Abstract
Purpose:
We investigated the feasibility of delivering the proinflammatory cytokine interleukin (IL)-12 into tumor using T cells genetically engineered to express a chimeric antigen receptor (CAR) against the VEGF receptor-2 (VEGFR-2).
Experimental Design:
Two different strains of mice bearing five different established subcutaneous tumors were treated with syngeneic T cells cotransduced with an anti–VEGFR-2 CAR and a constitutively expressed single-chain murine IL-12 or an inducible IL-12 gene after host lymphodepletion. Tumor regression, survival of mice, and persistence of the transferred cells were evaluated.
Results:
Adoptive transfer of syngeneic T cells cotransduced with an anti–VEGFR-2 CAR and a constitutively expressing single-chain IL-12 resulted in the regression of five different established tumors of different histologies without the need for IL-2 administration. T cells transduced with either anti–VEGFR-2 CAR or single-chain IL-12 alone did not alter the tumor growth indicating that both of them had to be expressed in the same cell to mediate tumor regression. Anti–VEGFR-2 CAR and IL-12–cotransduced T cells infiltrated the tumors, expanded, and persisted for prolonged periods. The antitumor effect did not require the presence of host T and B cells but was dependent on host IL-12R–expressing cells. The anti–VEGFR-2 CAR changed the immunosuppressive tumor environment by altering/reducing both the systemic and the intratumoral CD11b+Gr1+ myeloid suppressor cell subsets that expressed VEGFR-2.
Conclusions:
These results suggest that targeted delivery of IL-12 into the tumor environment with T cells redirected against VEGFR-2 is a promising approach for treating patients with a variety of solid tumor types.
Introduction
Adoptive cell transfer (ACT)–based immunotherapy with autologous tumor infiltrating lymphocytes has mediated dramatic tumor regressions in patients with melanoma (1, 2). Extending this approach to other common solid cancers has been hampered by difficulties in obtaining naturally occurring tumor-specific T cells and by the inability to identify shared antigens on cancers suitable for immunologic attack.
We have thus taken an alternate approach to ACT immunotherapy by genetically engineering lymphocytes to destroy tumor vasculature using a chimeric antigen receptor (CAR) specific for VEGF receptor-2 (VEGFR-2) overexpressed on tumor vasculature. Because most solid tumors depend on blood supply for growth, survival, and metastasis (3), this approach could potentially overcome problems common to targeting specific cancer antigens such as intratumor heterogeneity of antigen expression and genetic instability leading to downregulation of antigen/MHC molecules (4–6). Recently, we have shown that adoptive transfer of syngeneic mouse T cells genetically engineered with a CAR against VEGFR-2, the primary functional receptor mediating the angiogenic activity of VEGF, caused inhibition of growth of 5 different established tumor types from different histologic origins in 2 different strains of mice, when administered in conjunction with IL-2 (7). Although, occasional long-term tumor-free survival could be achieved through administration of multiple doses of anti–VEGFR-2 CAR T cells along with exogenous IL-2, most mice exhibited regrowth of tumor 2 to 3 weeks after treatment.
In our earlier studies in mouse tumor models (7), we showed a specific traffic of T cells transduced with an anti-VEGFR-2 CAR to tumor vasculature and expansion and persistence of the adoptively transferred anti-VEGFR-2 CAR-transduced T cells at the tumor site that was associated with an antitumor response. These results provided us with a rationale to enhance the therapeutic index of the transferred cells by exploring the potential of anti-VEGFR-2 CAR-transduced T cells to deliver therapeutic cytokines to the tumor environment that are otherwise toxic if administered systemically.
We chose interleukin-12 (IL-12), a known potent proinflammatory cytokine that mediates both adaptive and innate immunity (8, 9). IL-12 exhibits many biological effects on multiple immune cells, including a stimulatory effect on natural killer (NK) cells, CD4+ (type 1) helper T cells, and cytotoxic lymphocytes (CTL) and IFN-γ production as well as antitumor, antiangiogenic, and antimetastatic activities (8–11). Nevertheless, systemic administration of IL-12 can be highly toxic thus limiting its clinical use at therapeutically effective doses (12–14).
Recently, Kerkar and colleagues (2010) reported that the adoptive transfer of T cells specifically targeting the gp100 tumor antigen and genetically engineered to express a single-chain IL-12 gene could mediate destruction of large vascularized B16 melanomas in mice without the need for vaccine or IL-2 (15). We have now extended these findings to 2 important ways. The constitutive secretion of IL-12 is toxic and thus we developed a vector encoding an inducible IL-12 that was released only upon T-cell receptor (TCR) engagement in the tumor lesion, thus enabling potent antitum or effects with lesser toxicity (16). Furthermore, unlike targeting specific tumor antigens which are expressed only on a particular type of tumor, we have now targeted the genetically stable tumor vasculature, which offers the potential to treat a broad spectrum of solid tumors irrespective of their histologic origin or immunogenicity. Furthermore, access of effectors to the tumor is facilitated by targeting the vascular endothelium exposed to the circulation rather than the requirement for deep infiltration into the tumor stroma required of cells targeting conventional tumor antigens.
Therefore in this study, we investigated the feasibility of targeted delivery of IL-12 protein to tumor through adoptive transfer of anti–VEGFR-2 CAR–engineered T cells cotransduced with an inducible murine single-chain IL-12. Here, we show that in multiple mouse tumor models, adoptive transfer of syngeneic T cells cotransduced with anti–VEGFR-2 CAR and a gene encoding IL-12 induced curative regressions of all 5 established solid tumors tested in C57BL/6 and BALB/c mice without the need for exogenous recombinant IL-2 infusion. Effective tumor treatment was associated with enhanced infiltration, expansion, and persistence of the adoptively transferred anti–VEGFR-2 CAR plus IL-12–engineered T cells into the tumor stroma compared with cells transduced with anti–VEGFR-2 CAR alone and was dependent on the expression of IL-12 receptor on host cells. Furthermore, the dramatic reduction in the myeloid-derived cell subsets in the tumor appeared to play a role in the efficacy of anti–VEGFR-2 and IL-12–engineered T cells.
Materials and Methods
Mice and tumor lines
Inbred female C57BL/6 mice were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center. Female C57BL/6 CD45.1 (Ly5.1+), C57BL/6 Rag1−/−, C57BL/6 IL12rb2−/−, C57BL/6 β2m−/−, C57BL/6 TCRα−/−, and BALB/c mice were obtained from Jackson Laboratories. Details of the source and culture conditions of the cell lines used in this study were described previously (7).
Retrovirus production and transduction of mouse T cells
The retroviral vector constructs used in this study are schematically illustrated in Fig. 1A and are described in the corresponding figure legend. Details of the retroviral constructs, methods used to generate retroviral viral particles, and mouse T-cell transductions have been previously described (7, 15, 16) and has been included as Supplementary Data.
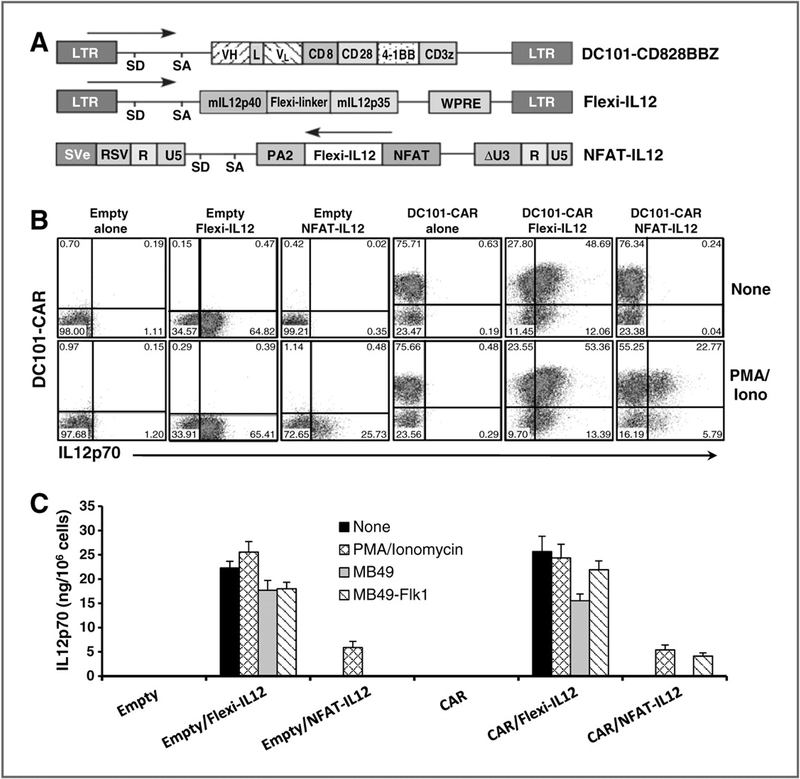
Figure 1.
Construction and characterization of recombinant retroviral vectors expressing CAR targeted against mouse VEGFR-2 and mouse single-chain IL-12. A, schematic representation of recombinant retroviral vectors used in this study. In DC101-CD828BBZ vector, the DC101 ScFv is made up of the variable regions of heavy (VH) and light chains (VL) of a rat IgG against mouse VEGFR-2 joined by a 218 linker that is linked to the hinge and transmembrane regions of the mouse CD8α chain, and the intracellular signaling sequences derived from mouse CD28, 4–1BB, and CD3Ϛ molecules. The Flexi-IL12 vector encodes the single-chain IL-12 comprised of p40 and p35 subunits of the murine IL-12 linked by (Gly4Ser)3 flexible linker. The NFAT-IL-12 vector expresses the murine single-chain Flexi-IL12 under the transcriptional control of the inducible minimal IL-2 promoter that contains 6 NFAT-binding motifs. SD, splice donor; SA, splice acceptor; PA2, polyadenylation signal. B, enriched splenic CD3þ T cells were stimulated for 2 days with ConA and IL-7 and then transduced with an empty or anti–VEGFR-2 CAR retroviral vector. The next day, cells were transduced with retroviral vector expressing Flexi-IL12 or NFAT-IL12 or left untransduced. Two days posttransduction, cells were analyzed for transgene expression by flow cytometry. Expression of IL-12 in transduced T cells was determined by intracellular FACS staining with or without PMA and ionomycin stimulation. Representative FACS data showing the percentage of T cells in each quadrant are presented. C, mouse T cells were transduced as described in Fig. 1B and 2 days later stimulated with PMA and ionomycin for 4 hours or cocultured with indicated target cell lines for 18 hours. Culture supernatants were assayed for secreted IL-12 by ELISA. Results are presented as the mean values ± SEM of triplicates. The data shown in Fig. B and C are representative of 3 independent experiments.
Fluorescence-activated cell-sorting analysis of anti–VEGFR-2 CAR and IL-12 expression in transduced T cells
Expression of the anti–VEGFR-2 CAR and IL-12 in retrovirally transduced mouse T cells was determined by flow cytometry as described previously (7, 15, 16). A detailed description of the immunostaining and flow cytometry methods is provided as Supplementary Data.
Cytokine release assay
Transduced mouse T cells were tested for specific reactivity against target cells expressing VEGFR-2 using cytokine release assays using a commercially available ELISA kits (mouse IL-12p70 and IFN-γ: Endogen; TNF-α: R&D Systems). A detailed description of these assays is provided as Supplementary Data.
Tumor models, adoptive transfer, and analysis of transferred and tumor-infiltrating cells
A detailed description of ACT methodology and analysis of transferred and tumor-infiltrating cells is described in the Supplementary Data. All experiments were conducted with the approval of the National Cancer Institute Animal Use and Care Committee.
Statistical analysis
Tumor growth slopes at each data point were compared with Wilcoxon rank-sum test. Single-measurement comparisons between 2 groups were tested using the unpaired 2-tailed Student t test. P values of less than 0.05 were considered statistically significant.
Results
Expression and secretion of recombinant proteins encoded by various retroviral expression vectors
The schematic representation of the retroviral vector constructs used in this study are shown in Fig. 1A and have been described elsewhere (7, 15, 16). Retroviral vector expressing the DC101-CD828BBz CAR was designated as DC101 CAR (anti–VEGFR-2 CAR) and an empty vector devoid of transgenes was used as a control. We have also used a vector encoding a single-chain bioactive IL-12 obtained by fusing the p35 and p40 murine IL-12 subunits with a flexible (Gly4Ser)3 linker designated as Flexi-IL12 (15) and a retro-viral vector construct SERS11-NFAT-IL12.PA2 expressing the murine single-chain IL-12 gene under the transcriptional control of NFAT-responsive minimal IL-2 promoter that drives IL-12 expression only in activated T cells (16) and was designated as NFAT-IL12 in this study.
Murine T cells were engineered to express anti–VEGFR-2 CAR (DC101 CAR) using a retroviral vector expressing DC101-CD828BBz (Fig. 1A). The specificity of anti–VEGFR2 CAR–engineered T cells in recognizing VEGFR-2+ endothelial cells and tumor cell lines but not of VEGFR-2–negative cells as indicated by proliferation and IFN-γ secretion and the cytolysis of the target cells in an antigen restricted fashion has been described previously (7). To assess the ability of VEGFR2–specific T cells to coexpress bioactive IL-12, we sequentially cotransduced mouse T cells with retroviral vectors expressing anti–VEGFR-2 CAR or an empty vector and/or Flexi-IL12 (constitutive) or NFAT-IL12 (inducible). A representative flow cytometric analysis showing the expression of anti–VEGFR-2 CAR and/or IL-12 in transduced T cells is presented in Fig. 1B. Expression of retroviral long terminal repeat (LTR)-driven Flexi-IL12 was detectable at high frequencies (range 56.4%–72%) in transduced T cells independent of T-cell activation. However, using the inducible NFAT-IL12 vector, IL-12 was expressed (range 25.3%–35.2%) strictly upon cell activation with phorbol 12-myristate 13-acetate (PMA) and ionomycin.
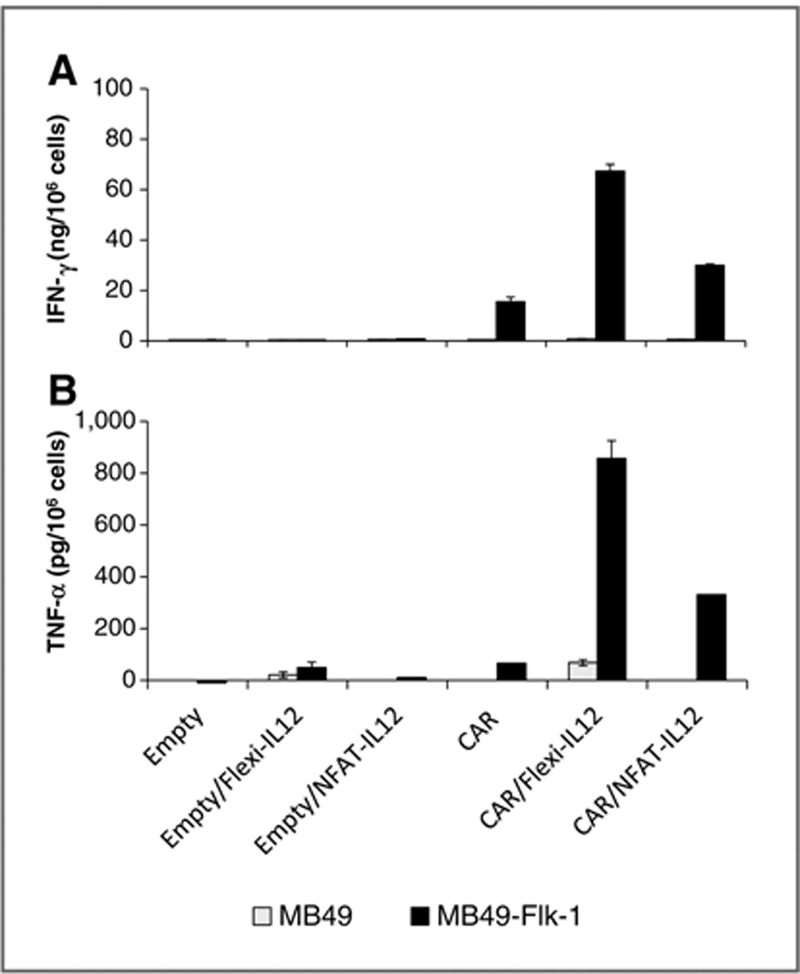
Flexi-IL12–transduced T cells secreted 5 to 6 times more IL-12 than cells transduced with NFAT-IL12 when stimulated with PMA/ionomycin (Fig. 1C). T cells coexpressing anti–VEGFR-2 CAR and the inducible NFAT-IL-12, but not those cotransduced with an empty retroviral vector and inducible NFAT-IL12, specifically released bioactive IL12p70 upon CAR engagement with the MB49-Flk1 VEGFR-2–expressing target cells but not MB49 cells that did not express VEGFR-2 (3.6–5.3 ng/1 × 106 cells; Fig. 1C). Furthermore, when cocultured with the VEGFR-2 gene– transduced MB49 tumor line (MB49-Flk1), the syngeneic mouse T cells cotransduced with anti–VEGFR-2 CAR and Flexi-IL12 or NFAT-IL12 secreted 4.35- and 1.6-fold more IFN-γ respectively (Fig. 2A) and 13- and 5.7-fold more TNF-α respectively than T cells expressing VEGFR-2 CAR only (Fig. 2B). T cells transduced with empty vector alone or cotransduced with empty vector and Flexi-IL12 failed to secrete IFN-γ (Fig. 2A) or secreted low levels of TNF-α upon stimulation with MB49-Flk1 cells (Fig. 2B).
Figure 2.
IL-12 cotransduction enhanced the antigen-specific immune responses of anti–VEGFR-2 CAR–transduced mouse T cells. Enriched CD3+ mouse T cells were transduced with the indicated retroviral vectors as described in Fig. 1. Two days later, cells were cocultured with VEGFR-2–negative MB49 tumor line or MB49 cells stably expressing mouse VEGFR-2 (referred as MB49-Flk1) for 18 to 24 hours. Culture supernatants were assayed for secreted IFN-γ (top) and TNF-α (bottom) by ELISA. Results are presented as the mean ± SEM of triplicates. The data shown are representative of 2 independent experiments.
Importantly, in accord with the known antiproliferative activity of IL-12, lymphocytes engineered with constitutively expressed Flexi-IL12 failed to expand in vitro beyond 3.8- to 4.3-fold and underwent apoptosis after 6 days in culture irrespective of the coexpression of anti–VEGFR-2 CAR, likely due to the accumulation of IL-12 in the culture medium. In contrast, T cells transduced with NFAT-IL12 expanded 7.5- to 8.2-fold in 6 days culture and sustained their growth for up to 8 days similar to anti–VEGFR-2 CAR or empty vector alone transduced T cells. This was also seen when attempting to grow human T cells transduced to secrete IL-12. Human T cells transduced with the Flexi-IL12 gene grew very poorly in vitro compared with cells transduced with the NFAT-IL12 gene (16).
Anti–VEGFR-2 and IL-12–engineered T cells induced the regression of 5 different vascularized syngeneic tumors without the addition of exogenous IL-2
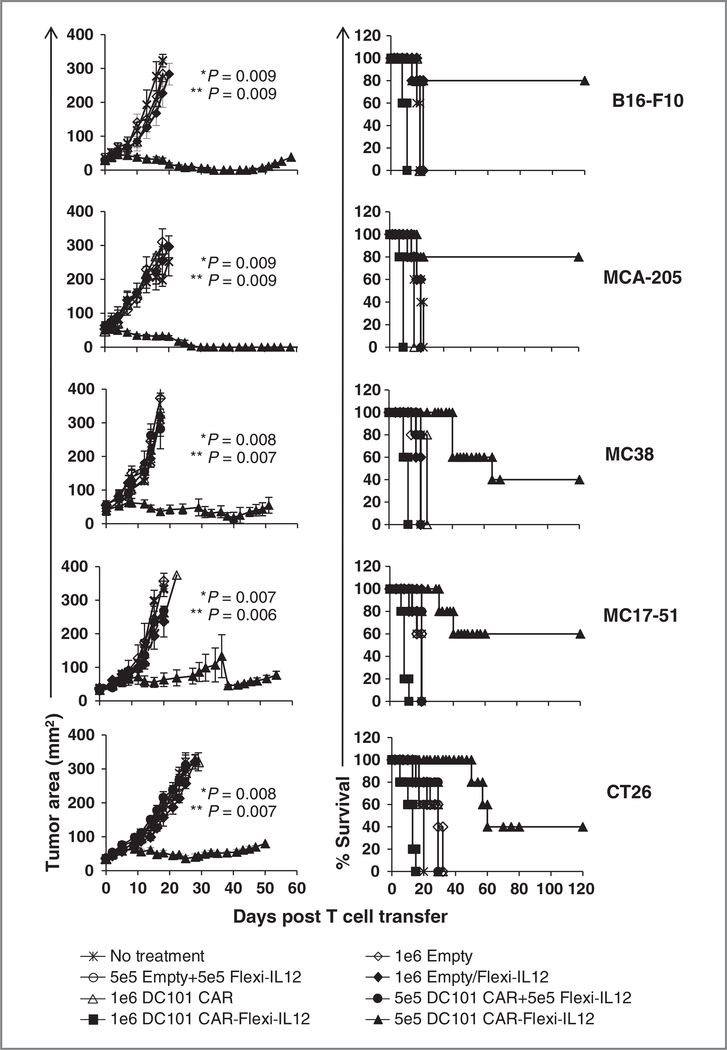
Our previous studies showed that a single dose of 2 × 107 anti–VEGFR-2 CAR–engineered T cells in conjunction with high dose IL-2 administration delayed time to progression and improved survival in various tumor models in mice though very few cures were seen and relapse of progressive tumor growth typically ensued 2 to 3 weeks posttreatment (7). The cell therapy had little to no impact in the absence of IL-2 administration. To assess the in vivo therapeutic efficacy of anti–VEGFR-2 CAR and IL-12–cotransduced T cells, we adoptively transferred them at 2 different doses (5 × 105 and 1 × 106) into sublethally irradiated (5 Gy) mice bearing a variety of vascularized tumors established subcutaneously for 10 to 12 days before therapy. The B16F10 (B16) melanoma, the MCA205 and MC17–51 sarcomas, and the MC38 colon cancers grew in C57BL/6 mice and the CT26 colon cancer grew in BALB/c mice. As shown in Fig. 3, 1 × 106 anti–VEGFR-2 CAR (DC101 CAR)-transduced cells had no impact on tumor growth and 1 × 106 cells cotransduced with the anti–VEGFR-2 CAR and with the gene for Flexi-IL12 were lethal. However, lowering the dose to 5 × 105 cells cotransduced with anti–VEGFR-2 CAR and Flexi-IL12 prolonged the survival and mediated cures of mice in all 5 tumor models tested in 2 different mouse strains. Most importantly, no exogenous IL-2 administration was required to mediate this effect. T cells engineered to express either the anti–VEGFR-2 CAR or single-chain IL-12 alone or given in a 1:1 mixture of these single gene transduced cells did not alter tumor growth in sublethally irradiated tumor-bearing mice (Fig. 3) indicating that both the anti–VEGFR-2 CAR and IL-12 had to be expressed in the same cell to mediate the antitumor effect. Moreover, the predefined tumor specificity of the IL-12–transduced T cells dictated the antitumor effect because T cells cotransduced with an empty vector and IL-12 were unable to control tumor growth. These results were consistent with our previous observations seen with gp100-specific TCR transgenic pmel-1 T cells and IL-12 (15), which required tumor specificity to mediate tumor regression. In these experiments, however we targeted a protein broadly expressed on tumor vasculature in contrast to a tumor antigen unique to 1 tumor.
Figure 3.
Adoptively transferred anti–VEGFR-2 CAR and Flexi-IL12–engineered mouse T cells induced regression of multiple types of established syngeneic tumors in mice without exogenous IL-2 administration and increased the survival of tumor-bearing mice. Ten to 12 days old B16 (melanoma), MCA-205 (sarcoma), MC38 (colorectal adenocarcinoma), or MC17–51 (sarcoma) tumor-bearing C57BL/6 mice and 12 to 14 days old CT26 colon tumor-bearing BALB/c mice were sublethally irradiated at 5 Gy TBI and treated with 1 × 106 or 5 × 105 syngeneic T cells transduced with various retroviral vectors as indicated in the figure. Control group received no treatment. Each treatment group included a minimum 5 mice. Serial, blinded tumor measurements were obtained and the products of perpendicular diameters were plotted± SEM. P values are shown for anti–VEGFR-2 CAR and IL-12–transduced T cells versus no treatment (∗) or anti–VEGFR-2 CAR alone transduced T cells (∗∗). Mice treated with 1 × 106 anti–VEGFR-2 CAR and Flexi-IL12–transduced T cells died by day 14 postcell transfer irrespective of the tumo type and mouse strains used.
The significant treatment related mortality seen in mice treated with more than 5 × 105 cells was likely attributed to the high levels of systemic IL-12 constitutively produced by the proliferating anti–VEGFR-2 CAR and Flexi-IL12–cotransduced T cells as suggested previously (15). Taken together our data show that multiple types of established vascularized epithelial tumors from different histologic origin can be eliminated through targeting of IL-12 to the tumor environment using the CAR directed against VEGFR2 expressed on tumor vasculature.
T cells engineered with anti–VEGFR-2 CAR and inducible IL-12 caused durable antitumor regressions of B16 melanoma without dose-limiting toxicities
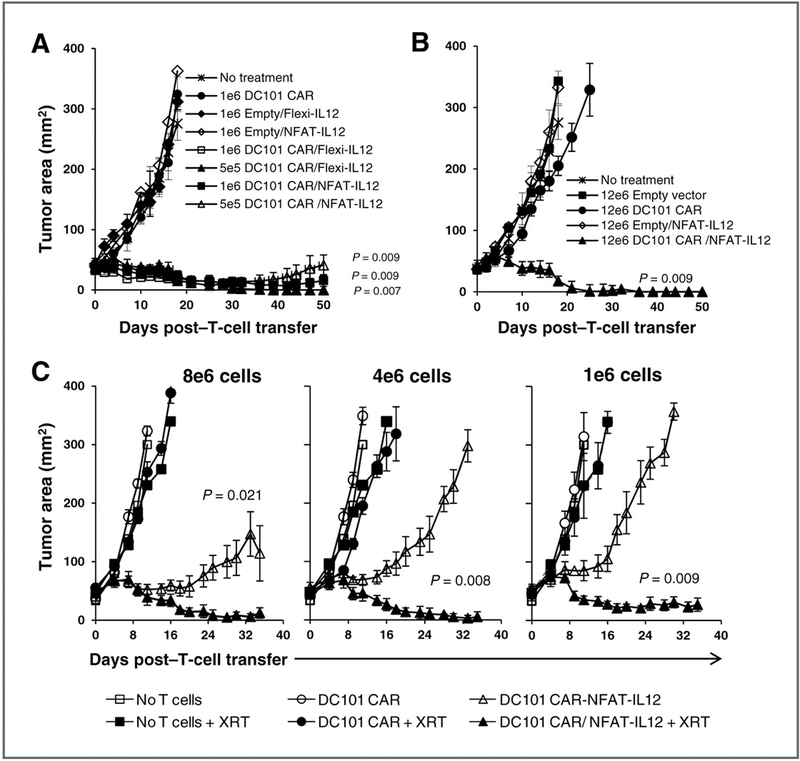
Because of the toxicity seen by cells constitutively secreting IL-12 produced by the Flexi-IL12 gene, we constructed a new vector whereby expression of IL-12 was controlled by an NFAT-responsive promoter, which could drive IL-12 secretion only upon cell activation resulting from TCR engagement (16). To evaluate the relative efficacy of the anti–VEGFR-2 CAR–transduced cells alone or cotransduced with either the Flexi-IL12 or the inducible NFAT-IL-12, we adoptively transferred decreasing doses of syngeneic T cells cotransduced with anti–VEGFR-2 CAR and the constitutive Flexi-IL12 or inducible NFAT-IL12 vectors into sublethally irradiated (5 Gy) mice bearing vascularized subcutaneous B16 melanoma established for 12 days. Control groups received T cells transduced with either empty vector or anti–VEGFR-2 CAR alone or empty vector and Flexi-IL12 or NFAT-IL12 in various numbers as indicated in Fig. 4A. Five hundred thousand T cells engineered with anti– VEGFR-2 CAR and constitutively expressing Flexi-IL12, mediated dramatic tumor regression; however, mortality of mice was again seen if cell numbers were increased to 1 × 106. In contrast, cells expressing the anti–VEGFR-2 CAR and the inducible NFAT-IL12 coexpressing T cells induced long-term tumor regression at all doses from 5 × 105 to 12 × 106 (Fig. 4A and B) in the absence of any apparent toxicity as measured by body weight loss and pathologic examination of tissues from treated mice (data not shown).
Figure 4.
T cells engineered with anti–VEGFR-2 CAR and inducible IL-12 caused durable regressions of B16 melanoma without dose limiting toxicities, and host lymphodepletion is required for their enhanced antitumor effect. C57BL/6 mice bearing 12-day-old subcutaneous B16 melanoma were treated with indicated numbers of syngeneic T cells transduced with various retroviral vectors or left untreated as shown in the figures.Aand B, all mice received 5 Gy TBI before T-cell transfer. C, only some groups received TBI (+XRT) before T-cell transfer. Each treatment group included a minimum 5 mice. Serial, blinded tumor measurements were obtained and the products of perpendicular diameters were plotted ± SEM. P values shown in A and B: anti–VEGFR-2 CAR and IL-12–cotransduced T cells versus anti–VEGFR-2 CAR alone transduced T cells. P values shown in C: anti–VEGFR-2 CAR and NFAT-IL12–transduced T cells with 5 Gy TBI versus without TBI (–XRT). A, mice treated with 1 × 106 anti–VEGFR-2 CAR and Flexi-IL12–transduced T cells died by day 14 post-cell transfer (open square symbols).
Taken together, these data show that toxicities associated with the constitutively expressed IL-12 gene transduced VEGFR-2–specific T cells could be eliminated without compromise to their antitumor effects by restricting IL-12 expression and accumulation locally to the tumor site using an inducible NFAT-IL12. Therefore, in all of our experiments described in the remainder of the study the NFAT-IL12 rather than the Flexi-IL12 vector was used to transduce T cells along with the anti–VEGFR-2 CAR.
Host lymphodepletion before adoptive transfer improved the antitumor effect of anti–VEGFR-2 CAR and IL-12–cotransduced T cells
In prior study targeting the gp100 antigen on the B16 melanoma using pmel-1 TCR transgenic T cells, the therapeutic effect was enhanced if endogenous cells were depleted before ACT with 5 Gy total body irradiation (TBI; refs. 15–17). It has been shown that host lymphodepletion facilitated the function and persistence of the adoptively transferred T cells by increasing the availability of homeostatic cytokines (17) as well as eliminating immune suppressor cell populations in the tumor microenvironment (18). As shown in Fig. 4C, anti–VEGFR-2 CAR and NFATIL12–cotransduced T cells induced a significant tumor regression compared with the control groups in the absence of host preconditioning but this therapeutic effect was significantly enhanced and caused durable cancer regressions if mice received 5 Gy TBI before ACT.
Anti–VEGFR-2 CAR–transduced T cells effectively infiltrated the tumor when cotransduced with IL-12
We have previously shown that anti–VEGFR-2 CAR–transduced T cells could effectively infiltrate, expand in the tumor, and mediate tumor growth inhibition if adoptively transferred into preconditioned mice bearing established tumors. Importantly, administration of exogenous IL-2 was absolutely required to achieve this effect (7). However, in our study, in multiple experiments we consistently documented regression of established tumor seven with as few as 5 × 105 anti–VEGFR-2 CAR and IL-12–cotransduced T cells in the absence of IL-2 administration.
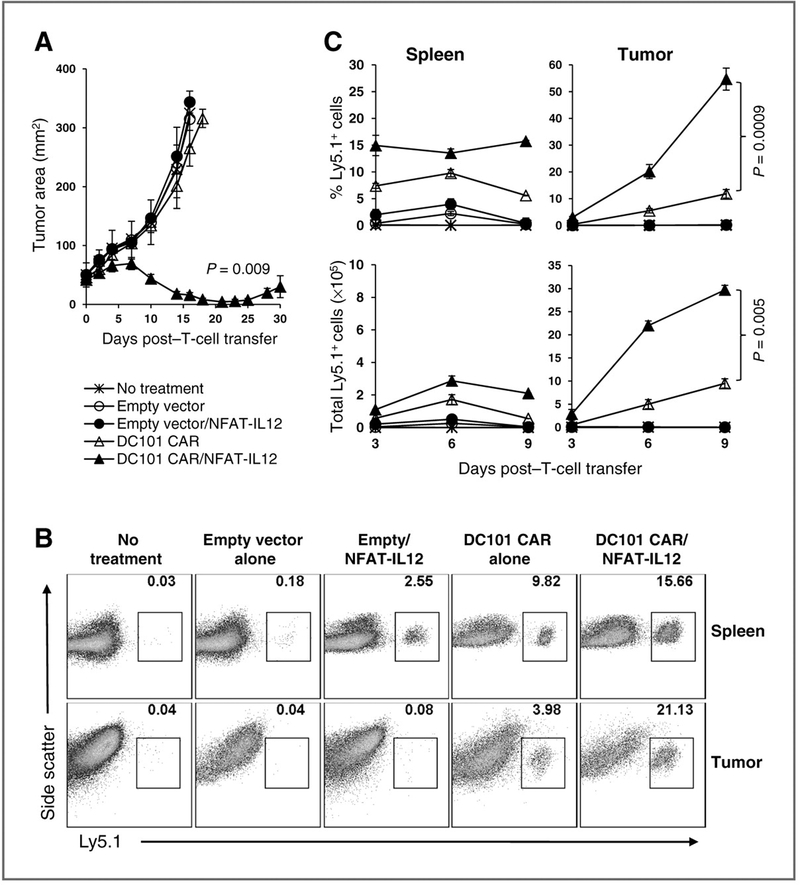
To gain further insights into the mechanisms of enhanced tumor eradication by the adoptively transferred cells cotransduced with the anti–VEGFR-2 CAR and IL-12, we studied the ability of these cells to infiltrate and persist in the tumor. We adoptively transferred 1 × 106 Ly5.1 congenically gene marked T cells engineered with an empty vector or anti–VEGFR-2 CAR alone or cotransduced with NFAT-IL12 into sublethally irradiated tumor-bearing mice and harvested the spleens and tumors at 3, 6, and 9 days after transfer. Only the cotransduced cells mediated tumor regression as seen in Fig. 5A. Single-cell preparations of the spleen and tumor tissues of mice from different groups were analyzed for the percentage and total number of viable adoptively transferred T cells expressing Ly5.1+ by flow cytometry.
Figure 5.
Enhanced tumor infiltration of adoptively transferred anti–VEGFR-2 CAR and IL-12–cotransduced T cells in mice bearing established B16-F10 tumor. C57BL/6 mice–bearing B16 melanoma tumor were sublethally irradiated with 5 Gy TBI and treated with 1 × 106 Ly5.1+ syngeneic T cells transduced with anti–VEGFR-2 CAR or empty vector alone or either of these vectors cotransduced with NFAT-IL12 vector. Control group received no treatment. Each group included minimum of 14 mice. A, serial, blinded tumor measurements were obtained from 5 mice per group and the products of perpendicular diameters were plotted ± SEM. Tumors and spleens of 3 mice from each group were excised at different time points post-therapy and processed to obtain single-cell suspensions and analyzed by flow cytometry. Percentage of the Ly5.1+ lymphocytes was determined in total viable fraction of the cell preparations by flow cytometry. Absolute numbers of Ly5.1+ cells were determined by multiplying the percentage of Ly5.1+ cells by the total number of viable cells. B, representative FACS data from single-cell preparations of spleen and tumor tissues from 1 mouse in each group obtained on day 6 post-ACT showing the percentage Ly5.1+ cells gated in the total viable cell population. C, pooled data obtained from 3 mice from each group collected at indicated time points post-ACT showing the percentage and total number of Ly5.1+ cells in spleen and tumor tissues. Data represented as mean ± SEM.
Representative fluorescence-activated cell sorting (FACS) data showing the frequency of adoptively transferred Ly5.1+ T cells in the total viable cell populations of spleen and tumor tissues from 1 mouse in each group on 6 days post–T-cell transfer is presented in Fig. 5B. The mean percentage and the total number of Ly5.1+ cells in the spleen and tumor tissues obtained from 3 mice per group at different time point post–T-cell transfer are shown in Fig. 5C. There was an increase in both the percentage and number of adoptively transferred Ly5.1+ T cells in the spleen and in the tumor tissues of mice treated with the anti–VEGFR-2 CAR-transduced cells as well as cells cotransduced with anti–VEGFR-2 CAR and IL-12 at all time points studied. Whereas, T cells transduced with an empty vector only or cotransduced with NFAT-IL12 were found in the spleen and tumor in very small numbers on day 3 posttransfer. The anti–VEGFR-2 CAR and NFAT-IL12–cotransduced T cells infiltrated tumors rapidly and significantly expanded in larger numbers than T cells expressing anti–VEGFR-2 CAR alone, possibly due to the combined impact on tumor vasculature by the CAR and IL-12. This was associated with a rapid tumor regression in mice receiving anti–VEGFR-2 CAR and NFAT-IL12–cotransduced T cells as shown in Fig. 5A.
IL-12–responsive host cells other than T and B cells are required for tumor regression mediated by anti–VEGFR-2 CAR and IL-12–engineered T cells
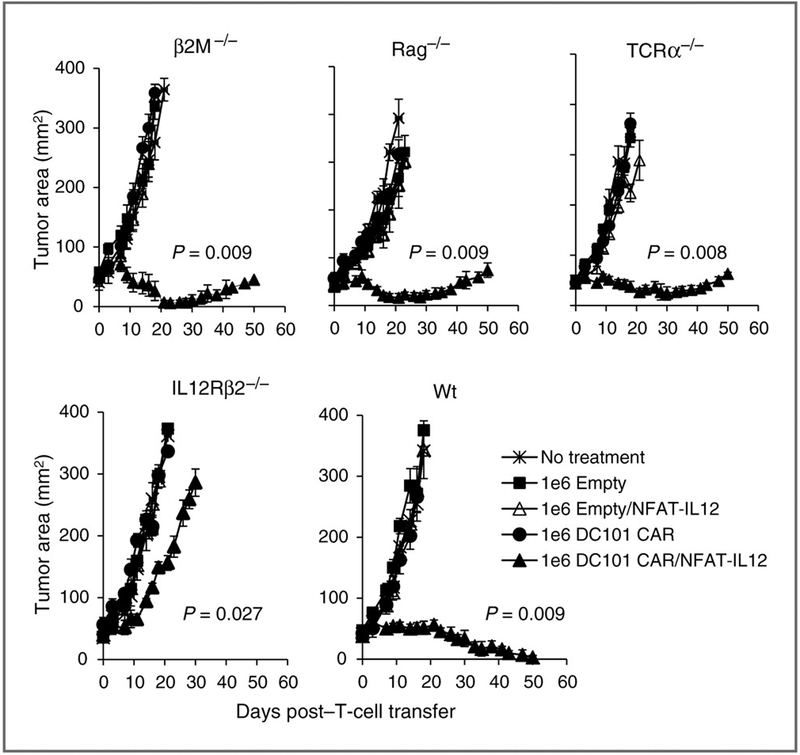
We next undertook a series of in vivo experiments with various endogenous immune cell knockout mice to dissect the mechanism(s) underlying the antitumor effect of adoptively transferred T cells coexpressing anti–VEGFR-2 CAR and IL-12. We established 12-day-old subcutaneous B16 melanomas in wild-type (WT), Rag1−/− (which are devoid of endogenous T and B cells), β2m−/− (which express very low levels of MHC class I molecules and are deficient in CD8+ T lymphocytes), TCRα−/− (lack TCRαβ T cells), and IL12rb2−/− mice (defective in IL-12–mediated signaling) in C57BL/6 background. We adoptively transferred 1 × 106 syngeneic T cells cotransduced with anti–VEGFR-2 CAR and NFAT-IL12 vectors into these sublethally irradiated mice (5 Gy TBI) and followed the tumor growth. Control groups received no T cells or either empty vector or anti–VEGFR-2 CAR alone or empty vector and NFAT-IL12–engineered T cells.
As shown in Fig. 6, adoptively transferred T cells coexpressing anti–VEGFR-2 CAR and IL-12 were effective in mediating tumor regression in mice lacking endogenous T or B cells or host cells lacking expression of MHC Class I. However, tumor regression was severely impaired in IL12rb2−/− mice. Similar results were observed in 2 independent experiments. These data suggest that host cell responsiveness to IL-12 was critical in mediating tumor regression. Our results clearly show that endogenous MHC-restricted T-cell responses are not necessary to induce tumor regression, whereas host cell responsiveness to transgenic IL-12 released by the antigen-experienced anti–VEGFR-2 CAR T cells is critically required for tumor regression.
Figure 6.
Enhanced tumor regression mediated by anti–VEGFR-2 CAR and IL-12 T–cotransduced T cells was independent of host T and B cells or MHC class I expression of host cells but required a host response to transgenic IL-12. Mice that were genetically knocked out for indicated immune cell components/function in C57BL/6 background were subcutaneously implanted with B16 melanoma cells 12 days before therapy and treated with 1 × 106 T cells retrovirally modified to express anti–VEGFR-2 CAR or an empty vector alone or together with NFAT-IL12 vector. No treatment groups served as control. All mice received 5 Gy TBI before T-cell therapy. Serial, blinded tumor measurements were obtained from 5 mice per group and the products of perpendicular diameters obtained. All data are expressed as a mean ± SEM and is representative of at least 2 independent experiments. P values shown are compared with group treated with anti–VEGFR-2 CAR only transduced T cells.
Anti–VEGFR-2 CAR–transduced T-cell treatment effectively reduced the intratumoral myeloid-derived suppressor cells
Tumor infiltration by immune suppressive cells coupled with acquired T-cell nonresponsiveness play critical roles in tumor-associated immune evasion. Accumulating evidence suggests that host myeloid-derived suppressor cells (MDSC) can be present in tumors and promote immune evasion in cancer patients and tumor-bearing mice (19–24). Recently, it has been shown that there is a high level of expression of VEGFR-2 on several cell types in the tumor stroma including the CD11b+ immature myeloid suppressor cell subsets comprising macrophages, immature monocytes and immature dendritic cells and CD4+CD25+ immunosuppressive regulatory T cells as well as the pericytes in the tumor vessels and tumor endothelial cells (25–31). Importantly, these VEGFR2+CD11b+ myeloid cell subsets have been shown to express the plasticity to transform into endothelial cells, participate in tumor angiogenesis (29, 32) and thereby enhance tumor growth and metastasis, induce differentiation of regulatory suppressor cells in the tumor, and contribute to tumor resistance to radiotherapy, antiangiogenic therapy, and immunotherapy (29, 32, 33). Accumulating evidence suggests that myeloid cells in the tumor vasculature as well as some tumor cells express functional receptor for IL-12 (8, 34–37). These findings prompted us to determine whether anti–VEGFR-2 CAR and IL-12–engineered T-cell therapy caused changes in the CD11b+Gr1+ MDSC suppressor cell populations. Therefore, we analyzed the frequency and total number of CD11b+Gr1+ MDSC in single-cell preparations of the spleen and tumor tissues of B16 tumor–bearing mice treated with T cells described in the previous experiment shown in Fig. 5.
Representative FACS data showing the frequency of CD11b+Gr1+ cells in the total viable cell fractions of spleen and tumor tissues from 1 mouse in each group analyzed on day 6 is presented in Supplementary Fig. S1A. The mean percentage and the total number of CD11b+Gr1+ cells in the spleen and tumor tissues obtained from 3 mice per group at different time point post–T-cell transfer are shown in Supplementary Fig. S1B. Both the percentage and the absolute numbers of MDSCs were dramatically decreased in both the spleen and tumor tissues of mice treated with T cells expressing either anti–VEGFR-2 CAR with or without NFAT-IL12 at all the time points analyzed (Supplementary Fig. S1B) compared with groups that received T cells transduced with either an empty vector alone or empty vector plus NFAT-IL12 gene, or no T cells. There were no significant differences, either in the frequency or the absolute numbers of CD11b+Gr1+ MDSC between groups treated with T cells expressing anti–VEGFR-2 CAR alone or CAR plus NFATIL12 suggesting that this effect was predominantly mediated by the anti–VEGFR-2 CAR. Moreover, reduction in MDSCs ubiquitously occurred both in the spleen as well as in the tumor indicating that the anti-VEGFR CAR–engineered T-cell treatment modulated the systemic as well as intratumoral accumulation and/or expansion of MDSCs.
In aggregate, these results indicate that the antitumor effect of anti–VEGFR-2 CAR and IL-12–transduced T cells was dependent on targeting one or more of the IL-12–responsive host cell subset(s) in the tumor environment with the ability to provide antigen-specific activation of anti–VEGFR-2CAR–transduced T cells and release of inducible IL-12.
Discussion
IL-12 is a heterodimeric Th1-associated cytokine produced by activated macrophages and dendritic cells, B cells, and possibly other cells and is involved in regulation of NK cells, peripheral blood mononuclear cells, and B cells, either directly or through stimulation of secretion of secondary cytokines (8–11). Evidence suggests that IL-12 induces proliferation of T cells only in combination with a strong antigen signal and plays a role in the amplification of an antigen-specific T-cell response (38). In prior studies the antitumor, antimetastatic, and antiangiogenic activities of recombinant IL-12 have been extensively studied using a variety of murine tumor models and the critical roles of T cells, NK cells, macrophages, and IFN-γ in IL-12–induced antitumor effects have been established (8–11). Recombinant IL-12 therapy, however, is restricted by severe toxicities including deaths that prevent its systemic administration to achieve therapeutic levels in solid tumor lesions in patients with cancer (12–14), thus limiting its potential therapeutic applications. Attempts to sequester the effects of IL-12 in the tumor environment by recombinant molecules comprising IL-12 protein conjugated to antibodies against either tumor-specific molecules such as HER2 (39) and tumor vasculature– specific peptides such as ED-B domain of fibronectin (40) CNGRC (41), RGD (42), and VNTANST (43) resulted in minimal improvements in the efficacy of treatments.
Wagner and colleagues (2004) first suggested a gene therapy approach to locally deliver IL-12 to tumor sites using antigen-specific T cells (44). This study showed that transduction of single-chain IL-12 to Hodgkin tumors by Epstein Barr virus–specific CTL significantly improved their effector function in vitro through enhancing the secretion of Th1 cytokines, including IFN-γ and TNF-α, and reducing the secretion of Th2 cytokines, IL-4, and IL-5 as well as through antagonizing the immune suppressive effects of TGF-β produced by the tumors. Following this report, our group (15, 16) and others (45) have further established the potential of adoptive transfer of tumor-specific CTLs engineered with either a constitutively expressed single-chain IL-12 or inducible IL-12 to achieve delivery of IL-12 to the tumor site and induce the regression of large established tumors in mice.
The ability of the T cell–mediated delivery of IL-12 to the tumor described in these tumor-targeting strategies is limited by the ability to identify T cells specific for tumor-associated antigens. To expand the potential of this gene therapy approach to the treatment of patients with multiple tumor types, we investigated the feasibility of targeting IL-12 to the tumor microenvironment using T cells redirected against VEGFR-2 that is overexpressed on the tumor vasculature and stroma of most solid tumors (3, 25–31). In this study, murine T cells were successfully transduced with retroviral vectors expressing an anti–VEGFR-2 CAR (DC101 CAR) and a single-chain IL-12 gene that was expressed either constitutively under the transcriptional control of the retroviral LTR (Flexi-IL12) or an inducible NFAT promoter that drove IL-12 expression strictly in activated T cells (NFAT-IL12). Murine T cells transduced with the Flexi-IL12 vector constitutively secreted IL-12. However, IL-12 secretion using the NFAT-IL12 vector was obtained only after T-cell activation either through nonspecific stimulation or antigen-specific stimulation if the cells were cotransduced with anti–VEGFR-2 CAR (Fig. 1B and C). In addition, these cells produced high levels of IFN-γ and TNF-α upon antigen-specific activation (Fig. 2A and B).
Furthermore, we have shown that adoptive transfer of a small number (5 × 105) of T cells genetically engineered to coexpress anti–VEGFR-2 CAR and murine single-chain Flexi-IL-12 exhibited enhanced antitumor activity by mediating regression of multiple types of established subcutaneous solid tumors in 2 different strains of mice without the need for exogenous IL-2 administration (Fig. 3). Importantly many mice in this treatment group showed prolonged tumor-free survival and were probably cured (Fig. 3). Although robust tumor regression was seen in mice bearing subcutaneous tumors if treated with low numbers of 5 × 105 T cells coexpressing anti–VEGFR-2 CAR and single-chain Flexi-IL12, severe morbidity, and mortalities were consistently documented if the number of transferred T cells was increased to 1 × 106 or more (Fig. 4A).
In contrast to these results, T cells coexpressing the anti– VEGFR-2 CAR and inducible NFAT promoter driven IL-12 were able to mediate significant tumor regression when adoptively transferred into tumor-bearing mice and did not cause toxicity even at doses as high as 12 × 106 cells (Fig. 4B). Although we have not tested the systemic levels of IL-12 in mice treated with anti–VEGFR-2 CAR and Flexi-IL12- or NFAT-IL12–transduced T cells, data from the in vitro experiments shown in Figs. 1B and 2 suggest that the anti–VEGFR-2 CAR and Flexi-IL12–transduced T cells secrete high levels of IL-12, IFN-γ, and TNF-α, which could likely be attributed to their dose-dependent toxicity.
Furthermore, to achieve enhanced therapeutic efficacy of this adoptive therapy with anti–VEGFR-2 CAR and IL-12–transduced T cells, pretreatment lymphodepletion of host was required as shown previously (16). It was necessary for the genes encoding anti–VEGFR-2 CAR and IL-12 to be expressed in the same cell to achieve therapeutic effectiveness.
Systemic trafficking of adoptively transferred T cells is a physiologic characteristic independent of antigen specificity. However, the presence of predefined tumor specificity in the transferred T-cell population is required for their retention in the tumor and initiation of an immune response. Our results showed that provision of a CAR with a predefined specificity against VEGFR-2 was absolutely required to redirect the adoptively transferred cells specifically to the tumor site. Most importantly cotransduction of anti–VEGFR-2 CAR and IL-12 resulted in a substantial increase of the expansion and long-term persistence of the transferred T cells compared with cells transduced with either the anti–VEGFR-2 CAR alone or cotransduced with an empty vector and IL-12 (Fig. 5A–C), which may account in part for their improved effectiveness in inducing tumor regression. Similarly, T cells transduced with an empty vector and NFAT-IL12 were found in the spleen and tumor on day 3 and 6 posttransfer, albeit at low numbers, but they failed to expand or mediate antitumor immunity suggesting that without the antigen-specific activation they were unable to release IL-12 and sustain their homeostatic proliferation and survival (Fig. 5B and C). Thus, anti–VEGFR-2 CAR and NFAT-IL12–engineered T cells were capable of delivering IL-12 at therapeutic levels locally in the tumor microenvironment upon antigen-specific stimulation, leading to increased infiltration and/or expansion of transferred cells in the tumor and enhanced tumor regression (Fig. 5A). Moreover, the transgenic IL-12 seemed to act upon the host cells to induce an antitumor immune response. The effectiveness of the adoptive therapy conducted in T and B cell defective mice showed that the antitumor impact of the anti–VEGFR-2 CAR and IL-12–cotransduced T cells was not dependent on endogenous T and B cells. However, host cell expression of IL-12 receptor was required to mediate robust tumor regression (Fig. 6). In some experiments, recurrence of tumor growth was observed in some animals treated with anti–VEGFR-2 CAR and NFAT-IL12–transduced T cells irrespective of the presence or absence of endogenous hose immune cells. Because NFAT-IL12 expression in transduced cells requires T-cell activation, it is possible that in some animals reduced tumor recognition by these transferred T cells resulted in decreased local concentration of IL-12 leading to tumor regrowth.
The mechanism of the therapeutic effect of IL-12 has not yet been completely understood. IL-12 has been described to stimulate the immune system by enhancing the immune activity of NK cells and CD8 T lymphocytes (8–11, 38). In previous studies, adoptive therapy of IL-12–engineered gp100-specific transgenic pmel-1 T cells into a lymphodepleted B16 tumor-bearing host resulted in a dramatic increase in endogenous NK and CD8+ T cells within the tumor and indirectly suggested their role in facilitating tumor destruction (16). Notably, depletion of NK cells did not alter the antitumor effect of IL-12–transduced tumor-specific T cells in the subsequent study (47). In our experiments, ACT infusion cell products were negative for the presence of NK cells as determined by FACS analysis (data not shown) ruling out the contribution of donor-derived NK cells in mediating tumor regression. However, a recent study showed that IL-12 initiated local antitumor immunity by stimulating a subset of NKp46+ lymphoid tissue-inducer (LTi) cells, independent of T lymphocytes or NK cells (46). Although our data indicated the independence of the anti–VEGFR-2 CAR and IL-12–cotransduced T cells for endogenous T and B cells and MHC class I restricted antigen presentation by host cells to inducing tumor regression, involvement of host NK cells, or NKp46+ LTi cells in our therapeutic setting has not yet been ruled out and requires further studies.
Recently, a detailed study in a mouse tumor model showed that IL-12–dependent enhancement of the antitumor function of CD8+ T cells expressing defined antigen-specific TCR occurred through induction of an inflammatory response and sensitization of bone marrow–derived stromal cells within tumors that changed the antigen presentation properties of bone marrow–derived myeloid infiltrating cells within regressing lesions (47). In corroboration of these findings, a recent study showed that adoptive transfer of T cells redirected against CEA-expressing malignancies through a CAR against CEA and further engineered to release inducible IL-12 upon CAR engagement in the tumor lesion, resulted in destruction of both antigen positive and antigen-loss cancer lesions (45). This study also documented that IL-12–mediated accumulation of activated macrophages and elevated levels of TNF-α in the tumor site were critical to the antitumor efficacy against antigen loss tumor lesions. Similarly, in patients with cancer, recombinant IL-12 treatment was shown to mediate antitumor effects through induction of Th1-type responses and infiltration of both NK cells and macrophages in tumor lesions (48).
The direct antiangiogenic effect of IL-12, in addition to its proinflammatory and immunostimulatory effects on both adoptive and innate immune cells have been well established (8–11). Also IL-12 receptor (IL-12R) expression on myeloid cells in the tumor stroma as well as some tumor cells has been reported (8, 34–37). Accumulating evidence suggest that tumor-infiltrating immunosuppressive immature myeloid cells and regulatory T cells, as well as the endothelial cells and pericytes in the tumor vessels express VEGFR-2 (29, 32, 33) and significantly contribute to tumor angiogenesis, growth, and metastasis, and induce resistance to therapy (19–24, 29, 32, 33). Here, we showed that adoptive transfer of anti–VEGFR-2 CAR–transduced T cells into tumor-bearing mice caused significant reduction in the frequency and total number of CD11b+Gr1+ MDSCs both in systemic as well as at the intratumoral sites (Supplementary Fig. S1A and S1B). We speculate that these changes in the tumor environment and/or clearance of this immunosuppressive tumor microenvironment facilitated productive tumor regression for which IL-12-mediated enhancement in infiltration/expansion and persistence of the adoptively transferred T cells in the tumor site was required. This does not seem to be due to any specific antigen presentation by host cells. In contrast to results seen using antigen-specific T cells cotransduced with IL-12, which required the expression of class I molecules on host cells (15, 47), T cells cotransduced with anti–VEGFR-2 CAR and IL-12 were fully effective in the absence of host expression of MHC class I. Although we do not have any direct evidence to support our observation on the impaired tumor regression by anti–VEGFR-2 CAR and IL-12 cotransduced T cells in mice lacking IL-12 signaling, we are tempted to speculate that the immune cellular components of the tumor microenvironment is different in these mice compared with wild-type mice and suggest the existence of one or more of the host cellular component(s) that required IL-12–mediated signaling and probably were involved in the tumor regression. These observations illustrate the complexity of the biological reactions following systemic adoptive immunotherapy with anti–VEGFR-2 CAR and IL-12–transduced T cells because the expression of VEGFR2 as well as IL-12 receptor was not just limited to endothelial cells and other immune cells. Other cell types including cancer stem cells and epithelial cells surrounding lung tumor lesions have been shown to express these molecules (49, 50). Recently, we showed that the adoptively transferred anti–VEGFR-2 CAR–transduced cells specifically localized to tumor endothelium when adoptively transferred in vivo into tumor-bearing mice (7) and mediated tumor regression likely through targeting the VEGFR-2–expressing cells in the tumor vasculature. However, the extensive tumor necrosis following our treatment has precluded our ability to dissect out the host cellular components in the tumor and to directly observe the destruction of tumor vasculature in vivo. A better understanding of these unknown in vivo cellular components and their interactions and the biological mechanisms that lead to tumor eradication by anti–VEGFR-2 CAR and IL-12 will help design therapeutic strategies with increased efficacy.
Together, our data show that targeting local expression of IL-12 in the tumor environment through adoptive transfer of T cells cotransduced with the anti–VEGFR-2 CAR and an inducible IL-12 may be a desirable therapeutic approach in the treatment of solid malignancies in which antigenic signatures have not yet been identified and provide a new perspective to adoptive immunotherapy of cancer.
Supplementary Material
Translational Relevance.
This article provides preclinical evidence that adoptive transfer of less than 1 × 106 T cells cotransduced with a chimeric antigen receptor targeted against the VEGF receptor-2, which is overexpressed in tumor vasculature and the interleukin-12 gene could effectively mediate regression of established syngeneic tumors of different histologies without the need for exogenous IL-2 administration. Our results suggest that this may be a promising approach for treating patients with a variety of solid tumor types and provide a rationale for exploring this approach in clinical trials.
Acknowledgments
The authors thank Douglas Palmer, Robert Reger, Zulmarie Franco, Dorina Frasheri, and David Jones (Surgery Branch, NCI) for help with the animal studies.
Grant Support
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following nonmyeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005; 23:2346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008;26:5233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999;5:1359–64. [DOI] [PubMed] [Google Scholar]
- 4.Maeurer MJ, Gollin SM, Storkus WJ, Swaney W, Karbach J, Martin D, et al. Tumor escape from immune recognition: loss of HLA-A2 melanoma cell surface expression is associated with a complex rearrangement of the short arm of chromosome 6. Clin Cancer Res 1996;2:641–52. [PubMed] [Google Scholar]
- 5.Ganss R, Limmer A, Sacher T, Arnold B, Hammerling GJ. Autoaggression and tumor rejection: it takes more than self-specific T-cell activation. Immunol Rev 1999;169:263–72. [DOI] [PubMed] [Google Scholar]
- 6.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol 2000;74:181–273. [DOI] [PubMed] [Google Scholar]
- 7.Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest 2010;120:3953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinchieri G Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003;3:133–46. [DOI] [PubMed] [Google Scholar]
- 9.Trinchieri G Immunobiology of interleukin-12. Immunol Res 1998;17:269–78. [DOI] [PubMed] [Google Scholar]
- 10.Hendrzak JA, Brunda MJ. Antitumor and antimetastatic activity of interleukin-12. Curr Top Microbiol Immunol 1996;213 (Pt 3):65–83. [DOI] [PubMed] [Google Scholar]
- 11.Wigginton JM, Gruys E, Geiselhart L, Subleski J, Komschlies KL, Park JW, et al. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest 2001;108:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen J IL-12 deaths: explanation and a puzzle. Science 2005;270:908. [DOI] [PubMed] [Google Scholar]
- 13.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 1997;90:2541–8. [PubMed] [Google Scholar]
- 14.Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol 1999;27:58–63. [DOI] [PubMed] [Google Scholar]
- 15.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res 2010;70:6725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther 2011;19:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005;202:907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 1982;155:1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almand B, Clark JI, Nikitina E, van BJ, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 2001;166:678–89. [DOI] [PubMed] [Google Scholar]
- 20.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 2005;65:3044–8. [DOI] [PubMed] [Google Scholar]
- 21.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 2007;25:2546–53. [DOI] [PubMed] [Google Scholar]
- 22.Bronte V Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol 2009;39:2670–2. [DOI] [PubMed] [Google Scholar]
- 23.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev 2006;213:131–45. [DOI] [PubMed] [Google Scholar]
- 25.Udagawa T, Puder M, Wood M, Schaefer BC, D’Amato RJ. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J 2006;20:95–102. [DOI] [PubMed] [Google Scholar]
- 26.Larrivee B, Pollet I, Karsan A. Activation of vascular endothelial growth factor receptor-2 in bone marrow leads to accumulation of myeloid cells: role of granulocyte-macrophage colony-stimulating factor. J Immunol 2005;175:3015–24. [DOI] [PubMed] [Google Scholar]
- 27.Reddy K, Zhou Z, Schadler K, Jia SF, Kleinerman ES. Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing’s tumor vessels. Mol Cancer Res 2008;6:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang N, Fang Z, Contag PR, Purchio AF, West DB. Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood 2004;103:617–26. [DOI] [PubMed] [Google Scholar]
- 29.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8:618–31. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Onishi H, Wada J, Yamasaki A, Tanaka H, Nakano K, et al. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol 2010;40:197–203. [DOI] [PubMed] [Google Scholar]
- 31.Duignan IJ, Corcoran E, Pennello A, Plym MJ, Amatulli M, Claros N, et al. Pleiotropic stromal effects of vascular endothelial growth factor receptor 2 antibody therapy in renal cell carcinoma models. Neoplasia 2011;13:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004;6:409–21. [DOI] [PubMed] [Google Scholar]
- 33.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice.J Clin Invest 2010;120: 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krawczyk P, Wojas K, Milanowski P, Rolinski J. Myeloid and lymphoid dendritic cells and cytotoxic T lymphocytes in peripheral blood of non-small cell lung cancer patient–a pilot study. Adv Med Sci 2006;51:160–3. [PubMed] [Google Scholar]
- 35.Pistoia V, Cocco C, Airoldi I. Interleukin-12 receptor beta2: from cytokine receptor to gatekeeper gene in human B-cell malignancies. J Clin Oncol 2009;27:4809–16. [DOI] [PubMed] [Google Scholar]
- 36.Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology 2011;133:221–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Airoldi I, Di CE, Cocco C, Taverniti G, D’Antuono T, Ognio E, et al. Endogenous IL-12 triggers an antiangiogenic program in melanoma cells. Proc Natl Acad Sci U S A 2007;104:3996–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertagnolli MM, Lin BY, Young D, Herrmann SH. IL-12 augments antigen-dependent proliferation of activated T lymphocytes. J Immunol 1992;149:3778–83. [PubMed] [Google Scholar]
- 39.Peng LS, Penichet ML, Morrison SL. A single-chain IL-12 IgG3 antibody fusion protein retains antibody specificity and IL-12 bioactivity and demonstrates antitumor activity. J Immunol 1999;163:250–8. [PubMed] [Google Scholar]
- 40.Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L, et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol 2002;20:264–9. [DOI] [PubMed] [Google Scholar]
- 41.Colombo G, Curnis F, De Mori GM, Gasparri A, Longoni C, Sacchi A, et al. Structure-activity relationships of linear and cyclic peptides containing the NGR tumor-homing motif. J Biol Chem 2002;277:47891–7. [DOI] [PubMed] [Google Scholar]
- 42.Dickerson EB, Akhtar N, Steinberg H, Wang ZY, Lindstrom MJ, Padilla ML, et al. Enhancement of the antiangiogenic activity of interleukin-12 by peptide targeted delivery of the cytokine to alphavbeta3 integrin. Mol Cancer Res 2004;2:663–73. [PubMed] [Google Scholar]
- 43.Cutrera J, Dibra D, Xia X, Hasan A, Reed S, Li S. Discovery of a linear peptide for improving tumor targeting of gene products and treatment of distal tumors by IL-12 gene therapy. Mol Ther 2011;19:1468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner HJ, Bollard CM, Vigouroux S, Huls MH, Anderson R, Prentice HG, et al. A strategy for treatment of Epstein-Barr virus-positive Hodgkin’s disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther 2004;11:81–91. [DOI] [PubMed] [Google Scholar]
- 45.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011;71:5697–706. [DOI] [PubMed] [Google Scholar]
- 46.Eisenring M, vom BJ, Kristiansen G, Saller E, Becher B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol 2010;11:1030–8. [DOI] [PubMed] [Google Scholar]
- 47.Kerkar SP, Goldszmid R, Muranski PP, Chinnasamy D, Yu Z, Reger RN, et al. IL-12 triggers an acute-inflammatory environment that reverses dysfunctional antigen-presentation by myeloid-derived cells within murine tumors. J Clin Invest 2011;121:4746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Herpen CM, Looman M, Zonneveld M, Scharenborg N, de Wilde PC, van de Locht L, et al. Intratumoral administration of recombinant human interleukin 12 in head and neck squamous cell carcinoma patients elicits a T-helper 1 profile in the locoregional lymph nodes. Clin Cancer Res 2004;10:2626–35. [DOI] [PubMed] [Google Scholar]
- 49.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010;468:829–33. [DOI] [PubMed] [Google Scholar]
- 50.Airoldi I, Di CE, Cocco C, Caci E, Cilli M, Sorrentino C, et al. IL-12 can target human lung adenocarcinoma cells and normal bronchial epithelial cells surrounding tumor lesions. PLoS One 2009;4:e6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.