Abstract
Balance task performance is affected in persons with multiple sclerosis (PwMS), but the control strategies used to perform specific tasks are not well understood. The purpose of this study was to evaluate segmental control during quiet standing in PwMS and controls to understand whether MS alters use of the ankle and hip strategies to manage postural sway. Coherence of acceleration between the trunk and legs was evaluated with accelerometers placed on the sacrum and lower leg. Thirty six PwMS and 20 healthy control subjects performed quiet standing with eyes open and closed while center of pressure (CoP) and acceleration of postural sway was measured. Acceleration frequencies were divided into lower frequencies (≤1.0 Hz) and higher frequencies (>1.0 Hz) to categorize sway characteristics. With eyes open, coherence was significantly lower in PwMS compared to controls at lower frequencies only. With eyes closed, coherence was significantly lower in PwMS compared to controls, who use an ankle strategy at lower frequencies only, at both lower and higher frequencies. Both groups showed decreased coherence with increasing frequency when eyes were open and closed. Coherence was significantly correlated with CoP sway area in PwMS during the eyes closed condition only. The reduced coherence in PwMS during both lower and higher frequency sway indicates PwMS utilize a mixed ankle-hip sway strategy regardless of sway frequency. This is in contrast to sway in healthy subjects which utilizes an ankle strategy at lower frequencies and a mixed strategy at higher frequencies. Lack of adaptability in segmental control strategy likely contributes to abnormal postural control, as reflected by CoP sway patterns, in PwMS.
Keywords: postural control, neurological disorder, acceleration, center of pressure
1. Introduction
Persons with multiple sclerosis (PwMS) have altered postural control, as reflected by increased postural sway while standing (Karst, Venema, Roehrs, & Tyler, 2005; Van Emmerik, Remelius, Johnson, Chung, & Kent-Braun, 2010), reaching (Karst, et al., 2005), and in response to external perturbations (Cameron, Horak, Herndon, & Bourdette, 2008; Huisinga, St George, Spain, Overs, & Horak, 2014). The relationship between altered postural sway and increased incidence of falls is often cited in PwMS, as well as other populations (Jacobs & Kasser, 2012; Kasser, Jacobs, Foley, Cardinal, & Maddalozzo, 2011; Rocchi, Chiari, & Horak, 2002). However, it is not clear how sway abnormalities reflect dysfunctional motor coordination patterns in PwMS. Postural sway is usually measured as center of pressure displacement in the antero-posterior or medio-lateral directions on a force plate or more recently from inertial sensors on the lower trunk. Increase in sway area, especially with eyes closed in PwMS, is thought to be related to slowed sensory and motor axonal conduction (Cameron, et al., 2008; Huisinga, et al., 2014). In addition, slowed propriospinal conduction time in PwMS has been related to prolonged latencies of automatic postural responses to surface perturbations (Cameron, et al., 2008). While center of pressure sway measures are strong indicators of dysfunctional postural control, these measures do not directly reflect the postural movement strategies employed by PwMS during quiet stance. Directly examining the kinematics of body segments during standing in PwMS will allow for a better understanding of how persons with MS apply adaptive postural strategies to compensate for imbalance due to loss of somatosensory feedback, muscle weakness due to slowed axonal conduction velocities, and other central nervous system deficits (Cameron, et al., 2008; Noseworthy, Lucchinetti, Rodriguez, & Weinshenker, 2000).
When standing quietly, human stance is often modeled as a combination of a single (ankle strategy) and double (hip strategy) inverted pendulum which pivots around the ankle primarily, with increasing contribution of hip motion with larger postural sway (Horak & Macpherson, 1996; Jeka, Oie, Schoner, Dijkstra, & Henson, 1998; Peterka, 2002). Previous sway modeling (Creath, Kiemel, Horak, Peterka, & Jeka, 2005) found that at sway oscillations below 1 Hz, healthy young individuals move with the trunk and legs in-phase simulating an ankle strategy while at frequencies greater than 1 Hz, trunk and leg motion is anti-phase, simulating a hip or mixed (hip-ankle) strategy. This in-phase or anti-phase strategy was identified by examining the coherence between the angles of the two segments but coherence can be examined between any signals which represent segment motion, including segment acceleration and can be thought of as a correlation coefficient in the frequency domain between two signals (Creath, et al., 2005). Selection of in-phase (single segment inverted pendulum, ankle strategy) and antiphase (dual segment pendulum, hip or mixed strategy) movement patterns, and transitions between patterns, may depend on loss of stability as well as pre-selected movement strategy based on the task (Bardy, Marin, Stoffregen, & Bootsma, 1999; Bardy, Oullier, Bootsma, & Stoffregen, 2002; Creath, et al., 2005).
In PwMS, the segmental coordination patterns utilized during quiet standing are unknown. It has been speculated based on variability analysis of center of pressure sway patterns, that PwMS are less adaptable in their movement patterns (Huisinga, Yentes, Filipi, & Stergiou, 2012). In the present study, we evaluated the postural strategies in PwMS using accelerometry during quiet standing. Specifically, we evaluated the coherence of the acceleration patterns between the trunk and the legs. Coherence analysis allows for the calculation of the relationship between two segments across frequencies (Zhang, Kiemel, & Jeka, 2007). We hypothesized that PwMS would display coherence patterns between the trunk and the legs that differ from healthy controls across a range of frequencies. We also expected that within PwMS, coherence would be similar across both low (≤ 1.0 Hz ) and higher (> 1.0Hz) frequencies since PwMS may be more inflexible in adapting movement patterns (Huisinga, Yentes, et al., 2012). Similar coherence across low and higher frequencies in PwMS would not follow the coherence seen in healthy controls who display more of an ankle strategy at low frequencies and mixed hip-ankle strategy at higher frequencies. Finally, to help further understand the ankle and hip strategies, we hypothesized that lower coherence of trunk-leg would be related to longer latencies of postural responses and to larger sway area during quiet stance in PwMS.
2. Methods
2.1. Participants
All participants were recruited through the Oregon Health & Science University MS Clinic. All participants provided informed consent according to the Oregon Health & Science University Institutional Review Board. Inclusion criteria for all MS subjects were 1) diagnosis of MS made by a neurologist, 2) ability to perform the Timed 25 Foot Walk test without a walking aid, 3) no clinical relapses within the previous 60 days, 4) free from any other problems which may affect gait such as vestibular issues, orthopedic problems, and diabetic neuropathy. Healthy control subjects were also free of any conditions that could affect their walking. On the day of testing, all PwMS completed the self-reported Expanded Disability Status Scale (srEDSS) as a general classification of global MS-related disability level. The EDSS is a standard and heavily used disability classification scale for patients with MS (Kurtzke, 1983). The srEDSS correlates strongly (Bowen, Gibbons, Gianas, & Kraft, 2001) with the clinician administered version and was utilized in this study to reduce participant burden due to the remote location of the testing laboratory.
2.2. Standing protocol and data analysis
Subjects were equipped with 6 MTX Xsens inertial sensors (49A33G15, Xsens, Enschede, NL, USA), containing 3D accelerometers, gyroscopes, and magnetometers mounted on: (i) sternum, 2cm below the sternal notch, (ii) sacrum (L5 level, approximately at the body’s center of mass), (iii) on the dorsum of the right and left wrist, (iv) right and left shin. Only accelerometry data from the sacrum and lower leg, collected at 50Hz, were used for analysis.
2.2.1. Task 1 - Standing posture
Subjects stood with arms folded across the chest with their feet at a fixed heel-to-heel distance of 10 cm (Craig, Bruetsch, Lynch, Horak, & Huisinga, 2017; Huisinga, et al., 2014). Concurrently, subjects stood on a double force plate with one foot on each plate while ground reaction forces were sampled at 100 Hz. Ground reaction forces were filtered at 20 Hz and used to calculate center of pressure (CoP) sway area. Three, 30 second trials of quiet standing were performed for each condition: eyes open (EO) and eyes closed (EC). In the EO condition, patients were asked to focus on one point on the wall, 5 meters away, directly in front of them for the duration of the trial.
Accelerometry data collected by the Xsens sensors in the three coordinate axes were imported into Matlab. A trigonometric correction was used in order to adjust the acceleration data to a horizontal-vertical coordinate system (Moe-Nilssen & Helbostad, 2002). The algorithm (Moe-Nilssen & Helbostad, 2002) is based on the assumption that the best estimate of the mean acceleration over a period of time, during undisturbed, quiet standing, will be zero along any axis. After correcting for the tilt, accelerometry data for each axis was filtered with a 3Hz cut-off, zero-phase, low-pass Butterworth filter.
The power spectral and cross-power spectral densities (PSD and CPSD respectively) were independently calculated from the filtered anterior-posterior accelerations using Matlab’s built in functions, specifically with the Welch’s averaged method (Creath, et al., 2005). A Hanning window size of 4 seconds with an overlap of 50% was used for both calculations. Welch’s method is a spectral density estimation used for estimating the power of a signal at different frequencies. Having overlapping windows of 50% reduces the variance by about a factor of 2, owing to doubling the number of windows. The complex coherence was then calculated using the PSDs and CPSD by the following equation:
Where Pxy(f) is the CPSD and Pxx(f) and Pyy(f) are the PSDs for the signals being compared. When calculating the coherence between the trunk and leg acceleration signals, Pxx(f) represents the PSD of the leg acceleration, Pyy (f) the PSD of the trunk acceleration, and Pxy(f) the CPSD of the trunk and leg accelerations. Means of the coherence of each of the three trials for each condition were taken (ex: average of trials 1–3 for EO) and then means of each condition across every patient in a particular group were taken. Coherence was measured at all frequencies between 0–3.9262 Hz in 0.0336 Hz increments. We combined the coherence data into two groups: frequencies ≤ 1.0 Hz (mean of coherence of all frequencies below or equal to 1.0 Hz) & frequencies > 1.0 Hz (mean of coherence of all frequencies above 1.0 Hz) since Creath et al (Creath, et al., 2005) showed that 1.0 Hz was the cut-off between in-phase and anti-phase segmental motion during stance.
2.2.2. Task 2 - Perturbation
To measure postural response latency, subjects stood on two side-by-side computer-servo controlled, custom-made, hydraulic platforms that translated backward together causing forward body sway (Horak & Nashner, 1986). Subjects stood with arms folded across the chest, eyes open, with their feet at a fixed heel-to-heel distance of 10 cm. Foot placement at the beginning of each trial was controlled by marking the outlines of their feet with tape. Subjects stood on a platform that translated 4 cm forward at a rate of 15 cm/s, which required an in-place response with no stepping.
Surface EMG was recorded in all subjects in the dominant, right tibialis anterior using two 2.5 cm2 surface electrodes place approximately 2 cm apart with a ground electrode on the lateral condyle. Amplified EMG signals were band-pass filtered (70–2000 Hz), rectified, and stored for off-line analysis. Although no attempt was made to calibrate EMGs on an absolute scale, amplifier gains were fixed throughout all experimental sessions for all subjects. The postural response latency was defined as the time between the onset of surface translation to the first measurable increase in activity of the tibialis anterior muscle greater than 2 SD from baseline that was sustained for at least 50 ms (Cameron, et al., 2008; Huisinga, et al., 2014). Three translation trials were completed and the average postural response latency for each subject was used for analysis.
2.3. Statistical Analysis
Shapiro Wilks normality tests showed that the coherence data were not normally distributed. To measure main effects of group on coherence at different frequencies between the legs and trunk, a nonparametric general linear model (Friedman’s test) for Group (PwMS vs Controls) x Frequency (≤ 1.0 Hz vs > 1.0 Hz) was applied. To examine the relationship between coherence and postural response latencies as well as coherence and sway area, Spearman correlations were performed between coherence at frequencies ≤ 1.0 Hz and postural latencies and sway area. Spearman correlations were also performed between coherence at frequencies >1.0 Hz and postural latencies and sway area. Cohen’s d effect size is reported in Table 2 defined as the difference between two means divided by a standard deviation for the data. Alpha level was set at 0.05 and all analyses were performed with SPSS software (IBM SPSS Statistics, v. 20).
Table 2:
Mean, standard deviation, cohen’s d of the coherence between sagittal trunk and leg accelerations
| Frequencies ≤ 1.0 Hz mean (SD) cohen’s d |
Frequencies > 1.0 Hz mean (SD) cohen’s d |
||||
|---|---|---|---|---|---|
|
Eyes Open |
PwMS | 0.378 (0.073) | 0.88 | 0.245 (0.018) | 0.31 |
| Control | 0.435 (0.056) | 0.239 (0.021) | |||
|
Eyes Closed |
PwMS | 0.481 (0.034) | 0.82 | 0.300 (0.021) | 0.58 |
| Control | 0.527 (0.071) | 0.317 (0.036) | |||
PwMS: persons with multiple sclerosis
3. Results
Thirty six persons with multiple sclerosis and 20 healthy controls of similar age participated in this study (Table 1).
Table 1:
Demographic subject information
| PwMS | Control | |
|---|---|---|
| Age (yrs) | 45.6 ± 11.7 | 41.8 ± 10.7 |
| Height (cm) | 166.4 ± 18.4 | 167.9 ± 15.5 |
| Mass (kg) | 78.1 ± 19.9 | 78.7 ± 27.7 |
| Sex (F/M) | 32 / 8 | 17 / 3 |
| srEDSS | 4.3 ±1.2 | - |
PwMS: persons with multiple sclerosis
srEDSS: Self-reported Expanded disability status scale
3.1. Coherence in the Eyes open condition
Figure 1 illustrates how control subjects had higher coherence than MS subjects between trunk and leg motion during stance, especially at lower frequencies with mean values listed in Table 2. Overall, coherence between trunk and leg segments was lower in PwMS compared to healthy controls. Results show a significant main effect of Group (F=6.79, p=0.010) and of Frequency (F=288.86, p<0.001) where coherence was lower in PwMS compared to healthy controls, and, coherence was lower at frequencies >1.0 Hz compared to frequencies ≤ 1.0 Hz in both groups. The interaction between Group and Frequency was also significant (F=10.7, p=0.001). Post-hoc, paired tests indicate that coherence was lower in PwMS compared to controls at lower frequencies (≤ 1.0 Hz) but not at higher frequencies (> 1.0 Hz).
Figure 1:
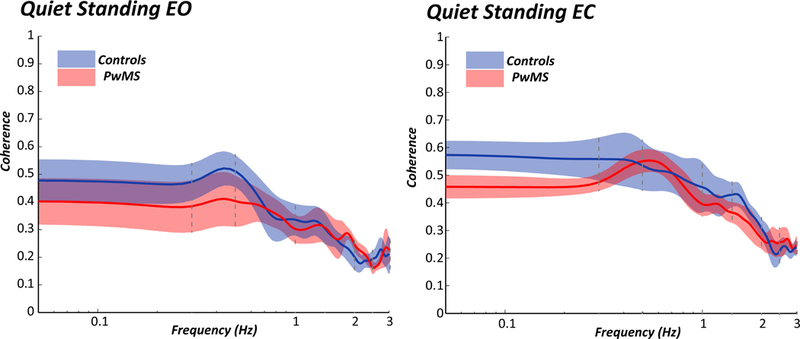
Mean and standard error of the coherence between sagittal trunk and leg accelerations across frequencies comparing the control (blue) and PwMS (red). Left plot shows coherence during standing with eyes open and right plot shows coherence during standing with eyes closed.
3.2. Coherence in the eyes closed condition
Like the eyes open condition, overall coherence between trunk and leg segments was lower in PwMS compared to healthy controls when standing with eyes closed. There was a significant main effect of Group (F=15.1, p=0.001) and a significant main effect of Frequency (F=591.5, p<0.001). PwMS also had lower coherence compared to healthy controls and, coherence between segments decreased as frequency was increasing in both groups. The interaction between Group and Frequency was not significant (F=3.33, p=0.071).
3.3. Relationship between coherence and postural response latency
We previously reported that postural response latencies in response to surface translations were significantly longer in this cohort of PwMS compared to healthy control subjects (Huisinga, et al., 2014). In the present study, these postural response latencies in PwMS were not significantly correlated with trunk-leg coherence at frequencies ≤ 1.0 Hz during the eyes open (p=0.582) or the eyes closed (p=0.196) quiet standing. Postural response latencies were also not significantly correlated with trunk-leg coherence at frequencies >1.0 Hz during the eyes open (p=0.698) or eyes closed (p=0.192) quiet standing. In healthy controls, there were also no significant correlations between postural response latency and trunk-leg coherence at frequencies ≤ 1.0 Hz during the eyes open (p=0.732) or eyes closed (p=0.932) quiet standing or at frequencies > 1.0 Hz during the eyes open (p=0.839) or eyes closed (p=0.912) quiet standing.
3.4. Relationship between coherence and CoP sway area in PwMS
We previously reported that CoP sway area was significantly greater in this cohort of PwMS compared to healthy controls (Huisinga, et al., 2014). During eyes closed quiet standing, there is a significant correlation between CoP sway area and trunk-leg coherence at frequencies ≤ 1.0 Hz (r=−0.395, p=0.017) and at frequencies >1.0 Hz (r=−0.374, p=0.025) (Figure 2). However, PwMS show no significant correlations between CoP sway area and trunk-leg coherence during eyes open quiet standing at frequencies ≤ 1.0 Hz (p=0.699) or at frequencies >1.0 Hz (p=0.210). Healthy control subjects show no correlations between CoP sway and trunk-leg coherence at any frequency.
Figure 2:
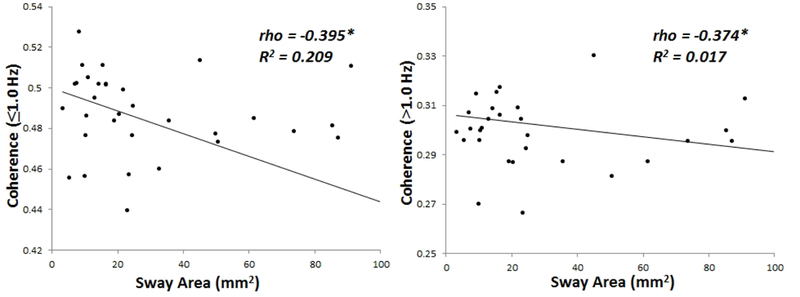
Relationship between center of pressure sway area and coherence at lower (p = 0.017) and higher frequencies (p = 0.025) in persons with multiple sclerosis only. There was no relationship between these variables in healthy control subjects. *Significant correlation
4. Discussion
The purpose of this study was to examine segment coherence during quiet standing in PwMS in order to understand segmental control strategies that are related to altered postural sway. As expected, coherence between the trunk and leg sagittal motion decreased as sway frequency increased from 0 to 3.0 Hz in both PwMS and healthy controls. Results also indicated that PwMS showed lower coherence between the trunk and legs than healthy controls during quiet standing at frequencies ≤ 1.0 Hz and at frequencies >1.0 Hz. These findings confirm our hypothesis that PwMS would display lower coherence compared to healthy controls. However, our second hypothesis that PwMS would demonstrate similar coherence across frequencies was not substantiated since coherence decreased as frequency increased. Our results also showed that coherence was not correlated with postural response latencies to external perturbations but coherence was significantly correlated with sway area during quiet standing with eyes closed in PwMS.
Decreasing coherence as the frequency of sway acceleration increases was demonstrated by both PwMS and healthy controls in the eyes open and eyes closed conditions. These findings are in agreement with previous work (Creath, et al., 2005) which demonstrated that healthy control subjects have higher trunk-leg coherence (in-phase) at low frequencies (< 1 Hz) and lower trunk-leg coherence (anti-phase) at higher frequencies. In the present study, all subjects appear to follow the expected transition from higher coherence to lower coherence as frequency increases. Coherence was lower in PwMS compared to healthy controls at lower frequencies (≤ 1.0 Hz) indicating PwMS utilize less of an ankle strategy and more of a mixed hip-ankle strategy at these frequencies. In contrast, at lower frequencies- healthy subjects move with the trunk and legs in-phase simulating an ankle strategy (Creath, et al., 2005). It is at higher frequencies only that healthy subjects utilize a mixed hip-ankle strategy (Creath, et al., 2005), and the present study shows that PwMS utilize a mixed strategy at both higher and lower frequencies. To summarize, these results indicate that PwMS used the same mixed hip-ankle strategy regardless of the sway frequency (low or high) which is a different segment control pattern, relative to sway frequency, than is_seen in healthy subjects .
These results agree with previous studies showing that patients with poor proprioception use more of a hip strategy to control posture compared to control subjects (Horak & Nashner, 1986; Horak, Nashner, & Diener, 1990). Adequate postural control occurs as a function of somatosensory information being integrated with vestibular information necessary for an adequate motor response to maintain control of stance (Creath, Kiemel, Horak, & Jeka, 2008). PwMS can demonstrate both somatosensory deficits (Cameron, et al., 2008; Huisinga, et al., 2014) and muscle weakness and asymmetry (Chung, Remelius, Van Emmerik, & Kent-Braun, 2008). Because upright stance requires a mixture of biomechanical and neural control (Zhang, et al., 2007), it is’s likely that both somatosensory deficits and strength asymmetry contribute to the altered coherence between legs and trunk in PwMS.
This study also showed that coherence was significantly negatively correlated with postural sway during eyes closed quiet standing where larger the postural sway area indicated lower coherence (mixed ankle-hip strategy) between trunk and legs (Figure 2). The use of a mixed hip-ankle strategy when sway is large is helpful for equilibrium control since the hip strategy moves the body CoM faster than the ankle strategy (Kuo, Speers, Peterka, & Horak, 1998). In PwMS, the mixed strategy is used at both lower and higher frequencies indicating that PwMS rely on a similar control strategy regardless of task demands and effectively lack adaptability in postural control. Previous studies have also demonstrated that PwMS have reduced adaptability of COP sway during postural sway tasks (Huisinga, Yentes, et al., 2012; Hunt, Widener, & Allen, 2014) and of trunk sway during gait (Huisinga, Mancini, St George, & Horak, 2013). The present study demonstrated that this reduced adaptability could be due to similar control strategies being used across tasks rather than using adaptive strategies which are task dependent.
Postural response latencies were not correlated with coherence in PwMS or in healthy controls. Previous studies have demonstrated that postural response latencies are significantly correlated with sway area (Cameron, et al., 2008; Huisinga, et al., 2014) and the present study demonstrated that coherence was correlated with sway area. Lack of correlation between latencies and coherence may indicate that latencies are reflected more directly in task performance, i.e. COP sway, rather than performance strategy, i.e. coherence. Since there was also no correlation between latencies and coherence in healthy controls, it seems that even in a healthy system, individual somatosensory and motor conduction times may not be directly reflected in segmental control strategy. Alternatively, other symptoms in PwMS such as muscle weakness (de Haan, de Ruiter, van Der Woude, & Jongen, 2000) and strength asymmetry (Chung, et al., 2008) could be more related to segmental control strategy than muscle latencies but those symptoms were not measured in the present study. One limitation of the present study is that the postural response latencies were measured during a postural perturbation task while coherence was measured during quiet standing. If the experimental set up was different, it would be possible to directly measure latencies and coherence at the same time.
5. Conclusions
Using body-worn inertial sensors, we demonstrated that PwMS display an abnormally coordinated sway pattern that is similar regardless of task difficulty (low frequency versus high frequency sway accelerations) which agrees with previous studies examining sway with nonlinear measures (Huisinga, Filipi, & Stergiou, 2012; Huisinga, Yentes, et al., 2012). Because segment coherence reflects motor coordination for postural control strategies, it would be of interest to monitor leg and trunk coherence in PwMS who have intentionally received a balance-training intervention. As previously recommended, PwMS would likely benefit from a training intervention aimed at incorporating variability in the training task (Huisinga, Yentes, et al., 2012). In this way, the consistent use of mixed segmental control strategy during standing in PwMS, which is related to in abnormal postural sway, may be addressed.
Highlights.
Postural sway is abnormal in persons with multiple sclerosis (PwMS)
Abnormal trunk and leg coordination, as represented by coherence, during standing is related to postural sway deficits
Abnormal coherence of segment acceleration could be measured in a clinic as an indicator of postural control changes in neurological pathologies
Coherence of trunk and leg in PwMS could be abnormal due to sensorimotor delays which could be modifiable with therapy
Acknowledgements
We would like to acknowledge the assistance of Rebecca St. George for her help with data collection. This work was supported by grants from the National Multiple Sclerosis Society (RG4914A1/2; PI-JH) (MB 0011; PI-FH), the United States Department of Veteran’s Affairs Merit Award (E1075-R; PI-FH), and the National Institutes of Health (R01 AG00645729; PI-FH).
References
- Bardy BG, Marin L, Stoffregen TA, & Bootsma RJ (1999). Postural coordination modes considered as emergent phenomena. J Exp Psychol Hum Percept Perform, 25, 1284–1301. [DOI] [PubMed] [Google Scholar]
- Bardy BG, Oullier O, Bootsma RJ, & Stoffregen TA (2002). Dynamics of human postural transitions. J Exp Psychol Hum Percept Perform, 28, 499–514. [PubMed] [Google Scholar]
- Bowen J, Gibbons L, Gianas A, & Kraft GH (2001). Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Mult Scler, 7, 201–206. [DOI] [PubMed] [Google Scholar]
- Cameron MH, Horak FB, Herndon RR, & Bourdette D (2008). Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosensory & motor research, 25, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung LH, Remelius JG, Van Emmerik RE, & Kent-Braun JA (2008). Leg power asymmetry and postural control in women with multiple sclerosis. Med Sci Sports Exerc, 40, 1717. [DOI] [PubMed] [Google Scholar]
- Craig JJ, Bruetsch AP, Lynch SG, Horak FB, & Huisinga JM (2017). Instrumented balance and walking assessments in persons with multiple sclerosis show strong test-retest reliability. J Neuroeng Rehabil, 14, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creath R, Kiemel T, Horak F, & Jeka JJ (2008). The role of vestibular and somatosensory systems in intersegmental control of upright stance. J Vestib Res, 18, 39. [PMC free article] [PubMed] [Google Scholar]
- Creath R, Kiemel T, Horak F, Peterka R, & Jeka J (2005). A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes. Neurosci Lett, 377, 75–80. [DOI] [PubMed] [Google Scholar]
- de Haan A, de Ruiter CJ, van Der Woude LH, & Jongen PJ (2000). Contractile properties and fatigue of quadriceps muscles in multiple sclerosis. Muscle & nerve, 23, 1534. [DOI] [PubMed] [Google Scholar]
- Horak FB, & Macpherson JM (1996). Postural orientation and equilibrium In Handbook of Physiology, Section 12: Exercise: Regulation and Integration of multiple systems. In Rowell LB & Shepherd JJ (Eds.). New York: Oxford University Press. [Google Scholar]
- Horak FB, & Nashner LM (1986). Central programming of postural movements: adaptation to altered support-surface configurations. Journal of Neurophysiology, 55, 1369–1381. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM, & Diener HC (1990). Postural strategies associated with somatosensory and vestibular loss. Experimental brain research.Experimentelle Hirnforschung.Experimentation cerebrale, 82, 167. [DOI] [PubMed] [Google Scholar]
- Huisinga JM, Filipi ML, & Stergiou N (2012). Supervised resistance training results in changes in postural control in patients with multiple sclerosis. Motor control, 16, 50–63. [DOI] [PubMed] [Google Scholar]
- Huisinga JM, Mancini M, St George RJ, & Horak FB (2013). Accelerometry reveals differences in gait variability between patients with multiple sclerosis and healthy controls. Ann Biomed Eng, 41, 1670–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisinga JM, St George RJ, Spain R, Overs S, & Horak FB (2014). Postural response latencies are related to balance control during standing and walking in patients with multiple sclerosis. Arch Phys Med Rehabil, 95, 1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisinga JM, Yentes JM, Filipi ML, & Stergiou N (2012). Postural control strategy during standing is altered in patients with multiple sclerosis. Neurosci Lett. [DOI] [PubMed] [Google Scholar]
- Hunt CM, Widener G, & Allen DD (2014). Variability in postural control with and without balance- based torso- weighting in people with multiple sclerosis and healthy controls. Phys Ther, 94, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, & Kasser SL (2012). Effects of dual tasking on the postural performance of people with and without multiple sclerosis: a pilot study. J Neurol, 259, 1166–1176. [DOI] [PubMed] [Google Scholar]
- Jeka J, Oie K, Schoner G, Dijkstra T, & Henson E (1998). Position and velocity coupling of postural sway to somatosensory drive. J Neurophysiol, 79, 1661. [DOI] [PubMed] [Google Scholar]
- Karst GM, Venema DM, Roehrs TG, & Tyler AE (2005). Center of pressure measures during standing tasks in minimally impaired persons with multiple sclerosis. J Neurol Phys Ther, 29, 170. [DOI] [PubMed] [Google Scholar]
- Kasser SL, Jacobs JV, Foley JT, Cardinal BJ, & Maddalozzo GF (2011). A prospective evaluation of balance, gait, and strength to predict falling in women with multiple sclerosis. Arch Phys Med Rehabil, 92, 1840–1846. [DOI] [PubMed] [Google Scholar]
- Kuo AD, Speers RA, Peterka RJ, & Horak FB (1998). Effect of altered sensory conditions on multivariate descriptors of human postural sway. Experimental brain research.Experimentelle Hirnforschung.Experimentation cerebrale, 122, 185. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF (1983). Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology, 33, 1444. [DOI] [PubMed] [Google Scholar]
- Moe-Nilssen R, & Helbostad JL (2002). Trunk accelerometry as a measure of balance control during quiet standing. Gait Posture, 16, 60–68. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, & Weinshenker BG (2000). Multiple sclerosis. N Engl J Med, 343, 938. [DOI] [PubMed] [Google Scholar]
- Peterka RJ (2002). Sensorimotor integration in human postural control. J Neurophysiol, 88, 1097. [DOI] [PubMed] [Google Scholar]
- Rocchi L, Chiari L, & Horak FB (2002). Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry, 73, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Emmerik RE, Remelius JG, Johnson MB, Chung LH, & Kent-Braun JA (2010). Postural control in women with multiple sclerosis: effects of task, vision and symptomatic fatigue. Gait Posture, 32, 608. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kiemel T, & Jeka J (2007). The influence of sensory information on two-component coordination during quiet stance. Gait Posture, 26, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]


