Abstract
We screened for novel circuits in the mouse brain that promote wakefulness. Chemogenetic activation experiments and EEG recordings pointed to glutamatergic/nitrergic (NOS1) and GABAergic neurons in the VTA. Activating glutamatergic/NOS1 neurons, which were wake- and REM-sleep-active, produced wakefulness through projections to the nucleus accumbens and the lateral hypothalamus. Lesioning the glutamate cells impaired the consolidation of wakefulness. By contrast, activation of GABAergic VTA neurons elicited long-lasting NREM-like sleep resembling sedation. Lesioning these neurons produced an increase in wakefulness that persisted for at least 4 months. Surprisingly, these VTA GABAergic neurons were wake-and REM-sleep-active. We suggest that GABAergic VTA neurons may limit wakefulness by inhibiting the arousal-promoting VTA glutamatergic and/or dopaminergic neurons and through projections to the lateral hypothalamus. Thus, in addition to its contribution to goal- and reward-directed behaviours, the VTA has a role in regulating sleep and wakefulness.
We still do not know all the circuitry in mammals regulating wakefulness and sleep1–4. Broadly, wakefulness is promoted by ascending aminergic and peptidergic systems1,5–8. GABAergic and glutamatergic pathways can also induce wakefulness and physical activity9–17. On the other hand, sleep is promoted by GABAergic/peptidergic and glutamatergic/nitrergic neurons that inhibit the wake-promoting neurons3,18–23.
Here, we describe a non-hypothesis driven chemogenetic search for further circuitry controlling vigilance states, and unexpectedly converge on the ventral tegmental area (VTA). The VTA is intensively investigated for its regulation of goal-, and reward-directed and social behaviors24–28. As well as dopamine neurons (VTADA), the VTA contains GABAergic and glutamatergic neurons, which independently project out of the VTA24,29,30. These GABA and glutamate neurons are believed to control reward-, goal-directed and social behaviour31,32. These behaviours require wakefulness, and indeed VTADA neurons are selectively wake and REM-sleep active, and actually promote wakefulness5,8,33. Complimenting this work, we find that VTA glutamate/NOS1 and GABA neurons increase and decrease wakefulness respectively. The VTA is, therefore, a node whose circuitry potently influences vigilance state.
Results
A chemogenetic search for glutamatergic circuitry enhancing wakefulness
We searched for glutamatergic neurons in the posterior hypothalamic/midbrain area (PH/MB) that could promote wakefulness (Fig. 1). By injecting AAV-DIO-hM3Dq-mCherry into Vglut2-ires-Cre mice, we expressed the excitatory hM3Dq DREADD receptor in Vglut2 neurons in progressively more defined locations (Fig. 1). [note: for the following series of experiments, CNO (1 mg/kg) was injected i.p. at the start of “lights on”, when the mice had their maximal sleep drive. Injecting CNO (1 mg/kg) i.p. into AAV-naïve Vglut2-ires-Cre mice - i.e. mice not injected with AAV - did not produce any changes in the amounts of sleep or wakefulness (Supplementary Fig. 1a)].
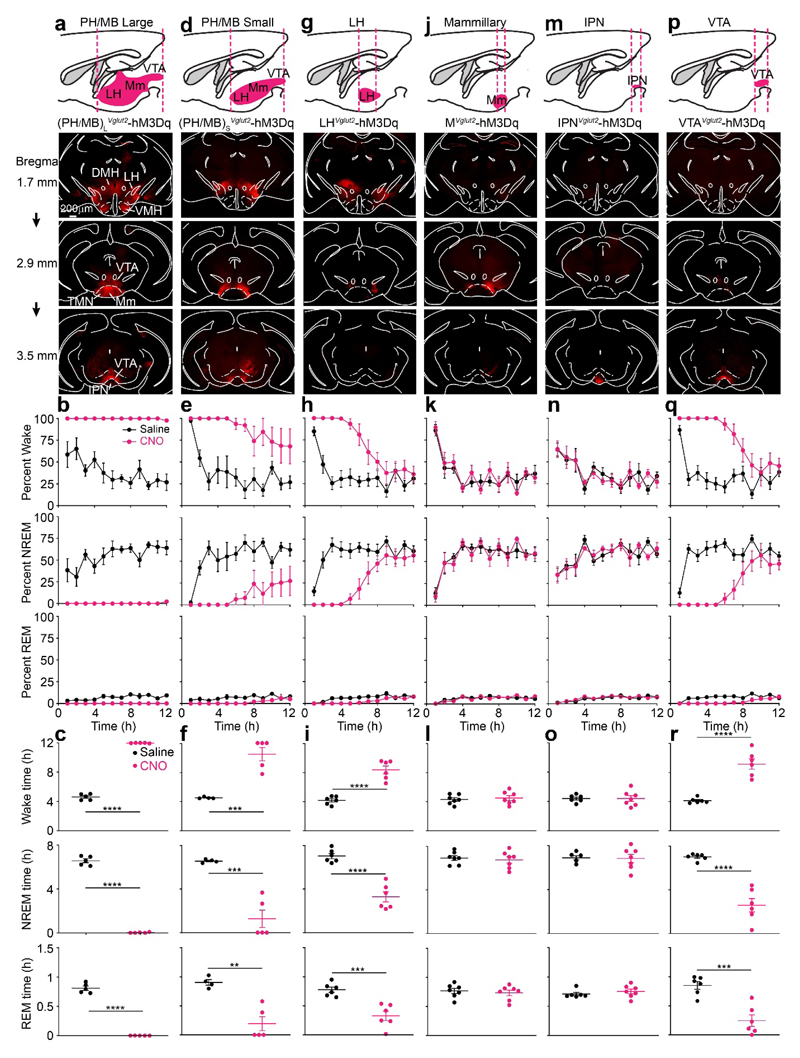
Fig. 1. Chemogenetic mapping for novel glutamatergic areas in the posterior hypothalamus and midbrain that promote wakefulness identifies the VTA.
AAV-DIO-hM3Dq-mCherry was injected into different areas of the brain of Vglut2-ires-Cre mice. AAV expression was determined by immunocytochemistry for mCherry (red). The images show the actual mCherry staining.
(a, b and c) AAV injection into a large volume of posterior hypothalamus and midbrain (PH/MB)L. The experiment in (a) was repeated independently 5 times. The graphs show percent of wake, NREM and REM sleep and how these states vary with saline (n=5 mice) or CNO (n=5 mice) i.p. injections.
(d, e and f) AAV injection into a smaller volume of posterior hypothalamus and midbrain, and sleep-wake states scored as above after saline (n=4 mice) or CNO (n=5 mice) i.p. injections. The experiment in (d) was repeated independently 5 times.
(g, h and i) AAV injection was restricted to the LH, and sleep wake states scored following saline and CNO injection after saline (n=6 mice) or CNO (n=6 mice) i.p. injections. The experiment in (g) was repeated independently 6 times.
(j, k and l) AAV injection was restricted to the mammillary area, and sleep wake states scored following saline (n=7 mice) and CNO (n=7 mice) i.p. injection. The experiment in (j) was repeated independently 5 times. See Supplementary Fig. 2a for examples of hM3Dq-mCherry expression in individual mice.
(m, n and o) AAV injection was restricted to the interpeduncular nucleus (IPN), and sleep wake states scored following saline (n=6 mice) and CNO (n=7 mice) i.p. injection. The experiment in (m) was repeated independently 6 times. See Supplementary Fig. 2b for examples of individual hM3Dq-mCherry expression.
(p, q and r) AAV injection was restricted to the VTA, and sleep wake states scored following saline (n=6) and CNO (n=6) i.p. injection. The experiment in (p) was repeated independently 6 times. See Supplementary Fig. 2c for examples of hM3Dq-mCherry expression in individual mice.
DMH, dorsomedial hypothalamus; LH, lateral hypothalamus; PH, posterior hypothalamus; IPN, interpeduncular nucleus; MM, medial mammillary area; TMN, tuberomammillary area; VMH, ventromedial hypothalamus; VTA ventral tegmental area. All error bars represent the SEM. **p<0.01, ***p<0.001, ****p<0.0001; two-sided unpaired t-test. For detailed statistics information, see Supplementary Table1.
We obtained dramatic results from large volume injections of AAV-DIO-hM3Dq-mCherry into the PH/MB of Vglut2-ires-Cre mice [(PH/MB)LVglut2-hM3Dq mice] (Fig. 1a). Following CNO injections, 100% wakefulness was induced for 12 hours compared with saline-injected control mice (Fig. 1b, c). In these (PH/MB)LVglut2-hM3Dq mice, hM3Dq-mCherry receptor expression (determined by mCherry staining) was found throughout the lateral hypothalamus (LH), dorsal medial hypothalamus (DMH), ventral medial hypothalamus (VMH), mammillary area (MM), tuberomammillary (TMN) area, supramammillary area (SuM), VTA and interpeduncular nucleus (IPN). A smaller AAV injection volume into the PH/MB of Vglut2-ires-Cre mice resulted in hM3Dq expression in the LH, MM and VTA (PH/MB)SVglut2-hM3Dq mice, Fig. 1d). CNO i.p. injection into these (PH/MB)SVglut2-hM3Dq mice produced continuous wakefulness for approximately 5 hours, followed by enhanced wakefulness for another 7 hours (Fig. 1e, f). Restricting hM3Dq expression to the LH and activating with systemic CNO also produced extended wakefulness (Fig. 1g, h, i).
By contrast, restricting hM3Dq expression to glutamatergic neurons in the mammillary area of Vglut2-ires-Cre mice (Fig. 1j, Supplementary Fig. 2a, MVglut2-hM3Dq mice), and giving CNO did not produce arousal above that resulting from baseline saline injections (Fig. 1k, l). Restricting hM3Dq receptor expression to the IPN of Vglut2-ires-Cre mice (Fig. 1m, Supplementary Fig. 2b, IPNVglut2-hM3Dq mice), and subsequent injection of CNO also did not elicit arousal compared with saline injection (Fig. 1n, o).
Glutamatergic neurons in the VTA produce wakefulness
We next limited hM3Dq receptor expression to the VTA of Vglut2-ires-Cre mice to make VTAVglut2-hM3Dq mice (Fig. 1p, Supplementary Fig. 2c). Following 1 mg/kg CNO i.p. injection, there was 5 hours of 100% wakefulness; the extent of wakefulness remained elevated for nearly the entire “lights on” period (Fig. 1q, r). [note: as a further control for the specificity of CNO’s actions, we injected AAV-DIO-mCherry into the VTA of Vglut2-ires-Cre mice; CNO injection (i.p.) into these VTAVglut2-mCherry mice had no effect on the amounts of sleep or wakefulness (Supplementary Fig. 1c)].
Of the brain regions that we injected, the VTAVglut2 population that promotes wakefulness had not previously been identified in this role, and so we decided to study these cells in detail. We first confirmed that these neurons were excited by CNO in VTAVglut2-hM3Dq mice. One hour after CNO i.p. injection into VTAVglut2-hM3Dq mice, cFOS protein was elevated in hM3Dq-expressing VTAVglut2 neurons (saline: 41 ± 4, CNO: 378 ± 36 cFOS-positive cells), confirming excitation of VTAVglut2 neurons (Supplementary Fig. 2d). Looking at the CNO-evoked EEG spectra of VTAVglut2-hM3Dq mice (Supplementary Fig. 2e), the excitation of VTAVglut2 neurons produced higher theta (8 Hz) activity (Supplementary Fig. 2f) but the EMG signal did not change (Supplementary Fig. 2f). Activation of VTAVglut2 neurons also strongly increased the latency to both NREM and REM sleep (Supplementary Fig. 2g). However, excitation of VTAVglut2 neurons did not cause hyperactivity (Supplementary Fig. 2h). In an open field assay, the CNO-injected (i.p.) mice did not move further than saline-injected mice, but stayed awake for an extended period.
Confirming the behavioral effects of the chemogenetic activation of VTAVglut2 neurons, optogenetic activation of VTAVglut2 neurons with ChR2 also increased wakefulness (Supplementary Fig. 3a, 3b, c).
VTAVglut2 neurons consolidate wakefulness during the sleep-wake cycle
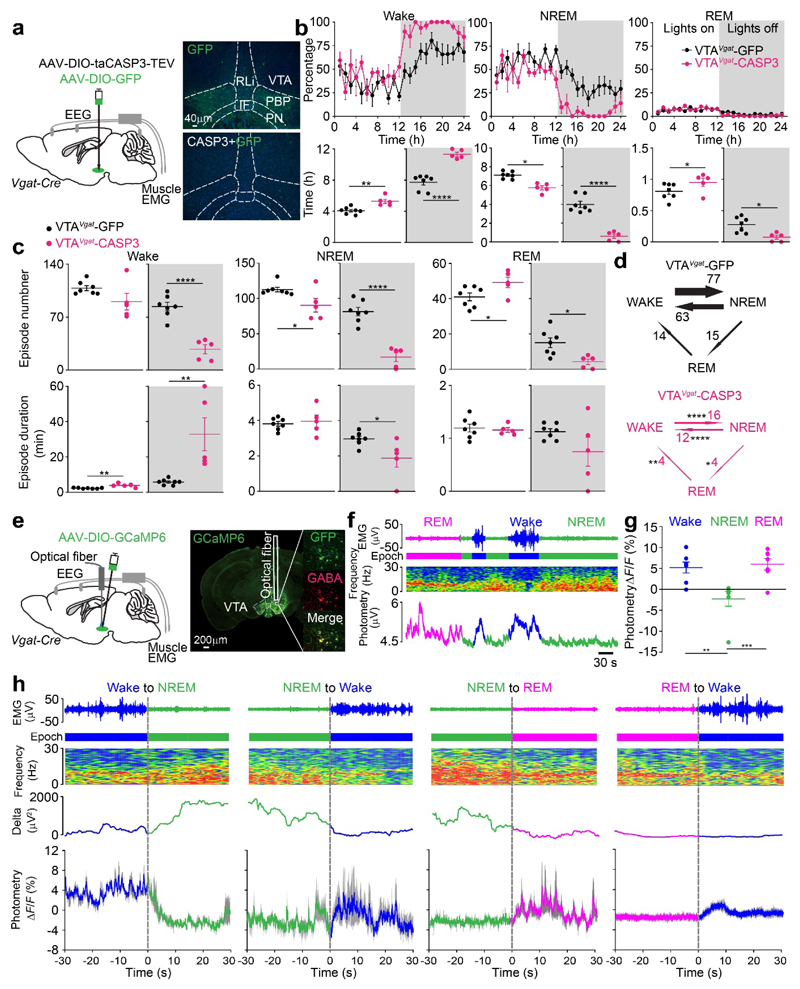
To investigate how VTAVglut2 neurons influence the sleep-wake cycle over 24 hours, we genetically ablated VTAVglut2 neurons using AAV-DIO-CASP3 to produce VTAVglut2-CASP3 mice (Fig. 2a, Supplementary Fig. 3d). About 80% of the VTAVglut2 neurons were destroyed (Supplementary Fig. 3d). Chronic lesioning of VTAVglut2 cells reduced wakefulness, and increased NREM sleep, but only during the “lights off” phase (Fig. 2b). Looking at the sleep-wake microarchitecture, wake consolidation was impaired, with more episodes and shorter episode durations of wake (Fig. 2c), and with more transitions between wake and NREM sleep (Fig. 2d), again with the phenotype appearing selectively during lights off”.
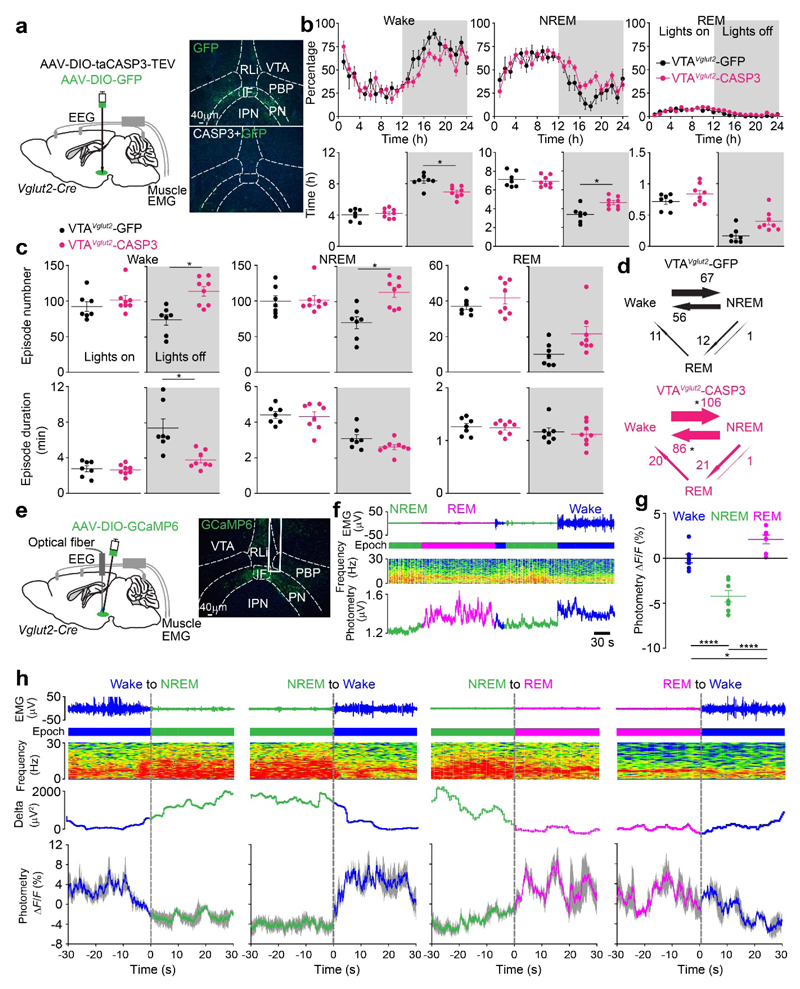
Fig. 2. VTAVglut2 neurons consolidate wakefulness and are selectively wake- and REM sleep-active.
(a) Lesioning of VTAVglut2 neurons. Injection of AAV-DIO-GFP (control) or AAV-DIO-GFP and AAV-DIO-taCASP3-TEV into the VTA area of Vglut2-ires-Cre mice. Pictures show GFP control expression in the VTA area of VTAVglut2-GFP mice and that this GFP expression has been greatly diminished in the VTAVglut2-CASP3 mice. The experiment was repeated independently 6 times. IF, interfasicular nucleus; IPN, interpeduncular nucleus; PBP, parabrachial pigmented nucleus; PN, paranigral nucleus; PBP, parabrachial pigmented nucleus; RLi, rostral linear nucleus.
(b) Lesioning of VTAVglut2 neurons. Percentage of wake, NREM and REM sleep in control VTAVglut2-GFP mice (n=7 mice) and VTAVglut2-CASP3 mice (n=8 mice), and the total vigilance times in the “lights on” and “lights off” periods.
(c, d) Lesioning of VTAVglut2 neurons. Episode number and duration for wake, NREM and REM sleep, and vigilance state transitions during the “lights off” periods in VTAVglut2-GFP control mice (n=7 mice) and VTAVglut2-CASP3 mice (n=8 mice).
(e) Fiber photometry for VTAVglut2 neurons. Injection of AAV-DIO-GCaMP6 into the VTA of the Vglut2-ires-Cre mice. The experiment was repeated independently 7 times. GCaMP6 expression can be detected in the VTA area and the trace of where the optical fiber was placed is marked.
(f) Fiber photometry Ca2+spectra (bottom trace) recorded in the VTA of VTAVglut2-GCaMP6 mice aligned with the EEG spectra (middle trace) and EMG (top trace) during wakefulness, NREM and REM sleep. “Epoch” indicates vigilance state: blue, wake; green, NREM sleep; magenta, REM sleep.
(g) Fiber photometry ΔF/F ratio of the Ca2+ signal in VTAVglut2-GCaMP6 mice during wakefulness, NREM sleep and REM sleep (n=7 mice; 38 sessions).
(h) Detail of how the Ca2+ photometry signal in Vglut2 neurons of VTAVglut2-GCaMP6 mice changes at the boundaries of the vigilance states (n=7 mice). Ca2+ photometry ΔF/F ratio (bottom trace) in the VTAVglut2-GCaMP6 mice aligned with the extracted δ power in the EEG, the EEG spectra itself and EMG during wakefulness, NREM and REM sleep. “Epoch” indicates vigilance state: blue, wake; green, NREM sleep; magenta, REM sleep. Grey shaded regions represent SEM.
*p<0.05, **p<0.01, ****p<0.0001; For b-d, two-sided unpaired t-test, for g, one-way ANOVA. All error bars represent the SEM. For detailed statistics information, see Supplementary Table1.
VTAVglut2 neurons are selectively wake- and REM sleep-active
To determine when during the natural sleep-wake cycle the VTAVglut2 neurons were active, we made VTAVglut2-GCaMP6 mice (Fig. 2e), then recorded Ca2+ signals by fiber photometry (Fig. 2f; Supplementary Fig. 3e). (Note: we used GCaMP6s for all this and all subsequent photometry experiments). The Ca2+ signal increased selectively during wakefulness and REM sleep (Fig. 2f, g), and with novel objects and female scents (Supplementary Fig. 3e). During NREM sleep, the VTAVglut2 neurons had lower Ca2+ signals (Fig. 2f, g). At the transitions between the vigilance states, the ΔF/F ratio increased from NREM to wake and from NREM to REM sleep (Fig. 2h), but decreased from wakefulness to NREM. The ΔF/F ratio changed little from REM sleep to wake transitions (Fig. 2h). [As controls, no changes in the ΔF/F ratio were found by photometry between vigilance states in VTAVglut2-GFP mice (Supplementary Fig. 3f, g); furthermore, there was no bleaching of the signal in VTAVglut2-GCaMP6s mice, as the ΔF/F ratios stayed constant over 6 hours (Supplementary Fig. 3h)].
VTAVglut2 neurons promote wakefulness independently of dopamine
Dopamine neurons in the VTA promote wakefulness5,8, so we tested if the wake-promoting effect of VTAVglut2 neurons depended on dopamine. We first verified that VTAVglut2 neurons are largely distinct from VTA dopamine neurons. Immunohistochemistry confirmed that only 26 ± 1.6% of the Vglut2 cells were TH-positive (Supplementary Fig. 4a, b), as shown previously24. Unlike dopamine neurons, VTAVglut2 cells were mostly located in the midline VTA nuclei - the rostral linear nucleus (RLi) and the interfasicular nucleus (IF) (Supplementary Fig. 4b)24,34. Thus, the majority of VTAVglut2 and VTADA neurons are distinct populations. We next chemogenetically activated the VTAVglut2 neurons in VTAVglut2-hM3Dq mice with CNO in the presence of systemically administered dopamine antagonists SCH-23390 and raclopride for D1 and D2/D3 receptors respectively (Supplementary Fig. 4c, d) (note: mice were injected i.p. with the dopamine receptor antagonists 30 mins before CNO i.p. injection – please see Methods for drug concentrations). Even when dopamine receptors were blocked, VTAVglut2 neurons still promoted wakefulness (Supplementary Fig. 4d).
VTAVglut2 neurons promote wakefulness via the nucleus accumbens and the lateral hypothalamus
To identify the brain areas that participate in generating the VTAVglut2-mediated wakefulness, we mapped cFOS activity in VTAVglut2-hM3Dq mice. Following CNO i.p. injection, increased numbers of cFOS-positive cells were identified in multiple brain regions (Fig. 3a and Supplementary Fig. 5a, b). cFOS expression was particularly activated in the lateral hypothalamus (LH), nucleus accumbens (NAc) and ventral pallidum (VP) (Fig. 3a and Supplementary Fig. 5a, b). In the LH, 60% of cFOS-expressing cells that were activated by CNO i.p. injections were also orexin-positive compared with the low number of cFOS expressing cells following saline injection (Supplementary Fig. 5c).
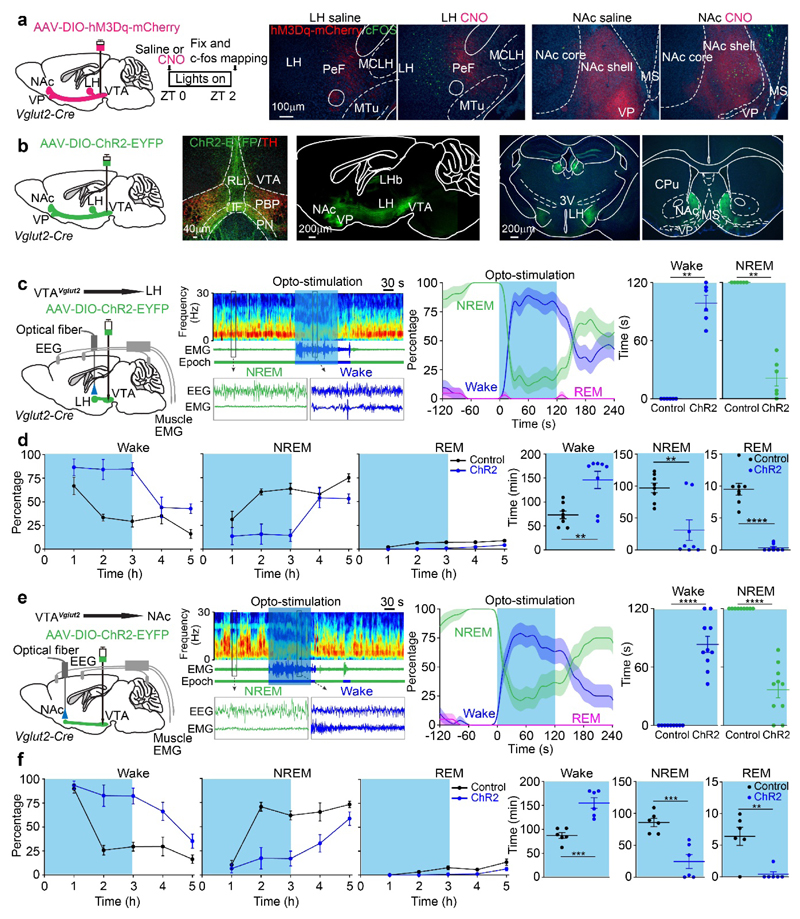
Fig. 3. VTAVglut2 neurons promote wakefulness by their projections to the LH and NAc.
(a) cFOS-based activity mapping of brain regions after exciting VTAVglut2 neurons. In VTAVglut2-hM3Dq mice, labelled axons mainly project from the VTA to the LH and NAc. cFOS protein expression in neurons of the LH and NAc of VTAVglut2-hM3Dq mice 2 hours after saline or CNO i.p. injection at ZT0. The red in the histology figure is the primary fluorescence of the hM3Dq-mCherry-positive axons coming from the VTA area, the cFOS immunohistochemistry is shown in green. The experiment was repeated independently 6 times.
CPu, caudate-putamen; IF, interfasicular nucleus; LH, lateral hypothalamus; LHb, lateral habenula; MS, medial septum; NAc, nucleus accumbens; PeF, perifornical area; PBP, parabrachial pigmented nucleus; PN, paranigral nucleus; PBP, parabrachial pigmented nucleus; RLi, rostral linear nucleus; 3V, third ventricle; VP, ventral pallidum
(b) Axonal projections of VTAVglut2 neurons. AAV-DIO-ChR2-EYFP was delivered into the VTA of Vglut2-ires-Cre mice, and axons projecting to the LH and NAc were strongly labelled. The experiment was repeated independently 4 times.
(c, d) To functionally investigate the VTAVglut2→LH projection, an optical fiber was placed into the LH area of VTAVglut2-ChR2-EYFP mice. (c) Mice were given 120 s of optostimulation (20 Hz) during NREM sleep (“lights on” period) and the percentage and time of wake and NREM were scored (control: n=6 mice; 23 trials; ChR2: n=6 mice; 21 trials). (d) VTAVglut2-ChR2-EYFP mice (control: n=8 mice; ChR2: n=8 mice) were given 3 hours of opto-stimulation at the start of the sleep period (“lights on” period) and the percentage and time of wake, NREM and REM sleep were scored.
(e, f) To functionally investigate the VTAVglut2→NAc projection, an optical fiber was placed into the NAc area of VTAVglut2-ChR2-EYFP mice. (e) Mice were given 120 s of opto-stimulation (20 Hz) during NREM sleep (“lights on” period) and the percentage and time of wake and NREM were scored (control: n=9 mice; 21 trials; ChR2: n=10 mice; 20 trials). (f) VTAVglut2-ChR2-EYFP mice (control: n=6 mice; ChR2: n=6 mice) were given 3 hours of opto-stimulation at the start of the sleep (“lights on” period) and the percentage and time of wake, NREM and REM sleep were scored.
**p<0.01, ***p<0.001, ****p<0.0001, for c and e, two-sided mann-whitney u test. For d and f, two-sided unpaired t-test. All error bars represent the SEM. For (c) and (e), the shaded region represents SEM. For detailed statistics information, see Supplementary Table1.
We then undertook ChR2-based circuit mapping of the VTAVglut2 neurons in VTAVglut2-ChR2-EYFP mice (Fig. 3b, Supplementary Fig. 5d). Consistent with the cFOS activation data, there was a dense projection of VTAVglut2 neurons to the LH and NAc (Fig. 3b and Supplementary Fig. 5c), as described previously29,30,35,36. To test whether the VTAVglut2→LH or VTAVglut2→NAc projections can promote wakefulness, we placed optical fibers into the LH and NAc of VTAVglut2-ChR2-EYFP mice to stimulate the terminals (Fig. 3c, d, e, f). Optically stimulating VTAVglut2 fibers in the LH strongly and consistently promoted waking from NREM sleep (Fig. 3c; Supplementary Fig. 6a); and chronic optical stimulation for 3 hours maintained wakefulness (Fig. 3d). Similarly, optogenetic stimulation of the VTAVglut2 terminals in the NAc promoted waking from NREM sleep (Fig. 3e; Supplementary Fig. 6b) and chronic optogenetic stimulation for 3 hours increased wakefulness and reduced NREM and REM sleep (Fig. 3f). Thus the LH and NAc are two areas contributing to wakefulness when excited by VTAVglut2 neurons.
Nitric oxide synthase marks wake-promoting VTAVglut2 neurons
To confirm the wake-promoting actions of the VTAVglut2→LH and VTAVglut2→NAc projections, we used retrograde-labeling by injecting AAV-Retro-DIO-Chronos-GFP into the LH and NAc of Vglut2-ires-Cre mice. We identified many GFP-positive soma in the VTA (Supplementary Fig. 7a, b). We optogenetically activated the Chronos-GFP retrolabeled cells by inserting optical fibers into the VTA (Supplementary Fig. 7b). Tonic opto-activation increased cFOS in these retro-labelled neurons (control: 31 ± 5, stimulation: 206 ± 41) (Supplementary Fig. 7c) Activation of the retro-labeled NAc→VTAVglut2 neurons increased wakefulness (Supplementary Fig. 7d). To profile these retro-labeled VTAVglut2 neurons, we stained VTAVglut2 neurons with a panel of antibodies recognizing neurochemical markers (nitric oxide synthase NOS1, tyrosine hydroxylase TH, glutamic acid decarboxylase GAD67, parvalbumin PV, somatostatin SOM) (Fig. 4a, Supplementary Fig. 7e). Of these markers, double-labelling of retrolabeled NAc→VTAVglut2 and LH→VTAVglut2 neurons identified that about 68 ± 9 % of the GFP-positive cells were immuno-positive for NOS1 (Fig. 4a, Supplementary Fig. 7f), mostly in the midline VTA. This was confirmed by direct double-labelling with VTAVglut2-ChR2-EYFP neurons and VTANos1 neurons (Supplementary Fig. 8a, b). About 75 ± 3% of VTANos1 cells were VTAVglut2-positive (Supplementary Fig. 8b), as also seen independently34.
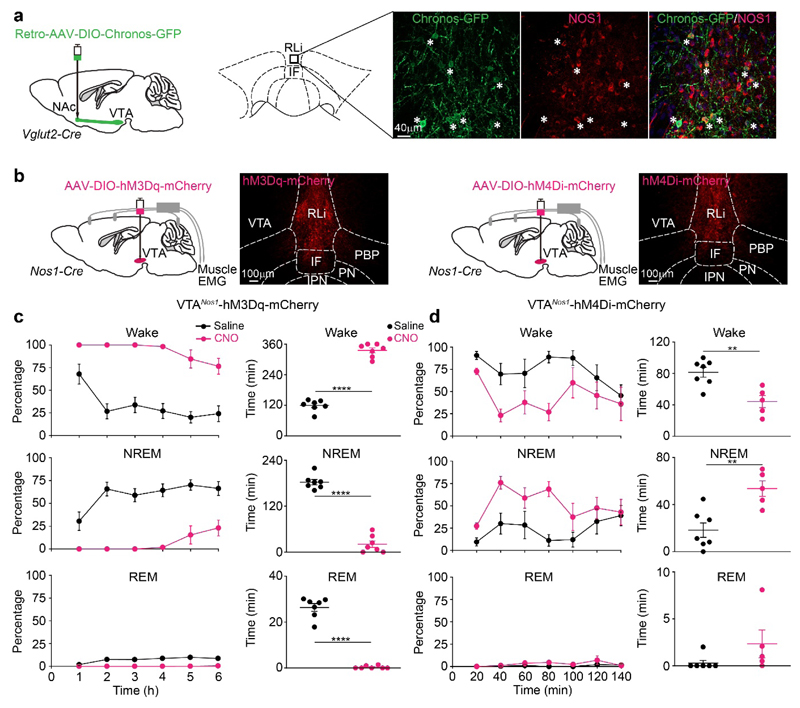
Fig. 4. VTAVglut2/Nos1 neurons promote wakefulness.
(a) Retro-mapping of VTAVglut2→NAc connections. Retro-AAV-DIO-Chronos-GFP was injected into the NAc of Vglut2-ires-Cre mice. Chronos-GFP expression was detected in cells of the VTA and Chronos-GFP retro-labeled VTA midline soma (from the NAc injection) were doubled-labelled by immunocytochemistry with NOS1 antisera. The experiment was repeated independently 3 times.
(b) Testing how VTANos1 neurons influence vigilance state. AAV-DIO-hM3Dq-mCherry or AAV-DIO-hM4Di-mCherry was injected into the VTA area of Nos1-ires-Cre mice. Images show the expression of hM3Dq-mCherry or hM4Di-mCherry in the VTA. The experiment was repeated independently 6 times.
(c) Excitation of VTANos1 neurons induces wakefulness. Percentage and time of wake, NREM and REM sleep after saline (n=7 mice) or CNO (n=7 mice) i.p. injection at the start of sleep period (“lights on” period) into VTANos1-hM3Dq mice.
(d) Inhibition of VTANos1 neurons induces NREM sleep. Percentage and time of wake, NREM and REM sleep after saline (n=7 mice) or CNO (n=5 mice) i.p. injection during wake period (“lights off” period) into VTANos1-hM4Di mice.
IF, interfasicular nucleus; PBP, parabrachial pigmented nucleus; PN, paranigral nucleus; PBP, parabrachial pigmented nucleus; RLi, rostral linear nucleus; VTA, ventral tegmental area
**p<0.01, ****p<0.0001; two-sided unpaired t-test. All error bars represent the SEM. For detailed statistics information, see Supplementary Table1.
To test if the VTANos1 neurons are functionally the same as the VTAVglut2 neurons in producing wakefulness, we chemogenetically activated the VTANos1 neurons by delivering AAV-DIO-hM3Dq-mCherry into the VTA of Nos1-ires-Cre mice (Fig. 4b, c). As for the VTAVglut2-hM3Dq mice, giving 1 mg/kg CNO i.p. to the VTANos1-hM3Dq mice produced sustained wakefulness (100%) for 4 hours (Fig. 4c, Supplementary Fig. 8c). Also similar to the VTAVglut2-hM3Dq mice, the wakefulness produced by i.p. administered CNO activation of VTANos1 neurons was not blocked by systemic i.p. administered dopamine D1 and D2/D3 receptor antagonists (Supplementary Fig. 8d) (note: mice were injected i.p. with the dopamine receptor antagonists 30 mins before CNO i.p. injection – see Methods for drug concentrations). Thus, the wake-promoting VTANos1 neurons are a subset of VTAVglut2 neurons. Chemogenetic inhibition of these VTAVglut2/Nos1 neurons, by delivering AAV-DIO-hM4Di-mCherry into the VTA of Nos1- ires-Cre mice and giving CNO i.p., decreased wakefulness and produced more NREM sleep (Fig. 4b, Fig. 4d, Supplementary Fig. 8e). Therefore, VTAVglut2/Nos1 neurons can bidirectionally regulate wakefulness. We next mapped projections of VTANos1 neurons by injecting AAV-DIO-ChR2-EYFP into the VTA of Nos1-ires-Cre mice. Similar to VTAVglut2 neurons, VTANos1 neurons project to the NAc, VP and LH (Supplementary Fig. 8f), again implying that the NOS1-expressing neurons are a subset of the glutamatergic ones.
VTAVgat neurons limit wakefulness and induce NREM sleep
We next looked for neurons in the VTA that could potentially restrain the wake-promoting activity of the VTAVglut2/Nos1 neurons. The VTA contains many GABAergic neurons (Supplementary Fig. 9a, b)24,30. These can be detected by expression of the vesicular GABA transporter (VGAT). We first confirmed that Vgat gene-expressing neurons were mostly distinct from dopamine or glutamate neurons: only 0.3 ± 0.1% of these GABAergic neurons (as defined by VTAVgat-ChR2-EYFP staining) stained with TH (Supplementary Fig. 9a), and 12 ± 0.6% of these VTAVgat neurons were NOS1-positive; however, these cells were not in the midline but mostly in the lateral part of the VTA (PBP), which are distinct from VTANos1/Vglut2 populations in the midline (Supplementary Fig. 9b)34.
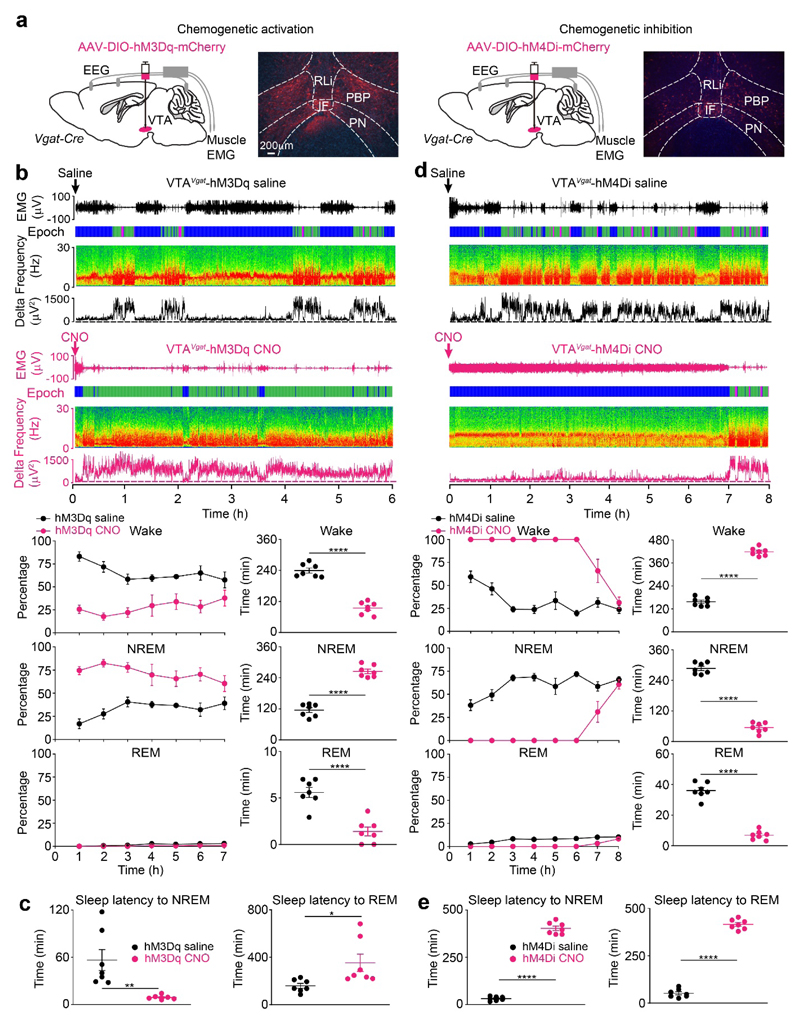
To examine if VTAVgat neurons contribute to sleep-wake regulation, AAV-DIO-hM3Dq-mCherry or AAV-DIO-hM4Di-mCherry was injected into the VTA of Vgat-ires-Cre mice to generate VTAVgat-hM3Dq and VTAVgat-hM4Di mice respectively (Fig. 5a, Supplementary Fig. 9c, d). CNO injection i.p. into VTAVgat-hM3Dq mice produced sustained (80%) NREM sleep for 6 hours (Fig. 5b), with continuous δ power in the EEG. Following CNO administration i.p., the latency to NREM sleep was reduced to about 10 minutes compared with saline administration (Fig. 5c). However, latency to REM sleep was significantly increased - there was no REM sleep in the first 6 hours (Fig. 5c) [note: we did several further controls for the specificity of CNO’s actions to ensure it was not acting as a sedative after conversion to clozapine37. We injected 1 mg/kg CNO i.p. into both AAV-naïve Vgat-ires-Cre mice - i.e. mice that had not received any AAV injections (Supplementary Fig. 1b), and into VTAVgat-mCherry mice (produced by injecting AAV-DIO-mCherry into the VTA of Vgat-ires-Cre mice). In neither of these types of mice did CNO alter the amounts of sleep or wakefulness compared with saline-injected controls, (Supplementary Fig. 1d). In a separate study21, we similarly found that CNO systemically injected i.p. at the higher concentration of 5 mg/kg into AAV-naïve Vgat-ires-Cre mice also did not induce NREM sleep above the background of sleep occurring following saline injection21].
Fig. 5. Excitation of GABAergic neurons in the VTA induces sleep and their inhibition produces continuous wakefulness.
(a) Excitation and inhibition of VTAVgat neurons. AAV-DIO-hM3Dq-mCherry or AAV-DIO-hM4Di-mCherry was injected into the VTA of Vgat-ires-Cre mice. Images show the expression of hM3Dq-mCherry or hM4Di-mCherry in the VTA. The experiment was repeated independently 6 times. IF, interfasicular nucleus; PBP, parabrachial pigmented nucleus; PN, paranigral nucleus; PBP, parabrachial pigmented nucleus; RLi, rostral linear nucleus;
(b) Excitation of VTAVgat neurons induces NREM sleep and suppresses REM sleep. Two individual EEG/EMG spectra and extracted delta power from the EEG are shown for VTAVgat-hM3Dq mice that received saline (n=7 mice) or CNO (n=7 mice) i.p. injection during wake period (“lights off” period). The percentage and time in wake, NREM and REM sleep after saline (n=7 mice) or CNO (n=7 mice) injection are shown. “Epoch” indicates vigilance state: blue, wake; green, NREM sleep; magenta, REM sleep.
(c) Excitation of VTAVgat neurons reduces latency to NREM sleep. Latencies to NREM and REM sleep after saline (n=7 mice) or CNO (n=7 mice) i.p. injection into VTAVgat-hM3Dq mice.
(d) Inhibition of VTAVgat neurons induces wakefulness. Two individual EEG/EMG spectra and extracted delta power from the EEG are shown for VTAVgat-hM4Di mice that received saline (n=7 mice) or CNO (n=7 mice) i.p. injection at the start of sleep period (“lights on” period). The percentage and time in wake, NREM and REM sleep after saline (n=7 mice) or CNO (n=7 mice) i.p. injection are shown. “Epoch” indicates vigilance state: blue, wake; green, NREM sleep; magenta, REM sleep.
(e) Inhibition of VTAVgat neurons increases latency to sleep. Latencies to NREM (n=7 mice) and REM sleep (n=7 mice) after CNO or saline i.p. injection into VTAVgat-hM4Di mice.
*p<0.05, **p<0.01, ****p<0.0001; two-sided unpaired t-test. All error bars represent the SEM. For detailed statistics information, see Supplementary Table1.
In converse experiments with chemogenetic inhibition of VTAVgat neurons, injecting CNO i.p. into VTAVgat-hM4Di mice produced 100% wakefulness for 6 hours with sustained theta frequencies in the EEG (Fig. 5d). There was an increased latency to the first NREM and REM sleep bouts to over 6 hours post-injection of CNO i.p. compared with saline i.p.-injected mice (Fig. 5e).
We next examined if subtypes of GABAergic neuron can induce NREM sleep. Subtypes of GABAergic neurons in the VTA include those expressing parvalbumin (Pv), somatostatin (Som), and in the PBP region, Nos1/Vgat34 (see also Supplementary Fig. 9b). We chemogenetically activated VTAPv, VTASom and VTA-PBPNos1/Vgat populations in the VTA (Supplementary Fig. 10) by expressing hM3Dq-mCherry in these cells. Activation of VTAPv and VTASom neurons each produced 3 hours of NREM sleep (Supplementary Fig. 10). However, activating VTA-PBPNos1/Vgat neurons did not induce either sleep or wakefulness (Supplementary Fig. 10). Thus, several GABAergic subtypes in the VTA can contribute to induction of NREM sleep, although each group activated individually does not give the full effect obtained with activating VTAVgat neurons.
Lesioning of VTAVgat neurons produces continuous wakefulness
To look at VTAVgat function in the sleep-wake cycle over 24 hours, we chronically lesioned VTAVgat neurons with AAV-DIO-CASP3 (Fig. 6a, Supplementary Fig. 11a). About 88% of the VTAVgat neurons were destroyed (Supplementary Fig. 11a). In these VTAVgat-CASP3 mice, there was an increase in wakefulness in both the “lights on” and “lights off” periods, but especially during the “lights off” period – the VTAVgat-CASP3 animals slept for only 40 minutes, whereas non-lesioned VTAVgat-GFP control mice slept 4 hours in the 12 hours of the “lights off” period (Fig. 6b). During “lights on”, the least active period of the mice, the control mice slept for about 7 hours, and the VTAVgat-CASP3 mice slept for about 6 hours (Fig. 6b). During “lights-off” the average NREM episode duration of VTAVgat-CASP3 mice was much shorter, and transitions between vigilance states were dramatically decreased (Fig. 6c, d). The reduced sleep phenotype of VTAVgat-CASP3 persisted unchanged for at least 4 months, resulting in the mice having a permanent and substantial sleep deficit (Supplementary Fig. 11b).
Fig. 6. VTAVgat neurons inhibit wakefulness. Lesioning of VTAVgat neurons produces extended wakefulness, but VTAVgat neurons are selectively wake- and REM-active.
(a) Lesioning of VTAVgat neurons. Injection of AAV-DIO-GFP (control) or AAV-DIO-taCASP3-TEV into the VTA area of Vgat-ires-Cre mice. Pictures show GFP control expression in the VTA area of VTAVgat-GFP mice and that this GFP expression has been greatly diminished in the caspase treated mice. The experiment was repeated independently 5 times. IF, interfasicular nucleus; PBP, parabrachial pigmented nucleus; PN, paranigral nucleus; PBP, parabrachial pigmented nucleus; RLi, rostral linear nucleus
(b) Lesioning of VTAVgat neurons increases wakefulness. Percentage of wake, NREM and REM sleep in control VTAVgat-GFP mice (n=7 mice) and VTAVgat-CASP3 mice (n=5 mice), and the total vigilance times in the “lights on” and “lights off” periods.
(c, d) Lesioning of VTAVgat neurons reduces the transitions between vigilance states and stabilizes wakefulness. Episode number and duration for wake, NREM and REM sleep, and vigilance state transitions during the “lights off” periods in VTAVgat-GFP control mice (n=7 mice) and VTAVgat-CASP3 mice (n=5 mice).
(e) Fiber photometry for Ca2+ levels in VTAVgat neurons. Injection of AAV-DIO-GCaMP6 into the VTA of Vgat-ires-Cre mice. GCaMP6 expression can be detected in the VTA area and was co-stained with GABA. The trace of where the optical fiber was placed is illustrated. The experiment was repeated independently 7 times.
(f) Fiber photometry for VTAVgat neurons. Neurons are more active in wake and REM sleep. Ca2+ photometry spectra (bottom trace) recorded in the VTA of VTAVgat-GCaMP6 mice aligned with the EEG spectra (middle trace) and EMG (top trace) during wakefulness, NREM and REM sleep. “Epoch” indicates vigilance state: blue: wake; green: NREM sleep; magenta, REM sleep.
(g) Fiber photometry for VTAVgat neurons. ΔF/F ratio of the Ca2+ photometry signal in VTAVgat-GCaMP6f mice during wakefulness, NREM sleep and REM sleep (n=7 mice; 41 trials).
(h) Fiber photometry for VTAVgat neurons. Detail of how the Ca2+ signal in Vgat neurons of VTAVgat-GCaMP6 mice changes at the boundaries of the vigilance states (n=7 mice). “Epoch” indicates vigilance state: blue, wake; green, NREM sleep; magenta: REM sleep. Grey shaded regions represent SEM.
*p<0.05, **p<0.01, ****p<0.0001; For b-d, two-sided unpaired t-test, for g, one-way ANOVA. All error bars represent the SEM. For detailed statistics information, see Supplementary Table1.
VTAVgat neurons are selectively wake- and REM-active
Based on the chemogenetic and lesioning results, we might have expected VTAVgat neurons to be NREM sleep-active. To test this, we made VTAVgat-GCaMP6 mice and measured the activity of VTAVgat neurons by fiber photometry during different vigilance states of freely moving mice (Fig. 6e). Surprisingly, as for the Vglut2 neurons, we found that the Ca2+ signal for the VTAVgat neurons actually increased selectively during wakefulness and REM sleep (Fig. 6f). During NREM sleep the VTAVgat cells had a lower Ca2+ signal (Fig. 6f, g). At the transitions between the vigilance states (Fig. 6h), the ΔF/F ratio decreased from wakefulness to NREM sleep, and increased from NREM sleep to wake, and from NREM to REM sleep (Fig. 6h). Within an individual bout of wake or REM sleep, the Ca2+ signal of the VTAVgat cells did not differ between the earlier and later parts of the bouts (Supplementary Fig. 11c).
To search for NREM sleep-active VTAVgat neurons that might have been missed by fiber photometry, we used micro-endoscopic calcium imaging of these neurons in VTAVgat-GCaMP6f mice (Supplementary Fig. 12). We placed a gradient refractive index (GRIN) lens above the VTA, and recorded fluorescence of VTAVgat neurons during NREM sleep or wakefulness of freely moving mice (Supplementary Fig. 12a). For the single cells that were tracked (16 cells from 4 mice), Ca2+ levels in VTAVgat neurons increased during wake and decreased during NREM sleep (Supplementary Fig. 12b, c, d, e, f). This result further confirmed that VTAVgat neurons are selectively wake-active.
VTAVgat neurons reduce wakefulness and induce NREM sleep by both local inhibition and via projections to the LH
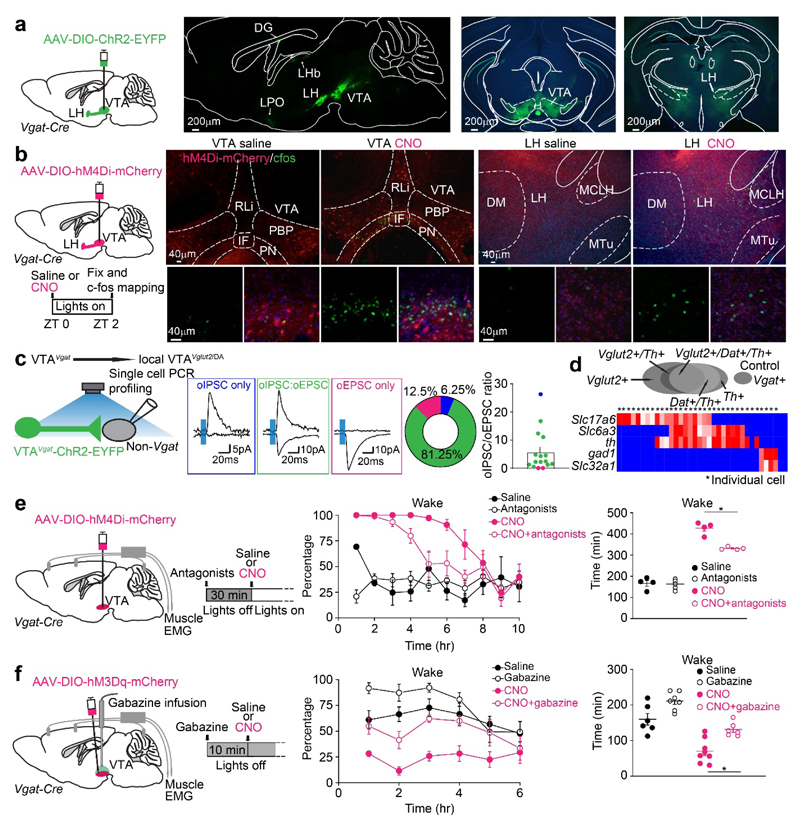
Because VTAVgat neurons are selectively wake- and REM-on, we hypothesized that they are not physiologically triggering NREM sleep, but they are instead limiting wakefulness. To explore how VTAVgat neurons do this, we conducted ChR2-based mapping of the projections of VTAVgat axons by delivering AAV-DIO-ChR2-EYFP into the VTA area of Vgat-ires-Cre mice. As published previously38, dense VTAVgat ChR-positive fibers were found locally in the VTA (Fig. 7a), but also in the LH area (Fig. 7a), and to some extent in the lateral habenula and dentate granule cells of the hippocampus (Supplementary Fig. 13a); a few fibers were also in the PFC and lateral preoptic area (Supplementary Fig. 13a). The especially dense VTAVgat fibers in the VTA indicate strong local inhibition. To test these connections, we did cFOS activity-mapping by inhibiting VTAVgat neurons with 1 mg/kg CNO injected i.p. into VTAVgat-hM4Di mice to examine which areas of the brain expressed cFOS protein (by disinhibition). After CNO injection i.p., which promoted wakefulness (Fig. 5d), cFOS was induced strongly in the VTA and LH (Fig. 7b, Supplementary Fig. 13b, c). Thus, based on cFOS expression as a readout of neuronal excitation, VTAVgat neurons cause inhibition by projecting to the LH, as well as local inhibition in the VTA.
Fig. 7. VTAVgat neurons limit wakefulness in part by locally inhibiting dopamine and Vglut2 neurons in the VTA.
(a) Mapping axonal projections of VTAVgat neurons. AAV-DIO-ChR2-EYFP was delivered into the VTA of Vgat-ires-Cre mice, and axons in the local VTA and projecting to the LH were strongly labelled. The experiment was repeated independently 4 times. DG: detent granule cells; IF, interfasicular nucleus; LH, lateral hypothalamus; LHb: lateral habenula; LPO: lateral preoptic area; MCLH, magnocellular nucleus, lateral hypothalamus; MTu, medial tuberomammillary nucleus; PBP, parabrachial pigmented nucleus; PN, paranigral nucleus; RLi, rostral linear nucleus; VTA: ventral tegmental area.
(b) cFOS-based activity mapping of brain regions after inhibiting VTAVgat neurons. In VTAVgat-hM4Di mice, cFOS protein expression is found in neurons of the VTA and LH 2 hours after saline or CNO i.p. injection at ZT0. The red in the histology figure is the primary fluorescence of the hM4Di-mCherry-positive axons, the cFOS immunohistochemistry is shown in green. The experiment was repeated independently 6 times.
(c, d) (c) Investigating the local transmitter properties of VTAVgat neurons in the midline VTA. Acute brain slice electrophysiology was performed on non-Vgat neurons in the midline VTA area in VTAVgat-ChR2-EYFP mice. Non-Vgat cells were visually selected by YFP negative signals, and after whole-cell status was successfully achieved, a 5ms single blue LED light pulse was given to the local VTA area. The percentages of recorded non-Vgat cells which had either oIPSCs only, or oEPSCs only, or both oIPSCs and oEPSCs were: oIPSC only: 6.25% (n=1); oIPSC and oEPSC (oIPSC: oEPSC), 81.25% (n=13); oEPSC only, 12.5% (n=2). The relative amplitude ratio of the oIPSC peaks versus the oEPSC peaks of non-Vgat cells was 5.71±1.8 (n=16). (d) Heat map for the single-cell PCR of patched cells. The genes tested for were: Slc17a6 (vglut2); Slc6a3 (dat), Slc32a1 (vgat), th, and gad1.
(e) VTAVgat neurons inhibit wakefulness in part by inhibiting dopamine neurons. Dopamine receptor D1 and D2/3 antagonists (SCH23390 and raclopride respectively) were injected into VTAVgat-hM4Di mice 30 min before saline or CNO injection. Percentage and time of wake was scored after saline or CNO injection (saline: n=4 mice; antagonists: n=5 mice; CNO: n=4 mice; CNO+antagonists: n=4 mice).
(f) Local inhibition from VTAVgat neurons limits wakefulness. A cannula was placed into the VTA of VTAVgat-hM3Dq mice and mice were given gabazine 10 min before saline or CNO i.p. injection. Percentage and time of wake was scored (saline: n=6 mice; gabazine: n=7 mice; CNO: n=8 mice; CNO+gabazine: n=6 mice).
*p<0.05, for e and f, repeated measures two-way ANOVA and Bonferroni-Holm post hoc test. All error bars represent the SEM. For detailed statistics information, see Supplementary Table1.
We confirmed directly that VTAVgat neurons can mediate local inhibition. We prepared acute brain slices containing the midline VTA from VTAVgat-ChR2-EYFP mice, optogenetically activated the VTAVgat neurons (Fig. 7c), and observed the postsynaptic responses in non-Vgat cells. Whole cell patch-clamping confirmed that 87.5% of postsynaptic cells (14 out of 16 cells) received either optogentically-evoked (o) oIPSCs only, or both oIPSCs and oEPSCs (Fig. 7c); 12.5% of cells (2 out of 16 cells) had oEPSCs only (Fig. 7c). From the relative peak oIPSC and oEPSC ratios, most non-VGAT cells had significantly larger oIPSCs than oEPSCs (Fig. 7c). From single-cell PCR profiling, these non-Vgat neurons with oIPSCs were a mixture of VTAVglut2, VTADA, and VTAVglut2/DA neurons (Fig. 7d); the two cells that responded with only oEPSCs were VTAVglut2 and VTADA cells. Thus, the majority of non-Vgat cells in the midline VTA have a large density of inhibitory input from local VTAVgat neurons.
Because midline VTAVgat neurons inhibit midline dopamine neurons, we examined the effect of this local inhibition on wakefulness. We gave D1 and D2/3 receptor antagonists to CNO-injected VTAVgat-hM4Di mice (mice were injected i.p. with the dopamine receptor antagonists 30 mins before CNO i.p. injection – see Methods for drug concentrations). Without dopamine antagonists, CNO inhibition of VTAVgat neurons caused 6 hours of sustained wakefulness (as shown previously in Fig. 5d). With dopamine receptor antagonists, CNO-induced wakefulness was blocked by 20% (Fig. 7e), implying that the sustained wakefulness originating from the inhibited VTAVgat neurons could be partially due to the activation (disinhibition) of VTA dopamine neurons.
To test if VTAVgat neurons use local inhibition to restrict wakefulness, we infused the GABAA receptor antagonist Gabazine (SR95531) into the VTA of CNO i.p.-injected VTAVgat-hM3Dq mice (please see Methods for Gabazine concentration) (Fig. 7f). In the CNO-injected VTAVgat-hM3Dq mice, Gabazine reduced CNO-induced NREM sleep by 40% (Fig. 7f). Thus, the sustained NREM sleep originating from the activated VTAVgat neurons was partially due to local inhibition of VTAVglut2, VTADA, and VTAVglut2/DA neurons.
As blocking local GABA transmission with Gabazine did not completely abolish the ability of activated VTAVgat neurons to induce sustained NREM sleep, the projections of these VTAVgat neurons might also contribute. We found that following CNO inhibition of VTAVgat neurons in VTAVgat-hM4Di mice, the number of cFOS-expressing cells in the LH was strongly elevated compared with saline injections (Fig. 7b). About 50% of those FOS-positive LH cells were orexin neurons (Supplementary Fig. 13d). By injecting Retro-AAV-DIO-Chronos-GFP into the LH area of Vgat-ires-Cre mice, we also detected dense retro-labeled soma in the VTA (Supplementary Fig. 13e).
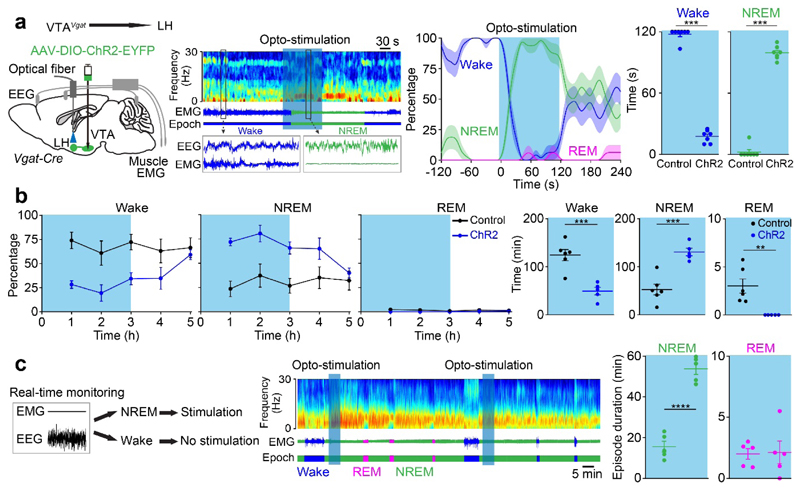
The results imply that the VTAVgat→LH projection participates in NREM sleep induction. We therefore placed optical fibers into the LH of VTAVgat-ChR2-EYFP mice to stimulate the terminals of VTAVgat neurons (Fig. 8a). Opto-activating VTAVgat fibers in the LH strongly and consistently initiated wake to NREM sleep transitions (Fig. 8a); Chronic opto-stimulation for 3 hours increased NREM sleep and reduced wakefulness and REM sleep (Fig. 8b). Moreover, activation could also maintain NREM sleep, with much longer episode duration but without affecting REM sleep duration (Fig. 8c), indicating that the VTAVgat→LH projection is sufficient to promote and maintain NREM sleep. However, the EEG power did not differ (Supplementary Fig. 14). Because cFOS expression is highly elevated in the DG when we inhibited VTAVgat neurons (Supplementary Fig. 13c), we also opto-stimulated the VTAVgat→DG projection, but we did not observe any effects on sleep or wakefulness (Supplementary Fig. 15a, b). The above results imply that VTAVgat neurons limit wakefulness by both inhibiting locally VTA glutamatergic and dopaminergic neurons, but also via projection targets (orexin neurons) in the lateral hypothalamus.
Fig. 8. VTAVgat neurons inhibit wakefulness in part via projections to the lateral hypothalamus.
(a, b) To functionally test the VTAVgat→LH projections, an optical fiber was placed into the LH area of VTAVgat-ChR2-EYFP mice. (a) Mice were given 120 s of opto-stimulation (20 Hz) during their active period (during the “lights off” period) and the percentage and time of wake and NREM sleep were scored (control: n=7 mice; 16 trials; ChR2: n=7 mice; 16 trials) the sem; (b) VTAVgat-ChR2-EYFP mice (control: n=6 mice; ChR2: n=5 mice) were given 3 hours of opto-stimulation during the active period (“lights-off” period) and the percentage and time of wake, NREM and REM sleep were scored.
(c) Mice were given 5 min of opto-stimulation when NREM sleep occurred (control: n=5 mice; 5 trials; ChR2: n=5 mice; 5 trials). The duration of NREM and REM sleep was scored.
**p<0.01, ***p<0.001, ****p<0.0001, for a, two-sided mann-whitney u test. For b and c, two-sided unpaired t-test. All error bars represent the SEM. Shaded regions represent SEM. For detailed statistics information, see Supplementary Table1.
Discussion
Our search for novel circuits that promote wakefulness identified wake- and REM sleep-active glutamatergic/NOS1 neurons in the VTA (see Supplementary Fig. 16 for schematic summary). By contrast, we found that VTA GABAergic neurons, when artificially activated, produce a profound sedative state, but surprisingly these neurons, like VTADA and VTAVglut2 cells, are selectively wake- and REM-active during normal sleep5. Because of this mismatch between physiological activity and the outcome of artificial activation, we speculate that VTAVgat neurons do not physiologically promote natural NREM sleep, but instead restrain wakefulness. This speculation is, in our view, supported by the results of lesioning the VTAVgat neurons (see below). Alternatively, there could be rare VTA GABAergic neurons, not detected by fiber photometry or in vivo Ca2+ imaging, that are NREM sleep-active. Such a cell type might actively induce NREM sleep. At the moment there is no direct evidence for either idea, limiting wakefulness or rare NREM-active GABA cells. There are certainly subtypes of GABA neuron in the VTA e.g. Pv- and Som-expressing cells, but we found that activating these also induced NREM sleep, although each subtype activated individually does not give the full effect obtained with activating the complete set of VTAVgat neurons. Future work needs to clarify how the subtypes of VTAVgat neurons interact to influence sleep and wakefulness.
When VTAVgat neurons are lesioned this causes permanent sleep loss that persists for months (Supplementary Fig. 11b). It will be interesting to identify metabolic changes produced by this long-term loss of sleep. The effects of the VTAVgat and VTAVglut2 lesions, i.e. more and less wakefulness respectively, manifest selectively during the “lights-off”/active phase of the mice (Fig. 2b, Fig. 6a and Supplementary Fig. 11). This fits with multiunit recordings from mouse VTA neurons, where most cells are under circadian control and fire more during “lights off”39.
Loss of VTAVglut2 neurons doubles the number of NREM sleep episodes, and so fragments wakefulness. Nevertheless, the effects of the VTAVgat lesions on wakefulness (Fig. 6a) are greater than those of lesioning VTAVglut2 cells (Fig. 2b). It is difficult to predict the effects of ablations. For example, histamine neurons, cholinergic neurons and noradrenergic neurons can be triply lesioned without influencing the baseline amounts of sleep-wake40, yet the acute activation or inhibition of these cell groups induces larges changes in vigilance state1. We speculate that VTAVgat neurons are strategically important as they influence diverse targets including VTAVglut2, VTADA and LH-orexin neurons. Hence the loss of VTAVgat neurons produces large effects. The persistence of this phenotype (chronic wakefulness) post-lesion, suggests no compensation is possible, and perhaps emphasizes the importance of VTAVgat neurons in regulating vigilance state.
We propose that VTAVgat neurons limit wakefulness both via projections to arousal-promoting orexin neurons in the LH (Supplementary Fig. 16), and by inhibiting glutamate and dopamine neurons locally in the VTA. As expected41, we found some midline VTAVgat neurons co-release glutamate, although the ratio of optically-evoked IPSCs to EPSCs was about 5:1 in favor of inhibition. In the LH, many (60%) of the VTAVgat targets are orexinergic neurons, but VTAVgat terminals could also inhibit wake-promoting GABAergic projection neurons as well11,15.
When VTAVgat neurons are chemogenetically excited the duration of the evoked NREM sleep is remarkable, lasting some 6 hours, similar to sedation. Thus, VTAVgat neurons could, in principle, be a target for novel sedatives that promote a sustained NREM-like sleep (Supplementary Fig. 16). It is surprising that this strong sedative effect arising from activating VTAVgat neurons has not been noticed. Previous work emphasized that activating VTA GABAergic neurons effects motivational states by inhibiting dopamine neurons in the VTA42, or by inhibiting cholinergic neurons in the NAc31. But in none of these experiments was the EEG recorded, so it is unclear how to interpret the behaviors, especially when decreases in a particular behavior were reported, which could, in fact, be caused by sedation.
Inputs or modulators that physiologically excite VTAVgat neurons will tend to decrease wakefulness. The lateral habenula (LHb) is one such nucleus that sends many excitatory glutamatergic projections to GABAergic neurons in the VTA43. Thus, strong activation of this LHb pathway would be predicted to induce NREM-like sleep. We have found that the anesthetic propofol requires activation of glutamatergic neurons of the lateral habenula to induce sedation i.e. slow wave (delta) power in the EEG and motor immobility44. Downstream of the LHb, this mechanism could include the activation of the VTAVgat neurons, including those GABAergic cells in the rostromedial tegmental nucleus at the posterior end of the VTA45.
As with VTAVgat neurons, the extreme wakefulness produced by stimulating VTAVglut2/Nos1 neurons may not have been noticed before because no EEG analysis was performed. We found that the midline VTAVglut2/Nos1 neurons promote wakefulness, in part, through the NAc and in part through the LH. The VTAVglut2/Nos1 terminals in the LH could excite GABAergic projection neurons that in turn promote wakefulness11,15, as well as exciting orexin neurons. On the other hand, because lesioning the NAc increases wakefulness46,47, this implies NAc GABAergic projection neurons limit wakefulness, or are actively inducing NREM sleep. If terminals of VTADA neurons are stimulated in the NAc, wakefulness is produced5. This is similar to stimulating the VTAVglut2/Nos1 terminals. However, since the wake-promoting effect of VTAVglut2/Nos1 neurons is not blocked by dopamine receptor antagonists, it could be that the wake-promoting dopamine terminals do so by promoting glutamate release in the NAc. Similar to our findings, the paraventricular thalamus also promotes wakefulness by sending glutamatergic projections to the NAc17. It could be that glutamate inputs local GABA neurons in the NAc, which then inhibit the NREM sleep-promoting GABAergic projection neurons. In any case, the NAc is probably a core part of the wake-promoting circuitry.
Similar to GABA and glutamate, NOS1 associates with neurons regulating both wakefulness and sleep21,48. A recent report found Vglut2/Nos1 projection neurons in the supramammillary nucleus promote wakefulness when chemogenetically activated14. Our CNO injections to activate mammillary Vglut2 neurons did not increase wakefulness (Fig. 1j). The reason for the difference is unclear, but in principle there could be a continuous population of Vglut2/Nos1 cells from the VTA through to the supramammillary nucleus.
What is the significance of the VTAVglut2/Nos1 or the VTAVgat neurons being REM active? Activating the VTAVglut2/Nos1 or the VTAVgat neurons did not produce REM sleep, and activating VTAVgat neurons during NREM sleep did not alter REM sleep duration (Fig. 8c). Therefore, the VTAVglut2 and VTAVgat neurons respond to the primary REM sleep-inducing circuitry, but do not induce or maintain REM sleep.
In summary, our findings on VTAVglut2/Nos1 and VTAVgat neurons, and other recent discoveries on dopamine VTA neurons5 identify the VTA as a critical center regulating wakefulness. The VTA is exceptionally well-connected, receiving glutamate and GABA inputs from nearly all brain areas42,43,49,50, making it well suited to serve as an integrator of vigilance state. This should be considered when designing experiments to look at the role of the VTA in reward, goal-directed and social behaviours.
Methods
Mice
All experiments were performed in accordance with the UK Home Office Animal Procedures Act (1986); all procedures were approved by the Imperial College Ethical Review Committee and the Ethics Committee for Animal Experimentation of Xijing Hospital, Xi’an, and was conducted according to the Guidelines for Animal Experimentation of Chinese Council institutes. The following strains of mice were used: Vglut2-ires-Cre: Slc17a6tm2(cre)Lowl/J, kindly provided by B.B. Lowell, JAX stock 01696351; Vgat-ires-Cre: Slc32a1tm2(cre)Lowl/J kindly provided by B.B. Lowell, JAX stock 01696251; Nos1-ires-Cretm1(cre)Mgmj/J, JAX stock 01752652; Som-ires-Cre: Ssttm2.1(cre)Zjh/J, JAX stock 01304453; and Pv-Cre B6;129P2-Pvalbtm1(cre)Arbr/J, JAX stock 00806954. All mice used in the experiments were male and aged 8 weeks at the start of the stereotaxic injections and experiments. Mice were maintained on a 12 hr:12 hr light:dark cycle at constant temp and humidity with ad libitum food and water.
AAV transgene plasmids
pAAV-hSyn-DIO-hM3Dq-mCherry and pAAV-hSyn-DIO-hM4Di-mCherry were gifts from Bryan L. Roth (Addgene plasmids 44361 and 44362)55; pAAV-CBA-DIO-GFP was a gift from Edward Boyden (Addgene plasmid 28304); pAAV-EF1α-DIO-taCASP3-TEV was a gift from Nirao Shah (Addgene plasmid 45580)56. pAAV-EF1α-DIO-hChR2(H314R)-EYFP was a gift from Karl Deisseroth (Addgene plasmid 20298). AAV2/9-CAG-DIO-GCaMP6f was a gift from HanBio Co., Ltd (Shanghai, China), and packaged by BrainVTA (Wuhan, China).
To create pAAV-hSyn-DIO-GCaMP6s, we used the GCaMP6s reading frame from pGP-CMV-GCaMP6s-EGFP (gift of Douglas Kim57, Addgene plasmid 40753). The plasmid pAAV-hSyn-DIO-hM3Dq-mCherry (see above) was digested with AscI and NheI, to remove the hM3Dq-mCherry reading frame but keeping both sets of loxP sites to give the pAAV-DIO backbone. The modified GCaMP6s reading frame (in-frame-mutated to remove AscI and NheI sites) was amplified by PCR from pAAV-hsyn-GCaMP6s, digested with AscI and NheI, and then ligated into the pAAV-DIO backbone to give pAAV-hSyn-DIO-GCaMP6s.
Adeno-associated virus (AAV) preparation and stereotaxic injections
To produce AAV (capsid serotype 1/2), the adenovirus helper plasmid pFΔ6, capsid-plasmids pH21 (AAV1) and pRVI (AAV2), and the relevant pAAV transgene plasmid (see section “AAV transgene plasmids”) were co-transfected into HEK293 cells and the subsequent AAV particles were harvested on heparin columns, as described previously58,59.
To produce retro-AAV-DIO-rc(Chronos-GFP), we used the rAAV2 packaging plasmid, a gift from Alla Karpova and David Schaffer60 (Addgene plasmid 81070), and the helper plasmid pFΔ6, together with pAAV-EF1α-DIO-rc[Chronos-GFP], a gift from Edward Boyden61 (Addgene plasmid 62725). These plasmids were co-transfected into HEK293 cells. To purify the retro-AAV we used the AAVpro Purification Kit (all Serotypes) (Takara-Clontech, Cat#: 6666).
Surgery
Mice were anesthetized 2% isoflurane in oxygen by inhalation and received buprenorphine injection and placed on a stereotaxic frame (Angle Two, Leica Microsystems, Milton Keynes, Buckinghamshire, UK). The AAV was injected through a stainless steel 33-gauge/15mm/PST3 internal cannula (Hamilton) attached to a 10 µl Hamilton syringe, at a rate of 0.1 µl min−1.
The injection co-ordinates and volume were (PH/MB)L: ML (±0.85 mm), AP (-2.7 mm), DV (-5.05 mm), 1.5 ul + 1.5 ul;
(PH/MB)S: ML (±0.85 mm), AP (-2.7 mm), DV (-5.05 mm), 1 ul + 1 ul;
LH: ML (±1.00 mm), AP (-1.56 mm), DV (-5.20 mm), 100 nl + 100 nl;
M: ML (±0.86 mm), AP (-2.7 mm), DV (-5.04 mm), 100 nl + 100 nl;
IPN: ML (-0.02mm), AP (-3.52 mm), DV (-4.67 mm), 20 nl + 20 nl;
VTA: ML (±0.35 mm), AP (-3.3 mm), DV (-4.25 mm), 50 nl + 50 nl;
PBP of the VTA: ML (±0.54 mm), AP (-3.52 mm), DV (-4.29 mm), 20 nl + 20 nl
After injection, the cannula was left at the injection site for at 5 min and then slowly pulled out. After injections, mice were implanted with 3 gold-plated miniature screw electrodes (–1.5 mm Bregma, +1.5 mm midline; +1.5 mm Bregma, –1.5 mm midline;–1 mm Lambda, 0 mm midline – reference electrode) with two EMG wire (AS634, Cooner Wire, CA). The EMG electrodes were inserted between the neck musculature. The EEG-EMG device was affixed to the skull with Orthodontic Resin power and Orthodontic resin liquid (Tocdental, UK).
For the telemetry EEG and EMG surgery, a TL11M2-F20-EET device (Data Science International, USA) was implanted in the abdominal cavity in mice, and four wires of which were subcutaneously lead to mouse’s neck by a guiding cannula. Mice were then fixed onto the stereotaxic apparatus in a prone position. A pair of wires was imbedded into the bilateral parietal skulls (AP 0.2 mm, ML 1.5 mm, DV -0.1 mm; AP - 1.7 mm, ML -0.2 mm, DV -0.1 mm) by the dental cement to record EEG. The other pair of wires was implanted in the neck muscles to monitor the EMG.
For fiber optogenetic experiments, mice received surgical implantation of a monofiberoptic cannula (200 µm; Doric Lenses, Inc., Quebec, Canada) after virus injection, above the VTA (AP −3.3 mm; ML 0.13 mm; DV −3.93 mm), NAc (AP 1.1 mm; ML 0.6 mm; DV −4.2 mm), DG (AP -1.94 mm; ML 1 mm; DV -2 mm) and LH (AP-1.4 mm; ML 1.0 mm; DV -5.16 mm). For fiber photometry experiments, mice received surgical implantation of a monofiberoptic cannula (200 µm, respectively; Doric Lenses, Inc., Quebec, Canada), after virus injection above the VTA (AP −3.3 mm; ML 0.13 mm; DV −3.93 mm) to target VTAVglut2 neurons and (AP −3.3 mm; ML 0.35 mm; DV −4.15 mm) to target VTAVgat neurons and EEG/EMG implants.
The placements of the fibers from all the experiments are shown in Supplementary Fig. 17.
For the microendoscopic calcium imaging, Vgat-ires-Cre mice, which had already been injected with AAV2/9-CAG-DIO-GCaMP6f into the VTA were allowed to recovery from surgery for three weeks. They were then re-anesthetized with isoflurane and had TL11M2-F20-EET telemetry devices fitted (see other protocols in the Methods section). The skull was coated with UV curable resin. After 20 s of exposure to ultraviolet light, a protective coating was formed on the skull. A small hole (1 mm diameter) was drilled on the skull (AP -3.45 mm, ML 1.25 mm). To avoid bleeding or drying of the meninges and brain tissue, saline (0.9% NaCl) was superfused constantly. The tissue drill with a PCB bit (500 μm diameter) was fixed on the stereotaxic frame carefully to remove the brain tissue over the target area. By using a micromanipulator (MP285, Sutter, USA), a GRIN lens (diameter 500 μm, long 7.6 mm) was slowly implanted into the VTA (AP -3.3 mm, ML 0.35 mm, DV -4.15 mm). During the insertion process, the lens stayed still for 10 minutes for every 1 mm insertion. The lens was then secured by dental cement and the part left outside the skull was covered by tissue glue (Kwik-Sil). One week later, the tissue glue was removed. The camera (nVista, Inscopix) was connected with the lens to check the fluorescence signals, and then the camera base (baseplate) was secured to the skull with dental cement. After surgery, mice were allowed to recover for at least 2 weeks before experiments. The positions of the GRIN lens placements for all mice are shown in Supplementary Fig. 17f.
Behavioural protocols and drug treatments
For chemogenetic experiments, clozapine-N-oxide (C0832, Sigma-Aldrich, dissolved in saline, 1 mg/kg) or saline was injected i.p. and the vigilance states recorded. Mice were split into random groups that received either saline or CNO injection. To test for sleep promoting effects, we injected saline or CNO during the “lights off” period when the mice were likely to be most active; and to test for wake-promoting effects, we injected saline or CNO at the start of the “lights on” period when the mice had their maximum sleep drive. For the PH/MBVglut2-hM3Dq mice, LHVglut2-hM3Dq mice, MVglut2-hM3Dq mice, IPNVglut2-hM3Dq mice, VTAVglut2-hM3Dq mice, VTANos1-hM3Dq mice, VTAVgat-hM4Di mice, CNO or saline were injected at the start of the “lights on” sleep phase. For the VTANos1-hM4Di mice, VTAVgat-hM3Dq mice, VTAPv-hM3Dq mice, VTASom-hM3Dq mice, VTA-PBPNos1/Vgat-hM3Dq mice, CNO or saline were injected i.p. during the “lights off” active phase. For the optogenetic experiments, VTAVglut2-ChR2-EYFP mice or VTAVglut2-GFP were opto-stimulated (20 Hz, 1 min) during the “lights on” phase; VTAVglut2-ChR2-EYFP→LH or VTAVglut2-ChR2-EYFP→NAc mice were opto-stimulated (20 Hz, 2 min or 5 Hz, 1 min) during the “lights on” sleep phase. For chronic optogenetic stimulation: VTAVglut2-ChR2-EYFP→LH or VTAVglut2-ChR2-EYFP→NAc mice or NAcVglut2-Chronos-GFP→VTA mice were opto-stimulated (20 Hz, 2 s with 58 s interval) at the start of “lights on” sleep phase for 3 hours;
VTAVgat-ChR2-EYFP→LH mice were stimulated (20 Hz, 2 min) during “lights off” active phase. For chronic optogenetic stimulation: VTAVgat-ChR2-EYFP→LH mice were opto-stimulated (20 Hz, 2 s with 58 s interval) at the start of “lights on” sleep phase for 3 hours;
To examine the maintenance of NREM sleep, stimulation were performed during “lights on” sleep phase and EEG/EMG tracing were observed in a real-time window. Laser was turned on for 5 min when NREM sleep occurred.
Note: in all the Ca2+ photometry experiments we used GCaMP6s, and for the in vivo microscopy experiments for Ca2+ imaging we used GCaMP6f. For the photometry experiments, the Ca2+ signal of VTAVglut2-GCaMP6 or VTAVgat-GCaMP6 mice were measured for 2 to 6 hours during both the “lights off” wake phase and “lights on” sleep phase. To challenge the mice with novel objects or female, novel objects or female mice were put in the home cage during the “lights off” wake phase and the Ca2+ signal of the VTAVglut2-GCaMP6 mice was measured.
For the chemogenetic pharmacological experiments, VTAVglut2-hM3Dq, VTANos1-hM3Dq or VTAVgat-hM4Di mice were injected (i.p.) with dopamine receptor D1 (SCH23390 0.03 mg/kg) and D2/3 receptor antagonists (raclopride 2 mg/kg) injection and 30 min after the antagonists’ injection, the mice were received a saline or CNO (1 mg/kg) i.p. injection, as previously reported8. VTAVgat-hM3Dq mice, gabazine (0.001 µg) or saline (300 nl) was infused through a gilded cannula according to a previous study62. 10 min after infusion, the mice were received saline or CNO (1 mg/kg) by i.p. injection.
Locomotor activity
The locomotor activity was detected in an activity test chamber (Med Associates, Inc) with ANY-maze video tracking system. We injected saline or CNO (1 mg/kg) i.p. at the start of the “lights on” period when the mice had their maximum sleep drive, and we performed the locomotion experiment 30 min after injection. The behavior was recorded by a video tracking system (ANY-maze) using a camera (FUJIFILM co.) and measured by ANY-maze software (Stoelting Co. US.).
EEG analysis, fiber photometry, microendoscopic calcium imaging, and sleep-wake behavior
EEG and EMG
EEG and EMG signals were recorded using Neurologger 2A devices63 or using TL11M2-F20-EET telemetry devices and dataquest ART (version 4.33). NREM sleep, REM sleep and wake states were first automatically classified using a sleep analysis software Spike2 or NeuroScore and then manually scored.
For the fiber photometry27, a Grass SD9 stimulator was used to control a 473-nm Diode-pumped solid state (DPSS) blue laser with fiber coupler (Shanghai Laser & Optics century Co., Shanghai, China). The laser light was passed through a single source fluorescence cube (FMC_GFP_FC, Doric Lenses, Quebec, Canada) through an optical fiber patch cord (Ø 200 µm, 0.22 NA, Doric Lenses). From the filter cube, a multimodal optical patch cord (Ø 200 µm, 0.37 NA, Doric Lenses) was connected to the mouse chronically implanted fiber (Ø 200 µm, 0.37 NA) with a ceramic split mating sleeves ferrules (Thorlabs, Newton, New Jersey). The GCaMP6 output was then filtered at 500-550 nm using a second dichroic in the fluorescence cube and converted to voltage by an amplified photodiode (APD-FC, Doric Lenses). The photodiode output was amplified by a lock-in amplifier (SR810, Stanford Research Systems, California, USA), also used to drive the laser at 125 Hz with an average power of 80 µW at the fiber tip. The signal was then digitized using a CED 1401 Micro box (Cambridge Electronic Design, Cambridge, UK) and recorded at 1 kHz using Spike2 software (Cambridge Electronic Design, Cambridge, UK).
Photometry
The photometry signal was matched with the EEG and EMG recordings. For each experiment, the photometry signal F was converted to ΔF/F by ΔF/F(t)=(F(t)-median (F))/median (F)5. In some recordings, we observed a decay of photometry signal at the beginning of the recordings. All the sessions were selected after the photometry signal became stable. For the sleep–wake analysis, we performed the recording 3-4 sessions per mouse, each 1-6 hours long and 1 session for 8 hours. To analyze vigilance states for wake, NREM and REM sleep, we selected the all the sessions that mice had all three states5, and we calculated the ΔF/F photometry ratio during the contiguous three vigilance states. To analyse the transitions for vigilance states, we selected one randomly chosen session per mouse.
For the micorendoscopic Ca2+ imaging64, the signal was recorded with the nVista HD system (Inscopix). We analyzed the Ca2+ imaging data using ImageJ plug-ins and custom MATLAB script. Video acquisitions were corrected for movement artifacts using TurboReg. Mosaic (Inscopix) was used to analyse the data using independent component analyses (PCA-ICA) as described in previous studies65,66. The ΔF/F ratio was calculated as ΔF/F(t)=(F(t)-median (F))/median (F). Ca2+ traces and ΔF/F were matched to the EEG/EMG, which was simultaneously recorded with the Ca2+ imaging data.
Immunohistochemistry
Mice were transcardially perfused with 4% paraformaldehyde (Thermo scientific) in phosphate buffered saline (Sigma). Brains were removed and left in 30% sucrose/PBS. 40 or 60-μm-thick coronal sections were cut using a Leica VT1000S vibratome. Free-floating sections were washed in PBS three times for 5 min, permeabilized in PBS plus 0.4% Triton X-100 for 30 min, blocked by incubation in PBS plus 5% normal goat serum (NGS) (Vector), 0.2% Triton X-100 for 1 hour.
Sections were incubated with primary antibody diluted in PBS plus 2% NGS overnight at 4°C in a shaker. Incubated slices were washed three times in PBS for 10 min, and incubated for 2 hours with secondary antibody (Molecular Probes) in PBS and subsequently washed 4 times in PBS for 10 min (all at room temperature).
Primary antibodies used: rabbit polyclonal cFOS (1:4000, Santa Cruz Biotechnology, UK); rat monoclonal mCherry (1:2000, ThermoFisher); rabbit polyclonal GFP (1:1000, ThermoFisher); mouse monoclonal TH (1:2000, Sigma); mouse monoclonal NOS1 (1:200, Santa Cruz, UK); mouse monoclonal NOS1 (1:200, Sigma); rat monoclonal somatostatin (1:1000, Merck); mouse monoclonal parvalbumin (1:1000, Merck); mouse monoclonal Orexin-A (1:200, Santa Cruz, UK). Secondary antibodies were Alexa Fluor 488 goat anti-rabbit, Alexa Fluor 488 goat anti-mouse, Alexa Fluor 594 goat anti-rabbit, Alexa Fluor 594 goat anti-mouse, Alexa Fluor 594 goat anti-rat (1:1000, Invitrogen Molecular Probes, UK).
Slices were mounted on slides, embedded in Mowiol (with DAPI), cover-slipped, and analyzed using an upright fluorescent microscope (Nikon Eclipse 80i, Nikon Corporation, JAPAN) or a Zeiss LSM 510 inverted confocal microscope or a Leica SP5 MP confocal microscope (Facility for Imaging by Light Microscopy, FILM, Imperial College London). Images were acquired using Z-scan.
Acute brain slice electrophysiology and single-cell RT-PCR from the midline VTA area of VTAVgat-ChR2-EYFP mice
Slice preparation
VTAVgat-ChR2-EYFP mice were killed by cervical dislocation. Following decapitation, the brains were quickly removed and placed into cold oxygenated N-Methyl-D-glucamine (NMDG) solution (in mM: NMDG 93, HCl 93, KCl 2.5, NaH2PO4 1.2, NaHCO3 30, HEPES 20, glucose 25, sodium ascorbate 5, Thiourea 2, sodium pyruvate 3, MgSO4 10, CaCl2 0.5). Coronal brain slices (220-μm thickness) encompassing the midline VTA were obtained using a vibratome (Vibrating Microtome 7000smz-2; Campden Instruments LTD, UK). Slices were kept in NMDG solution at 33°C for 15min with constant oxygenation, and transferred to fully oxygenated standard aCSF (in mM: NaCl 120, KCl 3.5, NaH2PO4 1.25, NaHCO3 25, glucose 10, MgCl2 1, CaCl2 2) and were maintained in a chamber that was gently and continuously aerated with carbogen gas for at least 90 min at room temperature (20–22 °C) before use for electrophysiology.
Electrophysiological recording from midline VTA neurons innervated by VTAVgat neurons in VTAVgat-ChR2-EYFP mice
Slices were transferred to a submersion recording chamber and were continuously perfused at a rate of 4-5 ml/ min with fully oxygenated aCSF at room temperature. For whole-cell recording, patching pipettes at 4-6 MΩ were pulled from autoclaved borosilicate glass capillaries (1.5mm OD, 0.86mm ID, Harvard Apparatus, #GC150F-10) and filled with RNase-free intracellular solution containing (in mM): 140 K-gluconate, 5 NaCl, 10 HEPES, 0.1 EGTA, 2 MgCl2, 2 Mg-ATP, and 0.3 Na-GTP (pH 7.35, osmolality 285 mOsm) or 125 KCl, 20 NaCl, 10 HEPES, 1 EGTA, 1 CaCl2, 1 MgCl2, 2 Mg-ATP and 0.5 Na-GTP (pH 7.35, osmolality 285 mOsm). 0.1% Neurobiotin was included in the intracellular solutions to identify the cell position and morphology following recording. Recordings were performed in current clamp or voltage clamp mode using a Multiclamp 700B amplifier (Molecular Devices, CA). Access and input resistances were monitored throughout the experiments using a 5-mV voltage step. The access resistance was typically <20 MΩ, and results were discarded if resistance changed by more than 20%. Membrane capacitance (Cm) was measured under voltage clamp at -50 mV using a hyperpolarizing 10 mV, 250 ms step. Cm was measured from the change in membrane charge taken from the integrated capacity transients (pClamp, Molecular Devices).
Non YPF+ neurons (presumed non VTAVgat neurons) were visually identified and randomly selected. To maximize RNA recovery, the internal solution in the patch pipette was limited up to 1 µl. A blue light (470 nM) was delivered by TTL-control LED to the entire field through the objective. After the stable voltage clamp was achieved, a single 5 ms or 25 Hz (5 ms) were given at the 30 s and 60 s inter-sweep interval, subsequently. The light intensity was adjusted according to the magnitude of its response.
At the end of each recording, cytoplasm was aspirated into the patch pipette, and expelled into a PCR tube contained lysate buffer. The single cell RT-PCR assays were performed using the Single-Cell to-CT Kit (Ambion). The content of the neuron was aspirated into the recording pipette and expelled into cell lysis/DNase I solution. Reverse transcription and cDNA pre-amplification were performed according to the kit protocol. qPCR was performed using the TaqMan Gene Expression Assay system (Applied Biosystems machine, Foster City, USA). The mouse TaqMan assay probes were designed by, and purchased from Invitrogen (Thermofisher): m18srRNA, Mm03928990_g1; mSlc17a6 (Vglut2) Mm00499876_m1; mSlc6a3 (dat) Mm00438388_m1; mTh Mm00447557_m1; mGad1: Mm04207432_g1; mSlc32a1 (Vgat) Mm00494138_m1; The single cell gene expression matrix was made in Origin.
Quantification and statistics
All statistical tests were run in “Origin 2015” (Origin Lab). The individual tests we used are given in the figure legends and the details are supplied in Supplementary Table 1. All data are given as mean ± SEM unless otherwise stated in the figure legends. No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications21,44. The data met the assumptions of the statistical tests used. Before using any given statistical test we formally tested for normality and equal variances. When we found the data were non-normal we used non-parametric tests (details in the relevant figure legends and in in Supplementary Table 1). All t-tests were two-sided.
We excluded mice where it was subsequently found that the placement of the opto-fibers was misplaced or that there was no AAV transgene expression or when this expression was in the wrong place. Mice were assigned randomly to the experimental and control groups. When possible, experimental treatments were also randomized. When mice were given drugs verses saline, for example, they received the drug or saline in random order. All experimental data analysis was blinded, including cFOS counting, the analysis of EEG data and animal behaviour that was scored from videos.
Supplementary Material
Acknowledgements
We thank M. Ungless (Faculty of Medicine, Imperial College London) for comments on the manuscript. Our work was supported by the Wellcome Trust (107839/Z/15/Z, N.P.F. and 107841/Z/15/Z, W.W); the UK Dementia Research Institute (WW and NPF), and the Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (Grant No. 81620108012, H.D. and N.P.F.); the China Scholarship Council (YM), a Rubicon Fellowship (019.161LW.010) from the Netherlands Organization for Scientific Research (WB), an Imperial College Schrödinger Scholarship (G.M.), and an Imperial College Junior Research Fellowship (JJH). DB and JJH were also supported by The Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001055), the Medical Research Council (FC001055), and the Wellcome Trust (FC001055). The Facility for Imaging by Light Microscopy (FILM) at Imperial College London is in part supported by funding from the Wellcome Trust (grant 104931/Z/14/Z) and BBSRC (grant BB/L015129/1).
Footnotes
Reporting Summary. Further information on research design is available in the Life Sciences Reporting Summary linked to this article.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Author contributions
N.P.F. and W.W. conceived, and with X.Y. and H.D. designed the experiments, X.Y., W.L., Y.M., K.T., J.J.H., E.C.H., W.B., G.M., D.W., L.L., J.G., M.C., Y.L., R.Y., D.B. and Q. Y. performed the experiments and/or data analysis, A.L.V. provided the Neurologgers, N.P.F. and W.W. contributed to the data analysis and with H.D. supervised the project. N.P.F., X.Y. and W.W. wrote the paper.
Competing interests
The authors declare no competing interests.
References
- 1.Scammell TE, Arrigoni E, Lipton JO. Neural Circuitry of Wakefulness and Sleep. Neuron. 2017;93:747–765. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber F, Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538:51–59. doi: 10.1038/nature19773. [DOI] [PubMed] [Google Scholar]
- 3.Saper CB, Fuller PM. Wake-sleep circuitry: an overview. Curr Opin Neurobiol. 2017;44:186–192. doi: 10.1016/j.conb.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gent TC, Bassetti C, Adamantidis AR. Sleep-wake control and the thalamus. Curr Opin Neurobiol. 2018;52:188–197. doi: 10.1016/j.conb.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci. 2016;19:1356–1366. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X, et al. Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron. 2015;87:164–178. doi: 10.1016/j.neuron.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho JR, et al. Dorsal Raphe Dopamine Neurons Modulate Arousal and Promote Wakefulness by Salient Stimuli. Neuron. 2017;94:1205–1219 e1208. doi: 10.1016/j.neuron.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Oishi Y, et al. Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D2-like receptors in mice. Brain Struct Funct. 2017;222:2907–2915. doi: 10.1007/s00429-017-1365-7. [DOI] [PubMed] [Google Scholar]
- 9.Kosse C, Schone C, Bracey E, Burdakov D. Orexin-driven GAD65 network of the lateral hypothalamus sets physical activity in mice. Proc Natl Acad Sci U S A. 2017;114:4525–4530. doi: 10.1073/pnas.1619700114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schone C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7:697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera CG, et al. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat Neurosci. 2016;19:290–298. doi: 10.1038/nn.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anaclet C, et al. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6 doi: 10.1038/ncomms9744. 8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18:1641–1647. doi: 10.1038/nn.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen NP, et al. Supramammillary glutamate neurons are a key node of the arousal system. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01004-6. 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venner A, Anaclet C, Broadhurst RY, Saper CB, Fuller PM. A Novel Population of Wake-Promoting GABAergic Neurons in the Ventral Lateral Hypothalamus. Curr Biol. 2016;26:2137–2143. doi: 10.1016/j.cub.2016.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gent TC, Bandarabadi M, Herrera CG, Adamantidis AR. Thalamic dual control of sleep and wakefulness. Nat Neurosci. 2018;21:974–984. doi: 10.1038/s41593-018-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren S, et al. The paraventricular thalamus is a critical thalamic area for wakefulness. Science. 2018;362:429–434. doi: 10.1126/science.aat2512. [DOI] [PubMed] [Google Scholar]
- 18.Weber F, et al. Regulation of REM and Non-REM Sleep by Periaqueductal GABAergic Neurons. Nat Commun. 2018;9 doi: 10.1038/s41467-017-02765-w. 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung S, et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature. 2017;545:477–481. doi: 10.1038/nature22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 21.Harding EC, et al. A neuronal hub binding sleep initiation and body cooling in response to a warm external stimulus. Current Biology. 2018;28:2263–2273. doi: 10.1016/j.cub.2018.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anaclet C, et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci. 2014;17:1217–1224. doi: 10.1038/nn.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uygun DS, et al. Bottom-Up versus Top-Down Induction of Sleep by Zolpidem Acting on Histaminergic and Neocortex Neurons. J Neurosci. 2016;36:11171–11184. doi: 10.1523/JNEUROSCI.3714-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- 25.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luscher C. The Emergence of a Circuit Model for Addiction. Annu Rev Neurosci. 2016;39:257–276. doi: 10.1146/annurev-neuro-070815-013920. [DOI] [PubMed] [Google Scholar]
- 27.Gunaydin LA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung LW, et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357:1406–1411. doi: 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor SR, et al. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol. 2014;522:3308–3334. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creed MC, Ntamati NR, Tan KR. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front Behav Neurosci. 2014;8:8. doi: 10.3389/fnbeh.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan KR, et al. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor NE, et al. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1614340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul EJ, et al. nNOS-expressing neurons in the ventral tegmental area and substantia nigra pars compacta. eNeuro. 2018 doi: 10.1523/ENEURO.0381-18.2018. 0381-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi J, et al. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat Neurosci. 2016;19:725–733. doi: 10.1038/nn.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez JL, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fifel K, Meijer JH, Deboer T. Circadian and Homeostatic Modulation of Multi-Unit Activity in Midbrain Dopaminergic Structures. Sci Rep. 2018;8:7765. doi: 10.1038/s41598-018-25770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–14048. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Root DH, et al. Single rodent mesohabenular axons release glutamate and GABA. Nat Neurosci. 2014;17:1543–1551. doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieh EH, et al. Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron. 2016;90:1286–1298. doi: 10.1016/j.neuron.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faget L, et al. Afferent Inputs to Neurotransmitter-Defined Cell Types in the Ventral Tegmental Area. Cell Rep. 2016;15:2796–2808. doi: 10.1016/j.celrep.2016.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gelegen C, et al. Excitatory Pathways from the Lateral Habenula Enable Propofol-Induced Sedation. Curr Biol. 2018;28:580–587 e585. doi: 10.1016/j.cub.2017.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur J Neurosci. 2010;31:499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu MH, et al. The role of nucleus accumbens core/shell in sleep-wake regulation and their involvement in modafinil-induced arousal. PLoS One. 2012;7:e45471. doi: 10.1371/journal.pone.0045471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morairty SR, et al. A role for cortical nNOS/NK1 neurons in coupling homeostatic sleep drive to EEG slow wave activity. Proc Natl Acad Sci U S A. 2013;110:20272–20277. doi: 10.1073/pnas.1314762110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beier KT, et al. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell. 2015;162:622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG., Jr Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med. 2012;18:820–823. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taniguchi H, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang CF, et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klugmann M, et al. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Murray AJ, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tervo DG, et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron. 2016;92:372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klapoetke NC, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jennings JH, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anisimov VN, et al. Reconstruction of vocal interactions in a group of small songbirds. Nat Methods. 2014;11:1135–1137. doi: 10.1038/nmeth.3114. [DOI] [PubMed] [Google Scholar]
- 64.Flusberg BA, et al. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat Methods. 2008;5:935–938. doi: 10.1038/nmeth.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Groessl F, et al. Dorsal tegmental dopamine neurons gate associative learning of fear. Nat Neurosci. 2018;21:952–962. doi: 10.1038/s41593-018-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen KS, et al. A Hypothalamic Switch for REM and Non-REM Sleep. Neuron. 2018;97:1168–1176 e1164. doi: 10.1016/j.neuron.2018.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.