Abstract
Extracellular nucleotides might influence aspects of the biology of reproduction in that ATP affects smooth muscle contraction, participates in steroidogenesis and spermatogenesis, and also regulates transepithelial transport, as in oviducts. Activation of cellular nucleotide purinergic receptors is influenced by four plasma membrane-bound members of the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family, namely NTPDase1, NTPDase2, NTPDase3, and NTPDase8 that differ in their ecto-enzymatic properties. The purpose of this study was to characterize the expression profile of the membrane-bound NTPDases in the murine female and male reproductive tracts by immunological techniques (immunolabelling, Western blotting) and by enzymatic assays, in situ and on tissue homogenates. Other than the expected expression on vascular endothelial and smooth muscle cells, NTPDase1 was also detected in Sertoli cells and interstitial macrophages in testes, in ovarian granulosa cells, and in apical cells from epididymal epithelium. NTPDase2 was largely expressed by cells in the connective tissue; NTPDase3 in secretory epithelia, and finally, NTPDase8 was not detected in any of the tissues studied here. In addition, NTPDase6 was putatively detected in Golgi-phase acrosome vesicles of round spermatids. This descriptive study suggests close regulation of extracellular nucleotide levels in the genital tract by NTPDases that may impact specific biological functions.
Keywords: NTPDases, P2 receptors, ATP, Female, Male
Introduction
Extracellular nucleotides (ATP, ADP, UTP, and UDP), as well as the derivative nucleosides (e.g. adenosine), serve as autocrine and/or paracrine signalling molecules, acting on receptors collectively named purinoceptors. Nucleotides act on two major widely expressed receptor subfamilies: P2X receptors, which are ligand-gated channels, and P2Y G-protein-coupled receptors (reviewed in Boeynaems et al. 2005; Burnstock 2006a). Adenosine also triggers signal transduction that involves A1, A2A, A2B and/or A3 receptors (reviewed in Benarroch 2008).
The activation of nucleotide receptors, also called purinoceptors, is influenced by cell surface ectonucleotidases that modulate the concentration of their ligands. Amongst these, the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family constitutes the most abundant one and includes four plasma membrane bound members: NTPDase1, NTPDase2, NTPDase3, and NTPDase8 (Robson et al. 2006; Yegutkin 2008; Zimmermann 2001). These all hydrolyze nucleoside triphosphates and diphosphates; mainly differing in the hydrolysis rates for nucleoside diphosphates. Whereas NTPDase1 (CD39) dephosphorylates ATP and ADP at a similar rate, NTPDase3 and 8 degrade ATP with a significant preference over ADP, and NTPDase2 strongly prefers nucleoside triphosphates over diphosphates (Robson et al. 2006). In mammals, these enzymes are differentially expressed. NTPDase1 is expressed by leukocytes (Kansas et al. 1991; Pulte et al. 2007), vascular endothelial cells (Enjyoji et al. 1999; Kaczmarek et al. 1996), smooth muscle cells (Sévigny et al. 1997, 2002), dendritic cells (Mizumoto et al. 2002), and in a variety of other cell types (reviewed in Robson et al. 2006). NTPDase2 is particularly associated with the adventitial surface of blood vessels (Sévigny et al. 2002), type I cells of taste buds (Bartel et al. 2006), and with different types of glial cells (Braun et al. 2004; Wink et al. 2006). NTPDase3 is expressed by neurons in the brain (Belcher et al. 2006) and the last cloned and characterized plasma membrane NTPDase, NTPDase8, is predominantly expressed by hepatocytes in bile canaliculae (Bigonnesse et al. 2004; Fausther et al. 2007).
There is growing evidence that extracellular nucleotides are involved in the regulation of the physiology of the reproductive system as these have been demonstrated to influence diverse aspects of the biology of reproduction (Burnstock 2007). ATP plays a role in several functions requiring smooth muscle contraction, such as in penile erection, ejaculation, and lactation (reviewed in Burnstock 2006b). It also participates in endocrine responses, increasing testosterone secretion by Leydig cells (Foresta et al. 1996), modulating the response to follicle-stimulating hormone by Sertoli cells (Filippini et al. 1994; Ko et al. 2003), and participating in the ovarian follicle cycle (Park et al. 2003, Tai et al. 2005). Spermatogenesis involves differential P2X receptor subtype expression (Glass et al. 2001) and exogenous ATP has been reported to improve human sperm motility (Edwards et al. 2007). ATP also regulates transepithelial transport in oviductal epithelium (Keating and Quinlan 2008) while changes in the expression pattern of purinergic receptor were observed in the uterus during implantation (Slater et al. 2002) and pregnancy (Khanam and Burnstock 2007; Slater et al. 2000). It is noteworthy that enhanced plasma ATP levels with associated decreases in ecto-apyrase activity in tissue-samples were found in pregnant women experiencing preeclampsia (Bakker et al. 2007), a leading cause of maternal and infant illness and death.
Therefore, expression of ectonucleotidases, such as NTPDases could presumably regulate nucleotide-mediated signalling within the reproductive system. However, aside from the documented ATPase activity in Sertoli cells (Barbacci et al. 1996; Casali et al. 2001; Zamoner et al. 2006), there is a lack of information about NTPDase expression and activity in this system. The aim of the present work was to characterize the expression profile and enzymatic activity of the plasma membrane-bound members of the E-NTPDase family in the murine female and male reproductive tracts.
Materials and methods
Animals and antibody production
This study was carried out in accordance with the guidelines of the Institutional Ethical Committee for Experimental Animals. Entpd1−/− mice (Enjyoji et al. 1999), originally from 129 SVJ × C57BL/6 background, were backcrossed seven generations onto C57BL/6 background. Mice and Sprague–Dawley rats were killed and the tissues biopsied.
For antibody production (mN1-2C and mN3-3C), Hartley guinea pigs were obtained from Charles River Laboratories (Québec, QC, Canada) and genetic immunization was carried out with plasmids (pcDNA3.1) encoding each of the mouse proteins.
Antibodies
Unless indicated otherwise, all the primary antibodies used in this study have been previously characterized and validated: rabbit C9F (Enjyoji et al. 1999; Heine et al. 2001) and guinea pig mN1-2C (characterized here) to mouse NTPDase1, rabbit BZ3-4F to rat (Dranoff et al. 2002; Sévigny et al. 2002; Vlajkovic et al. 2004) and mN2-36L to mouse (Bartel et al. 2006) NTPDase2, guinea pig mN3-3C (characterized here) to mouse NTPDase3, guinea pig rN8-8C to rat NTPDase8 (Fausther et al. 2007), as well as the monoclonal antibody to CD31 (also called PECAM, a marker of endothelial cells; BD Pharmingen, San Diego, CA, USA) and rat monoclonal antibody to F4/80 (a macrophage marker; clone A3-1, Serotec Ltd, Oxford, UK). Secondary antibodies used were: biotin-conjugated goat anti-rabbit, goat anti-guinea pig (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) and goat anti-rat (Vector Laboratories Inc., Burlington, ON, Canada); horseradish peroxidase-conjugated donkey anti-rabbit (Jackson ImmunoResearch Laboratories Inc.) and goat anti-guinea pig (Santa Cruz Biotechnology, Santa Cruz, CA, USA); Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 594 goat anti-guinea pig (Invitrogen, Burlington, ON, Canada).
ATPase and ADPase activity measurement
Tissues were homogenised with a polytron in the following buffer: 95 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride (PMSF) and 45 mM Tris–HCl, pH 7.6. The homogenates were centrifuged at 850 g for 5 min and the resulting supernatants used for ATPase and ADPase activity measurements. The enzyme activity of protein extract was measured at 37°C in 0.5 ml of 5 mM CaCl2, 80 mM Tris buffer pH 7.4 as described previously (Kukulski et al. 2005; Sévigny et al. 1997). Briefly, reaction was started with 0.5 mM substrate (ATP or ADP), stopped after 20 min by the addition of 0.125 ml of malachite green reagent, and the liberated inorganic phosphate was measured at 630 nm according to Baykov et al. (1988). The results were expressed as nmols Pi/min/mg protein. All experiments were performed in triplicate with appropriate controls.
Statistical analysis was performed with Statgraphics Plus v5.1 (StatPoint, Inc, Herndon, VA), which also included means and standard errors of the means. For each tissue the significance of differences in means between two groups was determined by the unpaired Student t test. Significance level was set at p < 0.05.
Cell transfection and Western blot
COS-7 cells were cultured and transiently transfected with mouse NTPDase1, NTPDase2 or NTPDase3 cDNA constructs as described previously (Kukulski et al. 2005). For Western blot assays, cell protein homogenates and tissue particulate fractions, obtained by further centrifuging the homogenates at 100,000 g for 45 min, were resuspended in NuPAGE lithium dodecyl sulfate sample buffer (Invitrogen, Burlington, ON, Canada). Particulate protein fraction corresponding to 100 μg of protein homogenates or 5 μg of protein from lysates of transfected cells, were added per lane, separated on NuPAGE 4–12% Bis-Tris gels (Invitrogen, Burlington, ON, Canada) under nonreducing conditions, according to Laemmli (1970), and transferred to an Immobilon-P membrane (Millipore, Bedford, MA, USA) by electroblotting according to the manufacturer’s recommendation. Membranes were then blocked with 2.5% nonfat milk in PBS containing 0.15% Tween®20 (pH 7.4) O/N at 4°C and subsequently probed by incubation with the primary antibodies. Appropriate secondary horseradish peroxidase-conjugated antibodies were used, and the membranes developed with the Western Lightning™ Plus-ECL system (PerkinElmer Life and Analytical Sciences, CT, USA).
Immunohistochemistry, immunofluorescence and enzyme histochemistry
For histochemical studies, freshly dissected tissues were embedded in O.C.T. freezing medium (Tissue-Tek®, Sakura Finetk, USA) and snap-frozen in isopentane in dry ice and stored at −80°C until used. Sections of 6 μm were prepared and fixed in 10% phosphate-buffered formalin mixed with cold acetone (Fisher Scientific, Ottawa, ON, Canada). Alternatively, the testes were excised, fixed with 4% paraformaldehyde and immersed in sucrose before being included in O.C.T. freezing medium. Immunohistochemistry (peroxidase-based activity) and immunofluorescence experiments were performed as previously described (Dranoff et al. 2002; Fausther et al. 2007; Sévigny et al. 2002). Briefly, tissue sections or fixed cells grown on coverslips were incubated O/N at 4°C with the indicated primary antibodies and then with the appropriate secondary antibodies. Preimmune sera were routinely included as controls for the immunolabelling experiment.
Localization of ectonucleotidase activities was determined using the Wachstein/Meisel lead phosphate method (Braun et al. 2003). Fixed slices were preincubated for 30 min at RT in 50 mM Tris–maleate buffer, pH 7.4, containing 2 mM CaCl2, 250 mM sucrose, and 2.5 mM levamisole, as an inhibitor of alkaline phosphatase. Enzymatic reaction was performed for 1 h at 37°C in the same buffer supplemented with 5 mM MnCl2, 2 mM Pb(NO3)2, 3% Dextran T-250 and in the presence of 200 μM ATP or ADP as substrate. For control experiments the substrate was either omitted or added in the absence of CaCl2 and MnCl2 as source of divalent cations needed for the NTPDase’s enzymatic function, and in presence of 2 mM EDTA. The reaction was revealed by incubation with 1% (NH4)2S v/v for exactly 1 min.
Samples were counterstained with aqueous haematoxylin or DAPI, mounted with Mowiol mounting medium and observed and photographed under a BX51 Olympus microscope.
Results
ATPase and ADPase biochemical activities in tissue homogenates
As shown in Table 1, high levels of ATPase activity were detected in the male tissue homogenates, with no significant changes in Entpd1−/− mice. In contrast, specific ADPase activity was substantially diminished in these mice, by about 30%. Epididymal biopsies displayed the highest ATPase activity among all the tissues analysed here.
Table 1.
ATPase and ADPase activities in mouse tissue homogenates of wild type and Entpd−/− mice
| Tissues | Wild-type mice
|
Entpd1−/− mice
|
||||
|---|---|---|---|---|---|---|
| ATP | ADP | ATP/ADP | ATP | ADP | ATP/ADP | |
| Epididymis | 818 ± 48 | 116 ± 16 | 7.4 ± 1.4 | 870 ± 78 | 91 ± 9* | 9.7 ± 0.6 |
| Testis | 333 ± 14 | 64 ± 2 | 5.2 ± 0.1 | 318 ± 20 | 42 ± 5* | 7.7 ± 0.4 |
| Uterus | 154 ± 20 | 72 ± 16 | 2.2 ± 0.1 | 123 ± 13 | 17 ± 2* | 7.4 ± 0.9* |
| Ovary | 171 ± 22 | 37 ± 2 | 4.7 ± 0.9 | 112 ± 8* | 8 ± 2* | 15.7 ± 2.8* |
| Oviduct | 292 ± 1 | 118 ± 11 | 2.5 ± 0.2 | 115 ± 13* | 16 ± 1* | 7.4 ± 0.7* |
Results are the means ± standard deviations of three to five experiments on tissues from different animals, each in triplicate, and are expressed as nmols Pi/min/mg protein
Significant difference between the NTPDase1 deficient and the wild type samples with p < 0.05
In the female genital tract, ATPase activity was significantly decreased in oviducts of Entpd1−/− mice. Changes in ADPase activities were more pronounced with significantly lowest activity in all Entpd1−/− tissues analysed. Note that the oviducts displayed the highest ADPase activity in WT mice but were amongst the lowest in Entpd1−/− mice.
The ratios of ATPase and ADPase activities increased in the Entpd1−/− tissues, also supporting the view that NTPDase1 is the dominant ADPase.
Generation and specificity of NTPDases antibodies
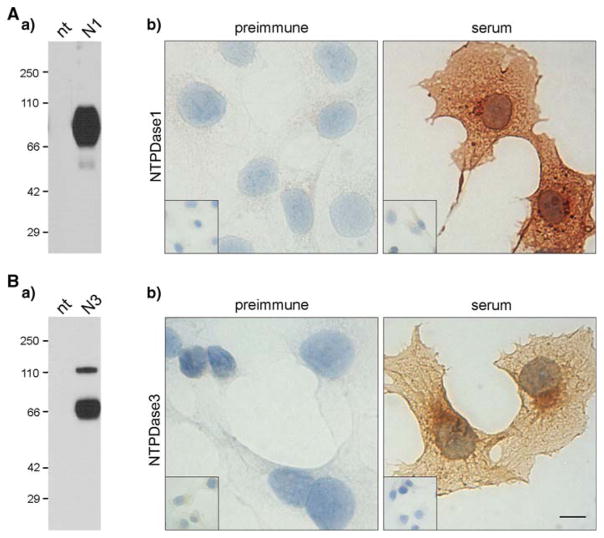
Specificity of polyclonal antibodies to mouse NTPDase1 (mN1-2C) and NTPDase3 (mN3-3C), further used in this work for immunolabelling experiments, was clearly established by Western blot (Fig. 1Aa, Ba) and immunocytochemistry (Fig. 1Ab, Bb).
Fig. 1.
Specificity of the antibodies to mouse NTPDases. Specificity of the polyclonal sera to (A) mouse NTPDase1 (mN1-2C) and (B) mouse NTPDase3 (mN3-3C) were tested by Wes000000tern blot (a) and immunocytochemistry (b). a Western blot on 5 μg of protein extract from COS-7 cells transfected or not (nt) with plasmid encoding for mouse NTPDase1 (N1) or NTPDase3 (N3). Specific bands were detected at the expected molecular weight in the NTPDase-transfected cell samples for both antibodies while no band was revealed in the nontransfected ones. Molecular weights are indicated in kDa (left). Note that the weak band at about 55 kDa (Aa) corresponds to a truncated form of NTPDase1 that is usually observed for the enzyme (Lemmens et al. 2000; Schulte am Esch et al. 1999) and that mN3-3C antibody recognized the NTPDase3 in the form of both monomer and dimer (Ba). b Immunocytochemistry on transfected COS-7 cells with plasmid encoding for mouse NTPDase1 or NTPDase3 and nontransfected cells. A specific staining for both antibodies was obtained with sera on transfected cells (serum) while no staining was detected with the preimmune sera (preimmune). Insets show the absence of immunoreactivity in nontransfected COS-7 cells with either the specific or the preimmune sera. Scale bar 10 μm. Insets: four-time lower magnification
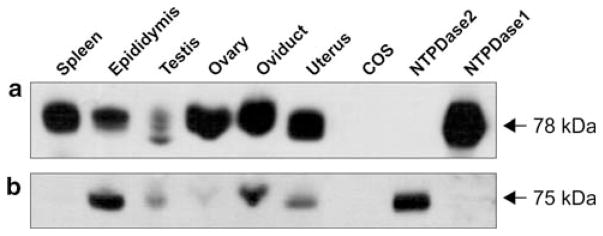
NTPDase1 and 2 protein expression by Western blot
All the tissues analysed expressed NTPDase1, especially those from the female reproductive system (Fig. 2a). A single specific band of 78 kDa was detected in all the tissues analysed except for the testes in which two additional bands, slightly smaller, could be seen. These bands may come from different post-translational modifications in this tissue, such as different level of glycosylation. NTPDase2 was mainly detected in the samples from the male reproductive system, especially in epididymis. The specific 75-kDa band also appeared in the tissues of the female reproductive system, although very faintly in the ovaries (Fig. 2b). Due to probably both, low expression of NTPDase3 and weak binding of this antibody in the presence of SDS, Western blot of NTPDase3 was not successful in these conditions.
Fig. 2.
Western blot showing expression of NTPDase1 (a) and NTPDase2 (b) in mouse tissue membrane fractions. Primary antibodies used were C9F and mN2-36L, respectively. Particulate protein fraction corresponding to 100 μg of protein homogenates or 5 μg of proteins from cell lysates was added per lane. a Spleen and protein extracts of NTPDase1-transfected COS-7 cells were used as positive controls. b Protein extracts of NTPDase2-transfected COS-7 cells were used as positive control. Nontransfected COS-7 cells (COS) were used as negative controls for both antibodies
NTPDases immunolocalization and enzymatic histochemistry
NTPDase1, NTPDase2, and NTPDase3 were detected in murine male and female reproductive tissues. Unless indicated otherwise this section describes the results obtained in mouse. For NTPDase8, due to the higher specificity of the antibody to the rat protein (Fausther et al. 2007), all the experiments were done in this species; however, as NTPDase8 was not detected either in male or female reproductive tracts it will therefore not be further commented on.
Female reproductive system
Ovaries
NTPDase1 labelling was detected in the granulosa of the largest follicles, mainly being preovulatory and corpora lutea (Fig. 3) and also in blood vessels, especially abundant in the ovarian medullae. NTPDase2 was detected in the ovarian connective tissue. The zona pellucida was nonspecifically stained. No NTPDase3 staining could be detected in these organs (data not shown).
Fig. 3.
Immunolocalization of NTPDase1 (N1), NTPDase2 (N2), NTPDase3 (N3), and ATPase enzyme histochemistry (ATP) in the mouse female reproductive organs. In the ovary (left column) NTPDase1 labelling was especially intense in the granulosa of some larger follicles (asterisk), where ATPase activity was also specifically detected. NTPDase2 was immunolocalized upon connective tissue cells between follicles. In the oviduct (middle column) NTPDase1 labelling was found in blood vessels (arrows) and in the cells of the smooth muscle layer (bar). NTPDase2 was immunolocalized at the connective tissue cells from the lamina propria, surrounding blood vessels and muscle fibres. ATPase activity was detected in these structures and also in the apical side of the epithelium where NTPDase3 expression was abundant (arrow heads). In the uterus (right column), NTPDase1 immunolabelling was detected in endometrial blood vessels (arrows) as well as within the myometrium (bar) where intense ATPase activity was also revealed. NTPDase2 was immunolocalized at the connective tissue cells lining the endometrial glands (arrow heads) and surrounding the myometrial muscle fibres. Anti-CD31 labelling was used as a marker for endothelial cells of blood vessels in uterus. Nuclei were counterstained with Haematoxylin. Scale bar 50 μm
In agreement with the expression pattern of NTPDase1 and NTPDase2 described above, ATPase activity was detected in granulosa cells of large follicles, and in blood vessels (Fig. 3).
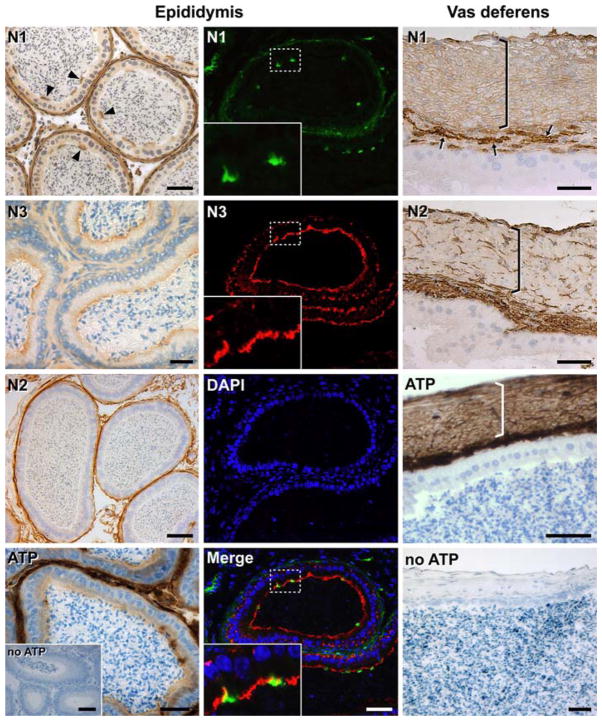
Oviducts
NTPDase1 was detected in smooth muscle cells of the tunica muscularis (Fig. 3) as well as in blood vessels’ muscular layer of oviductal serosa (not shown). NTPDase1 was also detected in the endothelium of blood vessels of all the tissue, as confirmed with the anti-CD31 staining (not shown), being especially evident in the lamina propria mucosae. NTPDase2 expression was detected in the connective tissue and NTPDase3 at the apical pole of the epithelial cells lining the lumen of the oviducts.
ATPase activity was detected in all structures and cells stained with NTPDase1, 2, and 3 antibodies (Fig. 3).
Uterus
Strong expression of NTPDase1 was detected on uterine blood vessel endothelium (Fig. 3); an observation that was confirmed with the anti-CD31 staining. Smooth muscle cells from the myometrium were also NTPDase1-positive. NTPDase2 staining was mainly associated with endometrial connective tissue, lining glands and surrounding myometrial muscle fibres (Fig. 3). No NTPDase3 staining could be detected (data not shown).
Again, ATPase activity was detected in all the NTPDase1 and NTPDase2-positive structures reported above (Fig. 3).
Male reproductive system
Testes
NTPDase1 labelling was detected in myoepithelial cells surrounding seminiferous tubules as well as in blood vessels of the interstitial tissue. Select cells within the interstitial tissue were also positive (Fig. 4). Immunostaining in serial sections with the macrophage marker F4/80 showed that these cells were interstitial macrophages. In addition, NTPDase1 was detected in the seminiferous epithelium, coinciding with the diffuse distribution of Sertoli cell plasma membranes. NTPDase2 labelling was detected in the connective tissue surrounding interstitial blood vessels, in peritubular cells and also in Sertoli cells. No NTPDase3 staining was detected (data not shown). Comparable results were also obtained in rat testis (data not shown).
Fig. 4.
Immunolocalization of NTPDase1 (N1), NTPDase2 (N2) and enzyme histochemistry (ATP, GDP) in the testes of wild type or Entpd1−/− (−/−) mice. NTPDase1 was immunodetected within blood vessels, on myoepithelial cells surrounding the seminiferous tubules and on Sertoli cells (N1 inset; 100×; note distinctive large irregularly shaped nucleus with apparent nucleolus); the absence of immunolabelling in Entpd1−/− testes demonstrated the specificity of the antibody (N1 −/− inset). All these structures displayed an intense ATPase activity (ATP in right column) while no signal was seen in absence of substrate (no ATP, inset). Serial section assays indicated that some cells at the interstitial tissue displaying ATPase activity were NTPDase1-positive (left column N1 and ATP rectangles); these cells were identified as interstitial macrophages by serial staining using the anti-NTPDase1 and anti-F4/80 antibodies (left column N1 and F4/80 rectangles). NTPDase2 was immunolocalized to peritubular cells (N2), coinciding with the intense ATPase activity still present in Entpd1−/− testes (ATP −/−). NTPDase2 was also immunolocalized in Sertoli cells (N2 inset; 100×). In addition, ATPase activity was seen in the Golgiphase acrosome vesicles of the round spermatids (arrow heads), being more intense with GDP (GDP −/− inset) as substrate. Nuclei were counterstained with Haematoxylin. Black scale bar 50 μm. White scale bar 10 μm
ATPase activity was detected in interstitial tissue, surrounding seminiferous tubules, and into the seminiferous epithelium, coinciding with NTPDase1 and NTPDase2 labelling (Fig. 4). Activity pattern was similar when using ADP as a substrate (data not shown). In addition to the activities corresponding to NTPDase1 and NTPDase2 immunolabelling, brown lead deposits were abundantly found in association with the round spermatids. This activity was also detected in Entpd1−/− mice (Fig. 4) albeit more intense when UDP (not shown), and especially GDP, were used as substrates. The activity gradient for the different nucleotides assayed was GDP > UDP > ADP > UTP > ATP, with no activity for AMP. This suggests the presence of another NTPDase-like enzyme expressed in an intracellular compartment. Similar results were obtained in rat testis (not shown).
Excurrent ducts
Epididymis
NTPDase1 was detected in the smooth muscle cell layers surrounding the duct, in blood vessels, and at the luminal surface of the apical cells from the epithelium (Fig. 5), mainly present in caput and corpus and almost absent in cauda. NTPDase2 was immunolocalized in cells of the connective tissue. NTPDase3 was detected as a continuous labelling lining the lumen, coinciding with the widely distributed epididymal principal cells (Fig. 5 and Supplementary Fig. 1). Immunofluorescence and double staining experiments showed that NTPDase1 and NTPDase3 do not colocalize in the epididymal epithelium, each enzyme being expressed by a different cell type (Fig. 5).
Fig. 5.
Localization of NTPDase1 (N1), NTPDase2 (N2), NTPDase3 (N3) by immunohistochemistry, immunofluorescence and enzyme histochemistry (ATP) in the mouse male excurrent ducts. In the epididymis (left and middle columns) NTPDase1 was immunodetected in the smooth muscle cells surrounding the duct, in the blood vessels and at the luminal surface of apical cells from the epididymal epithelium (arrow heads in left column and green fluorescence in middle column). NTPDase2 was immunolocalized to cells lining the basal side of the duct. ATPase activity was detected in these structures as well as at the luminal side of the epithelium, whereas NTPDase3 expression was also abundant, coinciding with the distribution of principal cells in this epithelium (red fluorescence in middle column). Fluorescence merge image (Merge in middle column) confirmed that different epithelial cell types express NTPDase1 and NTPDase3; DAPI (blue) was used for nuclei counterstaining. In the vas deferens (longitudinal section; right column) NTPDase1 was immunodetected in the smooth muscle layers surrounding the duct (bracket) and in blood vessels, especially abundant in the lamina propria (arrows). NTPDase2 was immunolocalized at the connective tissue cells of both tunica mucosae and tunica muscularis. Intense ATPase activity was revealed in all these structures while any brown deposits were seen in the absence of substrate (no ATP). Nuclei were counterstained with Haematoxylin. Scale bar 50 μm
ATPase activity staining correlated with the immunolabelling of NTPDase1, NTPDase2, and NTPDase3 indicated above (Fig. 5) suggesting again that these ectonucleotidases are dominant in this system. Similar data were obtained with ADP as substrate (not shown).
Vas deferens
NTPDase1 expression was detected in the thick muscle cell layers surrounding the duct as well as in blood vessels, especially abundant in the lamina propria mucosae (Fig. 5). NTPDase2 immunolabelling was detected in the connective tissue of lamina propria and between muscle fibres. NTPDase3 was not detected in this tissue.
ATPase activity confirmed again that the immunodetected NTPDase1 and NTPDase2 were enzymatically active (Fig. 5).
Male reproductive accessory glands
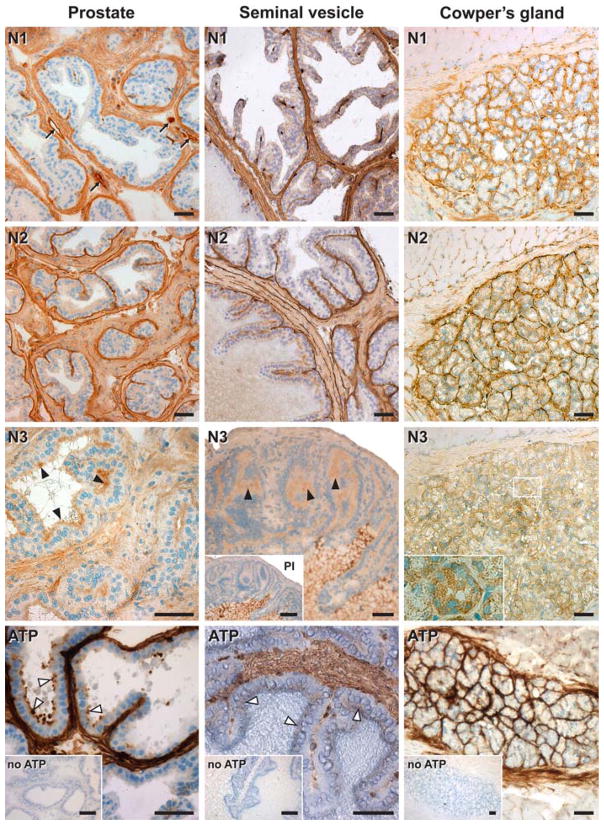
Prostate
NTPDase1 was detected in both smooth muscle cells and blood vessels of the prostate parenchyma. NTPDase2 was also immunolocalized in the parenchyma, in association with the connective tissue and with cells lining the basal side of the prostate glands. NTPDase3 was detected at the apical pole of epithelial cells lining the lumen of the secretory portion of the glands (Fig. 6).
Fig. 6.
Localization of NTPDase1 (N1), NTPDase2 (N2), NTPDase3 (N3) by immunohistochemistry and enzyme histochemistry (ATP) in the mouse male reproductive tract accessory glands. In the prostate (left column) NTPDase1 was immunodetected in smooth muscle cells and in blood vessels (arrows) of the gland parenchyma. NTPDase2 was immunolocalized at the connective tissue cells between muscle fibres and also lining the basal side of the prostatic glands. NTPDase3 was detected at the luminal side of epithelial prostatic secretory glands (black arrow heads), coinciding with the ATPase activity (white arrow heads). Nonspecific NTPDase3 staining was detected in prostate stroma as confirmed with the preimmune control (not shown). In the seminal vesicle (middle column) similar labelling was found as for the prostate. Notice that the luminal side of the epithelium was positively and specifically stained with the anti-NTPDase3 antibody (black arrow heads), coinciding with a sparse ATPase activity (white arrow heads). Note that the secretion was nonspecifically stained with the NTPDase3 antibody (see inset with the preimmune serum, PI). In the Cowper’s gland (right column), NTPDase1 was immunodetected in the myoepithelial cells, NTPDase2 in cells surrounding the acini and NTPDase3 at the acinar lumen as well as in the acinar cells (see magnification in N3). ATPase activity was detected on all the above NTPDase-positive structures. Control experiments in the absence of substrate are indicated as “no ATP”. Nuclei were counterstained with Haematoxylin. Scale bar 50 μm
ATPase activity was noted exclusively in the NTPDase1, NTPDase2, and NTPDase3 expressing structures indicated above (Fig. 6).
Seminal vesicles
NTPDase1 was immunolocalized in both smooth muscle cells and blood vessels, and NTPDase2 in the connective tissue of these glands. Weak staining for NTPDase3 was detected at the luminal side of epithelial cells from the mucosae (Fig. 6). The apparent staining of the glandular secretions appeared nonspecific (see inset in Fig. 6).
ATPase activity was found in the above-indicated structures (Fig. 6) although this activity was sparsely visualized in the epithelium, in agreement with the low expression and weak activity of NTPDase3 in situ.
Bulbourethral (Cowper’s) glands
NTPDase1 was immunodetected in cells surrounding the secretory acini, coinciding with the localization of myoepithelial cells in these glands (Fig. 6). NTPDase2 was strongly detected at the basal layer of glandular acini. Finally, acinar secretory cells and excretory ducts cells were immunopositive for NTPDase3 antibodies. A punctuate labelling was seen in secretory cells.
ATPase activity was strongly detected in cells surrounding the gland acini of the glands and only a sparse activity was found in the secretory cells (Fig. 6), which is in perfect agreement with the above immunolocalization results. ADPase activity distribution was similar although less intense (not shown).
Table 2 summarizes the immunohistochemical staining for all the mouse tissues analysed.
Table 2.
Resume of the immunohistochemical staining for NTPDase1, NTPDase2, and NTPDase3 in mouse male and female reproductive tracts
| NTPDase1 | NTPDase2 | NTPDase3 | |
|---|---|---|---|
| Male | |||
| Testis | + | + | − |
| Seminiferous epithelium | + | − | − |
| Sertoli cells | + | + | − |
| Peritubular cells | + | + | − |
| Interstitial tissue | + | + | − |
| Interstitial macrophages | + | − | − |
| Epididymis | + | + | + |
| Epithelium | + | − | + |
| Principal cells | − | − | + (a) |
| Apical cells | + (a) | − | − |
| Vas deferens | + | + | − |
| Prostate | + | + | + |
| Secretory epithelium | − | − | + (a) |
| Seminal vesicle | + | + | + |
| Epithelium | − | − | + (a) |
| Cowper’s gland | + | + | + |
| Acinar secretory cells | − | − | + |
| Myoepithelial cells | + | + | − |
| Female | |||
| Ovary | + | + | − |
| Granulosa | + | − | − |
| Oviduct | + | + | + |
| Epithelial cells | − | − | + (a) |
| Uterus | + | + | − |
| All tissues | |||
| Smooth muscle cells | + | − | − |
| Vascular endothelium | + | − | − |
| Connective tissue | − | + | − |
+ positive reaction, (a) apical staining, − no staining
Note that no immunostaining was observed for NTPDase8 in any of the rat tissues from the reproductive tract studied
Discussion
In this descriptive study, NTPDase1, NTPDase2, and NTPDase3 have been detected in the murine reproductive tracts. In all cases, enzymatic in situ results at the cell surface correlated with the immunolabelling of these three NTPDases. As these ectoenzymes regulate extracellular nucleotide levels at the cell surface, we will discuss their potential function in these structures. NTPDase8 has not been detected in any of the rat tissues tested here and will not be further commented. Also, since the role of NTPDase1 and NTPDase2 in blood vessels has already been largely documented (Enjyoji et al. 1999; Robson et al. 2006; Sévigny et al. 2002), it will not be repeated in this discussion.
Female reproductive tract
The biochemical results presented in this work with tissue homogenates showed high NTPDase1 expression levels in mouse female reproductive tract. This is consistent with the abundance of smooth musculature in these organs, especially in the oviducts and the uterus, where a purinergic signalling is well documented (Bardini et al. 2000; Gillman and Pennefather 1998; Khanam and Burnstock 2007). A failure of the contractility of these organs, acting, among other functions, as a functionally united peristaltic pump, has been proposed as a cause of infertility (Zervomanolakis et al. 2007). Immunolocalization of NTPDase1 in the smooth muscle fibres and NTPDase2 within the connective tissue surrounding the fibres, suggest a role of both enzymes in the control of contraction. In agreement, Ziganshin et al. (2006) reported the expression of both P2X and P2Y purinoceptors in the human pregnant uterus with changes in their relative expression during pregnancy. The presence of P2Y receptors would help maintaining the pregnancy, while the P2X receptors would potentiate the contractility in labour. It is, thus, conceivable that NTPDase1 and 2 may also play a role during pregnancy, although further investigations are required.
Interestingly, NTPDase3 expression was detected at the oviductal epithelium. ATP contributes to the fluid formation by inducing ion secretion by oviduct epithelial cells (Keating and Quinlan 2008), which, in turn, influences events such as oocyte and embryo transport, fertilization, and early stages of embryo development. NTPDase3 may therefore modulate ATP-regulated transepithelial transport in these cells.
Finally, in the ovary, aside from the expected expression of NTPDase1 in blood vessels, this protein was also expressed in granulosa from large follicles, mainly preovulatory and corpora lutea; NTPDase1 expression being undetected in primordial, primary, or secondary follicles. This suggest that the presence of this enzyme could be related to the developmental stage of the follicle, as it has also been pointed for the P2X expression pattern (Bardini et al. 2000). Moreover, expression of P2 purinergic receptors has been demonstrated in granulosa cells (Kamada et al. 1994) and an apoptotic effect has been reported for ATP on these cells (Park et al. 2003, Tai et al. 2005). This suggests a role of this nucleotide in follicular atresia, a process biologically and morphologically resembling apoptosis. In this context, NTPDase1 may be involved in maintaining granulosa cellular viability. Interestingly, fluid from larger follicles approximate one-tenth of the ATP concentration of that from smaller ones (Park et al. 2003). This is in agreement with the late appearance of NTPDase1 expression.
Male reproductive tract
Testes
In testes, NTPDase1 was detected on the interstitial tissue macrophages, which are in intimate physical and functional association with Leydig cells (Niemi et al. 1986), and therefore, modulators of steroidogenesis (Hales 2002). In Leydig cells, extracellular ATP modulates steroidogenesis, increasing testosterone secretion (Foresta et al. 1996; Poletto Chaves et al. 2006). Although functional data point to the involvement of the P2X2 receptor in this response, the P2 expression pattern displayed by these cells is not completely defined. By NTPDase1 expression, testicular macrophages would be well equipped to control the extracellular ATP levels and, in turn, hormone production by Leydig cells.
NTPDase1 and NTPDase2 were immunolocalized to Sertoli cells; an observation in agreement with a previous ecto-ATPase activity identified in these cells (Barbacci et al. 1996). ATP influences follicle-stimulating hormone (FSH) responsiveness (Filippini et al. 1994), and both P2X and P2Y receptors have been identified in these cells (Ko et al. 2003). Another report also showed expression of NTPDase1, NTPDase2 as well as of NTPDase3 in rat Sertoli cell cultures by means of RT-PCR (Zamoner et al. 2006). Different experimental approaches may explain the discrepancy. Alternatively, the mRNA levels may not lead to a sufficient amount of NTPDase3 to be detected by immunolocalization.
Interestingly, an intense nucleotidase activity was detected in the Golgi-phase acrosomal vesicle of round spermatids. The pattern of nucleoside hydrolysis, with a clear preference for GDP, and also for UDP and ADP over UTP and ATP, with the absence of both AMP hydrolysis and immunoreactivity for NTPDase1, 2, 3 and 8, suggests expression of NTPDase6, an enzyme expressed in the Golgi apparatus that may be involved in glycosylation reactions (Braun et al. 2000).
Excurrent ducts
Contraction of the epididymis and especially the vas deferens is required for sperm transport and excretion. In these organs the influence of extracellular ATP is well documented (reviewed in Zhou et al. 2007). In the vas deferens, ATP, secreted either from nerve terminals, the epithelium or the sperm itself, modulates noradrenaline release (Queiroz et al. 2003) and also induces smooth muscle contraction through P2X receptors located on smooth muscle cells (Banks et al. 2006). Expression of both NTPDase1, in association with muscle cells, and NTPDase2, between the fibres, along with a strong ATPase activity, points to the implication of these enzymes in the control of extracellular ATP levels influencing contraction. Moreover, similar to P2X immunostaining (Lee et al. 2000), no regional differences in NTPDase1 and NTPDase2 labelling intensities were found between the epididymal and the prostatic regions of the vas deferens.
In the epididymis, aside from the effect on smooth muscle cells determining sperm transport, ATP stimulates anion secretion across the epithelium (Zhou et al. 2007) and Ca2+ signalling through activation of P2X and P2Y purinoceptors (Shariatmadari et al. 2003), modifying fluid composition. Maturation of the spermatozoa, a main function of epididymis, is dependent on this local fluid environment. Moreover, ATP alters motility and improves fertilizing capability of the sperm (Rodríguez-Miranda et al. 2008). Results shown in this report revealed an important ATPase activity at the luminal side of the epididymal epithelium, where NTPDase3 expression was associated with the principal cells, and NTPDase1 expression at the apical cells. Principal cells, the predominant cell type in the epididymal epithelium, are involved in fluid reabsorption from the lumen and also control sperm maturation through not only merocrine but also apocrine secretion that involves the presence of epididymosomes (reviewed by Sullivan et al. 2007). Apical cells are much less abundant and contribute to transepithelial fluid transport processes and are involved in luminal acidification (Breton et al. 1999). The significance of the particular distribution of NTPDases on these cells needs further analysis, but the presence of both ectonucleotidases suggests a role in the fine regulation of the P2X and P2Y receptors that are also differentially expressed along epididymal epithelium (Shariatmadari et al. 2003).
Accessory glands
Purinergic signalling has been studied in the prostate and smooth muscles of seminal vesicles (Lee et al. 2000), where NTPDase1 and NTPDase2 were also identified. Interestingly, NTPDase3 was expressed at the luminal side of the secretory epithelial cells of prostate, seminal vesicles, and Cowper’s glands. This enzyme has already been localized in association with other exocrine glands (Lavoie and Sévigny 2006), pointing to a key role in the modulation of exocrine secretion, where its activity could influence the extent of activation of the purinergic receptors expressed by these cells. Interestingly, as for the epididymis, where NTPDase3 is highly expressed, an apocrine secretion has also been described in the prostate and seminal vesicles leading to the formation of “prostasomes” and “seminosomes”, respectively. Finally, in Cowper’s glands, NTPDase3 was also detected inside cells contained into vesicles. Whether this enzyme will reach the plasma membrane after vesicle fusion or will be released into the glandular secretion, as for rat NTPDase1 in the pancreatic juice (Sorensen et al. 2003; Yegutkin et al. 2006), still remains to be defined. It is difficult at the moment to determine the potential functions played by NTPDases as the purinoceptor expression pattern in these glands is still lacking.
Taken together, the biochemical and histochemical results presented in this work demonstrate the presence of NTPDase1, NTPDase2, and NTPDase3 in murine male and female reproductive tracts. Their differential distributions might contribute to clarify their respective roles in the regulation of the purinergic signalling pathways. Because of their specific localisation, NTPDase1 and NTPDase2 would be mainly involved in functions requiring contraction resulting in fluid transits. As NTPDase3 is the main NTPDase expressed by secretory epithelia, this points to a role of the enzyme in the regulation of glandular exocrine secretion. Interestingly, important reproductive cells such as ovarian granulosa cells, Sertoli cells, and epididymal epithelial cells have been shown to express NTPDases. Further investigations are in progress to define the physiological role of these ecto-enzymes in these systems.
Supplementary Material
Acknowledgments
This work was supported by grants to J. Sévigny from the Canadian Institutes of Health Research (CIHR) and to S.C. Robson from NIH. M. Martín-Satué was recipient of a fellowship from the Spanish Ministry of Education and Science (MEC-Programa José Castillejo), E.G. Lavoie of a scholarship from the Fonds de Recherche en Santé du Québec and J. Sévigny of a New Investigator award from the CIHR. We thank Mr. Gilles Grondin for histology analysis services. We are also grateful to Dr. Robert Sullivan and Dr. Mohamed El-Alfy for their advice and help with the analysis of the male reproductive system.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00418-008-0551-3) contains supplementary material, which is available to authorized users.
Contributor Information
M. Martín-Satué, Centre de Recherche en Rhumatologie et Immunologie, Centre Hospitalier Universitaire de Québec, Université Laval, 2705 Blvd Laurier, Local T1-49, Quebec, QC G1V 4G2, Canada
É. G. Lavoie, Centre de Recherche en Rhumatologie et Immunologie, Centre Hospitalier Universitaire de Québec, Université Laval, 2705 Blvd Laurier, Local T1-49, Quebec, QC G1V 4G2, Canada
J. Pelletier, Centre de Recherche en Rhumatologie et Immunologie, Centre Hospitalier Universitaire de Québec, Université Laval, 2705 Blvd Laurier, Local T1-49, Quebec, QC G1V 4G2, Canada
M. Fausther, Centre de Recherche en Rhumatologie et Immunologie, Centre Hospitalier Universitaire de Québec, Université Laval, 2705 Blvd Laurier, Local T1-49, Quebec, QC G1V 4G2, Canada
E. Csizmadia, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
O. Guckelberger, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
S. C. Robson, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
Jean Sévigny, Centre de Recherche en Rhumatologie et Immunologie, Centre Hospitalier Universitaire de Québec, Université Laval, 2705 Blvd Laurier, Local T1-49, Quebec, QC G1V 4G2, Canada.
References
- Bakker WW, Donker RB, Timmer A, van Pampus MG, van Son WJ, Aarnoudse JG, van Goor H, Niezen-Koning KE, Navis G, Borghuis T, Jongman RM, Faas MM. Plasma hemopexin activity in pregnancy and preeclampsia. Hypertens Pregnancy. 2007;26:227–239. doi: 10.1080/10641950701274896. [DOI] [PubMed] [Google Scholar]
- Banks FC, Knight GE, Calvert RC, Thompson CS, Morgan RJ, Burnstock G. The purinergic component of human vas deferens contraction. Fertil Steril. 2006;85:932–939. doi: 10.1016/j.fertnstert.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Barbacci E, Filippini A, De Cesaris P, Ziparo E. Identification and characterization of an ecto-ATPase activity in rat Sertoli cells. Biochem Biophys Res Commun. 1996;222:273–279. doi: 10.1006/bbrc.1996.0734. [DOI] [PubMed] [Google Scholar]
- Bardini M, Lee HY, Burnstock G. Distribution of P2X receptor subtypes in the rat female reproductive tract at late pro-oestrus/early oestrus. Cell Tissue Res. 2000;299:105–113. doi: 10.1007/s004419900138. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Zsarnovszky A, Crawford PA, Hemani H, Spurling L, Kirley TL. Immunolocalization of ecto-nucleoside triphosphate diphosphohydrolase 3 in rat brain: implications for modulation of multiple homeostatic systems including feeding and sleepwake behaviors. Neuroscience. 2006;137:1331–1346. doi: 10.1016/j.neuroscience.2005.08.086. Epub 2005 Dec 7. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Adenosine and its receptors: multiple modulatory functions and potential therapeutic targets for neurologic disease. Neurology. 2008;70:231–236. doi: 10.1212/01.wnl.0000297939.18236.ec. [DOI] [PubMed] [Google Scholar]
- Bigonnesse F, Lévesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJ, Sévigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. doi: 10.1021/bi0362222. [DOI] [PubMed] [Google Scholar]
- Boeynaems JM, Communi D, Gonzalez NS, Robaye B. Overview of the P2 receptors. Semin Thromb Hemost. 2005;31:139–149. doi: 10.1055/s-2005-869519. [DOI] [PubMed] [Google Scholar]
- Braun N, Fengler S, Ebeling C, Servos J, Zimmermann H. Sequencing, functional expression and characterization of rat NTPDase6, a nucleoside diphosphatase and novel member of the ecto-nucleoside triphosphate diphosphohydrolase family. Biochem J. 2000;351(Pt 3):639–647. [PMC free article] [PubMed] [Google Scholar]
- Braun N, Sévigny J, Mishra SK, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J NeuroSci. 2003;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- Braun N, Sévigny J, Robson SC, Hammer K, Hanani M, Zimmermann H. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia. 2004;45:124–132. doi: 10.1002/glia.10309. [DOI] [PubMed] [Google Scholar]
- Breton S, Tyszkowski R, Sabolic I, Brown D. Postnatal development of H + ATPase (proton-pump)-rich cells in rat epididymis. Histochem Cell Biol. 1999;111:97–105. doi: 10.1007/s004180050339. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling. Br J Pharmacol. 2006a;147(Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006b;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Casali EA, da Silva TR, Gelain DP, Kaiser GR, Battastini AM, Sarkis JJ, Bernard EA. Ectonucleotidase activities in Sertoli cells from immature rats. Braz J Med Biol Res. 2001;34:1247–1256. doi: 10.1590/s0100-879x2001001000003. [DOI] [PubMed] [Google Scholar]
- Dranoff JA, Kruglov EA, Robson SC, Braun N, Zimmermann H, Sévigny J. The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology. 2002;36:1135–1144. doi: 10.1053/jhep.2002.36823. [DOI] [PubMed] [Google Scholar]
- Edwards SE, Buffone MG, Knee GR, Rossato M, Bonanni G, Masiero S, Ferasin S, Gerton GL, Moss SB, Williams CJ. Effects of extracellular adenosine 5′-triphosphate on human sperm motility. Reprod Sci. 2007;14:655–666. doi: 10.1177/1933719107306227. [DOI] [PubMed] [Google Scholar]
- Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- Fausther M, Lecka J, Kukulski F, Levesque SA, Pelletier J, Zimmermann H, Dranoff JA, Sévigny J. Cloning, purification, and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol. 2007;292:G785–G795. doi: 10.1152/ajpgi.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini A, Riccioli A, De Cesaris P, Paniccia R, Teti A, Stefanini M, Conti M, Ziparo E. Activation of inositol phospholipid turnover and calcium signaling in rat Sertoli cells by P2-purinergic receptors: modulation of follicle-stimulating hormone responses. Endocrinology. 1994;134:1537–1545. doi: 10.1210/endo.134.3.8119196. [DOI] [PubMed] [Google Scholar]
- Foresta C, Rossato M, Nogara A, Gottardello F, Bordon P, Di Virgilio F. Role of P2-purinergic receptors in rat Leydig cell steroidogenesis. Biochem J. 1996;320:499–504. doi: 10.1042/bj3200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman TA, Pennefather JN. Evidence for the presence of both P1 and P2 purinoceptors in the rat myometrium. Clin Exp Pharmacol Physiol. 1998;25:592–599. doi: 10.1111/j.1440-1681.1998.tb02257.x. [DOI] [PubMed] [Google Scholar]
- Glass R, Bardini M, Robson T, Burnstock G. Expression of nucleotide P2X receptor subtypes during spermatogenesis in the adult rat testis. Cells Tissues Organs. 2001;169:377–387. doi: 10.1159/000047905. [DOI] [PubMed] [Google Scholar]
- Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. 2002;57:3–18. doi: 10.1016/s0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- Heine P, Braun N, Sévigny J, Robson SC, Servos J, Zimmermann H. The C-terminal cysteine-rich region dictates specific catalytic properties in chimeras of the ectonucleotidases NTPDase1 and NTPDase2. Eur J Biochem. 2001;268:364–373. doi: 10.1046/j.1432-1033.2001.01896.x. [DOI] [PubMed] [Google Scholar]
- Kaczmarek E, Koziak K, Sévigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- Kamada S, Blackmore PF, Oehninger S, Gordon K, Hodgen GD. Existence of P2-purinoceptors on human and porcine granulosa cells. J Clin Endocrinol Metab. 1994;78:650–656. doi: 10.1210/jcem.78.3.8126137. [DOI] [PubMed] [Google Scholar]
- Kansas GS, Wood GS, Tedder TF. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J Immunol. 1991;146:2235–2244. [PubMed] [Google Scholar]
- Keating N, Quinlan LR. Effect of basolateral adenosine triphosphate on chloride secretion by bovine oviductal epithelium. Biol Reprod. 2008;78:1119–1126. doi: 10.1095/biolreprod.107.065508. Epub 2008 Mar 5. [DOI] [PubMed] [Google Scholar]
- Khanam T, Burnstock G. Changes in expression of P2X1 receptors and connexin 43 in the rat myometrium during pregnancy. Fertil Steril. 2007;88:1174–1179. doi: 10.1016/j.fertnstert.2007.01.130. Epub 2007 Jun 11. [DOI] [PubMed] [Google Scholar]
- Ko WH, Au CL, Yip CY. Multiple purinergic receptors lead to intracellular calcium increases in cultured rat Sertoli cells. Life Sci. 2003;72:1519–1535. doi: 10.1016/s0024-3205(02)02410-4. [DOI] [PubMed] [Google Scholar]
- Kukulski F, Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, Kirley TL, Sévigny J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavoie ÉG, Sévigny J. Localization of nucleoside triphosphate diphosphohydrolase-3 in mouse digestive associated glands. Purinergic Signalling. 2006;2(2):245. [Google Scholar]
- Lee HY, Bardini M, Burnstock G. P2X receptor immunoreactivity in the male genital organs of the rat. Cell Tissue Res. 2000;300:321– 330. doi: 10.1007/s004410000207. [DOI] [PubMed] [Google Scholar]
- Lemmens R, Vanduffel L, Kittel A, Beaudoin AR, Benrezzak O, Sévigny J. Distribution, cloning, and characterization of porcine nucleoside triphosphate diphosphohydrolase-1. Eur J Biochem. 2000;267:4106–4114. doi: 10.1046/j.1432-1327.2000.01462.x. [DOI] [PubMed] [Google Scholar]
- Mizumoto N, Kumamoto T, Robson SC, Sévigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- Niemi M, Sharpe RM, Brown WR. Macrophages in the interstitial tissue of the rat testis. Cell Tissue Res. 1986;243:337–344. doi: 10.1007/BF00251049. [DOI] [PubMed] [Google Scholar]
- Park DW, Cho T, Kim MR, Kim YA, Min CK, Hwang KJ. ATP-induced apoptosis of human granulosa luteal cells cultured in vitro. Fertil Steril. 2003;80:993–1002. doi: 10.1016/s0015-0282(03)01118-x. [DOI] [PubMed] [Google Scholar]
- Poletto Chaves LA, Pontelli EP, Varanda WA. P2X receptors in mouse Leydig cells. Am J Physiol Cell Physiol. 2006;290:C1009– C1017. doi: 10.1152/ajpcell.00506.2005. Epub 2005 November 16. [DOI] [PubMed] [Google Scholar]
- Pulte ED, Broekman MJ, Olson KE, Drosopoulos JH, Kizer JR, Islam N, Marcus AJ. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb Res. 2007;121:309–317. doi: 10.1016/j.thromres.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz G, Talaia C, Goncalves J. ATP modulates noradrenaline release by activation of inhibitory P2Y receptors and facilitatory P2X receptors in the rat vas deferens. J Pharmacol Exp Ther. 2003;307:809–815. doi: 10.1124/jpet.103.054809. [DOI] [PubMed] [Google Scholar]
- Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Miranda E, Buffone MG, Edwards SE, Ord TS, Lin K, Sammel MD, Gerton GL, Moss SB, Williams CJ. Extracellular adenosine 5′-triphosphate alters motility and improves the fertilizing capability of mouse sperm. Biol Reprod. 2008;79:164–171. doi: 10.1095/biolreprod.107.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte am Esch JII, Sévigny J, Kaczmarek E, Siegel JB, Imai M, Koziak K, Beaudoin AR, Robson SC. Structural elements and limited proteolysis of CD39 influence ATP diphosphohydrolase activity. Biochemistry. 1999;38:2248–2258. doi: 10.1021/bi982426k. [DOI] [PubMed] [Google Scholar]
- Sévigny J, Lévesque FP, Grondin G, Beaudoin AR. Purification of the blood vessel ATP diphosphohydrolase, identification and localisation by immunological techniques. Biochim Biophys Acta. 1997;1334:73–88. doi: 10.1016/s0304-4165(96)00079-7. [DOI] [PubMed] [Google Scholar]
- Sévigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, Imai M, Zimmermann H, Robson SC. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood. 2002;99:2801–2809. doi: 10.1182/blood.v99.8.2801. [DOI] [PubMed] [Google Scholar]
- Shariatmadari R, Sipila P, Vierula M, Tornquist K, Huhtaniemi I, Poutanen M. Adenosine triphosphate induces Ca2+ signal in epithelial cells of the mouse caput epididymis through activation of P2X and P2Y purinergic receptors. Biol Reprod. 2003;68:1185– 1192. doi: 10.1095/biolreprod.102.007419. Epub 2002 Oct 30. [DOI] [PubMed] [Google Scholar]
- Slater NM, Barden JA, Murphy CR. Distributional changes of purinergic receptor subtypes (P2X 1–7) in uterine epithelial cells during early pregnancy. Histochem J. 2000;32:365–372. doi: 10.1023/a:1004017714702. [DOI] [PubMed] [Google Scholar]
- Slater M, Murphy CR, Barden JA. Purinergic receptor expression in the apical plasma membrane of rat uterine epithelial cells during implantation. Cell Calcium. 2002;31:201–207. doi: 10.1016/S0143-4160(02)00033-7. [DOI] [PubMed] [Google Scholar]
- Sorensen CE, Amstrup J, Rasmussen HN, Ankorina-Stark I, Novak I. Rat pancreas secretes particulate ecto-nucleotidase CD39. J Physiol. 2003;551:881–892. doi: 10.1113/jphysiol.2003.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–491. doi: 10.1111/j.1745-7262.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Tai CJ, Chang SJ, Chien LY, Leung PC, Tzeng CR. Adenosine triphosphate induces activation of caspase-3 in apoptosis of human granulosa-luteal cells. Endocr J. 2005;52:327–335. doi: 10.1507/endocrj.52.327. [DOI] [PubMed] [Google Scholar]
- Vlajkovic SM, Housley GD, Munoz DJ, Robson SC, Sévigny J, Wang CJ, Thorne PR. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience. 2004;126:763–773. doi: 10.1016/j.neuroscience.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Wink MR, Braganhol E, Tamajusuku AS, Lenz G, Zerbini LF, Libermann TA, Sévigny J, Battastini AM, Robson SC. Nucleoside triphosphate diphosphohydrolase-2 (NTPDase2/CD39L1) is the dominant ectonucleotidase expressed by rat astrocytes. Neuroscience. 2006;138:421–432. doi: 10.1016/j.neuroscience.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Jalkanen S, Novak I. ATP-consuming and ATP-generating enzymes secreted by pancreas. J Biol Chem. 2006;281:29441–29447. doi: 10.1074/jbc.M602480200. [DOI] [PubMed] [Google Scholar]
- Zamoner A, Bruno AN, Casali EA, Corbelini PF, Diniz GP, Barreto-Chaves ML, Silva FR, Sarkis JJ, Pessoa-Pureur R. Genomic-independent action of thyroid hormones on NTPDase activities in Sertoli cell cultures from congenital hypothyroid rats. Life Sci. 2006;80:51–58. doi: 10.1016/j.lfs.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Zervomanolakis I, Ott HW, Hadziomerovic D, Mattle V, Seeber BE, Virgolini I, Heute D, Kissler S, Leyendecker G, Wildt L. Physiology of upward transport in the human female genital tract. Ann NY Acad Sci. 2007;1101:1–20. doi: 10.1196/annals.1389.032. [DOI] [PubMed] [Google Scholar]
- Zhou WL, Zuo WL, Ruan YC, Wang Z, Du JY, Xiong Y, Chan HC. The role of extracellular ATP in the male reproductive tract. Sheng Li Xue Bao. 2007;59:487–494. [PubMed] [Google Scholar]
- Ziganshin AU, Zaitcev AP, Khasanov AA, Shamsutdinov AF, Burnstock G. Term-dependency of P2 receptor-mediated contractile responses of isolated human pregnant uterus. Eur J Obstet Gynecol Reprod Biol. 2006;129:128–134. doi: 10.1016/j.ejogrb.2005.11.018. Epub 2005 Dec 15. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev Res. 2001;52:44–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.