The combined use of conventional synthetic DMARDs (csDMARDs) and biologic DMARDs (bDMARDs) as well as the application of the treat-to-target strategy has revolutionized treatment of RA, and clinical remission or low disease activity are now realistic targets, achieved by the majority of RA patients [1]. However, only 30–50% of patients treated with the combination of csDMARDs and bDMARDs achieved remission assessed by composite measures within 6 months, and unmet needs remain for the treatment of RA. In addition, bDMARDs can potentially induce immunogenicity by developing anti-drug antibodies, which leads to loss of efficacy of the drug. Moreover, bDMARDs require intravenous or subcutaneous use because they are easily digested following oral application.
Under such circumstances, targeted synthetic DMARDs—orally available low molecular mass products—are attracting attention, because they enter into cytoplasm and directly regulate intracellular signals. The multiple cytokines and cell-surface-functioning antigens bind to receptors and activate various signalling pathways, including phosphorylation of kinase proteins. Today, 518 kinases are recognized in the human kinome and they are divided into eight families. Janus kinases (JAKs) belong to the tyrosine protein kinase family and play pivotal roles in intracellular signalling. After the engagement of cytokine receptors constitutively bound to JAK, JAK is activated and, in turn, phosphorylates the cytokine receptors, resulting in phosphorylation of the signal transducers and activators of transcription (STATs), which subsequently translocate into the nucleus where they regulate gene expression. The JAK family consists of four members, JAK1, JAK2, JAK3 and TYK2, and >40 different cytokines and growth factors are shown to activate specific combinations of JAKs and STATs, which are implicated in the pathogenesis of RA [2, 3]. O’Shea et al. [4] also document the downstream biological effects of various JAKs, focusing on safety as well as efficacy.
Based on this background, multiple clinical trials using orally available JAK inhibitors in patients with RA have been undertaken or are ongoing globally. Results of clinical trials using tofacitinib, relatively selective for JAK1/3, and baricitinib, selective for JAK1/2, showed tolerable safety in the short term, and high clinical efficacy in patients with active RA and naïve to csDMARDs or showing an inadequate response to csDMARDs or bDMARDs. According to the results, tofacitinib and baricitinib were launched in 2012 and 2017, respectively, in some countries. As Taylor has documented here, both tofacitinib and baricitinib demonstrated rapid improvements in disease activity, functional status and patient-reported outcomes as well as disease modification in the patients [5]. It is noteworthy that the efficacy of tofacitinib was comparable to that of adalimumab, a bDMARD targeting TNF, and that baricitinib was superior to adalimumab at week 12 for the ACR20 response [6, 7]. The most commonly observed adverse events were related to infection, haematological, hepatic and renal disorders, and hypercholesterolaemia. Unlike bDMARDs targeting particular molecules, kinase inhibitors regulate signalling induced by multiple cytokines and their safety concerns are sometimes complicated and are still under discussion. As Harigai described, no signs of increased risk for malignancy and opportunistic infection have been reported except for herpes zoster in patients with RA treated with JAK inhibitors [8]. Their association with rare serious adverse events, including thromboembolic events, gastrointestinal perforation and interstitial lung disease, remains debated. Further investigation by post-marketing surveys is needed to determine their risk–benefit ratio and selection of the most appropriate patients for such therapy, which would help us understand the positioning of these drugs [8].
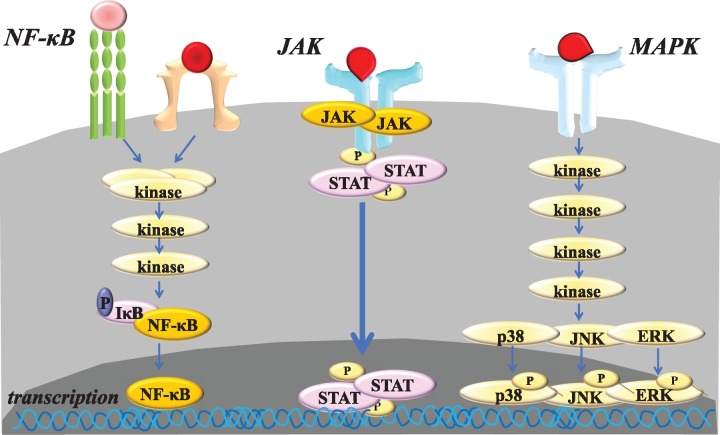
Westhovens writes that clinical phase III trials using filgotinib and upadacitinib, specific JAK1 inhibitors, as well as decernotinib and peficitinib, JAK3-selective inhibitors, are underway and that targeted selectivity of the JAK inhibitors is a complex interplay between selectivity tested in vitro, dosing, drug-to-drug interaction, role of eventual metabolites, and pharmacokinetics and pharmacodynamics [9]. On the other hand, it is well known that thousands of low-molecular mass products targeting kinases such as mitogen-activated protein kinase and nuclear factor-κB have failed in development during the long history of treatments. The success of JAK inhibitors may have many reasons. Different from other signalling pathways, the JAK–STAT pathway is simple, namely, after ligation of receptors, JAK–STAT associated with receptors is phosphorylated and STAT in turn translocates into the nucleus and induces transcription of target genes. Such a structural simplicity may lead to the simplicity of tolerable safety and marked efficacy of the inhibitors, although adverse events probably involving off-target effects of these JAK inhibitors remain unresolved (Fig. 1). Fragoulis et al. [10] document that the clinical studies using JAK inhibitors are ongoing in their application for other rheumatic, autoimmune and allergic diseases, including psoriatic arthritis, spondyloarthropathies, inflammatory bowel diseases, skin disorders such as psoriasis, alopecia areata and atomic dermatitis, non-infectious uveitis, giant cell arteritis, systemic lupus erythematosus, systemic sclerosis, Sjogren’s syndrome and dermatomyositis. Moreover, the success of JAK inhibitors affects the research on intracellular signalling and its relevance to pathological processes as well as development of the subsequent compounds, including targeted synthetic DMARDs targeting spleen tyrosine kinase and Bruton’s tyrosine kinase, which are in clinical trials. Although JAK inhibitors are novel, they have new modes of action and may, therefore, effectively close the unmet medical needs. Thus, JAK inhibitors have the potential to bring the next paradigm shift in treatment strategy and will provide not only clues to advance the elucidation of the pathological and clinical characteristics of RA, but also benefits to patients as a result.
Fig. 1.
Structural simplicity of the JAK–STAT signalling pathway
After the engagement of cytokine receptors constitutively bound to JAK, JAK is activated and in turn phosphorylates the cytokine receptors, resulting in phosphorylation of STAT, which subsequently translocate into the nucleus, where it regulates gene expression. Different from other kinase such as MAPK and NF-kB, such a structural simplicity of the JAK–STAT signalling pathway may lead to the simplicity of tolerable safety and marked efficacy of the JAK inhibitors, which have led to the success of their development. JAK: Janus kinase; MAPK: mitogen-activated protein kinase; NF-κB: nuclear factor-κB; STAT: signal transducers and activators of transcription.
Supplement: This supplement is supported by a grant from Gilead Sciences, Inc.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: Y.T. has received speaking fees and/or honoraria from Daiichi-Sankyo, Astellas, Eli Lilly, Chugai, Sanofi, Abbvie, Pfizer, YL Biologics, Bristol-Myers, Glaxo-Smithkline, UCB, Mitsubishi-Tanabe, Novartis, Eisai, Takeda, Janssen, Asahi-kasei and has received research grants from Mitsubishi-Tanabe, Bristol-Myers, Eisai, Chugai, Takeda, Abbvie, Astellas, Daiichi-Sankyo, Ono, MSD, Taisho-Toyama, Japan.
References
- 1. Smolen JS, Aletaha D, McInnes IB.. Rheumatoid arthritis. Lancet 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka Y. Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. J Biochem 2015;158:173–9. [DOI] [PubMed] [Google Scholar]
- 3. O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A.. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 2013;72:ii111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gadina M, Le MT, Schwartz DM. et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology 2019;58:i4–i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor PC. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology 2019;58:i17–i26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Vollenhoven RF, Fleischmann R, Cohen S. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 7. Taylor PC, Keystone EC, van der Heijde D. et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376: 652–62. [DOI] [PubMed] [Google Scholar]
- 8. Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology 2019;58:i34–i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westhovens R. Clinical efficacy of new JAK inhibitors under development. Just more of the same? Rheumatology 2019;58:i27–i33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fragoulis GE, McInnes IB, Siebert S.. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology 2019;58:i43–i54. [DOI] [PMC free article] [PubMed] [Google Scholar]