Summary:
Adoptive cell transfer using autologous tumor infiltrating lymphocytes or lymphocytes transduced with antitumor T-cell receptor (TCR) is an effective therapy for patients with metastatic melanoma. A limiting factor in the effectiveness of this treatment is the apoptosis of the transferred cells when Interleukin-2 (IL-2) administration is withdrawn. In an attempt to improve persistence of the transferred lymphocytes, we cotransduced human peripheral blood lymphocytes with retroviruses encoding Bcl-2 or Bcl-xL, antiapoptotic genes of the BCL2 family, and the MART-1 melanoma tumor antigen-specific TCR, DMF5. Lymphocytes were cotransduced with 38% to 64% cotransduction efficiency, and exhibited a marked delay in apoptosis after IL-2 withdrawal. Cotransduction with Bcl-2 or Bcl-xL did not affect cytokine secretion or lytic ability of the DMF5-transduced lymphocytes. After 5 days of IL-2 withdrawal, cotransduced lymphocytes produced similar levels of IFN-γ per cell as DMF5-alone transduced lymphocytes in response to tumor cells. Cotransduction did not alter the phenotype of lymphocytes with respect to a panel of T-cell differentiation markers. In a mouse model of melanoma, adoptively transferred T cells transduced with Bcl-2 persisted better in vivo at the site of tumor, 13 and 21 days after adoptive transfer (P = 0.0064 and 0.041, respectively), with evidence of enrichment of the Bcl-2-transduced population over time (P <0.0001). Thus, by coexpressing Bcl-2 or Bcl-xL with a tumor-specific TCR, we have engineered a lymphocyte that resists apoptosis owing to IL-2 withdrawal without altering its tumor-specific function or phenotype, and thus may show improved antitumor effectiveness in vivo after cell transfer.
Keywords: immunotherapy, adoptive transfer, gene therapy, tumor immunology, melanoma, IL-2 withdrawal
Adoptive immunotherapy for cancer using either tumor-infiltrating lymphocytes (TIL) or peripheral blood lymphocytes (PBL) genetically modified to express an antitumor T-cell receptor (TCR) can mediate tumor regression in patients with metastatic melanoma. Administration of TIL after a lymphodepleting regimen mediates objective responses in 49% to 72% of patients,1 and early studies with gene-modified lymphocytes resulted in objective responses of 30%.2
Before reinfusion to the patient, cells are expanded to large numbers ex vivo (109 to 1010) by culturing with interleukin-2 (IL-2). IL-2 is a requisite lymphocyte growth factor and cells grown in IL-2 are dependent on its presence for survival. Withdrawal of IL-2 in vitro induces rapid apoptosis of lymphocytes, so IL-2 is administered to patients at the time of cell administration.3–5. However, owing to toxicities such as vascular leak syndrome, IL-2 therapy is limited to the first few days of therapy.6 Once adoptively transferred cells are no longer exposed to IL-2 in vivo they can undergo apoptosis.
In patients treated with TIL or gene-modified PBL, persistence of adoptively transferred lymphocytes correlates with antitumor response.2,7 In vivo apoptosis owing to IL-2 withdrawal may be a major obstacle to persistence, and, consequently, to the antitumor efficacy of adoptively transferred lymphocytes. Transducing tumor-specific lymphocyte clones with the antiapoptotic gene Bcl-2 can inhibit IL-2 withdrawal-mediated apoptosis without interfering with their function.8 Similarly, Bcl-xL has been shown to inhibit apoptosis owing to IL-2 withdrawal.9 Studies in primates have indicated that the persistence of effector T cells derived from a central memory component may be related to augmented expression of Bcl-2 and Bcl-xL and inhibition of apoptosis.10
Recent studies of adoptive immunotherapy have emphasized the use of cells retrovirally transduced with antitumor TCRs.11–13 Here, we show the feasibility of cotransducing PBL with the gene encoding anti-MART-1 melanoma tumor antigen TCR, DMF5, recently shown to mediate cancer regression in patients with metastatic melanoma,2 along with the gene encoding either Bcl-2 or Bcl-xL. Our findings indicate that the cotransduced lymphocytes maintain their full, specific effector function and phenotype while inhibiting apoptosis owing to IL-2 withdrawal. Furthermore, translation of this work in a mouse model shows that the addition of an antiapoptotic gene can lead to enhanced survival of lymphocytes at tumor sites in vivo.
MATERIALS AND METHODS
Cell Lines
All of the PBL used in this study were cryopreserved peripheral blood mononuclear cells (PBMC) obtained by leukapheresis of metastatic melanoma patients treated in approved clinical protocols at the Surgery Branch, National Cancer Institute, NIH, Bethesda, MD. T2 is a lympho-blastoid cell line deficient in TAP function, whose HLA class I proteins can be easily loaded with exogenous peptides.14 Melanoma lines 938mel, 888mel, 624mel, and 526mel were generated in the Surgery Branch from resected tumor lesions, as described earlier.15 The cell lines described above were maintained in RPMI1640 (Invitrogen Corp, Grand Isle, NY) supplemented with 10% heat-inactivated FBS (Gemini Bio-Products, West Sacramento, CA). Culture medium (CM) for established human T-lymphocyte lines was 50% RPMI1640, 50% AIM-V medium (Invitrogen Corp) supplemented with 0.05-mM mercap-toethanol, 300-IU/mL interleukin-2 (IL-2) (Chiron Corp. Emerville, CA) plus 10% human AB serum (Gemini Bio-Products). PG13 producer clone and 293GP cell lines were cultured in DMEM (Invitrogen Corp) with 10% heat inactivated FBS. Mouse T cells were cultured in RPMI1640+10% heat inactivated FBS supplemented with 0.05-mM β-mercaptoethanol, 1-mM sodium pyruvate (Invitrogen Corp), MEM nonessential amino acids (Invitrogen Corp), and 2-mM glutamine (Invitrogen Corp).
Construction of Retroviral Vectors
The retroviral vector backbone used in this study, pMSGV1, is a derivative of pMSGV [murine stem cell virus-based splice-gag vector] that uses a murine stem cell virus long terminal repeat.16 DMF5 TCR-α and DMF5 TCR-β cDNA, divided by a furin 2A cleavage segment, were introduced into pMSGV1.2
Human Bcl-2 cDNA after GFP cDNA and an internal ribosomal entry site (GFP.Bcl-2) were cloned into pMSGV from p-Bcl-2.8 Human Bcl-xL cDNA followed by internal ribosome entry site (IRES) and green fluorescent protein (GFP) (Bcl-xL.GFP) were cloned into the pMSGV from pMIG-Bcl-xL (Addgene plasmid 8790)17 as well. Cloning details can be provided upon request.
Production of Retroviral Supernatants
MSGV1.DMF5TCR (hereafter referred to as DMF5) retroviral supernatant was obtained from a stable PG13 packaging clone as described earlier.18
MSGV1.GFP.IRES.Bcl-2 and MSG1.Bcl-xL.IRES. GFP retroviral supernatants were produced transiently by cotransfection of the retroviral packaging cell line 293GP with 10 mg of vector DNA and 5 μg of envelope DNA (RD114) using Lipofectamine 2000 (Invitrogen Corp) transfection reagent and Opti-MEM Reduced-Serum Media (Invitrogen Corp). Transfections were carried out on poly-D-lysine-coated 10-cm plates (BD Biosciences, San Jose, CA). Media was changed to DMEM with 10% heat inactivated FBS after 6 hours, and viral supernatants were harvested at the 48 hours time point. These supernatants were diluted 1:1 with DMEM with 10% FCS, and then used to transduce PBL.
Retroviral Cotransduction
Cryopreserved PBL were thawed, washed, and resuspended at 2 × 106/mL in 20-mL CM with 50 ng/mL of anti-CD3 mAb (OKT3) and 300IU/mL of IL-2 (hereafter referred to as Stim1). The lymphocytes were then cultured for 48 hours before transduction. Before transduction, retroviral supernatants were spun (2000 × g for 2h at 32°C) onto 6-well plates pretreated with 20 μg/mL of RetroNectin (Takara Bio Inc, Shiga, Japan). For cotransduction wells, equal volumes of each retroviral supernatant were added. For the first transduction, 2 × 106 lymphocytes in 4 mLs of CM with 300 IU/mL IL-2 were added to 6-well plates preloaded with retroviral vector and spun for 10 minutes at 1500 RPM. After 24 hours culture at 37°C, PBL were transferred to a second set of 6-well plates preloaded with retroviral vector. Between 5 and 7 days after the second transduction (7 to 9 days after initial stimulation), the PBL cultures were analyzed for expression of GFP and TCR DMF5 by flow cytometry. Mock-transduced cells were taken through the same procedure as transduced cells, except that the retroviral supernatant was substituted with DMEM+10% FBS.
Growth and Survival of T Cells in the Presence and Absence of IL-2
At 6 to 10 days after the second transduction, transduced PBL were washed 3 times with PBS, resuspended in CM with or without 300-IU/mL IL-2 at 1.5 × 106/mL and plated in quadruplicate in a 24-well plate. Survival of cells after IL-2 withdrawal after a rapid expansion protocol (REP) was also evaluated. In brief, 10 days after the initial stimulation (Stim1), cells were expanded ex vivo in the presence of allogeneic feeders, 50-ng/mL OKT3, and 6000 IU/mL IL-2, as is done clinically.19,20 These cells were then tested for sensitivity to IL-2 withdrawal, as described previously.
Every 4 days, 1 mL of media was removed from the wells and fresh CM with or without IL-2 was added. Cultures with IL-2 were maintained at 0.5 to 3.0 × 106/mL. Viable cells were counted by trypan blue exclusion in duplicate wells at each time point and then one well was harvested at each time point for Fluorescence Activated Cell Sorting analysis and coculture assays. Cell growth and survival of each transduction condition was ennumerated by multiplying the percentage of transduced or cotransduced population by the total viable cell number at each time point. Averages and SEM are based on data from duplicate wells.
Cytokine Release Assays and Evaluation of Tumor-induced Apoptosis
Duplicate cocultures were set up at 105:105 (E:T) ratio cells in a final volume of 200-mL R10 medium in individual wells of 96-well plates, and incubated overnight at 37°C. Supernatants were tested for cytokine secretion by IFN-γ ELISA (Pierce Endogen). After collection of supernatants, cells were washed twice with PBS and resuspended in staining buffer containing fluorochrome-conjugated Abs for measurement of CD3 and MART-1 tetramer. After staining, cells were washed and resuspended in 100 μL of Annexin V binding buffer containing 5 μ each of Annexin V and 7AAD. After 15 minutes, 400 μL of Annexin V binding buffer was added and cells were analyzed by flow cytometry.
51Cr Release Assay
Cytolytic assays with melanoma cell line targets were carried out as described earlier.21 In brief, target cells were pulsed with 100 mCi (1Ci = 37GBq) 51Cr (GE Healthcare Life Sciences, Piscataway, NJ) for 2 hours at 37°C and washed. We plated 1 × 103 labeled target cells with effector cells at indicated ratios in a 96-well, U-bottomed plate for 4 hours at 37°C. The amount of 51Cr released was determined using a g-counter. Percentage of specific lysis was calculated from triplicate samples by using the following formula:
Intracellular Cytokine Induction
Cocultures were setup at 5 × 105:5 × 105 (E:T) ratio in a final volume of 1 mL, and incubated for 6 hours at 37°C in the presence of brefeldin A (GolgiPlug, BD Biosciences). PBL stimulated with PMA (2ng/mL) and ionomycin (1 μM) was used as a positive control. Cells were then washed and stained for surface antigens before being fixed and permeabilized (Cytofix/Cytoperm, BD Biosciences) for intracellular staining.
Flow Cytometry
MART-1-specific TCR DMF5 was stained with a tetrameric HLA-A2/MART-1:27–36(27L)-fluorochrome conjugate (iTAg MHC Tetramer, Beckman Coulter, Fullerton, CA) according to the manufacturer’s recommendations. Cell surface expression levels of human CD3, CD4, CD8, CD25, CD27, CD28, CD45RO, CD62L, and CCR7 (BD Biosciences) were measured by immunofluorescence using PE, PE-Cy7, APC, and APC-Cy7. Cell surface expression levels of mouse CD3, CD8, and Thy1.1 were measured by immunofluorescence using APC-Cy7, PE, and APC. Cells were stained in PBS (Invitrogen Corp) with 1% FBS. Intracellular staining for IFN-g (anti-IFN-γ APC, BD Biosciences) was done in a permeabilization and washing buffer (Perm/Wash, BD Biosciences). Staining for Annexin V (PE-conjugate, BD Biosciences) and 7-AAD (BD Biosciences) using Annexin V Binding Buffer (BD Biosciences) was conducted after cell surface staining and just before flow cytometric analysis. Annexin V-only and 7AAD-only staining controls were used.
Immunofluorescence was measured using a FACS-Canto II flow cytometer (BD Biosciences). A combination of forward angle light scatter, side angle light scatter, and propidium iodide staining was used to gate out dead cells. Quadrants were based on staining with appropriate isotype control antibodies.
Real-time Reverse Transcription-polymerase Chain Reaction (RT-PCR)
RNA was isolated from 4 × 106 cells using the RNeasy Mini Kit with QIAShredder (Qiagen, Germantown) and frozen at −80°C. Generation of cDNA by reverse transcription and RT-PCR were carried out using the commercially available Quantitative Real-Time PCR Human Apoptosis RT2 PCR Array Kit based on SYBR Green (SABiosciences, Frederick, MD) and a 7500 Fast RealTime PCR System (Applied Biosystems, Carlsbad, CA). The levels of gene expression were calculated relative to the housekeeping genes β2M, HPRT1, RPL13A, GAPDH, and ACTB, using the ΔCt method:
Mouse and Tumor Cells
The pmel-1 TCR transgenic mouse model has been described earlier.22 C57BL/6 mice and Thy1.1 + mice were obtained from The Jackson Laboratory and bred at the National Institutes of Health animal facility. B16F10, a gp100 + spontaneous murine melanoma, was maintained in DMEM with 10% heat inactivate FBS, 0.05-mM β-mercap- toethanol, 1-mM sodium pyruvate, and 2-mM glutamine.22
Stimulation and Transduction of Thy1.1+ pmel-1 T Cells
Seven days before adoptive cell transfer, CD8 + splenocytes from pmel-1 TCR transgenic mice expressing the Thy1.1 congenic marker were sorted by positive selection (Miltenyi Biotec, Auburn, CA). CD8 sorted cells were stimulated for 48 hours with 2-μg/mL plate-bound anti-CD3 (BD Biosciences), 1-μg/mL soluble anti-CD28 (BD Biosciences) and 30-IU/mL IL-2. After 48 hours, CD8 + enriched cells were transduced once with retroviral vector expressing GFP.Bcl-2 and GFP-alone, as described before. Transduction efficiency was evaluated 3 days later and also on the day of adoptive cell transfer (Day 0).
Adoptive Cell Transfer
In vivo mice studies were carried out according to the guidelines set by the NCI and NIH Animal Ethics Committee. C57BL/6 female mice at 6 to 8 weeks of age were injected with 5 × 105 B16 cells 10 to 12 days before adoptive cell transfer. On the day of cell transfer, mice were irradiated at 500 cGy and treated i.v. with 1 × 106 Thy1.1 + pmel-1 T cells transduced with GFP.Bcl-2 or GFP alone, as described previously. Concomitantly, mice were treated i.v. with recombinant vaccinia virus expressing human gp100 peptide 25 to 33 (hgp10025−33), followed by i.p. injections of 6 doses of IL-2 (300,000 IU/0.5mL/dose/mouse) at intervals of 8 to 12 hours for 3 days. Each treatment group included 5 mice. Serial, blinded tumor measurements were obtained and the products of perpendicular diameters were plotted ± SEM. Statistics for tumor treatment were calculated using Wilcoxon Rank Sum Test based on linear slopes of the tumor growth curves at each data point.
Evaluation of In Vivo Persistence of Adoptively Transferred T Cells
Tumors from 3 mice in treatment groups receiving vaccine, IL-2, and cells transduced with either GFP.Bcl-2 or GFP-alone were harvested at 3 time points. Single cell suspensions of tumors were made using 40-mm nylon cell strainer (BD Biosciences), and lymphocytes were further separated by density gradient centrifugation using Lympholyte M (Cedarlane Laboratories, Burlington, Canada). Tumor lymphocytes were counted and the percentage of Thy1.1 + GFP + lymphocytes (CD8 gate) was determined by flow cytometry. Student paired t test was used to determine P values for the frequency of lymphocytes expressing Thy1.1 + GFP + within tumor. A 2-way analysis of variance (ANOVA) was used to determine the P value for enrichment of GFP expression in Thy1.1+ lymphocytes within tumor over time.
RESULTS
Cotransduction of PBL With DMF5 and Bcl-2 or Bcl-xL
The retroviral vector for the clinical TCR DMF5 is based on an MSGV1 backbone (Fig. 1A). To transduce PBMC with both this TCR and either Bcl-2 or Bcl-xL, we constructed vectors for these 2 antiapoptotic genes based on the MSGV backbone (Fig. 1B). In both cases, the antiapoptotic gene is flanked by an IRES element and GFP for more efficient identification of transduced populations, especially when considering selective growth or survival. For selective outgrowth of T lymphocytes, PBL were stimulated for 2 days in the presence of OKT3 (50 ng/mL) and IL-2 (300IU/mL) before 2 rounds of retroviral transduction with DMF5, GFP.Bcl-2, Bcl-xL.GFP, or both DMF5 and either GFP.Bcl-2 or Bcl-xL.GFP. For cotransductions, exposing PBL to both retroviral supernatants on each day of transduction versus performing transductions sequentially with each vector over 2 days did not alter the cotransduction efficiency (data not shown).
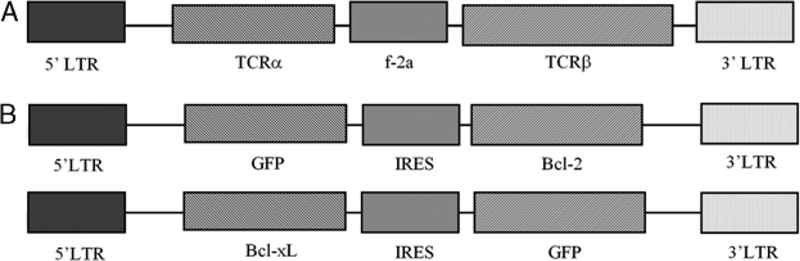
FIGURE 1.
Retroviral vectors for TCR DMF5 and antiapoptotic genes, Bcl-2 and Bcl-xL. Linearized representation of 3 retroviral vectors containing the MSGV1 vector backbone and a LTR-driven expression system. A, Retroviral vector containing the α- and β-chains of the MART-1 antigen-specific TCR, and an f-2A linker sequence to couple the translation of the 2 chains.2 B, Retroviral vectors containing human Bcl-2 or Bcl-xL cDNA, GFP, and an IRES element to couple translation. GFP coexpression allowed for efficient identification of selective growth or survival of transduced populations. GFP indicates green fluorescent protein; IRES, internal ribosome entry site; LTR, long terminal repeat; TCR, T-cell receptor.
For clinical purposes, sufficient transduction efficiency of the TCR is necessary to maximize the number of tumor reactive lymphocytes transferred after ex vivo expansion. DMF5 transduction efficiency was not diminished in cotransduction with GFP.Bcl-2 or Bcl-xL.GFP compared with DMF5 transduction alone (Fig. 2A). Cotransduction efficiencies of DMF5 and GFP.Bcl-2 or Bcl-xL.GFP varied from 37.6% to 49.5% and 51% to 64%, respectively (Table 1, last column). Although largely reproducible, existing variations in transduction efficiency can be partly attributed to the use of transiently produced retroviral supernatant for GFP.Bcl- 2 and Bcl-xL.GFP transductions. Using a producer clone, as was done for the production of DMF5 retroviral supernatant, leads to more reliable retroviral titers.23
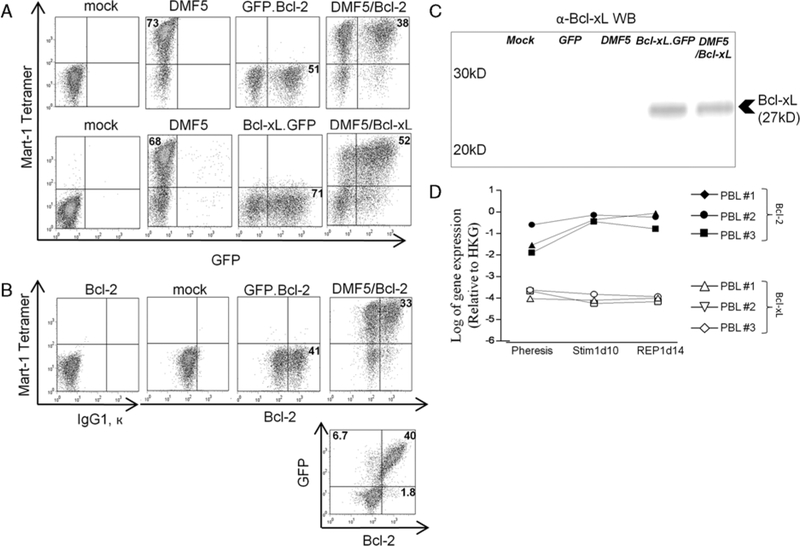
FIGURE 2.
Cotransduction of human PBL with tumor-specific T-cell receptor and Bcl-2 or Bcl-xL. A, Flow cytometry of human PBL 7 days after stimulation with OKT3 (50 ng/mL) and IL-2 (300 IU/mL) and 5 days after mock transduction or retroviral cotransduction with indicated vectors. B, Intracellular stain for Bcl-2 in human PBL after retroviral transduction with GFP.Bcl-2 to test GFP as a surrogate for ectopic Bcl-2 expression. C, Western blot analysis of Bcl-xL expression in human PBL after 5 different transduction conditions. Bcl-xL.GFP is denoted as Bcl-xL. D, Basal level of gene expression of Bcl-2 and Bcl-xL in unmodified PBL before (pheresis) and after stimulation (Stim1, REP) as determined by RT-PCR. GFP indicates green fluorescent protein; HKG, house-keeping genes; PBL, peripheral blood lymphocytes; REP, rapid expansion protocol.
TABLE 1.
Reproducible Cotransduction With DMF5 T-cell Receptor (TCR) and Bcl-2 or Bcl-xL in Peripheral Blood Lymphocytes (PBL) From Several Patients
| PBL# | %MART-1 Tetramer + | %GFP + | %MART-1 Tetramer+ /GFP+ |
|---|---|---|---|
| DMF5/GFP.Bcl-2 cotransduced | |||
| 1 | 72.8 | 51.2 | 37.6 |
| 2 | 76.7 | 57.7 | 49.5 |
| 3 | 73.7 | 56 | 45.9 |
| 4 | 78.3 | 60.2 | 47.4 |
| DMF5/Bcl-xL.GFP cotransduced | |||
| 1 | 64 | 74 | 51 |
| 2 | 64 | 72.5 | 52.7 |
| 3 | 78.3 | 75.7 | 64 |
| 4 | 65.5 | 75.7 | 54 |
To test whether GFP+ populations were an accurate surrogate of Bcl-2 or Bcl-xL-transduced populations, we examined gene expression in mock-transduced and transduced PBL by intracellular staining (Bcl-2) and western blot (Bcl-xL). In GFP.Bcl-2 or DMF5/GFP.Bcl-2- transduced lymphocytes, a subset of the population had augmented Bcl-2 expression (Fig. 2B, upper panels) and over 96% of this subset was GFP+ (Fig. 2B, lower panel). In western blot analysis, Bcl-xL expression was present only in samples transduced with the Bcl-xL.GFP vector (Fig. 2C).
The entire population of mock-transduced PBL showed basal expression of Bcl-2 compared with isotype control. As noted, western blot analysis did not indicate any basal level of Bcl-xL expression in mock-transduced PBL. Using RT-PCR, we confirmed that Bcl-2, but not Bcl-xL, was basally expressed in PBL from 3 donors before and after stimulation (Fig. 2D).
Lymphocytes Cotransduced With DMF5 and Bcl-2 or Bcl-xL Escape Apoptosis and Remain Viable After IL-2 Withdrawal
We evaluated the growth and survival of cotransduced lymphocytes in the presence and absence of IL-2. For adoptive cell transfer of gene-modified lymphocytes, PBL are stimulated with OKT3 and IL-2 on day 0, transduced on days 2 and 3, and expanded ex vivo in 300-IU/mL IL-2 until days 9 to 12, at which time they are administered to the patient. The patient then receives high-dose (720,000 U/kg) IL-2 every 8 hours to tolerance (typically 2 to 3 days). For an accurate in vitro model of lymphocyte survival after IL-2 is withdrawn from the patient, PBL were stimulated, transduced, and cultured ex vivo in IL-2 until days 8 to 12 (hereafter referred to as Stim1 PBL). After Stim1, cultures were 96.7 ±1.8% CD3+, confirming the selective outgrowth of T lymphocytes. At this point, we withdrew IL-2 or not from in vitro cultures and assessed growth and survival of transduced populations of PBL by viable cell counts and flow cytometry.
Proliferation of DMF5-transduced lymphocytes in the presence of 300-IU/mL IL-2 was not inhibited by cotransduction with GFP.Bcl-2 (Fig. 3A) or Bcl-xL.GFP (data not shown) in multiple donor PBL. Mock-transduced PBL grew better than transduced PBL in 2 out of 3 PBL donors (Fig. 3A). However, this growth advantage of mock- transduced PBL was not seen in comparison to GFP-alone transduced PBL (data not shown).
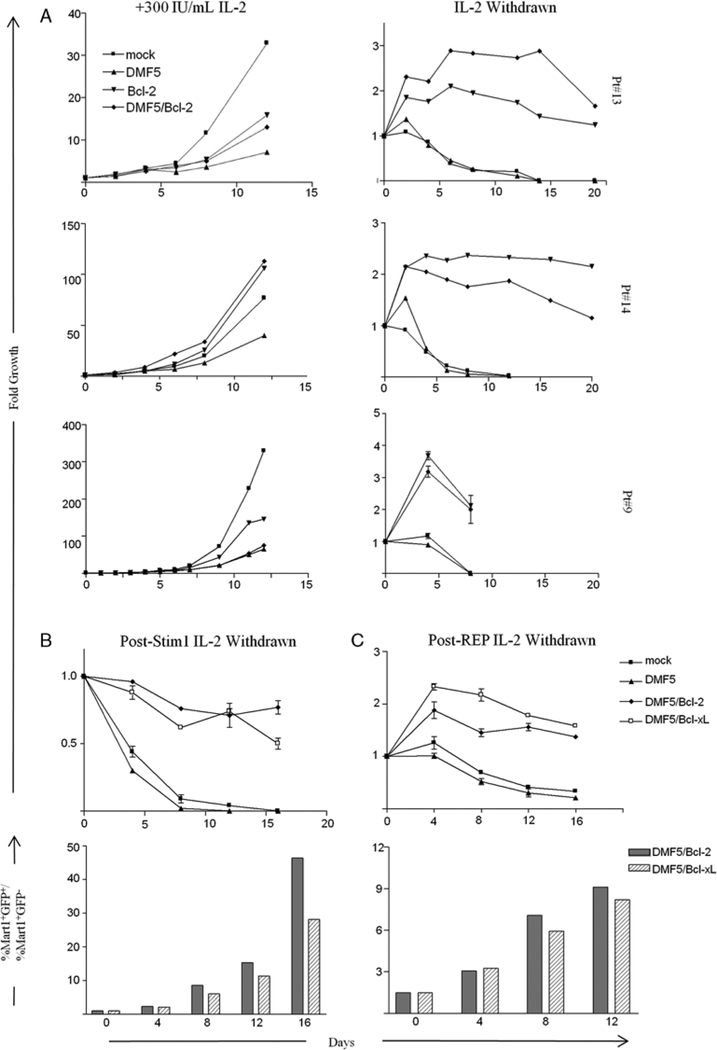
FIGURE 3.
PBL transduced with DMF5 and Bcl-2 or Bcl-xL survive in the absence of IL2 and grow comparable with DMF5-alone transduced lymphocytes in the presence of IL2. A, Mock-transduced PBL or PBL transduced with DMF5 TCR, Bcl-2, or DMF5 TCR and Bcl-2 were cultured in 300-IU/mL IL-2 for 7 days after transduction and then plated in CM with 300-IU/mL IL-2 or without IL-2 (day 0). Each time point represents fold growth or survival of the specific transduced population based on viable cell counts and Fluorescence Activated Cell Sorting analysis of GFP+ and MART-1 tetramer+ populations. B, Comparison of survival of PBL transduced with DMF5 TCR and either Bcl-2 or Bcl-xL after IL-2 withdrawal post-Stim1 (upper panel). Ratio of survival of MART-1 tetramer+GFP+ cells to MART-1 tetramer+GFP cells within a bulk cotransduced population after IL-2 withdrawal post-Stim1. C, Same as in B, except IL-2 was withdrawn after a REP where transduced cells were expanded with a second OKT3 stimulation, allogeneic feeders, and 1000C.U. IL-2 for 12 days and plated in CM with or without 1000C.U. IL-2. GFP indicates green fluorescent protein; PBL, peripheral blood lymphocytes; REP, rapid expansion protocol.
After 8 days of IL-2 withdrawal, only negligible numbers of mock-transduced and DMF5-transduced PBL remained viable (Fig. 3A). In comparison, GFP.Bcl- 2-transduced or DMF5/GFP.Bcl-2-transduced PBL remained viable beyond 3 weeks after IL-2 withdrawal (Fig. 3A) but then slowly died. However, aside from an initial growth phase likely owing to residual effects of IL-2, Bcl.2.GFP and DMF5/Bcl2.GFP-transduced lymphocytes did not proliferate after IL-2 withdrawal.
We later compared effects of Bcl-2 with the effects of Bcl-xL on survival after IL-2 withdrawal of Stim1 PBL. Furthermore, we studied effects of IL-2 withdrawal after a REP that is used clinically to obtain large numbers of transduced lymphocytes (Materials and Methods). Co- transducing either gFp.BcI-2 or Bcl-xL.GFP with DmF5- transduced cells conferred a similar survival advantage after IL-2 withdrawal in PBL both after Stim1 and REP (Figs. 3B and C). It is interesting to note that, mock- transduced and DMF5-alone transduced PBL survived longer after IL-2 withdrawal post-REP compared with post-Stim1—albeit, and not PBL cotransduced with Bcl-2 or Bcl-xL.
To examine the competitive survival advantage of cotransduced lymphocytes, we quantified the ratio of MART-1 tetramer+GFp+ to mArT-1 tetramer+GFP− populations (normalized to day 0) after IL-2 withdrawal in cotransduced PBL post-Stim1 or post-REP1. In both cases, the ratio increased over time, indicating enrichment of the double-positive population after IL-2 withdrawal (Figs. 3B and C, lower panels). The enrichment was more pronounced after IL-2 withdrawal in Stim1 PBL, reiterating the more rapid loss in viability of DMF5-alone transduced cells in Stim1 versus REP1 PBL.
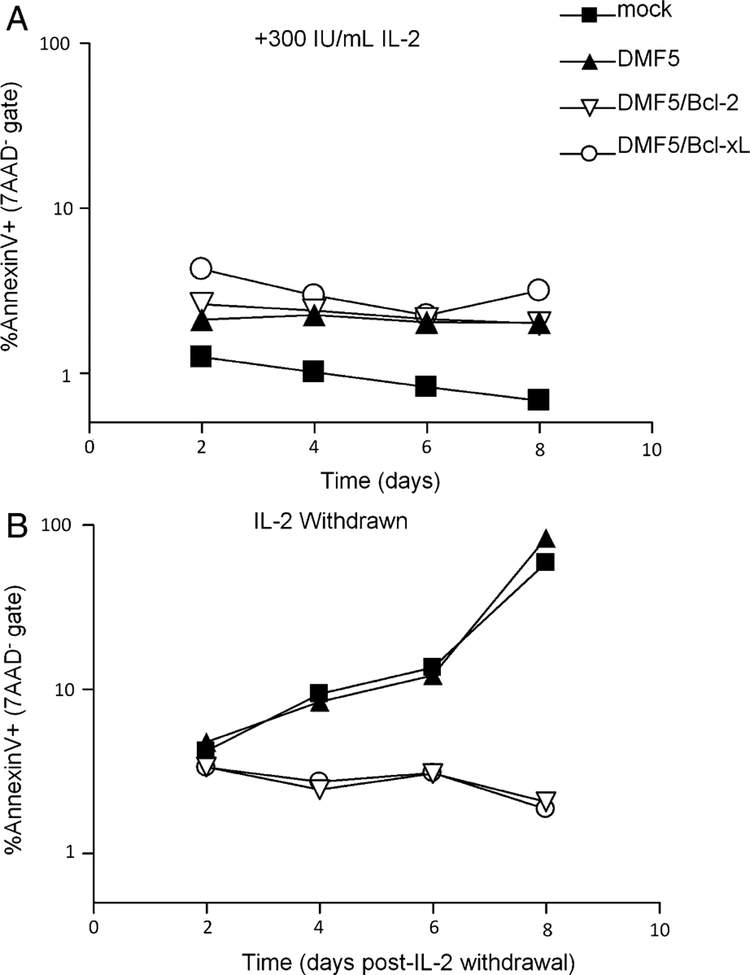
The role of Bcl-2 and Bcl-xL as inhibitors of the intrinsic pathway of apoptosis, which is induced by cytokine or growth factor withdrawal, has been well described.24 To test that the ability of lymphocytes transduced with DMF5 and Bcl-2 or Bcl-xL to survive after IL-2 withdrawal is due to an inhibition of apoptosis, we measured levels of Annexin V staining in viable PBL (7AAD−) after IL-2 withdrawal or not. In the presence of 300-IU/mL IL-2, Bcl-2 and Bcl-xL did not have any effect on the level of Annexin V staining, which remained under 5% for both mock transduced and transduced samples (Fig. 4A). After IL-2 withdrawal, Annexin V staining increased to 58.9% and 83.5% by day 8 in mock-transduced and DMF5-transduced PBL, respectively (Fig. 4B). Meanwhile, less than 3% of PBL cotransduced with DMF5 and Bcl-2 or Bcl-xL at this time point stained with Annexin V. These results are representative of similar experiments with PBL from 3 different donors.
FIGURE 4.
Survival advantage of PBL cotransduced with DMF5 and Bcl-2 correlates with inhibition of apoptosis. A, At day 0, IL-2 was withdrawn or not from mock-transduced PBL or PBL transduced with DMF5 TCR, or DMF5 TCR and Bcl-2 or Bcl-xL. At indicated time points, bulk cultures were analyzed by flow cytometry for Annexin V and 7AAD. Graphs indicate percentage of early apoptotic cells (Annexin V+) in the viable gate (7AAD−). PBL indicates peripheral blood lymphocytes.
Cotransduction of PBL With DMF5 and Bcl-2 or Bcl-xL Does Not Alter Specific Lymphocyte Cytokine Release or Lysis
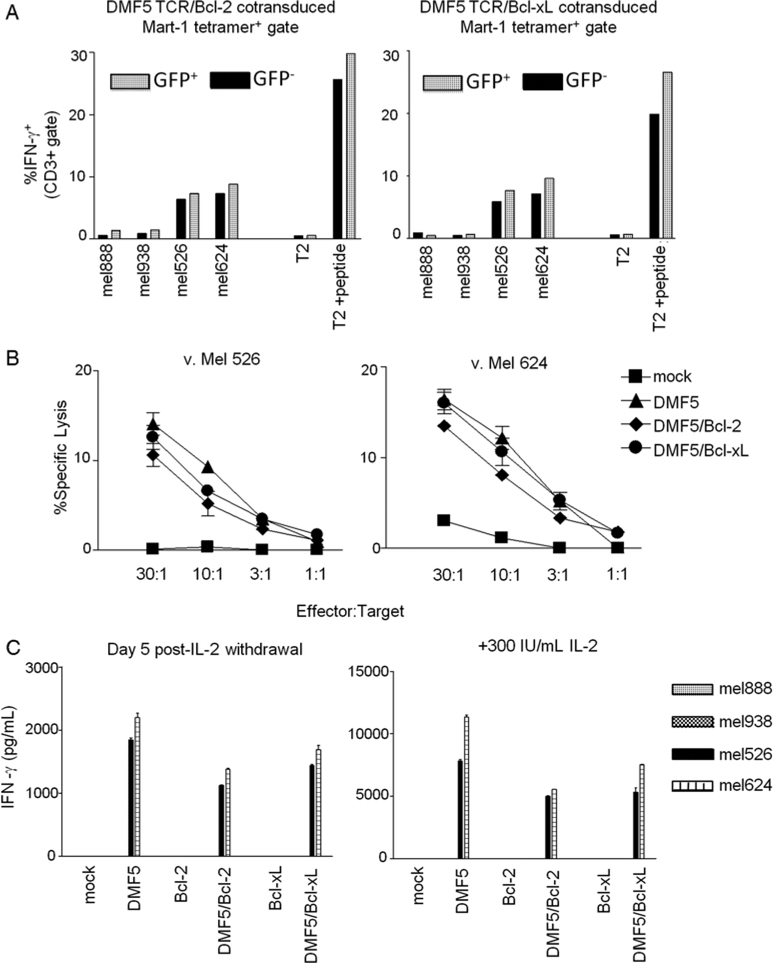
To test the ability of PBL cotransduced with DMF5 and Bcl-2 or Bcl-xL to respond to specific stimulation, cotransduced PBL were assessed for intracellular IFN-γ after 6 hours coculture with a panel of melanoma cell lines and T2 cells pulsed with MART-1 peptide or not. Intracellular IFN-γ staining allowed for a direct comparison of reactivity between DMF5-transduced populations (MART-1 tetramer +) that were cotransduced with Bcl-2 or Bcl-xL (GFP +) or not (GFP−). DMF5-transduced PBL only stained for IFN-γ when stimulated with tumors expressing both MART-1 antigen and HLA-A*0201 (mel526 and mel624), or T2 cells pulsed with MART-1 peptide (Fig. 5A). DMF5-transduced PBL did not stain for IFN-g when cocultured with tumors expressing MART-1 antigen and not HLA*0201, or with T2 cells that were not pulsed with MART-1 peptide. No significant differences were seen in the percentage of GFP+ or GFP− subpopulations producing IFN-γ (Fig. 5A). Next, we examined the lytic ability of bulk transduced populations of mock-transduced or transduced PBL in a standard 4 hours 51Cr release assay. As reported earlier,2 DMF5-transduced PBL did not lyse HLA-mismatched MART-1 expressing melanoma cell lines (mel888, mel938; data not shown). Cotransduction with GFP.Bcl-2 or Bcl-xL.GFP did not affect the ability of DMF5-transduced PBL to lyse mel526 and mel624 (Fig. 5B).
FIGURE 5.
Addition of Bcl-2 or Bcl-xL transgene does not alter the specificity or function of DMF5-transduced PBL. A, PBL cotransduced with DMF5 TCR and Bcl-2 or Bcl-xL were cocultured for 6 hours with MART-1+/HLA*0201+ (mel526+, mel624+) and HLA*0201-negative (mel888−, mel938−) melanoma tumors and T2 target cells either coincubated with 1 μM MART-1 peptide or not. MART-1 tetramer+ lymphocytes (CD3+ gate) were analyzed for GFP expression and intracellular IFN-γ release by flow cytometry. Bar values indicate percentage of IFN-g+ cells in the GFP− or GFP+ subset. Results are representative of experiments from 2 different patients. B, Bulk transduced or mock-transduced PBL were cocultured with mel526+ and mel624+ in a 4 hours 51Cr-release assay. Values are plotted as average of triplicate samples ± SEM. Results are representative of PBL from 2 different patients. C, After 5 days of IL-2 withdrawal or not, transduced or mock-transduced PBL were cocultured overnight with HLA-matched (mel526+, mel624+) and HLA-mismatched (mel888−, mel938−) melanoma cell lines. Supernatants were assessed for IFN-γ by ELISA. Values are averages of duplicate samples ± SEM. Results are representative of PBL from 3 different patients. GFP indicates green fluorescent protein; HLA, human leukocyte antigen; PBL, peripheral blood lymphocytes.
Cotransduced Lymphocytes Surviving After IL-2 Withdrawal Continue to Produce IFN-γ in Response to Specific Tumor Stimulation
To test whether cotransduced cells surviving after IL-2 withdrawal are still able to function in response to specific tumor, IL-2 was withdrawn or not from culture for 5 days and then PBL was cocultured with melanoma cell lines. Although the amount of IFN-γ released from PBL in response to specific tumor stimulation after IL-2 withdrawal was diminished, the amount of IFN-γ released per viable cell after IL-2 withdrawal was not significantly different in DMF5-transduced cells versus DMF5 and Bcl-2 or Bcl-xL- cotransduced PBL (Fig. 5C). This experiment was repeated in PBL from 3 different donors with similar results. Furthermore, IL-2 withdrawn lymphocytes maintain their ability to lyse tumor targets specifically (data not shown).
Bulk-Cotransduced PBL Uniformly Upregulate Annexin V After Overnight Coculture With Tumor Targets
To model in vivo T-cell interaction with tumor-antigen at the tumor site independent of stromal effect, mock-transduced and transduced PBL were cocultured overnight with mel888, mel526, T2 cells, and T2 cells pulsed with 1uM MART-1 peptide as described previously. After coculture, cells were stained with CD3 and MART-1 tetramer and washed thoroughly before staining with Annexin V and 7AAD. We evaluated expression of Annexin V after gating on viable (7AAD−) lymphocytes by side scatter. CD3 and MART-1 staining does not accurately identify lymphocytes due to the internalization of CD3 and TCR upon antigenic recognition. Mock-transduced cells did not upregulate Annexin V in response to any tumor lines. Annexin V expression was uniformly increased in DMF5 transduced, DMF5/GFP.Bcl-2 and DMF5/Bcl-xL.GFP-transduced PBL in response to coculture with mel526 and T2 cells pulsed with peptide, but not with mel888 or T2 cells alone (data not shown). Importantly, the expression of GFP in Annexin V− 7AAD− cells did not vary between cotransduced PBL after coculture with relevant tumors (mel526 and T2 cells pulsed with peptide) versus cotransduced PBL cocultured with irrelevant tumors (mel888 and T2 cells alone) (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/JIT/A57).
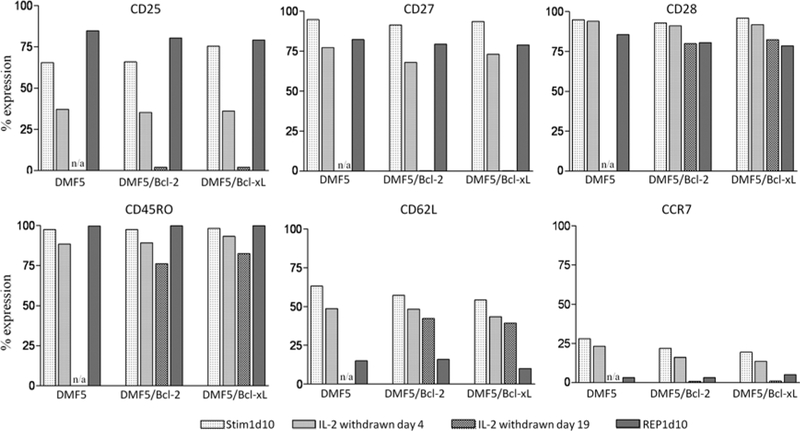
Cotransduction of PBL With DMF5 TCR and Bcl-2 or Bcl-xL Does Not Alter Its Phenotype at Clinically Relevant Time Points
We compared the phenotype of DMF5-transduced PBL with DMF5 and GFP.Bcl-2 or Bcl-xL.GFP-cotransduced PBL with respect to a panel of differentiation markers: CD25, CD27, CD28, CD45RO, CD62L, and CCR7. Four relevant time points were selected: Stim1d10 and REP1d10 correspond to possible time points for adoptive transfer of cells to a patient. We also evaluated phenotype after 4 and 19 days of IL-2 withdrawal (initiated on Stim1d10) to determine the phenotype of surviving cells expressing Bcl-2 or Bcl-xL. With respect to all markers, there were no differences between DMF5 TCR-alone transduced PBL and PBL cotransduced with both DMF5 TCR and Bcl-2 or Bcl-xL (Fig. 6). From Stim1d10 to REP1d10, there was an upward trend for CD25 expression, whereas CD27, CD28, CD62L, and CCR7 expression decreased (Fig. 6). Whereas after Stim1 there was a mixture of CD45RO1o and CD45ROhi populations, after the REP, all cells were CD45ROhi (data not shown). CD25 and CD27 expression were diminished after 4 days of IL-2 withdrawal, and neither was expressed after 19 days of IL-2 withdrawal. Expression of CD28, CD45RO, CD62L, and CCR7 were also all decreased after IL-2 withdrawal. None of the phenotypic evaluations after 19 days of IL-2 withdrawal include the DMF5 TCR-alone transduced PBL, as these cells did not survive to this point.
FIGURE 6.
Addition of Bcl-2 or Bcl-xL transgene does not alter the phenotype of DMF5-transduced PBMC. DMF5 TCR alone transduced PBMC and DMF5 TCR and Bcl-2 or Bcl-xL-transduced PBMC were analyzed for surface expression of CD25, CD27, CD28, CD45RO, CD62L, and CCR7. Each transduction condition was analyzed at 4 time points. All cells were stimulated with OKT3 and IL-2 and grown in IL-2 for 10 days (Stim1d10). Then, cells were either withdrawn from IL-2 for 4 or 19 days, or they had undergone a rapid expansion protocol (Materials and Methods) for 10 days (REP1d10). DMF5 TCR-alone transduced cells were not viable 19 days after IL-2 withdrawal (N/A). Results are representative of experiments with PBL from 2 patients. PBL indicates peripheral blood lymphocytes; PBMC, peripheral blood mononuclear cells.
Intratumoral Persistence of Tumor-specific Mouse T Cells Transduced With Human Bcl-2
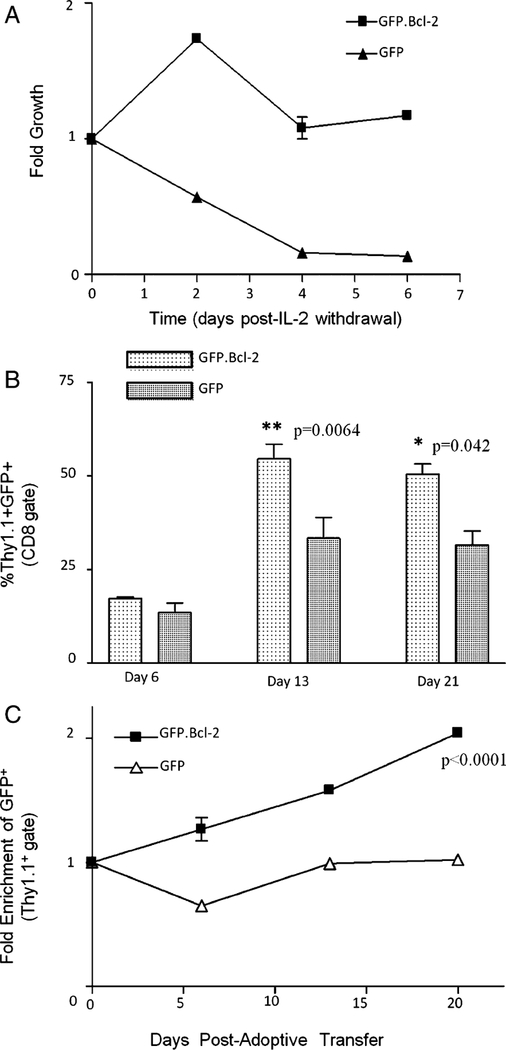
To evaluate whether inhibition of apoptosis owing to cytokine withdrawal increases persistence of tumor-specific cells in vivo, we adoptively transferred Thy1.1+ pmel-1 TCR transgenic T cells transduced with human Bcl-2 (GFP.Bcl-2) or GFP alone into C57BL/6 mice bearing B16 melanoma. Thy1.1+ pmel-1 T cells transduced with human Bcl-2, similar to human PBL, are protected against IL-2 withdrawal mediated cell death in vitro (Fig. 7A). On days 6, 13, and 21 after adoptive transfer, we harvested tumors from mice receiving Thy1.1 + pmel-1 cells transduced with either GFP.Bcl-2 or GfP alone. CD8+ lymphocytes within the tumors were evaluated for percentage of Thy1.1 + GFP+ lymphocytes. At days 13 and 21, more tumor infiltrating CD8+ lymphocytes were Thy1.1 + GFP + in mice receiving GFP.Bcl-2 versus GFP-alone transduced Thy1.1+ pmel-1 T cells (P = 0.0064 and 0.041, respectively; Fig. 7B). To test whether Thy1.1+ pmel-1 cells that received the gene of interest were selectively surviving within B16 tumors of C57BL/6 mice, we evaluated the percentage of intratumoral Thy1.1+ pmel-1 CD8+ cells expressing GFP at days 6, 13, and 21—relative to the percentage of GFP expression in the cells administered to the mice. GFP expression was enriched over time in tumor infiltrating Thy1.1 + T cells from mice receiving GFP.Bcl-2 transduced versus GFP-alone transduced pmel-1 cells (P <0.001, Fig. 7C) in repeated experiments.
FIGURE 7.
Intratumoral persistence of adoptively transferred pmel-1 cells transduced with human Bcl-2. A, Pmel-1 T cells were stimulated with 2-μg/mL plate-bound anti-CD3 and 1 μg/mL of soluble anti-CD28 and cultured in 30 IU/mL of IL-2 for 2 days, transduced with either GFP.Bcl-2 or GFP alone, and cultured for another 3 days before being washed and plated in CM without IL-2 (day 0). Cells were counted by trypan blue exclusion and analyzed by flow cytometry at each time point to calculate the number of viable transduced T cells. Data are expressed as a fold growth relative to day 0 and is the average of 2 wells ± SEM. B and C, Tumors were harvested from C57BL/6 mice on days 6, 13, and 21 after treatment with vaccine, IL-2, and Thy1.1+ pmel-1 T cells transduced with either GFP.Bcl-2 or GFP alone. Cells were stained for CD3, CD8 and Thy1.1 and analyzed by flow cytometry. B, Bar graphs indicate the percentage of Thy1.1+GFP+ cells within the CD8+ lymphocyte gate. Each data point represents the average of 3 mice ± SEM. Student paired t test was used to calculate P values. C, Percentage of Thy1.1+ cells expressing GFP+ was enumerated and normalized the expression of GFP in the administered cells to obtain fold enrichment of transduced cells in tumors. P values were obtained using a 2-way ANOVA with respect to enrichment and time. GFP indicates green fluorescent protien.
DISCUSSION
The use of PBL genetically modified to express a TCR has opened new possibilities for the adoptive immunotherapy of cancer.25 One enticing possibility involves modifying PBL with both a TCR and a secondary gene that enhances the ability of lymphocytes to mediate tumor regression. One major limitation of adoptive immunotherapy is the IL-2- dependent growth and survival of T cells, and the limited amount of IL-2 that can be administered to patients in conjunction with cell therapy due to IL-2-induced patient toxicities.6 In this study, we modified PBL with both a TCR and an antapoptotic gene to address the IL-2-dependent survival of adoptively transferred lymphocytes.
Using the TCR DMF5, which has recently been shown to mediate tumor regression in metastatic melanoma,2 and either Bcl-2 or Bcl-xL, we reproducibly cotransduced 38% to 64% of PBL with the TCR and antiapoptotic gene (Table 1, Fig. 2A). Bcl-2 and Bcl-xL were tagged with GFP for efficient monitoring of transduced populations—and the validity of GFP as a surrogate was confirmed by intracellular staining and western blot (Figs. 2B and C). These cells were successfully expanded in IL-2 to quantities sufficient for adoptive cell transfer, while retaining specificity, function, and phenotype. Importantly, cotransduced PBL survived beyond 3 weeks after withdrawal of IL-2, and retained specificity and function after IL-2 withdrawal. Finally, survival of T cells after IL-2 withdrawal was shownto improve in vivo persistence of T cells at tumor sites in a mouse model of melanoma.
The death of T cells after IL-2 withdrawal is mediated by the intrinsic apoptotic pathway and is inhibited by Bcl-2 and Bcl-xL.8,9,24 Whereas lymphocytes have basal Bcl-2 expression, Bcl-xL is inducible upon specific types of stimulation.26 This was confirmed by RT-PCR analysis of Bcl-2 and Bcl-xL expression in PBL prior to and after Stim1 and REP1 (Fig. 2D). We considered that this may result in differences in the effect on the survival of the cells after IL-2 withdrawal. However, our experiments indicated no differences; cotransducing PBL with DMF5 and Bcl-2 or Bcl-xL conferred an equal survival advantage after IL-2 withdrawal (Figs. 3B and C). As expected, this survival advantage correlated with an inhibition of apoptosis (Fig. 4B).
Specifically, lymphocytes transduced with both a TCR and antiapoptotic gene survived beyond 3-weeks after IL-2 withdrawal. Although these cells proliferated shortly after IL-2 withdrawal (likely owing to residual effects of IL-2), their growth plateaued in the absence of exogenous stimulation. This pattern of growth after IL-2 withdrawal mimics that of lymphocytes transduced with the IL-2 gene.3
Although the survival advantage of cotransduced PBL in the absence of IL-2 was clearly shown, their ability to retain function is the basis for any potential improvement in tumor treatment. Using flow cytometry to assess intracellular IFN-γ, we compared cotransduced populations (MART-1 + GFP +) with single-transduced populations (MART-1+GFP—) that existed within the same culture of cotransduced PBL. Both of these populations only produced IFN-γ in response to specific tumor stimulation. Furthermore, no differences were seen in the ability of these 2 populations to respond to specific tumor stimuli. Perhaps most importantly, in a coculture with tumor targets after 5 days of IL-2 withdrawal, cotransduced cells produced similar amounts of IFN-γ as DMF5-alone transduced cells. Thus, since cotransduced cells have greater viability after 5 days of IL-2 withdrawal and continue to express the same amount of IFN-γ per cell, the effective amount of IFN-γ release is amplified.
In adoptive immunotherapy, stimulated and transduced PBL can be expanded in IL-2 for 9–12 days (Stim1) or additionally expanded using allogeneic feeders, a second dose of OKT3, and high dose IL-2 (REP). In cells that underwent a REP, the presence of Bcl-2 or Bcl-xL also sustained increased survival after IL-2 withdrawal. Our data indicated that mock-transduced or TCR-alone transduced PBL were more resistant to IL-2 withdrawal after REP than after Stim1. This resistance was not explained by differential expression of Bcl-2, Bcl-xL, or Bax as determined by RT-PCR (Fig. 2D; data not shown). The increased resistance to IL-2 withdrawal may be a result of higher doses of IL-2 used for the REP versus Stim1 (6000 vs. 300IU/mL) or, as shown in Figure 6, the development of a memory phenotype (increased CD45RO and decreased CD62L) in PBL after rEp versus Stim1.
Recent work by Gattinoni et al27 described the increased proliferative capacity and antitumor efficacy of lymphocytes with a central memory compared to effector memory phenotype. We therefore assessed the effect of Bcl-2 and Bcl-xL on the phenotype of the transduced cells. Neither Bcl-2 nor Bcl-xL altered the phenotype of DMF5- transduced PBL with respect to a panel of differentiation markers (Fig. 6). In addition, the ability of DMF5 and Bcl-2 or Bcl-xL cotransduced PBL to survive beyond 8 days of IL-2 withdrawal allowed for a more prolonged analysis of the effects of IL-2 withdrawal on T-cell activation and differentiation markers. An extended period of IL-2 withdrawal results in a loss of CD25 expression as expected. However, surprisingly, CD27 expression is lost completely—contrary to CD27 expression in TIL after IL-2 withdrawal in vitro (Fig. 6).28 Furthermore, IL-2 withdrawal does not result in a drastic change in differentiation of T-cell memory subsets. That is, IL-2 withdrawn cells are highly CD45RO+ and only begin to lose CD62L expression, consistent with a gradual differentiation from the central to effector memory phenotype (Fig. 6).
In addition to inhibiting the intrinsic pathway of apoptosis, Bcl-2 and Bcl-xL can also provide partial resistance against the extrinsic pathway; specifically, they mediate partial inhibition of Fas-mediated apoptosis.9,29 This has implications in the ability of Bcl-2 and Bcl-xL to inhibit activation-induced cell death—that could improve survival of T cells encountering tumor-antigen. After coculture of DMF5 TCR-transduced cells overnight with specific tumor targets or T2 cells pulsed with peptide, there was an upregulation of Annexin V in the viable lymphocyte gate that was not observed in coculture with HLA- mismatched tumors (data not shown). This shows that activation of T cells through the TCR overnight induces apoptotic pathways, either directly through TCR stimulation, or indirectly through the cytokine millieu produced by TCR activation. With PBL cotransduced with the TCR and Bcl-2 or Bcl-xL, we were able to test the role of these antiapoptotic genes in the inhibition of activation-induced cell death by examining the phenotype of the viable, Annexin V— subset after stimulation. In cotransduced PBL stimulated through the TCR by tumor targets, the percentage of Bcl-2 or Bcl-xL expressing cells (marked by GFP) was not enriched in the Annexin V— subset, indicating that the subset of cells expressing Bcl-2 or Bcl-xL were not able to selectively escape apoptosis (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/JIT/A57). This suggests that Bcl-2 and Bcl-xL, in the setting of tumor stimulation, either fail to confer resistance to the extrinsic pathway of apoptosis or fail to protect against the toxic millieu created by TCR recognition of tumor cells, which includes high concentrations of IFN-g. This reinforces the role of inhibition of cytokine withdrawal as the primary mechanism for Bcl-2 and Bcl-xL in T-cell survival.
To test whether inhibiting IL-2 withdrawal-mediated apoptosis can lead to increased persistence in vivo, we used T cells transduced with Bcl-2 in a mouse model of melanoma. Charo et al8 earlier showed improved tumor treatment with pmel-1 TCR/Bcl-2 transgenic mice. However, the possible effects of Bcl-2 expression during T-cell development make that tumor treatment model less than ideal. As shown in Figure 7A, human Bcl-2 confers the same survival advantage after IL-2 withdrawal to mouse T cells as it does to human T cells. And in 2 independent experiments, the fraction of adoptively transferred T cells within the tumor infiltrating lymphocyte population was significantly greater in Bcl-2-transduced versus GFP-transduced treatment group at days 13 and 21 after transfer. Furthermore, the percentage of transduced cells was enriched in the tumor for the Bcl-2-transduced group but not the GFP-transduced group. In 1 of 2 independent experiments, the Bcl-2-transduced group mediated a statistically significant improvement in tumor treatment (P < 0.05; data not shown). The improved survival of Bcl-2-transduced tumor-specific T cells within the tumor can be attributed to a multitude of factors related to the inhospitable tumor microenvironment, including inhibition of apoptosis mediated by cytokine-withdrawal, hypoxia, AICD, and FAS expression in the tumor microenvironment.
One possible problem with using Bcl-2 to transduce tumor specific lymphocyte clones is the potential for malignant transformation of the transduced cells. Bcl-2 overexpression plays an important role in lymphoma development. However, experience with gene therapy of T cells has not resulted in any cases of malignant transformation, and neither Bcl-2 nor Bcl-xL has been implicated in the development of T-cell leukemias.30,31 We have not seen immortalization of any Bcl-2 or Bcl-xl culture. Although the starting PBL population includes B lymphocytes, culturing cells in OKT3 and high-dose IL-2 induces a selective outgrowth of CD3 + T cells. Thus, the initial transduced population is primarily T lymphocytes, which are further cultured in high-dose IL-2, thereby eliminating any remnant B lymphocytes. Furthermore, all of our cultures transduced with Bcl-2 or Bcl-xL eventually died after IL-2 withdrawal.
The ability to attain high levels of cotransduction of lymphocytes with genes encoding a TCR with other genes opens the door to many gene modifications that could potentially improve the adoptive immunotherapy of cancer. Possible additional gene modification of lymphocytes include genes to alter traffic of cells, provide constitutive secretion of growth factors, provide costimulatory molecules, and inhibit the expression of negative regulatory molecules.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank L. Johnson, J. Riley, D. Palmer, C. Hinrichs, L. Church, R. Reger, D. Frasheri, S. Farid, A. Mixon, and other members of the National Cancer Institute Surgery Branch for their generous gifts of reagents, discussions, and technical assistance in this study.
This research was supported by the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
All authors have declared there are no conflicts of interest in regards to this work.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.immunotherapy-journal.com.
REFERENCES
- 1.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu K, Rosenberg SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J Immunol. 2001; 167:6356–6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotze MT, Matory YL, Ettinghausen SE, et al. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985;135: 2865–2875. [PubMed] [Google Scholar]
- 5.Duke RC, Cohen JJ. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986;5:289–299. [PubMed] [Google Scholar]
- 6.Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–484. discussion 484–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charo J, Finkelstein SE, Grewal N, et al. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 2005; 65:2001–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton D, Gilham DE, O’Neill A, et al. Retroviral transduction of human peripheral blood lymphocytes with Bcl-X(L) promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 2002;9:527–535. [DOI] [PubMed] [Google Scholar]
- 10.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T-cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossig C, Brenner MK. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol Ther. 2004;10:5–18. [DOI] [PubMed] [Google Scholar]
- 13.Kessels HW, Wolkers MC, van den Boom MD, et al. Immunotherapy through TCR gene transfer. Nat Immunol. 2001;2:957–961. [DOI] [PubMed] [Google Scholar]
- 14.Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–246. [DOI] [PubMed] [Google Scholar]
- 15.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- 16.Zhao Y, Zheng Z, Robbins PF, et al. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng EH, Wei MC, Weiler S, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8: 705–711. [DOI] [PubMed] [Google Scholar]
- 18.Robbins PF, Li YF, El-Gamil M, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T-cell functions. J Immunol. 2008;180:6116–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddell SR, Greenberg PD. The use of anti-CD3 and anti- CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. [DOI] [PubMed] [Google Scholar]
- 20.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8 + T cells. J Exp Med. 2003;198:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuss S, Biese P, Cosset FL, et al. Suspension packaging cell lines for the simplified generation of T-cell receptor encoding retrovirus vector particles. Gene Ther. 2007;14:595–603. [DOI] [PubMed] [Google Scholar]
- 24.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broome HE, Dargan CM, Krajewski S, et al. Expression of Bcl-2, Bcl-x, and Bax after T-cell activation and IL-2 withdrawal. J Immunol. 1995;155:2311–2317. [PubMed] [Google Scholar]
- 27.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Kerstann KW, Ahmadzadeh M, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawahara A, Kobayashi T, Nagata S. Inhibition of Fas- induced apoptosis by Bcl-2. Oncogene. 1998;17:2549–2554. [DOI] [PubMed] [Google Scholar]
- 30.Baliga BC, Kumar S. Role of Bcl-2 family of proteins in malignancy. Hematol Oncol. 2002;20:63–74. [DOI] [PubMed] [Google Scholar]
- 31.Kitada S, Pedersen IM, Schimmer AD, et al. Dysregulation of apoptosis genes in hematopoietic malignancies. Oncogene. 2002;21:3459–3474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.