Abstract
Renal cell carcinomas (RCC) with overlapping histomorphologic features poses diagnostic challenges. This is exemplified in RCCs with eosinophilic cytoplasm that include, eosinophilic, solid and cystic RCC (ESC RCC), RCCs in germline aberrations of tuberous sclerosis complex (TSC) genes mutated (TSC RCC) individuals and other RCC subtypes. Here we used next-generation sequencing (NGS) technology to molecularly profile 7 ESC RCC tumors. Mutational and copy number analysis of NGS data revealed mutually exclusively somatic bi-allelic loss of TSC1 or TSC2 genes both negative regulators of the mammalian target of rapamycin (mTOR) pathway in 85% (6 / 7) of evaluated cases. Thus, lack of germline TSC aberration in matched non-neoplastic renal parenchyma distinguishes ESC RCC from TSC RCC. Immunohistochemistry data shows mTOR pathway activation in all tumors, thus supporting a pathognomonic role for TSC aberrations in ESC RCC. Our study clarifies the molecular identity of ESC RCC, provide basis for the revision of current RCC classification, and may guide future therapeuties.
Patient Summary
Molecular characterization of ESC RCC revealed recurrent and mutually exclusive somatic homozygous loss of TSC family genes. This observation provides greater insight into the unique biology of this novel type of tumor and potentially expands the therapeutic options for ESC RCC patients.
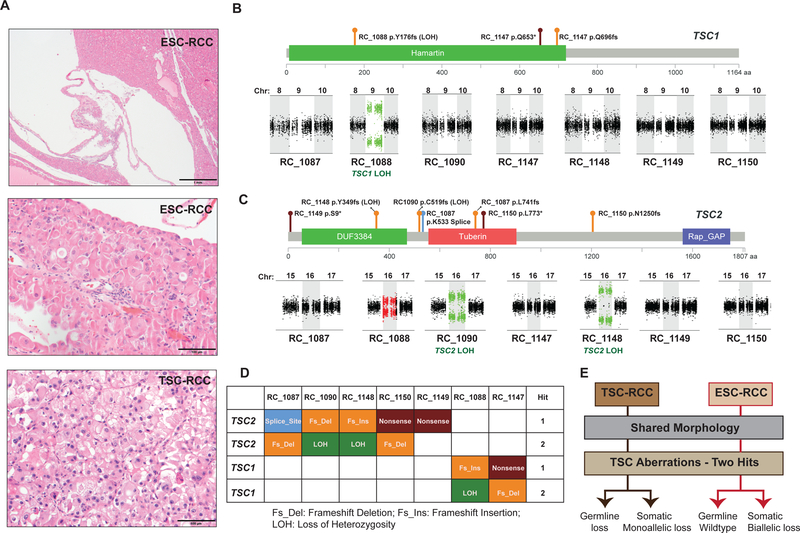
A unique renal neoplasm with eosinophilic cytoplasm and solid and cystic growth pattern[1] (Figure 1A) named eosinophilic solid and cystic renal cell carcinoma (ESC RCC), may present sporadically or associated with the tuberous sclerosis complex (TSC RCC) [2]. While TSC RCC is associated with germline and accompanied somatic mutations of TSC1 or TSC2 genes, the molecular drivers of sporadic ESC RCC remains unknown.
FIGURE 1.
Somatic bi-allelic loss of TSC genes in ESC RCC. A) Low (top) and high (middle) magnification of the histology images of a representative ESC RCC case with solid and cystic growth pattern and cells with abundant eosinophilic cytoplasm; high (bottom) magnification of a TSC RCC case with similar morphology to ESC RCC. Scale bars low: 1mm and high: 100μm. B) Schematic representation of TSC1 protein domains with the observed mutational events in ESC RCC (top) and copy number LOH plots for chromosomes 8, 9, and 10 (bottom). TSC1 is located on chromosome 9 in the human genome. C) Schematic representation of TSC2 protein functional domains with the observed mutational events in ESC RCC (top) and copy number LOH plots for chromosomes 15, 16, and 17 (bottom). TSC2 is located on chromosome 16 in the human genome. Deleterious somatic mutations, including frameshift indels (orange), nonsense (deep red), and splice site (blue) events in TSC genes are depicted. Copy number plots green: LOH; red: gain; black: diploid. D. Table summarizes the mutually exclusive TSC gene aberrations in ESC RCC samples. E. Flow chart indicates the type of TSC aberrations that characterizes and distinguishes TSC RCC from ESC.
Genomic profiling of major RCC including clear cell (ccRCC), papillary (pRCC), and chromophobe (chRCC) note recurrent aberrations in at least 16 different genes at appreciable frequencies [3]. In contrast, recurrent molecular aberrations among rare RCCs remain poorly understood. Using next-generation sequencing (NGS) we discovered frequent bi-allelic loss of Hippo pathway genes, such as protein tyrosine phosphatase 14 (PTPN14), in the rare mucinous, tubular, and spindle cell carcinoma (MTSCC) of the kidney[4]. These results indicated that RCC subtypes with a unique morphology, may harbor distinct genomic landscapes. Hence, we employed a similar sequencing approach to interrogate ESC RCC.
Firstly, we identified 7 ESC RCC cases confirmed to have arisen sporadically (Figure 1A) and obtained formalin fixed paraffin embedded (FFPE) tissue from the tumor and the matched non-neoplastic renal parenchyma. All ESC RCC demonstrated classic morphologic features, including a solid and cystic growth pattern and cells with abundant eosinophilic cytoplasm (Figure 1A). As previously reported, such ESC RCC are virtually identical to a subset of TSC RCC[2]. Genomic DNA isolated from tissue samples were subjected to whole exome NGS using validated methodology[4]. Available clinical information and various quality metrics of the sequencing data in terms of alignment rate, genomic coverage and PCR duplication rate were as expected for FFPE samples[4], and presented in Supplemental Table 1. Mutations were identified as previously described[4], and ESC RCC demonstrated a low mutational burden similar to the other RCC subtypes characterized thus far (Supplemental Figures 1a and 1b and Supplemental Table 2)[3]. Mutation analysis of ESC RCC identified somatic deleterious mutations (frame shift indels, nonsense, and splice site mutations) in both TSC2 (5 cases) and TSC1 (2 cases). In addition, we observed 3 cases a bi-allelic loss, 2 with unique mutations in TSC2 (cases RC_1087, RC_1150) and 1 in TSC1 (case RC_1147) (Figures 1B and 1C). Importantly these aberrations are tumor specific as the adjacent non-neoplastic renal parenchyma showed only the wildtype sequence in all seven cases, thus confirming a somatic event without any germline involvement. We also interrogated for copy number variations (CNV) in the tumor samples to investigate for a potential second hit in the 4 cases (TSC2: cases RC_1090, RC_1148 and RC_1149; TSC1: case RC_1088) that displayed deleterious mutations in only one allele. CNV analysis revealed loss of heterozygosity (LOH) in TSC2 (cases RC_1090 and RC_1148) on chromosome 16 and in TSC1 (case RC_1088) on chromosome 9, thus indicating a biallelic loss in these cases (Figures 1B and 1C). As expected, cases with LOH also showed high variant allele fraction (close to the estimated tumor content) for TSC mutations (Supplemental Table 2). In case RC_1149, we were unable to account for a second hit with existing analysis, but at present could not eliminate epigenetic inactivation; this case also had a lower tumor content upon morphologic evaluation (Supplementary Table 1). In summary, exome sequencing analysis revealed mutually exclusive somatic loss of either TSC1 or TSC2 genes in a mutually exclusive fashion in the majority of ESC RCC cases (Figure 1D and 1E).
We next interrogated for TSC gene mutations in the publically available pan-RCC NGS dataset generated by The Cancer Genome Atlas consortium (TCGA)(n=1316 patients). In the TCGA pan-RCC cohort, deleterious mutations in TSC1 were found in 17/1316 cases (8 ccRCC, 2 chRCC, 2 pRCC, 2 unRCC and 3 ncRCC) and TSC2 aberrations in 22/1316 cases (8 ccRCC, 3 chRCC, 6 pRCC, 3 unRCC and 2 ncRCC). Only 2/39 cases, however, showed any evidence of somatic bi-allelic loss (Supplemental Tables 3 and 4), thus indicating that the TSC bi-allelic loss within RCC is a rare event.
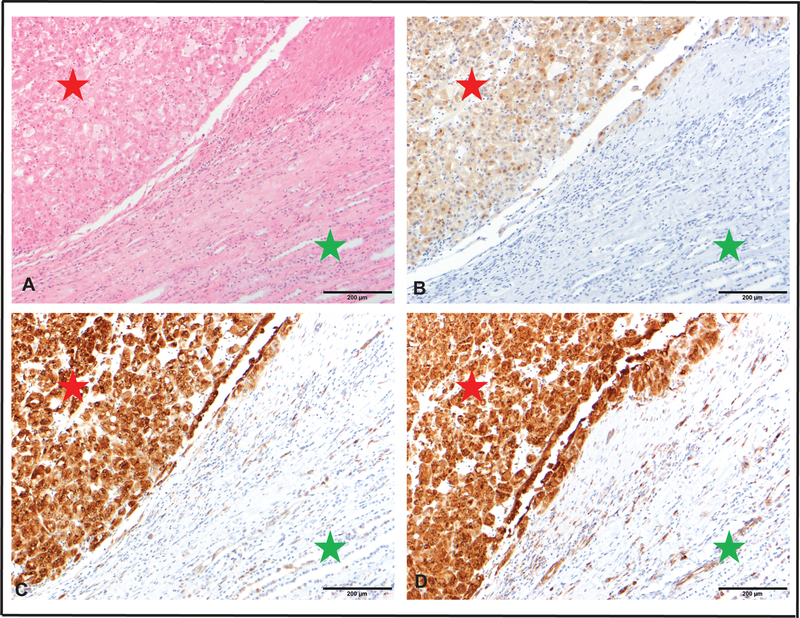
TSC1/2 and TBC1D7 (TBC1 Domain Family Member 7) constitute the heterotrimeric TSC complex that activates the small GTPase Rheb (Ras Homolog enriched in brain) which directly activates mTOR complex 1 (mTORC1) [5, 6]. TSC genes regulate mTOR signaling which coordinates cell growth by altering mRNA translation, metabolism, and protein turnover[7]. Regulation of protein synthesis by mTORC1 is largely effected by protein phosphorylations that activates p70S6 kinase 1 (S6K1) and inhibits eIF4E binding protein 1(4EBP1)[7]. To determine if the TSC1/2 aberrations in ESC RCC lead to mTOR pathway activation, we monitored phosphorylation status of both S6K1 (at two sites) and 4EBP1 by immunohistochemistry. We noted significantly increased phosphorylation in ESC RCC compared to the adjacent non-neoplastic renal parenchyma for these effector proteins (Figure 2 and Supplemental Table 5). This functional readout strongly supports the pathognomonic role of TSC1/TSC2 mutations in these tumors. Overall, the combination of the previously reported features, (e.g. distinct morphology and a predominant CK7-negative/CK20-positive phenotype associated with an indolent behavior) and the frequent TSC mutations coupled with the activation of the mTOR signaling pathway within these tumors suggest that ESC RCC represents a novel subtype of RCC. ESC RCC demonstrates indolent behavior with rare metastases[8, 9] and is typically cured by surgical excision. Our findings suggest that TSC mutations in such tumors have the potential to be tailored as mTOR inhibitors.
FIGURE 2.
Bi-allelic loss of TSC genes activates mTOR pathway in ESC-RCC A. H&E stained section from ESC-RCC showing characteristic morphological features (red star) and background benign renal parenchyma (green star). Scale bar 200 μm. B. Tumor cells diffusely and moderately express phospho-4E-BP1 (red star) with negative expression in the background renal parenchyma (green star). Scale bar 200 μm. C. Tumor cells diffusely and strongly express phospho-S6 Rib P D68.F8 (red star) with weak expression in certain cell types in the background renal parenchyma (green star). Scale bar 200 μm. D. Tumor cells diffusely and strongly express phospho-S6 Rib P D57.2.2E (red star) with weak to moderate expression in certain cell types within the background renal parenchyma (green star). Scale bar 200 μm.
Given the relative rarity of the disease, our limited number of cases should be considered to be a moderate cohort size. Furthermore, while many levels of indirect evidence support the clonal nature of the TSC1/2 mutations (immunohistochemistry data, relationship between tumor purity and VAF), multi-regional analysis was not performed, and is a limitation of this study.
ESC RCC is currently not a formal renal tumor subtype in the 2016 World Health Organization classification[10]. However, our findings define specific genomic insults that link somatic aberrations in TSC genes to the majority of ESC RCC cases, and thus support the inclusion of these tumors as a distinct entity within the contemporary classification of RCC.
Supplementary Material
REFERENCES
- [1].Trpkov K, Hes O, Bonert M, Lopez JI, Bonsib SM, Nesi G, et al. Eosinophilic, Solid, and Cystic Renal Cell Carcinoma: Clinicopathologic Study of 16 Unique, Sporadic Neoplasms Occurring in Women. The American journal of surgical pathology. 2016;40:60–71. [DOI] [PubMed] [Google Scholar]
- [2].Guo J, Tretiakova MS, Troxell ML, Osunkoya AO, Fadare O, Sangoi AR, et al. Tuberous sclerosis-associated renal cell carcinoma: a clinicopathologic study of 57 separate carcinomas in 18 patients. The American journal of surgical pathology. 2014;38:1457–67. [DOI] [PubMed] [Google Scholar]
- [3].Chen F, Zhang Y, Senbabaoglu Y, Ciriello G, Yang L, Reznik E, et al. Multilevel Genomics-Based Taxonomy of Renal Cell Carcinoma. Cell reports. 2016;14:2476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mehra R, Vats P, Cieslik M, Cao X, Su F, Shukla S, et al. Biallelic Alteration and Dysregulation of the Hippo Pathway in Mucinous Tubular and Spindle Cell Carcinoma of the Kidney. Cancer discovery. 2016;6:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–13. [DOI] [PubMed] [Google Scholar]
- [6].Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012;47:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169:361–71. [DOI] [PubMed] [Google Scholar]
- [8].McKenney JK, Przybycin CG, Trpkov K, Magi-Galluzzi C. Eosinophilic solid and cystic renal cell carcinomas have metastatic potential. Histopathology. 2018;72:1066–7. [DOI] [PubMed] [Google Scholar]
- [9].Li Y, Reuter VE, Matoso A, Netto GJ, Epstein JI, Argani P. Re-evaluation of 33 'unclassified' eosinophilic renal cell carcinomas in young patients. Histopathology. 2018;72:588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. European urology. 2016;70:93–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.