NIH S10 OD018132-01 to the UMass CCNI.
References
- [1].Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS, Functional connectivity in the motor cortex of resting human brain using echo-planar mri, Magn. Reson. Med 34 (1995) 537–541. [DOI] [PubMed] [Google Scholar]
- [2].Raichle ME, Snyder AZ, A default mode of brain function: a brief history of an evolving idea, Neuroimage 37 (2007) 1083–1090. [DOI] [PubMed] [Google Scholar]
- [3].Liang Z, King J, Zhang N, Uncovering intrinsic connectional architecture of functional networks in awake rat brain, J. Neurosci 31 (2011) 3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang N, Rane P, Huang W, Liang Z, Kennedy D, Frazier JA, et al. , Mapping resting-state brain networks in conscious animals, J. Neurosci. Methods 189 (2010) 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Murgatroyd CA, Nephew BC, Effects of early life social stress on maternal behavior and neuroendocrinology, Psychoneuroendocrinology 38 (2013) 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carini LM, Nephew BC, Effects of early life social stress on endocrinology, maternal behavior, and lactation in rats, Hormones Behav 64 (2013) 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carini LM, Murgatroyd CA, Nephew BC, Using chronic social stress to model postpartum depression in lactating rodents, J. Visualized Exp (2013) e50324. [DOI] [PMC free article] [PubMed]
- [8].Coverdill A, McCarthy M, Bridges R, Nephew B, Effects of chronic central arginine vasopressin (AVP) on maternal behavior in chronically stressed rat dams, Brain Sci 2 (2012) 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nephew BC, Bridges RS, Effects of chronic social stress during lactation on maternal behavior and growth in rats, Stress 14 (2011) 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liang Z, King J, Zhang N, Neuroplasticity to a single-episode traumatic stress revealed by resting-state fMRI in awake rats, Neuroimage 103 (2014) 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huang W, Tam K, Fernando J, Heffernan M, King J, DiFranza JR, Nicotine and resting state functional connectivity: effects of intermittent doses, Nicotine Tobacco Res (2015). [DOI] [PMC free article] [PubMed]
- [12].Caffrey MK, Nephew BC, Febo M, Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups, Neuropharmacology 58 (2010) 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nephew BC, Caffrey MK, Felix-Ortiz AC, Ferris CF, Febo M, Blood oxygen level-dependent signal responses in corticolimbic ‘emotions’ circuitry of lactating rats facing intruder threat to pups, Eur. J. Neurosci 30 (2009) 934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nephew B, Caffrey M, Felix-Ortiz A, Febo M, Cocaine sensitization increases kyphosis and modulates neural activity in adult nulliparous rats, Brain Sci 2 (2012) 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murgatroyd CA, Pen˜a CJ, Podda G, Nestler EJ, Nephew BC, Early life social stress induced changes in depression and anxiety associated neural pathways which are correlated with impaired maternal care, Neuropeptides 52 (2015) 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liang Z, King J, Zhang N, Anticorrelated resting-state functional connectivity in awake rat brain, Neuroimage 59 (2012) 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Williams KA, Magnuson M, Majeed W, LaConte SM, Peltier SJ, Hu X, et al. , Comparison of alpha-chloralose, medetomidine and isoflurane anesthesia for functional connectivity mapping in the rat, Magn. Reson. Imaging 28 (2010) 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nasrallah FA, To XV, Chen DY, Routtenberg A, Chuang KH, Resting state functional connectivity data supports detection of cognition in the rodent brain, Data Brief 7 (2016) 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schilbach L, Müller VI, Hoffstaedter F, Clos M, Goya-Maldonado R, Gruber O, et al. , Meta-analytically informed network analysis of resting state fMRI reveals hyperconnectivity in an introspective socio-affective network in depression, PLoS One 9 (2014) e94973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB, Definition and characterization of an extended social-affective default network, Brain Struct. Funct 220 (2015) 1031–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Milad MR, Quirk GJ Fear, Extinction as a model for translational neuroscience: ten years of progress, Annu. Rev. Psychol 63 (2011) 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nieuwenhuis ILC, Takashima A, The role of the ventromedial prefrontal cortex in memory consolidation, Behav. Brain Res 218 (2011) 325–334. [DOI] [PubMed] [Google Scholar]
- [23].Drevets WC, Price JL, Furey ML, Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression, Brain Struct. Funct 213 (2008) 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. , Deep brain stimulation for treatment-resistant depression, Neuron 45 (2005) 651–660. [DOI] [PubMed] [Google Scholar]
- [25].Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH, Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression, Biol. Psychiatry 64 (2008) 461–467. [DOI] [PubMed] [Google Scholar]
- [26].Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, et al. , Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression, Cereb. Cortex 18 (2008) 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. , Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus, Biol. Psychiatry 62 (2007) 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chase HW, Moses-Kolko EL, Zevallos C, Wisner KL, Phillips ML, Disrupted posterior cingulate?amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI, Social Cognit. Affect. Neurosci 9 (2014) 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS, Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder, Am. J. Psychiatry 156 (1999) 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Philip NS, Valentine TR, Sweet LH, Tyrka AR, Price LH, Carpenter LL, Early life stress impacts dorsolateral prefrontal cortex functional connectivity in healthy adults: informing future studies of antidepressant treatments, J. Psychiatric Res 52 (2014) 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. , Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ, Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity, Depress. Anxiety 31 (2014) 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Afonso VM, Sison M, Lovic V, Fleming AS, Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization, Behav. Neurosci 121 (2007) 515–526. [DOI] [PubMed] [Google Scholar]
- [34].Febo M, Felix-Ortiz AC, Johnson TR, Inactivation or inhibition of neuronal activity in the medial prefrontal cortex largely reduces pup retrieval and grouping in maternal rats, Brain Res 1325 (2010) 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hsu DT, Kirouac GJ, Zubieta J-K, Bhatnagar S, Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood, Front. Behav. Neurosci 8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S, Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression, Neuropharmacology 61 (2011) 336–346. [DOI] [PubMed] [Google Scholar]
- [37].Nollet M, Leman S, Role of orexin in the pathophysiology of depression: potential for pharmacological intervention, CNS Drugs 27 (2013) 411–422. [DOI] [PubMed] [Google Scholar]
- [38].Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV, Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls, Front. Neurosci 8 (2014) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].James MH, Campbell EJ, Walker FR, Smith DW, Richardson HN, Hodgson DM, et al. , Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats, Front. Behav. Neurosci 8 (2014) 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, et al. , The paraventricular thalamus controls a central amygdala fear circuit, Nature 519 (2015) 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Monroy E, Hernandez-Torres E, Flores G, Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring, J. Chem. Neuroanat 40 (2010) 93–101. [DOI] [PubMed] [Google Scholar]
- [42].Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R, Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat, J. Chem. Neuroanat 38 (2009) 266–272. [DOI] [PubMed] [Google Scholar]
- [43].Olazabal DE, Young LJ, Oxytocin receptors in the nucleus accumbens facilitate/’spontaneous/’ maternal behavior in female prairie voles, Hormones Behav 48 (2005) 177. [DOI] [PubMed] [Google Scholar]
- [44].Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ, Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat, J. Neurosci 24 (2004) 4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL 3rd, et al. , Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression, Am. J. Psychiatry 165 (2008) 90–98. [DOI] [PubMed] [Google Scholar]
- [46].Crowell A, Riva-Posse P, Garlow S, Mayberg H, Toward an understanding of the neural circuitry of major depressive disorder through the clinical response to deep brain stimulation of different anatomical targets, Curr. Behav. Neurosci. Rep 1 (2014) 55–63. [Google Scholar]
- [47].Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. , Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression, Biol. Psychiatry 67 (2010) 110–116. [DOI] [PubMed] [Google Scholar]
- [48].van der Werff SJ, Pannekoek JN, Veer IM, van Tol MJ, Aleman A, Veltman DJ, et al. , Resting-state functional connectivity in adults with childhood emotional maltreatment, Psychol. Med 43 (2013) 1825–1836. [DOI] [PubMed] [Google Scholar]
- [49].Strathearn L, Maternal neglect: oxytocin, dopamine and the neurobiology of attachment, J. Neuroendocrinol 23 (2011) 1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nephew BC, Murgatroyd C, Ittet FP, Febo M, Brain reward pathway dysfunction in maternal depression and addiction: a present and future transgenerational risk, J. Reward Defic. Syndr 1 (2015) 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kaiser S, Kruijver FP, Straub RH, Sachser N, Swaab DF, Early social stress in male Guinea-pigs changes social behaviour, and autonomic and neuroendocrine functions, J. Neuroendocrinol 15 (2003) 761–769. [DOI] [PubMed] [Google Scholar]
- [52].Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA, Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus, Mol. Psychiatry 7 (2002) 609–616. [DOI] [PubMed] [Google Scholar]
- [53].Andersen SL, Teicher MH, Delayed effects of early stress on hippocampal development, Neuropsychopharmacology 29 (2004) 1988–1993. [DOI] [PubMed] [Google Scholar]
- [54].Ovtscharoff W Jr., Braun K, Maternal separation and social isolation modulate the postnatal development of synaptic composition in the infralimbic cortex of Octodon degus, Neuroscience 104 (2001) 33–40. [DOI] [PubMed] [Google Scholar]
- [55].van der Werff SJA, Pannekoek JN, Andela CD, Meijer OC, van Buchem MA, Rombouts SARB, et al. , Resting-state functional connectivity in patients with long-term remission of Cushing/’s disease, Neuropsychopharmacology 40 (2015) 1888–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Klemm WR, Habenular and interpeduncularis nuclei: shared components in multiple-function networks, Med. Sci. Monit 10 (2004) 26. [PubMed] [Google Scholar]
- [57].Gaffori O, Le Moal M, Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions, Physiol. Behav 23 (1979) 317–323. [DOI] [PubMed] [Google Scholar]
- [58].Hansen S, Ferreira A, Food intake, aggression, and fear behavior in the mother rat: control by neural systems concerned with milk ejection and maternal behavior, Behav. Neurosci 100 (1986) 64–70. [DOI] [PubMed] [Google Scholar]
- [59].Factor EM, Mayer AD, Rosenblatt JS, Peripeduncular nucleus lesions in the rat: i. Effects on maternal aggression, lactation, and maternal behavior during pre- and postpartum periods, Behav. Neurosci 107 (1993) 166–185. [DOI] [PubMed] [Google Scholar]
- [60].Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA, Early life experience alters behavior during social defeat: focus on serotonergic systems, Neuroscience 136 (2005) 181–191. [DOI] [PubMed] [Google Scholar]
- [61].Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA, Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression, Brain Res 1305 (2009) 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yamamoto T, Takemura M, Inui T, Torii K, Maeda N, Ohmoto M, et al. , Functional organization of the rodent parabrachial nucleus, Ann. N. Y. Acad. Sci (2009). [DOI] [PubMed]
- [63].Simon MJ, Garcia R, Puerto A, Concurrent stimulation-induced place preference in lateral hypothalamus and parabrachial complex: differential effects of naloxone, Behav. Brain Res 225 (2011) 311–316. [DOI] [PubMed] [Google Scholar]
- [64].García R, Simon MJ, Puerto A, Rewarding effects of the electrical stimulation of the parabrachial complex: taste or place preference, Neurobiol. Learn. Mem 107 (2014) 101–107. [DOI] [PubMed] [Google Scholar]
- [65].Li J, Chen K, Yan J, Wang Q, Zhao X, Yang X, et al. , Increased sucrose intake and corresponding c-Fos in amygdala and parabrachial nucleus of dietary obese rats, Neurosci. Lett 525 (2012) 111–116. [DOI] [PubMed] [Google Scholar]
- [66].Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD, et al. , Depression and anxiety: role of the locus coeruleus and corticotropin-releasing factor, Brain Res. Bull 35 (1994) 561–572. [DOI] [PubMed] [Google Scholar]
- [67].Ben-Shimol E, Gass N, Vollmayr B, Sartorius A, Goelman G, Reduced connectivity and inter-hemispheric symmetry of the sensory system in a rat model of vulnerability to developing depression, Neuroscience 310 (2015) 742–750. [DOI] [PubMed] [Google Scholar]
- [68].Henckens MJAG, van der Marel K, van der Toorn A, Pillai AG, Fernández G, Dijkhuizen RM, et al. , Stress-induced alterations in large-scale functional networks of the rodent brain, Neuroimage 105 (2015) 312–322. [DOI] [PubMed] [Google Scholar]
- [69].Nephew BC. Behavioral roles of oxytocin and vasopressin. In: Sumiyoshi T, editor. Neuroendocrinology and Behavior: InTech; (2012). [Google Scholar]
- [70].Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F, et al. , Stress impact on resting state brain networks, PLoS One 8 (2013) e66500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sheline YI, Price JL, Yan Z, Mintun MA, Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus, Proc. Natl. Acad. Sci 107 (2010) 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Febo M, Technical and conceptual considerations for performing and interpreting functional MRI studies in awake rats, Front. Psychiatry 2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


