Abstract
We investigated the consequences of chronic social instability (CSI) during adulthood on social and maternal behavior in females and social behavior of their offspring in a rat model. CSI consisted of changing the social partners of adult females every 2–3 days for 28 days, 2 weeks prior to mating. Females exposed to CSI behaved less aggressively and more pro-socially towards unfamiliar female intruders. Maternal care was not affected by CSI in a standard testing environment, but maternal behavior of CSI females was less disrupted by a male intruder. CSI females were quicker to attack prey and did not differ from control females in their saccharin consumption indicating, respectively, no stress-induced sensory-motor or reward system impairments. Offspring of CSI females exhibited slower growth and expressed more anxiety in social encounters. This study demonstrates continued adult vulnerability to social challenges with an impact specific to social situations for mothers and offspring.
Keywords: epigenetics, maternal aggression, maternal care behavior, social behavior, social instability, stress
1 |. INTRODUCTION
The effect of the social environment on the behavioral ontogeny of organisms is a major concern for both humans and animals. Compared with common laboratory physical stress procedures (e.g., foot-shock, physical restraint), which are unlikely to mimic events naturally faced by animals or humans, challenging animals with an adverse social environment is considered as a more ethologically relevant way to investigate stress in laboratory animals, triggering evolutionarily selected behavioral and neuroendocrine responses (Martinez, Calvo-Torrent, & Pico-Alfonso, 1998; Palanza, Gioiosa, & Parmigiani, 2001; Tamashiro, Nguyen, & Sakai, 2005).
The effects of the social environment on behavioral development is of critical relevance for fields interested in animal behavior, especially considering the deleterious consequences of social stress on welfare (Minier, Capitanio, Gottlieb, & McCowan, 2012; Rault, 2012) and reproductive success which are mediated by maladaptive changes during gestation (Wingfield & Sapolsky, 2003) and postnatal care (Bahr, Pryce, Döbeli, & Martin, 1998). Because of ecological pressures (Creel, Dantzer, Goymann, & Rubenstein, 2013) or pressures due to human management for production (Proudfoot, Weary, & von Keyserlingk, 2012), research (Olsson & Westlund, 2007) or exhibition (Waples & Gales, 2002) animals may be chronically challenged with adverse social conditions including extreme changes in population density, forced social interactions, and repeated changes of social partners. Without consensus on the terminology of social stressors, we here refer to this later condition as “social partner instability” (Blanchard, McKittrick, & Blanchard, 2001; Proudfoot et al., 2012). The study of social stress also has translational significance since the vast majority of stimuli leading to psychopathology in humans are of a social nature (Brown & Prudo, 1981; Buwalda et al., 2005; Rygula et al., 2005). Specifically, adverse social environments and a lack of social support are key etiological factors in anxiety and depression in humans (Kendall-Tackett, 2007). In addition, the study of social stress in females is of critical interest due to their elevated susceptibility for affective mood disorders (Earls, 1986; Herzog et al., 2009; Tamashiro et al., 2005) as well as for the transgenerational consequences of stress on offspring.
Compared with early social experiences, extensively studied for the last two decades (see Champagne, 2010; Champagne & Curley, 2005), the effects of adverse adult social environments on adult social behavior and parental care remain underexplored in animal models. In particular, although this topic should focus on females for the above mentioned reasons, the vast majority of animal models of social stress have included only male subjects (Bhatnagar, Vining, Iyer, & Kinni, 2006; Klein et al., 1992), and/or used social stress procedures that are only or more stressful for males (Herzog et al., 2009; Palanza, 2001; Tamashiro et al., 2005). For instance, social defeat is an effective stressor for females of some monogamous rodent species such as the California mouse (Trainor et al., 2011) but appears to be far less effective for females of species which exhibit sexual dimorphism in aggressiveness, like humans (Archer, 2009) and rats, more sensitive to chronic social instability (Haller, Fuchs, Halàsz & Makara, 1999). Several studies demonstrate robust adverse effects of unstable social environment and/or disruption of social relationships on human health and coping abilities (Cornwell, 2015; German & Latkin, 2011; Gerstorf, Röcke, & Lachman, 2011; Perry, 2006) and given the reliance of women on social support (Plaisier et al., 2007; Tamres, Janicki, & Helgeson, 2002; Taylor, Klein, Lewis, Gruenewald & Updegraff, 2000; Walen & Lachman, 2000), social instability stress possesses substantial translational relevance.
The use of female models of social stress to understand intergenerational consequences has recently been developed in several empirical studies applying the social stress during specific reproductive periods, notably gestation (Elsenbruch et al., 2007; Kaiser & Sachser, 2005) and lactation (Babb, Carini, Spears, & Nephew, 2014; Murgatroyd & Nephew, 2013). By applying the social stress during gestation and lactation, these studies target very particular periods of reproduction when maternal females experience substantial changes in neuroplasticity (Numan & Insel, 2003) increasing their vulnerability to various stressors (Neumann, Toschi, Ohl, Torner, & Krömer, 2001; O’Hara & McCabe, 2013). The behavioral and transgenerational influence of chronic social partner instability has been explored in female mice during the socially vulnerable (McCormick, Smith, & Mathews, 2008) adolescent period (Saavedra-Rodríguez & Feig, 2013). In comparison, much less is known about adult female’s vulnerability to chronic social stress and consequences on social behavior, maternal care, and development of future offspring. Chronic social defeat in adults can have profound and long lasting consequences on social behavior and associated neuroendocrine factors (Champagne, 2010). Given the links between individual differences in social and maternal behavior (Budaev, Zworykin, & Mochek, 1999; Koski, 2014; Maestripieri, 1993; Pittet, Houdelier, et al., 2014) supported by neurophysiological studies illustrating the involvement of common mechanisms in these behaviors (Keverne & Curley, 2004; Maestripieri, Lindell, Ayala, Gold, & Higley, 2005; Nephew & Bridges, 2008), it is very likely that exposing adult females to chronic social instability can affect maternal care and subsequent offspring development.
The objective of the current study was to investigate the effects of chronic social partner instability during adulthood on female social behavior, maternal care, and the social behavior of their offspring. In addition to social and maternal behavior, we investigated predatory behavior (Kinsley et al., 2014) in adult females and offspring to determine whether potential differences in aggressive behaviors are specific to the social context or generalized to aggressive situations. Females were also tested for saccharin preference to determine whether influences of social instability on social and maternal behavior, two reward mediated behaviors (Ferris et al., 2005; Nephew, Murgatroyd, Pittet, & Febo, 2015; O’Connell & Hofmann, 2011), are also associated with abstract anhedonia, a known consequence of chronic social stress (Shimamoto, Holly, Boyson, DeBold, & Miczek, 2014). Previous studies on social instability used paradigms that exposed females to demographic variations including both social isolation and over-crowding as well as partner changes (Chaby, Sheriff, Hirrlinger, & Braithwaite, 2015; Haller et al., 1999; Herzog et al., 2009). The current study applied a less complex social partner instability paradigm incorporating frequent partner changes in uniform density social groups for four weeks. Our hypothesis was that social instability in F0 adult females will reduce social behavior, and this effect will be transmitted to F1 offspring through impaired maternal care.
2 |. METHODS
2.1 |. Animals
Animals in this study were maintained in accordance with the guidelines of the committee of the Care and Use of Laboratory Animal Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee. During the social instability procedure, the social interaction test and the maternal aggression test, some individuals expressed agonistic behaviors but care was taken to check for injuries and any signs of pain.
Animals involved in this experiment were Sprague Dawley rats (Rattus norvegicus) kept under a 12:12 light dark cycle (light switched on at 0700) at 23 ± 2 °C with food and water available ad libitum. Adults (60 females, 10 males used for reproduction and 20 males used as intruders) were provided by Charles River laboratories (Wilmington, MA), and were habituated to the colony for 2 weeks prior to experimental manipulation.
2.2 |. General schedule
The general schedule is described in the Figure 1.
FIGURE 1.
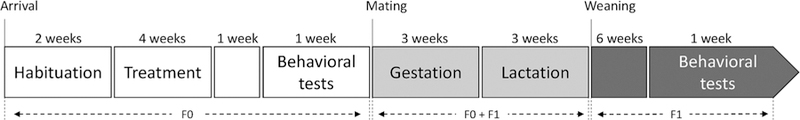
Timeline schedule of the experiment including F0 behavioral testing, mating, maternal care period and F1 testing are described. Thirty CON and thirty CSI females were tested for their social and predatory behavior. Ten CON and ten CSI were mated and tested for their maternal behavior. Twenty F1 CON and twenty F1 CSI were tested for their social behavior
Sixty adult females (60–70 days old, 175–200 g) were housed in groups of three in clear plastic cages (L 48 × W 126.5 × H 20 cm) for 2 weeks of habituation before the treatment started. During this habituation period, females were tested for their saccharine preference. They were also tested for their social behavior before treatment started as part of a separate ongoing study (the present study focuses on the effects of social instability treatment and these pre-treatment social behavior results are not included). Chronic Social Instability treatment was then applied (see Section 2.3) for 4 weeks. One week after the end of the CSI procedure, 30 control (CON) and 30 CSI females were tested for their social and predatory behavior. One week later, 15 CSI and 15 CON females, randomly selected, were mated by placing two females from similar treatment with one male for 4 days. The male was then removed and the two females were kept in the same cage for the rest of gestation until they were moved to individual cages 1–2 days prior to parturition. We obtained a sample of 10 CON and 10 CSI pregnant females. At birth (postnatal day 1, PND 1) pups were sexed using ano-genital distance and all litters were then culled to 10 pups (five males and five females). During lactation, maternal care, milk intake, and pup weight were measured at PND 2, 9, and 16 and maternal aggression was measured at PND 2. Pups were weaned at PND 21 by removing the mothers from the home cage and they remained in same sex sibling groups of three until one male and one female from each litter were tested for their social and predatory behavior (for a total of 20 F1CON and 20 F1CSI) between PND 65 and PND 72. The non-focal animals served as novel animals in the social interaction test.
2.3 |. Chronic social instability (CSI) procedure
Within the 60 F0 females, 30 were randomly attributed to the CSI treatment and 30 to the control (CON) treatment. The social instability procedure consisted in removing of one female per cage to place her with two new cagemates every 2 or 3 days on a random schedule. This procedure was applied over 28 days, while CON females remained in stable groups of three during this period. To prevent any bias due to more frequent handling in CSI compared with CON females, each CON female was also removed from her cage every 2 or 3 days and placed back several seconds later during the treatment period. Similarly, to prevent any bias due to a difference in cage familiarity between CSI and CON females, each time CSI females were transferred to new cages, the cages were changed for all CSI and CON animals.
2.4 |. Saccharin preference test
Adult females were tested for their saccharin consumption before and after treatment, as well as during gestation (day 15–18 of gestation). Testing occurred during the night between 1600 and 0800 and for the saccharin tests that occured before mating; females were isolated during this time. At 1600, female had access to two different bottles of either water or .02% saccharin solution. Bottles were weighed before and when they were removed the following morning. Saccharin intake proportion was calculated as follows: Weight of saccharin consumed/ total fluid intake.
2.5 |. Behavioral testing
Behavioral testing was videotaped to prevent human interference. In all the behavioral tests, the camera was placed laterally, in front of the large side of the cage, 20 cm higher than the cage floor level. Behaviors were later scored from videos by an observer naïve to treatment condition, using Odlog software (Macropod, Inc., Yarraville, Victoria, Australia), which records latencies, frequencies, and durations.
2.6 |. Social approach and social interaction
Thirty CON and 30 CSI F0 adult females were tested after the end of treatment, and 20 F1CON and 20 F1CSI offspring (10 males, 10 females in each group) were tested between PND 65 and PND 72. Tested rats were placed in clean breeding cages (L 48 × W 26.5 × H 20 cm) for 10 min. An empty clear plastic mouse cage cover with a plastic mesh top (L 19.7 × W 30.5 × H 9.5 cm) was placed on one side on the testing cage for an additional period of 10 min. These first 20 min allow the experimental animal to acclimate to the testing environment before the introduction of the social stimulus. A same-age, -sex, and -treatment unfamiliar rat was then placed under the cage top for 10 min to test for social approach. The cage top remained at the same place as during the habituation phase and allowed for olfactory inspection of the social stimulus but prevented direct physical interaction. Then, the cage top was removed and the animals were free to interact for 10 min to assess direct social interaction.
The entire 40 min test was videotaped. For social approach, scored behaviors included time spent distal to the cage top, in contact and on top of it, as well as olfactory investigation of the novel rat through the mesh and self-grooming. During the social interaction period, scored behaviors included olfactory investigation (distinctions between head, flank/back, and ano-genital investigations), aggression (launching towards the other rat, boxing, or biting), keeping the other rat down, allo-grooming, and self-grooming. Additionally, moving toward (total duration of time spent in locomotion reducing the distance with the partner) and away (total duration of time spent in locomotion extending distance with the partner) from the social stimulus were recorded to calculate an index of prosocial movement for all animals: total duration of moving towards the social stimulus/ total locomotion duration. The use of the proportion rather than the global amount of locomotion prevents the global activity level of test individuals to affect the measure of sociality.
Results obtained for F1 social behavior led to further investigation in their self-grooming behavior in a non-social context. Self-grooming was thus scored during the first 10 min that the F1 spent in the empty cage before the mouse cage top was introduced.
2.7 |. Predation test
The test rat was placed in a clean empty cage (L 48 × W 26.5 × H 20 cm) for 5 min before a cricket was released in the cage and the behavior of the rat was videotaped for 10 min.
Scored behaviors were the latency of first contact with the cricket, latency between first contact and first attack (head and/or paws fast movement oriented to the prey), latency between the first attack and the initial consumption of the prey, and the number of attacks (unsuccessful attacks lead to the flight of the cricket followed by new chase and attack behavior from the rat).
2.8 |. Maternal care, milk intake, and maternal aggression
Maternal behavior was tested on PND 2, 9, and 16 between 0900 and 1200. Pups were removed from the maternal cage and placed together in a clean cage with bedding for 1 hr. Videotaping started after the pups were weighed and placed back in their mother’s cage and lasted 30 min. This procedure is known to stimulate the typical pattern of maternal care that consists of retrieval to the nest, some nest building activity, grooming of the pups, and nursing (Nephew & Bridges, 2011). Pups and mothers were left undisturbed for an additional 90 min to assess milk intake over 120 min by weighing the pups again at the end of this time. Scored behaviors included all maternal behaviors (retrieving, nesting, pup grooming, nursing) as well as time spent in nest, time with all pups in nest, self-grooming, exploration (extension against walls of the cage), locomotion (moving in the cage without retrieving), and eating.
On PND 2, after maternal care testing and milk intake assessment, an unfamiliar male was introduced in the cage for 30 min to trigger maternal aggression behavior. Videotaping started when the male was placed in the cage and scored behaviors included defense behaviors towards intruder (threat, aggression, keeping-down), as well as maternal care (nesting, licking/grooming, and nursing), locomotion, and self-grooming. Additionally, the time with all pups in nest, mother in nest and intruder in nest were recorded.
2.9 |. Analyses
Statistics were performed using SPSS 22 (IBM, Chicago, IL). Most of the behavioral data did not reach the normality assumptions even after transformation. These data were analyzed using non-parametric two-tailed statistic tests. Mann–Whitney U tests compared means between the sets and Chi square tests compared proportions of animals that expressed or did not express targeted behaviors. Even though an important amount of nonparametric comparison tests is described, we here present uncorrected results for independent non-parametric tests to prevent the risk of conservative corrections to lead to type II errors, considering the mild character of our stress procedure. Weights of pups were analyzed using repeated measure ANOVAs with age as repeated measure and treatment and sex as fixed factors. Weights at each age were further compared using Bonferroni post hoc pair-comparison tests, corrected for multiple comparisons. Graphic and tables present results as mean ± SEM. The analysis of the behavior of F1 males and F1 females revealed no effects of sex and the results are presented with both sexes combined. Due to the large number of variables and relatively small sample size, a biologically significant description of effect sizes (Nakagawa, 2004) is also presented for results with a significant uncorrected p-value or a close to significance trend (p < .06). For behavioral data which do not meet normality assumptions, effects sizes associated with Mann–Whitney U tests were calculated by applying the formula from Rosenthal (1994): r = Z/√N and odds ratio are reported for significant Chi square results. Partial η2 (η2 p) are reported to detail effect sizes for the ANOVA exploring the influence of treatment, age and interaction on infant weight. Conventional interpretation of effect size is as follows: r: >0.1 small, >0.3 medium, >0.5 large; Odds Ratio (OR): >1.5 small, >2 medium, >3 large; η2p: >.01 small, >.06 medium, and 0.14 large (Cohen, 1988; Sullivan & Feinn, 2012).
3 |. RESULTS
3.1 |. F0 saccharin preference
Individuals that were later assigned to either CSI or CON groups did not differ in their saccharin preference before treatment, after treatment prior to mating, or during gestation (all p’s >.05).
3.2 |. F0 social behavior
During the social approach test, there were no differences between CON and CSI for time spent proximal to the stimulus animal (CON: 259.57 ± 2.72 s, CSI: 261.40 ± 3.44 s; Mann–Whitney U-test: U = 483.5, p = 0.62) or the time spent in olfactory investigation of the stimulus (CON: 71.77 ± 4.40 s, CSI: 76.20 ± 5.34 s; U = 493.5, p = 0.64). Nevertheless, during free social interactions with the intruder, CON and CSI females behaved differently. The proportion of females who expressed aggression toward the social stimulus was substantially lower in CSI than in CON (CON: 17/30, CSI: 8/30; χ2 = 5.55, p = .02, OR = 3.6). Additional social behavior data from F0 females and statistical results are presented in Table 1. CSI females investigated the back and flanks of the intruder more (p = .049), tended to express more prosocial movement (Mann–Whitney U-test: p = .058) and to held the intruder down earlier (p = .051) than CON females (Table 1). CON females initiated aggression towards the intruder sooner (p = .049) and spent significantly more time exhibiting aggressive behavior (p = .01; Table 1). Considering aggressive individuals only, the latency and duration of aggression did not differ between CON and CSI (latency: CON: 288.53 ± 35.45 s CSI: 230.00 ± 60.20 s; U = 52, p = 0.37; duration: CON: 3.00 ± 0.68 s, CSI: 1.63 ± 0.38 s; U = 49.5, p = 0.29). CON and CSI females did not differ in their self-grooming behavior with respect to frequency (CON: 5.03 ± 0.49, CSI: 5.90 ± 0.64; U = 504.4, p = 0.42) or duration (CON: 19.27 ± 2.29 s, CSI: 20.97 ± 2.68 s; U = 473.5 p = 0.73).
TABLE 1.
Results of social behavior of CON (N = 30) and CSI (N = 30) females during the social interaction test
| Unit | CON | CSI | U | r | p | |
|---|---|---|---|---|---|---|
| Proportion of prosocial moving | % | 50.50 ± 2.53 | 56.68 ± 2.64 | 578 | 0.24 | 0.058 |
| Head investigation duration | s | 12.27 ± 1.08 | 10.60 ± 1.36 | 348.5 | — | 0.13 |
| Head investigation frequency | Fq | 8.23 ± 0.62 | 7.43 ± 0.77 | 380 | — | 0.3 |
| Back/flank investigation duration | s | 7.00 ± 1.15 | 7.13 ± 0.87 | 489.5 | — | 0.56 |
| Back/flank investigation frequency | Fq | 4.23 ± 0.73 | 5.20 ± 0.56 | 582 | 0.18 | 0.049 |
| Ano-genital investigation duration | s | 30.50 ± 3.27 | 28.53 ± 2.91 | 435 | — | 0.82 |
| Ano-genital investigation frequency | Fq | 12.37 ± 1.06 | 14.40 ± 1.36 | 525.5 | — | 0.26 |
| Aggression duration | s | 1.70 ± 0.47 | 0.43 ± 0.16 | 301 | 0.33 | 0.01 |
| Aggression frequency | Fq | 1.27 ± 0.29 | 0.57 ± 0.21 | 313.5 | — | 0.24 |
| Aggression latency | s | 423.50 ± 34.85 | 501.33 ± 34.01 | 569 | 0.25 | 0.049 |
| Keep down duration | s | 9.93 ± 3.20 | 10.93 ± 3.46 | 462 | — | 0.86 |
| Keep down frequency | Fq | 2.83 ± 0.52 | 4.13 ± 0.93 | 484.5 | — | 0.6 |
| Keep down latency | s | 301.83 ± 36.31 | 224.97 ± 38.87 | 319 | 0.25 | 0.051 |
| Allo-grooming duration | s | 7.30 ± 1.24 | 10.57 ± 3.22 | 449 | — | 0.99 |
| Allo-grooming frequency | Fq | 3.97 ± 0.58 | 3.70 ± 0.64 | 420 | — | 0.66 |
Results are presented as mean ± SEM (s, seconds, Fq, total occurrences of behavioral expression during the test). Significant differences between CON and CSI are indicated in bold.
3.3 |. F0 predatory behavior
The latency for the first contact with the cricket did not differ between the groups (CON: 51.33 ± 19.63 s, CSI: 39.38 ± 4.49 s; U = 127.5, p = 0.17), but CSI females attacked the cricket faster after first contact (first contact to attack latencies: CON: 130.33 ± 41.89 s, CSI: 45.62 ± 30.85 s; U = 53.5, p = .04, r = 0.38) and tended to express more attacks (CON: 1.80 ± 0.38, CSI: 2.85 ± 0.36; U = 136.5, p = .07, r = 0.35). Results are illustrated in Figure 2.
FIGURE 2.
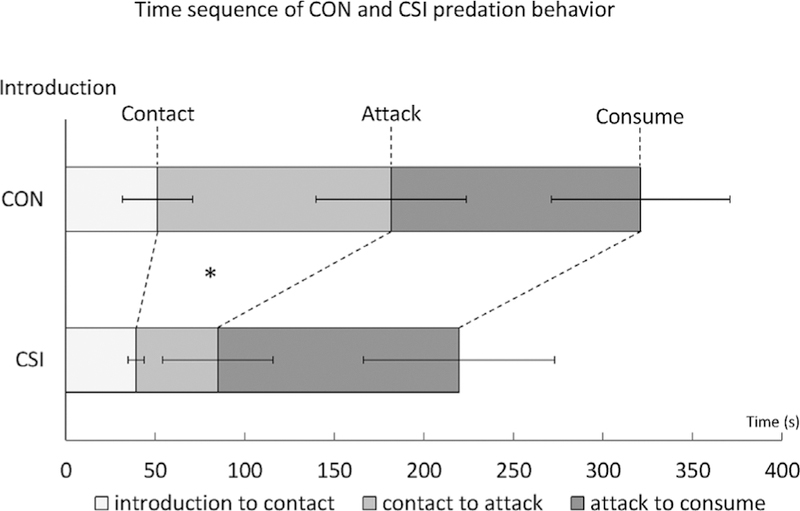
Mean ± SEM duration of the three predation phases: introduction to first contact, first contact to first attack and first attack to initial consumption of the prey. Mann–Whitney U-test, *p < .05
3.4 |. F0 maternal care, milk intake, and maternal aggression
Behavior of CON and CSI during the maternal care test and statistical results are reported in Table 2. After a 60 min mother–pup separation, CON and CSI females did not differ significantly in the time they spent retrieving, grooming, nursing, and nesting during the 30 min maternal care test on PND 2, 9, and 16 (all p’s >.05). The initial retrieving duration (latency to have all pups in nest after reunion) also did not differ between the CON and CSI at PND 2 and 9 (PND 2: CON: 254.81 ± 49.97 s, CSI: 354.0 ± 56.80; U = 75.5, p = 0.15; PND 9: CON: 174.10 ± 42.08, CSI: 329.30 ± 107.25; U = 75.50, p = 0.15). At PND 16, offspring were too mobile to accurately assess retrieving latencies. Overall time spent in nest did not differ between dams of the two sets at PND 2 (CON: 1,132.6 ± 68.44 s, CSI: 996.8 ± 105.17 s, U = 38.0, p = 0.39) and PND 9 (CON: 1,260.0 ± 78.51, CSI: 1098.1 ± 143.98; U = 46, p = 0.80) and that variable was not included in the analysis of PND 16 since a consistent nest could not be identified. Other behaviors (self-grooming, locomotion, exploration, and eating) did not differ significantly between CON and CSI (all p’s >.05).
TABLE 2.
Results of maternal care test of CON (N = 10) and CSI (N = 10) at PND 2, 9, and 16
| Unit | CON | CSI | U | p | |
|---|---|---|---|---|---|
| PND 2 | |||||
| Retrieving duration | s | 39.00 ± 9.75 | 46.50 ± 10.39 | 60.5 | 0.44 |
| Grooming duration | s | 208.70 ± 26.46 | 179.30 ± 34.84 | 43.0 | 0.63 |
| Nesting duration | s | 286.90 ± 36.94 | 191.40 ± 31.13 | 26.0 | 0.08 |
| Nursing duration | s | 679.70 ± 84.70 | 594.10 ± 130.19 | 44.0 | 0.68 |
| PND 9 | |||||
| Retrieving duration | s | 21.60 ± 5.12 | 35.1 ± 12.85 | 59.5 | 0.48 |
| Grooming duration | s | 404.40 ± 65.01 | 362.80 ± 85.90 | 41.0 | 0.53 |
| Nesting duration | s | 87.10 ± 24.35 | 102.60 ± 35.13 | 50.0 | >0.99 |
| Nursing duration | s | 961.80 ± 118.14 | 915.60 ± 154.29 | 50.0 | >0.99 |
| PND 16 | |||||
| Retrieving duration | s | 6.70 ± 2.42 | 3.70 ± 1.56 | 40.5 | 0.48 |
| Grooming duration | s | 304.80 ± 49.97 | 253.10 ± 37.65 | 35.0 | 0.28 |
| Nesting duration | s | 16.70 ± 8.03 | 42.50 ± 11.34 | 64.0 | 0.32 |
| Nursing duration | s | 896.10 ± 164.26 | 798.80 ± 136.51 | 37.0 | 0.35 |
Results are presented as mean ± SEM (s, seconds).
Milk intake over a 120 min reunion of F1 juveniles following a 60 min separation from the mother did not differ between CSI’s and CON’s litters (D2: CON: 1.41 ± 0.23 g, CSI: 1.06 ± 0.19 g; U = 34.0, p = 0.23; D9: CON: 5.36 ± 0.42 g, CSI: 4.61 ± 0.98 g; U = 43.5, p = 0.62; D16: CON: 3.05 ± 1.30 g, CSI: 4.71 ± 0.87 g; U = 60.0, p = 0.45).
In contrast, maternal care of CON and CSI dams significantly differed during the maternal aggression test. Results are reported in Table 3. After the introduction of the male, CSI females tended to resume maternal activities earlier than did CON females (p = .054). The time spent nursing and nesting did not differ significantly, but CSI females spent more time grooming the pups (p = .035). All the CON and CSI females were aggressive toward the male introduced in their cage and the time devoted to aggressive behaviors did not differ significantly between CON and CSI females (p > .05). Intruder males were observed in the nests of CSI dams for a longer duration compared to CON dams (p = .04), and the time with all pups in nest during this test tended to be shorter in CSI dams (p = .06).
TABLE 3.
Behavior of CON (N = 10) and CSI (N = 10) mothers during the maternal aggression test at PND 2
| Unit | CON | CSI | U | r | p | |
|---|---|---|---|---|---|---|
| Latency to return to maternal care | s | 1538.5 ± 123.11 | 948.5 ± 234.99 | 25 | 0.42 | 0.054 |
| Grooming duration | s | 4.88 ± 3.28 | 18.41 ± 7.17 | 78 | 0.50 | 0.035 |
| Nesting duration | s | 90.16 ± 21.11 | 114.56 ± 33.71 | 52.5 | — | 0.85 |
| Nursing duration | s | 57.39 ± 54.45 | 25.88 ± 34.02 | 50 | — | >0.99 |
| Threat duration | s | 244.49 ± 35.56 | 388.96 ± 65.16 | 72 | — | 0.1 |
| Aggression duration | s | 42.66 ± 8.02 | 40.08 ± 11.15 | 45 | — | 0.74 |
| Keep down duration | s | 12.93 ± 6.00 | 65.14 ± 30.90 | 71 | — | 0.12 |
| Mother in nest duration | s | 899.39 ± 166.63 | 953.07 ± 168.03 | 54 | — | 0.8 |
| All pups in nest duration | s | 1637.26 ± 158.63 | 1298.35 ± 197.38 | 25.5 | 0.41 | 0.06 |
| Males in nest duration | s | 6.63 ± 2.28 | 71.83 ± 46.59 | 77 | 0.45 | 0.04 |
Results are presented as mean ± SEM (s, seconds). Significant differences between CON and CSI are indicated in bold.
3.5 |. F1 development
The weight of pups (measured before separation) during lactation was affected by age (ANOVA: F1,38 = 8.013, p < .001, η2p= 0.97), treatment group (F1,38 = 8.013, p = .01, η2p= 0.18) and there was an interaction between age and treatment (F1,36 = 7.42, p = .01, η2p= 0.17). Overall, offspring of CSI mothers gained weight more slowly than did offspring of CON mothers. These results are illustrated in Figure 3. There were no effects of sex or interactions between sex and other factors (all p’s >.05).
FIGURE 3.
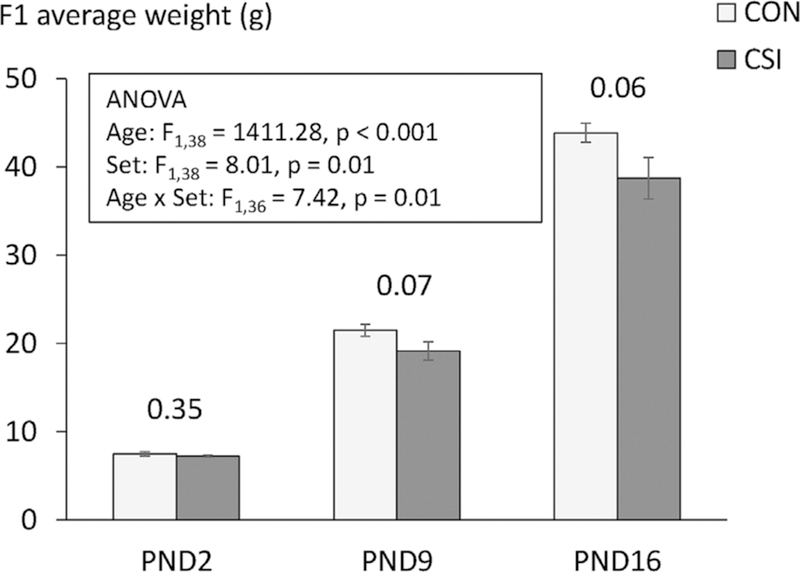
Mean ± SEM weights of F1 pups during the lactation period according to postnatal day (PND) and mother’s treatment. ANOVA revealed a main effect of age, a main effect of mother’s treatment (Set; CON: 24.30 ± 1.98, CSI: 21.70 ± 1.79) and a significant interaction between age and mother treatment due to faster weight gain of F1CON compared to F1CSI. Values on the top of bars refer to Bonferroni post hoc comparisons between F1CON and F1CSI weights at each age
3.6 |. F1 social behavior
During the social approach test, the behavior of F1CON and F1CSI did not differ significantly for time spent proximal to the stimulus (F1CON: 483.03 ± 36.25 s, F1CSI: 498.25 ± 31.17 s, U = 146.5, p = 0.46) or time spent in olfactory investigation of the stimulus (F1CON: 98.61 ± 12.51 s, F1CSI: 94.54 ± 14.26; U = 161.0, p = 0.77). During this phase of social approach, F1CSI expressed more self-grooming behaviors (F1CON: 2.79 ± 0.37, F1CSI: 4.56 ± 0.62, U = 241, p = .034, r = 0.35; Figure 4). The same difference appeared during the social interaction test (F1CON: 2.05 ± 0.39, F1CSI: 3.55 ± 0.61; U = 278.0, p = .035, r = 0.35; Figure 4). The behavior of F1CON and F1CSI oriented toward the intruder did not differ significantly during social interaction (p > .05, see Table 4). Only five individuals expressed aggression during this test and the proportion who expressed aggression did not differ between the two sets (F1CON: 1/20, F1CSI: 4/20, χ2 = 2.06, p = 0.15).
FIGURE 4.
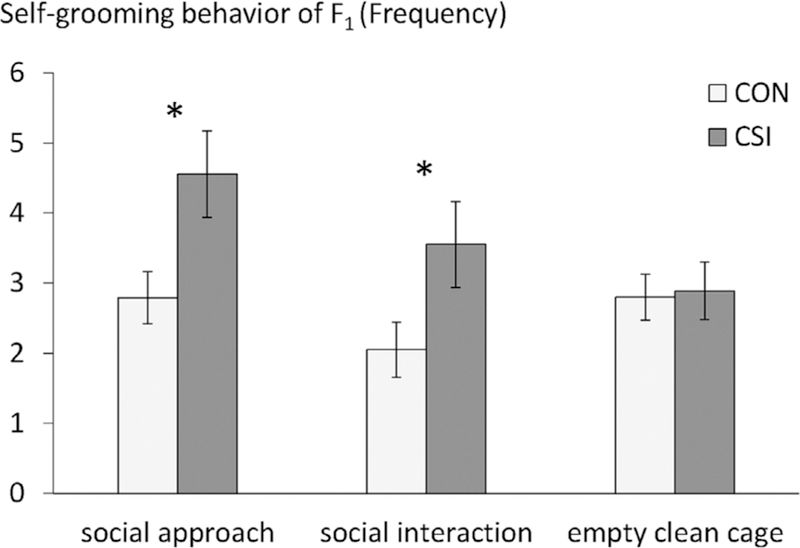
Mean ± SEM frequency of self-grooming in the social approach test of F1 animals, the social interaction test and when exposed to an empty clean cage. Mann–Whitney U-test, *p < .05
TABLE 4.
Behaviors of F1CON (N = 20) and F1CSI (N = 20) expressed during the social interaction test
| Unit | F1CON | F1CSI | U | p | |
|---|---|---|---|---|---|
| Proportion of prosocial moving | % | 43.50 ± 2.67 | 43.55 ± 3.19 | 205.5 | 0.90 |
| Head investigation duration | s | 24.04 ± 3.21 | 19.81 ± 2.31 | 165.0 | 0.36 |
| Head investigation frequency | Fq | 11.40 ± 1.26 | 10.00 ± 1.00 | 172.0 | 0.46 |
| Back/flank investigation duration | s | 15.95 ± 1.77 | 13.88 ± 1.36 | 161.0 | 0.30 |
| Back/flank investigation frequency | Fq | 8.75 ± 0.91 | 7.30 ± 0.61 | 154.5 | 0.22 |
| Ano-genital investigation duration | s | 31.32 ± 3.88 | 47.17 ± 6.81 | 258.0 | 0.12 |
| Ano-genital investigation frequency | Fq | 11.70 ± 1.30 | 15.35 ± 1.94 | 250.0 | 0.18 |
| Aggression duration | s | 0.18 ± 0.18 | 0.95 ± 0.45 | 239.5 | 0.29 |
| Aggression frequency | Fq | 0.10 ± 0.10 | 0.45 ± 0.22 | 239.0 | 0.30 |
| Aggression latency | s | 579.50 ± 20.5 | 552.50 ± 23.15 | 162.5 | 0.31 |
| Keep down duration | s | 0.72 ± 0.53 | 3.68 ± 2.29 | 222.0 | 0.57 |
| Keep down frequency | Fq | 0.25 ± 0.18 | 0.45 ± 0.25 | 219.5 | 0.60 |
| Keep down latency | s | 560.75 ± 27.75 | 544.75 ± 26.41 | 182.0 | 0.64 |
| Allo-grooming duration | s | 6.63 ± 2.55 | 5.30 ± 1.53 | 208.0 | 0.84 |
| Allo-grooming frequency | Fq | 1.70 ± 0.35 | 2.30 ± 0.61 | 206.5 | 0.86 |
Results are presented as mean ± SEM (s, seconds, Fq, Frequency of behaviors expressed during the test). Comparisons were tested using Mann–Whitney U tests.
To determine whether differences between F1CON and F1CSI in their self-grooming behavior only appears in a social context, we compared frequency of self-grooming during the exploration of the empty cage before the social stimulus was introduced and found no differences (F1CON: 2.80 ± 0.33, F1CSI: 2.89 ± 0.41; U = 190, p = 0.78; Figure 4).
3.7 |. F1 predation behavior
The behavior of the F1CON and F1CSI did not differ in the predation test, including the latency of first contact (F1CON: 136.30 ± 41.98 s, F1CSI: 8350 ± 39.97 s, U = 142.5, p = 0.12), the time between first contact and first attack (F1CON: 183.61 ± 58.41 s, F1CSI: 143.56 ± 43.71 s, U = 154.0, p = 0.82), the time between first attack and initial consumption of the prey (F1CON: 232.67 ± 54.90 s, F1CSI: 272.50 ± 61.59 s, U = 120.5, p = 0.51) or the number of attacks (F1CON: 4.0 ± 0.99, F1CSI: 3.30 ± 0.87, U = 180.0, p = 0.60).
4 |. DISCUSSION
This study investigated the influence of social partner instability in female rats during adulthood on subsequent social behavior, maternal care, predatory aggression, and offspring development and behavior. Our results demonstrate that chronic social partner instability has persistent effects on social behavior, maternal aggression, and the morphological and social development of offspring. Females exposed to social instability were less aggressive and displayed more social exploration of unfamiliar female intruders, but were faster to attack a prey. During lactation, CSI dams were less disrupted by exposure to a male intruder; they tended to resume maternal activities more quickly and spent more time grooming the pups. Offspring of females exposed to social instability exhibited impaired growth and displayed robust signs of social anxiety when exposed to unfamiliar conspecifics.
4.1 |. Influence of CSI procedure on adult social and maternal behavior
The social behavior of adult females was affected by CSI procedure but contrary to our initial expectations, there was no global reduction of social behavior by CSI exposed females. A smaller proportion of CSI females expressed aggression toward a female intruder compared with control females. CSI females also devoted less time to aggression and acted more pro-socially than control females. Even though the social challenge applied in our study is fundamentally different from social defeat paradigms, this suppression of aggression by CSI is in accordance with results reported from male studies of social defeat (Blanchard et al., 1995; Blanchard & Caroline, 1989; Meerlo, Overkamp, Daan, Van den Hoofdakker, & Koolhaas, 1996; Sandi & Haller, 2015). CSI females were quicker to attack a cricket than CON females despite similar latencies to make contact with the cricket, suggesting that the reduced aggressiveness of CSI during social interaction is not due to defective sensory-motor skills, known to be critical for prey catching (Kinsley et al., 2014).
Studies of social defeat in male rats report a general reduction of social behavior (Haller et al., 1999). In contrast, we did not observe any reduction in social behavior in CSI females in this study. In social mammals the establishment of bonds with social partners provides a buffering effect, increasing the ability of individuals to cope with stressful environments (Kikusui, Winslow, & Mori, 2006). Stressed animals have been noted to be more attracted to social partners (Kikusui et al., 2006; Taylor, 1981), a result which may be responsible for the higher prosocial behavior expressed by CSI females in our test. In contrast to our results, in female mice exposed to social partner instability during adolescence, Saavedra-Rodríguez and Feig (2013) reported decreased sociality. They nevertheless assessed social behavior by presenting a juvenile to an adult focal mouse, potentially preventing the emergence of differences in prosocial behavior, which may otherwise have appeared between animals of similar ages.
Unexpectedly, we did not find differences in the maternal care expressed by CON and CSI females in our study. We were expecting such alterations given the strong influence of social and stressful experience on maternal care (Gonzalez, Lovic, Ward, Wainwright, & Fleming, 2001; Melo et al., 2006) as well as consistent correlations between social behavior and maternal styles across taxa (fish: Budaev et al., 1999; birds: Pittet, Houdelier, et al., 2014; mammals: Maestripieri, 1993). However, it is hard to compare our results with previous studies since most studies involving prenatal influences of social environment focus on physiological and behavioral consequences on offspring and do not investigate maternal behavior (Kaiser & Sachser, 2005; Siegeler, Sachser, & Kaiser, 2011). Additionally, stressors are usually applied during gestation, a vulnerable developmental period for social behaviors (Braastad, 1998; Takahashi, Baker, & Kalin, 1990), including maternal behavior (Marino, Cronise, Lugo, & Kelly, 2002). Nevertheless, even when applied during pregnancy, chronic social stress does not seem to highly impact postnatal care of female rats (Neumann, Krömer, & Bosch, 2005). Together, these results suggest that social instability during adulthood does not have a major effect on maternal care without the inclusion of an additional social factor, such as a novel male intruder.
The current data indicate that CSI treatment overall did not greatly impact behavior during a maternal aggression test. CSI females threatened and acted aggressively toward an intruder male to a similar degree as control females. However, CSI females did appear somewhat more tolerant to an intruder male than control females, as evidenced by an increased duration of the intruder male in the nest of CSI females and the expression of more maternal care when a male intruder was in the cage compared to control females. We acknowledge the risk for these results to be potentially due to type I errors, but our analysis identified medium to large effect sizes and a higher tolerance of rodent females toward male intrusion following stress was also reported in a different study. Pardon, Gérardin, Joubert, Pérez-Diaz, and Cohen-Salmon (2000) reported decreased maternal aggression and normal sequences of maternal behavior in the “inappropriate situation” of infanticide danger following gestation stress. Exposure to chronic stress has been postulated to induce behavioral and neurophysiological changes that either enhance coping with later acute or short term stressors (Núñez, Ferré, Escorihuela, Tobeña, & Fernández-Teruel, 1996; Tamashiro et al., 2005) or induce exaggerated responses to acute stressors (McEwen, 2007), depending on the severity of initial stress procedure (Anisman, Zaharia, Meaney, & Merali, 1998). In the current study, our results suggest that the CSI procedure reduced the reactivity of females exposed to an intruder during lactation, considered an acute stressor (Neumann et al., 2001). This result highlights that the consequences of social instability can be observed beyond the specific context of female/female interactions in a non-reproductive period. Here, while potentially adaptive in an unstable social environment, the higher tolerance of CSI mothers to intruders appears to be a maladaptive consequence of CSI, considering the high risk of infanticide presented by males (Lonstein & Gammie, 2002).
The behavioral differences observed across context between CSI and CON females suggest a neurophysiological impact of our procedure. First, exposure to chronic social stress can induce depression-like behavior in related studies of stress during lactation (Carini, Murgatroyd, & Nephew, 2013; Murgatroyd et al., 2015). Here, the saccharin preference data indicate that CSI did not induce anhedonia, which is consistent with the lack of effect on maternal care, an ethologically and translationally relevant reward mediated behavior. However endocrine changes such as a disruption of the HPA axis (DeVries, 2002; Haller et al., 1999; Herzog et al., 2009; Johnson & Young, 2015), may have mediated the behavioral responses observed in CSI animals through interaction with the neuropeptides arginine vasopressin (AVP) and oxytocin (OXT) (Champagne, 2010) which can affect the behavioral responses to stressors (Neumann & Landgraf, 2012) and mediate affiliative and aggressive behavior (Nephew, 2012; Nephew & Bridges, 2008).
4.2 |. Intergenerational influence of CSI procedure
The offspring of CSI exposed dams displayed a slight delayed growth throughout lactation suggesting either impaired milk production or let down. A similar delayed growth was reported for offspring of mothers exposed to social stress during lactation (Nephew & Bridges, 2011). The weight gain difference between F1CSI and F1CON was likely too gradual to be detected by a 2 hr milk intake assessment, particularly considering the important inter-individual differences in nursing. F1 offspring of CSI and control mothers did not exhibit changes in aggression behavior in the social interaction test but offspring of CSI mothers expressed more self-grooming behaviors, a powerful index of anxiety (Castles, Whiten, & Aureli, 1999; Spruijt, Van Hooff & Gispen, 1992) in social situations but not during the exploration of a clean cage, known to be stressful for rodents (Castelhano-Carlos & Baumans, 2009; Rasmussen, Miller, Filipski, & Tolwani, 2011). These data indicate that exposure to social instability stimulated prosocial behaviors in the F0 generation while triggering increased social anxiety in the F1, supporting the hypothesis that behavioral consequences of unstable social environments may not always be transmitted in the same direction due to generational differences in the timing and nature of the social instability exposure.
Contrary to our initial hypothesis, the transgenerational consequences of CSI on offspring social behavior were not associated with major maternal behavior impairment. Variations in maternal care are known to have profound consequences on the behavioral development of offspring, particularly concerning social behavior (Pittet, Le Bot, Houdelier, Richard-Yris, & Lumineau, 2014b; Schino, Speranza, & Troisi, 2001; Spokas & Heimberg, 2008). Nevertheless, if slight differences in maternal care might have been undetected by our maternal behavior observation procedure, such discrete differences are unlikely to have mediated the transgenerational consequence of the chronic social instability procedure. Two “silent” alternative mechanisms may have supported the transmission of behavioral consequences to the F1 generation. First, a prenatal effect may have mediated the transmission from F0 to F1. Results from animal and human studies provide a robust foundation on the influence of prenatal stress (intrauterine exposure to high CORT levels) on offspring’s social behavior (Clarke & Schneider, 1993), generally accompanied with lower birth weight (Weinstock, Fride, & Hertzberg, 1988), while we report reduced growth in F1 CSI. A second mechanism to consider is the possibility of epigenetic modifications following the social instability procedure that may have been transmitted through the F0 germ-line. For example, following a chronic mild physical/social stress, Zaidan, Leshem, and Gaisler-Salomon (2013) have reported changes in gene expression of the corticotrophin releasing factor receptor type I (CRF1) not only in maternal brain but also in oocytes and brain of offspring from the two next generations.
5 |. CONCLUSION
This study was designed to investigate the influence of chronic social partner instability in adult females before reproduction, a relatively underexplored period. Our results indicate that adult chronic social instability exposure affects subsequent adult female behavior, including the expression of social and predatory aggression. These results support the existence of socially mediated behavioral plasticity in adults (Champagne, 2010). The presence of effects of social instability on maternal care only during exposure to an intruder demonstrates a unique degree of behavioral specificity. The results concerning maternal aggression additionally support the hypothesis that behavioral modifications following adverse environmental conditions are adaptive in this particular adverse environment but not when there is a substantial mismatch between the development environment and the test environment (Chaby et al., 2015). Finally, our results also indicate that social events, experienced by adult females outside of gestation and lactation, are also likely to present transgenerational consequences on offspring behavior.
ACKNOWLEDGMENTS
This work was supported by NICHD grant R00 HD059943 and National Center for Advancing Translational Sciences, National Institutes of Health, grant UL1TR001064 to B.C.N. and a postdoctoral research grant from the Fyssen Foundation to F.P. We would like to thank the Laboratory Animals Medicine Service of Tufts Cummings School of Veterinary Medicine for its exceptional animal care. We dedicate this work to the memory of Dr. Craig Kinsley, who provided essential guidance and feedback on the predation behavior testing procedure and analysis.
Funding information
NICHD, Grant number: R00 HD059943; National Center for Advancing Translational Sciences; National Institutes of Health, Grant number: UL1TR001064; Fyssen Foundation
REFERENCES
- Anisman H, Zaharia MD, Meaney MJ, & Merali Z (1998). Do early-life events permanently alter behavioraland hormonal responses to stressors? International Journal of Developmental Neuroscience, 16(3–4), 149–164. [DOI] [PubMed] [Google Scholar]
- Archer J (2009). The nature of human aggression. International Journal of Law and Psychiatry, 32(4), 202–208. [DOI] [PubMed] [Google Scholar]
- Babb JA, Carini LM, Spears SL, & Nephew BC (2014). Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Hormones and Behavior, 65(4), 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr NI, Pryce CR, Döbeli M, & Martin RD (1998). Evidence from urinary cortisol that maternal behavior is related to stress in gorillas1 1. Physiology & Behavior, 64(4), 429–437. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, & Kinni V (2006). Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. Journal of Neuroendocrinology, 18(1), 13–24. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, & Sakai RR (1995). Visible burrow system as a model of chronic social stress: Behavioral and neuroendocrine correlates. Psychoneuroendocrinology, 20(2), 117–134. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, & Caroline D (1989). Antipredator defensive behaviors in a visible burrow system. Journal of Comparative Psychology, 103(1), 70–82. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, & Blanchard DC (2001). Animal models of social stress: Effects on behavior and brain neurochemical systems. Physiology & Behavior, 73(3), 261–271. [DOI] [PubMed] [Google Scholar]
- Braastad BO (1998). Effects of prenatal stress on behaviour of offspring of laboratory and farmed mammals. Applied Animal Behaviour Science, 61(2), 159–180. [Google Scholar]
- Brown GW, & Prudo R (1981). Psychiatric disorder in a rural and an urban population: 1. Aetiology of depression. Psychological Medicine, 11(3), 581–599. [DOI] [PubMed] [Google Scholar]
- Budaev SV, Zworykin DD, & Mochek AD (1999). Individual differences in parental care and behaviour profile in the convict cichlid: A correlation study. Animal Behaviour, 58(1), 195–202. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MHP, Veenema AH, Huininga M, de Boer SF, Korte SM, & Koolhaas JM (2005). Long-term effects of social stress on brain and behavior: A focus on hippocampal functioning. Neuroscience & Biobehavioral Reviews, 29(1), 83–97. [DOI] [PubMed] [Google Scholar]
- Carini LM, Murgatroyd CA, & Nephew BC (2013). Using chronic social stress to model postpartum depression in lactating rodents. Journal of Visualized Experiments, (76). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelhano-Carlos MJ, & Baumans V (2009). The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats. Laboratory Animals, 43(4), 311–327. [DOI] [PubMed] [Google Scholar]
- Castles DL, Whiten A, & Aureli F (1999). Social anxiety, relationships and self-directed behaviour among wild female olive baboons. Animal Behaviour, 58(6), 1207–1215. [DOI] [PubMed] [Google Scholar]
- Chaby LE, Sheriff MJ, Hirrlinger AM, & Braithwaite VA (2015). Does early stress prepare individuals for a stressful future? Stress during adolescence improves foraging under threat. Animal Behaviour, 105, 37–45. [Google Scholar]
- Champagne FA (2010). Epigenetic influence of social experiences across the lifespan. Developmental Psychobiology, 52(4), 299–311. [DOI] [PubMed] [Google Scholar]
- Champagne FA, & Curley JP (2005). How social experiences influence the brain. Current Opinion in Neurobiology, 15(6), 704–709. [DOI] [PubMed] [Google Scholar]
- Clarke AS, & Schneider ML (1993). Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Developmental Psychobiology, 26(5), 293–304. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences Hillsdale, NJ: Lawrence Earlbaum Associates. [Google Scholar]
- Cornwell B (2015). Social disadvantage and network turnover. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70(1), 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel S, Dantzer B, Goymann W, & Rubenstein DR (2013). The ecology of stress: Effects of the social environment. Functional Ecology, 27(1), 66–80. [Google Scholar]
- DeVries AC (2002). Interaction among social environment, the hypothalamic-pituitary-adrenal axis, and behavior. Hormones and Behavior, 41(4), 405–413. [DOI] [PubMed] [Google Scholar]
- Earls F (1986). Sex differences in psychiatric disorders: Origins and developmental influences. Psychiatric Developments, 5(1), 1–23. [PubMed] [Google Scholar]
- Elsenbruch S, Benson S, Rücke M, Rose M, Dudenhausen J, Pincus-Knackstedt MK, … Arck PC (2007). Social support during pregnancy: Effects on maternal depressive symptoms, smoking and pregnancy outcome. Human Reproduction, 22(3), 869–877. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Harder JA, Messenger TL, & Febo M (2005). Pup suckling is more rewarding than cocaine: Evidence from functional magnetic resonance imaging and three-dimensional computational analysis. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(1), 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German D, & Latkin CA (2011). Social stability and health: Exploring multidimensional social disadvantage. Journal of Urban Health, 89(1), 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Röcke C, & Lachman ME (2011). Antecedent–consequent relations of perceived control to health and social support: Longitudinal evidence for between-domain associations across adulthood. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 66B(1), 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Lovic V, Ward GR, Wainwright PE, & Fleming AS (2001). Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Developmental Psychobiology, 38(1), 11–32. [DOI] [PubMed] [Google Scholar]
- Haller J, Fuchs E, Halász J, & Makara GB (1999). Defeat is a major stressor in males while social instability is stressful mainly in females: Towards the development of a social stress model in female rats. Brain Research Bulletin, 50(1), 33–39. [DOI] [PubMed] [Google Scholar]
- Herzog CJ, Czéh B, Corbach S, Wuttke W, Schulte-Herbrüggen O, Hellweg R, … Fuchs E (2009). Chronic social instability stress in female rats: A potential animal model for female depression. Neuroscience, 159(3), 982–992. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, & Young LJ (2015). Neurobiological mechanisms of social attachment and pair bonding. Current Opinion in Behavioral Sciences, 3, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, & Sachser N (2005). The effects of prenatal social stress on behaviour: Mechanisms and function. Neuroscience & Biobehavioral Reviews, 29(2), 283–294. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett K (2007). A new paradigm for depression in new mothers: The central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. International Breastfeeding Journal, 2(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB, & Curley JP (2004). Vasopressin, oxytocin and social behaviour. Current Opinion in Neurobiology, 14(6), 777–783. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, & Mori Y (2006). Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 361(1476), 2215–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Blair JC, Karp NE, Hester NW, McNamara IM, Orthmeyer AL, & Lambert KG (2014). The mother as hunter: Significant reduction in foraging costs through enhancements of predation in maternal rats. Hormones and Behavior, 66(4), 649–654. [DOI] [PubMed] [Google Scholar]
- Klein F, Lemaire V, Sandi C, Vitiello S, Van der Logt J, Laurent PE, … Mormède P(1992). Prolonged increase of corticosterone secretion by chronic social stress does not necessarily impair immune functions. Life Sciences, 50, 723–731. [DOI] [PubMed] [Google Scholar]
- Koski SE (2014). Broader horizons for animal personality research. Behavioral and Evolutionary Ecology, 2, 70. [Google Scholar]
- Lonstein JS, & Gammie SC (2002). Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neuroscience & Biobehavioral Reviews, 26(8), 869–888. [DOI] [PubMed] [Google Scholar]
- Maestripieri D (1993). Maternal anxiety in rhesus macaques (Macaca mulatta). Ethology, 95(1), 19–31. [Google Scholar]
- Maestripieri D, Lindell SG, Ayala A, Gold PW, & Higley JD (2005). Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neuroscience & Biobehavioral Reviews, 29(1), 51–57. [DOI] [PubMed] [Google Scholar]
- Marino MD, Cronise K, Lugo JN, & Kelly SJ (2002). Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Developmental Psychobiology, 41(4), 341–351. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, & Pico-Alfonso MA (1998). Social defeat and subordination as models of social stress in laboratory rodents: A review. Aggressive Behavior, 24(4), 241–256. [Google Scholar]
- McCormick CM, Smith C, & Mathews IZ (2008). Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behavioural Brain Research, 187(2), 228–238. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87(3), 873–904. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJF, Daan S, Van den Hoofdakker RH, & Koolhaas JM (1996). Changes in behaviour and body weight following a single or double social defeat in rats. Stress, 1(1), 21–32. [DOI] [PubMed] [Google Scholar]
- Melo AI, Lovic V, Gonzalez A, Madden M, Sinopoli K, & Fleming AS (2006). Maternal and littermate deprivation disrupts maternal behavior and social-learning of food preference in adulthood: Tactile stimulation, nest odor, and social rearing prevent these effects. Developmental Psychobiology, 48(3), 209–219. [DOI] [PubMed] [Google Scholar]
- Minier D, Capitanio JP, Gottlieb D, & McCowan B (2012). One size may not fit all: The importance of taking an individual approach to behavior management. The Enrichment Record, 13, 21–23. [Google Scholar]
- Murgatroyd CA, & Nephew BC (2013). Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology, 38(2), 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Taliefar M, Bradburn S, Carini LM, Babb JA, & Nephew BC (2015). Social stress during lactation, depressed maternal care, and neuropeptidergic gene expression. Behavioural Pharmacology, 26, 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S (2004). A farewell to Bonferroni: The problems of low statistical power and publication bias. Behavioral Ecology, 15(6), 1044–1045. [Google Scholar]
- Nephew BC (2012). Behavioral roles of oxytocin and vasopressin. In Sumiyoshi T (Ed.), Neuroendocrinology and behavior Rijeka, Croatia: InTech. [Google Scholar]
- Nephew BC, & Bridges RS (2008). Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacology Biochemistry and Behavior, 91(1), 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, & Bridges RS (2011). Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress, 14(6), 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Murgatroyd CA, Pittet F, & Febo M (2015). Brain reward pathway dysfunction in maternal depression and addiction: A present and future transgenerational risk. Journal of Reward Deficiency Syndrome, 1(3), 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Krömer SA, & Bosch OJ (2005). Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology, 30(8), 791–806. [DOI] [PubMed] [Google Scholar]
- Neumann ID, & Landgraf R (2012). Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends in Neurosciences, 35(11), 649–659. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Toschi N, Ohl F, Torner L, & Krömer SA (2001). Maternal defence as an emotional stressor in female rats: Correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. European Journal of Neuroscience, 13(5), 1016–1024. [DOI] [PubMed] [Google Scholar]
- Numan M, & Insel TR (2003). The neurobiology of parental behavior Springer Science & Business Media. [Google Scholar]
- Núñez JF, Ferré P, Escorihuela RM, Tobeña A, & Fernández-Teruel A (1996). Effects of postnatal handling of rats on emotional, HPA-Axis, and prolactin reactivity to novelty and conflict. Physiology & Behavior, 60(5), 1355–1359. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2011). The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. The Journal of Comparative Neurology, 519(18), 3599–3639. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, & McCabe JE (2013). Postpartum depression: Current status and future directions. Annual Review of Clinical Psychology, 9(1), 379–407. [DOI] [PubMed] [Google Scholar]
- Olsson IAS, & Westlund K (2007). More than numbers matter: The effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Applied Animal Behaviour Science, 103(3–4), 229–254. [Google Scholar]
- Palanza P (2001). Animal models of anxiety and depression: How are females different? Neuroscience & Biobehavioral Reviews, 25(3), 219–233. [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, & Parmigiani S (2001). Social stress in mice: Gender differences and effects of estrous cycle and social dominance. Physiology & Behavior, 73(3), 411–420. [DOI] [PubMed] [Google Scholar]
- Pardon M-C, Gérardin P, Joubert C, Pérez-Diaz F, & Cohen-Salmon C (2000). Influence of prepartum chronic ultramild stress on maternal pup care behavior in mice. Biological Psychiatry, 47(10), 858–863. [DOI] [PubMed] [Google Scholar]
- Perry BL (2006). Understanding social network disruption: The case of youth in foster care. Social Problems, 53(3), 371–391. [Google Scholar]
- Pittet F, Houdelier C, de Margerie E, Le Bot O, Richard-Yris M-A, & Lumineau S (2014a). Maternal styles in a precocial bird. Animal Behaviour, 87, 31–37. [Google Scholar]
- Pittet F, Le Bot O, Houdelier C, Richard-Yris M-A, & Lumineau S (2014b). Motherless quail mothers display impaired maternal behavior and produce more fearful and less socially motivated offspring. Developmental Psychobiology, 56(4), 622–634. [DOI] [PubMed] [Google Scholar]
- Plaisier I, de Bruijn JGM, de Graaf R, ten Have M, Beekman ATF, & Penninx BWJH (2007). The contribution of working conditions and social support to the onset of depressive and anxiety disorders among male and female employees. Social Science & Medicine, 64(2), 401–410. [DOI] [PubMed] [Google Scholar]
- Proudfoot KL, Weary DM, & von Keyserlingk MAG (2012). Linking the social environment to illness in farm animals. Applied Animal Behaviour Science, 138(3–4), 203–215. [Google Scholar]
- Rasmussen S, Miller MM, Filipski SB, & Tolwani RJ (2011). Cage change influences serum corticosterone and anxiety-like behaviors in the mouse. Journal of the American Association for Laboratory Animal Science: JAALAS, 50(4), 479–483. [PMC free article] [PubMed] [Google Scholar]
- Rault J-L (2012). Friends with benefits: Social support and its relevance for farm animal welfare. Applied Animal Behaviour Science, 136(1), 1–14. [Google Scholar]
- Rosenthal R (1994). Parametric measures of effect size. In Cooper H, & Hedges LV (Eds.), The handbook of research synthesis (pp. 231–244). New York, NY: Russell Sage Foundation. [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, & Havemann-Reinecke U (2005). Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behavioural Brain Research, 162(1), 127–134. [DOI] [PubMed] [Google Scholar]
- Saavedra-Rodríguez L, & Feig LA (2013). Chronic social instability induces anxiety and defective social interactions across generations. Biological Psychiatry, 73(1), 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, & Haller J (2015). Stress and the social brain: Behavioural effects and neurobiological mechanisms. Nature Reviews Neuroscience, 16(5), 290–304. [DOI] [PubMed] [Google Scholar]
- Schino G, Speranza L, & Troisi A (2001). Early maternal rejection and later social anxiety in juvenile and adult Japanese macaques. Developmental Psychobiology, 38(3), 186–190. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Holly EN, Boyson CO, DeBold JF, & Miczek KA (2014). Individual differences in anhedonic and accumbal dopamine responses to chronic social stress and their link to cocaine self-administration in female rats. Psychopharmacology, 232(4), 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegeler K, Sachser N, & Kaiser S (2011). The social environment during pregnancy and lactation shapes the behavioral and hormonal profile of male offspring in wild cavies. Developmental Psychobiology, 53(6), 575–584. [DOI] [PubMed] [Google Scholar]
- Spokas M, & Heimberg RG (2008). Overprotective parenting, social anxiety, and external locus of control: Cross-sectional and longitudinal relationships. Cognitive Therapy and Research, 33(6), 543–551. [Google Scholar]
- Spruijt BM, Van Hooff JA, & Gispen WH (1992). Ethology and neurobiology of grooming behavior. Physiological Reviews, 72(3), 825–852. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, & Feinn R (2012). Using effect size—Or why the P value is not enough. Journal of Graduate Medical Education, 4(3), 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Baker EW, & Kalin NH (1990). Ontogeny of behavioral and hormonal responses to stress in prenatally stressed male rat pups. Physiology & Behavior, 47(2), 357–364. [DOI] [PubMed] [Google Scholar]
- Tamashiro KLK, Nguyen MMN, & Sakai RR (2005). Social stress: From rodents to primates. Frontiers in Neuroendocrinology, 26(1), 27–40. [DOI] [PubMed] [Google Scholar]
- Tamres LK, Janicki D, & Helgeson VS (2002). Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Personality and Social Psychology Review, 6(1), 2–30. [Google Scholar]
- Taylor G (1981). Fear and affiliation in domesticated male-rats. Journal of Comparative and Physiological Psychology, 95(5), 685–693. [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung R,A, & Updegraff JA (2000). Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411–429. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Landeros RV, Knoblauch NW, Takahashi EY, Silva AL, & Crean KK (2011). Sex differences in social interaction behavior following social defeat stress in the monogamous california mouse (Peromyscus californicus). PLoS ONE, 6(2), e17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walen HR, & Lachman ME (2000). Social support and strain from partner, family, and friends: Costs and benefits for men and women in adulthood. Journal of Social and Personal Relationships, 17(1), 5–30. [Google Scholar]
- Waples KA, & Gales NJ (2002). Evaluating and minimising social stress in the care of captive bottlenose dolphins (Tursiops aduncus). Zoo Biology, 21(1), 5–26. [Google Scholar]
- Weinstock M, Fride E, & Hertzberg R (1988). Prenatal stress effects on functional development of the offspring. Progress in Brain Research, 73, 319–331. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, & Sapolsky RM (2003). Reproduction and resistance to stress: When and how. Journal of Neuroendocrinology, 15(8), 711–724. [DOI] [PubMed] [Google Scholar]
- Zaidan H, Leshem M, & Gaisler-Salomon I (2013). Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biological Psychiatry, 74(9), 680–687. [DOI] [PubMed] [Google Scholar]


