Abstract
Oral anticoagulants are commonly used drugs in patients with CKD and patients with ESKD to treat atrial fibrillation to reduce stroke and systemic embolism. Some of these drugs are used to treat or prevent deep venous thrombosis and pulmonary embolism in patients with CKD who undergo knee and hip replacement surgeries. Warfarin is the only anticoagulant that is approved for use by the Food and Drug Administration in individuals with mechanical heart valves. Each oral anticoagulant affects the coagulation profile in the laboratory uniquely. Warfarin and apixaban are the only anticoagulants that are Food and Drug Administration approved for use in patients with CKD and patients with ESKD. However, other oral anticoagulants are commonly used off label in this patient population. Given the acquired risk of bleeding from uremia, these drugs are known to cause increased bleeding events, hospitalization, and overall morbidity. Each anticoagulant has unique pharmacologic properties of which nephrologists need to be aware to optimally manage patients. In addition, nephrologists are increasingly asked to aid in the management of adverse bleeding events related to oral anticoagulant use in patients with CKD and patients with ESKD. This article summarizes the clinical pharmacology of these drugs and identifies knowledge gaps in the literature related to their use.
Keywords: Anticoagulants, Apixaban, Atrial Fibrillation, chronic kidney disease, Dabigatran, Edoroxaban, End-stage Kidney Disease, Heart Valves, hospitalization, Humans, Kidney Failure, Chronic, Nephrologists, Off-label Use, Oral Anticoagulants, Pharmaceutical Preparations, Pulmonary Embolism, Pyrazoles, Pyridones, Renal Insufficiency, Chronic, Rivaroxaban, Stroke, United States Food and Drug Administration, uremia, Venous Thrombosis, Warfarin
Introduction
The number of patients with CKD and patients with ESKD is increasing in the United States on the basis of the National Health and Nutrition Examination Survey data from 1999 to 2014 (1). Although heart failure, thrombotic cardiovascular events, and sudden cardiac death are common in CKD and ESKD, this population is also at a disproportionately higher risk of nonvalvular atrial fibrillation (AF) compared with the general population. Prevalence of AF increases as kidney disease worsens, and it is close to 15% by the time that patients with CKD become dialysis dependent, which is more than three times that of age-matched controls (2). Use of oral anticoagulants is common, and these agents are among the top 15 drugs prescribed to patients with CKD and patients with ESKD enrolled in Medicare Part D, Medicare Advantage, or Managed Care prescription drug programs (1). Warfarin is one of the most commonly prescribed oral anticoagulants. In the general population, newer oral anticoagulants (dabigatran, rivaroxaban, apixaban, and edoxaban) reduce risk of stroke or systemic embolism and bleeding versus warfarin in patients with AF, and they are increasingly prescribed in patients with CKD and patients with ESKD. Newer anticoagulants may be favored over warfarin in patients with ESKD and calciphylaxis (3). The reader can refer to previous review articles that have discussed extensively the clinical utility of oral anticoagulants in CKD (4). This review article will focus on the pharmacology of commonly used oral anticoagulants that are important in nephrology practice. In addition, it will identify knowledge gaps regarding use of these drugs in this patient population.
Warfarin
Pharmacology
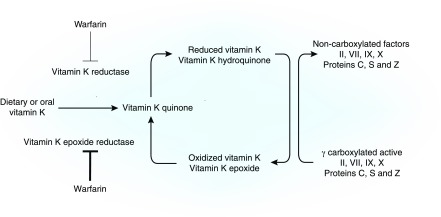
Warfarin is the oral anticoagulant with which clinicians have the most experience. It is a racemic mixture of two optically active isomers (R and S) in equal proportion (5). Its pharmacokinetic and pharmacodynamic (PK/PD) properties are shown in Tables 1 and 2. Common drug-drug interactions are shown in Table 3. Polymorphisms in vitamin K epoxide reductase gene and cytochrome P450 type 2C9 (CYP2C9) are not race specific, and they account for 25% and 10%, respectively, of the interindividual variability in warfarin dosing (6). Vitamin K epoxide reductase genotype may be the best predictor of warfarin dose, because it is responsible for the conversion of vitamin K epoxide to vitamin K (Figure 1) (6). CYP2C9 alleles (e.g., CYP2C9*2 and *3) are poor metabolizers, leading to prolonged t1/2 compared with the wild type (*1 allele) (5). The observed frequencies of CYP2C9*2 are 8%–19% in whites and <4% in blacks. The corresponding frequencies for *3 alleles are 6%–10% and <2%, respectively. The mechanism of action of warfarin is shown in Figures 1 and 2.
Table 1.
Summary of pharmacokinetic and pharmacodynamic properties of commonly used oral anticoagulants
| OAC | Type | Prodrug | Pharmacokinetics | Pharmacodynamics: Binding to Effector | ||
|---|---|---|---|---|---|---|
| Metabolism | Renal Dose Adjustment | Dialyzable | ||||
| Warfarin | Vitamin K–dependent factor inhibitor | No | Extensive metabolism by CYP2C9 | No | No | Irreversible |
| Dabigatran | Direct thrombin inhibitor | Yes | Metabolized by esterases, 80% excreted by kidney | Yes | Yes | Reversible |
| Apixaban | Free and clot-bound Xa inhibitor | No | Metabolized in liver by CYP3A4, then excreted in feces and kidney (25%), no active metabolite | No | Small | Reversible |
| Rivaroxaban | Free and clot-bound Xa inhibitor | No | 66% Excreted by kidney, 36% unchanged, minimal in feces | Yes | No | Reversible |
| Edoxaban | Free Xa inhibitor | No | 50% Excreted unchanged by the kidney, 10% hydrolyzed by carboxyesterase 1 | Yes | No | Reversible |
OAC, oral anticoagulant; CYP2C9, cytochrome P450 type 2C9; Xa, factor Xa; CYP3A4, cytochrome P450 type 3A4.
Table 2.
Additional pharmacokinetic properties in those with normal kidney function
| OAC | Cmax, h | t1/2, h | Protein binding, % | VD, L | Bioavailability, % |
|---|---|---|---|---|---|
| Warfarin | 2–6 | 42 | 97–99 | 10 | 99 |
| Dabigatran | 1–2 | 12–14 | 38 | 50–70 | 3–7 |
| Apixaban | 3–4 | 12 | 87 | 21 | 50 |
| Rivaroxaban | 2–4 | 6–13 | >90 | 50 | 66–100 |
| Edoxaban | 1–2 | 10–14 | 55 | 107 | 62 |
OAC, oral anticoagulant; Cmax, peak concentration; VD, volume of distribution.
Table 3.
Common drug-drug interactions of oral anticoagulants
| Drug | Increase Anticoagulant Effects | Decrease Anticoagulant Effects |
|---|---|---|
| Warfarin | Amiodarone, fluconazole, tigecycline, voriconazole, fluoroquinolones, verapamil, diltiazem, other anticoagulants, antiplatelet drugs, NSAIDs, and SSRIs | Rifampin, phenobarbital, carbamazepine, cigarette smoking |
| Dabigatran | Amiodarone, verapamil, ketoconazole, dronaderone, clopidogrel, enoxaparin, other anticoagulants, antiplatelet drugs | Rifampin |
| Apixaban | Ketoconazole, other anticoagulants, antiplatelet drugs | Rifampin |
| Rivaroxaban | Other anticoagulants, antiplatelet drugs, fluconazole, ketoconazole, erythromycin, and clarithromycin | Rifampin, phenytoin, carbamazepine, St. John’s Wort |
| Edoxaban | Other anticoagulants, antiplatelet drugs, | Rifampin |
NSAID, nonsteroidal anti-inflammatory drug; SSRI, serotonin reuptake inhibitor.
Figure 1.
Carboxylation of vitamin K–dependent proteins requires the reduced form of vitamin K, γ-glutamyl carboxylase enzyme, molecular oxygen, and carbon dioxide. Because body stores of vitamin K are low, the oxidized (inactive) form of vitamin K is recycled to the reduced (active) form by vitamin K epoxide reductase, which is inhibited by warfarin. Inhibition results in reduced hepatic synthesis of these clotting factors and reduction in their activities by 40%–50%.
Figure 2.
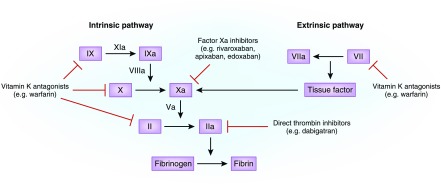
Oral anticoagulants act at different sites in the coagulation cascade for their anticoagulant effects.
Laboratory Measurement of Anticoagulant Effect
Internal normalized ratio (INR) is the most common test used to monitor warfarin response. Drugs, dietary changes, and disease processes alter warfarin effects. Therefore, its use requires frequent monitoring to maximize individual time spent in the therapeutic range on the basis of an INR between 2.0 and 3.0. Compared with individuals spending the least amount of individual time in the therapeutic range (<57%), those with the highest amount of individual time spent in the therapeutic range (>73%) experienced lower rates of stroke or systemic embolism (2% versus 1%), major bleeding (5% versus 3%), and all-cause mortality (7% versus 3%).
Pharmacology in Kidney Disease
The PK/PD of warfarin in CKD and ESKD is not well established (7). Clinical practice guidelines do not recommend dosage reduction for CKD or ESKD (5,8). Limdi et al. (9,10) found that mean (95% confidence interval [95% CI]) dose reductions of 10% (95% CI, 4% to 14%) and 19% (95% CI, 11% to 26%) were required in patients with eGFR=30–59 and <30 ml/min per 1.73 m2 compared with individuals with eGFR≥60 ml/min per 1.73 m2 to maintain therapeutic warfarin dosing. This cross-sectional analysis also adjusted for other confounders in the multivariable statistical model, and thus, interpretation of dose reductions solely on the basis of eGFR may be an oversimplified approach. Yet, it provides major evidence of increased exposure of drugs cleared by the liver in patients with CKD. With a single warfarin dose (0.75 mg/kg), individuals with GFR of 30–59 ml/min per 1.73 m2 had a shorter t1/2 at 29.9±5.0 versus 44.8±6.0 hours in healthy controls. An increase in warfarin clearance was observed from 2.6 ml/kg per hour in healthy controls to 3.7 ml/kg per hour in CKD (7). It remains to be established whether the dialysis procedure (hemodialysis or peritoneal dialysis) results in changes in warfarin kinetics and dynamics. Warfarin has significant drug-drug interactions that are especially important given the polypharmacy that is so prevalent in patients with CKD and patients with ESKD (Table 3).
Reversal of Antithrombotic Effects
Warfarin’s antithrombotic effects are reversed by low doses of vitamin K (Table 4). When pharmacologic doses of vitamin K (phytonadione 2.5–5 mg) are administered, reduced vitamin K is generated by a mechanism that bypasses epoxide reductase (via vitamin K reductase) that is less sensitive to warfarin (Figure 1) (5). Large vitamin K doses (10 mg) can result in warfarin resistance for >1 week (5). The American College of Chest Physicians guidelines recommend, for INRs≥9 and no bleed, a single oral 2.5- to 5-mg dose to bring the INR down in 1–2 days (5). For serious bleeding, regardless of INR value, 10 mg is administered parentally, and it is supplemented by fresh frozen plasma, prothrombin complex concentrate, or recombinant factor VIIa. These measures are repeated every 12 hours if the INR remains elevated (5). Because hemorrhagic effects can be prolonged in patients with CKD and patients with ESKD for a given INR value compared with in non-CKD individuals (11), clinicians should consider repeated therapy to ensure adequate reversal.
Table 4.
Reversal agents for oral anticoagulants and hemodialysis as an option to reverse antithrombotic effects
| Reversal modality | Warfarin | Dabigatran | Rivaroxaban | Apixaban | Edoxaban |
|---|---|---|---|---|---|
| Reversal by antidotes | |||||
| Prothrombin complex concentrate | Yes | No | Yes | Yes | Yes |
| Recombinant factor VIIa | Yes | No | Yes | Yes | Yes |
| Fresh frozen plasma | Yes | No | Yes | Yes | Yes |
| Factor VIII inhibitor bypass activity | Not reported | Yes | Yes | Yes | Yes |
| Specific antidote | No | Idaracizumab | Investigational (andexanet alfa) | ||
| Dialysis as a treatment option for major bleeding events | |||||
| Hemodialysis | No | Yes | No | No | No |
Efficacy and Safety
Compared with those with normal kidney function, CKD, especially GFR<30 ml/min per 1.73 m2, or ESKD complicates warfarin therapy. Specifically, lower doses are required to maintain therapeutic INR. Greater fluctuations in INR values with lower individual time in the therapeutic range and higher risks of major bleeding events for any given INR value are reported (9,10). In an observational study of 1273 long-term warfarin users, one third had a GFR of <60 ml/min per 1.73 m2 (11). Compared with individuals with GFR of >60 ml/min per 1.73 m2, those with GFR of 30–44 ml/min per 1.73 m2 and those with GFR<30 ml/min per 1.73 m2 had 2.2- and 5.8-fold higher risks, respectively, of major bleeding events at an INR value ≥4. GFR did not modify risk of hemorrhage for INR values <4 (11).
Because higher stroke rates were reported in patients with ESKD with versus without AF (4.57 versus 0.48 per 100 person-years, respectively) (12), previous cost utility analyses reported an increase in quality-adjusted life years with aspirin or warfarin treatment (13). However, warfarin increases bleeding risk, including intracranial hemorrhage, in patients with ESKD. In a retrospective study of patients with ESKD and AF, warfarin doubled stroke risk, presumably hemorrhagic, compared with no treatment (14). Another study evaluated patients with ESKD in the Fresenius Medical Care North America (FMCNA) database and reported 27% higher death risk with warfarin treatment (15). Observational studies are fraught with selection bias, especially because patients with ESKD and AF may be more likely to die compared with individuals with ESKD without AF. Data are limited to confirm or refute these concerns. There is concern of increased vascular calcification and calciphylaxis with warfarin given that it reduces function of vitamin K–dependent vascular calcification inhibitors, such as matrix Gla proteins (14,16). Finally, there are concerns about the possibility of AKI secondary to glomerular hemorrhage due to thrombin depletion in patients on warfarin with INR>3 in whom there is no other identifiable etiology of AKI (17). It is also believed to result in accelerated progression of CKD and worsen all-cause mortality in the short and long term (17). However, exact mechanisms and clinical presentation remain elusive to date.
Despite a Food and Drug Administration (FDA) black box warning for warfarin use in patients with kidney dysfunction due to increased risk of major bleeding, it is still commonly used. Furthermore, clinical practice guidelines continue to recommend warfarin in treating AF among patients with CKD and patients with ESKD (18). The American Heart Association 2014 updated guidelines for anticoagulation management in AF recommend warfarin as the drug of choice in patients with advanced CKD (creatinine clearance <30 ml/min) and patients with ESKD (8,19). The jury is still out regarding potential benefits and risks. If this high-risk patient population is not treated, it is estimated that stroke rate, including intracranial hemorrhage, would be approximately 7% (18). However, three distinct observational studies reported that warfarin did not reduce ischemic strokes among patients with ESKD. In addition, these studies reported an alarmingly higher intracranial hemorrhage rate compared with in the general population (3% versus 1% per year, respectively) (18).
Direct Thrombin Inhibitor—Dabigatran
Pharmacology
Dabigatran etexilate, 150 mg twice daily, is FDA approved to prevent stroke or systemic embolism in patients with AF. Nonspecific, ubiquitous esterases rapidly convert this nonpeptide prodrug into a potent, direct, and selective inhibitor of free and fibrin-bound thrombin (Table 1) (20). PK/PD properties are shown in Tables 1 and 2. Common drug-drug interactions are shown in Table 3. Its capsule (75 or 150 mg) contains dabigatran-coated pellets with a tartaric acid core to augment bioavailability at low pH. The core increases dyspepsia risk and gastrointestinal bleeding, especially with the 150-mg dose (20). Patients should not chew, break, or open capsules, because bioavailability increases dramatically (21). Substantial interindividual drug exposure variability exists (22). Dabigatran is approved at lower doses (75 mg twice daily), with a creatinine clearance of 15–30 ml/min (21).
Laboratory Measurement of Anticoagulant Effect
Activated partial thromboplastin time (APTT) is better than prothrombin time (PT) to detect dabigatran presence, but it cannot reliably distinguish between therapeutic and subtherapeutic concentrations (Table 4) (20,23). A normal thrombin time has the best negative predictive value to exclude the presence of dabigatran (20,23). Ecarin, a metalloproteinase, cleaves prothrombin to meizothrombin. Dabigatran inhibits this step. Ecarin-based assays, such as the ecarin clotting time, are highly sensitive and correlate strongly with drug concentrations. Studies showed that thrombin time and ecarin clotting time are linearly correlated with drug concentration measured by liquid chromatography tandem mass spectrometry (23).
Pharmacology in Kidney Disease
An open label, controlled study investigated PK/PD properties of a single 150-mg dabigatran dose in 23 patients with CKD and 50 mg in six patients with ESKD. The comparator group (six non-CKD controls) received two doses of 150 mg (standard dose) (24). Versus controls, areas under the plasma concentration-time curve (AUCs) were 1.5-, 3.2-, and 6.3-fold higher in patients with CKD and creatinine clearances of 50–80, 30–50, and ≤30 ml/min, respectively. Time to maximal plasma concentration (Cmax) was similar in patients with CKD and controls. Elimination t1/2 doubled in patients with CKD (creatinine clearance ≤30 ml/min) compared with non-CKD controls. Although six patients with ESKD received a reduced dose (50 mg), AUC was twofold higher than in non-CKD controls. A single hemodialysis session removed 62%–68% of the 50-mg dose. APTT and ecarin clotting time increased in correlation with changes in plasma drug concentration. Another PK/PD study was conducted in 15 patients with creatinine clearance of 15–30 ml/min. Participants received 75 mg twice daily, a dose resulting in mean steady-state drug exposure without drug accumulation (25). These studies suggest that drug exposure correlates with kidney disease severity and prescribed dose, which can be measured by APTT or ecarin clotting time.
Reversal of Antithrombotic Effects
There are patient reports using fresh frozen plasma and prothrombin complex concentrate to reverse dabigitran’s effects in patients with major bleeding (26). A recent randomized, controlled trial (RCT) in subjects with normal kidney function raised questions about the efficacy of prothrombin complex concentrate as an effective reversal agent (27). In another study in subjects with normal kidney function, nonspecific anti-inhibitor coagulant complex (e.g., factor VIII inhibitor bypass activity) but not recombinant factor VIIa reversed dabigatran’s anticoagulant effects (28). No studies have evaluated these agents in patients with CKD and patients with ESKD. A patient series of 11 life-threatening dabigatran-related major bleeding episodes reported use of hemodialysis and continuous venovenous hemofiltration (29). A PK/PD study of dabigatran 150 mg twice daily for 3 days in seven patients on hemodialysis reported 49% and 59% drug removal with blood flow rates of 200 and 400 ml/min, respectively, over a 4-hour treatment (30). Another study reported 62%–68% dabigatran removal with a single dialysis session (24). Although studies are limited by lack of control groups, randomization, and small sample size, available data suggest a possible role for kidney replacement therapy in reversal of dabigatran’s antithrombotic effects.
Recently, the FDA approved idarucizumab to reverse the antithrombotic effects of dabigatran (31). As a humanized mAb fragment directed against dabigatran and its acylglucuronide metabolites, its binding affinity to dabigatran is higher than dabigatran to thrombin, thus neutralizing the anticoagulant effect immediately after a single 5-g intravenous dose (32). Nearly one third (32%) of idarucizumab is excreted in urine, and the remainder undergoes metabolism primarily in kidney (32). In 12 subjects with creatinine clearance ≥60 to <90 ml/min and six subjects with creatinine clearance ≥30 to <60 ml/min, total antidote clearance was reduced, resulting in higher drug exposure by 44% and 84%, respectively (32). The package insert recommends no dose reduction for kidney dysfunction. More studies are needed to assess its efficacy in patients with CKD and patients with ESKD.
Efficacy and Safety
After FDA approval, patient reports of major bleeding were reported in frail elderly individuals, patients with CKD, and patients with ESKD (26,33). In the Randomized Evaluation of Long-Term Therapy Trial, 19% of patients had a baseline creatinine clearance <50 ml/min, and individuals with baseline creatinine clearance <30 ml/min were excluded (34). A subgroup analysis reported lower rates of stroke or systemic embolism with dabigatran 150 mg twice daily versus warfarin across all creatinine clearance categories (≥80, 50 to <80, and <50 ml/min) (35). Lower major bleeding rates were observed only in participants with creatinine clearance ≥80 ml/min. Table 5 summarizes four retrospective cohort studies and one meta-analysis reporting comparative effectiveness and safety data for dabigatran versus warfarin in CKD subgroups, and they concluded that dabigatran versus warfarin reduces risk of stroke or systemic embolism and intracranial hemorrhage, with an increased risk of gastrointestinal bleeding events (36–40). There is only one study in patients on hemodialysis using the FMCNA database; it reported a 1.5-fold higher risk of death or hospitalization from bleeding with dabigatran versus warfarin (Table 5) (41).
Table 5.
Comparative efficacy and safety data on dabigatran versus warfarin in patients with kidney disease and atrial fibrillation (36–41)
| Study Population | Sample Size of CKD Subgroup | Findings | Adjusted Risk Ratio (95% Confidence Interval) | |
|---|---|---|---|---|
| Dabigatran in patients with CKD | ||||
| Lauffenburger et al. (37) | Patients having commercial or Medicare supplemental insurance | 6727 | Reduced risk of S/SE | 0.74 (0.57 to 0.96)a |
| Increased risk of the composite of major GI bleeding, hemorrhagic stroke, ICH, or other bleeding | 1.52 (1.27 to 1.81)a | |||
| Hernandez et al. (38) | 5% Random sample of Medicare beneficiaries | 2964 | Increased risk of any bleeding | 1.11 (1.02 to 1.21)a |
| Increased risk of major bleeding | 1.55 (1.32 to 1.82)a | |||
| Majeed et al. (39) | Pooled analyses of five phase 3 RCTs | 1034 with any bleeding event, no mention of percentage with CKD | 30-d Mortality after the first bleeding event was lower for all of those who experienced any bleeding event in the five RCTs (no separate CKD subgroup analysis reported) | 0.66 (0.44 to 1.00)b |
| Graham et al. (40) | Medicare beneficiaries | 13% of 134,414 had CKD | No CKD subgroup analysis, results reported for the overall cohort | |
| Reduced risk of ischemic stroke | 0.80 (0.67 to 0.96)a | |||
| Reduced risk of ICH | 0.34 (0.26 to 0.46)a | |||
| Increased risk of major GI bleeding | 1.28 (1.14 to 1.44)a | |||
| Reduced risk of all-cause mortality | 0.86 (0.77 to 0.96)a | |||
| Romanelli et al. (36) | Meta-analysis | 348,750 Patients and no CKD subgroup analysis | No CKD subgroup analysis, results reported for the overall cohort | |
| Reduced risk of S/SE | 0.92 (0.84 to 1.01)a | |||
| Reduced risk of ICH | 0.44 (0.34 to 0.59)a | |||
| Increased risk of major GI bleeding | 1.23 (1.01 to 1.50)a | |||
| Dabigatran in patients on hemodialysis | ||||
| Chan et al. (41) | Fresenius Medical Care of North American database of patients on hemodialysis | 8345 | Increased risk of hospitalization or death from bleeding | 1.48 (1.21 to 1.81)c |
| Increased risk of hemorrhagic death | 1.78 (1.18 to 2.68)c |
S/SE, stroke or systemic embolism; GI, gastrointestinal; ICH, intracranial hemorrhage; RCT, randomized, controlled trial.
Adjusted hazard ratio.
Adjusted odds ratio.
Adjusted rate ratio.
Factor Xa Inhibitors
Rivaroxaban
Rivaroxaban is FDA approved in patients with AF to prevent stroke or systemic embolism (42). It is also FDA approved for deep venous thrombosis (DVT) and pulmonary embolism (PE) prophylaxis after knee and hip replacement (42,43). Like dabigatran, it is not approved in patients with mechanical heart valves. Oral bioavailability varies with dosing strength: 80%–100% with a 10-mg dose and 66% with a 20-mg dose. Other PK/PD properties are shown in Tables 1 and 2 (20). It is prescribed at a fixed oral dose with the evening meal: 20 mg/d for patients with a creatinine clearance of >50 ml/min and 15 mg/d for patients with a creatinine clearance of 30–50 ml/min (42). It should be avoided in patients with AF and a creatinine clearance of <15 ml/min (42). With a creatinine clearance of 15 to 50 ml/min the package insert recommends a reduced dose of 15 mg once daily with the evening meal in patients with nonvalvular atrial fibrillation. Rivaroxaban is not recommended for other indications with a creatinine clearance <30 ml/min. It does not interact with foods and interacts minimally with other drugs (Table 3). For DVT and PE prophylaxis, dosage is 10 mg/d. Rivaroxaban has a shorter t1/2 and more rapid onset of action than warfarin (43). Timing of initiation after procedures and daily adherence are prerequisites for clinical success (43). It is typically started 6–10 hours after surgery for DVT/PE prophylaxis, and it is continued for 35 days after hip replacement and 12 days after knee replacement (42). To transition from heparin to rivaroxaban, infusion is stopped, and rivaroxaban is started simultaneously. When transitioning from low molecular weight heparin, rivaroxaban is initiated within 2 hours of the next scheduled administration (42).
Pharmacology in Kidney Disease
A subgroup analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) with impaired creatinine clearance (<80 ml/min) reported no effect of kidney disease on rivaroxaban’s effectiveness and safety (44). A PK/PD study extended this finding by reporting similar AUCs (plasma concentration-time curve) in patients with ESKD and a 10-mg dose and healthy controls with a 20-mg dose (45). However, other controlled PK/PD studies challenged these findings and reported a 56% increase in AUC in patients with ESKD after a 15-mg dose administered postdialysis (46). Predialysis administration re-sulted in reduced drug exposure by only 5%. Finally, a PK/PD study of a single 10-mg dose was conducted in 24 patients with CKD (creatinine clearance <80 ml/min) and eight healthy controls (creatinine clearance ≥80 ml/min) (47). Compared with controls, the AUCs were 1.4-, 1.5-, and 1.6-fold higher with creatinine clearances of 50–80, 30–50, and <30 ml/min, respectively. The AUCs (factor Xa inhibition-time curve) were 1.5-, 1.9-, and 2.0-fold, respectively. This study suggests that reduced rivaroxaban clearance with worsening creatinine clearance resulted in increased drug exposure (47). Rivaroxaban is likely to accumulate in patients with CKD and patients with ESKD even at lower doses (10 or 15 mg/d), and it is poorly cleared by hemodialysis.
Apixaban
Apixaban is FDA approved for reduction of stroke or systemic embolism in patients with AF at 5 mg twice daily (48). With serum creatinine ≥1.5 mg/dl, age ≥80 years old, or body weight ≤60 kg, a reduced dose of 2.5 mg twice daily is recommended (48). It is also approved for DVT/PE prophylaxis after hip and knee replacement at 2.5 mg twice daily (48) and treatment of DVT/PE at 10 mg twice daily for a week followed by 5 mg twice daily (48). It is not approved for use with mechanical heart valves (48). PK/PD properties are shown in Tables 1 and 2. Drug-drug interactions are minimal (Table 3) (43).
Pharmacology in Kidney Disease
No significant kinetic changes were observed in peak plasma drug concentration (Cmax) or AUC among patients with CKD (creatinine clearance of 15–29 ml/min) and patients with ESKD (48). An open label, parallel group, single 5-mg dose PK/PD study was conducted in eight patients with ESKD and eight healthy controls (49). After 2 hours of drug administration, a 4-hour hemodialysis session was performed with dialysate flow rate of 500 ml/min and blood flow rate of 350–500 ml/min. The AUC in patients with ESKD was 36% higher versus controls (49). Because of its high degree of protein binding, dialysis clearance is low (18 ml/min), resulting in a 14% decrease in drug exposure (49). In a recent retrospective analysis of patients on hemodialysis, cumulative days of apixaban use in an outpatient setting, higher total daily apixaban doses, and total hemodialysis sessions were independent risk factors for bleeding events (adjusted odds ratio, 13.07; 95% CI, 1.54 to 110.54; adjusted odds ratio, 1.72; 95% CI, 1.20 to 2.48; and adjusted odds ratio, 2.04; 95% CI, 1.06 to 3.92, respectively) (50). Another PK/PD study prescribed a single 10-mg dose to 24 patients with CKD and various categories of creatinine clearance and eight healthy controls (51). Compared with controls, geometric mean AUCs increased by 16%, 29%, and 38% in patients with CKD and creatinine clearances of 50–80, 30–50, and <30 ml/min, respectively. Overall, elimination t1/2 was slightly increased in all subjects with CKD (17 hours) versus controls (15 hours). A direct linear relationship was observed between apixaban plasma concentration and antifactor Xa activity. These studies suggest that apixaban accumulates in patients with CKD and patients with ESKD and that it is poorly dialyzable. In another PD/PK study seven hemodialysis patients were given apixaban at 2.5 mg twice daily for eight days. The AUC, Cmax, and Cmin all increased when measured at day 8 compared to day 1 suggesting accumulation of the drug. At day 8 drug levels were still within the normal reference range. Drug levels comparing day 5 versus day 8 suggested that a steady state had been reached. Despite that it still would be of interest to examine levels with a longer duration of exposure (52).
Edoxaban
Edoxaban was FDA approved after a trial that established noninferiority compared with warfarin in patients with AF (53). It is also approved for treatment of DVT/PE only after an initial 5- to 10-day treatment with parenteral anticoagulation (19). It is recommended at 60 mg once daily for patients with creatinine clearance of 50–95 ml/min and 30 mg once daily for patients with creatinine clearance of 15–50 ml/min (54). PK/PD properties are shown in Tables 1 and 2. Common drug-drug interactions are shown in Table 3.
Pharmacology in Kidney Disease
Drug exposure increases by 32%, 74%, and 72% with creatinine clearances of 50–80, 30–50, and <30 ml/min, respectively (55). Although its molecular weight is 738 g/mol and it is only 55% protein bound, it is poorly cleared by dialysis (9% with a blood flow rate of 350 ml/min, a dialysate flow rate of 500 ml/min, and an F180NR dialyzer), possibly due to the large volume of distribution (107±20 L) (56).
Laboratory Measurement of Anticoagulant Effects
PT prolongation occurs to a greater degree than APTT prolongation with factor Xa inhibitors (Table 4) (20). A prolonged PT on warfarin does not equate to a similar anticoagulant effect on factor Xa inhibitors with the exact same PT value (23). Compared with PT and APTT assays, chromogenic anti-Xa activity assay (e.g., Rotachrom) may be more reliable and accurate (20,23). There is strong correlation between antifactor Xa activity and factor Xa inhibitor concentration (r2=0.95–1.00) (20). There are no FDA-approved kits that can be used for universal standardization of the anti-Xa activity assay.
Reversal of Antithrombotic Effects
Prothrombin concentrate complex, recombinant factor VIIa, and factor VIII inhibitor bypass activity can reverse their anticoagulant effects (Table 4) (27,28,57–60). There are no specific antidotes. Andexanet alfa, a modified recombinant human factor Xa molecule that acts as a decoy molecule, is under investigation (61).
Efficacy and Safety
The RCT (the ROCKET-AF) that led to FDA approval of rivaroxaban for AF included participants with CKD and excluded individuals with a creatinine clearance <30 ml/min (62). On the basis of studies in the general popula-tion, newer oral anticoagulants (dabigatran, rivaroxaban, or apixaban) compared with warfarin were more effective in reducing stroke or systemic embolism without an increased risk of intracranial hemorrhage and gastrointestinal bleeding (63). As a result, off-label use is increasing in patients with a creatinine clearance of <30 ml/min and ESKD (41). A study of the FMCNA database of patients with AF on chronic hemodialysis reported a 1.7-fold higher risk of death or hospitalization from bleeding with rivaroxaban versus warfarin (adjusted rate ratio, 1.71; 95% CI, 0.94 to 3.12) (41).
With apixaban, there is one published patient report of a major bleeding event noted in a patient on hemodialysis (64). Apixaban was superior to warfarin in reducing stroke or systemic embolism rates and major bleeding among participants with kidney dysfunction in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation Trial (65). A meta-analysis of RCTs comparing newer oral anticoagulants (dabigatran, rivaroxaban, and apixaban) with warfarin reported no difference in stroke, systemic embolism risk, or major bleeding in the CKD subgroup (relative risk, 0.64; 95% CI, 0.39 to 1.04 and relative risk, 0.89; 95% CI, 0.68 to 1.16, respectively)(66). Another meta-analysis reported reduced bleeding risk in the CKD subgroup (risk ratio, 0.80; 95% CI, 0.66 to 0.96) (67). In addition, bleeding rates were similar between individuals with creatinine clearance of 50–80 versus 30–50 ml/min on apixaban (67).
Compared with participants with creatinine clearance >50 ml/min, individuals with creatinine clearance of 30–50 ml/min in the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction Study 48 reported similar stroke or systemic embolism risk on edoxaban (68). Another subgroup analysis reported similar findings and a 24% reduction in bleeding risk (adjusted hazard ratio, 0.76; 95% CI, 0.58 to 0.98) (19). Finally, no difference in bleeding was reported between 15- and 30- to 60-mg/d doses in patients with GFR 15–30 ml/min per 1.73 m2 (19).
A recent Cochrane review reported reduced risk of stroke or systemic embolism and similar risk of major bleeding among patients with AF and CKD treated with factor Xa inhibitors versus warfarin (risk ratio, 0.81; 95% CI, 0.65 to 1.00 and risk ratio, 0.79; 95% CI, 0.59 to 1.04, respectively) (69). For both rivaroxaban and apixaban major clinical trials excluded patients on hemodialysis. With both drugs, at reduced dosages in hemodialysis patients, drug concentrations approximate those found in patients without kidney disease. However, the number of patients studied is very small and no conclusions can be drawn regarding their safety or efficacy, and caution should be exercised with their use in this patient population.
Gaps in the Literature
Although patients with CKD and patients with ESKD account for nearly 10% of the overall Medicare paid claims costs and although oral anticoagulant drugs are one of the top ten prescription drugs of Medicare prescription drug expenditure (70), comparative efficacy and safety data remain limited to support use of one oral anticoagulant over another in patients with CKD stages 4–5 or ESKD. Because these patients suffer from increased rates of hospitalization, adverse outcomes, and high health care–related costs (71), RCTs to investigate efficacy and safety of oral anticoagulants to improve hard clinical outcomes are critically important. Finally, there is lack of a standardized approach to assess kidney function in research, because debate continues regarding the preferred method for adjusting drug dosage. For example, the Modification of Diet in Renal Diseases eGFR calculation and the Cockcroft Gault creatinine clearance calculation were reported to over- or underestimate kidney function in various clinical settings (72,73).
Summary
Oral anticoagulants are commonly prescribed in patients with kidney disease. Understanding their clinical pharmacology and changes that occur as GFR declines is key to their effective use. Risks and benefits of oral anticoagulants are different in patients with CKD and patients with ESKD. All of these factors must be considered regardless of whether oral anticoagulants are prescribed for FDA-approved indications or used off label. Patients with GFR<30 ml/min per 1.73 m2, including those on dialysis, were systematically excluded from landmark trials. Extrapolation of comparative efficacy and safety in this patient population is difficult. Warfarin remains the most widely used oral anticoagulant. In our opinion, INR should be closely monitored in patients with ESKD. In our clinical practice, we check INR once a week in patients with ESKD. In our opinion, if the individual time in therapeutic INR range is <50% or if patients experience complications, such as calciphylaxis, we consider switching them to apixaban. Finally, until more data become available, we currently do not use dabigatran, rivaroxaban, and edoxaban in patients with CKD stage 5 and ESKD. Future studies are needed to establish whether use of oral anticoagulants result in net clinical benefit for individuals with CKD stages 4–5 and individuals with ESKD.
Disclosures
None.
Acknowledgments
This study was supported by American Heart Association Scientist Development grant 16SDG31000045 (to N.J.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.US Renal Data System: 2013 Annual Data Report. Available at: https://www.usrds.org/atlas13.aspx. Accessed December 11, 2017
- 2.Olesen JB, Lip GY, Kamper AL, Hommel K, Køber L, Lane DA, Lindhardsen J, Gislason GH, Torp-Pedersen C: Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 367: 625–635, 2012 [DOI] [PubMed] [Google Scholar]
- 3.King BJ, El-Azhary RA, McEvoy MT, Shields RC, McBane RD, McCarthy JT, Davis MDP: Direct oral anticoagulant medications in calciphylaxis. Int J Dermatol 56: 1065–1070, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Bansal N: Use of oral anticoagulation for patients with ESRD on hemodialysis with atrial fibrillation: Verdict 1. Clin J Am Soc Nephrol 11: 2093–2094, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G: Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133[6 Suppl]: 160S–198S, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Owen RP, Gong L, Sagreiya H, Klein TE, Altman RB: VKORC1 pharmacogenomics summary. Pharmacogenet Genomics 20: 642–644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harder S: Renal profiles of anticoagulants. J Clin Pharmacol 52: 964–975, 2012 [DOI] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64: e1–e76, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Limdi NA, Beasley TM, Baird MF, Goldstein JA, McGwin G, Arnett DK, Acton RT, Allon M: Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol 20: 912–921, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limdi NA, Limdi MA, Cavallari L, Anderson AM, Crowley MR, Baird MF, Allon M, Beasley TM: Warfarin dosing in patients with impaired kidney function. Am J Kidney Dis 56: 823–831, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limdi NA, Nolin TD, Booth SL, Centi A, Marques MB, Crowley MR, Allon M, Beasley TM: Influence of kidney function on risk of supratherapeutic international normalized ratio-related hemorrhage in warfarin users: A prospective cohort study. Am J Kidney Dis 65: 701–709, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vazquez E, Sanchez-Perales C, Garcia-Garcia F, Castellano P, Garcia-Cortes MJ, Liebana A, Lozano C: Atrial fibrillation in incident dialysis patients. Kidney Int 76: 324–330, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Quinn RR, Naimark DM, Oliver MJ, Bayoumi AM: Should hemodialysis patients with atrial fibrillation undergo systemic anticoagulation? A cost-utility analysis. Am J Kidney Dis 50: 421–432, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Yalamanchili V, Reilly RF: Does the risk exceed the benefit for anticoagulation in end-stage renal disease patients with nonrheumatic atrial fibrillation? Semin Dial 24: 387–388, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Chan KE, Lazarus JM, Thadhani R, Hakim RM: Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J Am Soc Nephrol 20: 872–881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eiser AR: Warfarin, calciphylaxis, atrial fibrillation, and patients on dialysis: Outlier subsets and practice guidelines. Am J Med 127: 253–254, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Wheeler DS, Giugliano RP, Rangaswami J: Anticoagulation-related nephropathy. J Thromb Haemost 14: 461–467, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Hart RG, Eikelboom JW, Brimble KS, McMurtry MS, Ingram AJ: Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can J Cardiol 29[Suppl]: S71–S78, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Chan KE, Giugliano RP, Patel MR, Abramson S, Jardine M, Zhao S, Perkovic V, Maddux FW, Piccini JP: Nonvitamin K anticoagulant agents in patients with advanced chronic kidney disease or on dialysis with AF. J Am Coll Cardiol 67: 2888–2899, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA: Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: A systematic review. Chest 151: 127–138, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradaxa drug label. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022512s007lbl.pdf. Accessed December 18, 2017
- 22.Cuker A, Siegal DM, Crowther MA, Garcia DA: Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 64: 1128–1139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adcock DM, Gosselin R: Direct Oral Anticoagulants (DOACs) in the laboratory: 2015 Review. Thromb Res 136: 7–12, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Stangier J, Rathgen K, Stähle H, Mazur D: Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: An open-label, parallel-group, single-centre study. Clin Pharmacokinet 49: 259–268, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Kooiman J, van der Hulle T, Maas H, Wiebe S, Formella S, Clemens A, van Buren M, Janssen M, Rabelink TJ, Huisman MV: Pharmacokinetics and pharmacodynamics of dabigatran 75 mg b.i.d. in patients with severe chronic kidney disease. J Am Coll Cardiol 67: 2442–2444, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Dumkow LE, Voss JR, Peters M, Jennings DL: Reversal of dabigatran-induced bleeding with a prothrombin complex concentrate and fresh frozen plasma. Am J Health Syst Pharm 69: 1646–1650, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M: Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: A randomized, placebo-controlled, crossover study in healthy subjects. Circulation 124: 1573–1579, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G: Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 108: 217–224, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Ross B, Miller MA, Ditch K, Tran M: Clinical experience of life-threatening dabigatran-related bleeding at a large, tertiary care, academic medical center: A case series. J Med Toxicol 10: 223–228, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khadzhynov D, Wagner F, Formella S, Wiegert E, Moschetti V, Slowinski T, Neumayer HH, Liesenfeld KH, Lehr T, Härtter S, Friedman J, Peters H, Clemens A: Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 109: 596–605, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Pollack CV Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Levy JH, Sellke FW, Stangier J, Steiner T, Wang B, Kam CW, Weitz JI: Idarucizumab for dabigatran reversal. N Engl J Med 373: 511–520, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Boehringer Ingelheim Pharmaceuticals Inc.: Praxbind package insert. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/761025lbl.pdf. Accessed October 26, 2015
- 33.Ribés-Cruz JJ, Torregrosa-Maicas I, Ramos-Tomás C, Solís-Salguero MA, Puchades-Montesa MJ, González-Rico MA, Juan-García I, Tomás-Simó P, Tejedor-Alonso S, Zambrano-Esteves P, Miguel-Carrasco A: Dabigatran-induced upper intestinal bleeding in a patient with chronic kidney disease. Nefrologia 33: 864–866, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators: Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Siegbahn A, Yusuf S, Wallentin L: Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: A RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 129: 961–970, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Romanelli RJ, Nolting L, Dolginsky M, Kym E, Orrico KB: Dabigatran versus warfarin for atrial fibrillation in real-world clinical practice: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 9: 126–134, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G: Effectiveness and safety of dabigatran and warfarin in real-world US patients with non-valvular atrial fibrillation: A retrospective cohort study. J Am Heart Assoc 4: 1–12, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez I, Baik SH, Piñera A, Zhang Y: Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med 175: 18–24, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majeed A, Hwang HG, Connolly SJ, Eikelboom JW, Ezekowitz MD, Wallentin L, Brueckmann M, Fraessdorf M, Yusuf S, Schulman S: Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation 128: 2325–2332, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA: Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 131: 157–164, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW: Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation 131: 972–979, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xarelto drug label. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202439s001lbl.pdf. Accessed December 18, 2017
- 43.Hylek EM: Therapeutic potential of oral factor Xa inhibitors. N Engl J Med 363: 2559–2561, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, Izumi T, Koretsune Y, Kajikawa M, Kato M, Ueda H, Iwamoto K, Tajiri M; J-ROCKET AF study investigators: Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study –. Circ J 76: 2104–2111, 2012 [DOI] [PubMed] [Google Scholar]
- 45.De Vriese AS, Caluwé R, Bailleul E, De Bacquer D, Borrey D, Van Vlem B, Vandecasteele SJ, Emmerechts J: Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis 66: 91–98, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Dias C, Moore KT, Murphy J, Ariyawansa J, Smith W, Mills RM, Weir MR: Pharmacokinetics, pharmacodynamics, and safety of single-dose rivaroxaban in chronic hemodialysis. Am J Nephrol 43: 229–236, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Kubitza D, Becka M, Mueck W, Halabi A, Maatouk H, Klause N, Lufft V, Wand DD, Philipp T, Bruck H: Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol 70: 703–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apixaban drug label. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s006lbl.pdf. Accessed December 22, 2017
- 49.Wang X, Tirucherai G, Marbury TC, Wang J, Chang M, Zhang D, Song Y, Pursley J, Boyd RA, Frost C: Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol 56: 628–636, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Steuber TD, Shiltz DL, Cairns AC, Ding Q, Binger KJ, Courtney JR: A multicenter analysis of factors associated with apixaban-related bleeding in hospitalized patients with end-stage renal disease on hemodialysis. Ann Pharmacother 51: 954–960, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Chang M, Yu Z, Shenker A, Wang J, Pursley J, Byon W, Boyd RA, LaCreta F, Frost CE: Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of apixaban. J Clin Pharmacol 56: 637–645, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Mavrakanas TA, Samer CF, Nessim SJ, Fisch G, Lipman ML: Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol 28: 2241–2248, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators: Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369: 2093–2104, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Edoxaban drug label. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf. Accessed December 22, 2017
- 55.Ridout G, de la Motte S, Niemczyk S, Sramek P, Johnson L, Jin J, He L, Mendell J, Salazar D: Effect of renal function on edoxaban pharmacokinetics and on population PK model. J Clin Pharmacol 49: 1124, 2009 [Google Scholar]
- 56.Parasrampuria DA, Marbury T, Matsushima N, Chen S, Wickremasingha PK, He L, Dishy V, Brown KS: Pharmacokinetics, safety, and tolerability of edoxaban in end-stage renal disease subjects undergoing haemodialysis. Thromb Haemost 113: 1124, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Fukuda T, Honda Y, Kamisato C, Morishima Y, Shibano T: Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost 107: 253–259, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Halim AB, Samama MM, Mendell J: Ex vivo reversal of the anticoagulant effects of edoxaban. Thromb Res 134: 909–913, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, Roquer J, Reverter JC, Sanz VV, Molina P, Lopez-Vilchez I, Diaz-Ricart M, Galan AM: Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates: Significance of studies in vitro with circulating human blood. PLoS One 8: e78696, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song Y, Wang Z, Perlstein I, Wang J, LaCreta F, Frost RJA, Frost C: Reversal of apixaban anticoagulation by four-factor prothrombin complex concentrate in healthy subjects: a randomized three-period crossover study. J Thromb Haemost 15: 2125–2137, 2017 [DOI] [PubMed] [Google Scholar]
- 61.Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, Mathur VS, Castillo J, Bronson MD, Leeds JM, Mar FA, Gold A, Crowther MA: Andexanet alfa for the reversal of factor Xa inhibition. N Engl J Med 17: 2413–2424, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators: Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883–891, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ: Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 110: 453–460, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Kufel WD, Zayac AS, Lehmann DF, Miller CD: Clinical application and pharmacodynamic monitoring of apixaban in a patient with end-stage renal disease requiring chronic hemodialysis. Pharmacotherapy 36: e166–e171, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, Parkhomenko A, López-Sendón JL, Lopes RD, Siegbahn A, Granger CB, Wallentin L: Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: Insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol 1: 451–460, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Harel Z, Sholzberg M, Shah PS, Pavenski K, Harel S, Wald R, Bell CM, Perl J: Comparisons between novel oral anticoagulants and vitamin K antagonists in patients with CKD. J Am Soc Nephrol 25: 431–442, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pathak R, Pandit A, Karmacharya P, Aryal MR, Ghimire S, Poudel DR, Shamoun FE: Meta-analysis on risk of bleeding with apixaban in patients with renal impairment. Am J Cardiol 115: 323–327, 2015 [DOI] [PubMed] [Google Scholar]
- 68.Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, Braunwald E: Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation 134: 24–36, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Kimachi M, Furukawa TA, Kimachi K, Goto Y, Fukuma S, Fukuhara S: Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev 11: CD011373, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.US Renal Data System: 2016 Annual data report. Available at: https://www.usrds.org/2016/view/Default.aspx Accessed July 24, 2017
- 71.Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, Banerjee S: Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol 109: 1510–1513, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Eppenga WL, Kramers C, Derijks HJ, Wensing M, Wetzels JF, De Smet PA: Individualizing pharmacotherapy in patients with renal impairment: The validity of the Modification of Diet in Renal Disease formula in specific patient populations with a glomerular filtration rate below 60 ml/min. A systematic review. PLoS One 10: e0116403, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spruill WJ, Wade WE, Cobb HH 3rd: Continuing the use of the Cockcroft-Gault equation for drug dosing in patients with impaired renal function. Clin Pharmacol Ther 86: 468–470, 2009 [DOI] [PubMed] [Google Scholar]