Abstract
Safflower (Carthamus tinctorius L.) is a multipurpose crop of dry land yielding very high quality of edible oil. Present study was aimed to investigate the genetic diversity and population structure of 131 safflower accessions originating from 28 different countries using 13 iPBS-retrotransposon markers. A total of 295 iPBS bands were observed among which 275 (93.22%) were found polymorphic. Mean Polymorphism information content (0.48) and diversity parameters including mean effective number of alleles (1.33), mean Shannon’s information index (0.33), overall gene diversity (0.19), Fstatistic (0.21), and inbreeding coefficient (1.00) reflected the presence of sufficient amount of genetic diversity in the studied plant materials. Analysis of molecular variance (AMOVA) showed that more than 40% of genetic variation was derived from populations. Model-based structure, principal coordinate analysis (PCoA) and unweighted pair-group method with arithmetic means (UPGMA) algorithms clustered the 131 safflower accessions into four main populations A, B, C, D and an unclassified population, with no meaningful geographical origin. Most diverse accessions originated from Asian countries including Afghanistan, Pakistan, China, Turkey, and India. Four accessions, Turkey3, Afghanistan4, Afghanistan2, and Pakistan24 were found most genetically distant and might be recommended as a candidate parents for breeding purposes. The findings of this study are most probably supported by the seven similarity centers hypothesis of safflower. This is a first study to explore the genetic diversity and population structure in safflower accessions using the iPBS-retrotransposon markers. The information provided in this work will therefore be helpful for scientists interested in safflower breeding.
1. Introduction
Safflower (Carthamus tinctorius L.) belongs to the Compositae family, it is self-pollinated and has a haploid genome size of about 1.4 GB and 2n = 24 chromosomes [1]. This crop is cultivated over wide geographical zones throughout the world for several uses including production of dyes, extraction of edible oil, and various medicinal utilizations [2]. Safflower has been in use since ancient times and the archeological remains of Carthamus spp. were found at sites in Syria about 7500 BC ago [3]. From these sites, cultivation of safflower spread to other regions like, Egypt, the Aegean, and southeastern Europe.
Safflower accessions specific to some regions show similarity on the basis of their morphological traits and such a region can be considered as a safflower similarity center. However, there is still a debate on the actual number of similarity centers in the world as ascertained by molecular markers [4]. Knowles [5] proposed seven similarity centers (1: Far East, 2: India-Pakistan, 3: Middle East, 4: Egypt, 5: Sudan, 6: Ethiopia, and 7: Europe) for safflower while, Ashri [6] identified ten similarity centers (1: Near East, 2: Iran/Afghanistan, 3: Turkey, 4: Egypt, 5: Ethiopia, 6: Sudan, 7: Far East, 8: India/Pakistan, 9: Europe, and 10: Kenya). Similarly, Chapman et al. [4] proposed five safflower similarity centers for safflower (1: Near East, 2: Iran & Afghanistan, Turkey, 3: Egypt, Ethiopia, (Sudan), 4: Far East, India/Pakistan, (Sudan), 5: Europe).
It is estimated that safflower is cultivated in nearly 20 countries with a total cultivated area of 1,140,002 hectares and the production of 948,516 tons [7]. Safflower major producer countries include, Russian Federation (286,351 tons), Kazakhstan (167,243 tons), Mexico (121,767 tons), USA (99,830 tons), Turkey (58,000 tons), and India (53,000 tons) which account for about 71% of the total world production [7]. In spite of containing good amount of polyunsaturated fatty acids and being resistant to dry conditions, still safflower did not gain the status of major oilseed crop. The primary factors which prevented its cultivation on large scale are low seed yield, low oil content, biotic stresses susceptibility, and spininess [8]. Therefore, the enhanced acceptability and utilization of safflower as an oilseed crop will require genetic improvement for the traits of interest. To this end, genetic diversity can be an effective approach by providing a good source of variations upon which breeding programs can build [9]. However, it is unfortunate that current safflower germplasm and breeding lines displayed low levels of genetic diversity, and were therefore of reduced usefulness in breeding programs. An extensive genetic and phenotypic diversity characterization among global safflower germplasm can help broaden the genetic base and diversity in the safflower crop, and identify elite accessions [10–11]. Safflower genetic diversity was investigated using different molecular markers; Random Amplified Polymorphic DNA (RAPD), Inter Simple Sequence Repeat (ISSR), Amplified Fragment Length Polymorphism (AFLP), Simple Sequence Repeats (SSRs), and Single Nucleotide Polymorphism (SNPs) [4–11–12–13–14–15–16–17–18–19–20], but so far, iPBS-retrotransposon markers have not been used to investigate the genetic diversity in safflower.
Retrotransposons are known as an important component of the plant genome in terms of structural evolution and have great potential of changing its position and copy number across plant genome [21]. Retrotransposons are genetic elements ranging from 50 to 90% in various plant genomes depending upon the plant species [22]. Long terminal repeat (LTR) and non- long terminal repeat (non-LTR) are the two classes of retrotransposons, and plant genome reveal higher proportions of LTR retrotransposons as compare to non-LTR [23]. Limitations in the retrotransposon marker systems resulted in the development of a new marker system named Inter-primer binding site (iPBS) retrotransposons having universal applicability [23–24]. iPBS is a PCR-based, universal marker system and depends upon the presence of tRNA as a reverse transcriptase primer binding site [25]. Minimum cost and high efficiency of iPBS-retrotransposons make them good marker system [23]. Various crops like, pea, chickpea, Lens, Turkish okra, Tobacco, and common bean have been studied efficiently using iPBS-retrotransposon markers system [26–27–28–29–30].
Several crop species have been improved utilizing molecular markers in various crop breeding programs [31]. However, for safflower, its genetics and genomics were less studied, which can explain the lack of reliable marker systems for use in the process of developing superior safflower cultivars [32–33]. This study was conducted to evaluate the genetic diversity and population structure of safflower accessions using iPBS-retrotransposons as a start for further scientific investigations and practical breeding use cases.
2. Materials and Methods
2.1. Plant materials and DNA isolation
Experimental materials comprising 131 safflower accessions collected from 28 different countries were evaluated in this study. Among these accessions, 94, 17, and 20 originated from the United States Department of Agriculture (USDA), Plant Genetic Resources Institute Pakistan, and from the Turkish Central Research Institute for Field Crops (Table 1). A total of 94 accessions from USDA and 17 from Pakistan used in this study were landraces. The 20 Turkish accessions were single plant selection among international germplasm from USDA and are candidate cultivars. Seeds of each accession were sown at the research and experimental area of Bolu Abant Izzet Baysal University. Fresh, young healthy leaves were harvested at proper time for the isolation of DNA, brought to laboratory and frozen at -80°C for later use. DNA extraction was performed using the bulk leaves of each accession, and followed CTAB protocol [34] with slight modifications [35]. DNA concentration of each accession was measured using agarose gel (0.8%) and was also confirmed with the help of NanoDrop (DeNovix DS-11 FX, USA). Final DNA concentration for the 131 accession samples to be used in polymerase chain reactions (PCR) was adjusted to 5 ng/μL; the samples were stored at -25 oC till the start of PCR amplifications.
Table 1. Passport data of 131 world safflower panel.
| Accession Number | Genotype Name | Accession No | Donor Organization | Location | Province/District | Country Origin | Plant ID | Continent |
|---|---|---|---|---|---|---|---|---|
| G1 | Isreal-1 | 30548 | USDA | - | - | Isreal | P1-198990 | Asia |
| G2 | Romania-1 | 30549 | USDA | - | - | Romania | P1-209287 | Europe |
| G3 | Morocco-1 | 30552 | USDA | - | - | Morocco | P1-239042 | Africa |
| G4 | Egypt-1 | 30563 | USDA | - | - | Egypt | P1-250082 | Africa |
| G5 | Pakistan-1 | 30564 | USDA | - | - | Pakistan | P1-250194 | Asia |
| G6 | Pakistan-2 | 30565 | USDA | - | - | Pakistan | P1-250201 | Asia |
| G7 | Pakistan-3 | 30567 | USDA | - | - | Pakistan | P1-250345 | Asia |
| G8 | Pakistan-4 | 30568 | USDA | - | - | Pakistan | P1-250346 | Asia |
| G9 | Pakistan-5 | 30569 | USDA | - | - | Pakistan | P1-250351 | Asia |
| G10 | Pakistan-6 | 30570 | USDA | - | - | Pakistan | P1-250353 | Asia |
| G11 | Pakistan-7 | 30573 | USDA | - | - | Pakistan | P1-250481 | Asia |
| G12 | Egypt-2 | 30574 | USDA | - | - | Egypt | P1-250528 | Africa |
| G13 | Egypt-3 | 30577 | USDA | - | - | Egypt | P1-250532 | Africa |
| G14 | Egypt-4 | 30578 | USDA | - | - | Egypt | P1-250540 | Africa |
| G15 | India-1 | 30579 | USDA | - | - | India | P1-250601 | Asia |
| G16 | Egypt-4 | 30580 | USDA | - | - | Egypt | P1-250605 | Africa |
| G17 | Egypt-6 | 30581 | USDA | - | - | Egypt | P1-250608 | Africa |
| G18 | Iran-1 | 30588 | USDA | - | - | Iran | P1-250720 | Asia |
| G19 | Jordan-1 | 30589 | USDA | - | - | Jordan | P1-251284 | Asia |
| G20 | Jordan-2 | 30590 | USDA | - | - | Jordan | P1-251285 | Asia |
| G21 | Isreal-2 | 30594 | USDA | - | - | Isreal | P1-253386 | Asia |
| G22 | Spain-1 | 30595 | USDA | - | - | Spain | P1-253388 | Europe |
| G23 | Spain-2 | 30596 | USDA | - | - | Spain | P1-253391 | Europe |
| G24 | Spain-3 | 30597 | USDA | - | - | Spain | P1-253394 | Europe |
| G25 | Spain-4 | 30598 | USDA | - | - | Spain | P1-253395 | Europe |
| G26 | Portugal-1 | 30604 | USDA | - | - | Portugal | P1-253553 | Europe |
| G27 | Portugal-2 | 30605 | USDA | - | - | Portugal | P1-253556 | Europe |
| G28 | Morocco-2 | 30606 | USDA | - | - | Morocco | P1-253560 | Africa |
| G29 | Portugal-3 | 30608 | USDA | - | - | Portugal | P1-253564 | Europe |
| G30 | Portugal-4 | 30610 | USDA | - | - | Portugal | P1-253569 | Europe |
| G31 | Portugal-5 | 30611 | USDA | - | - | Portugal | P1-253571 | Europe |
| G32 | Iraq-1 | 30612 | USDA | - | - | Iraq | P1-253761 | Asia |
| G33 | Iraq-2 | 30613 | USDA | - | - | Iraq | P1-253762 | Asia |
| G34 | Afghanistan-1 | 30614 | USDA | - | - | Afghanistan | P1-253764 | Asia |
| G35 | Isreal-3 | 3015 | USDA | - | - | Isreal | P1-253892 | Asia |
| G36 | Syria-1 | 30616 | USDA | - | - | Syria | P1-253898 | Asia |
| G37 | Syria-2 | 30617 | USDA | - | - | Syria | P1-253900 | Asia |
| G38 | Portugal-6 | 30620 | USDA | - | - | Portugal | P1-258412 | Europe |
| G39 | Uzbekistan-1 | 30623 | USDA | - | - | Uzbekistan | P1-262435 | Asia |
| G40 | China-1 | 30624 | USDA | - | - | China | P1-262452 | Asia |
| G41 | China-2 | 30625 | USDA | - | - | China | P1-262453 | Asia |
| G42 | Iran-2 | 30631 | USDA | - | - | Iran | P1-304444 | Asia |
| G43 | Iran-3 | 30633 | USDA | - | - | Iran | P1-304448 | Asia |
| G44 | Turkey-1 | 30646 | USDA | - | - | Turkey | P1-304498 | Asia |
| G45 | Turkey-2 | 30648 | USDA | - | - | Turkey | P1-304502 | Asia |
| G46 | Turkey-3 | 30650 | USDA | - | - | Turkey | P1-304504 | Asia |
| G47 | Turkey-4 | 30651 | USDA | - | - | Turkey | P1-304505 | Asia |
| G48 | Afghanistan-2 | 30653 | USDA | - | - | Afghanistan | P1-304592 | Asia |
| G49 | India-2 | 30662 | USDA | - | - | India | P1-305195 | Asia |
| G50 | Russia-1 | 30663 | USDA | - | - | Russia | P1-305535 | Asia |
| G51 | India-3 | 30673 | USDA | - | - | India | P1-306926 | Asia |
| G52 | India-4 | 30674 | USDA | - | - | India | P1-306941 | Asia |
| G53 | India-5 | 30677 | USDA | - | - | India | P1-306976 | Asia |
| G54 | Kazakhstan-1 | 30681 | USDA | - | - | Kazakhstan | P1-314650 | Asia |
| G55 | Turkey-5 | 30688 | USDA | - | - | Turkey | P1-340086 | Asia |
| G56 | Argentina-1 | 30695 | USDA | - | - | Argentina | P1-367833 | America |
| G57 | Uzbekistan-2 | 30696 | USDA | - | - | Uzbekistan | P1-369846 | Asia |
| G58 | Uzbekistan-3 | 30697 | USDA | - | - | Uzbekistan | P1-369853 | Asia |
| G59 | Syria-3 | 30700 | USDA | - | - | Syria | P1-386174 | Asia |
| G60 | Thailand-1 | 30701 | USDA | - | - | Thailand | P1-387821 | Asia |
| G61 | Iran-4 | 30713 | USDA | - | - | Iran | P1-405958 | Asia |
| G62 | Iran-5 | 30718 | USDA | - | - | Iran | P1-405967 | Asia |
| G63 | Bangladesh-1 | 31509 | USDA | - | - | Bangladesh | PI-401472 | Asia |
| G64 | Bangladesh-2 | 31510 | USDA | - | - | Bangladesh | PI-401478 | Asia |
| G65 | Bangladesh-3 | 31511 | USDA | - | - | Bangladesh | PI-401480 | Asia |
| G66 | India-6 | 33538 | USDA | - | - | India | PI 199878 | Asia |
| G67 | Afghanistan-3 | 33541 | USDA | - | - | Afghanistan | PI 220647 | Asia |
| G68 | Australia-1 | 33542 | USDA | - | - | Australia | PI 235660 | Oceania |
| G69 | Turkey-6 | 33543 | USDA | - | - | Turkey | PI 237538 | Asia |
| G70 | Pakistan-8 | 33547 | USDA | - | - | Pakistan | PI 250474 | Asia |
| G71 | Pakistan-9 | 33548 | USDA | - | - | Pakistan | PI 250478 | Asia |
| G72 | Iran-6 | 33556 | USDA | - | - | Iran | PI 250840 | Asia |
| G73 | Jordan-3 | 33559 | USDA | - | - | Jordan | PI 251265 | Asia |
| G74 | Jordan-4 | 33560 | USDA | - | - | Jordan | PI 251267 | Asia |
| G75 | Jordan-5 | 33561 | USDA | - | - | Jordan | PI 251268 | Asia |
| G76 | Israel-4 | 33564 | USDA | - | - | Israel | PI 251290 | Asia |
| G77 | Turkey-7 | 33565 | USDA | - | - | Turkey | PI 251978 | Asia |
| G78 | Turkey-8 | 33567 | USDA | - | - | Turkey | PI 251984 | Asia |
| G79 | Austria-1 | 33568 | USDA | - | - | Austria | PI 253519 | Europe |
| G80 | Hungary-1 | 33575 | USDA | - | - | Hungary | PI 288983 | Europe |
| G81 | Libya-1 | 33608 | USDA | - | - | Libya | PI 393499 | Africa |
| G82 | Bangladesh-4 | 33609 | USDA | - | - | Bangladesh | PI 401470 | Asia |
| G83 | Iran-7 | 33621 | USDA | - | - | Iran | PI 406010 | Asia |
| G84 | Turkey-9 | 33627 | USDA | - | - | Turkey | PI 406701 | Asia |
| G85 | Turkey-10 | 33628 | USDA | - | - | Turkey | PI 406702 | Asia |
| G86 | Pakistan-10 | 33635 | USDA | - | - | Pakistan | PI 426521 | Asia |
| G87 | China-3 | 33638 | USDA | - | - | China | PI 543979 | Asia |
| G88 | China-4 | 33639 | USDA | - | - | China | PI 543982 | Asia |
| G89 | China-5 | 33642 | USDA | - | - | China | PI 544001 | Asia |
| G90 | China-6 | 33651 | USDA | - | - | China | PI 568809 | Asia |
| G91 | China-7 | 33661 | USDA | - | - | China | PI 568874 | Asia |
| G92 | France-1 | 33662 | USDA | - | - | France | PI 576985 | Europe |
| G93 | Austria-2 | 33670 | USDA | - | - | Austria | BVAL-901352 | Europe |
| G94 | Pakistan-11 | Check | PGRI-Pakistan | - | - | Pakistan | Thori-78 | Asia |
| G95 | Pakistan-12 | 16266 | PGRI-Pakistan | Jacobabad | Sindh | Pakistan | - | Asia |
| G96 | Pakistan-13 | 16267 | PGRI-Pakistan | Shikarpur | Sindh | Pakistan | - | Asia |
| G97 | Pakistan-14 | 16268 | PGRI-Pakistan | Shikarpur | Sindh | Pakistan | - | Asia |
| G98 | Pakistan-15 | 16269 | PGRI-Pakistan | Larkana | Sindh | Pakistan | - | Asia |
| G99 | Pakistan-16 | 16270 | PGRI-Pakistan | Larkana | Sindh | Pakistan | - | Asia |
| G100 | Pakistan-17 | 16355 | PGRI-Pakistan | Dadu | Sindh | Pakistan | - | Asia |
| G101 | Pakistan-18 | 16356 | PGRI-Pakistan | Dadu | Sindh | Pakistan | - | Asia |
| G102 | Pakistan-19 | 16357 | PGRI-Pakistan | Karachi | Sindh | Pakistan | - | Asia |
| G103 | Pakistan-20 | 16358 | PGRI-Pakistan | Karachi | Sindh | Pakistan | - | Asia |
| G104 | Pakistan-21 | 16359 | PGRI-Pakistan | Gilgit | GB | Pakistan | - | Asia |
| G105 | Pakistan-22 | 19233 | PGRI-Pakistan | Gilgit | GB | Pakistan | - | Asia |
| G106 | Pakistan-23 | 20920 | PGRI-Pakistan | Islamabad | Federal Areas | Pakistan | - | Asia |
| G107 | Pakistan-24 | 21933 | PGRI-Pakistan | Karachi | Sindh | Pakistan | - | Asia |
| G108 | Pakistan-25 | 24779 | PGRI-Pakistan | Quetta | Balochistan | Pakistan | - | Asia |
| G109 | Pakistan-26 | 27549 | PGRI-Pakistan | Hyderabad | Sindh | Pakistan | - | Asia |
| G110 | Pakistan-27 | 30698 | PGRI-Pakistan | Hyderabad | Shindh | Pakistan | - | Asia |
| G111 | Pakistan-28 | 35803 | PGRI-Pakistan | Gakooch | Gilgit/Balistan | Pakistan | - | Asia |
| G112 | Afganistan-4 | 7-T | CRIFC-Turkey | - | - | Afganistan | - | Asia |
| G113 | Afganistan-5 | 9-T | CRIFC-Turkey | - | - | Afganistan | - | Asia |
| G114 | China-8 | 27-T | CRIFC-Turkey | - | - | China | - | Asia |
| G115 | China-9 | 29-T | CRIFC-Turkey | - | - | China | - | Asia |
| G116 | Turkey-11 | 36-T | CRIFC-Turkey | - | Tarme | Turkey | - | Asia |
| G117 | Turkey-12 | 37-T | CRIFC-Turkey | - | Tarme | Turkey | - | Asia |
| G118 | Turkey-13 | 57-T | CRIFC-Turkey | - | Elbistan | Turkey | - | Asia |
| G119 | Turkey-14 | 58-T | CRIFC-Turkey | - | Elbistan | Turkey | - | Asia |
| G120 | Canada-1 | 74-T | CRIFC-Turkey | - | Canada | - | America | |
| G121 | Canada-2 | 75-T | CRIFC-Turkey | - | Canada | - | America | |
| G122 | USA-1 | 80-T | CRIFC-Turkey | - | Montana | USA | - | America |
| G123 | Iran-8 | 116-T | CRIFC-Turkey | - | Iran | - | Asia | |
| G124 | USA-2 | 130-T | CRIFC-Turkey | - | USA | - | America | |
| G125 | USA-3 | 132-T | CRIFC-Turkey | - | USA | - | America | |
| G126 | Turkey-15 | 134-T | CRIFC-Turkey | - | Tarme | Turkey | - | Asia |
| G127 | USA-4 | 149-T | CRIFC-Turkey | - | İdoha | USA | - | America |
| G128 | Iran-9 | 152-T | CRIFC-Turkey | - | Iran | - | Asia | |
| G129 | USA-5 | 153-T | CRIFC-Turkey | - | İdoha | USA | - | America |
| G130 | Iran-10 | 177-T | CRIFC-Turkey | - | Iran | - | Asia | |
| G131 | Turkey-16 | 277-T | CRIFC-Turkey | - | Tarme | Turkey | - | Asia |
USDA: United States Department of Agriculture; PGRI: Plant Genetic Resources Institute; CRIFC: Central Research Institute for Field Crop;—Not known.
2.2. iPBS-retrotransposon PCR amplifications
Seventy iPBS-retrotransposon primers were initially screened using eight randomly selected accessions of safflower for PCR amplifications [25]. Out of the 70 iPBS-retrotransposon primers, 13 were found polymorphic and selected for PCR amplification, and produced strong bands (Table 2). A total reaction volume of 20 μL for PCR amplifications were comprised of 3 ng/ul template DNA, 2 μL dNTPs (Thermo Scientific), 0.2 μL U Taq DNA polymerase (Thermo Scientific), 3.2 μL primer, 2 μL 1x PCR buffer (Thermo Scientific), 2 μL MgCl2 and 7.6 μL distilled water. Reactions were performed in the sequence of denaturation at 95 oC for 3 min, subsequently followed by 30 denaturation cycles at 95 oC for 15 sec, annealing temperature 50–65 oC for one minute depending upon the primer, and a final extension for five minute at 72 oC [25]. The amplified fragments were electrophoresed on agarose gel 1.2% (w/v) using 0.5x TBE buffer at a constant voltage of 120 V for 230 minute. Staining of the gel was performed with ethidium bromide and visualized using UV Imager Gel Doc XR+ system (Bio-Rad, USA) light and photographed. A 100 bp+ DNA ladder was used as molecular weight marker.
Table 2. List of 13 iPBS-retrotransposon primers with their sequence and annealing temperature used to determine genetic diversity among 131 safflower accessions.
| Primer name | Sequence | Annealing temperature (oC) |
|---|---|---|
| iPBS2252 | TCATGGCTCATGATACCA | 52 |
| iPBS2376 | TAGATGGCACCA | 52 |
| iPBS2377 | ACGAAGGGACCA | 53 |
| iPBS2391 | ATCTGTCAGCCA | 52 |
| iPBS2398 | GAACCCTTGCCGATACCA | 51 |
| iPBS2228 | CATTGGCTCTTGATACCA | 53 |
| iPBS2374 | CCCAGCAAACCA | 53 |
| iPBS2399 | AAACTGGCAACGGCGCCA | 52 |
| iPBS2401 | AGTTAAGCTTTGATACCA | 53 |
| iPBS2239 | ACCTAGGCTCGGATGCCA | 52 |
| iPBS2375 | TCGCATCAACCA | 52 |
| iPBS2383 | GCATGGCCTCCA | 53 |
| iPBS2392 | TAGATGGTGCCA | 52 |
2.3. Data analysis
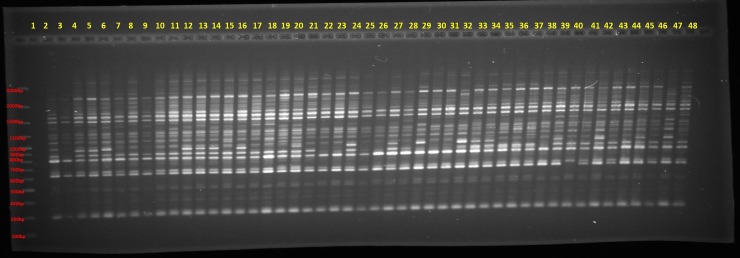
Strong, clear, and unambiguous bands were selected for scoring. iPBS-retrotransposon markers are dominantly inherited markers and were therefore scored using the binary system: 0 or 1, respectively, for the absence and presence of specific bands with respect to 100 bp+ DNA ladder (Fig 1). For individual iPBS-retrotransposon markers, PopGene ver. 1.32 [36] was used to estimate various important genetic diversity parameters including effective alleles number (Ne), Shannon's Information Index (I), and gene diversity (He) (Table 3). Polymorphism information content (PIC) was computed for each iPBS-retrotransposon marker following Baloch et al. [28] criteria. At the safflower samples level, the diversity metrics evaluated included the overall gene diversity (Ht), inbreeding coefficient (Fis) and the pair-wise FST (measure of genetic structure), all of which were determined using hierfstat R package [37] following the algorithms of Goudet et al. [38] and Yang, [39]. R statistical software was used to compute pairwise genetic distance (GDj) as measured by Jaccard’s coefficient [40]. The population structure was assessed using the Bayesian clustering model-based STRUCTURE software, unweighted pair group method with arithmetic mean (UPGMA), and Principle coordinate analysis (PCoA). The most suitable number of clusters (K subpopulations) was determined following the protocol of Evanno et al. [41] using STRUCTURE software. A total of ten independent runs were set for each K value, and for each run, the initial burn-in period was set to 500 with 500,000 MCMC (Markov chain Monte Carlo) iterations with no prior information on the origin of individuals. We plotted the clusters number (K) against logarithm probability relative to standard deviation (ΔK). Final assignment of individual accessions was based on the magnitude of the membership coefficient being greater than or equal to 50% as suggested by Habyarimana, [42] and Nadeem et al. [9]. R statistical software was used to analysis of molecular variance (AMOVA) for considering two main population strata: the model based structure and the country of origin of the accessions.
Fig 1. A representative gel imaging picture revealing genetic diversity among 131 safflower accessions using 13 iPBS-retrotransposon markers.
Table 3. List of various diversity parameters computed to evaluate genetic diversity among 131 safflower accessions using 13 iPBS- retrotransposon primers.
| Primers | Total Bands | Polymorphic Bands | Polymorphism (%) | PIC | Ne | I | He | Ht |
|---|---|---|---|---|---|---|---|---|
| iPBS2252 | 20 | 15 | 75 | 0.432 | 1.2399 | 0.2666 | 0.1609 | 0.16092 |
| iPBS2376 | 32 | 29 | 90.6 | 0.531 | 1.4461 | 0.4171 | 0.2746 | 0.26511 |
| iPBS2377 | 36 | 29 | 80.6 | 0.781 | 1.4935 | 0.4578 | 0.3011 | 0.28914 |
| iPBS2391 | 10 | 8 | 80 | 0.663 | 1.4672 | 0.4143 | 0.2745 | 0.27452 |
| iPBS2398 | 22 | 20 | 90.9 | 0.316 | 1.3023 | 0.2901 | 0.1835 | 0.18353 |
| iPBS2228 | 16 | 14 | 87.5 | 0.323 | 1.1813 | 0.1999 | 0.12 | 0.12005 |
| iPBS2374 | 27 | 26 | 96.3 | 0.374 | 1.2904 | 0.313 | 0.1939 | 0.19393 |
| iPBS2399 | 28 | 26 | 92.9 | 0.271 | 1.2293 | 0.2248 | 0.14 | 0.12747 |
| iPBS2401 | 22 | 19 | 86.4 | 0.231 | 1.1578 | 0.1998 | 0.1117 | 0.07055 |
| iPBS2239 | 28 | 26 | 92.9 | 0.623 | 1.324 | 0.3353 | 0.2084 | 0.18431 |
| iPBS2375 | 22 | 20 | 90.9 | 0.587 | 1.4451 | 0.4055 | 0.2655 | 0.25677 |
| iPBS2383 | 15 | 11 | 73.3 | 0.488 | 1.2603 | 0.2787 | 0.1693 | 0.12818 |
| iPBS2392 | 17 | 14 | 82.4 | 0.582 | 1.5053 | 0.4372 | 0.2909 | 0.27281 |
| Mean | 22.69 | 19.77 | 86.1 | 0.477 | 1.334 | 0.3261 | 0.2073 | 0.19441 |
| Total | 295 | 275 |
PIC: Polymorphism information content, Ne: effective alleles number, I: Shannon's Information Index, He: gene diversity, Ht: overall gene diversity
3. Results
3.1.iPBS-retrotransposon marker analysis and genetic diversity
Thirteen most polymorphic iPBS-retrotransposon primers produced a total of 295 clear and strong scorable bands with an average of 22.69 bands per primer across 131 safflower accessions. Out of the 295 scorable bands, 275 (93.22%) were polymorphic with an average of 19.77 bands per primer (Table 3). The highest (36) and lowest (10) number of scorable bands were observed for primers iPBS2377 and iPBS2391, respectively. The primers iPBS2376 and iPBS2377 revealed highest number of polymorphic bands (29) each and exhibited highest information content (PIC), while primer iPBS2391 revealed least number of polymorphic bands (8) and was least informative. The PIC value ranged from 0.23 (iPBS2401 primer) to 0.78 (iPBS2377 primer) with a mean of 0.48. Highest (1.51) and lowest (1.16) number of effective alleles were observed for primers iPBS2392 and iPBS2401, respectively with an average of 1.33 effective numbers of alleles. Similarly, maximum (0.46) and minimum (0.20) Shannon's information index was reported for primers iPBS2377 and iPBS2401 and iPBS2228 respectively, having an average value of 0.33. Highest (0.30) level of gene diversity was recorded for primer iPBS2377 while, lowest (0.11) level of gene diversity was observed for primer iPBS2401 with an average of 0.21. At the safflower accession samples level, the overall gene diversity (Ht), Fstatistic (Fst) and inbreeding coefficient (Fis) were 0.19, 0.21, and 1, respectively. The mean genetic diversity indices; observed number of alleles (1.86), effective number of alleles (1.34), Nei's gene diversity (0.21), Shannon's information index (0.33), and overall gene diversity (0.20) across four populations and one unclassified population were also determined (Table 4). Population A revealed observed number of alleles (1.68), effective number of alleles (1.28), Nei's gene diversity (0.17), Shannon's information index (0.28), and overall gene diversity (0.15). Population B revealed observed number of alleles (1.70), effective number of alleles (1.33), Nei's gene diversity (0.20), Shannon's information index (0.31), and overall gene diversity (0.19). Population C revealed observed number of alleles (1.63), effective number of alleles (1.26), Nei's gene diversity (0.16), Shannon's information index (0.26), and overall gene diversity (0.15). Population D revealed observed number of alleles (1.65), effective number of alleles (1.29), Nei's gene diversity (0.18), Shannon's information index (0.28), and overall gene diversity (0.17). Unclassified population revealed observed number of alleles (1.51), effective number of alleles (1.22), Nei's gene diversity (0.14), Shannon's information index (0.22), and overall gene diversity (0.13).
Table 4. Various diversity parameters computed to evaluate genetic diversity among 131 safflower across populations using 13 iPBS-retrotransposon primers.
| Populations | Na | Ne | H | I | Ht | Mean Jaccard Genetic distance (GD) | GD Range |
|---|---|---|---|---|---|---|---|
| Population A | 1.6814 | 1.2831 | 0.1748 | 0.2754 | 0.1498 | 0.222 | 0.05–0.339 |
| Population B | 1.6983 | 1.3255 | 0.1992 | 0.3096 | 0.1944 | 0.242 | 0.057–0.33 |
| Population C | 1.6305 | 1.2572 | 0.1616 | 0.2553 | 0.1459 | 0.238 | 0.126–0.357 |
| Population D | 1.6542 | 1.2931 | 0.1816 | 0.2840 | 0.1685 | 0.309 | 0.148–0.455 |
| UP | 1.5085 | 1.2150 | 0.1373 | 0.2176 | 0.1311 | 0.277 | 0.134–0.372 |
| Overall | 1.8644 | 1.3399 | 0.2106 | 0.3312 | 0.1971 | 0.288 | 0.05–0.507 |
Na: observed number of alleles, Ne: effective alleles number, I: Shannon's Information Index, h: gene diversity, Ht: overall gene diversity, UP: unclassified population
To clearly understand the broader picture of genetic diversity, pairwise genetic distance among 131 safflower accessions was measured with the Jaccard coefficient. The mean Jaccard genetic distance across the evaluated accessions was 0.288. The highest genetic distance (0.51) was observed between Turkey3 and Afghanistan4 accessions. Similarly, lowest genetic distance (0.05) was present between Afghanistan4 and Afghanistan5 accessions. Genetic distance was also calculated across the populations and mean genetic distance for population A (0.22), population B (0.24), population C (0.24), population D (0.31), and unclassified population (0.28).
Analysis of molecular variance (AMOVA) was carried out considering two main population strata: the model based structure and the country of origin of the accessions (Table 5). AMOVA revealed that the country of origin was not significant, while the model statistically significant effects on the molecular genotypic variability resulted from model-based structure (P = 0.005), country within model-based populations (P = 0.02), and model-based populations within country (P = 0.047). Variations between countries were not significant (P = 0.07), whereas variations within countries (P = 0.037) and between populations (P = 0.046) were significant (Table 6). The variations within and between populations explained 43 and 5 percent, respectively, of the genetic structure (Table 7). The country within population and the population within country explained 35 and 52 percent of the observed structure.
Table 5. Analysis of molecular variance (AMOVA) revealing genetic diversity in; (a) country within STRUCTURE populations, (b) populations within country.
| A | ||||||
| Source | Df | SS | MS | F.Model | R2 | Pr(>F) |
| Country | 27 | 9417 | 348.78 | 1.4789 | 0.22364 | 0.152 |
| country: group | 26 | 14531 | 558.89 | 2.3698 | 0.34509 | 0.02* |
| Residuals | 77 | 18160 | 235.84 | 0.43126 | ||
| Total | 130 | 42108 | 1 | |||
| B | ||||||
| Source | Df | SS | MS | F.Model | R2 | Pr(>F) |
| Structure | 4 | 2177 | 544.35 | 2.3081 | 0.05171 | 0.005 ** |
| group: country | 49 | 21771 | 444.3 | 1.8839 | 0.51703 | 0.047 * |
| Residuals | 77 | 18160 | 235.84 | 0.43126 | ||
| Total | 130 | 42108 | 1 |
“**” significance at the 0.1% nominal level and
“*” significance at the 1% nominal level; Country:group = country within STRUCTURE populations; Group:country = populations within country
Table 6. Analysis of molecular variance (AMOVA) revealing genetic diversity within the studied 131 safflower accessions.
| Test | Obs | Std.Obs | Alter | Pvalue |
|---|---|---|---|---|
| Variations within samples | 235.839 | -1.9713 | Less | 0.037 |
| Variations between samples | 92.2606 | 1.69048 | greater | 0.07 |
| Variations between group | -2.3109 | 2.06289 | greater | 0.046 |
Table 7. Analysis of molecular variance (AMOVA) revealing intra-genetic diversity within different Structure populations.
| Source | Df | SS | MS | F.Model | R2 | Pr(>F) |
|---|---|---|---|---|---|---|
| Populations | 4 | 278.63 | 69.658 | 8.3981 | 0.21049 | 0.001 *** |
| Within populations | 126 | 1045.11 | 8.295 | 0.78951 | ||
| Total | 130 | 1323.74 | 1 |
“***” corresponds to significance at the 0.05% nominal level
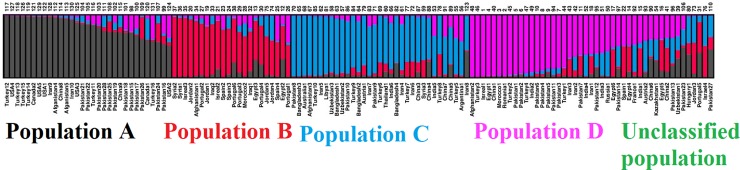
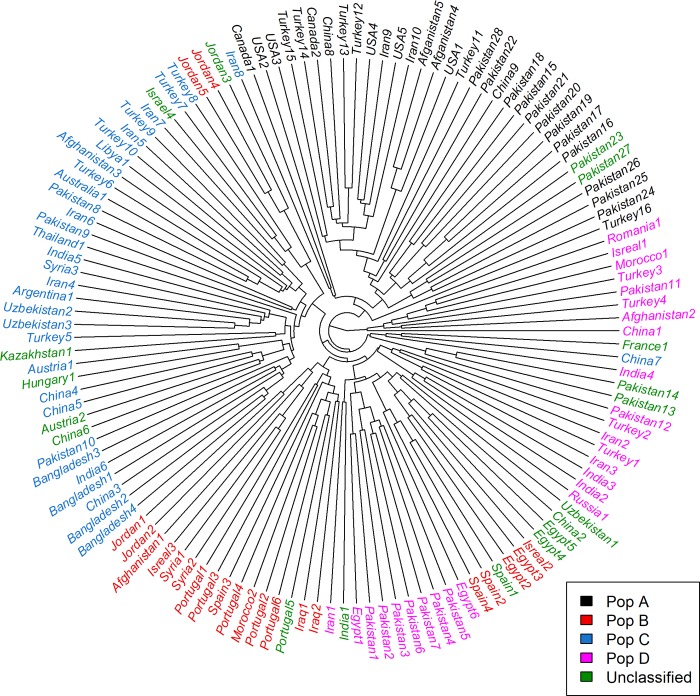
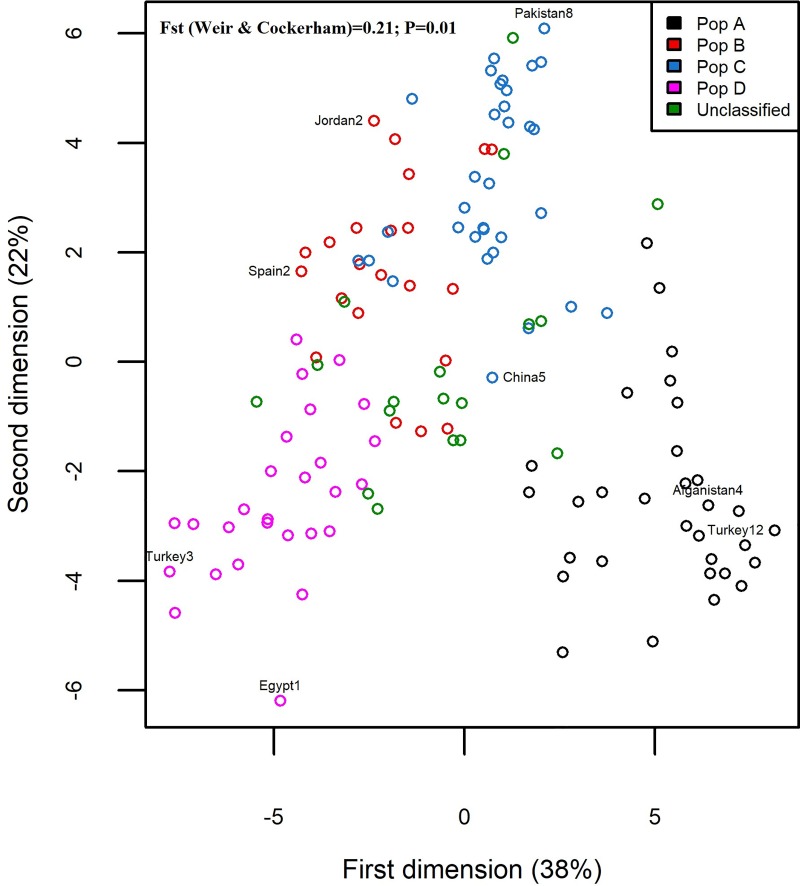
In accordance with the observed most suitable goodness of fit (K = 4), the Bayesian clustering model implemented in STRUCTURE software divided the evaluated safflower accessions into four main populations; 31 accessions (23.66% of the total accessions) in the population A (black), 22 accessions (16.79% of the total accessions) in the population B (red), 33 accessions (25.19% of the total accessions) in the population C (blue), 27 accessions (20.61% of the total accessions) in the population D (pink) (Fig 2). Eighteen accessions (on the right-most end of the structure graph) did not reach the membership threshold (50%) and were named unclassified population. The UPGMA based clustering divided 131 safflower accessions into four main clusters corresponding to the four populations (populations A, B, C, D) identified using the model-based structure. The unclassified accessions were dispersed throughout the four populations, particularly in population D where 9 (50%) of the unclassified accessions clustered. With the UPGMA algorithm, two (Jordan4, Jordan5) and five (Israel2, Egypt2, Egypt3, Spain2, Spain4) population B accessions clustered with population D and population C, respectively. Similarly, relative to model-based clustering algorithm, UPGMA discrepantly clustered the accession Iran8 (population C) in population A (Fig 3). PCoA divide all accessions into four populations; A, B, C, and D similar to structure based clustering, with the unclassified accessions being dispersed particularly throughout populations B, C, and D (Fig 4).
Fig 2. Structure-based clustering among 131 safflower accessions using 13 iPBS-retrotransposon markers.
Fig 3. UPGMA based clustering among 131 safflower accessions using 13 iPBS-retrotransposon markers.
Fig 4. Principal coordinate analysis (PCoA) among 131 safflower accessions using 13 iPBS-retrotransposon markers.
4. Discussion
4.1. iPBS-retrotransposons in assessing genetic diversity of safflower panel
To the best of our knowledge, the present investigation represents the first attempt to elucidate the genetic diversity and population structure of safflower accessions at DNA level using iPBS-retrotransposons. It was observed that retrotransposons are abundant and widely distributed throughout plant genome [43] and huge amount of error-prone retroviral replications lead to the accumulation of these genetic variations [44–45]. iPBS based markers have been used greatly for fingerprinting and genetic diversity investigation in plants [26–35–46–47]. A total of 13 polymorphic iPBS- retrotransposon markers were used in this study to carry out genetic diversity in a panel of 131 safflower accessions from 28 different countries, and 295 clear and strong bands were recorded. The average number of bands per primer was 22.69 while, 275 (93.22%) out of 295 bands were polymorphic. Mean polymorphism found in this study was higher than that of Yang et al. [20], as they reported 82.7% polymorphism using ISSRs in 48 safflower accessions. Furthermore, Sehgal et al. [19] obtained even lower polymorphism levels of 57.6, 68.0, and 71.2% using RAPD, SSR and AFLP markers, respectively. Polymorphism is one of the key requirements to determine good quality genetic markers; therefore, iPBS markers satisfy this requirement in safflower.
Polymorphism information content (PIC) is a widely used metric of the usefulness of molecular markers [48]. The PIC was found higher (0.48) in this work than in the findings by Ambreen et al. [12], Ambreen et al. [13], Barati and Arzani [49], Derakhshan et al. [50], Hamdan et al. [33], and Lee et al. [17], all of whom used SSR markers to evaluate the genetic diversity in safflower. In their works, Houmanat et al. [51] found lower PIC value of 0.23 relative to this study, using ISSRs markers in safflower. These results clearly suggest that more diverse iPBS-retrotransposon markers loci can be identified and effectively used as a tool for assessing genetic diversity and other investigations relying on genetic variants. Maximum number of effective alleles is desirable because it represent the presence of higher level of genetic variations. Number of effective alleles (1.16 to 1.51) found in this work was in the similar range (1.29 to 1.72) to that of Panahi and Neghab [52] using ISSR markers to assess the genetic diversity in Iranian safflower germplasm. Similarly, Sung et al. [53] obtained lower range of effective number of alleles (1.02 to 1.09) than us using RAPD markers. Possible reason behind the presence of higher number of effective alleles in this study might be the differences of experimental materials used during evaluation and also the different molecular marker system. Shannon's information index usually distinguishes the level of available genetic diversity in a population, combining abundance and evenness. Kumar et al. [54] reported lower range of Shannon's information index (0.24 to 0.44) than this study using AFLP markers, highlighting the safflower accessions evaluated in this work were more diverse with genetic variants being more evenly distributed throughout the population. This was confirmed also by the level of gene diversity which was found higher than that of Ambreen et al. [12] and Pearl and Burke [18].
To know the genetic diversity more clearly, diversity metrics like; overall gene diversity (0.24), Fst (0.21), and Fis (1) were also computed. The Fst (a measure of genetic differentiation) obtained in this work was comparable with the findings of Ambreen et al. [12] as they obtained Fst in the range 0.08 to 0.29. On the other hand, Mokhtari et al. [55] obtained mean Fis value of 0.01 which is lower than that (1.00) presented in this work. Safflower is a self-pollinated crop, higher Fis values are therefore expected. In this study, the estimated Fst value (0.21) was higher than the variation explained by the genetic population as evaluated by the analysis of molecular variance (AMOVA). The difference of magnitude between the two metrics was expected as Fst accounted only for genetic populations as a source of variation, while AMOVA accounted for genetic populations and the provenance of the accessions. To understand the variations level more clearly, various diversity indices were calculated at the population’s level and population B was found superior by representing higher values for these diversity indices. On the other hand, unclassified population reflected lesser level of diversity by accounting lower values for these diversity indices.
The evaluation of pairwise genetic distance showed a mean of 0.288, with the highest genetic distance between accessions Turkey3 and Afghanistan4, followed by Afghanistan2 and Pakistan24 with respective distance values of 0.51 and 0.49. Greater similarity was found between Afghanistan4 and Afghanistan5 accessions showing least genetic distance of 0.05. One understandable reason behind the presence of maximum genetic similarity might be due to their origin from the common parents. To explore the genetic diversity more clearly, genetic distances were also calculated at the population level and mean maximum genetic distance was reflected by the population D and minimum was resulted by population A. Within populations, Turkey16 and China9 reflected maximum genetic distance and minimum was present between Afghanistan4 and Afghanistan5 accessions belonging to population A. Within population B, maximum genetic distance was observed between accessions Iraq1 and Jordan4, while minimum genetic distance was shown between accessions Jordan4 and Jordan5. Argentina1 and Iran8 were the two most distinct accessions reflecting maximum genetic distance in the population C and Australia1 and Turkey6 were found two most genetically similar accession of population C representing minimum genetic distance. Within population D, Turkey3 and Iran9 were most diverse accessions and Kazakhstan1 and Pakistan14 were two genetically distinct accessions belonging to unclassified population. Germplasm containing desirable plant traits can be usefully integrated in breeding programs to develop superior cultivars [24], particularly through controlled hybridizations involving genetically distant parental lines. The above four most diverse accessions identified in this work can be recommended as a candidate parents for future safflower breeding programs.
The analysis of molecular variance (AMOVA) was used to determine the pattern of the partition of the total gene diversity among and within populations, and the countries of origin [56]. AMOVA showed that most of genetic structure was explained by variations from individuals within populations, the genetic populations within countries and the countries within genetic populations. These findings are in agreement with Wodajo et al. [57], as they reported more within-population (98.9%) importance on genetic structure than among populations (1.1%) using ISSR markers to evaluate 70 safflower accessions from Ethiopia. The discrepancy in terms of the magnitude of variance components explained by the differing sources of variation included in the AMOVA model. The authors included in their model only the population as a source of variation, while in this work two sources of variation were considered including the population and the country of origin.
The model-based structure application proved more robust and informative in previous investigations [58–59]. Structure was therefore used in this work as a benchmark for clustering algorithms. Using structure, the 131 safflower accessions were partitioned into four main populations (A, B, C, and D), and 18 individuals with poor membership coefficients across clusters were considered unclassified population (Fig 2). A total of 31, 22, 33, 27, and 18 safflower accessions were found in populations A, B, C, D, and unclassified population, respectively. Population A comprised of 31 safflower accessions from Turkey, USA, Canada, Iran, Afghanistan, China, and Pakistan. This population represents the accessions from the Asian and North American regions. Population B consisted of 22 safflower accessions from different countries including Syria, Israel, Jordan, Afghanistan, Portugal, Spain, Morocco, Iraq, and Egypt. Population B contained the accessions from the Mediterranean countries and all clustering of these accessions together represents their genetic similarity. The 33 safflower accessions found in population C were collected from Pakistan, Bangladesh, Australia, Afghanistan, Turkey, Iran, Libya, Uzbekistan, Thailand, India, China, Syria, and Argentina. Population C comprised of accessions from the Asian and Mediterranean countries and clustering of accessions from both regions proposed the distribution of safflower from Mediterranean region to Asia through Turkey. Population D comprised of 27 safflower accessions from Afghanistan, Turkey, Israel, Egypt, China, Morocco, Romania, Pakistan, India, Iran, and Russia. The unclassified population composed of 18 safflower accessions from Pakistan, Spain, Egypt, France, India, Austria, China, Kazakhstan, Uzbekistan, Hungry, Jordan, Portugal, and Israel. Clustering of accessions from Mediterranean countries confirmed this region as center of origin for safflower especially Syria [3] and from this region, it is distributed to other parts of the world. Turkey, represents a great level of biodiversity, differentiation center among the continents, and played a vital role to connect the continents with each other [24].
On continents basis, population A clustered a total of 7 and 24 accessions belonging to American and Asian continents respectively. In population B, 3, 11 and 8 accessions originated from Africa, Asia and Europe, respectively. Population C comprised accessions from America (2), Asia (29), Europe (1), and Oceania (1). In population D most of the accessions originated from Asia (23), while a few accessions came from Africa (3) and Europe (1). The unclassified population contained genotypes mostly from Asia (11), while the other few came from Africa (2) and Europe (5) accessions also made divergence from above four populations by making their separate group. Clearly, the clustering based on molecular markers did not discriminate the origins of the safflower accessions evaluated in this work, which was also confirmed by the AMOVA inferences. Accessions from different countries clustered together, implying that kinship was more determinant for the population structure than the geographical provenance. In addition to sharing common parentage, similarities of accessions in same group during clustering might also be due to convergent evolution and selection [60]. It can therefore be inferred that populations from different geographical regions shared a great proportion of genetic diversity. The design of the experiment in this work cannot provide explanation of the observed predominance of Asian safflower accessions. However, the above countries of origin are part of the seven "centers of similarity" (the Far East, India-Pakistan, the Middle East, Egypt, Sudan, Ethiopia and Europe) as recognized by Knowles [5]. Safflower accessions from Afghanistan, Pakistan, Turkey, India, and particularly from China were found more diverse as they were present in all populations. The higher diversity observed in the Asian safflower accessions is a strong evidence of their wider adaptability, which is supported by the findings of Yang et al. [20] and Zhang [61].
In 1969, Knowles recognized the existence of seven safflower similarity centers across the world. Overall, the centers of similarity were represented by several accessions in this study. However, the molecular marker data used in this study did not provide much support to the above Knowles’s hypothesis on the similarity centers. Indeed accessions belonging to different similarity centers were clustered together. This lack of importance of similarity centers in defining molecular-based populations was reported in scientific literature [62]. In population A, the safflower accessions locally collected from Pakistan were mostly (12 accessions) part of the India-Pakistan similarity center. Also, six accessions from Turkey, two from Afghanistan, and two from Iran were present in this population and can be assigned to the Middle East similarity center. Population B comprised of safflower accessions from Syria (2), Israel (2), Jordan (4), Afghanistan (1), and Iraq (2) belonging to the Middle East similarity center. Similarly, population B contains safflower accessions from Spain (3), Portugal (5), and Morocco (1) which are part of the Europe similarity center. Population C exhibited safflower accessions from Afghanistan (1), Turkey (6), Iran (5), and Syria (1) revealing the Middle East similarity center. Also, population C contains accessions from Pakistan (3), Bangladesh (4), and India (2) showing the India-Pakistan similarity center. Population D revealed the India-Pakistan similarity center by containing accessions from India (3) and Pakistan (9). Population D also exhibits the Middle East similarity center because it contains accessions from Afghanistan (1), Turkey (4), Israel (1), and Iran (3). The unclassified population revealed the presence of Europe similarity center as it contains one accession from each country; Spain, France, Austria, Hungry, and Portugal. In the same way, India-Pakistan similarity center was also available in the unclassified population due to the presence of safflower accessions from Pakistan (4) and India (1). There is a still need for more research in order to shed more light on the safflower similarity centers at molecular level by collecting and evaluating accessions from all known similarity centers.
The investigation of genetic relationships between the 131 accessions using UPGMA clustering algorithm resulted in a clustering pattern comparable with the model-based algorithm with a few exceptions as two and five population B accessions clustered with population D and population C, respectively, and UPGMA discrepantly clustered the accession Iran8 (population C) in population A (Fig 3). Since these accessions displayed mostly full membership coefficients in model-based Structure, the discrepancy observed in UPGMA clustering approaches can be explained by its reduced resolution power relative to the model-based Structure [58–59].
Principal coordinate analysis (PCoA) greatly supported the structure based clustering of 131 safflower accessions using 13 iPBS-retrotransposon primers (Fig 4). The four populations were clearly distinguishable, and the unclassified population was disseminated throughout the other populations, particularly throughout populations B, C, and D. These light discrepancies between PCoA and model-based structure can derive from differing clustering resolution, with model-based structure exhibiting more resolution. Indeed, 40% of the variation in the overall genetic structure was not accounted for by the first two PCoA dimensions presented in this work. The above-mentioned misclassifications of accessions in the principal coordinate space can be explained by the existence of genomic admixture. PCoA analysis revealed the same pattern of distribution of similarity centers as identified by structure based analysis. Population A, B, and D exhibited the Middle East similarity centers as they contain safflower accessions from Turkey, Afghanistan, Iran, Syria, Israel, Jordan, and Iraq. Population C comprised of India-Pakistan similarity center by containing safflower accession from India, Pakistan, and Bangladesh. Europe similarity center is present in population B and in the unclassified population of PCoA based analysis. It suggests more research work regarding the confirmation of safflower similarity centers at molecular level. Overall, iPBS-retrotransposons revealed a good spectrum of genome diversity in safflower and the explored genetic diversity can be used in future safflower breeding programs. As iPBS-retrotransposon marker system demonstrated competitive results in this work and in previous investigations, it is warranted to focus further attention on collecting and evaluating safflower germplasm at molecular level using iPBS-retrotransposons as an important tool for enhancing productivity. To contribute to the yet unending discussion on the safflower similarity centers, a robust sampling techniques including random sampling without replacement can be implemented on the accessions in major world safflower seed repositories; the sampled materials can be evaluated using clustering algorithms such as those implemented in this work.
5. Conclusion
A good level of genetic diversity was identified among 131 safflower accessions. The importance of genetic populations on the genetic structure was significant, but its magnitude was lesser than the importance the variations of individuals within genetic populations. The provenance of the samples showed no effects on the genetic structures in the 131 accessions. Our results most probably obey the seven similarity centers hypothesis of safflower but still there is need to conduct further research works to confirm these similarity centers at the molecular level. Generally, safflower accessions from Asian countries like Afghanistan, Pakistan, China, Turkey, and India were found diverse. Specifically, among 131 safflower germplasm, accessions Turkey3, Afghanistan4, Afghanistan2, and Pakistan24 were found most diverse at molecular level and might be recommended as a candidate parents for future safflower breeding programs.
Acknowledgments
The authors express their gratitude to TUBİTAK (The Scientific and Technological Research Council of Turkey) for providing a doctoral fellowship to Fawad Ali under the TUBITAK-2216 fellowship program for international researchers.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors express their gratitude to TUBİTAK (The Scientific and Technological Research Council of Turkey) for providing a doctoral fellowship to Fawad Ali under the TUBITAK-2216 fellowship program for international researchers.
References
- 1.Kumari S, Choudhary RC, Kumara Swamy RV, Saharan V, Joshi A, Munot J. Assessment of genetic diversity in safflower (Carthamus tinctorius L.) genotypes through morphological and SSR marker. J Pharmacogn Phytochem. 2017; 6(5):2723–31 [Google Scholar]
- 2.Weiss EA. Safflower: Oil seed crops. 2nd ed Blackwell Science, Oxford: 2000 [Google Scholar]
- 3.Marinova E, Riehl S. Carthamus species in the ancient Near East and south-eastern Europe: Archaeobotanical evidence for their distribution and use as a source of oil. Veg Hist Archaeobot. 2009; 18(4):341–349 [Google Scholar]
- 4.Chapman MA, Hvala J, Strever J, Burke JM. Population genetic analysis of safflower (Carthamus tinctorius L.; Asteraceae) reveals a near Eastern origin and five centers of diversity. Am J Bot. 2010; 97(5):831–840 10.3732/ajb.0900137 [DOI] [PubMed] [Google Scholar]
- 5.Knowles PF. Centers of plant diversity and conservation of crop germplasm: Safflower. Econ Bot. 1969; 23(4):324–329 [Google Scholar]
- 6.Ashri A. Evaluation of the germ plasm collection of safflower, carthamus tinctorius L. V. distribution and regional divergence for morphological characters. Euphytica. 1975; 24:651–659 [Google Scholar]
- 7.fao.org/faostat/en/#data/QD
- 8.Nimbkar N. Issues in safflower production in India. In: Safflower: Unexploited potential and world adaptability. Proceedings of the Seventh International Safflower Conference, Wagga Wagga, New South Wales, Australia. (2008)
- 9.Nadeem MA, Habyarimana E, Ciftci V, Nawaz MA, Karakoy T, Comertpay G,et al. Characterization of genetic diversity in Turkish common bean gene pool using phenotypic and whole-genome DArTseq-generated silicoDArT marker information. PlosONE. 2018a. 10.1371/journal.pone.0205363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica. 2005; 142(1–2):169–196 [Google Scholar]
- 11.Kumar S, Ambreen H, Murali TV, Bali S, Agarwal M, Kumar A et al. Assessment of genetic diversity and population structure in a global reference collection of 531 accessions of Carthamus tinctorius L. (Safflower) using AFLP markers. Plant Mol Biol Rep. 2015; 33(5):1299–1313 [Google Scholar]
- 12.Ambreen H, Kumar S, Kumar A, Agarwal M, Jagannath A, Goel S. Association mapping for important agronomic traits in safflower (Carthamus tinctorius L.) core collection using microsatellite markers. Front Plant Sci. 2018; 402(9) 10.3389/fpls.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambreen H, Kumar S, Variath MT, Joshi G, Bali S, Agarwal M, et al. Development of genomic microsatellite markers in Carthamus tinctorius L.(Safflower) using next generation sequencing and assessment of their cross-species transferability and utility for diversity analysis. PLoSONE. 2015; 10(8) 10.1371/journal.pone.0135443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amini F, Saeidi G, Arzani A. Study of genetic diversity in safflower genotypes using agro-morphological traits and RAPD markers. Euphytica. 2008; 163:21–30 10.1007/s10681-007-9556-6 [DOI] [Google Scholar]
- 15.Johnson RC, Kisha TJ, Evans MA. Characterizing safflower germplasm with AFLP molecular markers. Crop Sci. 2007; 47:1728–1736. 10.2135/cropsci2006.12.0757 [DOI] [Google Scholar]
- 16.Khan MA, von Witzke-Ehbrecht S, Maass BL, Becker HC. Relationships among different geographical groups, agro-morphology, fatty acid composition and RAPD marker diversity in safflower (Carthamus tinctorius L.). Genet Resour Crop Evol. 2009; 56(1):19–30 10.1007/s10722-008-9338-6 [DOI] [Google Scholar]
- 17.Lee GA, Sung JS, Lee SY, Chung JW, Yi JY, Kim YG, et al. Genetic assessment of safflower (Carthamus tinctorius L.) collection with microsatellite markers acquired via pyrosequencing method. Mol Ecol Resour. 2014; 14(1):69–78 10.1111/1755-0998.12146 [DOI] [PubMed] [Google Scholar]
- 18.Pearl SA, Burke JM. Genetic diversity in Carthamus tinctorius (Asteraceae; safflower), an underutilized oilseed crop. Am J Bot. 2014; 10(10):1640–1650 10.3732/ajb.1400079 [DOI] [PubMed] [Google Scholar]
- 19.Sehgal D, Rajpal VR, Raina SN, Sasanuma T, Sasakuma T. Assaying polymorphism at DNA level for genetic diversity diagnostics of the safflower (Carthamus tinctorius L.) world germplasm resources. Genetica. 2009; 135(3):457–470 10.1007/s10709-008-9292-4 [DOI] [PubMed] [Google Scholar]
- 20.Yang YX, Wu W, Zheng YL, Chen L, Liu RJ, Huang CY. Genetic diversity and relationships among safflower (Carthamus tinctorius L.) analyzed by inter-simple sequence repeats (ISSRs). Genet Resour Crop Evol. 2007; 54:1043–1051 10.1007/s10722-006-9192-3 [DOI] [Google Scholar]
- 21.Finnegan DJ. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989; 5:103–107 10.1016/0168-9525(89)90039-5 [DOI] [PubMed] [Google Scholar]
- 22.SanMiguel P, Tikhonov A, Jin YK, Motchoulskaia N, Zakharov D, Melake-Berhan A, et al. Nested retrotransposons in the intergenic regions of the maize genome. Science. 1996; 5288(274):765–768 10.1126/science.274.5288.765 [DOI] [PubMed] [Google Scholar]
- 23.Nadeem MA, Nawaz MA, Shahid MQ, Dogan Y, Comertpay G, Yıldız M, et al. DNA molecular markers in plant breeding: current status and recent advancements in the genomic selection and genome editing. Biotechnol Biotechnol Equip. 2018b; 32(2):261–285 [Google Scholar]
- 24.Arystanbekkyzy M, Nadeem MA, Aktas H, Yeken MZ, Zencirci N, Nawaz MA, et al. Phylogenetic and taxonomic relationship of turkish wild and cultivated emmer (triticum turgidum ssp. dicoccoides) revealed by iPBSretrotransposons markers. Int J Agric Biol. 2018; 10.17957/IJAB/15.0876 [DOI] [Google Scholar]
- 25.Kalendar R, Antoniu K, Smykal P, Schulman AH. iPBS: a universal method for DNA fingerprinting and retrotransposon isolation. Theor Appl Genet. 2010; 121(8):1419–1430 10.1007/s00122-010-1398-2 [DOI] [PubMed] [Google Scholar]
- 26.Andeden EE, Baloch FS, Derya M, Ozkan H. IPBS-Retrotransposon-based genetic diversity and relationship among wild annual Cicer species. J Plant Biochem Biotechnol. 2013; 22(4):453–466 [Google Scholar]
- 27.Aydın MF, Baloch FS. Exploring the genetic diversity and population structure of Turkish common bean germplasm by the iPBS-retrotransposons markers. Legume Res. 2018; 10.18805/LR-423 [DOI] [Google Scholar]
- 28.Baloch FS, Alsaleh A, de Miera LES, Hatipoglu R, Ciftci V, Karakoy T, et al. DNA based iPBS-retrotransposon markers for investigating the population structure of pea (Pisum sativum) germplasm from Turkey. Biochem Syst Ecol. 2015a; 61: 244–252. [Google Scholar]
- 29.Baloch FS, Derya M, Andeden EE, Alsaleh A, Cömertpay G, Kilian B, et al. Inter-primer binding site retrotransposon and inter-simple sequence repeat diversity among wild Lens species. Biochem Syst Ecol. 2015b; 58:162–168 [Google Scholar]
- 30.Yıldız M, Koçak M, Baloch FS. Genetic bottlenecks in Turkish okra germplasm and utility of iPBS retrotransposon markers for genetic diversity assessment. Genet Mol Res. 2015; 14(3):10588–10602 10.4238/2015.September.8.20 [DOI] [PubMed] [Google Scholar]
- 31.Varshney RK, Thudi M, Aggarwal R, Börner A (2007) Genic molecular markers in plants: development and applications. In: Varshney RK, Tuberosa R editors. Genomics-assisted crop improvement: genomics approaches and platforms; 2007. pp. 13–29. [Google Scholar]
- 32.Garcia-Moreno MJ, Velasco L, Perez-Vich B. Transferability of non-genic microsatellite and gene-based sunflower markers to safflower. Euphytica. 2010; 175(2):145–150 [Google Scholar]
- 33.Hamdan YAS, Garcia‐Moreno MJ, Redondo‐Nevado J, Velasco L, Perez‐Vich B. Development and characterization of genomic microsatellite markers in safflower (Carthamus tinctorius L.). Plant Breed. 2011; 130(2):237–241 [Google Scholar]
- 34.Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990; 12:13–15 [Google Scholar]
- 35.Baloch FS, Alsaleh A, Andeden EE, Hatipoglu R, Nachit M, Ozkan H. High levels of segregation distortion in the molecular linkage map of bread wheat representing West Asia and North Africa region. Turk J Agric For. 2016; 40:352–364 10.3906/tar-1508-27 [DOI] [Google Scholar]
- 36.Yeh FC, Yang R, Boyle TJ, Ye Z, Xiyan JM. PopGene32, Microsoft Windows-based Freeware for Population Genetic Analysis, Version 1.32 Molecular Biology and Biotechnology Centre, University of Alberta, Edmonton, Alberta, Canada: 2000 [Google Scholar]
- 37.Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: 2013; ISBN 3-900051-07-0, Available at: http://www.R-project.org/. [Google Scholar]
- 38.Goudet J, Raymond M, de Meeus T, Rousset F. Testing differentiation in diploid populations. Genetics. 1996; 144(4):1933–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang RC. Estimating hierarchical f statistics. Evolution. 1998; 52(4): 950–956. 10.1111/j.1558-5646.1998.tb01824.x [DOI] [PubMed] [Google Scholar]
- 40.Jaccard P. Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat. 1908; 144:223–270 [Google Scholar]
- 41.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005; 14:2611–2620 10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- 42.Habyarimana E. Genomic prediction for yield improvement and safeguarding of genetic diversity in CIMMYT spring wheat ('Triticum aestivum L.). Aust J Crop Sci. 2016; 10(1):127–136 [Google Scholar]
- 43.Kumar A, Bennetzen JL. Plant retrotransposons. Annu Rev Genet. 1999; 33: 479–532 10.1146/annurev.genet.33.1.479 [DOI] [PubMed] [Google Scholar]
- 44.Casacuberta JM, Vernhettes S, Audeon C, Grandbastien MA. Quasispecies in retrotransposons: a role for sequence variability in Tnt1 evolution. In: Capy P. editor. Evolution and Impact of Transposable Elements; 1997. pp. 109–117. [PubMed] [Google Scholar]
- 45.Cordaux R, Batzer MA. The impact of retrotransposons on human genome evolution. Nat Rev Genet. 2009; 10(10): 691–703 10.1038/nrg2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo DL, Guo MX, Hou XG, Zhang GH. Molecular diversity analysis of grape varieties based on iPBS markers. Biochem Syst Ecol. 2014; 52:27–32 10.1016/j.bse.2013.10.008 [DOI] [Google Scholar]
- 47.Jing-Yuan XU, Yan ZHU, Ze YI, Gang WU, Guo-Yong XIE, Min-Jian QIN. Molecular diversity analysis of Tetradium ruticarpum (WuZhuYu) in China based on inter-primer binding site (iPBS) markers and inter-simple sequence repeat (ISSR) markers. Chin J Nat Med. 2018; 16(1):1–9 10.1016/S1875-5364(18)30024-4 [DOI] [PubMed] [Google Scholar]
- 48.Anderson JA, Churchill GA, Autrique JE, Tanksley SD, Sorrells ME. Optimizing parental selection for genetic linkage maps. Genome. 1993; 36(1):181–6 [DOI] [PubMed] [Google Scholar]
- 49.Barati M, Arzani A. Genetic diversity revealed by EST-SSR markers in cultivated and wild safflower. Biochem Syst Ecol. 2012; 44:117–123 10.1016/j.bse.2012.04.013 [DOI] [Google Scholar]
- 50.Derakhshan E, Majidi MM, Sharafi Y, Mirlohi A. Discrimination and genetic diversity of cultivated and wild safflowers (Carthamus spp.) using EST-microsatellites markers. Biochem Syst Ecol. 2014; 54:130–136 10.1016/j.bse.2014.01.003 [DOI] [Google Scholar]
- 51.Houmanat K, Charafi J, Mazouz H, El Fechtali M, Nabloussi A. Genetic diversity analysis of safflower (Carthamus tinctorius) accessions from different geographic origins using ISSR markers. Int J Agric Biol. 2016; 18(6): 881–887 10.17957/IJAB/15.0144 [DOI] [Google Scholar]
- 52.Panahi B, Neghab MG. Genetic characterization of Iranian safflower (Carthamus tinctorius L.) using Inter Simple Sequence Repeats (ISSR) markers. Physiol Mol Bio Plants. 2013; 19(2):239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sung JS, Cho GT, Lee GA, Baek HJ, Hu MK. Phylogenetic Relationships and Genetic Diversity in Collected Resources of Carthamus tinctorius by Random Amplified Polymorphic DNA Markers.J Life Sci. 2010; 20(12): 1764–1771 [Google Scholar]
- 54.Kumar S, Ambreen H, Murali TV, Bali S, Agarwal M, Kumar A, et al. Assessment of genetic diversity and population structure in a global reference collection of 531 accessions of Carthamus tinctorius (safflower) using AFLP markers. Plant Mol Biol Rep. 2014; 10.1007/s11105-014-0828-8 [DOI] [Google Scholar]
- 55.Mokhtari N, Sayed-tabatabaei BE, Bahar M, Arabnezhad H. Assessment of genetic diversity and population genetic structure of Carthamus species and Iranian cultivar collection using developed SSR markers. J Genet. 2018; pp:1–12. 10.1007/s12041-018-0956-2 [DOI] [PubMed] [Google Scholar]
- 56.Lynch M, Milligan BG. Analysis of population genetic structure with RAPD markers. Mol Ecol. 1994; 3:91–99 [DOI] [PubMed] [Google Scholar]
- 57.Wodajo B, Mustefa FB, Tesfaye K. Clustering analysis of Ethiopian safflower (Carthamus tinctorius) using ISSR Markers. Int J Sci Res Publ. 2015; 5(3):434–440 [Google Scholar]
- 58.Bouchet S, Pot D, Deu M, Rami JF, Billot C, Perrier X, et al. Genetic structure, linkage disequilibrium and signature of selection in sorghum: lessons from physically anchored DArT markers. PLoS ONE. 2012; 7(3):e33470 10.1371/journal.pone.0033470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newell MA, Cook D, Hofmann H, Jannink JL. An algorithm for deciding the number of clusters and validation using simulated data with application to exploring crop population structure. Ann App Stat. 2013; 7:1898–1916 [Google Scholar]
- 60.Golkar P, Arzani A, Rezaei AM. Genetic variation in safflower (Carthamus tinctorious L.) for seed quality-related traits and inter-simple sequence repeat (ISSR) markers. Int J Mol Sci. 2011; 12(4):2664–2677 10.3390/ijms12042664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang ZW. Studies on genetic diversity and classification of safflower (Carthamus tinctorius L.) germplasm by isozyme techniques. J Plant Gene Res. 2000; 1(4):6–13 [Google Scholar]
- 62.Chapman MA, Burke JM. DNA sequence diversity and the origin of cultivated safflower (Carthamus tinctorius L.; Asteraceae). BMC Plant Biol. 2007; 7:60 10.1186/1471-2229-7-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.