Abstract
Mechanisms mediating protective effects of dietary restriction during aging are of great interest since activating such mechanisms protect against a wide range of age-related diseases. In mammals key metabolic responses to nutritional deprivation are mediated by the transcription factor PPAR-alpha, which is activated by free fatty acids and promotes lipid metabolism while inhibiting glucose metabolism. The C. elegans gene nhr-49 appears to function similarly in C. elegans. Here we report that protective effects of dietary restriction and inhibition of glucose metabolism to increase lifespan wild-type C. elegans and reduce toxicity in a polyQ model of Huntington’s disease in C. elegans are dependent on NHR-49 and its co-activator CREB-Binding Protein (CBP). We have previously demonstrated that inhibition of cbp blocks protective effects of dietary restriction and blocks the molecular switch from glucose metabolism to alternative substrates. Conversely, increased glucose concentration and inhibition of cbp reduce lifespan and increase proteotoxicity. Lactate and inhibition of ETC complex II mimicked toxic effects of glucose on proteotoxicity whereas pyruvate and inhibition of ETC complex I protected against glucose-enhanced proteotoxicity. These results support that PPAR-alpha-like activity mediates protective effects of dietary restriction by reducing glucose metabolism via reducing production of NADH, and corroborate and extend recent studies demonstrating that PPPAR-alpha agonists increase lifespan in C. elegans dependent on NHR-49.
Keywords: Dietary restriction, glucose metabolism, PPAR
Introduction
Dietary restriction increases lifespan, slows the rate of aging [1], and protects against many age-related impairments [2], including in models of Huntington’s disease [3,4]. The metabolic mechanisms by which dietary restriction is protective remain unclear, but studies from our laboratory have demonstrated that inhibition of CREB-binding protein (CBP) blocks all protective effects of dietary restriction, including in a model of Alzheimer’s disease proteotoxicity [5]. Inhibition of CBP also prevents the effect of dietary restriction to shift metabolism away from glucose metabolism and towards lipid metabolism [5]. These data suggest that at least some protective effects of dietary restriction are mediated by a decrease in glucose and an increase in lipid metabolism, leading to an increased FADH2:NADH ratio and thereby enhancing ETC complex II utilization relative to ETC complex I [6,7]. Since CBP is a transcriptional co-activator and does not directly bind DNA, these results raise the question of which transcription factors bind to the DNA of the relevant metabolic genes and thus mediate metabolic protection. This metabolic switch from glucose metabolism to lipid metabolism is thought to be protective since glucose metabolism promotes utilization of electron transport chain complex I, which produces reactive oxygen species, whereas lipid metabolism promotes utilization of complex II, which does not produce reactive oxygen species, [6,7]. Experimentally reducing complex II activity reduces lifespan, whereas experimentally reducing complex I activity increases lifespan [8]. Furthermore, Huntington’s Disease (HD) is characterized by relatively reduced complex II activity [9], and inhibition of complex II activity produces cellular phenotypes similar to those of HD [10].
In mammals the transcription factor PPAR-alpha is activated by free fatty acids during nutritional deprivation and mediates the switch from glucose metabolism to lipid metabolism [11]. This metabolic switch may mediate many of the protective effects of dietary restriction [6,7]. Furthermore a recent study has demonstrated that pharmacological activators of PPAR-alpha (e.g., fenofibrate) [12] increase lifespan in C. elegans, dependent on nhr-49, a functional homlog of ppar-alpha [13]. Here, we report that nhr-49 is required for protective effects of dietary and glucose restriction on lifespan and proteotoxicity.
Research Design and Methods
C. elegans strains
The AM140 strain with the rmIs132(P(unc-54)Q35::YFP) transgene [14], the N2 strain and the RB1716 strain with the ok2165 deletion in the nhr-49 gene. All strains were obtained from the Caenorhabditis Genetic Center (CGC) in Minneapolis, MN. Worms were maintained at 20°C with the E. coli OP50 strain as their food source.
RNAi strains
cbp-1, nhr-49, nuo-4 and mev-1 dsRNA expressing bacterial strains were from the genomic RNAi library produced by J. Ahringer at the Wellcome/CRC Institute Sequences for RNAi strains are below. The bacterial strain containing the empty L4440 vector was used for control.
cbp-1: forward primer- CAACGATCGAAACAACATGG; reverse primer- GGCTGGCTCATTCTGAAGAC
nhr-49: forward primer- ACCAACATCTGGAGCCAGTC; reverse primer- TGTCGTCGAGAATGAACTCG
nuo-4: forward primer- TTTTGGCCAGTTTCAATTCC; reverse primer- ACTTGCTGATCAACTCGGCT
mev-1: forwardprimer-AGAAAATCAGCATTGTTTCAGGA; reverse primer- ACGGAAAAGAGAGAAAATCGAAG
Paralysis and lifespan analysis
Eggs were collected via a standard axenization procedure whereby gravid worms were treated with hypochlorite. Worms were then grown on nematode growth media (NGM) plates (100 mM) with control OP50 bacteria until they reached day 1 of adulthood in all studies except the mitochondrial complex study. For the mitochondrial RNAi study, worms were grown on nuo-4 RNAi containing E. coli during development as well as during adulthood. AM140 worms were scored for paralysis and death every day starting on day 6-8 of adulthood. Paralysis was indicated by inability of the worm to travel forward after plate tapping and prodding with a platinum wire, but distinguished from death by waving of the head. N2 worms were scored starting on day 10 of adulthood and scored every 2-3 days.
RNA interference
Standard bacterial feeding methods were used for RNAi interference studies [15]. E.coli, which was transformed with respective RNAi construct, was grown overnight at 37°C. 3 5 mm NGM plates, supplemented with 5-fluorodeoxuridine (FUDR) (0.1 g/L) (Sigma), IPTG (0.24 g/L) (Sigma) and ampicillin (0.1 g/L) (CellGro), were seeded with bacteria expressing the dsRNA (180–200 μL) and incubated for several hours-overnight at 37°C. On day 1 of adulthood, worms were transferred to these plates. Worms were exposed to RNAi during adulthood with one exception. When treating with nuo-4 or mev-1 RNAi, worms were treated during development as well as adulthood.
Dietary restriction
Dietary restriction was accomplished by complete removal of food [16] since this is the protocol previously used to study effects of dietary restriction on polyQ proteotoxicity [17]. Thus worms were put on 35 mM NGM plates containing no bacteria. For RNAi + dietary restriction studies, worms were kept on plates containing RNAi for the first 4 days of adulthood and then transferred to dietary restriction plates or to control plates containing bacteria harboring the L4440 vector.
Drug Studies
For the drug and metabolite studies, the drug/metabolite was added to the standard NGM agar with a final concentration of 100 mM glucose (Fisher), 100 mM sodium lactate (Sigma), 200 mM sodium pyruvate (Sigma) and 4 mM 2DG (Sigma), L4440 was used as the control bacteria except for the pyruvate and lactae studies which uesd OP50. The 2-DG + glucose study and pyruvate+glucose study were performed with 50 worms.
Statistical analysis
Paralysis/survival curves were compared using the Kaplan-Meier statistical test. Onset of paralysis (or death) was statistically compared using a Wilcoxon rank sum test, a nonparametric comparison of medians between two groups. All statistical analyses were carried out using the GraphPad Prism 5 Software. Criterion for significance was set at p<0.05. Although the data presented here represents a single study with 60-80 worms each, each study was completely replicated 3 times unless otherwise specified [18].
Results
Protective effects of dietary restriction and glycolytic inhibition require nhr-49
Dietary restriction [19] and glycolytic inhibition [20] both increase lifespan in C. elegans, whereas elevated glucose reduces lifespan [21,22]. In mammals the transcription factor Ppar-alpha mediates the switch from glucose metabolism to lipid metabolism produced by nutritional deprivation [11,23] which may mediate protective effects of dietary restriction [6,7]. Furthermore, pharmacological activators of Ppar-alpha (e.g., fenofibrate) have also been shown to increase lifespan in C. elegans [12]. The transcription factor Creb-binding protein (CBP) serves as a transcriptional co-activator for PPARs [24]. Furthermore Cbp is required for protective effects of dietary restriction [5] and inhibition of miRNA 80 which produces responses similar to dietary restriction [25]. The transcription factor nhr-49 appears to serve the same function in worms as PPAR-alpha in mammals [13].
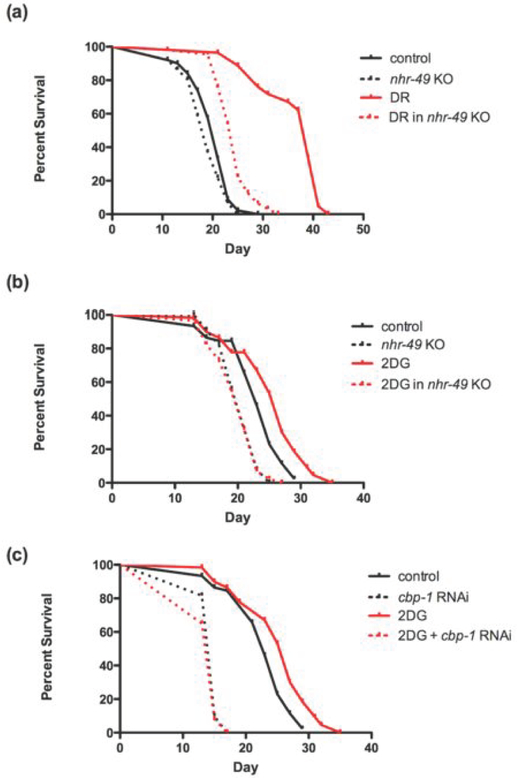
We therefore assessed if genetic ablation of nhr-49 would attenuate protective effects of dietary restriction and glycolytic inhibition. As shown in Figure 1a, ablation of nhr-49 largely (by 67%), though not quite completely, prevented the protective effects of dietary restriction to increase lifespan. Similarly, ablation of nhr-49 completely prevented the effect of glycolytic inhibition via 2-DG to increase lifespan (Figure 1b). Furthermore, inhibition of CBP with RNAi also completely prevented the effects of 2-DG to increase lifespan (Figure 1c). As indicated above, we have already demonstrated the inhibition of CBP prevents protective effects of dietary restriction to increase lifespan and protect against proteotoxicity [5].
Figure 1: Metabolic protection on lifespan is dependent on PPAR and CBP.
The nhr-49 knockout strain shows (a) almost complete prevention of lifespan extension by dietary restriction and (b) complete prevention of lifespan extension by glycolytic inhibition. Extension of lifespan by glycolytic inhibition using 2-DG is completely reversed in the (c) when cbp-1 is inhibited in the wild-type strain.
Inhibition of nhr-49 and cbp-1 blocks protective effect of dietary restriction on polyQ proteotoxicity
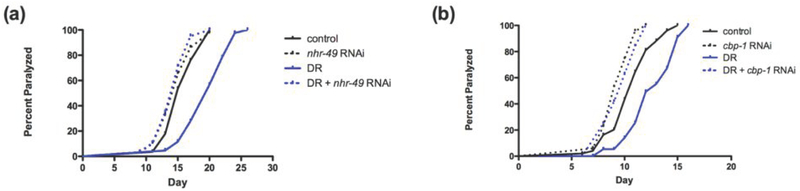
Several lines of investigation have suggested that proteotoxcity in polyQ models of Huntington’s Disease is mediated by the inhibition of CBP activity [26-28]. To assess the role of nhr-49 in polyQ proteotoxicity, we used the C. elegans AM140 strain which has a tract of 35 glutamine residues fused to YFP expressed in the body wall muscles. The readout of proteotoxicity is paralysis [29]. We assessed if inhibition of nhr-49 would block the protective effects of dietary restriction [17] in this model. In this study, nhr-49 was inhibited with RNAi to avoid the complications of crossing the polyQ transgenic worm with the nhr-49 knockout worm. As shown in Figure 2a, inhibition of nhr-49 completely prevented the protective effects of DR on proteotoxicity, as indicated by age-related paralysis (Figure 2a). Similarly, as shown in Figure 2b, inhibition of cbp-1 by RNAi also completely prevented the protective effects of DR on the polyQ proteotoxicity model.
Figure 2: PPAR and CBP are necessary for protection of dietary restriction on proteotoxicity.
Protection on proteotoxicity via dietary restriction is prevented with (a) nhr-49 inhibition or (b) cbp-1 inhibition.
Glucose accelerates polyQ toxicity while glycolytic inhibition delays proteotoxicity, dependent on nhr-49 and cbp-1
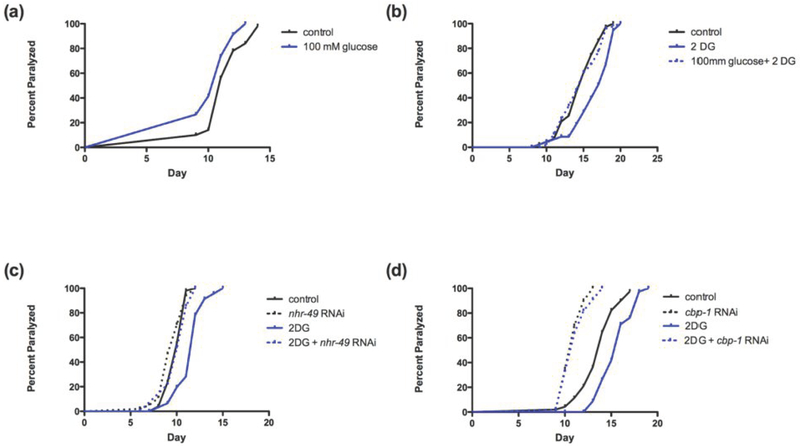
Consistent with previous studies on lifespan [21], as shown in Figure 3a, elevated glucose enhanced polyQ proteotoxicity. Conversely, inhibition of glycolysis by 2DG substantially protected against polyQ proteotoxicity (Figure 3b). Furthermore, elevated glucose completely prevented the protective effects of 2-DG on paralysis (Figure 3b). Similarly, inhibition of nhr-49 also completely prevented the protective effects of 2-DG on polyQ proteotoxicity (Figure 3c). Finally, inhibition of cbp-1 also prevented the protection of 2DG in the polyQ model (Figure 3d).
Figure 3: High glucose potentiates whereas glycolytic inhibition protects against polyQ proteotoxicity in a PPAR and CBP-dependent fashion.
(a) High glucose (100 mM) potentiates polyQ toxicity as indicated by accelerating the median onset of paralysis (p<0.005). (b) Glycolytic inhibition via 2-DG delays paralysis (p<0.0001). These protective effects are reversed by administration of high glucose (100 mM).Protection via glycolytic inhibiton is prevented with (c) nhr-49 or (d) cbp-1 inhibition.
NADH as enhancer of proteotoxicity
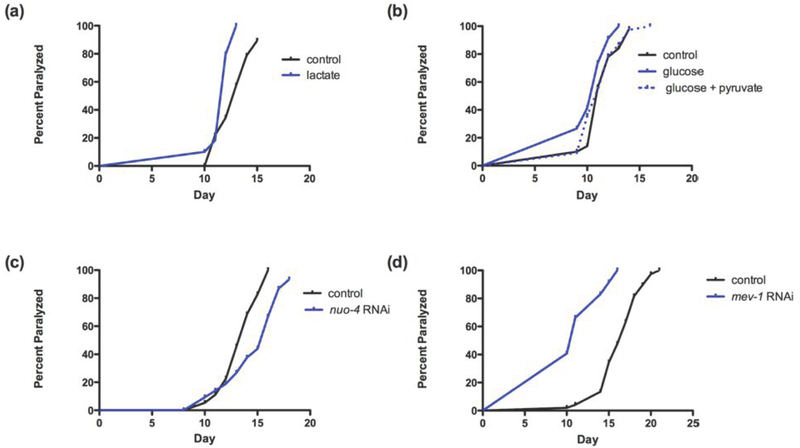
Several lines of evidence suggest that toxic effects of glucose metabolism are mediated in part by increasing NADH/NAD+ ratios [30] and protective effects of DR during aging are mediated by reducing this ratio [31]. NADH/NAD+ ratios are increased by lactate and reduced by pyruvate. As shown in Figure 4a, increased lactate enhanced polyQ-induced proteotoxicity (Figure 4a). In contrast, increased pyruvate produced a nonsignificant trend toward protection under standard conditions. However, increased pyruvate significantly protected against glucose-induced proteotoxicity (Figure 4b).
Figure 4: Role of NADH and ETC in proteotoxicity.
NADH can be manipulated by the addition of pyruvate or lactate. (a) Addition of lactate (100mM) exacerbates proteotoxicity as reflected by an acceleration of paralysis (p<0.005). (b) Addition of pyruvate (200 mM) protects against the toxic effect of glucose (100 mM) on the polyQ model. (c) RNAi inhibition of nuo-4, a homolog NADH ubiquinone reductase delays median day of paralysis by 2 days (p<0.001). (d) RNAi inhibition of mev-1, a homolog of cytochrome b of complex II of the ETC chain, exacerbates proteotoxicity as evidenced by a 6-day acceleration of paralysis.
The role of electron transport chain complexes in proteotoxicity
A possible mechanism of NADH toxicity involves enhanced activity of electron transport chain (ETC) complex I, which is the site of NADH oxidation for production of ATP and which produces reactive oxygen species [30]. Indeed, inhibition of ETC complex I increases lifespan, whereas inhibition of ETC complex II reduces lifespan [8]. Similarly, as shown in Figure 4c, RNAi inhibition of complex I protein NADH ubiquinone reductase homolog, nuo-4 significantly protected against proteotoxicity (Figure 4c). Conversely, inhibiting mev-1, a homolog of cytochrome b of complex II, enhanced proteotoxicity (Figure 4d).
Discussion
Mechanisms mediating the robust protective effects of dietary restriction on age-related diseases are the subject of great interest since they could lead to pharmacological treatments that mimic these effects. Previous studies suggested that the protective effects of dietary restriction on polyQ proteotoxicity require the activity of hsf-1 [17]. We had similarly reported that inhibition of hsf-1, as well as cbp-1, blocked protective effects of dietary restriction and the daf-2 mutation on lifespan [5]. Since HSF-1 interacts with and recruits CBP for transcription, we hypothesized that part of the protective effects of dietary restriction are mediated through this interaction [5]. However, since there is no evidence that HSF-1 regulates metabolic gene expression, especially the shift from glucose to lipid metabolism that appears to mediate at least some protective effects of dietary restriction [6,7,31], it was of interest to assess the role of other transcription factors, more relevant to metabolism. Since inhibition of CBP prevents this molecular switch produced by dietary restriction [5], whereas PPAR homologs promote this molecular switch [11], we hypothesized that this family of transcription factors mediate these protective effects of dietary restriction. This hypothesis was greatly supported by the report that pharmacological activators of Ppar-alpha (e.g., fenofibrate) [12] increase lifespan in C. elegans, dependent on nhr-49, the functional homolog of Ppar-alpha in C. elegans [13]. Here we report that genetic ablation of the ppar homolog nhr-49 largely blocks protective effects of dietary restriction, and completely blocks effects of glycolytic inhibition to increase lifespan. As expected inhibition of cbp-1 also completely blocked effects of glycolytic inhibition to increase lifespan.
Elevated glucose reduces lifespan [21,22] whereas inhibiting glucose metabolism by 2-DG increases lifespan [20] in C. elegans. The present studies confirm and extend these observations to polyQ proteotoxicity. Inhibition of the C. elegans homolog of AMPK prevented protective effects of 2-DG to increase lifespan [20]. Similarly in the present studies inhibiting cbp-1 also completely prevented the protective effects of 2-DG on lifespan as well as on proteotoxicity. Of particular interest, inhibiting the ppar homolog nhr-49 also completely blocked the protective effects of 2-DG on lifespan and proteotoxicity. Since increased glucose can reduce mammalian ppar gene expression by inhibiting AMPK [32], all of these studies are consistent with the hypothesis that 2-DG protects against proteotoxicity by activating AMPK activity, leading to increased PPAR activity and increased recruitment of CBP to genes that promote lipid metabolism and reduce glucose metabolism.
The mechanism by which glucose metabolism drives polyQ proteotoxicity remains to be established but our studies suggest that a key metabolic reaction is increased production of NADH (from NAD+). Our data support that one mechanism mediating toxic effects of this reaction is increased utilization of ETC complex I, consistent with studies implicating this same complex in limiting lifespan [8]. This hypothesis is particularly attractive since Huntington’s Disease is characterized by relatively reduced complex II activity vs. complex I activity [33]. On the other hand, the NAD+ mechanism also activates sirtuins, which are also implicated in mediating protective effects of dietary restriction [34], even in mammals [35]. A particularly attractive hypothesis is that some of the deleterious effects of NADH are mediated by inhibition of the NADH-dependent transcriptional co-repressor CtBP [36]. CtBP inhibits CBP function [37] and in turn inhibition of CtBP by RNAi increases lifespan [38]. Another mechanism by which glucose metabolism may lead to deleterious effects is inhibition of daf-16/FOXO [21], which also recruits CBP [39]. Conversely, we hypothesize that the profound effects of the insulin-like pathway on healthspan and lifespan [40] are mediated at least in part by reduced glucose metabolism. It is therefore of some interest that crossing the polyQ construct onto the daf-2-null background significantly reduces proteotoxicity (not shown).
Different protocols of DR entail somewhat different molecular mechanisms [19], although inhibition of cbp-1 blocks protective effects of all protocols examined as well as of the daf-2 mutation [5]. Therefore it will be of great interest to assess if inhibiting nhr-49 blocks protective effects of other protocols of DR and the daf-2 mutation. Furthermore, since reduction of lifespan by elevated glucose entails inhibition of daf-16 [21], the role of daf-16 in mediating effects of 2-DG on polyQ proteotoxicity is also of great interest. Similarly, the role of the metabolic switch away from glucose utilization in mediating protective effects of DR must also be assessed. Finally, it would be of great interest to determine if protective effects of DR on polyQ proteotoxicity specifically require induction of nhr-49 in ASI neurons, as appears to be the case for skn-1 [41].
In conclusion, we have demonstrated here that the PPAR family of transcription factors and CBP mediate at least some protective effects of dietary restriction and, especially, glycolytic inhibition on lifespan and proteotoxicity. These studies suggest that Ppar-alpha activators may be similarly protective to prevent age-related diseases in humans. Furthermore, these studies suggest that glucose toxicity on polyQ proteotoxicity is mediated, at least in part, by production of NADH and oxidation of NADH via ETC complex I. These studies also propose several targets for drug development: glycolytic inhibitors, pyruvate mimetics, and ETC complex I inhibitors.
Acknowledgements
We acknowledge Kimbie Casten in carrying out an early replicate of the nuo-4 RNAi study, Alex Lublin, Nicholas Barone, Che-Yu Tai, and Julia Fisher for assistance, and Michelle Ehrlich for advice. These studies were supported by NIH grant HD060914. The authors have no conflicts of interest in regard to this work.
References
- 1.Yen K, Mobbs CV. Evidence for only two independent pathways for decreasing senescence in Caenorhabditis elegans. Age (Dordr). 2010; 32: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010; 328: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999; 57: 195–206. [DOI] [PubMed] [Google Scholar]
- 4.Duan W, Guo Z, Jiang H, et al. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003; 100: 2911–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang M, Poplawski M, Yen K, et al. Role of CBP and SATB-1 in aging, dietary restriction, and insulin-like signaling. PLoS Biol. 2009; 7: e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mobbs CV, Mastaitis JW, Zhang M, et al. Secrets of the lac operon. Glucose hysteresis as a mechanism in dietary restriction, aging and disease. Interdiscip Top Gerontol. 2007; 35: 39–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarente L Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008; 132: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillin A, Hsu AL, Arantes-Oliveira N, et al. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002; 298: 2398–2401. [DOI] [PubMed] [Google Scholar]
- 9.Benchoua A, Trioulier Y, Zala D, et al. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol Biol Cell. 2006; 17: 1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beal MF, Brouillet E, Jenkins BG, et al. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993; 13: 4181–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez AM, Sanchez J, Tobaruela A, et al. Time-course effects of increased fatty acid supply on the expression of genes involved in lipid/glucose metabolism in muscle cells. Cell Physiol Biochem. 2010; 25: 337–346. [DOI] [PubMed] [Google Scholar]
- 12.Brandstadt S, Schmeisser K, Zarse K, et al. Lipid-lowering fibrates extend C. elegans lifespan in a NHR-49/PPARalpha-dependent manner. Aging (Albany NY). 2013; 5: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Gilst MR, Hadjivassiliou H, Jolly A, et al. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005; 3: e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satyal SH, Schmidt E, Kitagawa K, et al. Polyglutamine aggregates alter protein folding homeostasis in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2000; 97: 5750–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998; 395: 854. [DOI] [PubMed] [Google Scholar]
- 16.Kaeberlein TL, Smith ED, Tsuchiya M, et al. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006; 5: 487–494. [DOI] [PubMed] [Google Scholar]
- 17.Steinkraus KA, Smith ED, Davis C, et al. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008; 7: 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jafari M, Khodayari B, Felgner J, et al. Pioglitazone: an antidiabetic compound with anti-aging properties. Biogerontology. 2007; 8: 639–651. [DOI] [PubMed] [Google Scholar]
- 19.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009; 8: 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz TJ, Zarse K, Voigt A, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007; 6: 280–293. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009; 10: 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlotterer A, Kukudov G, Bozorgmehr F, et al. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes. 2009; 58: 2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyper SR, Viswakarma N, Yu S, et al. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal. 2010; 8: e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelman L, Zhou G, Fajas L, et al. p300 interacts with the N- and C-terminal part of PPARgamma2 in a ligand-independent and -dependent manner, respectively. J Biol Chem. 1999; 274: 7681–7688. [DOI] [PubMed] [Google Scholar]
- 25.Vora M, Shah M, Ostafi S, et al. Deletion of microRNA-80 activates dietary restriction to extend C. elegans healthspan and lifespan. PLoS Genet. 2013; 9: e1003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffan JS, Kazantsev A, Spasic-Boskovic O, et al. The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc Natl Acad Sci U S A. 2000; 97: 6763–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates EA, Victor M, Jones AK, et al. Differential contributions of Caenorhabditis elegans histone deacetylases to huntingtin polyglutamine toxicity. J Neurosci; 2006; 26: 2830–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Poirier MA, Liang Y, et al. Depletion of CBP is directly linked with cellular toxicity caused by mutant huntingtin. Neurobiol Dis. 2006; 23: 543–551. [DOI] [PubMed] [Google Scholar]
- 29.Morley JF, Brignull HR, Weyers JJ, et al. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002; 99: 10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ido Y Pyridine nucleotide redox abnormalities in diabetes. Antioxid Redox Signal. 2007; 9: 931–942. [DOI] [PubMed] [Google Scholar]
- 31.Imai S The NAD World: a new systemic regulatory network for metabolism and aging--Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009; 53: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joly E, Roduit R, Peyot ML, et al. Glucose represses PPARalpha gene expression via AMP-activated protein kinase but not via p38 mitogen-activated protein kinase in the pancreatic beta-cell. J Diabetes. 2009; 1: 263–272. [DOI] [PubMed] [Google Scholar]
- 33.Cai H, Cong WN, Ji S, et al. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr Alzheimer Res. 2012; 9: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000; 289: 2126–2128. [DOI] [PubMed] [Google Scholar]
- 35.Mercken EM, Hu J, Krzysik-Walker S, et al. SIRT1 but not its increased expression is essential for lifespan extension in caloric-restricted mice. Aging Cell; 2014; 13: 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q, Piston DW, Goodman RH. Regulation of corepressor function by nuclear NADH. Science. 2002; 295: 1895–1897. [DOI] [PubMed] [Google Scholar]
- 37.Senyuk V, Sinha KK, Nucifora G. Corepressor CtBP1 interacts with and specifically inhibits CBP activity. Arch Biochem Biophys. 2005; 441: 168–173. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Whetstine JR, Ghosh S, et al. The conserved NAD(H)-dependent corepressor CTBP-1 regulates Caenorhabditis elegans life span. Proc Natl Acad Sci U S A. 2009; 106: 1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nasrin N, Ogg S, Cahill CM, et al. DAF-16 recruits the CREB-binding protein coactivator complex to the insulin-like growth factor binding protein 1 promoter in HepG2 cells. Proc Natl Acad Sci U S A. 2000; 97: 10412–10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tissenbaum HA. Genetics, life span, health span, and the aging process in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci 2012; 67: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007; 447: 545–549. [DOI] [PubMed] [Google Scholar]