Abstract
Immunoglobulin E (IgE)-associated allergy is the most common immunologically-mediated hypersensitivity disease. It affects more than 25% of the population. In IgE-sensitized subjects, allergen encounter can causes a variety of symptoms ranging from hayfever (allergic rhinoconjunctivitis) to asthma, skin inflammation, food allergy and severe life-threatening anaphylactic shock. Allergen-specific immunotherapy (AIT) is based on vaccination with the disease-causing allergens. AIT is an extremely effective, causative and disease-modifying treatment. However, administration of natural allergens can cause severe side effects and the quality of natural allergen extracts limits its application. Research in the field of molecular allergen characterization has allowed deciphering the molecular structures of the disease-causing allergens and it has become possible to engineer novel molecular allergy vaccines which precisely target the mechanisms of the allergic immune response and even appear suitable for prophylactic allergy vaccination. Here we discuss recombinant allergy vaccines which are based on allergen-derived B cell epitopes regarding their molecular and immunological properties and review the results obtained in clinical studies with this new type of allergy vaccines.
Keywords: Allergy, Allergen, Allergen-specific immunotherapy, Allergy vaccines
1. Introduction
IgE-associated allergy is the most frequent immunologically-mediated hypersensitivity disease [1]. More than 25% of the population suffers from allergic symptoms ranging from allergic rhinoconjunctivitis (i.e., hayfever), allergic asthma, allergic skin manifestations (e.g., urticaria, atopic dermatitis), gastrointestinal manifestations of food allergy and severe, life-threatening systemic anaphylaxis. Several environmental factors contribute to the development of IgE sensitizations in subjects with a genetic predisposition for atopy [2]. In contrast to non-allergic subjects who mount a normal IgG response upon antigen exposure, the immune system of allergic subjects is biased towards the production of IgE antibodies [3–6]. Recently it has become possible to use comprehensive sets of micro-arrayed allergen molecules to monitor the longitudinal development of allergic sensitization in blood samples obtained from children after birth until adolescence. The results obtained for several birth cohorts have shown that allergic sensitization occurs early in life and then leads to the establishment of characteristic sensitization profiles which persist almost unaltered in adults [7,8].
Once allergen-specific IgE antibodies have been produced, they become loaded to a variety of immune cells via the high (FcεRI) and low-affinity receptor for IgE (FcεRII, CD23) [9,10]. Binding to these receptors is important for the triggering of the hypersensitivity reactions leading to allergic disease manifestations. When allergens crosslink the corresponding specific IgE antibodies bound via FcεRI, mast cells and basophils become activated to release inflammatory mediators, cytokines and proteases which lead to immediate allergic inflammation. Allergen uptake via IgE antibodies bound to FcεRII and FcεRI on antigen presenting cells has been shown to be much more efficient than non-IgE-facilitated allergen presentation for the activation of allergen-specific T cell responses in vitro [11]. Since allergic individuals encounter only very low doses of allergens it is conceivable that IgE-facilitated allergen presentation is also important mechanism for the activation of allergen-specific T cells in vivo. One must therefore assume that this mechanism is important for T cell activation and the release of inflammatory cytokines (e.g., IL-5) recruiting additional inflammatory cells (e.g., eosinophils) in allergic patients. In the majority of allergic patients, immediate inflammation induced by cross-linking of mast cell and basophil-bound IgE antibodies is a major mechanism responsible for allergic symptoms but also late-phase allergic symptoms caused by T cell activation and engagement of other innate inflammatory cells (e.g., eosinophils) contribute to allergic symptoms [12] Allergen avoidance is possible only for certain forms of allergy and also only to a limited extent (e.g., food allergy, certain indoor allergen sources such as house dust mites, pets). Therefore current forms of treatment are based mainly on the reduction of symptoms by pharmacotherapy neutralizing mediators which are released from mast cells and basophils and/or targeting cells involved in late-phase allergic inflammation (e.g., T cells, eosinophils). There are several “biologicals” in clinical evaluation [13,14] but currently only omalizumab, a monoclonal anti-IgE antibody which prevents IgE from binding to its receptors is in clinical use for the treatment of severe IgE-mediated asthma [15].
Both pharmacotherapy and biologicals only suppress allergic symptoms temporarily but do not affect the course of allergic disease. By contrast, allergen-specific immunotherapy (AIT) is the only treatment for IgE-associated allergy which prevents the progression of allergy from mild to severe symptoms and thus has a disease-modifying effect [16–18]. It has been also shown that AIT has long-lasting effects even after its discontinuation [19]. AIT has been used for the treatment of grass pollen-induced hay fever in 1911 for the first time [20]. It is based on the administration of the disease-causing allergens in the form of a therapeutic vaccine with the goal to modify the allergen-specific immune response in allergic patients. The clinical effects of AIT are accompanied by the production of allergen-specific IgG antibodies which are thought to interfere with the recognition of allergens by IgE antibodies and thus to suppress the allergic symptoms caused by IgE-allergen immune complexes. Furthermore a reduction of allergen-specific T cell activation and reduced boosts of IgE production have been observed in AIT-treated patients [17]. Major advantages of AIT are the disease-modifying and long-lasting effect which can be achieved by a therapeutic vaccine benefiting from the patient's immune system instead of administration of drugs and that AIT is usually more effective in reducing symptoms than pharmacotherapy. Moreover, AIT has been shown to be more cost-effective than pharmacotherapy and biologicals [21].
However, despite these important advantages the use of AIT is limited as compared to pharmacotherapy due to a variety of factors (Box 1). Pharmacotherapy can be broadly prescribed to patients suffering from different manifestations of allergy but AIT requires that the disease-causing allergens are determined in order to prescribe the correct vaccines. The administration of allergens in the course of AIT may induce local and also severe and systemic side effects. Due to the poor quality of natural allergen extracts used as sources for AIT efficacy of treatment may be low. Since the administration of allergen extracts can induce allergic reactions and thus side effects, AIT requires complicated up-dosing schedules and repeated administrations which lead to poor patient's compliance [22].
Box 1. Factors limiting the use of AIT and possible solutions to the problem.
Requires precise diagnosis to determine patients sensitization profile for accurate prescription of the correct vaccine Solution: Diagnosis based on allergen molecules allows identification of the sensitization allergen sources and thus allows precise prescription of the correct vaccines
Administration of allergens can induce side effects Solution: New technologies for production of hypoallergenic allergen-derivatives overcome side effects.
Poor quality of natural allergen extracts responsible for poor protection Solution: New vaccines based on defined recombinant molecules and synthetic peptides
Inconvenient administration schedules Solution: Hypoallergenic recombinant allergy vaccines can be engineered to reduce/eliminate side effects and to increase immunogenicity
Biomarkers needed for monitoring effects of AIT Solution: Mode of action for most forms of AIT, in particular for new targeted AIT, known and antibody-based biomarkers are available.
AIT with natural allergens may induce IgE- and thus allergic sensitization Solution: Use of recombinant allergen-derivatives with low or no sensitization potential
Progress made in the field of allergen characterization now allows to address each of the factors limiting the use of AIT (Box 1). Today most of the relevant disease-causing allergen molecules have been identified and can be used to decipher the molecular reactivity profiles of allergic patients [23]. Through molecular allergy diagnosis (i.e., component-resolved diagnosis) it is possible to identify precisely the disease-causing allergens and to prescribe the correct allergy vaccines [24,25]. Based on the detailed knowledge of the sequences, structures and immunological features of allergens it has become possible to engineer recombinant allergen derivatives and to produce allergen-derived synthetic peptides. Using such recombinant and synthetic allergen derivatives it is possible to improve the safety, immunogenicity, precision and convenience of AIT. For one of these approaches, i.e., B cell epitope-based recombinant allergy vaccines considerable progress has been made recently and promising results were obtained in a series of clinical trials ([26–29]; ClinicalTrials.gov Identifiers: NCT01538979; NCT02643641).
2. Characteristics of B cell epitope-based allergy vaccines
B cell epitope-based allergy vaccines represent a further development of recombinant hypoallergenic allergen derivatives which have been engineered to reduce IgE reactivity and thus allergenic activity of allergens to be used for AIT with the goal to make them as safe as possible [30]. Originally the reduction of IgE reactivity and allergenic activity of recombinant hypoallergenic allergen derivatives was achieved by disruption of their three-dimensional structure or alteration of their surface (reviewed in: [31,32]). For example, conformational IgE epitopes of the major birch pollen allergen, Bet v 1, were destroyed by fragmentation of the allergen into two fragments, each comprising approximately half of the protein [33]. Similar effects were obtained by mutation and reassembly to the allergens (reviewed in [32]). The reduction of allergenic activity of recombinant hypoallergenic allergen derivatives was documented by reduced IgE reactivity and low ability to activate basophils/mast cells by cross-linking of IgE and decreased induction of immediate type skin reactions [32]. At the same time the goal was to retain immunogenicity in order to guarantee that the vaccine induces upon immunization allergen-specific IgG antibodies which can block the interaction between patients IgE and the natural wildtype allergen. The blocking antibodies thus should protect the patient from allergic inflammation due to formation of allergen-IgE immune complexes which activate basophils and mast cells and also T cells via IgE-receptor interactions. Allergen-specific T cell epitopes were retained in the hypoallergenic allergen derivatives and were thought to provide T cell help for allergen-specific IgG production and eventually to induce also T cell tolerance [32]. When the first recombinant hypoallergenic allergen derivatives were evaluated in clinical immunotherapy studies in patients, it was found that they indeed had a reduced allergenic activity and induced allergen-specific blocking IgG antibodies leading to a reduction of allergic symptoms in the patients [34]. However, it turned out that even when IgE reactivity of hypoallergens was reduced or even completely abolished or when only T cell epitope-containing peptides without IgE reactivity were administered that patients developed late phase side effects due to activation of allergen-specific T cells [35–38]. These findings indicated that in addition to IgE-mediated allergic inflammation also non-IgE-mediated, only T cell – mediated mechanisms of allergic inflammation are operative in allergic patients which was verified by atopy patch testing with hypoallergens [39].
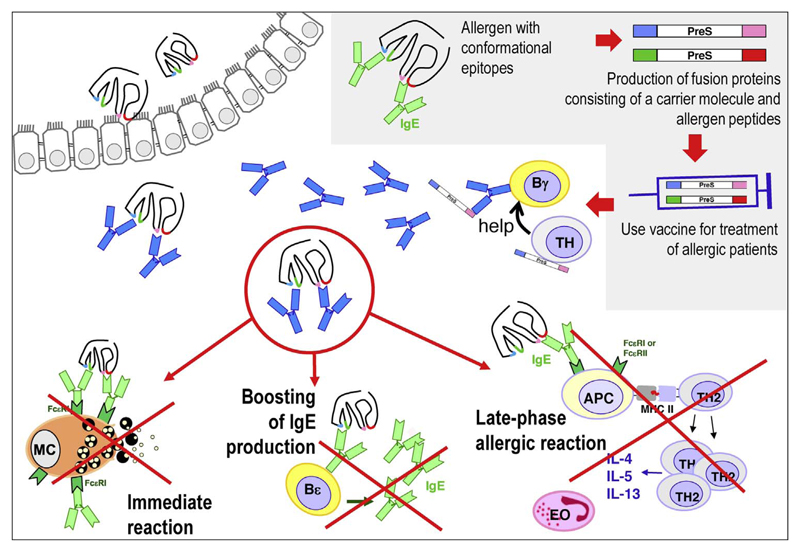
In order to reduce also the side effects caused by activation of allergen-specific T cells, B cell epitope containing vaccines were developed [40]. The principle of construction of this next generation hypoallergens is illustrated in the right upper corner of Fig. 1. In a first step, the IgE binding sites of the clinically relevant and frequently recognized allergens in a given allergen source are mapped. In the case of respiratory allergens the IgE epitopes are usually conformational epitopes which are recognized by IgE antibodies only in the context of the folded allergen. It is therefore possible to identify peptides derived from the IgE binding sites with a length of approximately 30 amino acids which due to lack of fold do not react with IgE antibodies [30,41,42]. These peptides can be rendered immunogenic by physical coupling to a carrier protein as already demonstrated by the classical work of the Nobel laureate Benacerraf who described the hapten-carrier principle [43,44]. The physical coupling to the carrier protein can be achieved by chemical coupling or via the production of a recombinant fusion protein consisting of the allergen-derived peptides and the carrier protein.
Fig. 1. Recombinant B cell epitope-based allergy vaccines: construction and mechanisms of AIT.
Table 1 [41,42,45–53] provides a summary of such peptide carrier-based allergy vaccines obtained by chemical coupling of allergen peptides to KLH or by production of recombinant fusion proteins in which viral proteins such as the VP1 coat protein from human rhinovirus (HRV) or the PreS domain from hepatitis B virus (HBV) were used [54]. The use of recombinant fusion proteins including viral carrier proteins was found to be of advantage over chemical coupling because the fusion proteins can be produced in a defined manner, allow the incorporation of several different allergen-derived peptides in a defined molarity and include a carrier protein which also induces a beneficial antiviral immune response [28]. The use of an carrier protein which does not contain allergen-specific T cell epitopes was intended to reduce the activation of allergen-specific T cells and thus of late phase side effects which actually was verified by atopy patch testing in allergic patients and in immunotherapy trials with a grass pollen allergy vaccine recently [26,27]. Moreover, the choice of an immunogenic viral carrier protein also offers the advantage that even peptides/allergen which per se are poor inducers of IgG antibodies can be rendered immunogenic [55]. The T cell help for the induction of allergen-specific IgG antibodies is thus derived from carrier-specific T cells without activation of allergen-specific T cells. The fusion proteins consisting of the viral carrier protein and the allergen-derived peptides can be produced as recombinant proteins in Escherichia coli in large quantities and low cost under controlled conditions (good manufacturing conditions: GMP) satisfying the requirements for modern vaccines [22]. They are well characterized regarding reduced IgE reactivity, allergenic activity and immunogenicity and it seems that they can be engineered for the most important allergens applying the same blue print technology of combining non-allergenic allergen peptides with a viral carrier protein (Table 1) [41,42,45–53]. The B cell epitope-based carrier vaccine designated BM32, which was constructed for the treatment of grass pollen allergy [53] has been already evaluated in several clinical trials and is therefore the most advanced vaccine.
Table 1. From prototypes to a universal tool box for generating recombinant B cell epitope-based allergy vaccines.
| Allergen source | Allergen | Carrier | Year | Reference |
|---|---|---|---|---|
| Peptide carrier vaccines based on chemically-coupled allergen-derived peptides | ||||
| Timothy grass pollen | Phl p 1 | KLH | 2001 | Focke et al., FASEB J. [41] |
| Birch pollen | Bet v 1 | KLH | 2004 | Focke et al., Clin. Exp. Allergy [42] |
| Olive pollen | Ole e 1 | KLH | 2011 | Twaroch et al., J. Allergy Clin. Immunol. [45] |
| House dust mite | Der p 2 | KLH | 2012 | Chen et al., Allergy [46] |
| Alternaria alternata | Alt a 1 | KLH | 2012 | Twaroch et al., Clin. Exp. Allergy [47] |
| Timothy grass pollen | Phl p 5 | KLH | 2014 | Focke et al., J. Allergy Clin. Immunol. [48] |
| Recombinant fusion proteins consisting of allergen-derived peptides fused to viral carrier proteins | ||||
| Timothy grass | Phl p 1 | VP1 (HRV) | 2009 | Edlmayr et al., J. Immunol. [49] |
| Cat | Fel d 1 | PreS (HBV) | 2011 | Niespodziana et al., J. Allergy Clin. Immunol. [50] |
| Birch pollen | Bet v 1 | PreS (HBV) | 2013 | Marth et al., J. Immunol. [51] |
| House dust mite | Der p 23 | PreS (HBV) | 2014 | Banerjee et al., J. Immunol. [52] |
| Timothy grass pollen | Phl p 1, 2, 5, 6 | PreS (HBV) | 2015 | Focke et al., J. Allergy Clin. Immunol. [53] |
Abbreviations: HRV: Human rhinovirus; HBV: Hepatitis B virus.
3. BM32, a recombinant B cell epitope-based grass pollen allergy vaccine
The allergens in grass pollen have been characterized in great detail regarding their sequences, molecular structures and immunological characteristics [56,57]. The panel of recombinant timothy grass pollen allergens shows extensive cross-reactivity with the allergens in other grasses and it has been shown that AIT with timothy grass pollen allergens is equally effective to AIT with grass pollen mixtures (reviewed in: [57]). For timothy grass pollen, four major allergens, i.e., Phl p 1, Phl p 2, Phl p 5 and Phl p 6, have been shown to represent the most important allergens [58,59]. Accordingly hypoallergenic derivatives for the latter four allergens were included in the grass pollen allergy vaccine BM32. The four derivatives were constructed by fusing peptides from the IgE binding sites of the allergens to HBV-derived PreS in the form of recombinant fusion proteins which were produced in E. coli [27,53]. The preclinical characterization showed that the four fusion proteins designated BM321, BM322, BM325 and BM326 showed almost no IgE reactivity and allergenic activity when tested in basophil activation assays using allergic patients basophils [53]. Furthermore, the BM fusion proteins showed a strongly reduced ability to induce T cell proliferation and release of pro-inflammatory cytokines in PBMC cultures of grass pollen allergic patients as compared to natural allergens [53]. Immunization with only three injections of BM32 induced IgG antibodies in rabbits which blocked IgE binding of grass pollen allergic patients similar as those induced with multiple injections of vaccines based on natural grass pollen allergen extracts [55]. Of note, by far the highest IgG antibodies to major grass pollen allergen Phl p 2 were induced by BM32 [55]. The lack of allergenic activity of BM32 was confirmed in a skin test study performed in grass pollen allergic patients [26]. In this study BM32 did not induce immediate type skin reactions due to mast cell degranulation and also no late phase allergic reactions due to T cell activation. A double-blind, placebo-controlled safety and dose-finding vaccination study which was then conducted in grass pollen allergic patients showed that BM32 was safe and well tolerated, induced robust allergen-specific IgG responses and reduced symptoms of grass pollen allergy upon grass pollen exposure in a pollen chamber [27]. This study demonstrated the immunological mechanisms underlying of vaccination with BM32 and identified the doses which were then used in a subsequent double-blind, placebo-controlled multicenter phase IIb study in which grass pollen allergic patients were treated for a period of two years (ClinicalTrials.gov Identifier: NCT01538979). In the latter study it could be shown that vaccination with BM32 improved symptoms of grass pollen allergy induced by natural pollen exposure. The results of the multicenter study were then confirmed in another phase IIb study which studied the effects of different numbers of pre-seasonal vaccination (three, four or five injections) (ClinicalTrials.gov Identifiers: NCT02643641). Both clinical field trials confirmed the good safety profile of BM32, showed that BM32 induces robust allergen-specific IgG blocking antibodies but does not boost allergenic IgE responses and improves symptoms of grass pollen allergy. Based on these clinical evaluation results of BM32 a final multicenter phase III study is under planning.
4. Mechanisms underlying AIT with recombinant B cell epitopebased allergy vaccines
Fig. 1 illustrates the mechanisms of treatment with B cell epitope-based allergy vaccines according to the data obtained from the preclinical characterization of these vaccines and the results obtained in clinical immunotherapy trials with BM32. In the immunotherapy trials BM32 was administered subcutaneously as Aluminum hydroxide-adsorbed vaccine similar as many vaccines which are used in infectious diseases and similar to many of the registered allergen extract-based vaccines for subcutaneous AIT. Vaccination with BM32 was found to induce IgG (IgG1 = IgG4 > IgG2) which are directed to the IgE binding sites on the natural allergens [27]. The allergen-specific IgG production is supported by carrier (i.e., PreS)-specific T cell help. Accordingly the vaccine does not only induce allergen-specific but also PreS-specific IgG antibodies [28]. Notably, the PreS-specific IgG antibodies were found to be directed against the N-terminal portion of PreS which is involved in infection of liver cells and it was found that BM32-induced antibodies also prevented HBV-infection of liver cells in vitro [28].
The allergen-specific IgG antibodies induced by vaccination with BM32 occur not only in the blood of vaccinated patients but can be found also in different body fluids such as in nasal secretions as was demonstrated earlier for AIT with hypoallergenic derivatives of the major birch pollen allergen, Bet v 1 ([60], Niederberger & Valenta, unpublished). BM32-induced allergen-specific IgG antibodies occupying the IgE binding sites of the natural allergens interfere with three major mechanisms responsible for allergic inflammation and progression of allergic disease in patients.
First, specific IgG captures allergen before and when it intrudes into the allergic patients mucosal surfaces and prevents the binding of the allergen to IgE residing on mast cells and basophils. As a result, allergen-specific blocking IgG prevents the degranulation of mast cells and basophils and thus the liberation of allergic mediators such as histamine, leukotrienes, proteases and inflammatory cytokines responsible for immediate allergic inflammation. Thus AIT with B cell epitope-based allergy vaccines reduces immediate allergic inflammation responsible for allergic rhinoconjunctivitis, asthma and possibly urticaria and anaphylaxis.
Second, allergen-specific IgG prevents the boosting of secondary IgE responses responsible for increases of allergen-specific IgE in allergic patients. In fact it has been shown that allergen exposure induces increases of allergen-specific IgE which leads to increased clinical sensitivity due to loading on mast cells and basophils and up-regulation of expression of receptors for IgE [61,62]. It has been also demonstrated that allergy vaccines based on extracts, recombinant hypoallergens and BM32 which induce allergen-specific IgG blunt the boosts of IgE production due to natural allergen exposure ([34,63,64], Niederberger & Valenta, unpublished). Although not yet proven, it is possible that the blunting of IgE responses induced by allergy vaccines may be involved in the sustained effect of AIT even after discontinuation of AIT when blocking antibodies have already declined. Furthermore, this effect may be responsible for the prevention of the progression of mild allergic symptoms (e.g., rhinitis) to severe symptoms (e.g., asthma) because there is evidence that the progression of allergy towards symptoms in childhood is associated with increases in allergen-specific IgE [65,66].
Third, blocking allergen-specific IgG induced by AIT with natural allergens, hypoallergens and BM32 has been shown to interfere with IgE-facilitated presentation of allergen by APCs to T cells and thus reduce T cell activation and the release of inflammatory cytokines by T cells [27,67,68]. The reduced T cell activation may likely be responsible for the reduction of T cell-mediated late phase allergic inflammation in allergic patients responsible for chronic allergic symptoms such as asthma and atopic dermatitis. It is also quite possible that the reduction of the release of pro-inflammatory cytokines from mast cells, basophils and T cells will lead to a reduced recruitment of eosinophils and other inflammatory cells responsible for chronic tissue damage in allergy.
The triple effect of AIT with the B cell epitope-based allergy vaccines on the pathomechanisms of allergy makes this new vaccine very attractive because other forms of treatment such as pharmacotherapy, anti-IgE therapy, T cell- and cytokine-targeting biologicals address only single pathomechanisms without having broad causative effects.
5. Advantages of recombinant B cell epitope-based allergy vaccines
The results obtained in the clinical studies performed with the B cell epitope-based grass pollen allergy vaccine ([27], ClinicalTrials.gov Identifiers: NCT01538979; NCT02643641) confirmed the high safety profile of the vaccine which was anticipated from the preclinical studies investigating IgE reactivity, ability to induce basophil as well as T cell activation [53] and from the clinical safety assessment done by skin testing in allergic patients [26]. In the course of the immunotherapy trials patients tolerated doses of 80 and 160 microgram of the mix of the four recombinant fusion proteins per subcutaneous injection well. No immediate type reactions were observed and only very few mild systemic late phase reactions occurred. Thus recombinant B cell epitope based allergy vaccines show a very high safety due to lack of allergenic activity (Table 2). The good safety profile should thus allow treating very sensitive patients such as patients suffering from severe allergic manifestations (e.g., asthma and atopic dermatitis) as well as of patients suffering from venom allergy and food allergy. Due to the high safety profile vaccination with the B cell epitope-based allergy vaccines does not require inconvenient up-dosing with multiple injections until the maintenance dose is reached because patients can be treated immediately with the therapeutically effective dose. According to results obtained in the clinical studies it is foreseen that the protective IgG response can be built up with five injections and maintained with single booster injections. The convenient application must be considered as important advantage over all existing allergen-extract-based treatments which require multiple injections in the case of subcutaneous immunotherapy (SCIT) or daily administration in the course of sublingual immunotherapy (SLIT) and therefore will likely improve the poor compliance of patients observed for current forms of AIT [69].
Table 2. Advantages and possible applications of recombinant B cell epitope-based allergy vaccines.
Advantages
|
Possible applications
|
Another important advantage of the recombinant B cell epitope-based vaccines is that they can be easily produced as recombinant proteins in E. coli under GMP conditions meeting the requirements of regulatory authorities for modern vaccines. The new technology therefore overcomes the severe limitations of vaccines based on natural allergen extracts (e.g., lack of batch consistency, impurities, lack and varying concentrations of individual allergens, degradation) [70]. Data obtained for several important allergens from different sources indicate that the technology of producing recombinant hypoallergenic versions is applicable to all allergen sources [49–53]. The possibility to produce the fusion proteins in E. coli not only circumvents problems of eukaryotic expression (e.g., comparably low yields, expensive growth media, possible viral infections, presence of unwanted mammalian proteins) but is also relatively inexpensive. In addition, the carrier proteins used for the construction of the fusion proteins determine to a large extent the biochemical properties of the recombinant fusion proteins which allows to apply similar purification strategies and facilitates the standardization and quality control of the proteins.
The mechanism of action of the B cell epitope-based allergy vaccines is mainly based on the induction of blocking IgG antibodies and thus is well defined. The development of the blocking antibodies and their ability to inhibit patients IgE binding to the allergens can be easily assessed in serum samples of treated patients by available surrogate assays [71]. Importantly, serological tests based on defined allergen molecules are available to identify patients for treatment with the correct vaccines [24,25]. There are therefore diagnostic kits available for the accurate prescription of the vaccines.
At present AIT vaccines are designed to cover complete allergen sources. This means that current vaccines are designed to cover the complete spectrum of relevant allergens in the given source. Once the vaccine has undergone the clinical trials necessary for registration, the composition of the vaccine cannot be altered. Thus patient-tailored treatment according to the individual sensitization profiles of patients is not possible. However, the mode of action of the B cell epitope-based allergy vaccines is very well defined and identical for each of the components in the vaccine. It will therefore be in principle possible to manufacture each of the individual components and to tailor the composition of vaccines according to individual patient's sensitization profiles without need for performing again extensive clinical studies for each of the combinations.
Finally and importantly, as shown for BM32 it seems that B cell epitope-based allergy vaccines do not boost IgE responses and do not sensitize unlike allergen extract-based vaccines [27]. For example, sublingual treatment with grass pollen allergen extracts induced an approximately five-fold increase of allergen-specific IgE in treated patients [72] whereas allergen-specific IgE levels in BM32-treated patients did not change [27]. The latter feature is of particular relevance for a possible preventive application of AIT in children because AIT vaccines should no sensitize especially when applied in a prophylactic setting. It therefore seems that another advantage of B cell epitope-based allergy vaccines is that they can be also used for prophylactic AIT without inducing IgE sensitizations and boosting of established IgE responses.
6. Possible applications of recombinant B cell epitope-based allergy vaccines
Currently BM32 the grass pollen allergy is the most advanced recombinant B cell epitope-based vaccine. It has been evaluated in three clinical immunotherapy trials in patients suffering from grass pollen-induced allergic rhinitis and mild asthma ([27], ClinicalTrials.gov Identifiers: NCT01538979; NCT02643641). As a next step, BM32 will be studied in a multicenter, double-blind, placebo-controlled phase III study to make it available for the treatment of grass pollen allergic patients. B cell epitope-based allergy vaccines have been also engineered for the treatment of birch pollen, cat, house dust mite and ragweed pollen allergy and underwent preclinical evaluation ([50,51], unpublished data). These vaccines will be the next to undergo clinical evaluation. The clinical AIT studies performed with BM32 have shown that the vaccine not only induced allergen-specific IgG antibodies but also antibodies specific for the HBV-derived PreS protein which in vitro inhibited HBV infection of cultured liver cells [28,29]. Approximately 10–20% of subjects who are vaccinated with currently registered HBV vaccines which contain only the HBV-S antigen are non-responders to these vaccines [29]. It is therefore planned to study if vaccination with BM32 can induce a protective immune response in subjects who are non-responders to available HBV vaccines. Furthermore studies are scheduled to investigate the usefulness of BM32 for therapeutic vaccination in HBV-infected subjects.
Regarding allergy vaccination several additional important questions need to be addressed: First it will be interesting to see if BM32 and B cell epitope-based allergy vaccines can be also used to treat safely patients suffering from severe manifestations of allergy such as asthma and atopic dermatitis. Furthermore, it needs to be studied if BM32 has a beneficial long-term effect on reducing symptoms of allergy. For this purpose, long-term studies are needed to investigate sustained effects of the vaccine. In addition, the safety of the vaccine has to be evaluated in a pediatric study to make it available for the treatment of grass pollen allergic children. In this context it will be especially of interest to study if BM32 can prevent the progression of allergic rhinitis to asthma in children similar as has been shown in the PAT study for allergen extract-based vaccines [18]. However, the analysis of the evolution of the development of allergic sensitization profiles in birth cohorts with micro-arrayed allergens has opened new exciting areas of research. In fact it has been shown that one can identify early in childhood by IgE serology children with clinically silent sensitizations and to predict in those children according to their IgE reactivity profiles and levels their likelihood of developing allergic symptoms later in life [65]. Since BM32 was found to induce preferentially a healthy IgG response and did not boost IgE sensitizations in the clinical studies performed so far it appears to be well suited for studying if early treatment of children with silent IgE sensitizations can prevent the development of allergic symptoms later in life. Finally, one may even consider to perform early vaccination with B cell epitope based allergy vaccines for preventive vaccination in terms of preventing the development of IgE sensitization [73]. Two scenarios may be considered in this context. One possibility would be early vaccination of children. The other possibility will be to explore if vaccination in pregnancy can induce high levels of allergenspecific antibodies in mothers which upon transmission to the child may prevent allergic sensitization. Data from experimental animal models provide already experimental support for both of these preventive vaccination scenarios [74,75] and it will be very exciting to see if the new vaccine technologies can indeed be used for specific allergy prevention.
Acknowledgements
This article is dedicated to all of those who have worked for improving the treatment of allergic patients with innovative molecular allergy vaccines.
Funding
This study was funded by Biomay AG, Vienna Austria and by research grants F4605 and F4613 of the Austrian Science Fund (FWF).
Footnotes
Conflicts of interest
Rudolf Valenta has received research grants from the Austrian Science Fund (FWF), Biomay AG, Vienna, Austria, Viravaxx, Vienna, Austria, Thermofisher, Uppsala, Sweden and Fresenius Medical Care, Bad Homburg, Germany. He serves as a consultant for Biomay, Virvaxx, Thermofisher and Fresenius. Verena Niederberger has no conflicts of interest to declare.
References
- [1].Kay AB, Kaplan AR, Bousquet J, Holt PG. Allergy and Allergic Diseases. 2nd ed. Wiley-Blackwell; Chichester, UK: 2008. [Google Scholar]
- [2].Anto JM, Bousquet J, Akdis M, Auffray C, Keil T, Momas I, Postma DS, Valenta R, Wickman M, Cambon-Thomsen A, Haahtela T, et al. Mechanisms of the Development of Allergy (MeDALL): introducing novel concepts in allergy phenotype. J Allergy Clin Immunol. 2017;139:388–399. doi: 10.1016/j.jaci.2016.12.940. [DOI] [PubMed] [Google Scholar]
- [3].Curin M, Swoboda I, Wollmann E, Lupinek C, Spitzauer S, van Hage M, Valenta R. Microarrayed dog, cat, and horse allergens show weak correlation between allergen-specific IgE and IgG responses. J Allergy Clin Immunol. 2014;133:918–921. doi: 10.1016/j.jaci.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siroux V, Lupinek C, Resch Y, Curin M, Just J, Keil T, Kiss R, Lødrup Carlsen K, Melén E, Nadif R, Pin I, et al. Specific IgE and IgG measured by the MeDALL allergen-chip depend on allergen and route of exposure: the EGEA study. J Allergy Clin Immunol. 2017;139:643–654. doi: 10.1016/j.jaci.2016.05.023. [DOI] [PubMed] [Google Scholar]
- [5].Schwarz A, Panetta V, Cappella A, Hofmaier S, Hatzler L, Rohrbach A, Tsilochristou O, Bauer CP, Hoffmann U, Forster J, Zepp F, et al. IgG and IgG4 to 91 allergenic molecules in early childhood by route of exposure and current and future IgE sensitization: results from the Multicentre Allergy Study birth cohort. J Allergy Clin Immunol. 2016;138:1426–1433. doi: 10.1016/j.jaci.2016.01.057. [DOI] [PubMed] [Google Scholar]
- [6].Resch Y, Michel S, Kabesch M, Lupinek C, Valenta R, Vrtala S. Different IgE recognition of mite allergen components in asthmatic and nonasthmatic children. J Allergy Clin Immunol. 2015;136:1083–1091. doi: 10.1016/j.jaci.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM, Bublin M, Curin M, Flicker S, Garmatiuk T, Hochwallner H, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–119. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lupinek C, Marth K, Niederberger V, Valenta R. Analysis of serum IgE reactivity profiles with microarrayed allergens indicates absence of de novo IgE sensitizations in adults. J Allergy Clin Immunol. 2012;130:1418–1420. doi: 10.1016/j.jaci.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoffmann HJ. News in cellular allergology: a review of the human mast cell and basophil granulocyte literature from January 2013 to May 2015. Int Arch Allergy Immunol. 2015;168:253–262. doi: 10.1159/000443960. [DOI] [PubMed] [Google Scholar]
- [10].Dema B, Suzuki R, Rivera J. Rethinking the role of immunoglobulin E and its high-affinity receptor: new insights into allergy and beyond. Int Arch Allergy Immunol. 2014;164:271–279. doi: 10.1159/000365633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Selb R, Eckl-Dorna J, Neunkirchner A, Schmetterer K, Marth K, Gamper J, Jahn-Schmid B, Pickl WF, Valenta R, Niederberger V. CD23 surface density on B cells is associated with IgE levels and determines IgE-facilitated allergen uptake, as well as activation of allergen-specific T cells. J Allergy Clin Immunol. 2017;139:290–299. doi: 10.1016/j.jaci.2016.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Campana R, Moritz K, Marth K, Neubauer A, Huber H, Henning R, Blatt K, Hoermann G, Brodie TM, Kaider A, Valent P, et al. Frequent occurrence of T cell-mediated late reactions revealed by atopy patch testing with hypoallergenic rBet v 1 fragments. J Allergy Clin Immunol. 2016;137:601–609. doi: 10.1016/j.jaci.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Heck S, Nguyen J, Le DD, Bals R, Dinh QT. Pharmacological therapy of bronchial asthma: the role of biologicals. Int Arch Allergy Immunol. 2015;168:241–252. doi: 10.1159/000443930. [DOI] [PubMed] [Google Scholar]
- [14].Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol. 2016;170:122–131. doi: 10.1159/000447692. [DOI] [PubMed] [Google Scholar]
- [15].Incorvaia C, Riario-Sforza GG, Ridolo E. IgE depletion in severe asthma: what we have and what could be added in the near future. EBioMedicine. 2017;17:16–17. doi: 10.1016/j.ebiom.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48–53. doi: 10.2500/ajra.2016.30.4253. [DOI] [PubMed] [Google Scholar]
- [17].Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- [18].The PAT investigator group. Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, Koivikko A, Norberg LA, Valovirta E, Wahn U, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- [19].Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, Till SJ, Hamid QA, Nouri-Aria KT. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- [20].Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–1573. [Google Scholar]
- [21].Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247–254. doi: 10.1097/MOO.0000000000000150. [DOI] [PubMed] [Google Scholar]
- [22].Valenta R, Campana R, Focke-Tejkl M, Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137:351–357. doi: 10.1016/j.jaci.2015.12.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, Vrtala S. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- [24].Kazemi-Shirazi L, Niederberger V, Linhart B, Lidholm J, Kraft D, Valenta R. Recombinant marker allergens: diagnostic gatekeepers for the treatment of allergy. Int Arch Allergy Immunol. 2002;127:259–268. doi: 10.1159/000057742. [DOI] [PubMed] [Google Scholar]
- [25].Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, Aalberse RC, Agache I, Asero R, Ballmer-Weber B, Barber D, et al. EAACI molecular allergology user's guide. Pediatr Allergy Immunol Suppl. 2016;23:1–250. doi: 10.1111/pai.12563. [DOI] [PubMed] [Google Scholar]
- [26].Niederberger V, Marth K, Eckl-Dorna J, Focke-Tejkl M, Weber M, Hemmer W, Berger U, Neubauer A, Stolz F, Henning R, Valenta R. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol. 2015;136:1101–1103. doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zieglmayer P, Focke-Tejkl M, Schmutz R, Lemell P, Zieglmayer R, Weber M, Kiss R, Blatt K, Valent P, Stolz F, Huber H, et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine. 2016;11:43–57. doi: 10.1016/j.ebiom.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cornelius C, Schöneweis K, Georgi F, Weber M, Niederberger V, Zieglmayer P, Niespodziana K, Trauner M, Hofer H, Urban S, Valenta R. Immunotherapy with the PreS-based grass pollen allergy vaccine BM32 induces antibody responses protecting against Hepatitis B infection. EBioMedicine. 2016;11:58–67. doi: 10.1016/j.ebiom.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gerlich WH, Glebe D. Development of an allergy immunotherapy leads to a new type of Hepatitis B vaccine. EBioMedicine. 2016;11:5–6. doi: 10.1016/j.ebiom.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–397. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- [31].Valenta R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol. 2002;2:446–453. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
- [32].Linhart B, Valenta R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine. 2012;19:4328–4335. doi: 10.1016/j.vaccine.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vrtala S, Hirtenlehner K, Vangelista L, Pastore A, Eichler HG, Sperr WR, Valent P, Ebner C, Kraft D, Valenta R. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J Clin Invest. 1997;99:1673–1681. doi: 10.1172/JCI119330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, Reisinger J, Pelzmann M, Hayek B, Kronqvist M, Gafvelin G, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;(Suppl 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Purohit A, Niederberger V, Kronqvist M, Horak F, Grönneberg R, Suck R, Weber B, Fiebig H, van Hage M, Pauli G, Valenta R, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38:1514–1525. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- [36].Haselden BM, Kay AB, Larché M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–1894. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Spertini F, Perrin Y, Audran R, Pellaton C, Boudousquié C, Barbier N, Thierry AC, Charlon V, Reymond C. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014;134:239–240. doi: 10.1016/j.jaci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- [38].Spertini F, DellaCorte G, Kettner A, de Blay F, Jacobsen L, Jutel M, Worm M, Charlon V, Reymond C. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1-derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: results of a phase IIb study. J Allergy Clin Immunol. 2016;138:162–168. doi: 10.1016/j.jaci.2016.02.044. [DOI] [PubMed] [Google Scholar]
- [39].Campana R, Mothes N, Rauter I, Vrtala S, Reininger R, Focke-Tejkl M, Lupinek C, Balic N, Spitzauer S, Valenta R. Non-IgE-mediated chronic allergic skin inflammation revealed with rBet v 1 fragments. J Allergy Clin Immunol. 2008;121:528–530. doi: 10.1016/j.jaci.2007.09.014. [DOI] [PubMed] [Google Scholar]
- [40].Valenta R, Vrtala S, Focke-Tejkl M, Bugajska-Schretter A, Ball T, Twardosz A, Spitzauer S, Grönlund H, Kraft D. Genetically engineered and synthetic allergen derivatives: candidates for vaccination against type I allergy. Biol Chem. 1999;380:815–824. doi: 10.1515/BC.1999.101. [DOI] [PubMed] [Google Scholar]
- [41].Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, Kraft D, Valenta R. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–2044. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- [42].Focke M, Linhart B, Hartl A, Wiedermann U, Sperr WR, Valent P, Thalhamer J, Kraft D, Valenta R. Non-anaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004;34:1525–1533. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- [43].Katz DH, Paul WE, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J Exp Med. 1970;132:261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Paul WE, Katz DH, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. II. Specific properties of carrier cells capable of enhancing anti-hapten antibody responses. J Exp Med. 1970;132:283–299. doi: 10.1084/jem.132.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Twaroch TE, Focke M, Civaj V, Weber M, Balic N, Mari A, Ferrara R, Quirce S, Spitzauer S, Swoboda I, Valenta R. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–184. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- [46].Chen KW, Focke-Tejkl M, Blatt K, Kneidinger M, Gieras A, Dall’Antonia F, Faé I, Fischer G, Keller W, Valent P, Valenta R, et al. Carrier-bound nonallergenic Der p 2 peptides induce IgG antibodies blocking allergen-induced basophil activation in allergic patients. Allergy. 2012;67:609–621. doi: 10.1111/j.1398-9995.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Twaroch TE, Focke M, Fleischmann K, Balic N, Lupinek C, Blatt K, Ferrara R, Mari A, Ebner C, Valent P, Spitzauer S, et al. Carrier-bound Alt a 1 peptides without allergenic activity for vaccination against Alternaria alternata allergy. Clin Exp Allergy. 2012;42:966–975. doi: 10.1111/j.1365-2222.2012.03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Focke-Tejkl M, Campana R, Reininger R, Lupinek C, Blatt K, Valent P, Pavkov-Keller T, Keller W, Valenta R. Dissection of the IgE and T-cell recognition of the major group 5 grass pollen allergen Phl p 5. J Allergy Clin Immunol. 2014;133:836–845. doi: 10.1016/j.jaci.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Edlmayr J, Niespodziana K, Linhart B, Focke-Tejkl M, Westritschnig K, Scheiblhofer S, Stoecklinger A, Kneidinger M, Valent P, Campana R, Thalhamer J, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–6306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- [50].Niespodziana K, Focke-Tejkl M, Linhart B, Civaj V, Blatt K, Valent P, van Hage M, Grönlund H, Valenta R. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–1570. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, Gieras A, Swoboda I, Zafred D, Keller W, Valent P, et al. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J Immunol. 2013;190:3068–3078. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Banerjee S, Weber M, Blatt K, Swoboda I, Focke-Tejkl M, Valent P, Valenta R, Vrtala S. Conversion of Der p 23, a new major house dust mite allergen, into a hypoallergenic vaccine. J Immunol. 2014;192:4867–4875. doi: 10.4049/jimmunol.1400064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, Stegfellner G, Maderegger B, Hauer M, Stolz F, Niederberger V, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207–1217. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Edlmayr J, Niespodziana K, Focke-Tejkl M, Linhart B, Valenta R. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. Curr Top Microbiol Immunol. 2011;352:121–140. doi: 10.1007/82_2011_130. [DOI] [PubMed] [Google Scholar]
- [55].Weber M, Niespodziana K, Linhart B, Neubauer A, Huber H, Henning R, Valenta R, Focke-Tejkl M. Comparison of the immunogenicity of BM32, a recombinant hypoallergenic B cell epitope-based grass pollen allergy vaccine with allergen extract-based vaccines. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.03.048. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Andersson K, Lidholm J. Characteristics and immunobiology of grass pollen allergens. Int Arch Allergy Immunol. 2003;130:87–107. doi: 10.1159/000069013. [DOI] [PubMed] [Google Scholar]
- [57].Gangl K, Niederberger V, Valenta R. Multiple grass mixes as opposed to single grasses for allergen immunotherapy in allergic rhinitis. Clin Exp Allergy. 2013;43:1202–1216. doi: 10.1111/cea.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Niederberger V, Laffer S, Fröschl R, Kraft D, Rumpold H, Kapiotis S, Valenta R, Spitzauer S. IgE antibodies to recombinant pollen allergens (Phl p 1, Phl p 2, Phl p 5, and Bet v 2) account for a high percentage of grass pollen-specific IgE. J Allergy Clin Immunol. 1998;101:258–264. doi: 10.1016/s0091-6749(98)70391-4. [DOI] [PubMed] [Google Scholar]
- [59].Westritschnig K, Horak F, Swoboda I, Balic N, Spitzauer S, Kundi M, Fiebig H, Suck R, Cromwell O, Valenta R. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008;38:260–267. doi: 10.1111/j.1365-2362.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- [60].Reisinger J, Horak F, Pauli G, van Hage M, Cromwell O, König F, Valenta R, Niederberger V. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005;116:347–354. doi: 10.1016/j.jaci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [61].Niederberger V, Ring J, Rakoski J, Jager S, Spitzauer S, Valent P, Horak F, Kundi M, Valenta R. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int Arch Allergy Immunol. 2007;142:133–144. doi: 10.1159/000096439. [DOI] [PubMed] [Google Scholar]
- [62].Saini SS, MacGlashan D. How IgE upregulates the allergic response. Curr Opin Immunol. 2002;14:694–697. doi: 10.1016/s0952-7915(02)00404-1. [DOI] [PubMed] [Google Scholar]
- [63].Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, Semper H, Valent P, Niederberger V, Kraft D, Valenta R. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–1208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- [64].Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D. Immune Tolerance Network Group Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- [65].Westman M, Lupinek C, Bousquet J, Andersson N, Pahr S, Baar A, Bergström A, Holmström M, Stjärne P, Lødrup Carlsen KC, Carlsen KH, et al. Mechanisms for the development of allergies consortium, early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. 2015;135:1199–1206. doi: 10.1016/j.jaci.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Posa D, Perna S, Resch Y, Lupinek C, Panetta V, Hofmaier S, Rohrbach A, Hatzler L, Grabenhenrich L, Tsilochristou O, Chen KW, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139:541–549. doi: 10.1016/j.jaci.2016.08.014. [DOI] [PubMed] [Google Scholar]
- [67].van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, Ipsen H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–2952. [PubMed] [Google Scholar]
- [68].Pree I, Shamji MH, Kimber I, Valenta R, Durham SR, Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin Exp Allergy. 2010;40:1346–1352. doi: 10.1111/j.1365-2222.2010.03548.x. [DOI] [PubMed] [Google Scholar]
- [69].Kiel MA, Röder E, Gerth van Wijk R, Al MJ, Hop WC, Rutten-van Mölken MP. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132:353–360. doi: 10.1016/j.jaci.2013.03.013. [DOI] [PubMed] [Google Scholar]
- [70].Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, Thomas WR, Alessandri C, Chen KW, de Blay F, Valenta R, et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol. 2012;159:253–262. doi: 10.1159/000337654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lupinek C, Wollmann E, Valenta R. Monitoring allergen immunotherapy effects by microarray. Curr Treat Opt Allergy. 2016;3:189–203. doi: 10.1007/s40521-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- [73].Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012;272:144–157. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Uthoff H, Spenner A, Reckelkamm W, Ahrens B, Wölk G, Hackler R, Hardung F, Schaefer J, Scheffold A, Renz H, Herz U. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J Immunol. 2003;171:3485–3492. doi: 10.4049/jimmunol.171.7.3485. [DOI] [PubMed] [Google Scholar]
- [75].Linhart B, Narayanan M, Focke-Tejkl M, Wrba F, Vrtala S, Valenta R. Prophylactic and therapeutic vaccination with carrier-bound Bet v 1 peptides lacking allergen-specific T cell epitopes reduces Bet v 1-specific T cell responses via blocking antibodies in a murine model for birch pollen allergy. Clin Exp Allergy. 2014;44:278–287. doi: 10.1111/cea.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]