Abstract
Objective
To review the current knowledge regarding recombinant and purified allergens and allergen-derived peptides.
Data Sources
PubMed, homepages relevant to the topic, and the National Institutes of Health clinical trial database were searched.
Study Selections
The literature was screened for studies describing purified and recombinant allergens and allergen-derived peptides. Studies relevant to the topic were included in this review.
Results
Advantages and drawbacks of pure and defined recombinant allergens and peptides over allergen extracts in the context of allergy research, diagnosis, and allergen immunotherapy are discussed. We describe how these molecules are manufactured, which products are currently available on the market, and what the regulative issues are. We furthermore provide an overview of clinical studies with vaccines based on recombinant allergens and synthetic peptides. The possibility of prophylactic vaccination based on recombinant fusion proteins consisting of viral carrier proteins and allergen-derived peptides without allergenic activity are also discussed.
Conclusion
During the last 25 years more than several hundred allergen sequences were determined, which led to a production of recombinant allergens that mimic biochemically and immunologically their natural counterparts. Especially in Europe, recombinant allergens are increasingly replacing allergen extracts in diagnosis of allergy. Despite many challenges, such as high cost of clinical trials and regulative issues, allergy vaccines based on recombinant allergens and peptides are being developed and will likely soon be available on the market.
Introduction
In 1988 and 1989, allergen-encoding complementary DNAs from hornet venom, birch, and house dust mites were first cloned, and this was actually the beginning of molecular allergology1–3 (Table 1). In 1992, cloning of allergen sequences for highly cross-reactive allergens helped to explain cross-reactivity among unrelated allergen sources, such as different tree pollens and fruits.4,5 Shortly after that, the first publications reporting that recombinant allergens could be useful for molecular allergy diagnosis appeared.6,7 In addition to diagnostic application, new forms of specific immunotherapy based on recombinant allergens and synthetic allergen–derived peptides have been explored,9,10,15–17 which led to the first immunotherapy trial performed with recombinant hypoallergenic allergen derivatives22 in 2000. In 1999, the concept of component-resolved diagnosis (CRD) with recombinant and purified natural allergens was introduced.20 CRD should enable the discrimination of cross-reactivity from genuine sensitization in allergic patients by using a multiplex testing.25 The original chip-based microarray contained 94 purified allergen molecules, which were covalently immobilized on a preactivated glass slide,25 and the IgE antibody profiles of allergic individuals were evaluated in a single analysis using 40 μL of serum. The repertoire of allergens has increased over time, and the commercially available version of microarray immune solid-phase allergen chip (ISAC) (Thermo Fisher Scientific, Waltham, Massachusetts, and Phadia, Uppsala, Sweden) currently contains 112 individual allergenic molecules. An extended version of the ISAC microarray containing 170 recombinant and natural allergens was developed in the frame of European Union project MEDALL to more broadly study IgE and IgG antibody development in children.27 The MEDALL chip has been shown to be superior to allergen extract testing regarding specificity and predictive value in several studies and allowed to investigate the evolution of the allergic immune response in birth cohorts and cohorts of allergic patients.34–36 In the last 10 years, several clinical trials of specific immunotherapy in which hypoallergenic derivatives were applied to patients gave hope that safer, more efficient, and patient-tailored forms of treatment will become available. These clinical trials are described in detail in this article.
Table 1. Milestones in the Developments in Recombinant Allergens and Peptides.
| Period | Milestones | Examples and references |
|---|---|---|
| 1988–1989 | Cloning of allergen encoding complementary DNAs | Hornet venom,1 house dust mite,3 birch2 |
| 1989–1992 | Complementary DNAs coding for highly cross-reactive allergens were isolated, explaining cross-reactivity between unrelated allergen sources | Homology of Bet v 1 with alder, hazel, hornbeam, and apple,4 profilins5 |
| 1991–1994 | Recombinant allergens are used in in vitro and in vivo allergy diagnosis | Three pollen,6 grass pollen allergy,7 Aspergillus fumigatus,8 house dust mite9 |
| 1993–1997 | Engineering of First recombinant hypoallergenic candidates for allergy vaccination | Hypoallergens in the form of isoforms, hapten,10 mutation,11 fragments12 |
| 1996 | First population studies using recombinant allergens | Skin testing with recombinant birch pollen allergens,13 recombinant Timothy grass pollen allergens in in vitro testing14 |
| 1996–1999 | Application of short peptides in humans | Intradermal administration of short peptides derived from Fel d 115–18 |
| 1999–2000 | Evaluation of recombinant hypoallergens in patients | Testing of hypoallergenic Bet v 1 derivatives in patients19 |
| 1999, 2002 | The concept of component-resolved diagnosis with recombinant allergens | Articles exemplify usefulness of recombinant allergens to discriminate cross reactivity from genuine sensitization20,21 |
| 2001–2004 | First immunotherapy trial with recombinant hypo allergens | Vaccination with hypoallergenic Bet v 1 prevents progression of allergy disease22 |
| 2002–2008 | First immunotherapy trial with recombinant wild type allergens | Immunotherapy with major grass and birch pollen allergens23,24 |
| 2002 | Development of first microarray for allergy diagnosis | Development of microarray containing 94 purified allergen molecules25 |
| 2008 | Recombinant allergens as reference materials for standardization of allergenic products | CREATE project of the European Union26 |
| 2008–2014 | Development of MEDALL microarray for allergy diagnosis | Extended version of ISAC microarray containing more than 170 recombinant and natural allergens27 |
| 2006–2016 | SLIT studies with recombinant allergens | Birch pollen allergy28 |
| 2009–2016 | Phase 2 and 3 specific immunotherapy studies with recombinant allergen derivatives and peptides for respiratory and food allergies completed | Grass pollen,29–31 fish,32 cat33 |
Abbreviations: ISAC, immune solid-phase allergen chip; MEDALL, Mechanisms of the Development of Allergy; SLIT, sublingual immunotherapy.
The objective of this review is to provide an overview of the current status of recombinant and purified allergens and peptides in the context of allergy research, diagnosis, and allergen immunotherapy (AIT) and should help allergologists and scientists to understand the advantages and drawbacks of using pure and defined recombinant allergens and peptides over allergen extracts. We describe several other aspects of recombinant allergens and synthetic peptides from manufacturing procedures to regulative issues to published and ongoing clinical studies.
Considerations Regarding Single Recombinant and Natural Allergens and Peptides
Potential Applications of Single Recombinant or Natural Allergens and Peptides
Single recombinant and natural allergens have many potential applications. They are useful tools in the research for immunologic, biochemical, and structural characterization of allergy-eliciting molecules and for understanding the mechanisms that are implicated in the development of allergy. Another important application is in allergy diagnostic assays. Two main types of IgE antibody assays are performed in the clinical immunology laboratory, singleplex assays in which IgE antibodies to only one single allergen are measured at a time (eg, ImmunoCAP, Thermo Fisher Scientific) and multiplex assays in which in parallel IgE reactivity to multiple allergens is determined (eg, microarray ISAC, Thermo Fisher Scientific).
Diagnostic tests based on microarrayed allergens can be useful in determining the correct prescription of AIT and can be used to monitor efficacy of AIT by monitoring the development of allergens specific IgGs.37 Furthermore, allergen components have the potential to be used in various ways to improve treatment options for allergic patients. Recombinant or purified natural allergens replacing the allergenic panel of natural extracts can be used in immunotherapy.23,24 Moreover, allergen derivatives with the goal of improving the efficacy and safety of vaccines and decreasing the number of injections are being intensively studied. These derivatives include hypoallergenic allergen mosaics,38 hypoallergenic hybrid proteins,39 allergen fragments,12 peptide carrier fusion proteins,29 and T-cell peptide–based vaccines.40
Peptides are primarily useful for scientific purposes and are used to search for immunodominant IgE, IgA, and IgG epitopes and T-cell epitopes. In food allergy, peptides are helpful in defining the sequential IgE epitopes of important wheat,41 milk,42 and peanut allergens.43 In celiac disease, immunodominant IgA epitopes of γ-gliadin were mapped by testing a series of overlapping peptides. For respiratory allergens, peptides are useful for defining conformational IgE epitopes by testing which linear sequences on coupling to a carrier molecule can induce good blocking IgG antibodies. Likewise, peptides derived from 4 major grass pollen allergens were used to define the sequences that can induce good blocking IgGs, and this was a basis for the allergy vaccine, which produced encouraging results in trials with grass pollen allergic patients.29–31 Finally, overlapping synthetic peptides were used to select the immunodominant T-cell epitopes of major cat allergen Fel d 1 that were included in therapeutic vaccine, which was tested in clinical studies for the ability to induce tolerance in cat allergic patients.40 Prophylaxis of allergy is also an emerging topic because AIT can prevent the progression of rhinitis to asthma when given in children.39,44 One could apply AIT in children who are sensitized (have allergen specific IgE) but do not have symptoms yet to prevent the development of atopy later in life. Prerequisite for this would be IgE testing by multiplex array in children at high risk for atopy. Another possible concept of prophylaxis is early intervention to prevent even the development of allergic sensitization by tolerance induction.39,45
Advantages and Disadvantages of Single Recombinant or Natural Allergens and Peptides Compared With Whole Allergen Extracts
The usefulness of commercial whole-allergen extracts for skin testing is limited by many factors (Table 2). They are very difficult to standardize and have variable content of major and minor allergens, and sometimes important allergens are even not present in the extracts. Five hundred different allergenic extracts for immunotherapy are marketed in the United States, but only 19 of them are standardized. Standardized allergenic extracts licensed for distribution in the United States include the pollens from short ragweed, 7 northern pasture grasses and Bermuda grass, cat hair and epithelia, the house dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), and the venoms from honey bees, yellow jackets, hornets, and paper wasps.46 In Europe, manufacturers use in-house reference preparations and create their own allergen extract units. Therefore, direct comparison of allergen extracts from different manufacturers regarding allergen content or potency is currently impossible. In 2012, the major allergens Bet v 1 and Phl p 5a were introduced by the European Pharmacopoeia Commission as biological reference materials, and the assays for quantification of major allergens in the extracts will likely become mandatory for allergen manufacturers in in-house reference preparation calibration in the near future.47 Several studies found that allergen extracts also contain nonallergenic compounds and can be contaminated by allergens from other sources, which can lead to false-positive results.48,49 Although appropriate measures should be in place at allergen manufacturing companies to prevent cross-contamination, standardization of allergen extracts is still a problem and contaminations still occur. However, allergen components are highly purified and defined molecules, and the amount of proteins is precisely known. Skin allergy testing and intradermal testing using extracts, although considered a relatively safe procedure, in rare occasions can lead to fatalities. Furthermore, using allergen components enables us to discriminate genuine sensitization from cross-reactivity, whereas testing with allergen extracts makes this impossible. This is especially important for exact prescription of immunotherapy. It was also shown that sensitization to certain allergenic components early in life can have a predictive role for development of symptoms later is life.34
Table 2. Advantages and Disadvantages of Single Recombinant and Purified Allergens and Peptides Compared With Whole Allergen Extracts in Diagnosis and Therapy.
| Recombinant allergens and allergen derivatives and peptides | Natural allergen extracts | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Highly pure proteins of defined quality (GMP production) | Initial establishment of the production process may be expensive but then easy to produce again and again | Technically easy preparation of extracts because no purifications are performed | Natural allergen sources may be limiting (eg, house dust mites), allergen contents cannot be influenced but only assessed for certain allergens |
| Defined amounts and reproducible quality | Initially complicated market authorization because the products are new | Contain multiple allergenic proteins from specific allergen source | Mixture of allergenic and nonallergenic components |
| Allergenic potential can be modified as needed: allergens mimicking wildtype allergens can be made for diagnosis and safe hypoallergens for AIT can be produced | Panel of allergens for certain sources not yet complete | Currently, easier market authorization because products are known | Variable content of allergens, batch to batch variations |
| Multiple advantages for educated allergologist (eg, enable to discriminate genuine sensitization from cross-reactivity) | Allergologists are more familiar with the use of extracts because of a long tradition | Important allergens are sometimes absent from the extract | |
| May contain contaminations from other sources | |||
Abbreviations: AIT, allergen immunotherapy; GMP, good manufacturing practices.
From a financial perspective, production of allergen extracts may seem to be inexpensive and simple compared with the production of single components, but in general, given that a diagnosis of allergy in a classic manner using extracts sometimes takes years, total costs might be lower if a patient with suspected allergy undergoes multiplex testing at the very beginning. However, for some allergen sources, a panel of purified or recombinant allergens is still incomplete, and in that case components cannot fully replace the extracts. Other issues include a more difficult market authorization and very high standards for allergen components. However, it turns out that allergen extracts might eventually disappear from the European market because they do not meet today’s quality requirements.50
Manufacturing of Single Recombinant or Natural Allergens and Peptides
How Single Recombinant or Natural Allergens and Peptides Are Manufactured
Recombinant proteins can be manufactured under good manufacturing practice (GMP) conditions in bacteria, yeast, insect cells, and mammalian cells. The most widespread expression system is Escherichia coli with the advantage that it is relatively cheap and easy to handle and proteins are produced in large amounts. The disadvantage is that certain proteins are found in the form of inclusion bodies and need a refolding step, which can be critical for the functionality of the allergen. Few proteins cannot be properly expressed in E coli because they require correct disulfide bond formation and/or posttranslational modifications and therefore need to be expressed in eukaryotic cells. No matter which of the expression system from the ones mentioned above is chosen, careful design, construction, and sequencing of the expression vector is important. Regarding antibiotic resistance gene of the expression vectors, kanamycin resistance should be favored over ampicillin because of reports about severe hypersensitivity reactions to ampicillin. Subsequently, master cell bank derived from one single transformed clone should be established and frozen as glycerol stock. This master cell bank will be used for all further production lots, thus ensuring consistency of the end product. For product expression and fermentation, high-cell density cultivation based on a fed-batch protocol is most common, and high-performance cultivation may result in a cell density of more than 100 OD of E coli and more than 200 OD units for Pichia pastoris, both resulting in recombinant protein titers of up to 10 g/L of culture broth. On expression, the cells are usually separated by continuous-flow centrifugation, followed by mechanical high-pressure homogenization. Expression and harvesting are referred as upstream processing. Proteins are subsequently purified by several chromatographic steps (eg, hydrophobic interaction, ion exchange, size-exclusion chromatography). In general, downstream processing (protein purification) is usually structured into the capture, intermediate purification, and polishing model. Purified proteins are then analyzed for their identity, quantity, homogeneity, fold stability, and aggregation by different analytical methods (eg, denaturing gel electrophoresis, mass spectrometry, circular dichroism spectroscopy, reverse-phase high-performance liquid chromatography, size exclusion chromatography).28,29,32 Quality control results usually contain information on protein concentration and appearance, endotoxin content, host cell DNA and protein content, confirmation of product’s identity by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, mass spectrometry, and immunoblot. For the registration of new allergen products, recombinant allergens are additionally compared with their natural counterparts regarding their biological activity by basophil activation and T-cell reactivity assays. Allergens produced in E coli are not glycosylated, which is considered an advantage because allergen’s glycosylation does not seem to play a role in allergy, and, even more, cross-reactive carbohydrate determinants on some fruit, vegetable, and venom allergens can be a problem for determining the exact elicitor of allergy because they can give rise to false-positive IgE test results.51,52 Likewise, yeast P pastoris overglycosylates the proteins, and such allergens could then yield false-positive results because of cross-reactive carbohydrates when used in in vitro testing. Therefore, glycosylated allergens for diagnosis are avoided. However, glycosylation can be avoided by mutating the glycosylation sites in the allergen sequences. The only carbohydrate with possible clinical relevance is α-1,3-galactose, which is implicated in meat allergy.53
The advantage of insect cells for allergen production are well-folded proteins that are secreted in the medium and are therefore easier to purify. However, insect cells and mammalian cells are more complicated to handle than bacteria or yeast, and the manufacturing costs are also higher. For these reasons, these 2 systems are more seldom used for recombinant allergen manufacturing. The end product is always a defined recombinant allergen or allergen derivative of high purity, free of contaminations for which a detailed physiochemical and immunologic characterization, including the potency of the molecule, is known and guaranteed for each batch because of the consistent production and purification process.
Traditional natural allergen extracts represent products that are different from pure recombinant allergens. They are obtained by extraction of natural allergen sources, such as pollen, house dust mites, animal dander, molds, and food. They represent undefined mixtures of allergens and nonallergenic materials. During extraction, there are not usually purification steps except for the removal of lipids and dialysis steps. The final product is always a crude allergen extract, and quality controls include at best the demonstration of the presence of one or a few allergens by antibody- or mass spectrometry–based assays. The concentration of the individual allergens is predetermined by the allergen source and cannot be influenced except by dilution or concentration of the whole extract. Some manufacturers still use potency assays to determine the allergenic activity of the extracts using reference serum samples from allergic patients, but the reactivity profiles and the titers in such reference serum samples may vary from patient to patient, depending on when samples are obtained from a given patient. Therefore, manufacturers in Europe have developed assays to measure the concentrations of major allergens in the natural extracts and supply allergologists with “standardized extracts” that are ready to use. Such assays are also used in the United States, but it is also common in the United States that allergologists buy stock extracts and individually assess the potency of the extracts in their patients by skin testing before use.
Diagnostic and therapeutic products based on purified natural allergens are rare or nonexisting. In principle, natural allergens can be purified from the natural allergen sources, such as pollen, animal dander, food, and house dust mites, using several chromatographic steps and are analyzed for their identity, quantity, homogeneity, folding, and stability similar to what is described above for recombinant allergens. The disadvantage of natural allergens is the large amount of starting material that is required for isolation of some allergens because these allergens sometimes constitute only a small percentage of total protein content (eg, some pollen or house dust mite allergens). However, some allergens, such as animal serum albumins or cow’s milk proteins, can be purified in sufficient amounts from their natural sources. A major concern regarding allergens derived from mammalian sources is that they may be contaminated with viruses.
Peptides can be manufactured using 2 major approaches, a solid-phase and a liquid-phase synthesis. Currently, 9-fluorenylmethyloxycarbonyl solid-phase peptide synthesis is the method of choice for peptide synthesis.54 Peptides are synthesized either on an automated peptide synthesizer or by manual synthesis. After synthesis, peptides are purified using high-performance liquid chromatography. Peptide products are finally analyzed for their identity and purity by reversed-phase high-performance liquid chromatography and by mass spectrometry. Purity of the peptides is dependent on the final application and is usually 70% to 98%, which strongly influences the costs of the peptide production. Usual contaminations in peptide production are so-called deletion sequences attributable to the slight inefficiencies of the coupling reaction that sometimes copurify with the target peptide. Peptides with purity between 70% and 85% can be used for generating polyclonal antibodies, testing antibody titer in enzyme-linked immunosorbent assay, or affinity purification of antibodies. Peptides used for drug studies in patients usually have purity greater than 98% and need to be produced under GMP conditions. Peptides with up to 60 amino acids are routinely synthesized, but it is also possible to synthesize longer peptides up to 100 amino acids.
Products Currently Available on the Market
Allergen components have been used in routine in vitro diagnostics for more than 10 years. Worldwide, 3 singleplex analyzers that use the classic allergosorbent design are dominant on the market. These are the ImmunoCAP (Thermo Fisher Scientific/Phadia), Immulite (Siemens Healthcare Diagnostics, Erlangen, Germany), and the HyTEC88 (Hycor Biomedical, Garden Grove, California).55 ImmunCAP technology offers allergen specific IgE testing with 70 allergen components produced as either recombinant or purified allergens (http://www.phadia.com). These components are from different foods, animals, trees, grass or weed pollens, fungi, mites, and venoms. The other 2 systems are allergen extract based. The example for reverse-phase assay for IgE antibody quantization in use is the ADVIA Centaur, which is also allergen extract based. The first multiplex in vitro allergy diagnostic tool based exclusively on allergen components is ImmunoCAP ISAC (Thermo Fisher Scientific/Phadia). This technology enables the measurement of IgE antibodies to a panel of 112 components from 51 allergen sources in a single step, using only 30 μL of serum or plasma. This system also holds great potential for improving the monitoring of the patients undergoing allergen immunotherapy.37 Initially, natural allergen extract–based diagnostic tests appeared less costly. However, the new multiplex tests using comprehensive panels of allergen molecules can resolve complicated cases of polysensitization more quickly56 and allow the selection of AIT more correctly than allergen extract–based tests.57,58 Therefore, recombinant allergen–based diagnostic tests seem to be more cost-effective than allergen extracts, and the advantages of molecular-based allergy diagnostics have been also recognized by the World Allergy Organization in a position paper.59 Regarding the vaccines based on single recombinant or natural allergens, no products so far are registered on the market. To register such a vaccine, appropriate clinical trials need to be performed before registration, and this is associated with huge costs and may take 5 to 10 years. Most of the efforts associated with the use of recombinant or purified allergens and peptides are being performed in Europe and less in the United States and other countries. However, some of the vaccines based on recombinant allergens and peptides successfully completed phases 2 clinical trials, and we hope the first recombinant-based vaccine is on the market soon. The introduction of new recombinant allergen–based therapies will obviously require considerable investments in the clinical trial, but once registered the vaccines can be produced in a consistent quality at low costs.
Regulatory Issues
In Europe, most AIT products have been marketed for decades as named patient products, which are primarily responsible for meeting GMP requirements. Since 1989, in the European Union, allergen extracts used for in vivo diagnostic tests or therapy have been defined as medicinal products and as a consequence have to be registered by national authorities. The marketing authorization procedures in different European countries are not homogeneous yet, but in general authorization requires clinical trials to demonstrate safety, sensitivity, and specificity of the allergen extracts.47,50 These procedures are of great value for allergologists and patients, but clinical trials may take years and have huge costs, and as a consequence manufacturers may significantly limit their allergen portfolios for economic reasons providing only the frequently used extracts.50 For the above mentioned reason, the future of in vivo allergy diagnosis based on allergen extracts in Europe is uncertain. As an alternative, serologic allergy tests represent so-called in vitro diagnostic medical devices, and because they are not applied directly on patients they do not need a marketing authorization as opposed to SPT extracts, extracts for provocation testing, and extracts for immunotherapy. This is one of the reasons why in vitro allergy diagnosis, such as CRD and basophil activation testing based on recombinant and purified allergens, is becoming increasingly important in allergy diagnosis in Europe. Regarding specific immunotherapy in Europe, in 2009, the European Medicines Agency issued a new guideline on the clinical development of products for AIT.60 Accordingly, a marketing authorization granted by a centralized procedure must be followed for recombinant allergen and peptide-based vaccines. Because such vaccines contain only a limited number of clinically important allergens from a certain allergen source, the applicant must justify the selected allergens and define and justify the selection of study population in regard to the included allergens. Vaccines based on recombinant allergens may not be suitable for all populations but should be designed to include all clinically relevant allergens from that allergen source, which are recognized by most patients. In the United States, the US Food and Drug Administration (FDA) is responsible for marketing approval of allergen products. For the standardized allergen extracts, manufacturers compare the allergen extract to a US reference standard for potency. An application for a new allergenic extract might not require extensive clinical data when the allergenic extract product is consistent with those reference standards. There are no FDA validated methods or requirements for testing the potency of nonstandardized extracts. The FDA is also responsible for approval of the new recombinant- or peptide-based allergy vaccines. A possible hurdle for approval in the United States is the use of aluminum hydroxide in some of vaccines for which the FDA requires costly, long-term safety studies. In addition, the FDA has set a high bar for efficacy—15% overall but 10% separation of the 95% confidence interval of the active product from the mean of the placebo. This means that large and therefore very expensive studies to narrow the confidence interval need to be performed.61
Clinical Studies Performed to Date
The first studies performed with synthetic peptides of the major cat allergen Fel d 1 did not yield satisfying clinical results15,16 (Table 3). Early studies with higher concentrations of longer Fel d 1–derived peptides (Allervax CAT) administered subcutaneously were only partially encouraging because improved tolerance to cats observed in cat allergic patients was often accompanied by late asthmatic responses in some of the study participants.17 A clinical study in patients with birch pollen allergy24 has shown that recombinant Bet v 1 (rBet v 1) was equally effective as birch pollen extract for subcutaneous immunotherapy (clinicaltrials.gov Identifier: NCT00410930). However, rBet v 1 is a fully IgE reactive protein, and therefore patients had to follow an inconvenient up-dosing schedule and monthly maintenance injections to avoid adverse effects. To increase the safety of sublingual immunotherapy, tablets that contained rBet v 1 for sublingual treatment were tested (clinicaltrials.gov Identifier: NCT00396149). The mean adjusted symptom scores were significantly decreased relative to placebo in patients receiving once-daily rBet v 1 tablets for 5 months.28 However, because the tablets need to be taken daily for many years, the adherence of the patients to the regimen is low, and it is also possible that sublingual application of rBet v 1 will induce oral allergy syndrome in some patients because an unmodified allergen that contains all IgE epitopes is applied. The need to reduce the potential adverse effects of AIT led in 2000 to the first AIT trial with recombinant allergen derivatives. Hypoallergenic recombinant fragments of rBet v 1 and rBet v 1 trimer were tested in 124 birch pollen allergic patients in a double-blind, placebo-controlled study.22 This treatment had clinical efficacy, induced allergen-specific blocking IgG antibodies, reduced seasonal boosts of IgE production, and eliminated IgE-mediated immediate adverse effects. However, because most Bet v 1 T-cell epitopes are still maintained in the vaccine, systemic late-phase adverse effects were observed despite eliminated IgE reactivity.22,70 Similar findings were made later with long peptides of the major birch pollen allergen Bet v 1.68,69
Table 3. Clinical Studies Performed to Date With Recombinant Allergens, Derivatives Thereof, and Peptides.
| Molecules (Approximate timeframe) | Vaccine description | Study design and clinical trial no. | References |
|---|---|---|---|
| Allervax CAT (1996–1999) | Two Fel d 1–derived peptides of 27 amino acids | SCIT, DBPC | 15, 16, 18 |
| Bet v 1 trimer, Bet v 1 fragments (2000–2001) | Hypoallergenic recombinant derivatives of Bet v 1 | Phase 2 completed, SCIT, DBPC | 22 |
| Recombinant Phl p 1, recombinant Phl p 2, recombinant Phl p 5aþb, recombinant Phl p 6 (2002–2013) | Recombinant grass pollen allergen cocktail | Phase 3 completed, SCIT, DBPC (Allergopharma and Worm), NCT00671268, NCT00309036 | 23 |
| Folding variant of Bet v 1 (2002–2013) | Hypoallergenic recombinant folding variant of the major birch pollen allergen (recombinant Bet v 1 folding variant) | Phase 3 completed, SCIT, DBPC (Allergopharma and Rak), NCT00266526, NCT00554983, NCT00841516 | 62, 63 |
| Recombinant Bet v 1 (2002–2008) | To compare recombinant Bet v 1 with natural Bet v 1 and birch pollen extract in SCIT in birch allergic patients | Phase 2 completed, SCIT, DBPC (Stallergenes and Pauli), NCT00410930 | 24 |
| Recombinant Bet v 1 tablets (2006–2013) | Recombinant Bet v 1 administered as sublingual tablets in birch pollen allergic subjects | Phase 2 completed (Stallergenes and Rak), SLIT, DBPC, NCT00901914, NCT00396149, NCT00889460 | 28 |
| ILIT with MAT-Fel d 1 (2008–2010) | Intralymphatic immunotherapy for cat allergy | Phase 1 (Senti and Kündig), NCT00718679 | 64 |
| Ara h 1, Ara h 2, and Ara h 3 (2009–2013) | Rectal application of Escherichia coli–encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 | Phase 1 completed, safety study (Allertein Therapeutics and Sicherer), NCT00850668 | 65 |
| Fcγ1-Fel d1 fusion protein (2011–2014) | Intradermal human Fcg1-Fel d1 fusion protein | Safety study (NIAID and Saxon), NCT01292070 | |
| BM32 (2012–2016) | Hypoallergenic vaccine for immunotherapy of grass pollen allergy consisting of four major allergens | Phase 2b completed, SCIT, DBPC (Biomay), NCT01538979, NCT02643641 | 29–31, 66 |
| ToleroMune Cat (2012–2016) | Fel d 1–derived synthetic peptides for induction of tolerance in cat allergic patients | Phase III completed, intradermal, DBPC (Circassia Ltd), NCT01620762 | 33, 67 |
| AllerT (2012–2015) | Bet v 1–derived contiguous overlapping peptides | Phase 2b completed, SCIT, DBPC (Anergis and Spertini), NCT01720251, NCT02143583, NCT02271009 | 68,69 |
| FAST-Fish (2013–2015) | Food allergy specific treatment for fish allergy based on subcutaneous application of mutated parvalbumin (recombinant Cyp p 1) | Phase 1/2 completed (FAST and Maling), NCT02017626 | 32 |
| ToleroMune Grass (2014–2016) | Short peptides from grass pollen allergens | Phase 2b/3 started, intradermal, DBPC (Circassia Ltd and Bernstein), NCT02795273, NCT02161107 | |
| ToleroMune HDM (2014–2016) | Short peptides derived from house dust mite allergens | Phase 2, intradermal, DBPC (Circassia Ltd), NCT02150343 | |
| ToleroMune Ragweed (2014–2016) | Short peptides from Amb a 1 | Phase 2 (Circassia Ltd), NCT02061709, NCT02396680 |
Abbreviations: DBPC, double-blind, placebo controlled; FAST, Food Allergy–Specific Immunotherapy; HDM, house dust mite; NIAID, National Institute of Allergy and Infectious Diseases; SCIT, subcutaneous immunotherapy.
Despite the discouragements of the early studies with Fel d 1–derived peptides, this line of study was followed by several research teams, which led to several interesting trials. One approach that used 7 synthetic T-cell epitope–containing peptides (13–17 amino acids) derived from major cat allergen Fel d 1 with the aim of inducing T-cell tolerance produced promising results up to phase 2 (Circassia Ltd, Oxford, United Kingdom). Treatment seemed to be clinically effective, and the effects could be long-lasting, with the advantage that this treatment requires only a few injections. It also seems that it has been possible to overcome the originally observed late-phase T-cell–mediated adverse effects.33 However, the recently released results from a phase 3 study found that there was no clinical benefit compared with placebo treatment.71 On the basis of the same principle of short peptides, several other vaccines developed by the same company were in clinical trials up to phase 2b (house dust mite, grass pollen, ragweed), but after the disappointing results of the phase 3 for cat vaccine, further trials will probably not be continued. A second approach with longer T-cell epitope peptides, named contiguous overlapping peptides, completed phase 2b clinical trials. A regimen that comprised 5 injections in 2 months resulted in improved nasal provocation scores, but, as already seen in the early cat peptide trials 17 years ago,17 higher concentrations of contiguous overlapping peptides frequently induced late asthmatic responses.68,69
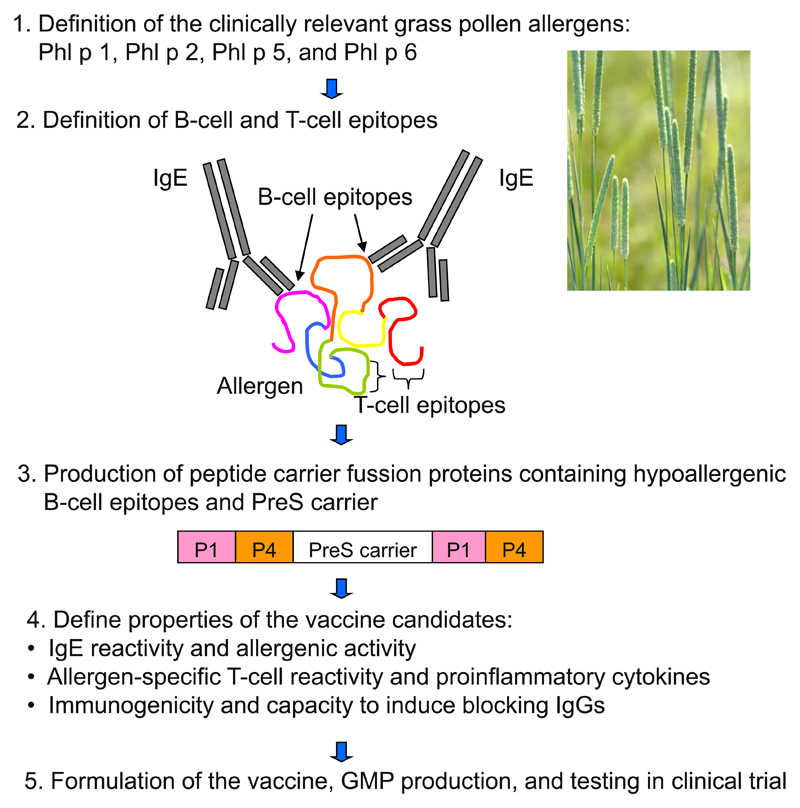
To overcome the problems of AIT-induced immediate-type reactions, T-cell–mediated late-phase reactions, and inconvenient treatment schedules based on large numbers of applications, a new B-cell epitope–based allergy vaccine, BM32, was developed29 (Fig 1) and tested in clinical trials (clinicaltrials.gov Identifiers: NCT01538979 and NCT02643641).30,31,66 The vaccine contains non–IgE-reactive peptides of a length of approximately 20 to 40 amino acids, which are derived from the IgE-binding sites of the 4 major allergens of grass pollen (Phl p 1, Phl p 2, Phl p 5, and Phl p 6). The peptides are covalently linked to a viral protein carrier that provides carrier-specific T-cell help. Because BM32 vaccine has a strongly reduced allergenic activity and reduced allergen specific T-cell reactivity,29,30 it is possible to inject high doses into patients so only 3 to 5 injections per year will be needed for treatment what makes this vaccine extremely convenient for the patient. Double-blind, placebo-controlled phase 2b was successfully completed, and good tolerability with no severe adverse effects and sustained relief of allergy symptoms were reported.31 As a carrier protein in BM32 vaccine, hepatitis B–derived PreS protein was used, and immunotherapy with BM32 in addition to beneficial effect for grass pollen allergy also induced antibody responses that protected against hepatitis B infection in vitro.66 This indicates that BM32 could also be useful for therapeutic vaccination of patients infected with hepatitis B virus.66,72
Figure 1.
Scheme of the development of recombinant grass pollen allergy vaccine (BM32) that is based on the fusion of hypoallergenic B-cell epitopes derived from 4 clinically relevant grass pollen allergens with viral carrier protein PreS. GMP, good manufacturing practice.
One of the most challenging areas of AIT is food allergies. The European Union–funded project Food Allergy–Specific Immunotherapy initiated in 2008 aimed at developing 2 hypoallergenic recombinant proteins for the sublingual (fruit) and subcutaneous (fish) route. The first aim of the project focused on the major allergen of peach, lipid transfer protein, and the second on the major allergen of fish, parvalbumin. Although the part regarding peach was less successful because of the difficulty of producing a hypoallergenic and immunogenic vaccine under GMP conditions, a vaccine for fish allergy based on mutant Cyp c 1 was tested in a phase 1/2a, 2-center, randomized, double-blind, placebo-controlled clinical trial (clinicaltrials.gov Identifier: NCT02017626).32,73 A low level of adverse effects and positive immunologic response were promising outcomes, and a phase 2b trial looking at the efficiency is currently planned. Other attempts to develop safe treatment for food allergy were made for peanut allergy. The trial with rectal application of E coli–encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 completed phase 1 and had a good safety (clinicaltrials.gov Identifier: NCT00850668).65
Relevance of Single Recombinant Allergens and Peptides
What Clinicians Should Know
CRD of allergy based on single recombinant or purified allergens offers several advantages over traditional allergen extracts. Products for in vitro diagnosis have been available on the market for almost 10 years and are continuing to develop. These methods are especially useful for testing children because only blood taking is required for testing patients who do not have clear results when tested with extracts. One of the major advantages is that CRD can discriminate genuine sensitization from cross-reactivity, and this information is especially valuable when deciding about immunotherapy. Testing with allergen extracts might reflect a more in vivo situation, but because of their poor quality, high diversity, and lack of standardization, results are not always reliable and clear.
Future Perspectives
New allergen sequences will be cloned and the panel of clinically relevant allergens will be completed. If introduced in the routine laboratory diagnosis of allergy, CRD will help us to discriminate between genuine sensitization and cross-reactivity and will be useful in predicting which patients will likely benefit from AIT and which patients will not. It will also be useful in identifying patients with polysensitization for the prescription of IgE-targeting therapies. Such patient-tailored forms of treatment could reduce the financial costs for health care system, save time for patients, and reduce adverse effects. Monitoring the course of immunotherapy by measuring allergen specific antibody responses developed during AIT will also be possible. Furthermore, successful immunotherapy studies, which were already performed or are still ongoing, with recombinant allergens and hypoallergenic allergen derivatives will lead to the registration of the first recombinant allergen–based vaccines in the near future. In addition to treatment, innovative vaccines based on recombinant fusion proteins that consist of viral carrier proteins and allergen-derived peptides without allergenic activity hold the promise of being useful for prophylactic vaccination for allergy.
Conclusion
Developments in the field of recombinant or purified allergen resulted in the in vitro tests that enable reliable diagnosis of allergy in a convenient way. New vaccines based on recombinant allergens, recombinant allergen derivatives, and synthetic peptides are currently being tested in clinical trials, and some of those might be applicable not only for therapeutic but also for prophylactic vaccination.
Funding Sources
This study was supported by research grant F4605 from the Austrian Science Fund and by a research grant from Biomay AG, Vienna, Austria.
Footnotes
Disclosures: Dr Valenta reported receiving grants and consulting fees or honoraria from Biomay AG, ThermoFisher, and Fresenius Medical Care. No other disclosures were reported.
References
- [1].Fang KS, Vitale M, Fehlner P, King TP. cDNA cloning and primary structure of a white-face hornet venom allergen, antigen 5. Proc Natl Acad Sci U S A. 1988;85:895–899. doi: 10.1073/pnas.85.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Breiteneder H, Pettenburger K, Bito A, et al. The gene coding for the major birch pollen allergen Betv1, is highly homologous to a pea disease resistance response gene. EMBO J. 1989;8:1935–1938. doi: 10.1002/j.1460-2075.1989.tb03597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chua KY, Stewart GA, Thomas WR, et al. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J Exp Med. 1988;167:175–182. doi: 10.1084/jem.167.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ebner C, Birkner T, Valenta R, et al. Common epitopes of birch pollen and apples—studies by Western and Northern blot. J Allergy Clin Immunol. 1991;88:588–594. doi: 10.1016/0091-6749(91)90152-e. [DOI] [PubMed] [Google Scholar]
- [5].Valenta R, Duchene M, Pettenburger K, et al. Identification of profilin as a novel pollen allergen; IgE autoreactivity in sensitized individuals. Science. 1991;253:557–560. doi: 10.1126/science.1857985. [DOI] [PubMed] [Google Scholar]
- [6].Valenta R, Duchene M, Vrtala S, et al. Recombinant allergens for immunoblot diagnosis of tree-pollen allergy. J Allergy Clin Immunol. 1991;88:889–894. doi: 10.1016/0091-6749(91)90245-j. [DOI] [PubMed] [Google Scholar]
- [7].Valenta R, Vrtala S, Ebner C, Kraft D, Scheiner O. Diagnosis of grass pollen allergy with recombinant timothy grass (Phleum mmunee) pollen allergens. Int Arch Allergy Immunol. 1992;97:287–294. doi: 10.1159/000236135. [DOI] [PubMed] [Google Scholar]
- [8].Moser M, Crameri R, Brust E, Suter M, Menz G. Diagnostic value of recombinant Aspergillus fumigatus allergen I/a for skin testing and serology. J Allergy Clin Immunol. 1994;93:1–11. doi: 10.1016/0091-6749(94)90227-5. [DOI] [PubMed] [Google Scholar]
- [9].Lynch NR, Thomas WR, Chua Y, García N, Di Prisco MC, López R. In vivo biological activity of recombinant Der p II allergen of house-dust mite. Int Arch Allergy Immunol. 1994;105:70–74. doi: 10.1159/000236805. [DOI] [PubMed] [Google Scholar]
- [10].Ball T, Vrtala S, Sperr WR, et al. Isolation of an immunodominant IgE hapten from an epitope expression cDNA library: dissection of the allergic effector reaction. J Biol Chem. 1994;269:28323–28328. [PubMed] [Google Scholar]
- [11].Smith AM, Chapman MD. Reduction in IgE binding to allergen variants generated by site-directed mutagenesis: contribution of disulfide bonds to the antigenic structure of the major house dust mite allergen Der p 2. Mol Immunol. 1996;33:399–405. doi: 10.1016/0161-5890(95)00150-6. [DOI] [PubMed] [Google Scholar]
- [12].Vrtala S, Hirtenlehner K, Vangelista L, et al. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J Clin Invest. 1997;99:1673–1681. doi: 10.1172/JCI119330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pauli G, Oster JP, Deviller P, et al. Skin testing with recombinant allergens rBet v 1 and birch profilin, rBet v 2: diagnostic value for birch pollen and associated allergies. J Allergy Clin Immunol. 1996;97:1100–1109. doi: 10.1016/s0091-6749(96)70264-6. [DOI] [PubMed] [Google Scholar]
- [14].Laffer S, Spitzauer S, Susani M, et al. Comparison of recombinant timothy grass pollen allergens with natural extract for diagnosis of grass pollen allergy in different populations. J Allergy Clin Immunol. 1996;98:652–658. doi: 10.1016/s0091-6749(96)70099-4. [DOI] [PubMed] [Google Scholar]
- [15].Norman PS, Ohman JL, Jr, Long AA, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–1628. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- [16].Simons FE, Imada M, Li Y, Watson WT, HayGlass KT. Fel d 1 peptides: effect on skin tests and cytokine synthesis in cat-allergic human subjects. Int Immunol. 1996;8:1937–1945. doi: 10.1093/intimm/8.12.1937. [DOI] [PubMed] [Google Scholar]
- [17].Haselden BM, Kay AB, Larché M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–1894. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maguire P, Nicodemus C, Robinson D, Aaronson D, Umetsu DT. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999;93:222–231. doi: 10.1006/clim.1999.4795. [DOI] [PubMed] [Google Scholar]
- [19].van Hage-Hamsten M, Kronqvist M, Zetterström O, et al. Skin test evaluation of genetically engineered hypoallergenic derivatives of the major birch pollen allergen, Bet v 1: results obtained with a mix of two recombinant Bet v 1 fragments and recombinant Bet v 1 trimer in a Swedish population before the birch pollen season. J Allergy Clin Immunol. 1999;104:969–977. doi: 10.1016/s0091-6749(99)70077-1. [DOI] [PubMed] [Google Scholar]
- [20].Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Grönlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT) Clin Exp Allergy. 1999;29:896–904. doi: 10.1046/j.1365-2222.1999.00653.x. [DOI] [PubMed] [Google Scholar]
- [21].Kazemi-Shirazi L, Niederberger V, Linhart B, Lidholm J, Kraft D, Valenta R. Recombinant marker allergens: diagnostic gatekeepers for the treatment of allergy. Int Arch Allergy Immunol. 2002;127:259–268. doi: 10.1159/000057742. [DOI] [PubMed] [Google Scholar]
- [22].Niederberger V, Horak F, Vrtala S, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101(suppl 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [24].Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- [25].Hiller R, Laffer S, Harwanegg C, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–416. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- [26].van Ree R, Chapman MD, Ferreira F, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63:310–326. doi: 10.1111/j.1398-9995.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- [27].Lupinek C, Wollmann E, Baar A, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergenchip. Methods. 2014;66:106–119. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nony E, Bouley J, Le Mignon M, et al. Development and evaluation of a sublingual tablet based on recombinant Bet v 1 in birch pollen-allergic patients. Allergy. 2015;70:795–804. doi: 10.1111/all.12622. [DOI] [PubMed] [Google Scholar]
- [29].Focke-Tejkl M, Weber M, Niespodziana K, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207–1217. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Niederberger V, Marth K, Eckl-Dorna J, et al. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol. 2015;136:1101–1103. doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zieglmayer P, Focke-Tejkl M, Schmutz R, et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EbioMedicine. 2016;11:43–57. doi: 10.1016/j.ebiom.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zuidmeer-Jongejan L, Huber H, Swoboda I, et al. Development of a hypoallergenic recombinant parvalbumin for first-in-man subcutaneous immunotherapy of fish allergy. Int Arch Allergy Immunol. 2015;166:41–51. doi: 10.1159/000371657. [DOI] [PubMed] [Google Scholar]
- [33].Couroux P, Patel D, Armstrong K, Larché M, Hafner RP. Fel d 1-derived synthetic peptide mmune-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45:974–981. doi: 10.1111/cea.12488. [DOI] [PubMed] [Google Scholar]
- [34].Westman M, Lupinek C, Bousquet J, et al. Mechanisms for the Development of Allergies Consortium. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. 2015;135:1199–1206. doi: 10.1016/j.jaci.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Curin M, Swoboda I, Wollmann E, et al. Microarrayed dog, cat, and horse allergens show weak correlation between allergen-specific IgE and IgG responses. J Allergy Clin Immunol. 2014;133:918–921. doi: 10.1016/j.jaci.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Skrindo I, Lupinek C, Valenta R, et al. The use of the MeDALL-chip to assess IgE sensitization: a new diagnostic tool for allergic disease? Pediatr Allergy Immunol. 2015;26:239–246. doi: 10.1111/pai.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lupinek C, Wollmann E, Valenta R. Monitoring Allergen Immunotherapy Effects by Microarray. Curr Treat Options Allergy. 2016;3:189–203. doi: 10.1007/s40521-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Curin M, Weber M, Thalhamer T, et al. Hypoallergenic derivatives of Fel d 1 obtained by rational reassembly for allergy vaccination and tolerance induction. Clin Exp Allergy. 2014;44:882–894. doi: 10.1111/cea.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Valenta R, Campana R, Focke-Tejkl M, Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137:351–357. doi: 10.1016/j.jaci.2015.12.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Worm M, Lee HH, Kleine-Tebbe J, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- [41].Baar A, Pahr S, Constantin C, et al. The high molecular weight glutenin subunit Bx7 allergen from wheat contains repetitive IgE epitopes. Allergy. 2014;69:1316–1323. doi: 10.1111/all.12464. [DOI] [PubMed] [Google Scholar]
- [42].Adams SL, Barnett D, Walsh BJ, Pearce RJ, Hill DJ, Howden ME. Human IgE-binding synthetic peptides of bovine beta-lactoglobulin and alpha-lactalbumin. In vitro cross-reactivity of the allergens. Immunol Cell Biol. 1991;3:191–197. doi: 10.1038/icb.1991.28. [DOI] [PubMed] [Google Scholar]
- [43].Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA. Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur J Biochem. 1997;245:334–339. doi: 10.1111/j.1432-1033.1997.t01-1-00334.x. [DOI] [PubMed] [Google Scholar]
- [44].Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- [45].Baranyi U, Farkas AM, Hock K, et al. Cell therapy for prophylactic tolerance in immunoglobulin E-mediated allergy. EbioMedicine. 2016;7:230–239. doi: 10.1016/j.ebiom.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Esch RE, Plunkett GA. Immunotherapy preparation guidelines, rules, and regulation. Curr Allergy Asthma Rep. 2013;13:406–4013. doi: 10.1007/s11882-013-0358-8. [DOI] [PubMed] [Google Scholar]
- [47].Jutel M, Agache I, Bonini S, et al. International Consensus on Allergen Immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137:358–368. doi: 10.1016/j.jaci.2015.12.1300. [DOI] [PubMed] [Google Scholar]
- [48].Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154:258–263. doi: 10.1159/000321113. [DOI] [PubMed] [Google Scholar]
- [49].Casset A, Mari A, Purohit A, et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol. 2012;159:253–262. doi: 10.1159/000337654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Klimek L, Hoffmann HJ, Renz H, et al. Diagnostic test allergens used for in vivo diagnosis of allergic diseases are at risk: a European Perspective. Allergy. 2015;70:1329–1331. doi: 10.1111/all.12676. [DOI] [PubMed] [Google Scholar]
- [51].Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- [52].Mittermann I, Zidarn M, Silar M, et al. Recombinant allergen-based IgE testing to distinguish bee and wasp allergy. J Allergy Clin Immunol. 2010;125:1300–1307. doi: 10.1016/j.jaci.2010.03.017. [DOI] [PubMed] [Google Scholar]
- [53].Commins SP, James HR, Stevens W, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2014;134:108–115. doi: 10.1016/j.jaci.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Behrendt R, White P, Offer J. Advances in Fmoc solid-phase peptide synthesis. J Pept Sci. 2016;22:4–27. doi: 10.1002/psc.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, et al. EAACI Molecular Allergology User’s Guide. Pediatr Allergy Immunol. 2016;27(suppl 23):1–250. doi: 10.1111/pai.12563. [DOI] [PubMed] [Google Scholar]
- [56].Fedenko E, Elisyutina O, Shtyrbul O, et al. Microarray-based IgE serology improves management of severe atopic dermatitis in two children. Pediatr Allergy Immunol. 2016;27:645–649. doi: 10.1111/pai.12572. [DOI] [PubMed] [Google Scholar]
- [57].Sastre J, Landivar ME, Ruiz-García M, et al. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy. 2012;67:709–711. doi: 10.1111/j.1398-9995.2012.02808.x. [DOI] [PubMed] [Google Scholar]
- [58].Stringari G, Tripodi S, Caffarelli C, et al. The effect of component-resolved diagnosis on specific immunotherapy prescription in children with hay fever: Italian Pediatric Allergy Network (I-PAN) J Allergy Clin Immunol. 2014;134:75–81. doi: 10.1016/j.jaci.2014.01.042. [DOI] [PubMed] [Google Scholar]
- [59].Canonica GW, Ansotegui IJ, Pawankar R, et al. A WAO-ARIA-GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6:17. doi: 10.1186/1939-4551-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].European Medicines Agency. [Accessed October 27, 2016]; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003605.pdf.
- [61].Nelson HS. Allergen immunotherapy: what’s new, what’s next? Expert Rev Clin Immunol. 2015;11:959–961. doi: 10.1586/1744666X.2015.1062726. [DOI] [PubMed] [Google Scholar]
- [62].Klimek L, Bachert C, Lukat KF, Pfaar O, Meyer H, Narkus A. Allergy immunotherapy with a hypoallergenic recombinant birch pollen allergen rBet v. 1-FV in a randomized controlled trial. Clin Transl Allergy. 2015;5:28. doi: 10.1186/s13601-015-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Meyer W, Narkus A, Salapatek AM, Häfner D. Double-blind, placebo-controlled, dose-ranging study of new recombinant hypoallergenic Bet v 1 in an environmental exposure chamber. Allergy. 2013;68:724–731. doi: 10.1111/all.12148. [DOI] [PubMed] [Google Scholar]
- [64].Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129:1290–1296. doi: 10.1016/j.jaci.2012.02.026. [DOI] [PubMed] [Google Scholar]
- [65].Wood RA, Sicherer SH, Burks AW, et al. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013;68:803–808. doi: 10.1111/all.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cornelius C, Schöneweis K, Georgi F, et al. Immunotherapy with the preS-Based grass pollen allergy vaccine BM32 induces antibody responses protecting against hepatitis B infection. EBioMedicine. 2016;11:58–67. doi: 10.1016/j.ebiom.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Patel D, Couroux P, Hickey P, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–109. doi: 10.1016/j.jaci.2012.07.028. [DOI] [PubMed] [Google Scholar]
- [68].Spertini F, Perrin Y, Audran R, et al. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014;134:239–240. doi: 10.1016/j.jaci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- [69].Spertini F, DellaCorte G, Kettner A, et al. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1-derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: results of a phase 2b study. J Allergy Clin Immunol. 2016;138:162–168. doi: 10.1016/j.jaci.2016.02.044. [DOI] [PubMed] [Google Scholar]
- [70].Campana R, Moritz K, Marth K, et al. Frequent occurrence of T cell-mediated late reactions revealed by atopy patch testing with hypoallergenic rBet v 1 fragments. J Allergy Clin Immunol. 2016;137:601–609. doi: 10.1016/j.jaci.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Circassia Ltd. [Accessed October 27, 2016]; http://www.circassia.com/media/press-releases/circassia-announces-top-line-results-from-cat-allergy-phase-iii-study.
- [72].Gerlich WH, Glebe D. Development of an allergy immunotherapy leads to a new type of hepatitis B vaccine. EBioMedicine. 2016;11:5–6. doi: 10.1016/j.ebiom.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Swoboda I, Balic N, Klug C, et al. A general strategy for the generation of hypoallergenic molecules for the immunotherapy of fish allergy. J Allergy Clin Immunol. 2013;132:979–981. doi: 10.1016/j.jaci.2013.04.027. [DOI] [PubMed] [Google Scholar]