Abstract
Approximately 7%–10% of patients with ESKD worldwide undergo peritoneal dialysis (PD) as kidney replacement therapy. The continuous nature of this dialytic modality and the absence of acute shifts in pressure and volume parameters is an important differentiation between PD and in-center hemodialysis. However, the burden of hypertension and prognostic association of BP with mortality follow comparable patterns in both modalities. Although management of hypertension uses similar therapeutic principles, long-term preservation of residual diuresis and longevity of peritoneal membrane function require particular attention in the prescription of the appropriate dialysis regimen among those on PD. Dietary sodium restriction, appropriate use of icodextrin, and limited exposure of peritoneal membrane to bioincompatible solutions, as well as adaptation of the PD regimen to the peritoneal transport characteristics, are first-line therapeutic strategies to achieve adequate volume control with a potential long-term benefit on technique survival. Antihypertensive drug therapy is a second-line therapeutic approach, used when BP remains unresponsive to the above volume management strategies. In this article, we review the available evidence on epidemiology, diagnosis, and treatment of hypertension among patients on PD and discuss similarities and differences between PD and in-center hemodialysis. We conclude with a call for randomized trials aiming to elucidate several areas of uncertainty in management of hypertension in the PD population.
Keywords: peritoneal dialysis; end stage kidney disease; icodextrin; blood pressure; renal dialysis; Sodium; Sodium, Dietary; Antihypertensive Agents; Prognosis; Glucans; Glucose; Kidney Failure, Chronic; hypertension; Peritonitis; Diuresis
Introduction
The continuous nature of kidney replacement therapy is considered as major advantage of peritoneal dialysis (PD) over hemodialysis (HD) (1). Nonetheless, epidemiology of hypertension appears to be similar in both modalities because elevated or uncontrolled BP is highly prevalent among patients on PD and is associated with mortality (2–4). Although hypertension in PD is managed using similar therapeutic strategies with those applied in HD (2), there are also some differences that require attention. For example, volume management in PD should take into consideration the value of preservation of residual kidney function and longevity of peritoneal membrane function (5).
In this article, we provide an overview of epidemiology, diagnosis, and management of hypertension in PD. We discuss similarities and differences between PD and in-center HD and provide directions for future research in these important areas.
Epidemiology
Prevalence and Control of Hypertension
Earlier studies suggested that hypertension might be more adequately controlled in PD than in HD. The 1995 Peritoneal Dialysis Core Indicators Study reported that among 1202 patients on PD, only 29% had systolic BP >150 mm Hg and 18% had diastolic BP >90 mm Hg (6). In contrast, a study enrolling 2535 patients on HD in the United States showed that hypertension affected up to 86% of study participants (7). Subsequent studies showed a high burden of hypertension in PD. The Italian Co-operative Peritoneal Dialysis Study Group reported that among 504 patients on PD, the prevalence of hypertension was 88%, using the World Health Organization/International Society of Hypertension (WHO/ISH) diagnostic criteria (8). Uncontrolled clinic BP ≥140/90 mm Hg was detected in 79% of drug-treated hypertensives. Using ambulatory BP monitoring, 77% of drug-treated hypertensives had poor BP control, indicating a high burden of hypertension (8).
The natural course of hypertension was retrospectively evaluated in 207 patients on incident PD (9). Using the WHO/ISH criteria, hypertension was prevalent in 93% of participants at initiation of PD. Longitudinally, BP was improved and reached a nadir during the first 6–12 months of follow-up; thereafter, a progressive deterioration in BP control was evident. Older age, severity of preexisting hypertension, and loss of residual kidney function were determinants of deterioration in BP control (9).
Ambulatory BP Patterns in Patients on PD versus Patients on HD
In a cosinor model analysis of 11,833 BP measurements obtained during 44-hour ambulatory BP monitoring in 125 patients on HD, increments in interdialytic weight gain were associated with higher linear increase and blunted circadian amplitude of BP between dialysis treatments (10). These volume-mediated alterations in the ambulatory BP rhythms may be less prominent in PD, owing to the “steady” volume state of these patients. Luik et al. (11) showed no difference in diurnal BP variation when 20 patients on continuous ambulatory peritoneal dialysis (CAPD) were compared with 20 patients on HD. Rodby et al. (12) showed that the circadian pattern of 48-hour BP was not different in a comparison of 27 patients on CAPD with 33 patients on HD. However, patients on HD had higher 48-hour BP levels and BP loads (12). By contrast, Tonbul et al. (13) showed that the average 44-hour BP levels and loads were similar between 24 patients on CAPD and 22 patients on HD. Daytime BP during the dialysis-on day was lower in patients on HD than in patients on CAPD; the reverse phenomenon occurred during the dialysis-off day (13). Larger head-to-head comparisons are warranted to elucidate similarities and differences in ambulatory BP between HD and PD.
BP Control in Patients on CAPD versus Patients on Automated Peritoneal Dialysis
Frequent night-time and long daytime dwells may interfere with peritoneal sodium removal, suggesting a less effective BP control in automated peritoneal dialysis (APD) compared with CAPD (1). Comparative studies showed that BP levels, antihypertensive drug use, the presence of pedal edema, and extracellular body water assessed with bioelectric impendence analysis (BIA) are not different between the two modalities (14). Studies using ambulatory BP monitoring suggested that 24-hour BP loads and diurnal BP variation are comparable between APD and CAPD (15). These observations, however, are derived from small, cross-sectional studies without appropriate matching between comparison groups.
The Association of BP with Mortality
Similar to the U-shaped association of peridialytic BP with mortality among patients on HD (16), cohort studies showed that clinic BP is inversely associated with mortality in patients on PD (Table 1) (4,17,18). This “reverse” epidemiology of hypertension may be explained by a number of factors that confound the association of high BP with mortality risk. In a cohort of 2770 patients on incident PD, each 10-mm Hg higher systolic BP was associated with 16% lower risk for all-cause mortality over the first year of follow-up (4). However, higher systolic BP was associated with excess risk for late mortal events, suggesting that dialysis vintage modifies the association of BP with mortality (4). The level of illness and severity of comorbidities with opposing effect on BP is another factor that affects the predictive value of hypertension. In a cohort of 77 patients on PD, when the analysis was adjusted for severity of congestive heart failure, the inverse association of lower systolic BP with excess mortality risk was mitigated (17). By contrast, the pulsatile component of BP provides a more direct mortality signal (3,19). The linear association of higher pulse pressure with mortality is possibly reflecting a state of accelerated arterial stiffening in ESKD. Lastly, the epidemiology of hypertension may vary considerably if out-of-office BP recordings are used as risk predictors. Among patients on HD, home and ambulatory BP recordings are linearly associated with excess mortality (16). Prospective studies are warranted to elucidate the prognostic significance of out-of-office BP recordings in PD.
Table 1.
Longitudinal studies exploring the association of BP with mortality among patients on peritoneal dialysis
| Author | Year | N | Follow-Up, mo | Age, yr | Baseline BP, mm Hg | % of Patients on Antihypertensive Therapy | Association of BP with all-cause mortality |
|---|---|---|---|---|---|---|---|
| Goldfarb-Rumyantzev et al. (18) | 2005 | 1053 | 23 | 57.2 | 141.7/79.9 | 82 | ↓ office systolic BP associated with ↑ all-cause mortality |
| Liu et al. (19) | 2008 | 153 | 30 | 54.5 | Median pulse pressure: 56 | NA | ↑ office pulse pressure associated with ↑ all-cause mortality |
| Fang et al. (3) | 2009 | 306 | 21 | 59.4 | Mean pulse pressure: 56.8 | 83.3 | ↑ office pulse pressure associated with ↑ all-cause mortality |
| Udayaraj et al. (4) | 2009 | 2770 | 45 | 58.0 | 143.1/81.4 | NA | ↑ office systolic BP associated with ↓ early all-cause mortality ↑ office systolic BP associated with ↑ late all-cause mortality |
| Afshinnia et al. (17) | 2016 | 77 | 35 | 51.0 | NA | 89.6 | ↑ office systolic BP associated with ↑ all-cause mortality after adjustment for the severity of congestive heart failure |
↓ , lower; ↑, higher; NA, not available.
The optimal BP targets among dialysis patients remain unknown. The reverse association of clinic BP with mortality in observational studies in contrasted by meta-analyses of randomized trials showing that deliberate BP lowering with antihypertensive drugs is associated with improvement in clinical outcomes (16). The feasibility and safety of BP lowering is also supported by the BP in Dialysis trial (20), in which 126 hypertensive patients on HD were randomized to an intensive, predialysis systolic BP target of 110–140 mm Hg versus a standard target of 155–165 mm Hg. Intensive BP lowering did not aggravate the incidence of major cardiovascular adverse events, hospitalizations, or vascular access thromboses (20). Larger, phase 3 trials evaluating “hard” clinical end points are warranted to define optimal BP targets. Until such evidence become available, we encourage the wider use of out-of-office BP monitoring as an approach to optimize risk stratification, and we support the current International Society of Peritoneal Dialysis (ISPD) recommended target of <140/90 mm Hg for self-measured home BP (5).
Diagnosis
The optimal method to diagnose hypertension, detect the presence of target organ damage, and prognosticate the risk of mortality in patients on PD is an area of controversy. The availability of home BP recordings routinely taken by patients on PD offers the advantage of using a theoretically superior BP-monitoring technique in hypertension management (2). Studies comparing the diagnostic accuracy of different BP-monitoring techniques are summarized in Table 2, and suggest that routine BP recordings taken by the patients themselves at home are worse than standardized clinic BP in approximating daytime ambulatory BP and in detecting evidence of target organ damage (21–23); this discrepancy is possibly explained by the absence of standardized protocols for home BP monitoring. Similarly to nondialysis populations, ambulatory BP monitoring is the gold-standard method in management of hypertension in PD (2). Using this method, the aforementioned Italian Co-operative Peritoneal Dialysis Study uncovered a nondipping status in 53% of 504 participants (8), confirming the high prevalence of nocturnal hypertension in this population. Owing to the low availability of ambulatory BP monitoring, we recommend the use of office BP measurements as the standard of care, and encourage the wide use of validated BP monitors as an approach to optimize the diagnostic accuracy of home BP recordings (2). Additional research is warranted in this area because currently available studies are small and underpowered.
Table 2.
Studies evaluating the diagnostic accuracy of different BP-monitoring techniques among patients on peritoneal dialysis
| Author | Year | N | Test Method | Reference Method | Study Results |
|---|---|---|---|---|---|
| Wang et al. (23) | 2001 | 31 | Clinic BP 10-d morning home BP | Ambulatory BP monitoring | The association of clinic systolic BP with daytime systolic BP (r=0.81; P<0.05) was stronger than the association of home systolic BP with daytime systolic BP (r=0.62; P<0.05) |
| Koc et al. (21) | 2002 | 74 | Clinic BP | Ambulatory BP monitoring | 24-h ambulatory BP could detect the presence of LV hypertrophy, whereas clinic BP had no association with indices of target organ damage |
| O’Shaughnessy et al. (22) | 2013 | 17 | Clinic BP BpTRU-derived BPa 7-d morning home BP | Ambulatory BP monitoring | Home systolic BP overestimated daytime systolic BP by 14.2 mm Hg (95% CI, 4.3 to 21.4 mm Hg); routine clinic BP and BpTRU-derived BP could better approximate daytime BP |
LV, left ventricular; 95% CI, 95% confidence interval.
BpTRU records BP automatically without an observer.
Management of Volume
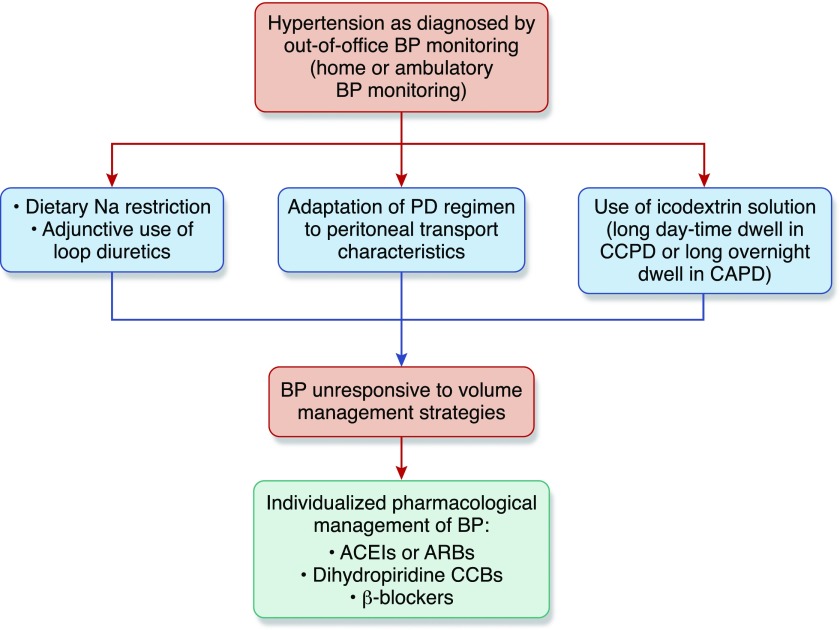
The first step in management of hypertension is the adequate clinical assessment of volume status (Figure 1). The ISPD guidelines (5) mandate the recognition and control of volume overload as first-line therapy of hypertension.
Figure 1.
Volume control as first-line therapeutic approach of hypertension among patients on PD. Diagnosis and management of hypertension among patients on PD should be on the basis of out-of-office BP monitoring. Given the poor diagnostic accuracy of conventional office BP recordings and limited availability of ambulatory BP monitoring, we recommend the wide use of home BP recordings taken by the patients themselves, with validated automated BP monitors as an alternative approach to confirm diagnosis and guide the overall management of hypertension. Management of hypertension should firstly be relied on adequate control of sodium and volume excess. Lowering dietary sodium intake, appropriate use of loop diuretics in the presence of substantial residual kidney function, adaptation of dialysis regimen to peritoneal transporting status, and use of icodextrin solutions during long daytime dwell in APD or during the long overnight dwell in CAPD are first-line volume management strategies. Antihypertensive drug therapy should be initiated when hypertension remains uncontrolled despite the adequate management of volume. Choice of the appropriate antihypertensive regimen should be individualized, taking into consideration the comorbidities and overall risk profile of each patient. Although comparative efficacy and safety of different antihypertensive drug categories among patients on PD remains elusive, some clinical studies suggest that angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) exert beneficial effects on a number of intermediate clinical end points, including long-term preservation of residual kidney function. CCB, calcium-channel-blockers; CCPD, continuous cyclic peritoneal dialysis; Na, sodium.
The Concept of Dry Weight
Definitions.
The concept of dry weight is introduced as an approach to recognize subclinical volume expansion (2). Management of dry weight in patients on HD is on the basis of the gentle and gradual reduction of postdialysis weight until patients reach an “ideal” weight at which there will be no signs/symptoms of hypervolemia or hypovolemia (2). In the Dry Weight Reduction in Hypertensive Hemodialysis Patients trial (24), dry weight reduction of 0.9 kg over an 8-week period provoked a reduction of 6.6/3.3 mm Hg in interdialytic BP. Despite the obvious differences between HD and PD, the concept of dry weight is similarly applicable to those on PD. Signs/symptoms reflecting hypovolemia should be carefully monitored when managing volume in patients on PD (2).
Assessment.
With the exception of patients presenting with clinically overt volume overload, the assessment of volume status using standard clinical diagnostic criteria is difficult. In the Brazilian Peritoneal Dialysis Multicenter study (25), the presence of pedal edema was able to detect volume expansion only in 28% out of 1089 patients on PD. Similarly, the presence of pedal edema is an insufficient tool to detect volume overload in asymptomatic patients on HD (26). Studies using more objective technologies showed that the prevalence of subclinical volume expansion among patients on PD is higher and clinical judgement is insufficient to determine whether patients are truly euvolemic. In the European Body Composition Monitoring study (27), BIA uncovered that only 40% out of 639 patients on PD were euvolemic (27). Standard clinical characteristics commonly used in dry weight assessment, such as urine output, peritoneal ultrafiltration, and BP levels, were unable to detect volume expansion (27). In a Chinese cohort of 307 patients on PD, BIA identified subclinical volume expansion in 67% of study participants (28). Among 1092 patients on PD participating in the Initiative of Patient Outcomes in Dialysis study (29), BIA showed that subclinical overhydration was detectable in 57% of participants (29). In a meta-analysis of 42 cohort studies (incorporating data from 60,790 patients on dialysis), a BIA-derived overhydration index >15% was associated with 2.28-fold higher risk for all-cause mortality (30). Among 88 patients on PD having their volume status assessed with lung ultrasound, moderate to severe lung congestion was detected in 46% of participants (31). The presence of pedal edema or dyspnea had poor accuracy in detecting lung congestion.
Whether the above technologies aid the achievement of euvolemia remains unclear. This hypothesis was explored in a pilot study, in which 160 patients on PD were randomized to BIA-guided volume management versus standard care (32). Significant reductions in extracellular-to-intracellular volume ratio were noted during the 3-month follow-up in the intervention group. By contrast, the United Kingdom-Shanghai and Control of Fluid Balance Guided by Body Composition Monitoring in Patients on Peritoneal Dialysis trials showed that BIA-guided volume management did not reduce extracellular body water and had no benefit on BP control or regression of left ventricular (LV) hypertrophy (33). In a 2017 meta-analysis of seven randomized trials, BIA-guided volume management was associated with a modest reduction of 2.73 mm Hg in systolic BP that was not translated into improvement in all-cause mortality (34). The ongoing Lung Water by Ultra-Sound Guided Treatment to Prevent Death and Cardiovascular Complications in High Risk ESKD Patients with Cardiomyopathy Trial is planning to recruit 500 high-risk patients on HD, aiming to compare the effect of guiding volume management with lung ultrasound versus standard care on a composite outcome of all-cause mortality, myocardial infarction, or worsening congestive heart failure. Randomized trials are warranted to elucidate the role of these techniques in patients on PD as well.
Dietary Sodium Restriction
Nonadherence to a sodium-restrictive diet is a major driver of thirst and fluid intake (1). Dietary sodium intake is recommended not to exceed 2 g daily (corresponding to 5 g of sodium chloride) (2). Improvement in total body sodium balance may facilitate the achievement of adequate volume control. Although this notion is not supported by clinical trial evidence, observational studies have shown significant BP lowering in response to a therapeutic strategy incorporating dietary sodium restriction alone or combined with enhanced ultrafiltration (35). Such an approach, however, may be associated with more rapid decline in residual kidney function, whereas other observational studies have associated low-sodium intake with higher mortality, possibly mediated through deficient protein and nutrient intake (36).
Adjunctive Use of Diuretics
Longer preservation of residual kidney function arising from gentle and continuous kidney replacement therapy is a potential advantage of PD over in-center HD. A post hoc analysis of the Canada-United States study showed that each 250-ml increment in urine volume was associated with 36% lower risk for all-cause mortality (37). Administration of loop diuretics may enhance urine output and fractional urinary excretion of sodium, facilitating the maintenance of fluid balance among patients on PD with preserved residual kidney function (1). In a randomized trial enrolling 61 incident patients on PD, compared with no treatment, administration of furosemide (250 mg/d) for 12 months increased 24-hour urine output and urinary sodium excretion, but had no protective effect on preservation of residual kidney function (38). Whether intensification of diuretic therapy is beneficial or incremental among patients on PD with residual diuresis warrants investigation in future trials.
Adaptation of the Dialysis Regimen to the Peritoneal Transport Characteristics
Volume overload and poor BP control may arise in patients receiving a PD regimen that is inappropriately adapted to their peritoneal transport characteristics (39). Longer dwells in fast transporters limit ultrafiltration because of a rapid dissipation of glucose gradient. Observational studies showed that fast transporters had higher 24-hour BP, abnormal circadian BP patterns, and higher LV mass index than low transporters (40). Observational studies showed that fast transporting status was independently associated with higher mortality risk (41), an association possibly explained by sodium and water reabsorption when long dwells with glucose-containing solutions are prescribed to fast transporters. In this setting, transfer to APD should be considered (1). By contrast, shorter dwells in low transporters limit net diffusive sodium removal because of sodium sieving. Midday exchanges with glucose-containing solutions or icodextrin during the long daytime dwell enhance sodium removal in APD (1).
Patients with substantial residual diuresis may benefit from less intensive regimens, such as dry night in CAPD and partially or fully dry day in APD (1). However, loss of residual kidney function represents a silent cause of volume overload. In this setting, intensification of PD, avoidance of dry periods, increasing dialysate glucose concentration, and appropriate use of icodextrin are therapeutic options to maintain euvolemia (1).
Icodextrin Solution
Hypertonic glucose-containing solutions, although possibly effective in providing short-term volume control, are associated with a number of adverse effects on residual kidney function, metabolic profile, peritoneal membrane function, and technique longevity (1). Icodextrin, a starch-derived glucose polymer, is widely used as alternative osmotic agent during the long overnight dwell in CAPD or during the long daytime dwell in APD (1). The advantages of icodextrin include the intensification of peritoneal ultrafiltration, protection of peritoneal membrane from the formation of advanced glycation end-products, and neutral effect on metabolic profile (1). In a meta-analysis of 12 randomized trials (42), compared with glucose-containing solutions, icodextrin enhanced net peritoneal ultrafiltration (weighted mean difference, 448.5 ml/d; 95% confidence interval, 289.3 to 607.8 ml/d) and reduced the incidence of overhydration (relative risk, 30%; 95% confidence interval, 15% to 59%) (42) without compromising residual kidney function.
Randomized trials tested also the hypothesis that the benefit of icodextrin on volume status may be accompanied by a parallel BP-lowering effect (43–47) (Table 3). A crossover study of 14 patients on APD compared icodextrin with standard 2.27% glucose solutions, both administered during the daytime dwell. Over a 4-week follow-up period, icodextrin reduced office BP by 19.5/6.0 mm Hg despite the reduction in antihypertensive medications in six out of 14 participants (47). In a double-blind trial, 50 hypertensive patients on PD were randomized to icodextrin or 2.27% glucose solutions during the long dwell for 6 months (43). Despite the absence of a significant reduction in ambulatory BP, icodextrin-treated participants required fewer antihypertensive medications to achieve BP control (43). Another trial randomized 59 diabetic patients on CAPD to icodextrin or 2.27% glucose solutions during the overnight dwell for 12 months (45). A BP-lowering effect was noted in icodextrin-treated participants, but not in those assigned to glucose-containing solutions; this effect was confirmed with ambulatory BP monitoring (48). Adequately powered randomized trials are required to elucidate the BP-lowering effect of icodextrin.
Table 3.
Randomized trials comparing the effect of icodextrin versus glucose-containing dialysate solutions on BP in patients on peritoneal dialysis
| Author | Year | N | Patient Characteristics | Design | Follow-Up, mo | Intervention | Effect on Body Weight | Effect on UF Volume | Effect on BP |
|---|---|---|---|---|---|---|---|---|---|
| Woodrow et al. (47) | 2000 | 14 | Patients on APD | Crossover | 1 | Icodextrin versus 2.27% glucose dialysate in daytime dwell | ↓ | ↓ | Icodextrin lowered BP by 19.5/6.0 mm Hg, despite the parallel reduction in antihypertensive drugs in six out of 14 participants |
| Plum et al. (46) | 2002 | 39 | Patients on APD | Parallel | 3 | Icodextrin versus 2.27% glucose dialysate in daytime dwell | ↓ | ↓ | No difference |
| Konings et al. (44) | 2003 | 40 | Patients on CAPD or APD | Parallel | 4 | Icodextrin versus standard dialysate glucose during the long dwell | ↓ | ↓ | No difference |
| Davies et al. (43) | 2003 | 50 | Hypertensive patients on CAPD or APD | Parallel | 6 | Icodextrin versus 2.27% glucose dialysate during the long dwell | ↓ | ↓ | No difference in BP levels, but icodextrin-treated participants required fewer antihypertensive medications during follow-up |
| Paniagua et al. (45) | 2008 | 59 | Diabetic patients on CAPD | Parallel | 12 | Icodextrin versus 2.27% glucose dialysate during the long night-time dwell | ↓ | ↓ | Icodextrin lowered BP by 10.4/6.2 mm Hg versus 2.27% glucose dialysate (P<0.01) |
UF, ultrafiltration; APD, automated peritoneal dialysis; ↓ , significant reduction in icodextrin groups relative to control group; CAPD, continuous ambulatory peritoneal dialysis.
Others
Low-Sodium Solutions.
Sodium removal in PD is achieved through convection with ultrafiltration as well as through diffusive transperitoneal sodium elimination (1). Dialysate sodium in currently available PD solutions is standardized to a concentration of 132–134 mmol/L, allowing only a small amount of diffusive sodium transport. Earlier interventional studies using low (120 mmol/L) or ultra-low (98 mmol/L) dialysate sodium showed that this approach enhances the diffusive removal but compromises convective elimination (49). Studies combining low-sodium dialysate with higher dialysate glucose concentrations to compensate for reduced osmolality showed that this intervention maintains peritoneal ultrafiltration, improves hydration status, and provokes significant reductions in ambulatory BP (50). The noninferiority of low-sodium (125 mmol/L) versus standard-sodium PD solutions (134 mmol/L) on dialysis adequacy (defined as a between-group difference of −0.5 in weekly total Kt/V urea) was tested in a recent trial enrolling 108 hypertensive patients on CAPD (51). This trial failed to prove the noninferiority of low-sodium solutions because total Kt/V urea was 2.53±0.89 in the low-sodium group versus 2.97±1.58 in the standard-sodium group (51). However, low-sodium dialysate enhanced diffusive sodium removal by 1.188 g/d. A significant between-group difference of −8.6/−4.6 mm Hg in office BP was noted at study completion. It has to be noted, however, that low-sodium dialysate solutions are not yet commercially available and the aforementioned results warrant investigation in phase 2 trials using ambulatory BP monitoring.
Biocompatible Solutions.
Neutral pH, low-glucose degradation product solutions are introduced as alternative to standard glucose-containing solutions on the basis that their higher biocompatibility may limit peritoneal membrane injury (1). In a meta-analysis of 18 randomized trials, biocompatible solutions were associated with longer preservation of residual kidney function, particularly when their administration was extended for >12 months (42). However, biocompatible solutions were ineffective in enhancing peritoneal ultrafiltration assessed in 4-hour peritoneal equilibration tests. Similarly, 24-hour peritoneal ultrafiltration was not different between biocompatible and glucose-containing solutions (42). Biocompatible solutions are not yet available in the United States.
Pharmacologic Management
Pathophysiology of hypertension in PD is complex and includes several nonvolume-dependent mechanistic pathways (2). When BP remains uncontrolled despite the adequate management of volume, antihypertensive therapy is the next treatment consideration to control BP.
The association of agents blocking the renin-angiotensin-aldosterone system (RAAS) with survival was evaluated in a prospective cohort of 306 incident patients on PD (52). Over a total follow-up of 8422 patient months, RAAS blockade use was associated with 62% lower risk for all-cause mortality (52). As shown in Table 4, pilot randomized trials are also suggestive of a beneficial effect of RAAS blockers on intermediate end points, including short-term BP variability, LV hypertrophy, and arterial stiffness (53–56). A 2014 meta-analysis of six randomized trials showed that compared with other antihypertensive drug categories, RAAS blockers were associated with slower decline in residual kidney function (57).
Table 4.
Randomized trials evaluating the effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients receiving peritoneal dialysis
| Author | Year | N | Characteristics | Design | Intervention | Follow-Up, mo | Primary Outcome | Overall Effect | Details |
|---|---|---|---|---|---|---|---|---|---|
| Li et al. (53) | 2003 | 60 | Patients on PD with residual kidney function | Open-label | Ramipril (5 mg/d) versus no treatment | 12 | Rate of decline in residual GFR or complete anuria | Better | Ramipril was superior to no treatment in reducing the incidence of complete anuria (HR, 58%; 95% CI, 36% to 94%) |
| Suzuki et al. (55) | 2003 | 24 | Patients on PD with LV hypertrophy | Double-blind | Valsartan (160 mg/d) versus placebo | 12 | Change in LV mass index | Better | Compared with placebo, valsartan therapy caused a greater regression of LV mass index (145±5 versus 121±4 g/m2; P<0.05) |
| Suzuki et al. (56) | 2004 | 34 | Patients on PD with hypertension and residual kidney function | Open-label | Valsartan (40–80 mg/d) versus other therapy not including ACEIs/ARBs | 24 | Rate of decline in residual GFR | Better | Valsartan retarded the loss of residual kidney function during follow-up (3.2±0.3 versus 4.3±0.7 ml/min per 1.73 m2), despite the absence of significant between-group difference in mean follow-up BP levels |
| Shigenaga et al. (54) | 2009 | 45 | Patients on PD with hypertension | Open-label | Candesartan (16 mg/d) or valsartan (160 mg/d) or other therapy not including ACEIs/ARBs | 6 | Change in LV mass index and baPWV | Better | Despite the absence of significant between-group difference in change of 24-h ambulatory BP, ARBs were superior to control therapy in causing regression of LV mass index and baPWV |
PD, peritoneal dialysis; HR, hazard ratio; 95% CI, 95% confidence interval; LV, left ventricular; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; baPWV, brachial-ankle pulse wave velocity.
The efficacy and safety of mineralocorticoid receptor antagonists among patients on PD was explored in three randomized trials, summarized in Table 5 (58–60). These pilot trials suggest that add-on therapy with mineralocorticoid receptor antagonists is associated with improvement in LV mass index and LV ejection fraction, without a significant increase in the incidence of hyperkalemia. The wide use of spironolactone and eplerenone among patients on PD should be avoided in anticipation of larger trials evaluating hard clinical end points.
Table 5.
Randomized trials evaluating the effect of mineralocorticoid receptor antagonists among patients on peritoneal dialysis
| Author | Year | N | Characteristics | Design | Intervention | Follow-Up, mo | Primary Outcome | Overall Effect | Details |
|---|---|---|---|---|---|---|---|---|---|
| Taheri et al. (60) | 2012 | 18 | Patients on PD with congestive heart failure and LV ejection fraction ≤45% | Double-blind | Spironolactone (25 mg every other day) versus placebo | 6 | Change in LV ejection fraction | Better | Spironolactone therapy improved LVEF relative to placebo (25.7%±7.3% versus 33.3%±7.8%; P=0.002) |
| Ito et al. (58) | 2014 | 158 | Patients on PD without congestive heart failure already treated with ACEIs or ARBs | Open-label | Add-on spironolactone (25 mg/d) versus no treatment | 24 | Change in LV mass index | Better | Compared with placebo, add-on spironolactone improved LV mass index at 6, 18, and 24 mo of follow-up |
| Lin et al. (59) | 2016 | 253 | Patients on HD or PD without congestive heart failure | Open-label | Add-on spironolactone (25 mg/d) versus placebo | 24 | Death from cardiovascular events, aborted cardiac arrest, or sudden death | Better | Spironolactone lowered the incidence of the primary outcome by 58% compared with placebo (HR, 42%; 95% CI, 26% to 78%) |
PD, peritoneal dialysis; LV, left ventricular; LVEF, left ventricular ejection fraction; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; HD, hemodialysis; HR, hazard ratio; 95% CI, 95% confidence interval.
In conclusion, epidemiology of hypertension in PD appears to follow similar patterns with the burden and prognostic significance of hypertension in in-center HD. The fact that PD is a home-based dialytic modality suggests that home BP monitoring should be widely applied as a technique with additive diagnostic and prognostic value to that of office BP recordings. Dietary sodium restriction, adjunctive use of diuretics, appropriate use of icodextrin, and adaptation of the PD regimen to the peritoneal transport characteristics are first-line volume management strategies with potential benefits on patient and technique survival. Antihypertensive therapy is recommended only when hypertension remains uncontrolled despite the adequate management of volume. Although small, randomized trials are suggestive of a beneficial effect of RAAS blockers on intermediate end points, the comparative effectiveness of different antihypertensive drug classes on hard clinical outcomes is unknown. Randomized trials rather than observational studies are needed to elucidate several areas of uncertainty in management of hypertension among patients on PD.
Disclosures
R.A. is a data safety monitoring committee member of Astra Zeneca and Ironwood Pharmaceuticals, a steering committee of randomized trials member of Akebia, Bayer, Janssen, Glaxo Smith Cline, Relypsa, Sanofi, and Genzyme US Companies, an adjudication committee member of Bayer, Boehringer Ingelheim, and Janssen, a scientific advisory board member or consultant for Celgene, Daiichi Sankyo, Inc., Eli Lilly, Relypsa, Reata, Takeda Pharmaceuticals USA, and ZS Pharma. V.L. has received honoraria from Amgen, Baxter, and Genesis. V.V. and P.I.G. declare no competing interests.
Acknowledgments
This work is supported by National Institutes of Health grant 5 R01 HL126903-02 and grant 5I01CX000829-04 from the US Department of Veterans Affairs Merit Review (to R.A.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kim YL, Biesen WV: Fluid overload in peritoneal dialysis Patients. Semin Nephrol 37: 43–53, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, Flynn J, Pogue V, Rahman M, Reisin E, Weir MR: Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol 25: 1630–1646, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang W, Yang X, Bargman JM, Oreopoulos DG: Association between pulse pressure and mortality in patients undergoing peritoneal dialysis. Perit Dial Int 29: 163–170, 2009 [PubMed] [Google Scholar]
- 4.Udayaraj UP, Steenkamp R, Caskey FJ, Rogers C, Nitsch D, Ansell D, Tomson CR: Blood pressure and mortality risk on peritoneal dialysis. Am J Kidney Dis 53: 70–78, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Wang AY, Brimble KS, Brunier G, Holt SG, Jha V, Johnson DW, Kang SW, Kooman JP, Lambie M, McIntyre C, Mehrotra R, Pecoits-Filho R: ISPD cardiovascular and metabolic guidelines in adult peritoneal dialysis patients part II - management of various cardiovascular complications. Perit Dial Int 35: 388–396, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocco MV, Flanigan MJ, Beaver S, Frederick P, Gentile DE, McClellan WM, Polder J, Prowant BF, Taylor L, Helgerson SD: Report from the 1995 core indicators for peritoneal dialysis study group. Am J Kidney Dis 30: 165–173, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG: Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115: 291–297, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cocchi R, Degli Esposti E, Fabbri A, Lucatello A, Sturani A, Quarello F, Boero R, Bruno M, Dadone C, Favazza A, Scanziani R, Tommasi A, Giangrande A: Prevalence of hypertension in patients on peritoneal dialysis: Results of an Italian multicentre study. Nephrol Dial Transplant 14: 1536–1540, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Menon MK, Naimark DM, Bargman JM, Vas SI, Oreopoulos DG: Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant 16: 2207–2213, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Light RP: Arterial stiffness and interdialytic weight gain influence ambulatory blood pressure patterns in hemodialysis patients. Am J Physiol Renal Physiol 294: F303–F308, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Luik AJ, Struijk DG, Gladziwa U, von Olden RW, von Hooff JP, de Leeuw PW, Leunissen KM: Diurnal blood-pressure variations in haemodialysis and CAPD patients. Nephrol Dial Transplant 9: 1616–1621, 1994 [PubMed] [Google Scholar]
- 12.Rodby RA, Vonesh EF, Korbet SM: Blood pressures in hemodialysis and peritoneal dialysis using ambulatory blood pressure monitoring. Am J Kidney Dis 23: 401–411, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Tonbul Z, Altintepe L, Sözlü C, Yeksan M, Yildiz A, Türk S: Ambulatory blood pressure monitoring in haemodialysis and continuous ambulatory peritoneal dialysis (CAPD) patients. J Hum Hypertens 16: 585–589, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Davison SN, Jhangri GS, Jindal K, Pannu N: Comparison of volume overload with cycler-assisted versus continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol 4: 1044–1050, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ataş N, Erten Y, Okyay GU, Inal S, Topal S, Öneç K, Akyel A, Çelik B, Tavil Y, Bali M, Arınsoy T: Left ventricular hypertrophy and blood pressure control in automated and continuous ambulatory peritoneal dialysis patients. Ther Apher Dial 18: 297–304, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Georgianos PI, Agarwal R: Blood pressure and mortality in long-term hemodialysis-time to move forward. Am J Hypertens 30: 211–222, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afshinnia F, Zaky ZS, Metireddy M, Segal JH: Reverse epidemiology of blood pressure in peritoneal dialysis associated with dynamic deterioration of left ventricular function. Perit Dial Int 36: 154–162, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldfarb-Rumyantzev AS, Baird BC, Leypoldt JK, Cheung AK: The association between BP and mortality in patients on chronic peritoneal dialysis. Nephrol Dial Transplant 20: 1693–1701, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Liu JH, Chen CC, Wang SM, Chou CY, Liu YL, Kuo HL, Lin HH, Wang IK, Yang YF, Huang CC: Association between pulse pressure and 30-month all-cause mortality in peritoneal dialysis patients. Am J Hypertens 21: 1318–1323, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Miskulin DC, Gassman J, Schrader R, Gul A, Jhamb M, Ploth DW, Negrea L, Kwong RY, Levey AS, Singh AK, Harford A, Paine S, Kendrick C, Rahman M, Zager P: BP in dialysis: Results of a pilot study. J Am Soc Nephrol 29: 307–316, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koc M, Toprak A, Tezcan H, Bihorac A, Akoglu E, Ozener IC: Uncontrolled hypertension due to volume overload contributes to higher left ventricular mass index in CAPD patients. Nephrol Dial Transplant 17: 1661–1666, 2002 [DOI] [PubMed] [Google Scholar]
- 22.O’Shaughnessy MM, Durcan M, Kinsella SM, Griffin MD, Reddan DN, Lappin DW: Blood pressure measurement in peritoneal dialysis: Which method is best? Perit Dial Int 33: 544–551, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang MC, Tseng CC, Tsai WC, Huang JJ: Blood pressure and left ventricular hypertrophy in patients on different peritoneal dialysis regimens. Perit Dial Int 21: 36–42, 2001 [PubMed] [Google Scholar]
- 24.Agarwal R, Alborzi P, Satyan S, Light RP: Dry-weight reduction in hypertensive hemodialysis patients (DRIP): A randomized, controlled trial. Hypertension 53: 500–507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira-Filho SR, Machado GR, Ferreira VC, Rodrigues CF, Proença de Moraes T, Divino-Filho JC, Olandoski M, McIntyre C, Pecoits-Filho R; BRAZPD study investigators: Back to basics: Pitting edema and the optimization of hypertension treatment in incident peritoneal dialysis patients (BRAZPD). PLoS One 7: e36758, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal R, Andersen MJ, Pratt JH: On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol 3: 153–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Biesen W, Williams JD, Covic AC, Fan S, Claes K, Lichodziejewska-Niemierko M, Verger C, Steiger J, Schoder V, Wabel P, Gauly A, Himmele R; EuroBCM Study Group: Fluid status in peritoneal dialysis patients: The European Body Composition Monitoring (EuroBCM) study cohort. PLoS One 6: e17148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Q, Yi C, Li J, Wu X, Yang X, Yu X: Prevalence and risk factors of fluid overload in Southern Chinese continuous ambulatory peritoneal dialysis patients. PLoS One 8: e53294, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronco C, Verger C, Crepaldi C, Pham J, De Los Ríos T, Gauly A, Wabel P, Van Biesen W; IPOD-PD Study Group: Baseline hydration status in incident peritoneal dialysis patients: The initiative of patient outcomes in dialysis (IPOD-PD study). Nephrol Dial Transplant 30: 849–858, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabinor M, Elphick E, Dudson M, Kwok CS, Lambie M, Davies SJ: Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): Systematic review and subgroup meta-analysis. Sci Rep 8: 4441, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panuccio V, Enia G, Tripepi R, Torino C, Garozzo M, Battaglia GG, Marcantoni C, Infantone L, Giordano G, De Giorgi ML, Lupia M, Bruzzese V, Zoccali C: Chest ultrasound and hidden lung congestion in peritoneal dialysis patients. Nephrol Dial Transplant 27: 3601–3605, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Luo YJ, Lu XH, Woods F, Wang T: Volume control in peritoneal dialysis patients guided by bioimpedance spectroscopy assessment. Blood Purif 31: 296–302, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Tan BK, Yu Z, Fang W, Lin A, Ni Z, Qian J, Woodrow G, Jenkins SB, Wilkie ME, Davies SJ: Longitudinal bioimpedance vector plots add little value to fluid management of peritoneal dialysis patients. Kidney Int 89: 487–497, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Covic A, Ciumanghel AI, Siriopol D, Kanbay M, Dumea R, Gavrilovici C, Nistor I: Value of bioimpedance analysis estimated “dry weight” in maintenance dialysis patients: A systematic review and meta-analysis. Int Urol Nephrol 49: 2231–2245, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Günal AI, Duman S, Ozkahya M, Töz H, Asçi G, Akçiçek F, Basçi A: Strict volume control normalizes hypertension in peritoneal dialysis patients. Am J Kidney Dis 37: 588–593, 2001 [PubMed] [Google Scholar]
- 36.Dong J, Li Y, Yang Z, Luo J: Low dietary sodium intake increases the death risk in peritoneal dialysis. Clin J Am Soc Nephrol 5: 240–247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bargman JM, Thorpe KE, Churchill DN; CANUSA Peritoneal Dialysis Study Group: Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Medcalf JF, Harris KP, Walls J: Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int 59: 1128–1133, 2001 [DOI] [PubMed] [Google Scholar]
- 39.van Biesen W, Heimburger O, Krediet R, Rippe B, La Milia V, Covic A, Vanholder R; ERBP working group on peritoneal dialysis: Evaluation of peritoneal membrane characteristics: Clinical advice for prescription management by the ERBP working group. Nephrol Dial Transplant 25: 2052–2062, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Tonbul Z, Altintepe L, Sözlü C, Yeksan M, Yildiz A, Türk S: The association of peritoneal transport properties with 24-hour blood pressure levels in CAPD patients. Perit Dial Int 23: 46–52, 2003 [PubMed] [Google Scholar]
- 41.Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG: Meta-analysis: Peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 17: 2591–2598, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Cho Y, Johnson DW, Craig JC, Strippoli GF, Badve SV, Wiggins KJ: Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev (3): CD007554, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimbürger O, Simonsen O, Davenport A, Tranaeus A, Divino Filho JC: Icodextrin improves the fluid status of peritoneal dialysis patients: Results of a double-blind randomized controlled trial. J Am Soc Nephrol 14: 2338–2344, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, Gerlag PG, Hoorntje SJ, Wolters J, van der Sande FM, Leunissen KM: Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: A randomized study. Kidney Int 63: 1556–1563, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Paniagua R, Orihuela O, Ventura MD, Avila-Díaz M, Cisneros A, Vicenté-Martínez M, Furlong MD, García-González Z, Villanueva D, Prado-Uribe MD, Alcántara G, Amato D: Echocardiographic, electrocardiographic and blood pressure changes induced by icodextrin solution in diabetic patients on peritoneal dialysis. Kidney Int Suppl 73(108): S125–S130, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Plum J, Gentile S, Verger C, Brunkhorst R, Bahner U, Faller B, Peeters J, Freida P, Struijk DG, Krediet RT, Grabensee B, Tranaeus A, Filho JC: Efficacy and safety of a 7.5% icodextrin peritoneal dialysis solution in patients treated with automated peritoneal dialysis. Am J Kidney Dis 39: 862–871, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Woodrow G, Oldroyd B, Stables G, Gibson J, Turney JH, Brownjohn AM: Effects of icodextrin in automated peritoneal dialysis on blood pressure and bioelectrical impedance analysis. Nephrol Dial Transplant 15: 862–866, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Paniagua R, Ventura MD, Avila-Díaz M, Cisneros A, Vicenté-Martínez M, Furlong MD, García-González Z, Villanueva D, Orihuela O, Prado-Uribe MD, Alcántara G, Amato D: Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int 29: 422–432, 2009 [PubMed] [Google Scholar]
- 49.Leypoldt JK, Charney DI, Cheung AK, Naprestek CL, Akin BH, Shockley TR: Ultrafiltration and solute kinetics using low sodium peritoneal dialysate. Kidney Int 48: 1959–1966, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Davies S, Carlsson O, Simonsen O, Johansson AC, Venturoli D, Ledebo I, Wieslander A, Chan C, Rippe B: The effects of low-sodium peritoneal dialysis fluids on blood pressure, thirst and volume status. Nephrol Dial Transplant 24: 1609–1617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rutkowski B, Tam P, van der Sande FM, Vychytil A, Schwenger V, Himmele R, Gauly A; Low Sodium Balance Study Group: Low-sodium versus standard-sodium peritoneal dialysis solution in hypertensive patients: A randomized controlled trial. Am J Kidney Dis 67: 753–761, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Fang W, Oreopoulos DG, Bargman JM: Use of ACE inhibitors or angiotensin receptor blockers and survival in patients on peritoneal dialysis. Nephrol Dial Transplant 23: 3704–3710, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Li PK, Chow KM, Wong TY, Leung CB, Szeto CC: Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med 139: 105–112, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Shigenaga A, Tamura K, Dejima T, Ozawa M, Wakui H, Masuda S, Azuma K, Tsurumi-Ikeya Y, Mitsuhashi H, Okano Y, Kokuho T, Sugano T, Ishigami T, Toya Y, Uchino K, Tokita Y, Umemura S: Effects of angiotensin II type 1 receptor blocker on blood pressure variability and cardiovascular remodeling in hypertensive patients on chronic peritoneal dialysis. Nephron Clin Pract 112: c31–c40, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Suzuki H, Nakamoto H, Okada H, Sugahara S, Kanno Y: A selective angiotensin receptor antagonist, Valsartan, produced regression of left ventricular hypertrophy associated with a reduction of arterial stiffness. Adv Perit Dial 19: 59–66, 2003 [PubMed] [Google Scholar]
- 56.Suzuki H, Kanno Y, Sugahara S, Okada H, Nakamoto H: Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis 43: 1056–1064, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Zeng X, Fu P, Wu HM: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for preserving residual kidney function in peritoneal dialysis patients. Cochrane Database Syst Rev 6: CD009120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito Y, Mizuno M, Suzuki Y, Tamai H, Hiramatsu T, Ohashi H, Ito I, Kasuga H, Horie M, Maruyama S, Yuzawa Y, Matsubara T, Matsuo S; Nagoya Spiro Study Group: Long-term effects of spironolactone in peritoneal dialysis patients. J Am Soc Nephrol 25: 1094–1102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin C, Zhang Q, Zhang H, Lin A: Long-term effects of low-dose spironolactone on chronic dialysis patients: A randomized placebo-controlled study. J Clin Hypertens (Greenwich) 18: 121–128, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taheri S, Mortazavi M, Pourmoghadas A, Seyrafian S, Alipour Z, Karimi S: A prospective double-blind randomized placebo-controlled clinical trial to evaluate the safety and efficacy of spironolactone in patients with advanced congestive heart failure on continuous ambulatory peritoneal dialysis. Saudi J Kidney Dis Transpl 23: 507–512, 2012 [PubMed] [Google Scholar]