Abstract
Muscle specific tyrosine kinase antibody positive myasthenia gravis (MuSK- MG) is characterized by autoantibodies against the MuSK protein of the neuromuscular junction resulting in weakness of bulbar and proximal muscles. We previously demonstrated that patients with MuSK-MG have increased pro-inflammatory Th1 and Th17 responses. Tacrolimus, an immunosuppressant used in AChR-MG and transplantation patients, inhibits T cell responses through interference with IL-2 transcription. The therapeutic efficacy and immunological effect of tacrolimus in MuSK-MG is unclear. In the current study we examined the proliferation, phenotype and cytokine production of CD4+ and CD8+ T cells in peripheral blood mononuclear cells of MuSK-MG following a 3-day in vitro culture with or without tacrolimus. We determined that tacrolimus profoundly suppressed CD4 and CD8 T cell proliferation and significantly suppressed Th1 and Th17 responses, as demonstrated by a reduced frequency of IFN-γ, IL-2, and IL-17 producing CD4 T cells and reduced frequencies of IFN-γ and IL-2 producing CD8 T cells. Tacrolimus also inhibits pathogenic Th17 cells coproducing IL-17 and IFN-γ. In addition, tacrolimus suppressed follicular T helper cell (Tfh) and regulatory T helper cell (Treg) subsets. These findings provide preliminary support for tacrolimus as a potential alternative immunosuppressive therapy for MuSK-MG.
Keywords: Myasthenia gravis, MuSK-myasthenia gravis, Tacrolimus, Th1, Th17, pathogenic Th17 cells, Follicular T helper cell, regulatory T helper cell
1. Introduction
Myasthenia gravis (MG) is a chronic autoimmune disease characterized by autoantibodies to postsynaptic proteins including acetylcholine receptor (AChR), muscle-specific tyrosine kinase (MuSK), lipoprotein receptor-related protein 4 (LRP4), and other components. By binding to their respective synaptic proteins, these autoantibodies inhibit neuromuscular transmission in the postsynaptic membrane resulting in skeletal muscle weakness. Approximately 5–8% of MG patients have autoantibodies against MuSK. These patients classically present with predominant bulbar, neck and proximal muscle weakness, and may have atrophy of involved muscles. MuSK MG patients tend to require more aggressive treatment, often with multiple immunosuppressives, and additional treatment options would be welcome to improve patient outcomes (Guptill et al., 2011b).
The presence of autoantibodies in MG supports a B cell mediated immunopathology and it has been shown that B cell maturation is dependent on CD4 T cells (Conti-Fine et al., 2008; Milani et al., 2003; Stathopoulos et al., 2018). CD4 T cells play a diverse role in adaptive immunity through their ability to differentiate into T helper subsets with defined roles to induce immune tolerance, promote inflammation, or support B cell function. In MuSK-MG, CD4 T cells exhibit enhanced inflammatory Th1 and Th17 responses (Yi et al., 2014; Yilmaz et al., 2015). Thus, targeted T cell therapies may be an effective and well tolerated treatment approach.
Tacrolimus is an immunosuppressant that inhibits T cell activation and proliferation. Several clinical trials have studied the efficacy and safety of tacrolimus in transplantation and AChR-MG (Evoli et al., 2002; Nagane et al., 2005; Ponseti et al., 2005; Ponseti et al., 2008). In transplant studies, tacrolimus inhibited Th1 and Th17 responses (Gallon et al., 2015). While tacrolimus is widely used in transplantation and in Asia for the treatment of AChR MG, no studies have reported the effect of tacrolimus in patients with MuSK MG or documented its immunological effects.
Given the reported activity of tacrolimus on Th1 and Th17 responses and strong evidence for involvement of these pathways in MuSK MG, the objective of this study was to characterize the inhibitory effects of tacrolimus on CD4 and CD8 T cell responses in MuSK-MG patients in order to provide preliminary support for the use of tacrolimus as a treatment option for MuSK-MG. Through a set of in vitro studies, we observed diminished T cell proliferation along with inhibition of Th1 and Th17 responses. Furthermore, tacrolimus inhibits the production of IL- 17+IFN-γ+ CD4 T cells, which has been characterized as the pathogenic subset of Thl7 cells (Gaublomme et al., 2015; Hirota et al., 2011). Collectively, this study demonstrates the ability of tacrolimus to inhibit proinflammatory Th1 and Th17 responses and provides rationale for pilot studies using tacrolimus to treat MuSK-MG.
2. Material and Methods
2.1. Study population and controls
Blood samples were obtained from 31 MuSK MG patients. MuSK MG patients were recruited during visits to the MG Clinics at Duke University and the University of North Carolina at Chapel Hill. All patients had detectable anti-MuSK antibodies according to commercially available testing (Athena Diagnostics, Worcester, MA), in addition to clinical and electrodiagnostic features consistent with MG. Key clinical information including demographics, disease duration, pharmacologic treatments, thymectomy status, Myasthenia Gravis Foundation of America (MGFA) severity class, and MG manual muscle testing (MG-MMT) (Table 1) were collected. Control subjects were matched for age and gender as closely as possible, weighed more than 110 pounds, were not receiving therapy for any chronic disease, and had no prior history of autoimmune disease. This study was approved by the Duke University Institutional Review Board and informed consent was obtained from each patient and normal donor.
Table 1:
Clinical characteristics of MuSK MG patients at the time of blood draw (N = 31).
| Age (yr) | F/M | Onset-age (yr) | Disease duration (mo) | Race | ThymX | MGFA | MMT | Treatment (daily dose) |
|---|---|---|---|---|---|---|---|---|
| 23 | Female | 22 | 13 | B | No | 2B | 3 | Pred 20 mg |
| 48 | Female | 36 | 150 | W | No | I | 1 | MMF 2000 mg |
| 60 | Female | 45 | 186 | B | No | 2B | 3 | Pred 10 mg + MMF2000mg |
| 23 | Female | 10 | 154 | B | No | 2A | 5 | MMF 1500mg |
| 46 | Female | 45 | 14 | B | No | I | 3 | Pred 20 mg |
| 29 | Female | 20 | 109 | B | No | 0 | 0 | None |
| 67 | Female | 49 | 218 | W | No | 2A | 3 | MMF 2000 mg |
| 56 | Female | 42 | 161 | W | Yes | 2B | 2 | None |
| 65 | Female | 53 | 139 | W | Yes | 1 | 3 | Pred 1.25 mg + MMF 2000 mg |
| 63 | Female | 51 | 146 | W | No | 3B | 23 | Pred 5 mg + AZA 150 mg |
| 64 | Female | 30 | 402 | W | Yes | 2B | 9 | MMF 500 mg |
| 31 | Female | 26 | 58 | B | Yes | 3B | 20 | Plasma exchange |
| 47 | Female | 34 | 157 | B | No | 2B | 3 | Pred 7.5 mg + MMF 2000 mg |
| 23 | Female | 21 | 20 | B | No | 2A | 2 | Pred 30 mg + MMF 2000 mg |
| 51 | Male | 43 | 94 | B | No | 1 | 1 | AZA 75 mg |
| 26 | Female | 17 | 112 | Mixed | Yes | 2B | 2 | Pred 20 mg + MMF 2500 mg |
| 58 | Female | 43 | 187 | B | Yes | 2B | 5 | MMF 2000 mg |
| 40 | Female | 34 | 78 | B | No | 2B | 5 | MMF 2000 mg |
| 31 | Female | 20 | 138 | W | No | 0 | 0 | None |
| 44 | Female | 29 | 185 | B | No | 2B | 6 | Pred 10 mg |
| 48 | Female | 32 | 184 | B | No | 2B | 7 | Pred 15 mg |
| 66 | Female | 48 | 215 | W | No | 2B | 4 | Pred 1 mg |
| 35 | Female | 33 | 28 | B | No | 3B | 26 | Mestinon 18mg + pred 30mg |
| 45 | Male | 37 | 104 | W | No | 2B | 9 | None |
| 28 | Female | 4 | 288 | W | No | 2A | 7 | Pred 7.5 mg + MMF 3000 mg |
| 48 | Female | 9 | 476 | W | No | 3B | 31 | Plasma exchange |
| 56 | Female | 36 | 235 | B | No | 2A | 21 | none |
| 58 | Female | 44 | 173 | B | No | 3B | 27 | Pred 20 mg |
| 53 | Male | 41 | 146 | B | Yes | 1 | 3 | Pred 5 mg |
| 28 | Female | 27 | 16 | W | No | 2B | 17 | MMF 2000 mg |
| 25 | Female | 5 | 239 | B | Yes | 3A | 4 | Pred 3.8 mg |
Abbreviations:AZA = azathioprine; B= black; d = day, F = female; mg = milligrams; M = male; MGFA =Myasthenia Gravis Foundation of America; MM = minimal manifestations; MMF = mycophenolate mofetil; MMT = myasthenia gravis manual muscle testing score at time of blood draw; Mo = months; Pred = predinison; ThymX = thymectomy; W = white; Yr =years.
2.2. Isolation and storage of peripheral blood mononuclear cells (PBMCs)
Peripheral blood was obtained by venipuncture and collected in acid-citrate-dextrose tubes (BD Vacutainer, Franklin Lake, NJ). PBMCs were separated by Ficoll (GE Healthcare, Uppsala, Sweden) density gradient centrifugation, washed and counted prior to storage. Cells were re-suspended in a 90% FBS (Gemini, West Sacramento, CA) and 10% DMSO (Sigma- Aldrich, St. Louis, MO) solution, and pro gressively cooled to −80°C in a CoolCell cell freezing container (BioCision, Larkspur, CA). The next day, the cells were stored in vapor phase liquid nitrogen for future use.
2.3. VPD450 labelling
PBMCs were thawed and washed with RPMI medium contained 10% FBS (R10) twice, and the number of cells and viability calculated. PBMCs were washed with phosphate buffered saline (PBS) twice to remove residual proteins from previous buffer and then re-suspended in PBS in a polypropylene test tube. Violet proliferation dye 450 (VPD450, BD Biosciences, San Jose, CA) (1μl per 1ml cells suspension) was added and cells were incubated for 15 min in a 37°C water bath. After incubation, PBMCs were washed once in PBS and once in R10. The sample was then re-suspended in R10 and used for culturing in different conditions for 72 hours before acquisition on LSRII flow cytometer (BD Biosciences, San Jose, CA). Proliferation index (the total number of divisions, divided by the number of cells that went into division) and division index (the average number of cell divisions) were determined using Flowjo software (Tree Star, Ashland, OR).
2.3. PBMC culture with Tacrolimus
PBMCs were equally split into four parts and plated in 96-well U bottom plates in RPMI with 10% FBS (R10). Cells were cultured with 1μg/ml anti-CD3 and 1μg/ml anti-CD28, in the absence or presence of 0, 10, or 100ng/ml tacrolimus (Sigma-Aldrich, St. Louis, MO). Concentrations were chosen based on published information showing that 5–10ng/ml is regarded as the therapeutic trough concentration for usual dosing in MG patients, while 100ng/ml is approximately the peak concentration (Kanai et al., 2017). Cells were cultured for 3 or 7 days at 37°C in 5% CO2 in a humidified incubator, and 5 hours prior to harvesting the cells, PBMCs were re-stimulated with 1 μg/mL phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) and 0.25μg/mL ionomycin (IONO, Sigma-Aldrich) in the presence of brefeldin A (BD Biosciences).Then, the phenotypic, molecular, and functional characterization of the PBMCs was performed as described below.
2.4. Surface and intracellular cytokine staining
Cultured cells were stained with 50uL of LIVE/DEAD violet dye (Life Technologies, Grand Island, NY) in PBS for 15 minutes at room temperature, to exclude dead cells from the analysis. Next, cell surface antigens were stained with a cocktail mix consisting of titrated volumes of anti-CD3 APC-Cy7, anti-CD4 BV785, anti-CD8 AlexaFluor 700, and anti-CXCR5 PE-Cy7 for 30 minutes at 4°C. Following cell surface staining, cells were treated with cytofix/cytoperm (BD Biosciences) according to the manufacturer’s recommendations. Intracellular staining was then performed for 30 min at 4°C using the following conjugates: anti- IFN-γ FITC, anti-IL-17A PerCP-Cy5.5, anti-IL-2 APC, and anti-IL-4 PE-Dazzle 594. Anti-IL-2 APC antibody was purchased from BD Biosciences, while other cytokine fluorescent antibodies were purchased from Biolegend. Cells were fixed with 1% paraformaldehyde (PFA) and acquired on a BD LSRII flow cytometer (BD Biosciences).
2.5. FOXP3 staining
PBMCs were plated in a 96-well round bottom plate and stained with LIVE/DEAD violet dye (described above), then cells were stained with anti-CD8 AlexaFluor 7000, anti-CD3 APC- Cy7, anti-CD4 BV785, anti-CD25 BV421 for 30 minutes in 4°C. Anti-CD3, anti-CD4, anti-CD8 and anti-CD25 were obtained from Biolegend. Following cell surface staining, cells were treated for 1 hour at 4°C with the FOXP3/Transcription Factor Fixation/Permeabilization buffer according to the manufacturer’s recommendations (eBioscience, San Diego, CA). Intra-nuclear staining was then performed for 30 min at 4°C with anti-FOXP3 PE (Biolegend). Cells were fixed with 1% PFA and acquired on a LSRII flow cytometer (BD Biosciences).
2.6. Data analysis and statistics
Flow cytometry analysis was performed using Flowjo software (Tree Star). Paired or unpaired student T-tests were used to determine statistical significance between two matched or unmatched groups, respectively. Analysis of variance (ANOVA) were used to compare the differences among three groups. Tukey HSD test were used for post-hoc analysis. The p values were calculated using Prism software (Graph Pad, La Jolla, CA).
3. Results
3.1. Study population
We included 31 MuSK-MG patients. The mean age of the MuSK-MG patients was 44.0 (range: 23–67 years) and the gender distribution was similar to prior reports (Table 1) (Guptill et al., 2011a; Guptill and Sanders, 2010). The duration from onset of symptoms to blood sample collection was > 1 year in all patients. Thymectomy was previously performed in 9 patients: one had thymic hyperplasia, and none had a thymoma. Patients were treated with prednisone, azathioprine, mycophenolate mofetil or combination immunosuppressant treatment. No patients had received rituximab.
3.2. Tacrolimus inhibits CD4 and CD8 T cell proliferation
To investigate the inhibitory effects of tacrolimus on the proliferation of CD4 and CD8 T cells, we cultured anti-CD3 and anti-CD28 stimulated PBMCs from MuSK-MG patients, for 3 and 7 days, in the absence or presence of 10ng/ml or 100ng/ml of tacrolimus. On days 3 and 7, proliferation of CD4 and CD8 T cells was assessed by the loss of VPD450 fluorescence. Both concentrations of tacrolimus suppressed CD4 T cell proliferation at day 3, but on day 7 only 100ng/ml of tacrolimus maintained significant suppression of CD4 T cell proliferation (Fig. 1A). Quantitatively, there was a significant decrease at day 3 in the proliferation (1.24±0.14 vs 0.94±0.12; p=0.0037) and division index (0.38±0.10 vs 0.21±0.06; p=0.001) following the addition of 100ng/mL of tacrolimus (Fig. 1B). For CD8 T cells, both concentrations of tacrolimus inhibited CD8 T cell proliferation at day 3, but the inhibition was not maintained at day 7 (Fig. 1A). The proliferation and division index at day 3 were significantly lower with tacrolimus 1.43±0.08 vs 1.18±0.09; p=0.019 and 0.60±0.12 vs 0.31±0.08; p=0.005, respectively (Fig. 1C). The decrease in T cell proliferation was supported by a reduction of blasting cells in the tacrolimus conditions (data not shown).
Fig. 1.
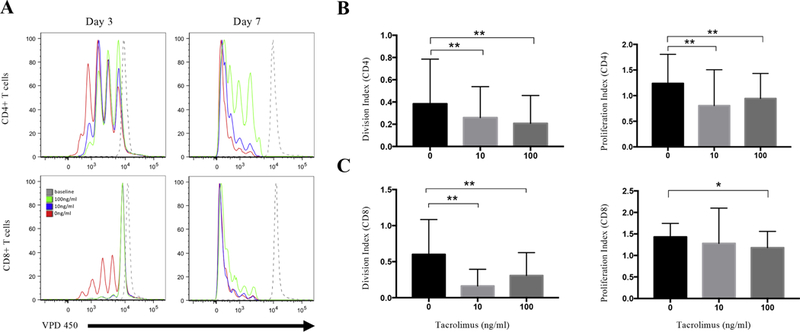
Tacrolimus decreases the proliferation of CD4+ and CD8+ T cells. PBMCs were isolated from 16 MuSK-MG patients, labeled with VPD450, and incubated with 1ug/ml anti-CD3 and 1ug/ml CD28 Abs in the absence or presence of 10ng/ml, 100ng/ml tacrolimus. (A) Comparison of CD4+ and CD8+ T cell proliferation in the presence of tacrolimus. The upper line shows CD4+ T cells proliferation after 3 days (left) and 7 days (right) of culture in the absence of tacrolimus (red), 10ng/ml tacrolimus (blue),100ng/ml tacrolimus (green), and the baseline was set on day 0 (grey, dashed line). The bottom histograms show CD8+ T cells proliferation after 3 days (left) and 7 days (right) of culture in the absence of tacrolimus (red), 10ng/ml tacrolimus (blue),100ng/ml tacrolimus (green), and the baseline was set on day 0 (grey, dashed line). (B) The division index (left) and proliferation index (right) in CD4+ T cells after 3 days of culture. (C) The division index (left) and proliferation index (right) in CD8+ T cells after 3 days of culture. *, p≤0.05; **, p≤0.01.
3.3. Tacrolimus suppresses Treg, Tfh-like, and Tfr cells
To explore the effect of tacrolimus on other Th subsets, we compared changes in the frequency of regulatory T cells (Treg; defined as CD4+CD25+FOXP3+), peripheral blood T follicular helper cells (Tfh-like cells; CD4+CXCR5+), and follicular regulatory T cells (Tfr; CD4+CXCR5+FOXP3+) after a 3 day incubation with 10ng/mL tacrolimus (Sage et al., 2014; Sage et al., 2013; Sakaguchi et al., 2006). Treg frequencies decreased dramatically with 10ng/ml tacrolimus (3.87±0.45% vs 10.85±1.85%, p=0.003) (Figure 2A, B) as did Tfh-like cell frequencies in MuSK-MG patients (9.74±1.07% vs 13.75±1.64%, P=0.0086) (Figure 2C, D). We also explored circulating Tfr changes in the same culture system and observed lower frequencies of Tfr cells in the presence of tacrolimus (0.84±0.15% vs 2.42±0.55%, p=0.006) (Figure 2E, F). Although the frequency of Tfh and Tfr cells decreased with 10ng/ml tacrolimus, the ratio of Tfh/Tfr increased from 7.205±1.686 to 14.1±2.777, p=0.005 (data not shown). Overall, tacrolimus has a suppressive effect on both regulatory and disease mediating CD4 T cell subsets.
Fig. 2.
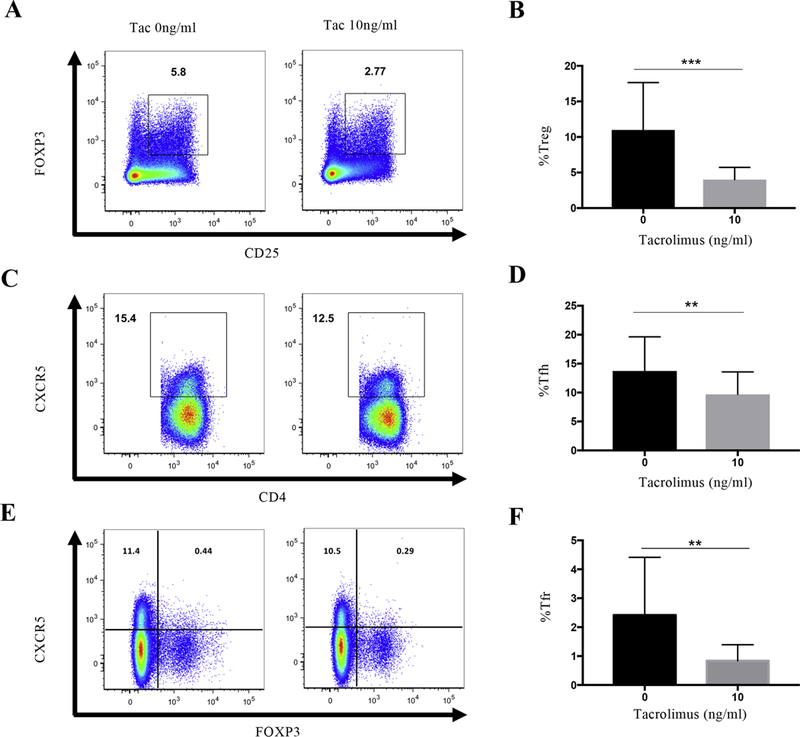
Tacrolimus reduces the frequency of Tfh and Tregs subsets in MuSK-MG. (A) Intranuclear staining for FOXP3+ Tregs after culturing without tacrolimus (left) and 10ng/ml tacrolimus (right) for 3 days. (B) Composite data from individual MuSK-MG patients showing the frequency of Tregs in the absence of tacrolimus (left column, black) and 10ng/ml tacrolimus (right column, grey). (C) Surface staining for CXCR5+ Tfh cells after culturing without tacrolimus (left) and 10ng/ml tacrolimus (right) for 3 days. (D) Composite data from individual MuSK-MG patients showing the frequency of Tfh cells in the absence of tacrolimus (left column, black) and 10ng/ml tacrolimus (right column, grey). (E) Intranuclear staining for FOXP3+CXCR5 Tfr cells after culturing without tacrolimus (left) and 10ng/ml tacrolimus (right) for 3 days. (F) Composite data from individual MuSK-MG patients showing the frequency of Tfr cells in the absence of tacrolimus (left column, black) and 10ng/ml tacrolimus (right column, grey). Statistical significance is represented as follows: ** p≤0.01; *** p≤0.001. Results are shown from four independent experiments, analyzing a total of 15 patients.
3.4. Tacrolimus suppresses Th1 and Th17, but not Th2 cell responses
To determine whether inhibition of T cell proliferation with tacrolimus is associated specific T cell subsets, we examined tacrolimus’ effect on the production of IFN-γ, IL-2, IL-4, and IL-17 in conjunction with proliferation. Two concentrations of tacrolimus (10ng/ml and 100ng/ml) were tested on PBMCs from MuSK-MG patients in a 3-day in vitro assay. Intracellular cytokine staining revealed that both concentrations of tacrolimus caused a significant decrease in the frequency ofCD4 T cells producing IFN-g, IL-2, and IL-17, and is associated with suppression of T cell proliferation (Fig 3A, B). Tacrolimus had no effect on IL-4 producing CD4 T cells. The ratio of Th1/Treg and Th17/Treg decreased with 10ng/ml Tacrolimus with a decrease from 0.714±0.697 to 0.147±0.158, p=0.0014, and 0.068±0.095 to 0.017±0.013 p=0.0233, respectively (data not shown). Tacrolimus had a similar effect on CD8 T cells by suppressing IFN-γ and IL-2 production (Fig 3C). Overall, tacrolimus inhibits Th1 (IFN-γ or IL-2 producing CD4+ T cells) and Th17 (IL-17 producing CD4+ T cells) associated cytokines, whereas IL-4, a Th2 associated cytokine, was not affected by tacrolimus.
Fig. 3.
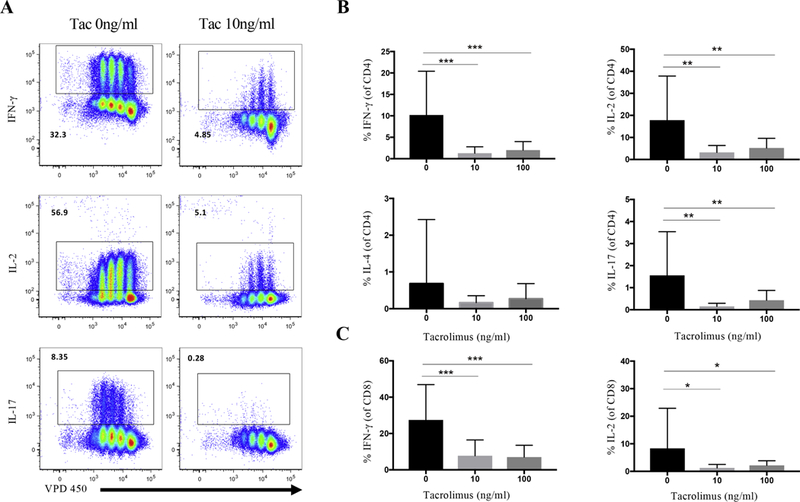
Tacrolimus suppresses CD4 and CD8 T cell function. (A) Gating on CD4+ T cells, intracellular cytokine analysis for IFN-γ, IL-2, IL-17A production (Y axis) and violet proliferation dye VPD 450 (X axis), in absence of tacrolimus (left) and 10ng/ml tacrolimus (right). (B) Comparison of CD4+IFN-γ+, CD4+IL-2+, CD4+IL-4+ and CD4+IL-17+ frequencies in CD4+ T cells among without tacrolimus (left black column), 10ng/ml tacrolimus (middle light grey column), and 100ng/ml tacrolimus (right dark grey column). (C) Comparison of CD8+IFN-γ+ and CD8+IL-2+ frequencies in CD8+ T cells among without tacrolimus (left, black column), 10ng/ml tacrolimus (middle, light grey column), and 100ng/ml tacrolimus (right, dark grey column). Statistical significance is represented as follows: * p≤0.05; ** p≤0.01; *** p≤0.001. Results are shown from six independent experiments, analyzing a total of 17–19 patients.
3.5. Tacrolimus suppresses IL-17+IFN-γ+CD4+ Tcell and IFN-γ+IL-2+ CD4+ Tcell subsets
Next, we analyzed whether tacrolimus could inhibit a subset of CD4+ T cells known as pathogenic Th17 cells in MuSK-MG patients. This subset was recently defined as Th17 cells that simultaneously produce IL-17 and IFN-γ (Gaublomme et al., 2015; Hirota et al., 2011). In the presence of tacrolimus, the frequency of IL-17+IFN-γ+CD4+ T cells were significantly suppressed with both the 10ng/ml and 100ng/ml concentrations of tacrolimus (Figure 4A, B) (P≤0.001, P≤0.001). Th1 cells that can produce IFN-γ and IL-2 have robust pro-inflammatory effects (Pantaleo and Harari, 2006). We found that IFN-γ+IL-2+CD4+ T cell frequencies were significantly decreased in the presence of tacrolimus (10ng/ml or 100ng/ml) (Figure 4C, D) (P≤0.001, P≤0.001).
Fig. 4.
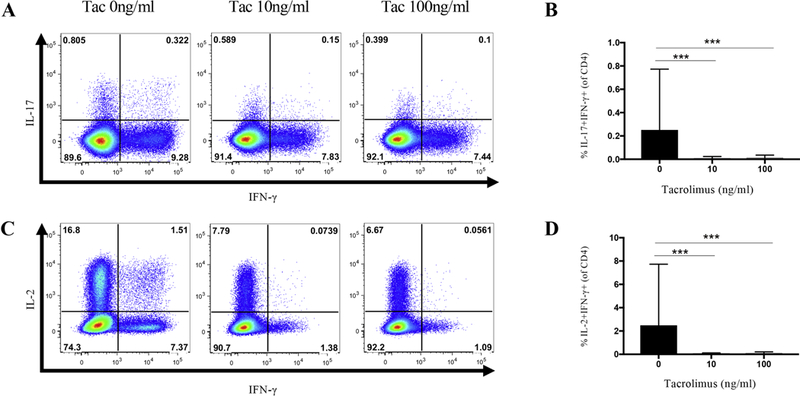
Comparison of CD4+IL-17+IFN-γ+ and CD4+IL-2+IFN-γ+ cells in the absence and presence of tacrolimus. (A) Intracellular cytokine staining for IL-17+IFN-γ+ pathogenic Th17 cells in the presence and absence of tacrolimus. (B) Composite data comparing the frequency of IL-17+IFN-γ+ pathogenic Th17 cells in 0ng/ml, 10ng/ml, and 100ng/ml of tacrolimus. (C) Intracellular cytokine staining for IL-2+IFN-γ+ CD4 T cells in the presence and absence of tacrolimus. (D) Composite data comparing the frequency of IL-2+IFN-γ+ CD4 T cells in 0ng/ml, 10ng/ml, and 100ng/ml of tacrolimus. Statistical significance is represented as follows: * p≤0.05; *** p≤0.001. Results are shown from five independent experiments, analyzing a total of 15–30 patients (0ng/ml N=30; 10ng/ml N=17; 100ng/ml N=15).
4. Discussion
MuSK-MG tends to be more resistant than AChR-MG to existing therapies with the exception of rituximab. Studies have consistently shown that many, but not all, MuSK-MG patients have excellent responses to rituximab (Diaz-Manera et al., 2012; Hehir et al., 2017; Nowak et al., 2011). Rituximab is not available for the treatment of MG worldwide and payor denials may also limit its use. Additional treatment options are needed, particularly for situations where rituximab is not available. Other treatment options may be limited by off target side effects (e.g., corticosteroids) or long latency to clinical effect (e.g., azathioprine). Tacrolimus is commonly used in Asia and Europe for the treatment of MG, but uncommonly prescribed in the United States, where other non-steroidal immunosuppressives such as mycophenolate mofetil and azathioprine are preferred. In this study, we explored the immunological impact of tacrolimus on CD4 and CD8 T cell responses in MuSK-MG. When cultured with PBMCs from MuSK-MG patients, tacrolimus demonstrated a broad effect on CD4 T cells notable for reducing the frequency of Th subsets including Th1, Th17, Tfh-like, and Treg cells, as well as a subset of Th17 cells described to have a pathogenic phenotype.
Tacrolimus is a T cell targeting immunosuppressant that binds to the intracellular protein FKBP- 12 to form a complex that inhibits calcineurin and nuclear translocation of NFATc. This results in the inhibition of IL-2 gene synthesis, T cell activation, and proliferation (Schreiber and Crabtree, 1992; Tocci et al., 1989). In renal transplantation, tacrolimus was associated with a reduced risk for acute rejection and better graft function compared with cyclosporine (Rath, 2013). The efficacy of tacrolimus has been evaluated in AChR-MG patients with doses ranging from 2mg/d to 5mg/d and a favorable clinical response observed within weeks (Nagane et al., 2005; Ponseti et al., 2005; Tao et al., 2017; Zhou et al., 2017). To this point, the immunological changes induced by tacrolimus therapy in patients with MG have been unclear and the clinical effects in patients with MuSK-MG have not been reported.
We initiated this study based on the known mechanism of tacrolimus combined with 1) recent descriptions of higher frequencies of Th1 and Th17 responses in MuSK-MG patients (Yi et al., 2014); 2) observations of IL-17 elevations in AChR MG patients (Roche et al., 2011; Xie et al., 2016); 3) experimental autoimmune MG models demonstrating IL-17 knock out mice did not develop clinical MG pathology (Aguilo-Seara et al., 2017); 4) apparent efficacy of tacrolimus in AChR-MG and 5) the impact of Th17 cells on the pathogenesis of other autoimmune diseases including multiple sclerosis, rheumatoid arthritis, and thyroiditis (Kuwabara et al., 2017; Tabarkiewicz et al., 2015). It is clear that Th1 and Th17 cells are involved in promoting MG immunopathology and T cell targeted therapies such as tacrolimus may enhance treatment efficacy. Given tacrolimus’ mechanism of action against T cell responses, we hypothesized that tacrolimus would suppress the Th1 and Th17 responses that are enhanced in MuSK-MG.
Prior studies in AChR-MG patients suggest that trough concentrations of >4.8ng/mL or 7– 8ng/mL have been associated with favorable clinical outcomes (Kanai et al., 2017; Ponseti et al., 2005). Our study supports that similar concentrations (10ng/ml) have profound inhibitory effects on proinflammatory Th1 and Th17 responses, while Th2 cells are not significantly affected. This suggests that future studies in MuSK-MG patients could use a similar drug concentration target to guide therapy.
Th17 cells can be subdivided into pathogenic and non-pathogenic Th17 cells based on their cytokine profile (Lee et al., 2012; Stockinger and Omenetti, 2017). Pathogenic Th17 cells are implicated in experimental models of autoimmunity and more recently in human disease (Hu et al., 2017). This cell subset is induced by IL-23 and simultaneously co-produces IL-17 and IFN-γ(Gaublomme et al., 2015; Hirota et al., 2011). Pathogenic Th17 cells are known to promote B cell responses and could be a therapeutic target in autoimmune disease (Kuwabara et al., 2017; Lee et al., 2014; Yamagata et al., 2015). In this study, tacrolimus exposure demonstrated remarkable inhibition of overall Thl7 cells, but also the IL-17+IFN-γ + pathogenic Till7 subset.
Tacrolimus also diminished the frequency of Tfh-like cells. Tfh cells are characterized by their ability to support somatic hypermutation B cells and antibody high-affinity maturation in germinal centers (Ueno, 2016). In the periphery, Tfh-like cells are recognized as memory subset of Tfh that have circulated out of the germinal center and with intact capacity to support antibody producing B cells (Sage et al., 2014; Schmitt et al., 2014). While we and others have shown an increase in the frequency of peripheral blood Tfh-like cells in AChR-MG, the role of Tfh-like cells in MuSK-MG remains undefined (Luo et al., 2013; Zhang et al., 2016). For future studies, it will be of interest to quantitatively and qualitatively examine the Tfh phenotypes between AChR and MuSK-Ab MG. If Tfh-like cell phenotype in MuSK-MG mirrors AChR-MG, the reduction in Tfh-like cell frequencies we observed with tacrolimus would be expected to have a favorable effect of disrupting Tfh-like interactions with B cells, ultimately reducing autoantibody production (Li et al., 2018).
An apparent consequence of adding tacrolimus is the suppression of regulatory cells, Tregs and Tfr cells. We demonstrated a decrease in Treg frequencies with tacrolimus, a finding that is consistent with transplantation studies (Gallon et al., 2015). The role of Tregs in the breakdown of self-tolerance in MG is uncertain. In AChR-MG, reports showing an alteration in Treg frequencies are not consistent (Fattorossi et al., 2005; Li et al., 2008; Masuda et al., 2010; Xu et al., 2012), and no changes were observed in Treg frequencies of MuSK-MG patients (Yi et al., 2014). Prior to this study, Tfr cell subsets have not been well reported in MG and the effect of tacrolimus on Tfr cells has not been reported in either MG or transplantation studies. The decrease in Tregs cells may be attributable to tacrolimus induced suppression of IL-2, a critical cytokine involved in the generation of Tregs (Wu et al., 2006). As a result, a decrease in Treg cells has consequential effects on Tfr cells, which originate from Treg precursors (Sage and Sharpe, 2016). Given the global suppression of the Th response, it may be more beneficial to evaluate the balance of the Th17 vs Treg response (Noack and Miossec, 2014). The dramatic inhibition of inflammatory Th1 and Th17 cells may outweigh any potential negative consequences of reduced Treg frequencies.
This study has several limitations. MuSK-MG patients received a variety of immunomodulatory treatments, including some with thymectomy, which could confound the results. Given the rarity and usual severity of the disease, this limitation is likely unavoidable as most patients are already receiving treatment when they are first evaluated at our centers. In addition, we expect that tacrolimus would be used as an adjunctive therapy in most cases, mimicking the experimental conditions in our study. Thymectomy is not commonly performed in MuSK-MG patients currently. For the patients in our study, thymectomy was performed prior to recognition of MuSK-MG as a separate clinical entity. All thymectomies were performed many years prior to collection of blood samples for our study, limiting the potential confounding effect on our results. Experimentally, this study used cytokine production as a determinant of T cell function, and an alternative measurement of function is the quantity of anti-MuSK autoantibodies following culture with tacrolimus.
Overall, the current study demonstrates tacrolimus’ ability to suppress proinflammatory Th1 and Th17 responses in MuSK-MG at concentrations that have been used to treat AChR-MG. Furthermore, Tfh-like cells known to support B cell proliferation, as well as the subset of pathogenic Th17 cells, appear very sensitive to tacrolimus. These data provide preliminary support for tacrolimus as a potential therapy for the treatment of MuSK-MG. Though these in vitro studies were conducted using samples from patients, further rigorous clinical assessment of tacrolimus in MuSK-MG patients are required to show an appropriate risk-benefit profile in this patient population. If successful, tacrolimus could become a welcome additional option to manage these often challenging patients.
Highlights.
Tacrolimus inhibits CD4+ and CD8+ T cell proliferation.
Tacrolimus suppresses Th1 and Th17 responses, but not Th2 responses, in lymphocytes of patients with MuSK-MG.
Pathogenic Thl7 cells, which are defined as IFN-γ and IL-17 double positive CD4 T cells, were highly decreased when exposed to tacrolimus.
The frequency of Tfh and Treg cells decreased in the presence of tacrolimus.
Acknowledgements
The authors will like to thank all patients who consented to participate in this study. We thank all the members of Dr. Yi’s laboratory for advice and critical reading of this manuscript. We also thank the Duke Immune Profiling Core (DIPC) for their flow cytometry services.
The study was supported by the National Natural Science Foundation Key International (Regional) Cooperation Research Project (No.81620108010) and International Program for Ph.D. Candidates, Sun Yat-sen University. JTG is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number K23NS085049.
Abbreviations
- MG
myasthenia gravis
- MuSK
muscle specific kinase
- AChR
acetylcholine receptor
- LRP4
lipoprotein receptor-related protein 4
- FKBP
FK506 binding protein
- NFAT
nuclear factor of activated T cell
- FOXP3
forkhead box P3 protein
- Th
helper T cell
- Tfh
follicular T helper cell
- Treg
regulatory T helper cell
- Tfr
regulatory follicular T helper cell
- PIS
post-intervention status
- MMT
manual muscle testing
- MGFA
Myasthenia Gravis Foundation of America
- PBMC
peripheral blood mononuclear cells
- FBS
fetal bovine serum
- DMSO
dimethyl sulfoxide
- PMA
phorbol 12-myristate 13-acetate
- IONO
ionomycin
- VPD450
violet proliferation dye 450
- CXCR5
C-X-C motif chemokine receptor type 5
Footnotes
Conflicts of interest
The author declare that they have no conflicts of interest related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilo-Seara G, Xie Y, Sheehan J, Kusner LL, Kaminski HJ, 2017. Ablation of IL-17 expression moderates experimental autoimmune myasthenia gravis disease severity. Cytokine 96, 279–285. [DOI] [PubMed] [Google Scholar]
- Conti-Fine BM, Milani M, Wang W, 2008. CD4+ T cells and cytokines in the pathogenesis of acquired myasthenia gravis. Annals of the New York Academy of Sciences 1132, 193–209. [DOI] [PubMed] [Google Scholar]
- Diaz-Manera J, Martinez-Hernandez E, Querol L, Klooster R, Rojas-Garcia R, Suarez- Calvet X, Munoz-Blanco JL, Mazia C, Straasheijm KR, Gallardo E, Juarez C, Verschuuren JJ, Illa I, 2012. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology 78, 189–193. [DOI] [PubMed] [Google Scholar]
- Evoli A, Di Schino C, Marsili F, Punzi C, 2002. Successful treatment of myasthenia gravis with tacrolimus. Muscle Nerve 25, 111–114. [DOI] [PubMed] [Google Scholar]
- Fattorossi A, Battaglia A, Buzzonetti A, Ciaraffa F, Scambia G, Evoli A, 2005Circulating and thymic CD4 CD25 T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunology 116, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallon L, Traitanon O, Yu Y, Shi B, Leventhal JR, Miller J, Mas V, L X, Mathew JM, 2015. Differential Effects of Calcineurin and Mammalian Target of Rapamycin Inhibitors on Alloreactive Th1, Th17, and Regulatory T Cells. Transplantation 99, 1774–1784. [DOI] [PubMed] [Google Scholar]
- Gaublomme JT, Yosef N, Lee Y, Gertner RS, Yang LV, Wu C, Pandolfi PP, Mak T, Satija R, Shalek AK, Kuchroo VK, Park H, Regev A, 2015. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell 163, 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptill JT, Marano A, Krueger A, Sanders DB, 2011a. Cost analysis of myasthenia gravis from a large U.S. insurance database. Muscle Nerve 44, 907–911. [DOI] [PubMed] [Google Scholar]
- Guptill JT, Sanders DB, 2010. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr. Opin. Neurol. 23, 530–535. [DOI] [PubMed] [Google Scholar]
- Guptill JT, Sanders DB, Evoli A, 2011b. Anti-MuSK antibody Myasthenia gravis: Clinical findings and response to treatment in two large cohorts. Muscle Nerve 44, 36–40. [DOI] [PubMed] [Google Scholar]
- Hehir MK, Hobson-Webb LD, Benatar M, Barnett C, Silvestri NJ, Howard JF Jr., Howard D, Visser A, Crum BA, Nowak R, Beekman R, Kumar A, Ruzhansky K, Chen IA, Pulley MT, LaBoy SM, Fellman MA, Greene SM, Pasnoor M, Burns TM, 2017. Rituximab as treatment for anti-MuSK myasthenia gravis: Multicenter blinded prospective review. Neurology 89, 1069–1077. [DOI] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B, 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Notarbartolo S, Croonenborghs T, Patel B, Cialic R, Yang TH, Aschenbrenner D, Andersson KM, Gattorno M, Pham M, Kivisakk P, Pierre IV, Lee Y, Kiani K, Bokarewa M, Tjon E, Pochet N, Sallusto F, Kuchroo VK, Weiner HL, 2017. Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat Commun 8, 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai T, Uzawa A, Kawaguchi N, Himuro K, Oda F, Ozawa Y, Kuwabara S, 2017. Adequate tacrolimus concentration for myasthenia gravis treatment. European journal of neurology 24, 270–275. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T, 2017. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Infamm. 2017, 3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK, 2012. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Collins M, Kuchroo VK, 2014. Unexpected targets and triggers of autoimmunity. J. Clin. Immunol. 34 Suppl 1, S56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xiao BG, X JY, Lu CZ, Lu JH, 2008. Decrease of CD4(+)CD25(high)Foxp3(+) regulatory T cells and elevation of CD19(+)BAFF-R(+) B cells and soluble ICAM-1 in myasthenia gravis. Clin. Immunol. 126, 180–188. [DOI] [PubMed] [Google Scholar]
- Li YM, Li Y, Shi YY, Yan L, Wu XJ, Tang JT, Bai YJ, Wang LL, 2018. Impact of immunosuppressive drugs on circulating Tfh cells in kidney transplant recipients: A pilot study. Transpl. Immunol. 46, 1–7. [DOI] [PubMed] [Google Scholar]
- Luo C, Li Y, Liu W, Feng H, Wang H, Huang X, Qiu L, Ouyang J, 2013. Expansion of circulating counterparts of follicular helper T cells in patients with myasthenia gravis. J. Neuroimmunol. 256, 55–61. [DOI] [PubMed] [Google Scholar]
- Masuda M, Matsumoto M, Tanaka S, Nakajima K, Yamada N, Ido N, Ohtsuka T, Nishida M, Hirano T, Utsumi H, 2010. Clinical implication of peripheral CD4+CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. J. Neuroimmunol. 225, 123–131. [DOI] [PubMed] [Google Scholar]
- Milani M, Ostlie N, Wang W, Conti-Fine BM, 2003. T cells and cytokines in the pathogenesis of acquired myasthenia gravis. Ann. N. Y. Acad. Sci. 998, 284–307. [DOI] [PubMed] [Google Scholar]
- Nagane Y, Utsugisawa K, Obara D, Kondoh R, Terayama Y, 2005. Efficacy of low-dose FK506 in the treatment of Myasthenia gravis--a randomized pilot study. European neurology 53, 146–150. [DOI] [PubMed] [Google Scholar]
- Noack M, Miossec P, 2014. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 13, 668–677. [DOI] [PubMed] [Google Scholar]
- Nowak RJ, Dicapua DB, Zebardast N, Goldstein JM, 2011. Response of patients with refractory myasthenia gravis to rituximab: a retrospective study. Ther. Adv. Neurol. Disord. 4, 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Harari A, 2006. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nature reviews. Immunology 6, 417–423. [DOI] [PubMed] [Google Scholar]
- Ponseti JM, Azem J, Fort JM, Lopez-Cano M, Vilallonga R, Buera M, Cervera C, Armengol M, 2005. Long-term results of tacrolimus in cyclosporine- and prednisone-dependent myasthenia gravis. Neurology 64, 1641–1643. [DOI] [PubMed] [Google Scholar]
- Ponseti JM, Gamez J, Azem J, Lopez-Cano M, Vilallonga R, Armengol M, 2008. Tacrolimus for myasthenia gravis: a clinical study of 212 patients. Ann. N. Y. Acad. Sci. 1132, 254–263. [DOI] [PubMed] [Google Scholar]
- Rath T, 2013. Tacrolimus in transplant rejection. Expert Opin. Pharmacother. 14, 115–122. [DOI] [PubMed] [Google Scholar]
- Roche JC, Capablo JL, Larrad L, Gervas-Arruga J, Ara JR, Sanchez A, Alarcia R, 2011. Increased serum interleukin-17 levels in patients with myasthenia gravis. Muscle Nerve 44, 278–280. [DOI] [PubMed] [Google Scholar]
- Sage PT, Alvarez D, Godec J, von Andrian UH, Sharpe AH, 2014. Circulating T follicular regulatory and helper cells have memory-like properties. J. Clin. Invest. 124, 5191–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage PT, Francisco LM, Carman CV, Sharpe AH, 2013. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nature immunology 14, 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage PT, Sharpe AH, 2016. T follicular regulatory cells. Immunol. Rev. 271, 246–259. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T, 2006. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27. [DOI] [PubMed] [Google Scholar]
- Schmitt N, Bentebibel SE, Ueno H, 2014. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 35, 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SL, Crabtree GR, 1992. The mechanism of action of cyclosporin A and FK506. Immunol. Today 13, 136–142. [DOI] [PubMed] [Google Scholar]
- Stathopoulos P, Kumar A, Heiden JAV, Pascual-Goni E, Nowak RJ, O’Connor KC, 2018. Mechanisms underlying B cell immune dysregulation and autoantibody production in MuSK myasthenia gravis. Annals of the New York Academy of Sciences 1412, 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Omenetti S, 2017. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 17, 535–544. [DOI] [PubMed] [Google Scholar]
- Tabarkiewicz J, Pogoda K, Karczmarczyk A, Pozarowski P, Giannopoulos K, 2015. The Role of IL-17 and Th17 Lymphocytes in Autoimmune Diseases. Arch. Immunol. Ther. Exp. (Warsz.) 63, 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Wang W, Jing F, Wang Z, Chen Y, Wei D, Huang X, 2017. Long-term efficacy and side effects of low-dose tacrolimus for the treatment of Myasthenia Gravis. Neurol. Sci. 38, 325–330. [DOI] [PubMed] [Google Scholar]
- Tocci MJ, Matkovich DA, Collier KA, Kwok P, Dumont F, Lin S, Degudicibus S, Siekierka JJ, Chin J, Hutchinson NI, 1989. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J. Immunol. 143, 718–726. [PubMed] [Google Scholar]
- Ueno H, 2016. T follicular helper cells in human autoimmunity. Curr. Opin. Immunol. 43, 24–31. [DOI] [PubMed] [Google Scholar]
- Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A, 2006. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 126, 375–387. [DOI] [PubMed] [Google Scholar]
- Xie Y, Li HF, Jiang B, Li Y, Kaminski HJ, Kusner LL, 2016. Elevated plasma interleukin-17A in a subgroup of Myasthenia Gravis patients. Cytokine 78, 44–46. [DOI] [PubMed] [Google Scholar]
- Xu WH, Zhang AM, Ren MS, Zhang XD, Wang F, Xu XC, Li Q, Wang J, Din BS, Wu YB, Chen GH, 2012. Changes of Treg-associated molecules on CD4+CD25 +Treg cells in myasthenia gravis and effects of immunosuppressants. J Clin Immunol 32, 975–983. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Skepner J, Yang J, 2015. Targeting Th17 Effector Cytokines for the Treatment of Autoimmune Diseases. Arch. Immunol. Ther. Exp. (Warsz.) 63, 405–414. [DOI] [PubMed] [Google Scholar]
- Yi JS, Guidon A, Sparks S, Osborne R, Juel VC, Massey JM, Sanders DB, Weinhold KJ, Guptill JT, 2014. Characterization ofCD4 and CD8 T cell responses in MuSK myasthenia gravis. J. Autoimmun. 52, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz V, Oflazer P, Aysal F, Durmus H, Poulas K, Yentur SP, Gulsen-Parman Y, Tzartos S, Marx A, Tuzun E, Deymeer F, Saruhan-Direskeneli G, 2015. Differential Cytokine Changes in Patients with Myasthenia Gravis with Antibodies against AChR and MuSK. PLoS One 10, e0123546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CJ, Gong Y, Zhu W, Qi Y, Yang CS, Fu Y, Chang G, Li Y, Shi S, Wood K, Ladha S, Shi FD, Liu Q, Yan Y, 2016. Augmentation of Circulating Follicular Helper T Cells and Their Impact on Autoreactive B Cells in Myasthenia Gravis. Journal of immunology 197, 2610–2617. [DOI] [PubMed] [Google Scholar]
- Zhou L, Liu W, Li W, Li H, Zhang X, Shang H, Zhang X, Bu B, Deng H, Fang Q,Li J, Zhang H, Song Z, Ou C, Yan C, Liu T, Zhou H, Bao J, Lu J, Shi H, Zhao C,2017. Tacrolimus in the treatment of myasthenia gravis in patients with an inadequate response to glucocorticoid therapy: randomized, double-blind, placebo-controlled study conducted in China. Therapeutic advances in neurological disorders 10, 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]


