Abstract
Today, in vivo allergy diagnosis and allergen-specific immunotherapy (AIT) are still based on allergen extracts obtained from natural allergen sources. Several studies analyzing the composition of natural allergen extracts have shown severe problems regarding their quality such as the presence of undefined nonallergenic materials, contaminants as well as high variabilities regarding contents and biological activity of individual allergens. Despite the increasing availability of sophisticated analytical technologies, these problems cannot be overcome because they are inherent to allergen sources and methods of extract production. For in vitro allergy diagnosis problems related to natural allergen extracts have been largely overcome by the implementation of recombinant allergen molecules that are defined regarding purity and biological activity. However, no such advances have been made for allergen preparations to be used in vivo for diagnosis and therapy. No clinical studies have been performed for allergen extracts available for in vivo allergy diagnosis that document safety, sensitivity, and specificity of the products. Only for very few therapeutic allergen extracts state-of-the-art clinical studies have been performed that provide evidence for safety and efficacy. In this article, we discuss problems related to the inconsistent quality of products based on natural allergen extracts and share our observations that most of the products available for in vivo diagnosis and AIT do not meet the international standards for medicinal products. We argue that a replacement of natural allergen extracts by defined recombinantly produced allergen molecules and/or mixtures thereof may be the only way to guarantee the supply of clinicians with state-of-the-art medicinal products for in vivo diagnosis and treatment of allergic patients in the future.
Keywords: Allergy, Allergen, Diagnosis, Allergen-specific immunotherapy, Allergen extract, Quality control, Recombinant allergen, Molecular allergy diagnosis
IgE-associated allergy is the most common and important immunologically mediated hypersensitivity disease affecting approximately 30% of the population worldwide.1,2 In the USA, allergies are a leading cause of chronic illness representing an immense burden for the health care system.3 The identification of the disease-causing allergens is critical for the accurate diagnosis of allergy and forms the basis for the treatment of allergic patients by allergen-specific interventions (eg, allergen avoidance, diet, allergen-specific immunotherapy [AIT]).4 AIT is in fact the only causal, disease-modifying, and long-lasting form of treatment.4,5 Therefore considerable efforts have been spent in the characterization of allergens beginning with the isolation of allergens from the natural allergen sources by biochemical means.2,6,7 A major breakthrough regarding allergen characterization has been achieved with the introduction of molecular cloning techniques for the isolation of the genes coding for allergens.8 Thirty years ago the genes coding for the first allergens were isolated and sequenced, and soon thereafter the first recombinant allergens were produced and used for in vitro diagnosis of allergy.9–11 In 1999, recombinant allergens were made available in a fully automated in vitro allergy diagnostic test system,12 and the first allergen chip containing micro-arrayed allergen molecules to be used as a multiallergen test was reported in 2002.13 Since then, in vitro allergy diagnosis has been revolutionized by molecular allergy diagnosis.6,14 However, recombinant allergen molecules have also been used for in vivo allergy diagnosis. More than 20 years ago the first skin prick test studies and also in vivo provocation test studies (eg, bronchial provocation, nasal provocation) were performed with recombinant allergens in patients and showed that recombinant allergens can be effective, safe, sensitive, and specific when used for in vivo allergy diagnosis.15–22 Despite the fact that several clinical studies have documented the advantages of recombinant allergen–based skin testing over allergen extract—based skin testing regarding specificity and clinical information,18,19,21,23 up to now no recombinant allergen—based in vivo tests are available. Presently only allergen extract—based tests that are not complying with the regulations for medicinal products are available for in vivo allergy diagnosis (ie, mainly skin testing). In fact, double-blind, placebo-controlled studies comparing sensitivity and specificity of the in vivo test allergen in patients for whom IgE reactivity profiles have been determined in parallel by serology would be desirable.
Likewise, recombinant allergen derivatives and recombinant allergens have been successfully evaluated for AIT more than 10 years ago.24–26 Unfortunately, only few molecular AIT approaches have been moved successfully into clinical evaluation,24–31 and there are therefore currently only allergen extract—based allergy vaccines available.
However, also only for few of the allergen extract—based AITs safety and efficacy have been documented according to the current rules for medicinal products as demanded by the European Directive 2001/83/EC in 2004.32 For most of the AIT products, no sufficient documentation in the form of properly randomized, double-blind, placebo-controlled clinical trials is available. We found only 2 subcutaneous immunotherapy (SCIT) products for grass pollen allergy,33,34 2 sublingual immunotherapy (SLIT) products for grass pollen allergy,35–37 1 SLIT product for ragweed pollen allergy,38 and 2 SLIT products for house dust mite allergy,39–43 which have been evaluated in large numbers of patients. Several large-scale clinical trials with allergen extracts are currently registered in the clinical trial database (https://clinicaltrials.gov/), but results are not yet published and it seems that a longer transition period is needed to implement European Directive 2001/83/EC. Regarding allergen extracts for in vivo diagnostic testing so far no studies and/or documentation satisfying the demands of European Directive 2001/83/EC are available and there are discussions ongoing if there should be a distinction between therapeutic and diagnostic allergen preparations.
In the next section, we discuss the problems that are associated with the preparation and characterization of allergen extracts from natural allergen sources to meet current requirements for medicinal products. Although allergen extracts to be used for in vivo allergy diagnosis and AIT need to be distinguished, they fall under the definition of medicinal products (ie, “any substance or combination of substances that may be used in or administered to human beings either with a view to restoring, correcting, or modifying physiological functions by exerting a pharmacological, immunological, or metabolic action, or to making a medical diagnosis”).
Allergen Extracts: Production and Quality Control
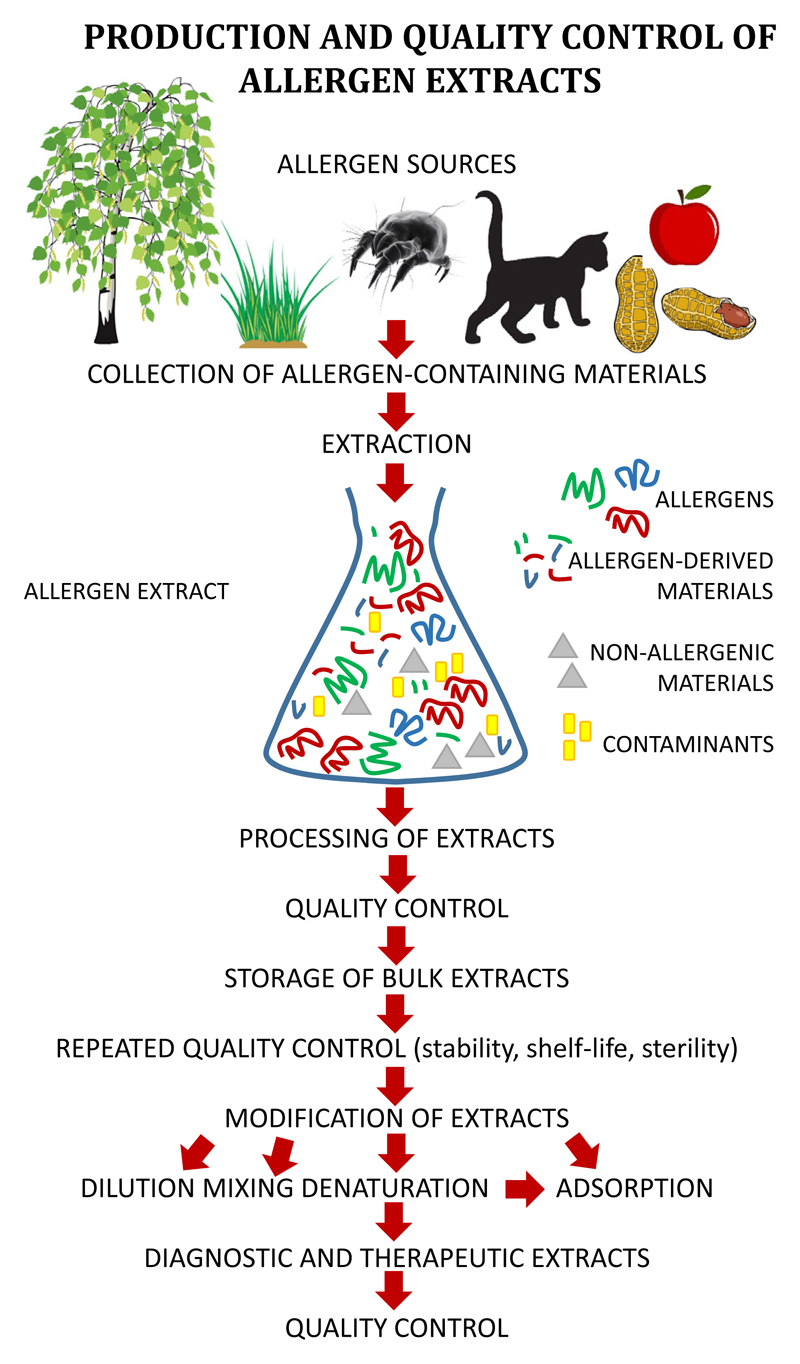
For a long time expert opinion was sufficient to place allergen products for in vivo diagnosis and therapy on the market and clinical studies following good clinical practice (GCP) standards were never performed. In many countries, especially in the European Union, the legal situation has dramatically changed during the last 2 decades. It is now required to demonstrate safety and efficacy for therapeutic allergen products as well as for allergen products used for in vivo application such as diagnostic allergen extracts used for provocation testing, including skin testing, bronchial, nasal, conjunctival, and food provocation testing.44,45 Although there are differences regarding the regulations in different continents and countries,44–49 the overall goal is that according to the “International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use” (http://www.ich.org/home.html), medicinal products that include also allergen products for in vivo diagnosis and treatment must be evaluated in clinical trials and undergo a thorough evaluation to be registered for use in patients. A major prerequisite for clinical trials and subsequent use in humans is that the medicinal product is produced following good manufacturing practice (GMP) and is shown to have consistent characteristics and quality. This requirement is already a major hurdle for allergen extracts that are produced from natural allergen sources. Figure 1 provides an overview of the steps leading to a diagnostic or therapeutic allergen extract. A first major problem relies in the allergen source that is used for the production of allergen extracts. The contents, concentrations, and ratios of the individual allergens have been reported to vary greatly depending on a large variety of factors.50–55 Only a few examples shall be mentioned in this context: for example, allergen contents in pollen vary depending on environmental factors such as ozone exposure,56 pollution,57 and plant species to name a few.58 The contents of house dust mite allergens and their ratios depend on the growth conditions of mites, how they are fed and cultivated, and what mite material (feces, bodies) is used as raw material for the production of extracts.59 In the case of animal allergens, allergen type and contents may vary depending on gender.60,61 Food allergens are expressed to a different extent in different parts of fruits and cultivars,62 and are extracted differently depending on the method used for extraction.63 Lipophilic allergens have therefore been overlooked for a long time.64,65 The spectrum of mold allergens shows great variation depending on mold strains and culture conditions.55 Furthermore, it has been shown that allergens occur in pollen as different isoforms with different allergenic activities and immunological properties in varying quantities, which means that no homogeneous single natural allergen preparation can be obtained from a natural allergen source.66–68 Therefore, only the recombinant expression of a defined isoform based on the corresponding gene can overcome this problem.
Figure 1.
Steps in the production and quality control of allergen extracts.
The presence of proteases in allergen extracts is another major problem because it may lead to the degradation of allergens that affects the allergenic activity, immunogenicity, and immunomodulatory capacity of an allergen extract.69–74 Because protease inhibitors that can help overcoming this problem are often toxic, it is impossible to prevent allergen degradation in extracts by the addition of such protease inhibitors. Certain allergens are proteases by themselves and therefore can digest not only other allergens but also may have effects on immune cells and tissues in the allergic patient on administration. This was shown for example for fungal allergens in mouse models but also for the major house dust mite allergen ex vivo for the human system.71–74
Some allergens are toxic and, at high concentrations, may induce inflammatory reactions per se in a nonsensitized subject.75
Another major problem is that allergen extracts contain a large variety of unknown nonallergenic materials that may have toxic and/or immunomodulatory effects. For example, it has been shown that nonallergenic components such as pollen-derived phytoprostanes may activate cells of the innate immune system (ie, dendritic cells) “unspecifically” and thus indirectly have effects on the adaptive immune response by inducing Th2 responses. Such effects have been observed for pollen and house dust mite allergen extracts.76,77 Another major concern is that allergen extracts may contain contaminants from other allergen sources. For example, the presence of house mite allergens in animal dander allergen extracts has been reported,78 pollen may be contaminated with unrelated pollen or fungi, and recently the presence of IgE-reactive bacterial antigens in house mite allergen extracts has been reported.79
When allergen extracts are prepared from natural allergen sources, one must therefore analyze not only the presence of intact allergens but also of allergen-derived materials exhibiting different properties as the intact allergens (eg, allergen peptides) (Figure 1). In this context, it should be mentioned that fractions of allergen extracts with different molecular weights have been shown to exhibit different allergenic and immunomodulatory properties as has, for example, been shown already early for grass pollen extracts.80 Furthermore, the presence of nonallergenic materials and possible contaminants requires analysis (Figure 1).
The analysis of the different materials in an extract (ie, allergen, allergen-derived materials, nonallergenic materials, contaminants) (Figure 1) must include many different parameters such as contents, concentrations, quality, ratios, activity parameters (eg, allergenic activity, immunogenicity, immunomodulatory activity), shelf-life, and stability; chemical and biological properties related to safety must be characterized for each of the different components, which is an extremely complex process. There are methods that in principle allow us to analyze the aforementioned parameters for single molecules with sufficient accuracy. Mass spectrometry has recently been proposed as a method for the standardization of allergen extracts.81 However, mass spectrometry can only demonstrate the presence of certain allergen-derived peptides in an extract but is not a real quantitative method and cannot tell anything about the allergenic or immunogenic properties of the molecules.82 Unfortunately, there is therefore no method that can analyze all important characteristics (physicochemical, structural, immunological properties) of the individual components present in complex mixtures such as allergen extracts at the same time.
The allergen extraction process is not a real purification process of certain allergen molecules but leads to a crude bulk allergen extract that can be further used for different purposes (Figure 1). Allergen extracts for in vivo diagnostic testing are usually prepared from the bulk allergen extracts by dilution and addition of certain preservatives. In this context, it has been found that certain allergen extracts contain also components that have been added during the manufacturing process. For example, human serum albumin is sometimes added by the manufacturers for stabilization purposes.50,51,54 Mixed allergen extracts are produced by mixing bulk allergen extracts from different allergen sources that may create several problems. For example, mixing of allergens dilutes the concentrations of allergens from each of the extracts that has been used for mixing to an unknown extent or may introduce proteases from other allergen sources leading to degradation.83–86 Denaturation of allergen extracts by various physicochemical procedures is performed for allergen extracts used for AIT to reduce the allergenic activity. There are different processes for denaturation such as aldehyde treatment, boiling, chemical denaturation, and various other treatments, but these procedures cannot be fully controlled and therefore affect to various degrees allergenic, immunogenic, and immunomodulatory properties of allergen extracts.87,88 Importantly, individual allergens cannot be traced any more as intact molecules in chemically modified or denatured allergen extracts, and one therefore can only try to assess the overall allergenic and/or immunogenic activity of a denatured extract, both of which may vary from one production batch to another.49,89
Finally, one has to consider that allergen extracts to be used for AIT are manufactured in different ways. Some allergen extracts are made as aqueous solutions without adjuvants, some are mixed with powders and excipients to form tablets, and some extracts are adsorbed to different adjuvants to which individual allergens may bind with different strength and stability.90–93 All these additional processes may affect individual allergens/immunogens to a different extent and thus introduce another layer of uncertainty in addition to those due to variations caused by allergen sources and methods of extraction, processing, denaturation, and mixing.
Methods for the Quality Control of Allergen Extracts
In Table I we have summarized advantages and disadvantages of different methods that can be used for the quality control of allergen extracts. For example, the determination of the total protein contents has been introduced as one of the first methods for the quality control of allergen extracts.94 It measures protein contents but does not identify specifically allergens and their properties. Methods for measuring the allergenic activity and IgE reactivity of allergen extracts (ie, potency assays) were introduced later as additional methods for quality control.95 These methods depend on reagents derived from patients because allergen extracts are assessed for reactivity with IgE antibodies, in basophil activation tests or by skin testing.95,96 Since allergic patients react with different allergens and have different sensitivities to these allergens, results obtained with potency assays depending on patients materials will vary widely. As a result, allergen preparations that are standardized according to such methods in different countries cannot be compared.48 Potency assays measuring the allergenic activity cannot be used for allergen extracts that have been modified to reduce allergenic activity, except one wants to measure the extent of reduction of allergenic activity in comparison with an unmodified allergen extract.
Table I. Advantages and disadvantages of methods for the quality control of allergen extracts.
| Measurement of protein contents (quantitative by nitrogen determination, qualitative by SDS-PAGE) |
| Advantage |
| Measures amount/quality of proteins, applicable to denatured allergen extracts |
| Disadvantage |
| Does not identify allergen molecules and does not discriminate between allergenic and nonallergenic components in extracts; does not inform about immunogenicity |
| Determination of IgE reactivity and allergenic activity (IgE reactivity, basophil activation, skin testing) |
| Advantage |
| Measures IgE reactivity and allergenic activity of an extract |
| Disadvantage |
| Does not identify allergen molecules and does not discriminate between allergenic and nonallergenic components in extracts; does not inform about immunogenicity |
| Determination of IgE reactivity and allergenic activity (IgE reactivity, basophil activation, skin testing) |
| Advantage |
| Measures IgE reactivity and allergenic activity of an extract |
| Disadvantage |
| Does not discriminate between allergens, shows IgE reactivity and allergenic reactivity only for 1 standard, only limiting amounts of the standard available; results may vary depending on the standard and do not reflect the situation in individual patients at different times, does not inform about immunogenicity, not applicable to denatured allergen extracts |
| Mass spectrometry |
| Advantage |
| Identifies allergen-derived materials according to characteristic mass |
| Disadvantage |
| Not suited for exact quantification, cannot discriminate between complete IgE-reactive allergens and nonallergenic allergen-derived materials such as allergen fragments/peptides, does not inform about immunogenicity |
| Circular dichroism, size exclusion |
| Advantage |
| Determine the fold of proteins and their aggregation behavior |
| Disadvantage |
| Usually only suitable for purified proteins, do no inform about IgE reactivity and allergenic activity, do not provide quantitative information, do not inform about immunogenicity, and not applicable to denatured allergen extracts |
| ELISA for quantification of allergens |
| Advantage |
| Allows quantifying of individual allergens |
| Disadvantage |
| Not available for each of the allergens, difficulty to discriminate between allergen isoforms and allergen-derived materials, does not necessarily measure IgE reactivity and allergenic activity, does not inform about immunogenicity, not applicable to denatured allergen extracts |
| Qualitative allergen detection (eg, immunoblotting) |
| Advantage |
| Visualizes the presence of allergens in an extract with specific antibody probes |
| Disadvantage |
| Does not allow a quantification of allergens, does not identify nonallergenic materials/contaminants, does not inform about allergenic activity or immunogenicity |
| Immunization |
| Advantage |
| Informs about the immunogenicity of allergen extracts and denatured allergen extracts regarding the induction of allergen-specific IgE and IgG antibodies on immunization of animals, applicable also for denatured/modified allergen extracts |
| Disadvantage |
| Does not allow quantifying individual allergens, does not identify allergens, does not inform about IgE reactivity and allergenic activity of the extract; results obtained for certain animals (eg, inbred mouse strains) do not necessarily reflect immunization of humans, may induce cross-reactive antibodies reacting also with other allergen sources |
| Safety and stability assays (chemical, biological) |
| Measurement of endotoxins and foreign nucleic acids: mandatory for in vivo use in humans, useful to determine contents of endotoxins, foreign nucleic acids, and immunomodulatory substances |
| Sterility tests: mandatory for in vivo use in humans, prevent administration of potentially infectious materials to humans |
| Toxicity tests: in vivo and in vitro tests (single-dose, repeated-dose, genotoxcitiy studies) to determine toxic effects, mandatory for in vivo use in humans, prevent administration of potentially toxic and mutagenic materials to humans |
| Stability tests: tests measuring the stability of the active ingredients in an extract (allergens, modified allergens) to ensure the desired activity, mandatory for in vivo use in humans, useful to prevent the administration of material with reduced or lost activity |
ELISA, Enzyme-linked immunosorbent assay; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
In addition, a series of biochemical and biophysical methods have been developed. They include, for example, mass spectrometry, circular dichroism, size exclusion that allows the detection of allergen peptides, and the analysis of the fold of proteins and of the aggregation behavior, respectively.97,98 In particular, mass spectrometry has been suggested as a powerful method for the standardization of allergen extracts.81,99,100 Although circular dichroism and gel filtration are very useful and suitable for the analysis of single purified molecules,101 these methods cannot be used for complex allergen mixtures. Sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotting allow the qualitative analysis of allergen extracts and may discriminate between intact allergens, aggregation, and degradation products according to molecular weight.102 Quantitative enzyme-linked immunosorbent assays performed with allergen-specific antibody probes permit the determination of the concentrations of intact allergens.103–105 The determination of the ability of an allergen extract to induce the production of allergen-specific IgG antibodies that block patients’ IgE binding can be obtained by immunization of animals with the formulated vaccine.106 Because antibodies induced in inbred mouse strains by allergy vaccines recognize other epitopes than those induced in allergic patients, it is recommended to perform immunization experiments in outbred animals such as rabbits.89 The IgG antibodies obtained in the animals can then be tested for their ability to inhibit allergic patients’ IgE binding to the allergens and allergen-induced effector cell activation. In fact, a recent study showing that cat allergy can be treated by passive immunization with recombinant allergenespecific antibodies emphasizes the importance of blocking antibodies for success of treatment and the need to test allergy vaccines for the induction of blocking antibodies in model systems.107 However, clinical studies in humans and postmarketing assessment will always be required to assess the immunogenic properties of AIT vaccines because there may be differences of immunogenicity in animals and man.
In addition to the assays characterizing allergens, allergen-derived materials, nonallergenic components and contaminants, additional tests are mandatory for safety, stability, toxicity, and sterility assessment. These methods are mandatory for the quality control of medicinal products (Table I).108,109
Allergen Extracts for Diagnosis and Treatment
Table E1 (available in this article’s Online Repository at www.jaci-inpractice.org) contains an overview of allergen extracts that we found to be registered or to be available in different continents and countries and, when available, the corresponding homepages of the regulatory agencies providing the information. As examples, we have analyzed a few countries, that is, the USA, Germany, Russia as well as Taiwan and Japan from Asia. However, already in this small selection of countries the heterogeneity of the regulations in different parts of the world becomes very clear.110 However, one common feature seems to be that allergens, regardless of whether they are used for therapy or as in vivo test allergens, are considered as biological medicinal products and therefore require marketing authorizations that are usually issued for the finished product. In the USA, so-called standardized and also nonstandardized injectable allergen extracts from a variety of manufacturers are available (Table E1). However, we were unable to find published state-of-the-art clinical studies that document the safety, specificity, and efficacy for the majority of these products. Randomized, double-blind, placebo-controlled clinical studies following the rules set for medicinal products have only been performed for few extracts available as tablets for sublingual therapy.35–43 The situation was similar for Germany. On the homepage of the Paul Ehrlich Institute that is responsible for the registration of medicinal products in Germany, extracts from several companies for skin testing and provocation testing are listed, but for none of these test allergen extracts we could find any documentation by clinical studies. For a handful of allergen extracts available for subcutaneous and sublingual AIT,33–43 clinical studies have been performed, whereas for the majority of therapeutic products, no evidence for efficacy and safety in the form of clinical studies could be found. In fact, in the European Union (EU), allergen products such as AIT vaccines and allergen products used for in vivo testing are defined as medicinal products according to Directive 2001/83/EC and are therefore required to obtain a market authorization that can follow different procedures in individual EU countries or via a centralized procedure that is valid for the whole EU.110 The current state-of-the-art approach for obtaining marketing authorization requires randomized, double-blind, placebo-controlled studies that are performed according to GCP guidelines, but in reality this has been fulfilled only for few AIT vaccines in the EU.33–43 A similar situation was found for Russia, Taiwan, and Japan where a quite limited number of allergen extracts are available (Table E1). One possibility for making allergen extracts available without fulfilling new rules for allergen products is based on so-called named patient products that can be prescribed by practitioners on an individual patient basis.32 However, it must be clear that the level of evidence for such products is very low (ie, expert opinion) and named patient prescription hence does not follow the current rules for medicinal products.
The legal situation in the USA is that allergen products are regulated as biological medicinal products under the Public Health Service Act and as drug product under the Federal Food, Drug and Cosmetics Act, and require a marketing authorization termed a biologics license application (BLA). The BLA has to demonstrate that the product is manufactured under GMP and is safe, pure, and effective. Thus randomized, double-blind, placebo-controlled studies according to the current GCP regulation are now required for marketing authorization. In the EU, the pharmaceutical industry has been requested to provide the necessary documentation for their products, and accordingly clinical studies are currently performed, however, mainly for AIT products but not for allergen extracts used for in vivo testing. It is therefore not surprising that there is a great risk that many natural allergen extracts will disappear in the EU, especially those for in vivo testing.44 Ultimately, also many AIT extracts may not be available any more in the future. Although the regulatory situation is different in other countries, it cannot be excluded that the pressure on quality control on allergen extracts will increase suddenly also there because the continuously rising costs for health care systems will demand that the safety and efficacy of medicinal products and drugs is documented by extensive clinical studies. It will therefore be necessary to intensify the discussions between major allergy societies and international control agencies to provide reliable, safe, efficient, and cost-effective options for therapy and in vivo diagnosis and eventually to distinguish between therapeutic and diagnostic allergen preparations.
Transition from Allergen Extracts to Molecules: The Only Solution?
If indeed the requirements for quality control and the documentation of safety and efficacy by clinical trials set by regulatory authorities increase for test and treatment allergens, there are basically at least 2 options. One option would be to fulfill the requirements with allergen extractebased technologies, whereas the other option would be to replace allergen extracts by defined recombinant allergen molecules and combinations of the 2 options can be envisaged. In Table II, we have performed a SWOT (Strength, Weakness, Opportunity, and Threat) analysis of the advantages and disadvantages of natural allergen extracts versus recombinant allergen molecules. One may argue that allergen extracts are traditional products that are known to the allergologist for a long time without requiring detailed knowledge regarding the individual allergen molecules. However, in the field of in vitro allergy diagnosis, molecular testing has become an important part of the diagnostic armamentarium of the allergologist, and it is argued that molecular testing will eventually completely replace extract-based testing.6,111,112 Of course, molecular testing requires detailed knowledge of the individual allergen molecules and hence continued medical education to enjoy the many advantages of molecular testing (eg, understanding of cross-reactivities, precise identification of the culprit allergen, resolution of complex cases, refinement of AIT prescription, identification of sensitization to high- or low-risk allergens) over allergen extractebased testing.7 One particular strength of molecular allergy diagnosis is that it allows us to identify precisely the culprit allergen sources in polysensitized patients that facilitate the accurate prescription of AIT.6,112 One may also consider the use of recombinant allergen mixes instead of natural allergen extracts. It is also possible that the knowledge gained from molecular allergen characterization may help to improve the quality of natural allergen extracts. The disadvantages of allergen extracts are mainly related to the fact that it is technically almost impossible to manufacture them in a way to satisfy the current requirements for medicinal products due to the limitations set by raw materials, extraction, and processing (Table I).
Table II. Advantages and disadvantages of natural allergen extracts and recombinant/synthetic allergen molecules.
| Natural allergen extracts |
| Advantages |
| • Preparation without extensive purification steps |
| • Contain several allergens of the allergen source |
| • Often reflect the allergen contents of the natural allergen sources |
| • Are already on the market with old authorizations |
| • Known to allergologists as traditional products |
| Disadvantages/limitations |
| • May contain nonallergenic components with different properties |
| • May be contaminated with allergens from other sources |
| • May present variable contents and ratios of allergens |
| • May present batch-to-batch variations due to manufacturing procedures and raw materials |
| • May be unstable and degrade |
| • Contents cannot be fully influenced by the manufacturer but are determined by the raw material |
| • Do not provide molecular information when used for diagnosis |
| • May cause allergic reactions on administration |
| • May not fulfill modern regulatory requirements for medicinal products |
| • May contain infectious materials |
| Recombinant/synthetic allergen molecules |
| Advantages |
| • Pure proteins/peptides of defined properties and quality |
| • Manufactured according to good manufacturing practice |
| • Can be produced in defined amounts and concentrations in reproducible manner |
| • Fulfill regulatory requirements for medicinal products, modern drugs, and vaccines |
| • Allergenic, immunogenic, and tolerogenic properties are predefined |
| • Allow specific targeting of immune mechanisms (eg, IgG induction, tolerance induction) |
| • Allow patient-tailored treatment |
| • Multiple advantages when used for diagnosis (ie, identification of culprit allergen molecules, revealing cross-reactivity, providing molecular profiles) |
| • Provide detailed diagnostic test information |
| • Production is independent of allergen raw materials |
| • Can be produced at costs comparable to natural allergen extracts |
| • Biologically safe due to GMP production |
| Possible disadvantages |
| • Require knowhow |
| • Require modern recombinant or synthetic production process |
| • Require new market authorization and clinical studies |
| • Need to produce different components |
GMP, Good manufacturing practice.
Recombinant allergens that exactly resemble the allergenic activity of the natural allergens as well as recombinant allergen derivatives with favorable properties for AIT are available now for decades and can be produced by controlled expression in appropriate host cells (eg, bacteria, eukaryotic cells) under defined conditions of GMP, which is the standard for medicinal products. Of course, the production of recombinant allergens and allergen derivatives requires a different know-how as compared with allergen extracts, but it is completely independent from natural and thus variable raw materials.7 Because recombinant allergenebased products are new kids on the block, they will require clinical studies and market authorizations, but ultimately such studies also need to be performed for allergen extracts; otherwise they may disappear.44 As a concrete example for the replacement of allergen extracts by recombinant allergenebased technologies, we would like to refer to grass pollen that is one of the most important allergen sources worldwide. It has been demonstrated that natural grass pollen allergen extracts show large variations regarding the contents of the individual allergens and therefore are highly heterogeneous.50 Likewise, grass pollen extractespecific AIT induces only partial protective immune responses against the individual major allergens.113,114 All these problems could be overcome with a recombinant hybrid allergen comprising the 4 major timothy grass pollen allergens: Phl p 1, Phl p 2, Phl p 5, and Phl p 6.115 This hybrid molecule can be easily produced in Escherichia coli in defined, reproducible quality and in a very large amount. The hybrid resembles the allergenic activity of grass pollen and can be used for in vivo diagnosis of grass pollen allergy.116 The single recombinant hybrid molecule could also be used to formulate AIT vaccines for grass pollen because AIT with Phl p 1, Phl p 2, Phl p 5, and Phl p 6 has been shown to be clinically effective.25 Moreover, several AIT approaches based on recombinant hypoallergenic molecules, recombinant allergens, and allergen-derived synthetic peptides have reached clinical application in controlled studies (Table III). One of these approaches is that a new recombinant B-cell epitope-based grass pollen allergy vaccine, termed BM32,30,31,130,131 which contains recombinant hypoallergenic fusion proteins consisting of nonallergen peptides from the 4 timothy grass pollen allergens fused to the hepatitis B-derived PreS protein as a carrier, has been shown to be hypoallergenic in vivo.132 In AIT trials, BM32 was safe and few injections were effective in reducing symptoms of grass pollen allergy (Table III).30,31,133,134 Thus grass pollen allergy is a very good and concrete example of how traditional allergen extracts used for in vivo testing and AIT can be replaced by modern recombinant technology.
Table III. Clinical trials with recombinant allergens, recombinant allergen derivatives and synthetic allergen-derived peptides.
| Molecules/approximate timeframe | Description of the vaccine | Study design and clinical trial number | References |
|---|---|---|---|
| AllervaxCAT, 1996-1999 | Two Fel d 1-derived peptides of 27 amino acids | SCIT, DBPC | 117, 118, 119 |
| Bet v 1 trimer, Bet v 1 fragments, 2000-2001 | Hypoallergenic recombinant derivatives of Bet v 1 | Phase II, SCIT/DBPC | 24 |
| rPhl p 1, rPhlp 2, rPhlp 5a + b, rPhl p 6, 2002-2013 | Recombinant grass pollen allergen cocktail | Phase II, SCIT/DBPC NCT00671268, NCT00309036 | 25 |
| Folding variant of Bet v 1, 2002-2013 | Hypoallergenic recombinant folding variant of the major birch pollen allergen (rBet v 1-FV) | Phase III, SCIT/DBPC NCT00266526, NCT00554983, NCT00841516 | 28, 120 |
| rBet v 1, 2002-2008 | Comparison of rBet v 1 with nBet v 1 and birch pollen extract for SCIT in birch pollen allergic patients | Phase II, SCIT/DBPC NCT00410930 | 26 |
| rBet v 1 tablets, 2006-2013 | r Bet v1 administered as sublingual tablets in birch pollen allergic subjects | Phase II, SLIT, DBPC NCT00901914 NCT00396149, NCT00889460 | 121 |
| ILIT with MAT-Fel d 1, 2008-2010 | Intralymphatic immunotherapy for cat allergy | Phase I, NCT00718679 | 122 |
| Ara h 1, Ara h 2, and Ara h 3, 2009-2013 | Rectal application of E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 | Phase I, safety study NCT00850668 | 123 |
| Fcγ1-Fel d1 fusion protein, 2011-2014 | Intradermal, human Fcγ1-Fel d 1 fusion protein | Safety study, NCT01292070 | 124 |
| BM 32, 2012-2017 | Hypoallergenic recombinant vaccine for immunotherapy of grass pollen allergy consisting of derivatives of the 4 major grass pollen allergens, phl p 1, Phl p 2, Phl p 5, and Phl p 6 | Phase IIa and 2 phase IIb studies, SCIT/DBPC NCT01350635, NCT01538979, NCT02643641 | 30, 31 |
| ToleroMune Cat, 2012-2016 | Fel d 1-derived synthetic peptides for induction of tolerance in cat allergic patients | Phase III, intradermal/DBPC NCT01620762 | 125, 126 |
| AllerT, 2012-2015 | Bet v 1-derived contiguous overlapping peptides | Phase IIb, SCIT/DBPC NCT01720251, NCT02143583, NCT02271009 Long-term follow-up of a phase IIb study AN004T |
29, 127, 128 |
| Sublingual immunotherapy of birch pollen-associated apple allergy, 2012-2016 | Recombinant Mal d 1 | Single-center, double-blind, placebo-controlled explorative study NCT01449786 | 129 |
| FAST-Fish, 2013-2015 | SCIT for fish allergy based on the subcutaneous application of mutated parvalbumin (rCyp p 1) | Phase IIa, NCT02017626 | 109 |
| ToleroMune Grass, 2014-2016 | Short peptides from grass pollen allergens | Phase IIb/III started intradermal/DBPC NCT02795273, NCT02161107 | |
| ToleroMune HDM, 2014-2016 | Short peptides derived from house dust mite allergens | Phase II, intradermal/DBPC NCT02150343 | |
| ToleroMune Ragweed, 2014-2016 | Short peptides from Amb a 1 | Phase II, NCT02061709, NCT02396680 |
DBPC, Double-blind, placebo-controlled; HDM, house dust mite; SCIT, subcutaneous immunotherapy.
Interestingly, recombinant allergenebased approaches seem to be not only applicable for the development of AIT approaches for respiratory allergies, but also for food and venom allergy. Regarding food allergy, a recombinant hypoallergenic mutant of the major fish allergen parvalbumin, mCyp c 1, has been expressed in E. coli and was shown to have strongly reduced allergenic activity in skin prick tests.109,135,136 The recombinant mCyp c 1 molecule was then formulated for subcutaneous AIT, and first clinical studies showed that treatment was safe and induced allergen-specific blocking IgG antibodies (ClinicalTrials.gov Identifiers: NCT02017626, NCT02365168, NCT02382718). Likewise, recombinant allergenebased strategies might be developed for venom allergy because the clinically relevant bee and wasp allergens have been expressed as recombinant proteins and could be used to develop recombinant AIT approaches.137,138
Summary
Recombinant hypoallergenic allergen derivatives comprising some of the most important allergen sources (eg, grass pollen, birch pollen, ragweed pollen, olive pollen, Parietaria pollen, cedar pollen, house dust mites, cat, dog, bee, and wasp venoms) have been characterized at a preclinical level and could be evaluated in clinical trials.130,131,139–155 For a few allergen sources, it may be challenging to prepare all the individual allergen molecules by recombinant technology to represent the complexity of the allergen source properly. However, so far recombinant AIT approaches have not reached wide-scale use in clinical practice. Because the molecules can be produced well in different expression systems, there are, in principle, no technical hurdles for their manufacturing. Some of the molecules are protected by international patents, but these are available for licensing. It rather seems that pharmaceutical companies were so far not willing to invest in their development because this would require the setting up of suitable production facilities and the conductance of clinical trials. With the implementation of regulations requesting the documentation of traditional allergen extracts by clinical trials during the last few years, the situation may change because the pharmaceutical industry is now requested to conduct GMP production and clinical trials to maintain their traditional allergen extracts on the market and/or may decide to develop recombinant allergenebased products. It is therefore likely that the pressure by the regulatory agencies will boost the development of high-quality allergens for in vivo use, and, accordingly, we may see the parallel development of allergen extract and recombinant allergenebased products for clinical use. Unfortunately, most of the current allergen extracts do not meet the criteria of medicinal products and are therefore at risk of disappearing. Even with the most advanced analytical methods, it is not possible to overcome all the quality problems that are due to the limits of allergen extractebased technologies. However, during the last 30 years, the most important allergen molecules from the most relevant allergen sources have been produced as defined recombinant molecules resembling the allergenic activity of the natural allergens. The recombinant allergen molecules can be produced at low costs, in consistent quality, and in large amounts for in vivo allergy testing and thus would meet easily the criteria set for medicinal products. Likewise, they could be used to formulate modern allergy vaccines. Moreover, recombinant hypoallergenic allergen derivatives have been produced for most of the important allergen sources and hold promise to improve safety, efficacy, and convenience of allergen-specific immunotherapy as well as to be useful for preventive allergy vaccination. It is thus argued that the time has come to implement recombinant technology for the production of new high-quality in vivo allergy tests and allergy vaccines.
Online Repository
Extended Data
Table E1.
Diagnostic and therapeutic allergen extracts registered in the USA, Germany, Russia, and Asia
| USA (https://www.fda.gov/BiologicsBloodVaccines/Allergenics/default.htm) |
| Injectable allergen extracts standardized (https://www.fda.gov/BiologicsBloodVaccines/Allergenics/ucm391514.htm) |
| Cat Hair (Felis domesticus): 7 manufacturers |
| Cat Pelt (Felis domesticus): 2 manufacturers |
| Mite D.f. (Dermatophagoides farinae): 6 manufacturers |
| Mite D.p. (Dermatophagoides pteronyssinus): 6 manufacturers |
| Bermuda Grass (Cynodon dactylon): 6 manufacturers |
| Kentucky (June) Bluegrass (Poa pratensis): 6 manufacturers |
| Orchard Grass (Dactylis glomerata): 6 manufacturers |
| Redtop Grass (Agrostis alba): 6 manufacturers |
| Perennial Ryegrass (Lolium perenne): 6 manufacturers |
| Sweet Vernal Grass (Anthoxanthum odoratum): 6 manufacturers |
| Timothy Grass (Phleum pratense): 6 manufacturers |
| Short Ragweed (Ambrosia artemisiifolia): 6 manufacturers |
| Honey Bee Venom (Apis mellifera): 2 manufacturers |
| Wasp Venom Protein (Polistes spp): 2 manufacturers |
| Yellow Hornet Venom Protein (Dolichovespula arenaria): 2 manufacturers |
| Yellow Jacket Venom Protein (Vespula spp): 2 manufacturers |
| Mixed Vespid Venom Protein (mixed yellow jacket, yellow hornet, and white-faced hornet): 2 manufacturers |
| Injectable allergen extracts, nonstandardized (https://www.fda.gov/BiologicsBloodVaccines/Allergenics/ucm391517.htm) |
| Six companies are licensed to manufacture and distribute such extracts |
| Allergen extracts: sublingual tablets for AIT (https://www.fda.gov/BiologicsBloodVaccines/Allergenics/ucm391505.htm) |
| GRASTEK Merck Sharp & Dohme Corp: Timothy grass pollen extract |
| ORALAIR Stallergenes S.A.L.: mix of 5 grass species |
| ODACTRA Merck Sharp & Dohme Corp: House dust mite (Dermatophagoides farinae and Dermatophagoides pteronyssinus) allergen extract |
| RAGWITEK Merck Sharp & Dohme Corp: Short ragweed pollen extract |
| Germany (https://www.pei.de/DE/arzneimittel/allergene/allergene-node.html) |
| Allergen extracts for skin prick testing: https://www.pei.de/DE/arzneimittel/allergene/test-allergene/pricktest/pricktest-node.html |
| • Grass-, corn-, weed pollen |
| • Tree pollen |
| • Food |
| • Molds and yeast |
| • House dust mites/storage mites |
| • Animal dander/hair |
| • Venoms |
| • Latex |
| Allergen extracts for provocation testing:https://www.pei.de/DE/arzneimittel/allergene/test-allergene/provokationstest/provokationstest-node.html |
| • Grass-, corn-, weed pollen |
| • Tree pollen |
| • Food |
| • Molds and yeast |
| • House dust mites/storage mites |
| • Animal dander/hair |
| For AIT: |
| For subcutaneous AIT: https://www.pei.de/DE/arzneimittel/allergene/therapie-allergene/subkutan/subkutane-therapie-node.html;jsessionid=FD6BE393DB5711476E2BC4D3FDCBF8C4.1_cid319 |
| Grass-, corn-, and weed pollen: https://www.pei.de/DE/arzneimittel/allergene/therapie-allergene/subkutan/graeser/graeser-getreide-kraeuter-pollen-node.html |
| 25 products |
| Tree pollen: https://www.pei.de/DE/arzneimittel/allergene/therapie-allergene/subkutan/baumpollen/baumpollen-node.html |
| 44 products |
| House dust mites: https://www.pei.de/DE/arzneimittel/allergene/therapie-allergene/subkutan/hausstaubmilben/hausstaubmilben-node.html |
| 28 products |
| Venoms: https://www.pei.de/DE/arzneimittel/allergene/therapie-allergene/subkutan/insektengifte/insektengifte-node.html |
| 18 products |
| Sublingual AIT: |
| Grass-, corn-, weed pollen: https://www.pei.de/DE/arzneimittel/allergene/therapie-allergene/sublingual/graeser/graeser-getreide-kraeuter-pollen-node.html |
| 27 products |
| Tree pollen: https://www.pei.de/DE/arzneimittel/allergene/therapie-allergene/sublingual/baumpollen/baumpollen-node.html |
| 4 products |
| House dust mites: https://www.pei.de/DE/arzneimittel/allergene/therapie-allergene/sublingual/hausstaubmilben/hausstaubmilben-node.html |
| 3 products |
| Russia |
| For in vivo diagnostic purposes: |
| Water-salt allergen extracts produced by AO “Biomed” Mechnikov |
| Water-salt allergen extracts produced by NPO Microgen |
| For AIT: |
| Water-salt allergen extracts produced by AO “Biomed” Mechnikov and by NPO Microgen |
| Subcutaneus AIT “Phostal,” “Alustal” (Stallergenes, France): tree pollen, grass pollen, HDM |
| Sublingual AIT “Staloral” (Stallergenes, France): HDM, birch pollen |
| Sublingual tablet “Oralair” (Stallergenes, France): grass pollen |
| Sublingual AIT by allergoids (Lopharma, Italy): HDM, grass pollen |
| Asia |
| Japan |
| In Japan allergen products are considered as biomedicines |
| For in vivo diagnostic purposes: |
| Extracts from Tori Pharmaceutical Co. https://www.torii.co.jp/en/ |
| Allergen extract for Scratch test: HDM “TORII” 100,000 JAU/mL, |
| Dermatophagoides farinae extract 10,000 AU/mL, |
| Dermatophagoides pteronyssinus extract 10,000 AU/mL. |
| Allergen Scratch Extract Positive control “TORII” Histamine dihydrochloride |
| For AIT: |
| Miticure House Dust Mite Sublingual Tablets 3,300 JAU Miticure House Dust Mite Sublingual Tablets 10,000 JAU (Torii Pharmaceutical Co., Ltd.) (Dermatophagoides farinae extract, Dermatophagoides pteronyssinus extract) |
| Actair 100 IR Sublingual Tablets-HDM Actair 300 IR Sublingual Tablets-HDM (Shionogi & Co., Ltd.) Dermatophagoides farinae extract bulk powder, Dermatophagoides pteronyssinus extract bulk powder. |
| Allergen extract for subcutaneous injection-HDM “TORII” 100,000 JAU/mL |
| Allergen extract for subcutaneous injection-HDM “TORII” 10,000 JAU/mL (Torii Pharmaceutical Co., Ltd.) |
| Cedartolen Sublingual Drop-Japanese Cedar Polllen 200 JAU/mL bottle |
| Cedartolen Sublingual Drop-Japanese Cedar Polllen 2,000 JAU/mL bottle |
| Standardized Japanese cedar pollen extract original solution 10,000 JAU/mL |
| Taiwan |
| Allergen extracts available from Allermed (USA), now merged by Greer Co. (USA). |
| China |
| Allergen extracts available from: |
| ALK (Horsholm, DenmarK), Stallergenes Greer. Co. (USA), WolwoPharma. Co. (China) (http://www.wolwobiotech.com/) |
AIT, Alergen-specific immunotherapy; HDM, house dust mite.
Acknowledgments
This study was supported by grants F4605, F4613 and P29991 of the Austrian Science Fund (FWF), by the Russian Academic Excellence Project 5-100 and by a Megagrant of the Government of the Russian Federation, grant No 14.W03.31.0024.
Abbreviations used
- AIT
Allergen-specific immunotherapy
- BLA
Biologics license application
- EU
European Union
- GCP
Good clinical practice
- GMP
Good manufacturing practice
Footnotes
Conflicts of interest: R. Valenta has received research grants from serves as a consultant for Biomay AG (Vienna) and Viravaxx (Vienna). The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Anto JM, Bousquet J, Akdis M, Auffray C, Keil T, Momas I, et al. Mechanisms of the Development of Allergy (MeDALL): introducing novel concepts in allergy phenotypes. J Allergy Clin Immunol. 2017;139:388–99. doi: 10.1016/j.jaci.2016.12.940. [DOI] [PubMed] [Google Scholar]
- 2.Valenta R, Karaulov A, Niederberger V, Gattinger P, van Hage M, Flicker S, et al. Molecular aspects of allergens and allergy. Adv Immunol. 2018;138:195–256. doi: 10.1016/bs.ai.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(Suppl):S43–70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 5.Curin M, Khaitov M, Karaulov A, Namazova-Baranova L, Campana R, Garib V, et al. Next-generation of allergen-specific immunotherapies: molecular approaches. Curr Allergy Asthma Rep. 2018;18:39. doi: 10.1007/s11882-018-0790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI molecular allergology user’s guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250. doi: 10.1111/pai.12563. [DOI] [PubMed] [Google Scholar]
- 7.Curin M, Garib V, Valenta R. Single recombinant and purified major allergens and peptides: how they are made and how they change allergy diagnosis and treatment. Ann Allergy Asthma Immunol. 2017;119:201–9. doi: 10.1016/j.anai.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–41. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 9.Fang KS, Vitale M, Fehlner P, King TP. cDNA cloning and primary structure of a white-face hornet venom allergen, antigen 5. Proc Natl Acad Sci U S A. 1988;85:895–9. doi: 10.1073/pnas.85.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua KY, Stewart GA, Thomas WR, Simpson RJ, Dilworth RJ, Plozza TM, et al. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J Exp Med. 1988;167:175–82. doi: 10.1084/jem.167.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breiteneder H, Pettenburger K, Bito A, Valenta R, Kraft D, Rumpold H, et al. The gene coding for the major birch pollen allergen Betv1, is highly homologous to a pea disease resistance response gene. EMBO J. 1989;8:1935–8. doi: 10.1002/j.1460-2075.1989.tb03597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Grönlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT) Clin Exp Allergy. 1999;29:896–904. doi: 10.1046/j.1365-2222.1999.00653.x. [DOI] [PubMed] [Google Scholar]
- 13.Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–6. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 14.Lupinek C, Wollmann E, Valenta R. Monitoring allergen immunotherapy effects by microarray. Curr Treat Options Allergy. 2016;3:189–203. doi: 10.1007/s40521-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser M, Crameri R, Brust E, Suter M, Menz G. Diagnostic value of recombinant Aspergillus fumigatus allergen I/a for skin testing and serology. J Allergy Clin Immunol. 1994;93:1–11. doi: 10.1016/0091-6749(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 16.Lynch NR, Thomas WR, Chua Y, García N, Di Prisco MC, López R. In vivo biological activity of recombinant Der p II allergen of house-dust mite. Int Arch Allergy Immunol. 1994;105:70–4. doi: 10.1159/000236805. [DOI] [PubMed] [Google Scholar]
- 17.Müller UR, Dudler T, Schneider T, Crameri R, Fischer H, Skrbic D, et al. Type I skin reactivity to native and recombinant phospholipase A2 from honeybee venom is similar. J Allergy Clin Immunol. 1995;96:395–402. doi: 10.1016/s0091-6749(95)70059-5. [DOI] [PubMed] [Google Scholar]
- 18.Menz G, Dolecek C, Schönheit-Kenn U, Ferreira F, Moser M, Schneider T, et al. Serological and skin-test diagnosis of birch pollen allergy with recombinant Bet v I, the major birch pollen allergen. Clin Exp Allergy. 1996;26:50–60. doi: 10.1111/j.1365-2222.1996.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 19.Pauli G, Oster JP, Deviller P, Heiss S, Bessot JC, Susani M, et al. Skin testing with recombinant allergens rBet v 1 and birch profilin, rBet v 2: diagnostic value for birch pollen and associated allergies. J Allergy Clin Immunol. 1996;97:1100–9. doi: 10.1016/s0091-6749(96)70264-6. [DOI] [PubMed] [Google Scholar]
- 20.Godnic-Cvar J, Susani M, Breiteneder H, Berger A, Havelec L, Waldhör T, et al. Recombinant Bet v 1, the major birch pollen allergen, induces hypersensitivity reactions equal to those induced by natural Bet v 1 in the airways of patients allergic to tree pollen. J Allergy Clin Immunol. 1997;99:354–9. doi: 10.1016/s0091-6749(97)70053-8. [DOI] [PubMed] [Google Scholar]
- 21.Niederberger V, Stübner P, Spitzauer S, Kraft D, Valenta R, Ehrenberger K, et al. Skin test results but not serology reflect immediate type respiratory sensitivity: a study performed with recombinant allergen molecules. J Invest Dermatol. 2001;117:848–51. doi: 10.1046/j.0022-202x.2001.01470.x. [DOI] [PubMed] [Google Scholar]
- 22.Niederberger V, Eckl-Dorna J, Pauli G. Recombinant allergen-based provocation testing. Methods. 2014;66:96–105. doi: 10.1016/j.ymeth.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heiss S, Mahler V, Steiner R, Spitzauer S, Schweiger C, Kraft D, et al. Component-resolved diagnosis (CRD) of type I allergy with recombinant grass and tree pollen allergens by skin testing. J Invest Dermatol. 1999;113:830–7. doi: 10.1046/j.1523-1747.1999.00796.x. [DOI] [PubMed] [Google Scholar]
- 24.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci. 2004;101(Suppl 2):14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Spertini F, Perrin Y, Audran R, Pellaton C, Boudousquié C, Barbier N, et al. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014;134:239–40. doi: 10.1016/j.jaci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Klimek L, Bachert C, Lukat KF, Pfaar O, Meyer H, Narkus A. Allergy immunotherapy with a hypoallergenic recombinant birch pollen allergen rBet v. 1-FV in a randomized controlled trial. Clin Transl Allergy. 2015;5:28. doi: 10.1186/s13601-015-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spertini F, DellaCorte G, Kettner A, de Blay F, Jacobsen L, Jutel M, et al. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1-derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: results of a phase 2b study. J Allergy Clin Immunol. 2016;138:162–8. doi: 10.1016/j.jaci.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 30.Zieglmayer P, Focke-Tejkl M, Schmutz R, Lemell P, Zieglmayer R, Weber M, et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine. 2016;11:43–57. doi: 10.1016/j.ebiom.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niederberger V, Neubauer A, Gevaert P, Zidarn M, Worm M, Aberer W, et al. Safety and efficacy of immunotherapy with the recombinant B-cell epitope-based grass pollen vaccine BM32. J Allergy Clin Immunol. 2018;142:497–509. doi: 10.1016/j.jaci.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonertz A, Roberts GC, Hoefnagel M, Timon M, Slater JE, Rabin RL, et al. Challenges in the implementation of EAACI guidelines on allergen immunotherapy: a global perspective on the regulation of allergen products. Allergy. 2018;73:64–76. doi: 10.1111/all.13266. [DOI] [PubMed] [Google Scholar]
- 33.Frew AJ, Powell RJ, Corrigan CJ, Durham SR. UK Immunotherapy Study Group. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:319–25. doi: 10.1016/j.jaci.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 34.DuBuske LM, Frew AJ, Horak F, Keith PK, Corrigan CJ, Aberer W, et al. Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32:239–47. doi: 10.2500/aap.2011.32.3453. [DOI] [PubMed] [Google Scholar]
- 35.Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;118:434–40. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Didier A, Worm M, Horak F, Sussman G, de Beaumont O, Le Gall M, et al. Sustained 3-year efficacy of pre- and coseasonal 5-grass-pollen sublingual immunotherapy tablets in patients with grass pollen-induced rhinoconjunctivitis. J Allergy Clin Immunol. 2011;128:559–66. doi: 10.1016/j.jaci.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 37.Valovirta E, Petersen TH, Piotrowska T, Laursen MK, Andersen JS, Sørensen HF, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. GAP investigators. J Allergy Clin Immunol. 2018;141:529–53. doi: 10.1016/j.jaci.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Creticos PS, Esch RE, Couroux P, Gentile D, D’Angelo P, Whitlow B, et al. Randomized, double-blind, placebo-controlled trial of standardized ragweed sublingual-liquid immunotherapy for allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2014;133:751–8. doi: 10.1016/j.jaci.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Nolte H, Bernstein DI, Nelson HS, Kleine-Tebbe J, Sussman GL, Seitzberg D, et al. Efficacy of house dust mite sublingual immunotherapy tablet in North American adolescents and adults in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2016;138:1631–8. doi: 10.1016/j.jaci.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 40.Bergmann KC, Demoly P, Worm M, Fokkens WJ, Carrillo T, Tabar AI, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–14. doi: 10.1016/j.jaci.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Emminger W, Hernández MD, Cardona V, Smeenk F, Fogh BS, Calderon MA, et al. The SQ house dust mite SLIT-tablet is well tolerated in patients with house dust mite respiratory allergic disease. Int Arch Allergy Immunol. 2017;174:35–44. doi: 10.1159/000478699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virchow JC, Backer V, Kuna P, Prieto L, Nolte H, Villesen HH, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715–25. doi: 10.1001/jama.2016.3964. [DOI] [PubMed] [Google Scholar]
- 43.Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine-Tebbe J. Effective treatment of house dust mite-induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2016;137:444–51. doi: 10.1016/j.jaci.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 44.Klimek L, Hoffmann HJ, Renz H, Demoly P, Werfel T, Matricardi PM, et al. Diagnostic test allergens used for in vivo diagnosis of allergic diseases are at risk: a European perspective. Allergy. 2015;70:1329–31. doi: 10.1111/all.12676. [DOI] [PubMed] [Google Scholar]
- 45.Klimek L, Hoffmann HJ, Kugler A, Muraro A, Hellings PW. Impact of changed legislation on skin tests: the present and future. Curr Opin Allergy Clin Immunol. 2016;16:465–8. doi: 10.1097/ACI.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 46.Jeong KY, Lee JH, Kim EJ, Lee JS, Cho SH, Hong SJ, et al. Current status of standardization of inhalant allergen extracts in Korea. Allergy Asthma Immunol Res. 2014;6:196–200. doi: 10.4168/aair.2014.6.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teshima R. Regulation of allergen products in Japan. Arb Paul Ehrlich Inst Bundesinstitut Impfstoffe Biomed Arzneim Langen Hess. 2009;96:224–9. [PubMed] [Google Scholar]
- 48.Larenas-Linnemann D, Cox LS. European allergen extract units and potency: review of available information. Ann Allergy Asthma Immunol. 2008;100:137–45. doi: 10.1016/S1081-1206(10)60422-X. [DOI] [PubMed] [Google Scholar]
- 49.Zimmer J, Vieths S, Kaul S. Standardization and regulation of allergen products in the European Union. Curr Allergy Asthma Rep. 2016;16:21. doi: 10.1007/s11882-016-0599-4. [DOI] [PubMed] [Google Scholar]
- 50.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–8. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 51.Focke M, Marth K, Valenta R. Molecular composition and biological activity of commercial birch pollen allergen extracts. Eur J Clin Invest. 2009;39:429–36. doi: 10.1111/j.1365-2362.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 52.Brunetto B, Tinghino R, Braschi MC, Antonicelli L, Pini C, Iacovacci P. Characterization and comparison of commercially available mite extracts for in vivo diagnosis. Allergy. 2010;65:184–90. doi: 10.1111/j.1398-9995.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 53.Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154:258–63. doi: 10.1159/000321113. [DOI] [PubMed] [Google Scholar]
- 54.Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol. 2012;159:253–62. doi: 10.1159/000337654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Twaroch TE, Curin M, Sterflinger K, Focke-Tejkl M, Swoboda I, Valenta R. Specific antibodies for the detection of Alternaria allergens and the identification of cross-reactive antigens in other fungi. Int Arch Allergy Immunol. 2016;170:269–78. doi: 10.1159/000449415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eckl-Dorna J, Klein B, Reichenauer TG, Niederberger V, Valenta R. Exposure of rye (Secale cereale) cultivars to elevated ozone levels increases the allergen content in pollen. J Allergy Clin Immunol. 2010;126:1315–7. doi: 10.1016/j.jaci.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Ackaert C, Kofler S, Horejs-Hoeck J, Zulehner N, Asam C, von Grafenstein S, et al. The impact of nitration on the structure and immunogenicity of the major birch pollen allergen Bet v 1.0101. PLoS One. 2014;9:e104520. doi: 10.1371/journal.pone.0104520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erler A, Hawranek T, Krückemeier L, Asam C, Egger M, Ferreira F, et al. Proteomic profiling of birch (Betula verrucosa) pollen extracts from different origins. J Proteomics. 2011;11:1486–98. doi: 10.1002/pmic.201000624. [DOI] [PubMed] [Google Scholar]
- 59.Erban T, Harant K, Hubert J. Detailed two-dimensional gel proteomic mapping of the feces of the house dust mite Dermatophagoides pteronyssinus and comparison with D. farinae: reduced trypsin protease content in D. pteronyssinus and different isoforms. J Proteomics. 2017;162:11–9. doi: 10.1016/j.jprot.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 60.Ramadour M, Guetat M, Guetat J, El Biaze M, Magnan A, Vervloet D. Dog factor differences in Can f 1 allergen production. Allergy. 2005;60:1060–4. doi: 10.1111/j.1398-9995.2005.00824.x. [DOI] [PubMed] [Google Scholar]
- 61.Mattsson L, Lundgren T, Everberg H, Larsson H, Lidholm J. Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol. 2009;123:362–8. doi: 10.1016/j.jaci.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Sancho AI, van Ree R, van Leeuwen A, Meulenbroek BJ, van de Weg EW, Gilissen LJ, et al. Measurement of lipid transfer protein in 88 apple cultivars. Int Arch Allergy Immunol. 2008;146:19–26. doi: 10.1159/000112499. [DOI] [PubMed] [Google Scholar]
- 63.Akkerdaas JH, Wensing M, Knulst AC, Krebitz M, Breiteneder H, de Vries S, et al. How accurate and safe is the diagnosis of hazelnut allergy by means of commercial skin prick test reagents? Int Arch Allergy Immunol. 2003;132:132–4. doi: 10.1159/000073714. [DOI] [PubMed] [Google Scholar]
- 64.Schwager C, Kull S, Behrends J, Röckendorf N, Schocker F, Frey A, et al. Peanut oleosins associated with severe peanut allergy-importance of lipophilic allergens for comprehensive allergy diagnostics. J Allergy Clin Immunol. 2017;140:1331–8. doi: 10.1016/j.jaci.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 65.Jappe U, Schwager C. Relevance of lipophilic allergens in food allergy diagnosis. Curr Allergy Asthma Rep. 2017;17:61. doi: 10.1007/s11882-017-0731-0. [DOI] [PubMed] [Google Scholar]
- 66.Swoboda I, Jilek A, Ferreira F, Engel E, Hoffmann-Sommergruber K, Scheiner O, et al. Isoforms of Bet v 1, the major birch pollen allergen, analyzed by liquid chromatography, mass spectrometry, and cDNA cloning. J Biol Chem. 1995;270:2607–13. doi: 10.1074/jbc.270.6.2607. [DOI] [PubMed] [Google Scholar]
- 67.Ferreira F, Hirtenlehner K, Jilek A, Godnik-Cvar J, Breiteneder H, Grimm R, et al. Dissection of immunoglobulin E and T lymphocyte reactivity of isoforms of the major birch pollen allergen Bet v 1: potential use of hypoallergenic isoforms for immunotherapy. J Exp Med. 1996;183:599–609. doi: 10.1084/jem.183.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spiric J, Engin AM, Karas M, Reuter A. Quality control of biomedicinal allergen products—highly complex isoallergen composition challenges standard MS database search and requires manual data analyses. PLoS One. 2015;10:e0142404. doi: 10.1371/journal.pone.0142404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chevigne A, Jacquet A. The emerging roles of the protease allergen Der p 1 in house dust mite-induced airway inflammation. J Allergy Clin Immunol. 2018;142:398–400. doi: 10.1016/j.jaci.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 70.Reithofer M, Jahn-Schmid B. Allergens with protease activity from house dust mites. Int J Mol Sci. 2017;18:E1368. doi: 10.3390/ijms18071368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sircar G, Saha B, Mandal RS, Pandey N, Saha S, Gupta Bhattacharya S. Purification, cloning and immuno-biochemical characterization of a fungal aspartic protease allergen Rhi o 1 from the airborne mold Rhizopus oryzae. PLoS One. 2015;10:e0144547. doi: 10.1371/journal.pone.0144547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snelgrove RJ, Gregory LG, Peiró T, Akthar S, Campbell GA, Walker SA, et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–92. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, et al. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hewitt CR, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995;182:1537–44. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Georgitis JW, Reisman RE. Venom skin tests in insect-allergic and insect-nonallergic populations. J Allergy Clin Immunol. 1985;76:803–7. doi: 10.1016/0091-6749(85)90752-3. [DOI] [PubMed] [Google Scholar]
- 76.Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, Ring J, et al. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med. 2005;201:1347. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacquet A. The role of innate immunity activation in house dust mite allergy. Trends Mol Med. 2011;17:604–11. doi: 10.1016/j.molmed.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Van der Veen MJ, Mulder M, Witteman AM, van Ree R, Aalberse RC, Jansen HM, et al. False-positive skin prick test responses to commercially available dog dander extracts caused by contamination with house dust mite (Dermatophagoides pteronyssinus) allergens. J Allergy Clin Immunol. 1996;98:1028–34. doi: 10.1016/s0091-6749(96)80187-4. [DOI] [PubMed] [Google Scholar]
- 79.Dzoro S, Mittermann I, Resch-Marat Y, Vrtala S, Nehr M, Hirschl AM, et al. House dust mites as potential carriers for IgE sensitization to bacterial antigens. Allergy. 2018;73:115–24. doi: 10.1111/all.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chakrabarty S, Ekramoddoullah AK, Kisil FT, Sehon AH. Allergens of Kentucky Blue Grass pollen. II. Isolation of hapten-like components from Kentucky Blue Grass pollen by preparative isoelectrofocussing. Int Arch Allergy Appl Immunol. 1980;63:369–82. doi: 10.1159/000232653. [DOI] [PubMed] [Google Scholar]
- 81.Spiric J, Reuter A, Rabin RL. Mass spectrometry to complement standardization of house dust mite and other complex allergenic extracts. Clin Exp Allergy. 2017;47:604–17. doi: 10.1111/cea.12931. [DOI] [PubMed] [Google Scholar]
- 82.Casset A, Valenta R, Vrtala S. Allergen content and in vivo allergenic activity of house dust mite extracts. Int Arch Allergy Immunol. 2013;161:287–8. doi: 10.1159/000347047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niemeijer NR, Kauffman HF, van Hove W, Dubois AE, de Monchy JG. Effect of dilution, temperature, and preservatives on the long-term stability of standardized inhalant allergen extracts. Ann Allergy Asthma Immunol. 1996;76:535–40. doi: 10.1016/S1081-1206(10)63274-7. [DOI] [PubMed] [Google Scholar]
- 84.Gangl K, Niederberger V, Valenta R. Multiple grass mixes as opposed to single grasses for allergen immunotherapy in allergic rhinitis. Clin Exp Allergy. 2013;43:1202–16. doi: 10.1111/cea.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grier TJ, Hall DM, Duncan EA, Gada SM. Allergen stabilities and compatibilities in immunotherapy mixtures that contain cat, dog, dust mite, and cockroach extracts. Ann Allergy Asthma Immunol. 2015;115:496–502. doi: 10.1016/j.anai.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Grier TJ, Hall DM, Duncan EA, Coyne TC. Mixing compatibilities of Aspergillus and American cockroach allergens with other high-protease fungal and insect extracts. Ann Allergy Asthma Immunol. 2015;114:233–9. doi: 10.1016/j.anai.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 87.Marsh DG, Lichtenstein LM, Campbell DH. Studies on “allergoids” prepared from naturally occurring allergens. I. Assay of allergenicity and antigenicity of formalinized rye group I component. Immunology. 1970;18:705–22. [PMC free article] [PubMed] [Google Scholar]
- 88.Haddad ZH, Marsh DG, Campbell DH. Studies on “allergoids” prepared from naturally occurring allergens. II. Evaluation of allergenicity and assay of antigenicity of formalinized mixed grass pollen extracts. J Allergy Clin Immunol. 1972;49:197–209. doi: 10.1016/0091-6749(72)90083-8. [DOI] [PubMed] [Google Scholar]
- 89.Weber M, Niespodziana K, Linhart B, Neubauer A, Huber H, Henning R, et al. Comparison of the immunogenicity of BM32, a recombinant hypoallergenic B cell epitope-based grass pollen allergy vaccine with allergen extract-based vaccines. J Allergy Clin Immunol. 2017;140:1433–6. doi: 10.1016/j.jaci.2017.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohashi-Doi K, Kito H, Du W, Nakazawa H, Ipsen H, Gudmann P, et al. Bioavailability of house dust mite allergens in sublingual allergy tablets is highly dependent on the formulation. Int Arch Allergy Immunol. 2017;174:26–34. doi: 10.1159/000479693. [DOI] [PubMed] [Google Scholar]
- 91.Mascarell L, Batard T, Cuiné JF, Nony E. The bioavailability of allergens in allergy tablets depends on several factors. Int Arch Allergy Immunol. 2018;175:252–3. doi: 10.1159/000486960. [DOI] [PubMed] [Google Scholar]
- 92.Heydenreich B, Bellinghausen I, Lund L, Henmar H, Lund G, Adler Würtzen P, et al. Adjuvant effects of aluminium hydroxide-adsorbed allergens and allergoids—differences in vivo and in vitro. Clin Exp Immunol. 2014;176:310–9. doi: 10.1111/cei.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moingeon P, Lombardi V, Baron-Bodo V, Mascarell L. Enhancing allergen-presentation platforms for sublingual immunotherapy. J Allergy Clin Immunol Pract. 2017;5:23–31. doi: 10.1016/j.jaip.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 94.Cox L. Standardized allergen extracts: past, present and future. Expert Rev Clin Immunol. 2005;1:579–88. doi: 10.1586/1744666X.1.4.579. [DOI] [PubMed] [Google Scholar]
- 95.Turner KJ, Stewart GA, Sharp AH, Czarny D. Standardization of allergen extracts by inhibition of RAST, skin test, and chemical composition. Clin Allergy. 1980;10:441–50. doi: 10.1111/j.1365-2222.1980.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 96.Kaul S, Lüttkopf D, Kastner B, Vogel L, Höltz G, Vieths S, et al. Mediator release assays based on human or murine immunoglobulin E in allergen standardization. Clin Exp Allergy. 2007;37:141–50. doi: 10.1111/j.1365-2222.2006.02618.x. [DOI] [PubMed] [Google Scholar]
- 97.Ferreira F, Wallner M, Gadermaier G, Erler A, Fritz G, Glatter O, et al. Physico-chemical characterization of candidate reference materials. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 2006;95:75–82. [PubMed] [Google Scholar]
- 98.Valenta R, Twardosz A, Vrtala S, Kraft D. Large scale production and quality criteria of recombinant allergens. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 1999;93:211–24. [PubMed] [Google Scholar]
- 99.Seppälä U, Dauly C, Robinson S, Hornshaw M, Larsen JN, Ipsen H. Absolute quantification of allergens from complex mixtures: a new sensitive tool for standardization of allergen extracts for specific immunotherapy. J Proteome Res. 2011;10:2113–22. doi: 10.1021/pr101150z. [DOI] [PubMed] [Google Scholar]
- 100.Chapman MD, Briza P. Molecular approaches to allergen standardization. Curr Allergy Asthma Rep. 2012;12:478–84. doi: 10.1007/s11882-012-0282-3. [DOI] [PubMed] [Google Scholar]
- 101.Verdino P, Keller W. Circular dichroism analysis of allergens. Methods. 2004;32:241–8. doi: 10.1016/j.ymeth.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 102.Anderson MC, Baer H. Immunoblotting of allergenic extracts. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 1988;82:95–106. [PubMed] [Google Scholar]
- 103.Chapman MD, Heymann PW, Wilkins SR, Brown MJ, Platts-Mills TA. Monoclonal immunoassays for major dust mite (Dermatophagoides) allergens, Der p I and Der f I, and quantitative analysis of the allergen content of mite and house dust extracts. J Allergy Clin Immunol. 1987;80:184–94. doi: 10.1016/0091-6749(87)90128-x. [DOI] [PubMed] [Google Scholar]
- 104.Van Ree R. Value of monoclonal antibody-based assays: advantages and drawbacks. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 1994;87:127–35. [PubMed] [Google Scholar]
- 105.Valenta R, Steinberger P, Laffer S, Dolecek C, Wiedemann P, Flicker S, et al. Cloning allergen-specific antibody fragments (Fabs); tools for allergen standardization and therapy of type I allergy. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 1997;91:222–9. [PubMed] [Google Scholar]
- 106.Mahler V, Vrtala S, Kuss O, Diepgen TL, Suck R, Cromwell O, et al. Vaccines for birch pollen allergy based on genetically engineered hypoallergenic derivatives of the major birch pollen allergen, Bet v 1. Clin Exp Allergy. 2004;34:115–22. doi: 10.1111/j.1365-2222.2004.01857.x. [DOI] [PubMed] [Google Scholar]
- 107.Orengo JM, Radin AR, Kamat V, Badithe A, Ben LH, Bennett BL, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun. 2018;9:1421. doi: 10.1038/s41467-018-03636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Batard T, Didierlaurent A, Chabre H, Mothes N, Bussières L, Bohle B, et al. Characterization of wild-type recombinant Bet v 1a as a candidate vaccine against birch pollen allergy. Int Arch Allergy Immunol. 2005;136:239–49. doi: 10.1159/000083950. [DOI] [PubMed] [Google Scholar]
- 109.Zuidmeer-Jongejan L, Huber H, Swoboda I, Rigby N, Versteeg SA, Jensen BM, et al. Development of a hypoallergenic recombinant parvalbumin for first-in-man subcutaneous immunotherapy of fish allergy. Int Arch Allergy Immunol. 2015;166:41–51. doi: 10.1159/000371657. [DOI] [PubMed] [Google Scholar]
- 110.Bonertz A, Roberts G, Slater JE, Bridgewater J, Rabin RL, Hoefnagel M, et al. Allergen manufacturing and quality aspects for allergen immunotherapy in Europe and the United States: an analysis from the EAACI AIT Guidelines Project. Allergy. 2018;73:816–26. doi: 10.1111/all.13357. [DOI] [PubMed] [Google Scholar]
- 111.Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. A WAO-ARIA-GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6:17. doi: 10.1186/1939-4551-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saltabayeva U, Garib V, Morenko M, Rosenson R, Ispayeva Z, Gatauova M, et al. Greater real-life diagnostic efficacy of allergen molecule-based diagnosis for prescription of immunotherapy in an area with multiple pollen exposure. Int Arch Allergy Immunol. 2017;173:93–8. doi: 10.1159/000477442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 114.Gadermaier E, Staikuniene J, Scheiblhofer S, Thalhamer J, Kundi M, Westritschnig K, et al. Recombinant allergen-based monitoring of antibody responses during injection grass pollen immunotherapy and after 5 years of discontinuation. Allergy. 2011;66:1174–82. doi: 10.1111/j.1398-9995.2011.02592.x. [DOI] [PubMed] [Google Scholar]
- 115.Linhart B, Hartl A, Jahn-Schmid B, Verdino P, Keller W, Krauth MT, et al. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J Allergy Clin Immunol. 2005;115:1010–6. doi: 10.1016/j.jaci.2004.12.1142. [DOI] [PubMed] [Google Scholar]
- 116.Metz-Favre C, Linhart B, Focke-Tejkl M, Purohit A, de Blay F, Valenta R, et al. Skin test diagnosis of grass pollen allergy with a recombinant hybrid molecule. J Allergy Clin Immunol. 2007;120:315–21. doi: 10.1016/j.jaci.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 117.Norman PS, Ohman JL, Jr, Long AA, Creticos PS, Gefter MA, Shaked Z, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–8. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 118.Simons FE, Imada M, Li Y, Watson WT, HayGlass KT. Fel d 1 peptides: effect on skin tests and cytokine synthesis in cat-allergic human subjects. Int Immunol. 1996;8:1937–45. doi: 10.1093/intimm/8.12.1937. [DOI] [PubMed] [Google Scholar]
- 119.Maguire P, Nicodemus C, Robinson D, Aaronson D, Umetsu DT. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999;93:222–31. doi: 10.1006/clim.1999.4795. [DOI] [PubMed] [Google Scholar]
- 120.Meyer W, Narkus A, Salapatek AM, Häfner D. Double-blind, placebo-controlled, dose-ranging study of new recombinant hypoallergenic Bet v 1 in an environmental exposure chamber. Allergy. 2013;68:724–31. doi: 10.1111/all.12148. [DOI] [PubMed] [Google Scholar]
- 121.Nony E, Bouley J, Le Mignon M, Lemoine P, Jain K, Horiot S, et al. Development and evaluation of a sublingual tablet based on recombinant Bet v 1 in birch pollen-allergic patients. Allergy. 2015;70:795–804. doi: 10.1111/all.12622. [DOI] [PubMed] [Google Scholar]
- 122.Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129:1290–6. doi: 10.1016/j.jaci.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 123.Wood RA, Sicherer SH, Burks AW, Grishin A, Henning AK, Lindblad R, et al. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013;68:803–8. doi: 10.1111/all.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu D, Kepley CL, Zhang K, Terada T, Yamada T, Saxon A. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat Med. 2005;11:446–9. doi: 10.1038/nm1219. [DOI] [PubMed] [Google Scholar]
- 125.Couroux P, Patel D, Armstrong K, Larché M, Hafner RP. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45:974–81. doi: 10.1111/cea.12488. [DOI] [PubMed] [Google Scholar]
- 126.Patel D, Couroux P, Hickey P, Salapatek AM, Laidler P, Larché M, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–9. doi: 10.1016/j.jaci.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 127.Spertini F, DellaCorte G, Kettner A, de Blay F, Jacobsen L, Jutel M, et al. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1-derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: results of a phase IIb study. J Allergy Clin Immunol. 2016;138:162–8. doi: 10.1016/j.jaci.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 128.Kettner A, DellaCorte G, de Blay F, Jacobsen L, Jutel M, Worm M, et al. Benefit of Bet v 1 contiguous overlapping peptide immunotherapy persists during first follow-up season. J Allergy Clin Immunol. 2018;142:678–680.e7. doi: 10.1016/j.jaci.2018.01.052. [DOI] [PubMed] [Google Scholar]
- 129.Kinaciyan T, Nagl B, Faustmann S, Frommlet F, Kopp S, Wolkersdorfer M, et al. Efficacy and safety of 4 months of sublingual immunotherapy with recombinant Mal d 1 and Bet v 1 in patients with birch pollen-related apple allergy. J Allergy Clin Immunol. 2018;141:1002–8. doi: 10.1016/j.jaci.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 130.Valenta R, Campana R, Focke-Tejkl M, Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137:351–7. doi: 10.1016/j.jaci.2015.12.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207–7.e1–11. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Niederberger V, Marth K, Eckl-Dorna J, Focke-Tejkl M, Weber M, Hemmer W, et al. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol. 2015;136:1101–3. doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valenta R, Campana R, Niederberger V. Recombinant allergy vaccines based on allergen-derived B cell epitopes. Immunol Lett. 2017;189:19–26. doi: 10.1016/j.imlet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cornelius C, Schöneweis K, Georgi F, Weber M, Niederberger V, Zieglmayer P, et al. Immunotherapy with the PreS-based grass pollen allergy vaccine BM32 induces antibody responses protecting against hepatitis B infection. EBioMedicine. 2016;11:58–67. doi: 10.1016/j.ebiom.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Swoboda I, Bugajska-Schretter A, Linhart B, Verdino P, Keller W, Schulmeister U, et al. A recombinant hypoallergenic parvalbumin mutant for immunotherapy of IgE-mediated fish allergy. J Immunol. 2007;178:6290–6. doi: 10.4049/jimmunol.178.10.6290. [DOI] [PubMed] [Google Scholar]
- 136.Douladiris N, Linhart B, Swoboda I, Gstöttner A, Vassilopoulou E, Stolz F, et al. In vivo allergenic activity of a hypoallergenic mutant of the major fish allergen Cyp c 1 evaluated by means of skin testing. J Allergy Clin Immunol. 2015;136:493–5. doi: 10.1016/j.jaci.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blank S, Bilò MB, Ollert M. Component-resolved diagnostics to direct in venom immunotherapy: important steps towards precision medicine. Clin Exp Allergy. 2018;48:354–64. doi: 10.1111/cea.13090. [DOI] [PubMed] [Google Scholar]
- 138.Gattinger P, Lupinek C, Kalogiros L, Silar M, Zidarn M, Korosec P, et al. The culprit insect but not severity of allergic reactions to bee and wasp venom can be determined by molecular diagnosis. PLoS One. 2018;13:e0199250. doi: 10.1371/journal.pone.0199250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marth K, Focke-Tejkl M, Lupinek C, Valenta R, Niederberger V. Allergen peptides, recombinant allergens and hypoallergens for allergen-specific immunotherapy. Curr Treat Options Allergy. 2014;1:91–106. doi: 10.1007/s40521-013-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hofer H, Asam C, Hauser M, Nagl B, Laimer J, Himly M, et al. Tackling Bet v 1 and associated food allergies with a single hybrid protein. J Allergy Clin Immunol. 2017;140:525–33. doi: 10.1016/j.jaci.2016.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, et al. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J Immunol. 2013;190:3068–78. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen KW, Marusciac L, Tamas PT, Valenta R, Panaitescu C. Ragweed pollen allergy: burden, characteristics, and management of an imported allergen source in Europe. Int Arch Allergy Immunol. 2018;176:163–80. doi: 10.1159/000487997. [DOI] [PubMed] [Google Scholar]
- 143.Jahn-Schmid B, Hauser M, Wopfner N, Briza P, Berger UE, Asero R, et al. Humoral and cellular cross-reactivity between Amb a 1, the major ragweed pollen allergen, and its mugwort homolog Art v 6. J Immunol. 2012;188:1559–67. doi: 10.4049/jimmunol.1102445. [DOI] [PubMed] [Google Scholar]
- 144.Villalba M, Rodríguez R, Batanero E. The spectrum of olive pollen allergens. From structures to diagnosis and treatment. Methods. 2014;66:44–54. doi: 10.1016/j.ymeth.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 145.Twaroch TE, Focke M, Civaj V, Weber M, Balic N, Mari A, et al. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–184.e7. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 146.Bonura A, Passantino R, Costa MA, Montana G, Melis M, Bondì ML, et al. Characterization of a Par j 1/Par j 2 mutant hybrid with reduced allergenicity for immunotherapy of Parietaria allergy. Clin Exp Allergy. 2012;42:471–80. doi: 10.1111/j.1365-2222.2011.03938.x. [DOI] [PubMed] [Google Scholar]
- 147.Curin M, Weber M, Thalhamer T, Swoboda I, Focke-Tejkl M, Blatt K, et al. Hypoallergenic derivatives of Fel d 1 obtained by rational reassembly for allergy vaccination and tolerance induction. Clin Exp Allergy. 2014;44:882–94. doi: 10.1111/cea.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fujimura T, Fujinami K, Ishikawa R, Tateno M, Tahara Y, Okumura Y, et al. Recombinant fusion allergens, Cry j 1 and Cry j 2 from Japanese Cedar Pollen, conjugated with polyethylene glycol potentiate the attenuation of Cry j 1-specific IgE production in Cry j 1-sensitized mice and Japanese cedar pollen allergen-sensitized monkeys. Int Arch Allergy Immunol. 2015;168:32–43. doi: 10.1159/000441141. [DOI] [PubMed] [Google Scholar]
- 149.Chen KW, Blatt K, Thomas WR, Swoboda I, Valent P, Valenta R, et al. Hypoallergenic Der p 1/Der p 2 combination vaccines for immunotherapy of house dust mite allergy. J Allergy Clin Immunol. 2012;130:435–43. doi: 10.1016/j.jaci.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Niespodziana K, Focke-Tejkl M, Linhart B, Civaj V, Blatt K, Valent P, et al. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–70. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martínez-Gómez JM, Johansen P, Rose H, Steiner M, Senti G, Rhyner C, et al. Targeting the MHC class II pathway of antigen presentation enhances immunogenicity and safety of allergen immunotherapy. Allergy. 2009;64:172–8. doi: 10.1111/j.1398-9995.2008.01812.x. [DOI] [PubMed] [Google Scholar]
- 152.Nilsson OB, van Hage M, Grönlund H. Mammalian-derived respiratory allergens—implications for diagnosis and therapy of individuals allergic to furry animals. Methods. 2014;66:86–95. doi: 10.1016/j.ymeth.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 153.Nilsson OB, Neimert-Andersson T, Bronge M, Grundström J, Sarma R, Uchtenhagen H, et al. Designing a multimer allergen for diagnosis and immunotherapy of dog allergic patients. PLoS One. 2014;9:e111041. doi: 10.1371/journal.pone.0111041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vinzón SE, Marino-Buslje C, Rivera E, Biscoglio de Jiménez Bonino M. A naturally occurring hypoallergenic variant of vespid Antigen 5 from Polybia scutellaris venom as a candidate for allergen-specific immunotherapy. PLoS One. 2012;7:e41351. doi: 10.1371/journal.pone.0041351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kussebi F, Karamloo F, Rhyner C, Schmid-Grendelmeier P, Salagianni M, Mannhart C, et al. A major allergen gene-fusion protein for potential usage in allergen-specific immunotherapy. J Allergy Clin Immunol. 2005;115:323–9. doi: 10.1016/j.jaci.2004.11.041. [DOI] [PubMed] [Google Scholar]