Abstract
Background
Radial probe endobronchial ultrasound using a guide sheath (EBUS-GS) is used to diagnose peripheral lung cancer. The aim was to identify the accuracy of molecular analysis that were performed with EBUS-GS specimens in patients with non-small cell lung cancer (NSCLC).
Method
From December 2015 to September 2017, we retrospectively studied 91 patients with peripheral NSCLC who underwent surgery after EBUS-GS. Epidermal growth factor receptor (EGFR) mutational and anaplastic lymphoma kinase (ALK) translocation status obtained from surgical specimens served as the references.
Results
Compared to the reference data, EGFR mutational testing of EBUS-GS specimens was in 97% agreement, and the κ coefficient was 0.931 (P< 0.001). In addition, on ALK translocation testing, the results of all 91 patients were in agreement with the reference data (concordance rate of 100%, κ coefficient 1.000; P< 0.001).
Conclusion
We found that EBUS-GS could be used for molecular diagnosis, such as EGFR mutational and ALK translocation status, in patients with peripheral NSCLC.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide [1]. In recent years, significant developments in the diagnosis and treatment of non-small cell lung cancer (NSCLC) have been made [2,3]. In particular, patient-tailored therapies with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors and anaplastic lymphoma kinase (ALK) inhibitors have improved progression-free survival in patients with inoperable NSCLC [4–8].
Patient-tailored therapy requires accurate molecular data, which in turn means that appropriate tissue must be acquired. It is ideal to harvest as much tissue as possible for use in pathologic evaluation and molecular testing. In addition, the remaining tissue should be preserved for further testing [9]. However, due to technical problems with tissue testing, there is a limit to the amount of tissue that can be harvested. [10]. To date, three lung biopsy modalities (surgical wedge resection, percutaneous core needle biopsy [PCNB], and bronchoscopy) have been used for both molecular analysis and histological confirmation [11]. Generally, a prompt and definitive diagnosis using a large amount of tissue can be made on video-assisted thoracoscopic wedge resection under general anesthesia; however, the mortality rate is 0.5% and the complications include persistent air leakage and pneumonia [12]. In addition, although PCNB has afforded good diagnostic performance over many decades, the procedure-related complications include iatrogenic pneumothorax, pleural seeding, and bleeding [13].
Peripheral bronchoscopic techniques, including virtual and electromagnetic navigation, and radial probe endobronchial ultrasound (EBUS) using a guide sheath (GS), have developed rapidly, and are now used to diagnose peripheral lung nodules [14–16]. Recently, transbronchial lung biopsy using a radial probe EBUS and a GS (EBUS-GS) has been shown to afford an acceptable diagnostic yield with a low complication rate [17,18]. However, the accuracy and reliability of molecular analyses of EBUS-GS specimens remain unclear. We retrospectively explored the accuracy of EGFR mutational and ALK translocation testing in small EBUS-GS tissue samples.
Materials and methods
Study population
Between December 2015 and September 2017, we retrospectively accessed the database of the EBUS-GS registry to explore the accuracies of EGFR mutational analysis and ALK fluorescence in situ hybridization (FISH) status performed on EBUS-GS specimens at Pusan National University Hospital (a university-affiliated, tertiary referral hospital in Busan, South Korea). During the study period, 97 consecutive patients who underwent surgical resection of peripheral NSCLC after a definitive histological EBUS-GS diagnosis were prospectively registered. When evaluating the mutational analyses, the surgical specimens served as the reference samples. Some of our clinical data included previous study conducted but not published [19]. Because of the retrospective nature of the study, the Institutional Review Board of Pusan National University Hospital approved this work without a requirement to obtain informed consent from each subject (approval no. 1711-023-061).
EBUS-GS procedure
Before each procedure, 4% lidocaine was sprayed into the oropharynx to create local anesthesia and the patient was sedated with intravenous midazolam and fentanyl. First, conventional bronchoscopy using a thin, 4-mm flexible bronchoscope (BF-P260F; Olympus, Tokyo, Japan) was performed to examine the bronchial tree. Next, the bronchoscope was moved as close as possible to the bronchus of interest, guided by the thin-section chest computed tomography (CT) image (0.625mm in both interval and thickness). Then, a radial probe EBUS (UM-S20-17S; Olympus) covered with a GS (K-201; Olympus) was advanced through a 2.0-mm-diameter working channel of the thin bronchoscope to target the peripheral lung lesion precisely. Once the lesion had been accurately identified, the radial probe EBUS was withdrawn, leaving the GS in place to allow brush cytology and forceps biopsy under fluoroscopic guidance [20–23]. Neither virtual bronchoscopy nor electromagnetic navigation was employed [14,15].
Molecular analyses
Both EGFR mutation and ALK FISH tests were performed using biopsy tissue and surgically resected samples. EGFR mutational tests were performed using an EGFR Mutation Detection Kit (PNA clamp; Panagene, Daejeon, South Korea) [24,25]. A commercial ALK FISH assay (Vysis ALK Break Apart FISH Probe Kit; Abbott Laboratories, Lake Bluff, IL, USA) was used to detect ALK translocation [26,27].
Procedure-related complications
Four hours after EBUS-GS, a plain chest film was taken to detect any procedure-related complication including iatrogenic pneumothorax, and a follow-up chest radiograph was taken the next morning. Severe procedure-related bleeding was defined as a need for intubation, radiological intervention, or transfusion. Any complication such as respiratory failure or pulmonary infection was recorded.
Statistical analysis
Data are presented as numbers (%) or medians (interquartile ranges [IQRs]) as appropriate. The extents of agreement between EGFR mutational tests and ALK FISH analyses (EBUS-GS vs. surgical specimens) were determined using Cohen’s κ statistic [28,29]. A two-sided P-value <0.05 was considered to indicate statistical significance. All statistical analyses were conducted using SPSS version 22.0 software for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patients
Of the 97 patients who underwent surgical resection of peripheral NSCLC after definitive diagnosis using EBUS-GS, 6 were excluded because their molecular analyses were incomplete. The baseline characteristics of the 91 subjects are shown Table 1. A total of 54 patients were male (59%), and the median age was 67 years (IQR, 60–72 years). The pathological diagnosis was as follows: adenocarcinoma in 68 patients (75%), squamous cell carcinoma in 18 (20%), and NSCLC not otherwise specified in 5 (5%).
Table 1. Baseline characteristics of 91 patients who underwent surgical resection after EBUS-GS.
| Characteristic | No. (%) or median (interquartile range) |
|---|---|
| Age, years | 67 (60–72) |
| Male gender | 54 (59) |
| Ever-smoker | 45 (50) |
| Pathological diagnosis | |
| Adenocarcinoma | 68 (75) |
| Squamous cell carcinoma | 18 (20) |
| Non-small cell lung cancer, NOS | 5 (5) |
EBUS-GS = endobronchial ultrasound using a guide sheath; NOS = not otherwise specified.
Molecular analysis
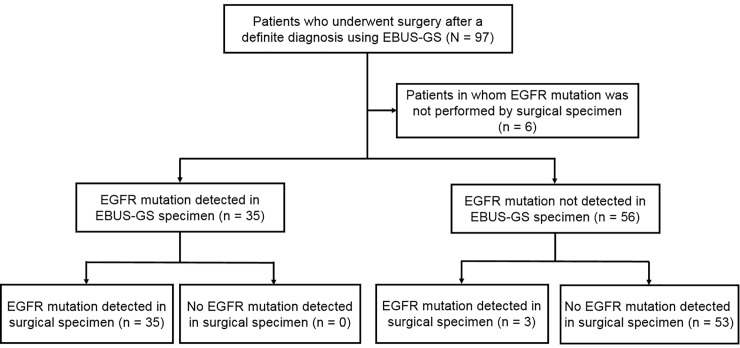
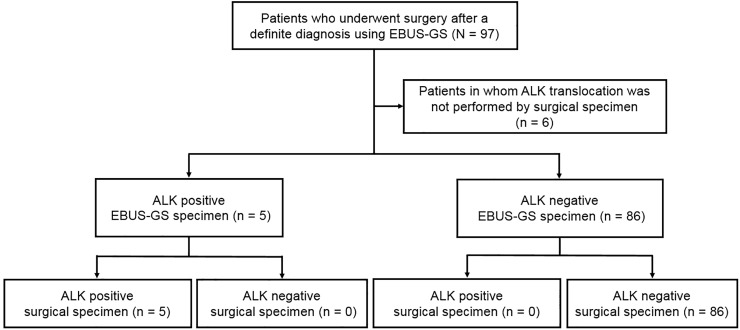
Using the EBUS-GS and surgical specimens, EGFR mutations were detected in 35 and 38 patients, respectively (38 and 42%). The results differed in three patients (3%) (Fig 1). The agreement rate was 97% and the κ coefficient was 0.931 (P< 0.001) (Table 2). In the ALK FISH test, 5 of 91 patients (5%) were positive on both surgical and EBUS-GS testing (Fig 2). The agreement rate was 100% and the κ coefficient was 1.000 (P< 0.001) (Table 2). Additional statistical analysis was performed except for squamous cell carcinoma patients. In 73 patients, the agreement rate of EGFR mutation was 96% and the κ coefficient was 0.918 (P = 0.046). In the ALK FISH test, agreement rate was 100% and the κ coefficient was 1.000 (P< 0.001).
Fig 1. Comparison of EGFR mutational analysis between the EBUS-GS and surgical specimens.
EBUS-GS = endobronchial ultrasound using a guide sheath; EGFR = epidermal growth factor receptor.
Table 2. Comparisons of the EGFR mutational and ALK translocation results between the EBUS-GS and surgical specimens.
| Specimens | Correlation analysis | ||||
|---|---|---|---|---|---|
| EBUS-GS (%) | Surgery (%) | Agreement rate | κ coefficient | P value | |
| EGFR mutation detected | 35/91 (38) | 38/91 (42) | 97% | 0.931 | <0.001 |
| ALK-positive | 5/91 (5) | 5/91 (5) | 100% | 1.000 | < 0.001 |
EGFR = epidermal growth factor receptor; ALK = anaplastic lymphoma kinase; EBUS-GS = endobronchial ultrasound using a guide sheath.
Fig 2. Comparison of ALK translocation analysis between the EBUS-GS and surgical specimens.
EBUS-GS = endobronchial ultrasound using a guide sheath; ALK = anaplastic lymphoma kinase.
Procedure-related complications
Only one patient (1%) developed procedure-related pneumothorax, but recovered spontaneously without chest tube insertion. No other complications were observedno severe hemorrhage, pulmonary infection, or respiratory failure was noted.
Disagreements in EGFR mutational analysis
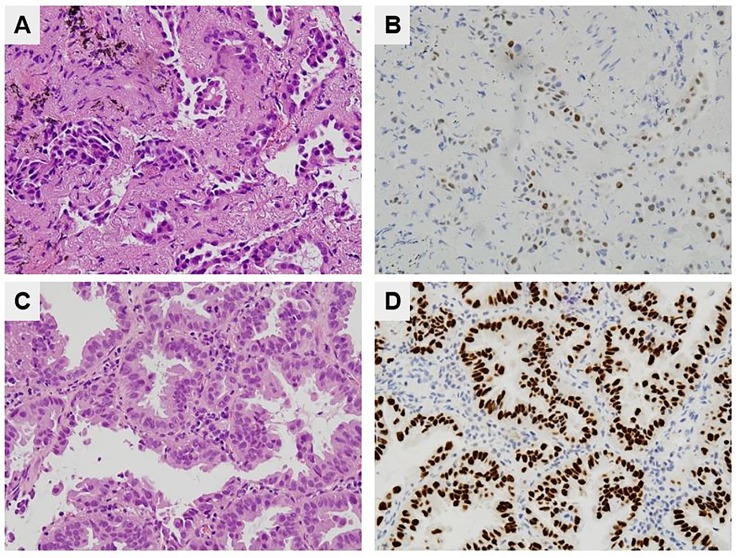
All three patients with inconsistent EGFR mutational results were pathologically diagnosed with adenocarcinomas. Compared to the EBUS-GS specimens that yielded correct results, the tumor cell numbers estimated by pathologists were lower on hematoxylin-and-eosin-stained slides of all incorrectly diagnosed EBUS-GS specimens. Moreover, only a few thyroid transcription factor-1-stained cells were observed in two of these EBUS-GS specimens (Fig 3A and 3B); one specimen could not be stained because the available tissue was insufficient (Case No. 2, Table 3). Higher numbers of thyroid transcription factor-1-and hematoxylin-and-eosin-stained cells were observed, at the same magnification, in EBUS-GS specimens that were correctly diagnosed (Fig 3C and 3D).
Fig 3. Comparison of an EBUS-GS specimen yielding false-negative EGFR results and a specimen yielding correct EGFR results.
(A) A few adenocarcinoma cells were clustered in the EBUS-GS specimen with the false-negative EGFR result (H&E stain, ×400). (B) The EBUS-GS specimen with false-negative EGFR result was weakly immunoactive for TTF-1 (×400). (C) Larger numbers of tumor cells were evident in the specimen yielding correct EGFR results (H&E stain, ×400). (D) The EBUS-GS specimen with correct EGFR result was strongly immunoactive for TTF-1(×400). EBUS-GS = endobronchial ultrasound using a guide sheath; EGFR = epidermal growth factor receptor; TTF-1 = thyroid transcription factor-1.
Table 3. Cases with discordant EGFR mutational results between the EBUS-GS and surgical specimens.
| Case No. | Age, years | Sex | Pathology | Lesion size, mma | Location | Bronchus sign | Probe location | TTF-1 IHC |
|---|---|---|---|---|---|---|---|---|
| 1 | 78 | Male | ADC | 36 | RLL | Positive | Adjacent to tumor | Positive |
| 2 | 74 | Female | ADC | 48 | RUL | Positive | Within tumor | Insufficientb |
| 3 | 70 | Female | ADC | 38 | RML | Positive | Within tumor | Positive |
EGFR, epidermal growth factor receptor; EBUS-GS, endobronchial ultrasound using a guide sheath; TTF-1, thyroid transcription factor-1; IHC, immunohistochemistry; ADC, adenocarcinoma; RLL, right lower lobe; RUL, right upper lobe; RML, right middle lobe.
a Largest tumor diameter.
b Insufficient EBUS-GS material for TTF-1 staining
Discussion
We found that EBUS-GS afforded very accurate EGFR mutational and ALK FISH diagnoses in NSCLC patients. To the best of our knowledge, this is the first report on the accuracy of molecular diagnosis using such specimens. In the 91 NSCLC patients, the accuracies of the EGFR mutational and ALK translocation tests were 97% and 100%, respectively. Our findings imply that appropriate decision-making in terms of anti-cancer drug selection (EGFR tyrosine kinase inhibitors, ALK inhibitors, or intravenous cytotoxic chemotherapy) is possible based on molecular data obtained from EBUS-GS specimens of patients with advanced NSCLC.
Tam et al. found that 83% of PCNB samples were suitable for molecular testing in 151 patients with NSCLC [30]. However, procedure-related complications occurred in 16% of the patients, of whom 57% required chest tube insertion to manage iatrogenic pneumothorax. Vanderlaan et al. reported that the accuracy of molecular analysis using PCNB samples was lower than noted in a previous study [31]; the accuracies of EGFR mutational and ALK FISH tests performed on PCNB samples were 68% and 65%, respectively, in 22 patients with NSCLC. Chen et al. found that all PCNB samples examined could be used for EGFR mutational testing [32]. However, this study feature relatively small group of 17 patients, and complications such as pneumothorax (18%) and hemoptysis (12%) were relatively common. In summary, although molecular diagnosis using PCNB samples is reliable, the incidence of procedure-related complications, such as iatrogenic pneumothorax, is relatively high.
In contrast, Steinfort et al. showed that EBUS-GS afforded similar pathological diagnostic accuracy compared with PCNB (87.5% vs. 93.3%, respectively) and good sensitivity (86% vs.92%, respectively), associated with considerably fewer procedure-related complications (3%vs. 27%, respectively) [33]. Hamaya et al. reported that the overall complication rate of EBUS-GS (pneumothorax or pneumonia) was 1.3% in 965 study subjects [17]. In the present study, the accuracies of EGFR mutational and ALK FISH testing were 97% and 100%, and the overall complication rate was only 1%. Thus, EBUS-GS is safe and reliable, and the tissue samples can be used for both pathological and molecular analyses.
Generally, molecular analysis proceeds using the tissue that remains after histological examination featuring hematoxylin-and-eosin staining. Therefore, molecular tests are usually performed employing less tissue than in histological examinations and molecular analysis of a small biopsy sample, such as that of PCNB or EBUS-GS, could yield false-negative results because of insufficient tumor tissue or a low tumor fraction. Eberhard et al. suggested that the tumor sectional area should be ≥1–2mm, except in non-tumor areas [34]. In addition, in terms of cell counts, >100 tumor cell nuclei should be assessed in terms of FISH. Lindeman et al. recommended that mutated cells should constitute ≥20% of all cells when EGFR mutational and ALK translocation statuses are evaluated [35]. In the present study, false-negative EGFR mutational data were obtained from three EBUS-GS specimens (3%). The tumor cell numbers in these specimens were lower than those of other specimens. Thus, insufficient tumor tissue available after histological examination explained the false-negative results. If the EGFR mutational status of an EBUS-GS specimen is negative, a false-negative should be considered when the sample volume is small, particularly if the patient is at risk of EGFR or ALK mutation (has an adenocarcinoma, is a female East Asian, or is a never-smoker) [36–39].
Our study had several limitations. First, although we used an EBUS-GS registry, selection bias may have occurred. Second, this was a single-center study with a relatively small number of subjects; our results can thus not be generalized to other institutions or geographical areas. Third, as we used data from surgical specimens as references, only patients with early-stage lung cancer who underwent surgery were included. Molecular analyses, such as EGFR mutational and ALK FISH tests, are required by patients with advanced NSCLC to guide the selection of anti-cancer drugs (EGFR tyrosine kinase or ALK inhibitors). Generally, patients with advanced NSCLC requiring molecular analysis have larger and more tumors than patients with early-stage lung cancer. Previous studies found that the accuracy of EBUS-GS evaluation was associated with lesion size [15,16,40]. Therefore, the accuracy of molecular diagnosis would be expected to be higher in actual clinical practice. Fourth, NGS data were unavailable in the present study. However, the sensitivity and specificity of PNA clamping are 97% and 100%, respectively, similar to the respective values of 95.83% and 98.11% for NGS [41–43]. If NGS is available for both small biopsy samples and surgical specimens, it is possible to compare the concordance of various kinds of mutational analyses. Fifth, given the retrospective nature of this study, it was not possible to quantitatively analyze the effect of sample volume on the molecular data. Generally, the cellularity of the specimen is important for interpretation of mutation analysis results [44]. Recent guidelines recommend mutation analysis of samples with an at-least 20% malignant cell content [35]. To address these issues, an additional prospective multicenter study with a large number of patients that incorporates methods to evaluate cellularity is needed.
Conclusion
The results of EGFR mutation and ALK gene rearrangement tests on EBUS-GS samples showed good agreement with those on surgical specimens of NSCLC patients.
Supporting information
ADC, adenocarcinoma; SqCC, squamous cell carcinoma; NSCLC, non-small cell lung cancer; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; RLL, right lower lobe; RUL, right upper lobe; LUL, left upper lobe; LLL, left lower lobe; RML, right middle lobe.
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Siegel RL, Miller KD, Jemal A.Cancer statistics, 2016. CA Cancer JClin.2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013; 382: 709–19. 10.1016/S0140-6736(13)61502-0 [DOI] [PubMed] [Google Scholar]
- 3.Moreira AL, Eng J. Personalized therapy for lung cancer. Chest. 2014; 146: 1649–57. 10.1378/chest.14-0713 [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13: 239–46. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 5.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361: 947–57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 6.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015; 16: 141–51. 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 7.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014; 371: 2167–77. 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 8.Wu YL, Saijo N, Thongprasert S, Yang JC, Han B, Margono B, et al. Post-hoc analyses from the phase III, randomized, multicenter, IPASS study of first-line gefitinib versus carboplatin/paclitaxel in Asian patients with EGFR mutation-positive advanced NSCLC. Lung Cancer. 2017; 104: 119–25. 10.1016/j.lungcan.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013. May;137(5):668–84. 10.5858/arpa.2012-0263-RA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reck M, Hermes A, Tan EH, Felip E, Klughammer B, Baselga J. Tissue sampling in lung cancer: a review in light of the MERIT experience. Lung Cancer. 2011. October;74(1):1–6. 10.1016/j.lungcan.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 11.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143: e93S–120S. 10.1378/chest.12-2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143: e142S–e65S. 10.1378/chest.12-2353 [DOI] [PubMed] [Google Scholar]
- 13.Lu CH, Hsiao CH, Chang YC, Lee JM, Shih JY, Wu LA, et al. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol. 2012; 7: 143–50. 10.1097/JTO.0b013e318233d7dd [DOI] [PubMed] [Google Scholar]
- 14.Eberhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med.2007; 176: 36–41. 10.1164/rccm.200612-1866OC [DOI] [PubMed] [Google Scholar]
- 15.Ishida T, Asano F, Yamazaki K, Shinagawa N, Oizumi S, Moriya H, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax. 2011; 66: 1072–7. 10.1136/thx.2010.145490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004; 126: 959–65. 10.1378/chest.126.3.959 [DOI] [PubMed] [Google Scholar]
- 17.Hayama M, Izumo T, Matsumoto Y, Chavez C, Tsuchida T, Sasada S. Complications with endobronchial ultrasound with a guide sheath for the diagnosis of peripheral pulmonary Lesions. Respiration. 2015; 90: 129–35. 10.1159/000431383 [DOI] [PubMed] [Google Scholar]
- 18.Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J. 2011; 37: 902–10. 10.1183/09031936.00075310 [DOI] [PubMed] [Google Scholar]
- 19.Eom JS, Mok JH, Kim I, Lee MK, Lee G, Park H, et al. Radial probe endobronchial ultrasound using a guide sheath for peripheral lung lesions in beginners. unpublish data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinagawa N, Yamada N, Asahina H, Kikuchi E, Oizumi S, Kurimoto N, et al. Transbronchial biopsy for peripheral pulmonary lesions under real-time endobronchial ultrasonographic guidance. J Bronchology Interv Pulmonol. 2009; 16: 261–5. 10.1097/LBR.0b013e3181bb8058 [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi E, Yamazaki K, Sukoh N, Kikuchi J, Asahina H, Imura M, et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur Respir J. 2004; 24: 533–7. 10.1183/09031936.04.00138603 [DOI] [PubMed] [Google Scholar]
- 22.Bonney A, Christie M, Beaty A, Lunke S, Taylor G, Irving L, el al. The feasibility of molecular testing on cell blocks created from brush tip washings in the assessment of peripheral lung lesions. J Thorac Dis. 2016. September; 8(9): 2551–2555. 10.21037/jtd.2016.08.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonney A, Beaty A, See K, Irving L, Steinfort D. Diagnostic Utility of Bronchial Brush-Tip Washings for the Immunohistochemical Assessment of Peripheral Lung Lesions. Acta Cytol. 2016; 60(1): 74–8. 10.1159/000444044 [DOI] [PubMed] [Google Scholar]
- 24.Kim HR, Lee SY, Hyun DS, Lee MK, Lee HK, Choi CM, et al. Detection of EGFR mutations in circulating free DNA by PNA-mediated PCR clamping. J Exp Clin Cancer Res. 2013; 32: 50 10.1186/1756-9966-32-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeo CD, Kim JW, Kim KH, Ha JH, Rhee CK, Kim SJ, et al. Detection and comparison of EGFR mutations in matched tumor tissues, cell blocks, pleural effusions, and sera from patients with NSCLC with malignant pleural effusion, by PNA clamping and direct sequencing. Lung Cancer. 2013; 81: 207–12. 10.1016/j.lungcan.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 26.Yi ES, Boland JM, Maleszewski JJ, Roden AC, Oliveira AM, Aubry MC, et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol. 2011; 6: 459–65. 10.1097/JTO.0b013e318209edb9 [DOI] [PubMed] [Google Scholar]
- 27.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363: 1693–703. 10.1056/NEJMoa1006448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012; 22: 276–82. [PMC free article] [PubMed] [Google Scholar]
- 29.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33: 159–74. [PubMed] [Google Scholar]
- 30.Tam AL, Kim ES, Lee JJ, Ensor JE, Hicks ME, Tang X, et al. Feasibility of image-guided transthoracic core-needle biopsy in the BATTLE lung trial. J Thorac Oncol. 2013; 8: 436–42. 10.1097/JTO.0b013e318287c91e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanderlaan PA, Yamaguchi N, Folch E, Boucher DH, Kent MS, Gangadharan SP, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014; 84: 39–44. 10.1016/j.lungcan.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CM, Chang JW, Cheung YC, Lin G, Hsieh JJ, Hsu T, et al. Computed tomography-guided core-needle biopsy specimens demonstrate epidermal growth factor receptor mutations in patients with non-small-cell lung cancer. ActaRadiol. 2008; 49: 991–4. [DOI] [PubMed] [Google Scholar]
- 33.Steinfort DP, Vincent J, Heinze S, Antippa P, Irving LB. Comparative effectiveness of radial probe endobronchial ultrasound versus CT-guided needle biopsy for evaluation of peripheral pulmonary lesions: a randomized pragmatic trial. Respir Med. 2011; 105: 1704–11. 10.1016/j.rmed.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 34.Eberhard DA, Giaccone G, Johnson BE. Biomarkers of response to epidermal growth factor receptor inhibitors in Non-Small-Cell Lung Cancer Working Group: standardization for use in the clinical trial setting. J Clin Oncol. 2008; 26: 983–94. 10.1200/JCO.2007.12.9858 [DOI] [PubMed] [Google Scholar]
- 35.Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn. 2018. March; 20(2):129–159. 10.1016/j.jmoldx.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Ren Y, Fang Z, Li C, Fang R, Gao B, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010; 28: 4616–20. 10.1200/JCO.2010.29.6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scagliotti GV, Longo M, Novello S.Non-small cell lung cancer in never smokers. Curr Opin Oncol. 2009; 21: 99–104. 10.1097/CCO.0b013e328321049e [DOI] [PubMed] [Google Scholar]
- 38.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006; 118: 257–62. 10.1002/ijc.21496 [DOI] [PubMed] [Google Scholar]
- 39.Yatabe Y, Mitsudomi T. Epidermal growth factor receptor mutations in lung cancers. Pathol Int. 2007; 57: 233–44. 10.1111/j.1440-1827.2007.02098.x [DOI] [PubMed] [Google Scholar]
- 40.Yang F, Chen H, Xiang J, Zhang Y, Zhou J, Hu H, et al. Relationship between tumor size and disease stage in non-small cell lung cancer. BMC Cancer. 2010; 10: 474 10.1186/1471-2407-10-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X, Yang Y, Li H, Chen Z, Jiang G, Fei K. Assessment of the clinical application of detecting EGFR, KRAS, PIK3CA and BRAF mutations in patients with non-small cell lung cancer using next-generation sequencing. Scand J Clin Lab Invest. 2016. September; 76(5): 386–92. 10.1080/00365513.2016.1183813 [DOI] [PubMed] [Google Scholar]
- 42.Jing C, Mao X, Wang Z, Sun K, Ma R, Wu J, et al. Next-generation sequencing-based detection of EGFR, KRAS, BRAF, NRAS, PIK3CA, Her-2 and TP53 mutations in patients with non-small cell lung cancer. Mol Med Rep. 2018. August; 18(2): 2191–2197. 10.3892/mmr.2018.9210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka T, Nagai Y, Miyazawa H, Koyama N, Matsuoka S, Sutani A, et al. Reliability of the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based test for epidermal growth factor receptor mutations integrated into the clinical practice for non-small cell lung cancers. Cancer Sci. 2007. February; 98(2): 246–52. 10.1111/j.1349-7006.2006.00377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han Y, Li J. Sample types applied for molecular diagnosis of therapeutic management of advanced non-small cell lung cancer in the precision medicine. Clin Chem Lab Med. 2017. October 26;55(12):1817–1833. 10.1515/cclm-2017-0112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ADC, adenocarcinoma; SqCC, squamous cell carcinoma; NSCLC, non-small cell lung cancer; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; RLL, right lower lobe; RUL, right upper lobe; LUL, left upper lobe; LLL, left lower lobe; RML, right middle lobe.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.