Abstract
Tofacitinib and baricitinib are the first orally available, small-molecule inhibitors of Janus kinase (JAK) enzymes to be approved for the treatment of RA. Tofacitinib is a selective JAK1, 3 inhibitor with less activity against JAK2 and TYK2 and baricitinib is a selective, oral JAK1, 2 inhibitor with moderate activity against TYK2 and significantly less activity against JAK3. Both drugs have undergone extensive phase III clinical trials in RA and demonstrated rapid improvements in disease activity, function and patient-reported outcomes as well as disease modification. Tofacitinib 5 mg bd, was approved by the Federal Drug Administration in 2012 for the treatment of RA in patients who are intolerant or unresponsive to MTX. An extended release formulation for the treatment of RA was approved by Federal Drug Administration in 2016. In 2017 the European Medicines Agency approved tofacitinib 5 mg bd in combination with MTX and baricitinib 4 mg and 2 mg once daily for the treatment of moderate to severe active RA in adult patients who are intolerant or unresponsive to one or more conventional synthetic DMARDs.
Keywords: Janus kinase, rheumatoid arthritis, efficacy, tofacitinib, baricitinib, small-molecule kinase inhibitor, symptoms and signs, structural damage, function, patient reported outcomes
Rheumatology key messages
Tofacitinib is an oral Janus kinase inhibitor with selectivity for Janus kinase 1 and 3.
Baricitinib is an oral Janus kinase inhibitor with selectivity for Janus kinase 1 and 2.
In RA clinical trials, tofacitinib and baricitinib demonstrated rapid improvements in multiple outcome measures.
Introduction
On a worldwide basis, rheumatoid arthritis (RA) is the commonest cause of inflammatory polyarthritis. It is now known to be a polygenic disorder in which gene–environment interactions play a role, and yet the aetiology of RA remains poorly understood. However, there have been significant advances in understanding RA’s pathology over the past generation and the role of several key pro-inflammatory cytokines, such as TNF and IL-6, and cell-associated targets, such as CD20 and co-stimulation molecules CD80/86, have been thoroughly validated by the advent of targeted biologic therapies [1].
Contemporary RA treatment recommendations [2, 3] emphasize the importance of early therapeutic intervention and treat-to-target strategies in which treatment adjustment is determined by measurement of therapeutic response with the target of remission or low disease activity [4]. Prior to the approval of the first targeted synthetic DMARDs (tsDMARDs), which will be the subject of this paper, clinical practice was to initiate treatment with conventional synthetic DMARDs (csDMARDs) such as MTX and a short period of glucocorticoids, followed in poor prognosis, refractory patients by parenterally administered biologic DMARDs (bDMARDs). The introduction of bDMARDs almost two decades ago revolutionized the long-term outcomes for many patients in terms of improved quality of life, reduced disability and mortality. But in spite of these advances, many unmet needs remain. For example, remission is only achieved and sustained in a minority of bDMARD-treated patients. And even then, troublesome symptoms may persist including pain, fatigue and morning joint stiffness [5]. Furthermore, loss of response to bDMARDs, in part as a consequence of the immunogenicity of administered protein, as well as drug discontinuations due to intolerance or adverse effects, emphasizes the ongoing need for a new generation of alternative therapies. Therefore, further advances remain necessary with a goal of restoring immune homeostasis, more complete symptom resolution and avoidance of the risks of immune suppression [1].
The first generation of protein-based, biologic therapies were large molecular mass molecules incapable of penetrating the lipid bilayer of the cellular membrane and were therefore directed against extracellular therapeutic targets. By contrast, low molecular mass, orally available, small molecules that target and inhibit components of the intracellular inflammatory signalling cascade have more recently been developed as an important alternative to biologic therapies for RA, and several have been thoroughly tested in clinical trials. Most successful among these to date have been inhibitors of the Janus kinase (JAK) enzymes. The JAK family comprises four members: JAK1, JAK2, JAK3 and TYK2.
A number of studies have demonstrated expression of different JAK isoforms and the downstream signal transducer and activator of transcription (STAT) proteins in synovial tissue and synovial cells [6–8]. Many proinflammatory cytokines implicated in RA pathogenesis bind to a specific group of so-called Type I and Type II cytokine receptors, which are structurally distinct from other receptors such as those that bind TNF and IL-1. Cytokines binding the Type I and II receptors are dependent on the JAK–STAT pathway for signal transduction. Therefore, several JAK inhibitors (jakinibs) with variable degrees of selectivity and specificity for the JAK enzymes have been investigated in RA.
To date, two JAK inhibitors or jakinibs, tofacitinib and baricitinib, have been approved for treatment of RA in certain regions. Tofacitinib, a first-generation, selective oral JAK1, 3 inhibitor with less activity against JAK2 and TYK2 [9], has undergone extensive clinical trials of twice daily dosing in RA [10–15]. It is approved for clinical use in over 80 countries. Baricitinib is a novel, potent and selective, first-generation, oral JAK1, 2 inhibitor with moderate activity against TYK2 and significantly less activity against JAK3 [16] that was generated by modifying the structure of tofacitinib. This was achieved by replacing the portion of the molecule that showed JAK1/JAK3 selectivity with a different chemical moiety, resulting in a new structure that exhibited specificity for JAK1 and JAK2 over JAK3 in kinase assays with IC50 values in the nanomolar range [9]. Baricitinib was recently approved in Europe for the treatment of RA following the completion of a large phase III clinical trial programme in which it was administered in a once daily dosing regimen [17–20]. A number of other investigational oral jakinibs with varying selectivity for the four JAK enzymes have been evaluated in phase II trials in patients with RA and these studies will be discussed in the article by Dr Westhovens [21].
It has been argued that a more selective JAK1 inhibitor might reduce dose-limiting toxicity, even though inhibition of JAK2 and JAK3 might contribute to efficacy [22]. For example, the γ chain cytokines signal through JAK1, 3 heterodimers and erythropoietin and thrombopoietin signal through JAK2 homodimers, as does granulocyte–macrophage colony-stimulating factor. However, to establish actual safety and benefit in vivo, careful scrutiny of clinical trial and real-world data for efficacy and risk will be necessary for each jakinib tested. In this paper, I will review the phase III clinical data for tofacitinib and baricitinib.
Evaluation of tofacitinib efficacy in clinical trials
Tofacitinib (Xeljanz) was the first jakinib developed for the treatment of autoimmune disease. Multiple randomized controlled trials (RCTs) have been completed in adult RA patients, including seven phase III trials (Table 1), demonstrating that tofacitinib is significantly efficacious for both early and established disease [23], as monotherapy, in combination with csDMARDs, including MTX, and in both treatment-naïve and treatment-refractory patients. Across the entire clinical trial programme, over 6000 patients received tofacitinib for up to 8.5 years for a total of over 19 400 years of patient exposure to drug. Tofacitinib, in a dose regime of 5 mg bd, was approved by the Federal Drug Administration (FDA) in 2012 for the treatment of RA in patients who are intolerant or unresponsive to MTX [24]. Subsequently, its effectiveness has been confirmed in over 4000 patients participating in clinical studies as well as in real-world experience. After review of the long term safety and efficacy data accrued, in January 2017 the European Medicines Agency recommended approval of tofacitinib for RA at the 5 mg bd dose. An extended release formulation of tofacitinib employing osmotic drug delivery was approved by the FDA for the treatment of RA in 2016 [25].
Table 1.
Tofacitinib: phase III clinical trials for moderate to severe RA
| ORAL Start [26] | ORAL Solo [11] | ORAL Sync [12] | ORAL Scan [14] | ORAL Standard [15] | ORAL Strategy [27] | ORAL Step [10] | |
|---|---|---|---|---|---|---|---|
| MTX-naïve | csDMARD-IR | csDMARD-IR | MTX-IR | MTX-IR | MTX-IR | TNF-IR | |
| (n = 958) | (n = 611) | (n = 795) | (n = 797) | (n = 717) | (n = 1146) | (n = 399) | |
| Duration, months | 24 | 6 | 12 | 24 | 12 | 12 | 6 |
| Background treatment | None | None | csDMARDs | MTX | MTX | None | MTX |
| Arms |
|
|
|
|
|
|
|
| Feature | X-ray with monotherapy | Monotherapy | Background DMARDs | X-ray | Active control (adalimumab) | Active control (adalimumab non-inferiority) | TNFi failure |
| Coprimary end points |
|
|
|
|
|
|
|
The table summarizes the broad range of RA patient types studied in tofacitinib phase III confirmatory studies. ADA: adalimumab; csDMARD: conventional synthetic DMARD; HAQ-DI: Health Assessment Disability Index; IR: inadequate response; mTSS: modified Total Sharp Score; SDAI, Simplified Disease Activity Index; TNFi: TNF inhibitor.
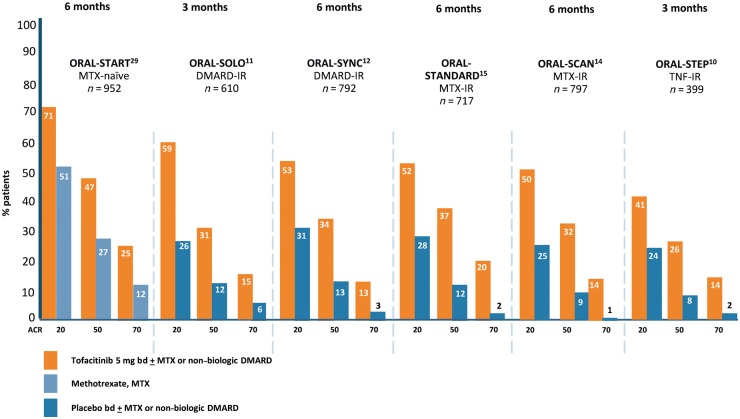
Across the phase III tofacitinib trial programme in RA (Table 1), patients achieved statistically significant and clinically meaningful improvements in disease activity as evaluated by the categorical criteria of the American College of Rheumatology (ACR20, 50 and 70) response criteria (Fig. 1) and other measures as well as improvements in functional status assessed by the Health Assessment Disability Index (HAQ-DI) and 36-Item Short Form Survey (SF-36). Furthermore, tofacitinib has been shown to be disease modifying and prevents progression of structural damage to joints as assessed both by conventional radiography and MRI. Importantly from a patient perspective, tofacitinib treatment also results in a rapid improvement in a range of patient-reported outcomes (PROs). Phase III clinical trials have established that tofacitinib in RA is superior to MTX, effective in MTX- and csDMARD-refractory, active RA, non-inferior in combination with MTX to the anti-TNF agent adalimumab plus MTX, and also efficacious in people with active RA who have failed to respond to multiple bDMARDs of different mechanisms of action. In the following paragraphs, the individual phase III trials and their key findings will be briefly described.
Fig. 1.
Tofacitinib: ACR responses at the time of primary end point for pivotal phase III clinical trials for moderate to severe RA
ORAL Solo was a 6-month RCT of 611 patients designed to evaluate the efficacy and safety of tofacitinib monotherapy in adults with active RA who had had an inadequate response (IR) to at least one csDMARD or bDMARD (DMARD-IR) and had discontinued all DMARDs except stable doses of antimalarial agents. Patients were randomized to tofacitinib 5 mg bd, 10 mg bd or placebo bd. At 3 months, patients randomized to placebo were blindly advanced to a predetermined dose of tofacitinib. Tofacitinib treatment was associated with statistically significant improvement in the co-primary end points of ACR20 (26.7% in placebo vs 59.8% for tofacitinib 5 mg bd; P < 0.001) and HAQ-DI (−0.19 in placebo vs −0.5 for tofacitinib 5 mg bd; P < 0.001) scores at month 3. There were also statistically significant improvements in ACR50 and ACR70 response criteria. The percentage of patients with a DAS28 of <2.6 was not significantly higher with tofacitinib (5.6 for the 5 mg bd dose) than with placebo (4.4) [11]. PROs provide quantitative data regarding the impact of RA to the individual that is of comparable and complementary value to the assessment of joint counts and laboratory tests. In the ORAL Solo study, tofacitinib demonstrated statistically significant and clinically meaningful improvements across multiple PROs. These included the SF-36 and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) at 3 months. Furthermore, there were statistically significant improvements in patient global assessment (PtGA), Pain and HAQ-DI that differentiated from placebo at week 2. The rapidity of benefit was striking with differentiation from baseline being recorded as early as 3 days after treatment initiation for PtGA and Pain [28].
ORAL Standard was a 12-month trial comparing tofacitinib both with placebo and with the anti-TNF biologic agent adalimumab in MTX-IR patients with active RA. In this double blind, double dummy study, patients taking background MTX were randomized to tofacitinib 5 mg bd, 10 mg bd, adalimumab 40 mg every other week, or placebo (to both tofacitinib and to adalimumab). At month 6, all placebo patients were blindly advanced to one of the two tofacitinib dose regimes. The three primary outcome measures were an improvement in ACR20 responses at month 6; the change from baseline to month 3 in HAQ-DI; and the percentage of patients meeting DAS28-4(ESR) remission criteria (<2.6) at month 6. At month 6, ACR20 response rates were significantly higher in the tofacitinib 5 mg or 10 mg arms (51.5% and 52.6%, respectively) and adalimumab arm (47.2%) than in the placebo arm (28.3%). There were also greater reductions in the HAQ-DI score at month 3 and higher percentages of patients with a DAS28-4(ESR) below 2.6 at month 6 in both the active-treatment groups than in the placebo group. The authors concluded that tofacitinib demonstrated superior efficacy to placebo with an efficacy that was numerically similar to adalimumab, although a formal non-inferiority comparison was not performed [15]. In the ORAL Standard study, a conservative non-responder imputation methodology was used for all data acquisition and analyses. The authors also examined the effect of an advancement penalty while using the non-responder imputation method. Under the advancement penalty, if a study subject fails to meet an end point at the pre-specified time of 3 months, he or she is declared a treatment failure for the duration of the study, even if achieving the end point at a later time. When analysis is undertaken using an advancement penalty method, the findings may tend to favour a drug with a faster kinetic of action. Importantly, the ORAL Standard trial was not designed to provide head-to-head comparative efficacy and should not be interpreted as evidence of tofacitinib superiority or non-inferiority to adalimumab. There were clinically meaningful improvements across a broad range of PROs with tofacitinib 5 and 10 mg bd and adalimumab that were significantly superior to placebo at 3 months and sustained to month 12. The greatest improvements were observed with tofacitinib 10 mg bd. The numbers needed to treat to achieve these PRO improvements were lowest for tofacitinib 10 mg bd and similar between tofacitinib 5 mg bd and adalimumab [29].
ORAL Sync was a 12-month RCT designed to evaluate the efficacy and safety of tofacitinib in combination with non-biologic DMARDs. To do this, 792 adults with active RA who had had an inadequate response to at least one csDMARD or bDMARD were randomized to tofacitinib 5 mg bd, 10 mg bd, or placebo bd in combination with various background non-biologic DMARDs. Of these, 79% of all patients were on background MTX. At month 6, all placebo patients were blindly advanced to one of the two tofacitinib dose regimes. Primary efficacy outcome measures were an improvement in ACR20 responses at month 6; the change from baseline to month 3 in HAQ-DI; and the percentage of patients meeting DAS28-4(ESR) remission criteria (<2.6) at month 6. Tofacitinib treatment was associated with statistically significant improvement in ACR20 response rates with a mean treatment difference for the 5 mg bd group compared with the combined placebo groups of 21.2% (95% CI, 12.2, 30.3%; P < 0.001). Tofacitinib demonstrated a rapid onset of benefit with significant ACR20 and ACR50 response rates observed by week 2 and ACR70 by week 4. The HAQ-DI scores at month 3 and DAS28-4(ESR) <2.6 response rates at month 6 were also superior in the tofacitinib groups vs placebo [12]. For all PROs assessed, by 3 months, a significantly greater proportion of patients in the higher dose tofacitinib 10 mg bd vs placebo dose reported improvements greater than the minimum clinically important differences. This was also true for the tofacitinib 5 mg bd dose with the exception of the SF‐36 role ‐emotional domain [30].
ORAL Scan was a 24-month trial designed to determine whether tofacitinib at 5 and 10 mg bd has an effect on structural progression in adult patients with active RA with an inadequate response to MTX. The study randomized 797 patients to tofacitinib 5 mg bd, 10 mg bd, or placebo bd, and all received background MTX. At 6 months, all patients on placebo were blindly advanced to one of the two tofacitinib regimes. At 3 months, non-responder placebo-treated patients were advanced in a blinded manner to receive tofacitinib. Both tofacitinib doses demonstrated clinical efficacy consistent with the findings of other studies. However, at 6 months, the observed structural damage progression of only 0.47 modified Total Sharp Score (mTSS) units in the placebo-treated arm was significantly less than the progression of radiographic joint damage expected in DMARD-inadequate responder populations based on historical data. In contrast, both tofacitinib arms showed negligible increases in mTSS (0.12 for 5 mg bd and 0.06 units for 10 mg bd) from baseline. However, it was not possible to demonstrate a statistical difference in structural damage inhibition by changes from baseline for tofacitinib 5 mg bd although there was statistically significant inhibition of radiographic progression (P < 0.05) with tofacitinib 10 mg bd [14].
ORAL Step was designed to test the efficacy of tofacitinib in patients with an inadequate response to at least one prior TNF inhibitor (TNFi). In this 6-month study, 399 subjects with active RA were randomized to receive tofacitinib 5 or 10 mg bd, or placebo, all in combination with MTX. At 3 months the patients randomized to placebo were blindly advanced to a predetermined dose of tofacitinib. Of the randomized patients, 64% had previously been treated with a single TNFi and 26% of patients had previously been treated with two TNFi. Of note this refractory RA population had a substantial burden of concomitant illness at screening. The primary end points, all at month 3, included ACR20 response rate, mean change from baseline in HAQ-DI and remission rates by (DAS)28-4(ESR) <2.6 [10]. ACR20 response rates were 41.7% (P = 0.0024) for tofacitinib 5 mg bd and 48.1% (P < 0.0001) for tofacitinib 10 mg bd vs 24.4% for placebo. Improvements from baseline in HAQ-DI were −0.43 (P < 0.0001) for tofacitinib 5 mg bd and −0.46 (P < 0.0001) for 10 mg bd vs −0.18 for placebo. Remission criteria by DAS28 < 2.6 were met for 6.7% (P = 0.0496) of patients on tofacitinib 5 mg bd and 8.8% (P = 0.0105) for 10 mg bd vs 1.7% for placebo. Tofacitinib treatment was also associated with statistically significant improvements in ACR50 and ACR70 response criteria and statistically significant and clinically meaningful improvements in PROs including PtGA, pain, physical function, Health related quality of life (HRQOL) and fatigue compared with placebo [31]. The percentage of patients with a DAS28 of <2.6 was significant in tofacitinib 10 mg bd group compared with placebo [10].
ORAL Start was a 24-month RCT designed to investigate the effects of tofacitinib monotherapy vs MTX in patients who were MTX-naïve (defined as no prior treatment or ⩽3 doses). In this study, 958 MTX naïve patients with active RA were randomized to tofacitinib 5 mg bd, 10 mg bd, or MTX starting at 10 mg/week with 5 mg/week increments every 4 weeks to 20 mg/week. Tofacitinib monotherapy resulted in clinically and statistically significant improvements in the primary outcomes that included reductions in signs and symptoms of RA assessed by ACR70 responses at month 6, achieved by 25.5% in the 5 mg bd group and 37.7% in the 10 mg bd group as compared with 12.0% of patients in the MTX group (P < 0.001 for both comparisons). Other primary outcomes included statistically significant improvements in physical function and inhibition of progression of structural damage compared with MTX. Mean changes in the mTSS from baseline to month 6 were significantly smaller in the tofacitinib groups than in the MTX group (0.2 in the 5 mg bd tofacitinib group and <0.1 in the 10 mg bd tofacitinib group, compared with 0.8 in the MTX group; P < 0.001 for both comparisons). Based on this trial, the FDA allowed the claim for slowing of radiographic progression [32]. There were also statistically significant differences between both tofacitinib doses and MTX with respect to multiple PROs with a rapid onset of effect. This was first evident at month 1 for PtGA, pain, HAQ-DI and fatigue by FACIT-F and at months 3 and 6 for other outcomes including HRQOL using Medical Outcomes Survey SF-36 questionnaire and quality of sleep using Medical Outcomes Survey-Sleep scale. The benefits persisted over the length of the trial [26].
ORAL Strategy was a phase IIIb/IV study designed to assess the comparative efficacy of tofacitinib monotherapy, tofacitinib plus MTX and adalimumab plus MTX for the treatment of MTX-IR RA. It was a 12-month head-to-head, non-inferiority double-blind, triple-dummy, active-controlled trial randomizing 1146 patients to tofacitinib 5 mg bd monotherapy, tofacitinib 5 mg bd plus MTX or adalimumab 40 mg every other week plus MTX. The primary end point was the proportion of patients who attained at least an ACR50 response rate in the full analysis set at month 6. A non-inferiority margin of 13% was set, the risk difference being based on half of the estimated difference in the ACR50 response rate between adalimumab and placebo (26%). ACR50 responses were observed in 38% receiving tofacitinib monotherapy, 46% receiving tofacitinib plus MTX and 44% receiving adalimumab plus MTX. Non-inferiority was declared for tofacitinib + MTX vs adalimumab + MTX but not for tofacitinib monotherapy vs either combination [27].
Evaluation of baricitinib efficacy in clinical trials
Baricitinib (Olumiant) was developed by Eli Lily and Incyte and approved by the European Medicines Agency in 2017 for use as 4 mg and 2 mg tablets once daily for the treatment of moderate to severe active RA in adult patients who have responded inadequately to, or who are intolerant to one or more csDMARDs. Baricitinib has not yet been approved by the FDA, with a request for additional clinical data to further determine the most appropriate doses and characterize safety concerns across treatment arms.
Baricitinib is a selective inhibitor of the JAK family without any effect on other kinase enzymes [33]. It inhibits JAK1 and JAK2, and to a much lesser extent TYK2. Baricitinib is considered a JAK3 sparing agent with 100-fold selectivity for JAK1 and JAK2 [9].
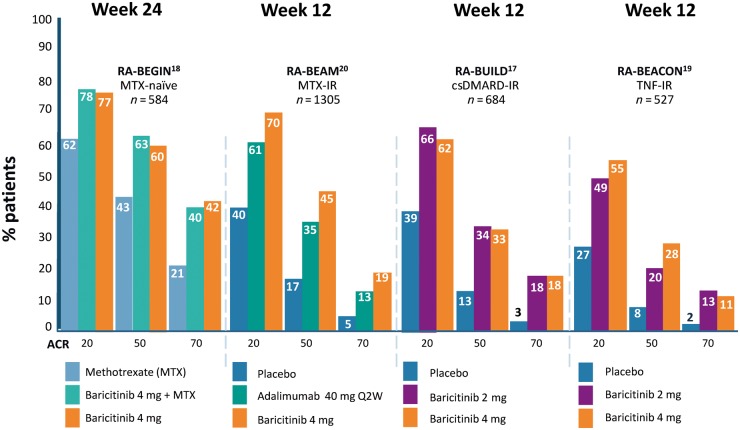
Baricitinib has demonstrated significant efficacy in phase II and III RCTs. Patients completing the RA-BEGIN, RA-BUILD, RA-BEAM and RA-BEACON phase III RCTs were eligible to enter long term extension studies including RA-BEYOND (Table 2, Fig. 2) and RA-BALANCE specifically for patients in Argentina, Brazil and China.
Table 2.
Baricitinib: phase III clinical trials for moderate to severe RA
| RA-BEGIN [18] | RA-BEAM [20] | RA-BUILD [17] | RA-BEACON [19] | RA-BEYOND [34] | |
|---|---|---|---|---|---|
| MTX-naïve | MTX-IR | csDMARD-IR | bDMARD-IR | OLE study | |
| (n = 588) | (n = 1307) | (n = 684) | (n = 527) | (n = 3073) | |
| Type of therapy | Monotherapy + combination therapy | Combination therapy | Combination therapy | Combination therapy | Monotherapy – patients who completed previous BARI RA study |
| Background | None/MTX | MTX | csDMARD | csDMARD | csDMARD |
| Active comparator | MTX | ADA + MTX | |||
| Arms |
|
|
|
|
|
| Duration, weeks | 24 | 52 | 24 | 24 | 52, with optional extension to 104 weeks |
| Primary end point | ACR20 (week 24) | ACR20 (week 12) | ACR20 (week 12) | ACR20 (week 12) | Safety & efficacy |
| Key secondary end point | Week 24: | Week 12: | Week 12: | Week 12: | Currently recruiting – estimated completion December 2020 |
|
|
|
|
The table summarizes the broad range of RA patient types studied in baricitinib phase III confirmatory studies. ADA: adalimumab; csDMARD: conventional synthetic DMARD; HAQ-DI: Health Assessment Disability Index; IR: inadequate response; OLE: open label extension; mTSS: modified Total Sharp Score; PBO: placebo; SDAI, Simplified Disease Activity Index.
Fig. 2.
Baricitinib: ACR responses at the time of primary end point for pivotal phase III clinical trials for moderate to severe RA
RA-BEGIN was a 52-week, phase III study trial designed to evaluate baricitinib as monotherapy or in combination with MTX vs MTX monotherapy in 588 patients with active RA and no prior treatment with csDMARDs (no or limited exposure to MTX) or bDMARDs. The study enrolled a population with a median disease duration of 0.2 years and with >91% of patients being DMARD naïve. Patients were randomized to baricitinib 4 mg monotherapy, MTX monotherapy or combination baricitinib 4 mg and MTX. The primary end point assessment was a non-inferiority comparison of ACR20 responses at 24 weeks between baricitinib monotherapy and MTX monotherapy. At 24 weeks, ACR20 response was significantly higher with baricitinib monotherapy (77%) and combination (78%) compared with MTX monotherapy (62%) and, in fact, baricitinib monotherapy met superiority criteria over MTX monotherapy with improvements observed as early as week 1. Baricitinib was also associated with reduction in radiographic progression compared with MTX monotherapy. However, the difference was only statistically significant for baricitinib in combination with MTX [18]. There was a greater magnitude and speed of pain relief in patients receiving baricitinib than in those on MTX monotherapy. A 70% improvement in pain, considered highly clinically relevant, was achieved by 50% of patients treated with baricitinib plus MTX in just 8 weeks, 12 weeks for baricitinib monotherapy and 20 weeks for MTX [13].
RA-BUILD was designed to assess the efficacy of baricitinib in patients with moderately to severely active RA who were refractory to or intolerant of csDMARDs. In a 24-week RCT, 684 csDMARD-IR patients were randomized to receive baricitinib 2 mg, 4 mg or placebo with background csDMARDs. The primary end point was met with more patients achieving ACR20 response at week 12 with baricitinib 4 mg than with placebo (62% vs 39%, P ⩽ 0.001). ACR20 responses were also significantly higher with baricitinib 2 mg and 4 mg compared with placebo as both 12 and 24 weeks. Statistically significant improvements were observed in DAS28, Simplified Disease Activity Index (SDAI) remission and HAQ-DI. A supportive assessment of the effect of baricitinib on radiographic progression of structural joint damage indicated a statistically significant reduction in mTSS progression at 24 weeks in both 2 mg (0.33) and 4 mg (0.15) arms compared with placebo (0.7) [17].
RA-BEAM was a 52-week, double-blind, randomized, double-dummy, placebo- and active-controlled, parallel-arm trial designed to assess improvements in disease activity, structural preservation and PROs including physical function, as well as safety and tolerability with oral baricitinib 4 mg once daily in 1307 MTX-IR RA patients with background MTX therapy. Comparisons were made with placebo and with the TNFi adalimumab, a standard-of-care bDMARD in this setting. The RA-BEAM statistical analysis plan used a pre-specified multiple-testing strategy to control for Type I error relating to the primary (ACR20 at week 12) and major secondary end points. Two gated assessments were made against adalimumab: a test of superiority with respect to change from baseline in DAS28-hsCRP and a test of non-inferiority with respect to ACR20 response at 12 weeks. In the plan for multiple comparisons, if non-inferiority was shown, the superiority of baricitinib to adalimumab was evaluated. ACR20 responses were significantly higher with baricitinib compared with placebo at 12 and 24 weeks. Baricitinib plus MTX was also found to be non-inferior to adalimumab plus MTX for the ACR20 response, with a margin of 12% (70% vs 61% for adalimumab), and was therefore considered to be significantly superior to adalimumab (P = 0.01). Starting as early as week 8, and sustained through week 52, a greater proportion of patients taking baricitinib achieved ACR50 and ACR70 responses. A significantly higher proportion of patients taking baricitinib had low disease activity, assessed by DAS28-CRP, compared with adalimumab at weeks 12 and 52. The proportion of patients achieving disease remission, measured by SDAI and Clinical Disease Activity Index (CDAI) scores, was also higher in the baricitinib group compared with the adalimumab group, at weeks 12, 24 and 52. Significantly greater inhibition of radiographic progression was also seen with baricitinib compared with placebo at 24 weeks (change from baseline in mTSS 0.41 vs 0.9) and 52 weeks (change from baseline in mTSS 0.71 vs 1.8). There was no significant difference between baricitinib plus MTX and adalimumab plus MTX in inhibition of radiographic progression. For the adalimumab group, change from baseline in mTSS was 0.33 at 24 weeks and 0.6 at 52 weeks. [20]. Baricitinib provided greater improvement in most PROs with statistical significance at several time points compared with placebo and adalimumab, including physical function, morning joint stiffness, pain, fatigue, overall work impairment and quality of life. These improvements were maintained to the end of the study at week 52 [35].
RA-Beacon was a 24-week, RCT in 527 patients designed to assess the efficacy of baricitinib in patients with moderately to severely active RA who had inadequate responses to bDMARDs, including at least one anti-TNF, or had unacceptable side effects. Of the recruited patients, 27% had at least three prior bDMARDs. Patients were randomized to baricitinib 2 mg, 4 mg or placebo, with background csDMARDs. For the primary end point, at week 12, ACR20 responses were 55% in the baricitinib 4 mg arm and 49% in the baricitinib 2 mg arm compared with 27% in the placebo group (P < 0.001). Statistically significant improvements were also observed between the higher-dose baricitinib group and placebo group in DAS28-CRP and HAQ-DI score, but not for a SDAI score of 3.3 or less [19]. Baseline PROs revealed severely impaired physical function and a high level of pain and fatigue in this highly refractory RA population. Both baricitinib-treated groups had significantly higher degrees of improvements in most PROs compared with placebo. Improvements were generally more rapid and of greater magnitude with baricitinib 4 mg than 2 mg and were maintained to week 24 [36].
The long-term safety and tolerability study, RA-BEYOND, which is ongoing at the time of writing, included people with moderate or severe RA who took part in a separate phase IIb study or one of the four trials described above. In this study, those who received baricitinib 4 mg once daily for at least 15 months and who achieved sustained low disease activity or remission (defined by CDAI score at two consecutive visits at least 3 months apart) were re-randomized in a double-blind manner to continue receiving baricitinib 4 mg once daily or to step down to 2 mg daily with or without csDMARDs [34]. Disease activity was assessed at 12 weeks. Most patients were able to sustain low disease or remission states in both baricitinib dose regimens. However, compared with the 4 mg group, the reduction to 2 mg at week 12 was associated with statistically significant increases in tender and swollen joint counts, physician global assessments, DAS28-CRP, CDAI and SDAI scores (all P-values <0.05). These data suggest that 4 mg once daily is the most efficacious baricitinib dose in keeping with other phase III studies.
Safety concerns
The evidence base for the clinical safety of JAK inhibitors is discussed in detail in the article by Dr Harigai [37]. Overall, as the phase III trials have emerged and long-term extension data have been evaluated, the absolute risk of serious adverse events appears comparable to that of bDMARDs. The most important difference appears to be an increased risk of herpes zoster infections, most marked in patients of Japanese and Korean ethnicity.
Approved JAK inhibitors and laboratory parameters
JAK inhibitors can cause anaemia and cytopenias but this is rarely of clinical significance for either tofacitinib or baricitinib at the approved doses. However, the fact that anaemia and cytopenias have been observed, particularly at higher than approved doses, may reflect the importance of JAK2 signalling in erythropoiesis and the involvement of JAK1 and JAK3 in lymphoid development. Unexpectedly, mild thrombocytosis has been observed in patients treated with baricitinib but not with tofacitinib. In a pooled analysis of tofacitinib from six phase III trials and two long term extension (LTE) studies (n = 4858), treatment with tofacitinib 5 and 10 mg bd resulted in decreased mean neutrophil counts, which generally stabilized during LTE studies. Tofacitinib was also associated with a gradual reduction in mean lymphocyte counts, which appeared to stabilize over time. Both 5 mg bd and 10 mg bd tofacitinib doses resulted in increases in haemoglobin levels as disease activity reduced, following which haemoglobin stabilized for up to 66 months. There were few cases of clinically meaningful haemoglobin reductions and the incidence of reported anaemia was low and similar to treatment with placebo and MTX [38]. In a pooled analysis of data from baricitinib phase II, III and LTE studies comprising 2451 patients, initial treatment-emergent shifts in haemoglobin were observed in one-third of subjects. However, reductions in haemoglobin below the lower limit of normal were not significantly different between placebo-treated patients or those taking 2 mg or 4 mg baricitinib: a surplus or placebo has been deleted. Furthermore, following an initial decline attributed to phlebotomy during the early weeks of the trials, dose-dependent increases in erythropoietin, iron and total iron binding capacity were observed, with a return to baseline or small rises in haemoglobin. Haemoglobin <8 g/dl was reported in <1% of patients in clinical trials [39].
Both tofacitinib and baricitinib treatment are associated with reductions in peripheral blood NK cell counts. In the case of tofacitinib, there is a dose-dependent decrease over the first 2 weeks of therapy while for baricitinib, there is a transient increase over the first 4 weeks of treatment before counts fall below baseline levels [40, 41]. However, there have been no reported associations between baseline or nadir NK cell counts and occurrence of serious infection, herpes zoster or malignancy [40].
Both tofacitinib and baricitinib are associated with increases in serum levels of low-density lipoprotein (LDL) and high-density lipoprotein (HDL) but without alteration in the LDL: HDL ratio. In pooled phase II tofacitinib studies, dose-dependent increases in total, HDL and LDL cholesterol of 16–30% were reported [42]. Similarly, dose-dependent increase in LDL, HDL and triglycerides was observed in a phase II study of baricitiinib in RA, in which the increase in HDL by week 12 correlated with improvement in DAS28 [43]. This may be related to modulation of signalling downstream of IL6 given that similar changes have been observed with biologic drugs targeting IL6R. Reviews of pooled data from late phase trials of tofacitinib and baricitinib indicate that like tocilizumab, this lipid change is not associated with major cardiovascular events [44, 45].
The place of tofacitinib and baricitinib in contemporary RA management
In accordance with the ACR guideline and EULAR recommendations for the management of RA, treatment should be initiated with MTX [2, 3]. In support of this recommendation, up to one-‐third of patients with early RA benefit from MTX monotherapy in controlling disease activity, improving patient function and limiting radiographic progression [46, 47]. In the event that patients with disease duration 6 months or more fail to achieve a satisfactory therapeutic response to MTX monotherapy and a short period of glucocorticoids, the ACR guidelines recommend consideration of either combination csDMARDs, a bDMARD ± MTX or tofacitinib ± MTX. Tofacitinib ± MTX can also be considered in bDMARD-refractory RA patients [2]. Similarly, EULAR recommendations for RA management suggest consideration of the addition to MTX of a JAK inhibitor, either tofacitnib or baricitinib, as treatment option in either MTX-IR or bDMARD-IR patients. There will doubtless continue to be close scrutiny of emerging real-world data for safety and sustained efficacy for the currently approved JAK inhibitors. However, the rapid clinical efficacy and absence of immunogenicity will be seen as beneficial. And for many patients, the perceived convenience of an oral dosing schedule will be advantageous. Furthermore, as tsDMARDs have a shorter half-life for biological inhibition of their therapeutic target than is the case for bDMARDs, a swifter reversal of any drug-related toxicity might be expected. Emerging real world data may shed further light on this possibility.
Supplement: This supplement is supported by a grant from Gilead Sciences, Inc.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: P.C.T. has received grants/research from Celgene, Eli Lilly and Company, Galapagos, UCB. Consultant for: AbbVie, Eli Lilly and Company, Galapagos, Pfizer, Gilead.
References
- 1. Smolen JS, Aletaha D.. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol 2015;11:276–89. [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, Saag KG, Bridges SL Jr. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewe R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Breedveld FC, Burmester GR. et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M.. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int 2016;36:685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Migita K, Izumi Y, Torigoshi T. et al. Inhibition of Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway in rheumatoid synovial fibroblasts using small molecule compounds. Clin Exp Immunol 2013;174:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker JG, Ahern MJ, Coleman M. et al. Changes in synovial tissue Jak-STAT expression in rheumatoid arthritis in response to successful DMARD treatment. Ann Rheum Dis 2006;65:1558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker JG, Ahern MJ, Coleman M. et al. Expression of Jak3, STAT1, STAT4, and STAT6 in inflammatory arthritis: unique JAK3 and STAT4 expression in dendritic cells in seropositive rheumatoid arthritis. Ann Rheum Dis 2006;65:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark JD, Flanagan ME, Telliez JB.. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem 2014;57:5023–38. [DOI] [PubMed] [Google Scholar]
- 10. Burmester GR, Blanco R, Charles-Schoeman C. et al. Tofacitinib (CP-690, 550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 11. Fleischmann R, Kremer J, Cush J. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 12. Kremer J, Li ZG, Hall S. et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Internal Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 13. Lee YCE, Emery P, Bradley JD. et al. Remaining pain in DMARD-naive rheumatoid arthritis patients treated with baricitinib and methotrexate [abstract]. Arthritis Rheumatol 2017;69(Suppl 10):https://acrabstracts.org/abstract/remaining-pain-in-dmard-naive-rheumatoid-arthritis-patients-treated-with-baricitinib-and-methotrexate/ (date last accessed 26 July 2018). [Google Scholar]
- 14. van der Heijde D, Tanaka Y, Fleischmann R. et al. Tofacitinib (CP-690, 550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 15. van Vollenhoven RF, Fleischmann R, Cohen S. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 16. Fridman JS, Scherle PA, Collins R. et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 2010;184:5298–307. [DOI] [PubMed] [Google Scholar]
- 17. Dougados M, van der Heijde D, Chen YC. et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017;76:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fleischmann R, Schiff M, van der Heijde D. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheum 2017;69:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Genovese MC, Kremer J, Zamani O. et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. [DOI] [PubMed] [Google Scholar]
- 20. Taylor PC, Keystone EC, van der Heijde D. et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376:652–62. [DOI] [PubMed] [Google Scholar]
- 21. Westhovens R. Clinical efficacy of JAK inhibitors under development. Just more of the same? Rheumatology 2019;58:i27–i33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaoka K. Janus kinase inhibitors for rheumatoid arthritis. Curr Opin Chem Biol 2016;32:29–33. [DOI] [PubMed] [Google Scholar]
- 23. Fleischmann RM, Huizinga TW, Kavanaugh AF. et al. Efficacy of tofacitinib monotherapy in methotrexate-naive patients with early or established rheumatoid arthritis. RMD Open 2016;2:e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Traynor K. FDA approves tofacitinib for rheumatoid arthritis. Am J Health Syst Pharm 2012;69:2120. [DOI] [PubMed] [Google Scholar]
- 25. Lamba M, Wang R, Fletcher T. et al. Extended-release once-daily formulation of tofacitinib: evaluation of pharmacokinetics compared with immediate-release tofacitinib and impact of food. J Clin Pharm 2016;56:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strand V, Lee EB, Fleischmann R. et al. Tofacitinib versus methotrexate in rheumatoid arthritis: patient-reported outcomes from the randomised phase III ORAL Start trial. RMD Open 2016;2:e000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fleischmann R, Mysler E, Hall S. et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390:457–68. [DOI] [PubMed] [Google Scholar]
- 28. Strand V, Kremer J, Wallenstein G. et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res Ther 2015;17:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strand V, van Vollenhoven RF, Lee EB. et al. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatol 2016;55:1031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strand V, Kremer JM, Gruben D. et al. Tofacitinib in combination with conventional disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: patient-reported outcomes from a phase III randomized controlled trial. Arthritis Care Res 2017;69:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strand V, Burmester GR, Zerbini CA. et al. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: patient-reported outcomes from a phase III trial. Arthritis Care Res 2015;67:475–83. [DOI] [PubMed] [Google Scholar]
- 32. Lee EB, Fleischmann R, Hall S. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 33. Richez C, Truchetet ME, Kostine M, Schaeverbeke T, Bannwarth B.. Efficacy of baricitinib in the treatment of rheumatoid arthritis. Expert Opin Pharmacother 2017;18:1399–407. [DOI] [PubMed] [Google Scholar]
- 34. Takeuchi T, Genovese M, Xie LI, et al. Baricitinib dose stepdown following disease control in patients with rheumatoid arthritis. Ann Rheum Dis 2016;75(Suppl 2):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keystone EC, Taylor PC, Tanaka Y. et al. Patient-reported outcomes from a phase 3 study of baricitinib versus placebo or adalimumab in rheumatoid arthritis: secondary analyses from the RA-BEAM study. Ann Rheum Dis 2017;76:1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smolen JS, Kremer JM, Gaich CL. et al. Patient-reported outcomes from a randomised phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON). Ann Rheum Dis 2017;76:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology 2019;58:i34–i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schulze-Koops H, Strand V, Nduaka C. et al. Analysis of haematological changes in tofacitinib-treated patients with rheumatoid arthritis across phase 3 and long-term extension studies. Rheumatol 2017;56:46–57. [DOI] [PubMed] [Google Scholar]
- 39. Kay J, Harigai M, Rancourt J. et al. Effects of baricitinib on haemoglobin and related laboratory parameters in rheumatoid arthritis patients. Ann Rheum Dis 2017;76(Suppl 2):513–4. [Google Scholar]
- 40. Emery P, McInnes I, Genovese M. et al. Characterisation of changes in lymphocyte subsets in baricitinib-treated patients with rheumatoid arthritis in two phase 3 studies. Ann Rheum Dis 2016;75(Suppl 1):A62–A. [Google Scholar]
- 41. van Vollenhoven RF, Tanaka Y, Lamba M. et al. Relationship between NK cell count and important safety events in rheumatoid arthritis patients treated with tofacitinib. Ann Rheum Dis 2015;74(Suppl 2):258–9. [Google Scholar]
- 42. McInnes IB, Kim HY, Lee SH. et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis 2014;73:124–31. [DOI] [PubMed] [Google Scholar]
- 43. Kremer JM, Genovese MC, Keystone E. et al. Effects of baricitinib on lipid, apolipoprotein, and lipoprotein particle profiles in a phase IIb study of patients with active rheumatoid arthritis. Arthritis Rheum 2017;69:943–52. [DOI] [PubMed] [Google Scholar]
- 44. Charles-Schoeman C, Wicker P, Gonzalez-Gay MA. et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum 2016;46:261–71. [DOI] [PubMed] [Google Scholar]
- 45. Taylor PC, Kremer J, Emery P. et al. Lipid profile and effect of statin treatment in pooled phase 2 and phase 3 baricitinib studies. Ann Rheum Dis 2018;77:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Breedveld FC, Weisman MH, Kavanaugh AF. et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. [DOI] [PubMed] [Google Scholar]
- 47. van Vollenhoven RF, Ernestam S, Geborek P. et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 2009;374:459–66. [DOI] [PubMed] [Google Scholar]