Abstract
The study examined whether psychosocial intervention for children diagnosed with a disruptive behavior disorder (DBD; n = 84) changed concentrations of cortisol and testosterone across a three-year follow-up when compared to a matched, non-clinical, healthy comparison (HC; n = 69) group,. Boys and girls (6 to 11 years) with a DBD were randomly assigned to one of two arms of a multi-method intervention. Hierarchical linear modeling revealed that children undergoing psychosocial intervention for a DBD experienced a significant decline in diurnal cortisol change over time (p < .05) when compared to the HC condition. However, boys with a DBD diagnosis had significantly lower mean cortisol concentrations prior to treatment (p < .05) and showed a significantly steeper increase in mean cortisol over time (p < .05) when compared to HC boys. Treatment effects for diurnal cortisol change were replicated in the boys-only analysis. No treatment effects were noted for testosterone in either analysis.
Keywords: conduct disorder, disruptive behavior disorders, cortisol, testosterone, longitudinal
Disruptive behavior disorders (DBD), including conduct disorder (CD) and oppositional defiant disorder (ODD) (American Psychiatric Association, 2000), begin in childhood and often extend into adolescence. These disorders may serve as the precursor for long-term problems such as criminality or substance use in adulthood (Farrington, 1991). Thus, determining successful prevention and intervention strategies is important to enhance both short- and long-term outcomes in youth with DBD.
Biological underpinnings of DBD have received far less attention than psychosocial, parental, or community factors. Knowing whether biological factors causally contribute to DBD and then whether they can be altered by treatment may be important for understanding the mechanisms involved in efficacious treatment modalities. To date, the most widely examined biological factors in DBD youth include the autonomic nervous system (Raine, 2002; van Goozen, Fairchild, Snoek, & Harold, 2007) and hormones, primarily cortisol (McBurnett, Lahey, Rathouz, & Loeber, 2000; Pajer, Gardner, Rubin, Perel, & Neal, 2001) and testosterone (Olweus, 1986; Popma, Vermeiren et al., 2007; Scerbo & Kolko, 1994; van Bokhoven et al., 2006).
Numerous studies report that cortisol, a stress hormone, is lower in youth diagnosed with DBD or in those with more DBD symptoms (McBurnett et al., 2000; Pajer et al., 2001; Shirtcliff, Granger, Booth, & Johnson, 2005; Susman, Dorn, Inoff-Germain, Nottelmann, & Chrousos, 1997; van Goozen et al., 1998). In non-diagnosed youth, lower cortisol also predicted later aggressive or disruptive behavior (Shoal, Giancola, & Kirillova, 2003; Sondeijker et al., 2008). However, findings regarding cortisol are not always consistent. Klimes-Dougan et al. (2001) reported no hypo- or hyperarousal in HPA axis activity in children with conduct disorder symptoms. Further, in those adolescents with high externalizing behavior scores, an association with low cortisol was not seen, although these children also exhibited internalizing behavior problems that may have masked group differences in cortisol (Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001).
Inconsistencies in findings may be a result of participant characteristics, the methodology, or the substance in which cortisol was measured. In addition to baseline cortisol, some studies have shown lower cortisol responsivity in DBD (van Goozen et al., 2000) as well as in youth with early- or adolescent-onset CD (Popma, Vermeiren et al., 2007). With respect to diurnal changes, no differences between early-onset and adolescent-onset CD groups were evident in slope of cortisol across the day, whereas evening cortisol was higher in CD youth compared to controls (Fairchild et al., 2008) whereas others showed that those with DBD had a slower decrease in cortisol across the diurnal cycle (Popma, Doreleijers et al., 2007). Low cortisol concentrations are hypothesized to be an adaptation to early negative social experiences, otherwise known as the attenuation hypothesis (Susman, 2006), although the exact mechanism involved in lower cortisol and disruptive behavior is unknown. Diurnal changes in cortisol are a normative physiological process, although rhythms that are disrupted may be a marker for psychological risk (Shirtcliff & Essex, 2008).
Higher testosterone has been shown to be related to aggressive behavior across different animal species for decades (Archer, 1991, 2006), although effect sizes are not always large. Studies with humans generally show a positive association between testosterone and disruptive behavior and dominance (Mazur & Booth, 1998), although many of these studies were conducted with adult men, some of whom were in the prison system (Dabbs et al., 1995; Ehrenkranz, Bliss, & Sheard, 1974). For youth with externalizing behavior problems or DBD, a positive association with testosterone has been reported (Book, Starzyk, & Quinsey, 2001; Granger et al., 2003; Olweus, 1986; Popma, Vermeiren et al., 2007; Scerbo & Kolko, 1994; van Bokhoven et al., 2004). Similar to cortisol, the direction of the associations with testosterone is not always consistent (Inoff-Germain et al., 1988; Schaal, Tremblay, Soussignan, & Susman, 1996; Susman et al., 1987).
The majority of these studies on hormones and DBD have been cross-sectional. Additionally, only a few studies have examined the impact that psychotherapeutic treatment for DBD has on hormones. If treatment assists in psychological change and, in turn, regulating behavior, then similar effects might also be reflected in hormone regulation as psychological and behavioral changes occur. In a hallmark study of DBD youth, more pre-treatment problem behaviors were noted in the low basal cortisol group compared to the high basal group, but these differences were not present following treatment (van de Wiel, van Goozen, Matthys, Snoek, & van Engeland, 2004). However, with respect to cortisol response after treatment, the low cortisol response group had significantly higher levels of oppositional behavior later in time than those in the high cortisol response group. Although the sample was small (n = 22) and the follow-up period was only 9 months, this study offers an important first step in understanding the role that hormones may play in DBD before and after treatment.
A second study, although not with DBD children, provides evidence that family intervention may alter cortisol in children aged 3 to 6 (Fisher, Stoolmiller, Gunnar, & Burraston, 2007). Across one year, cortisol activity in the treatment group became similar to the comparison group, whereas those with usual care had a progressive flattening of diurnal cortisol. Thus, examining diurnal variability in cortisol may be important in understanding the effects of treatment in DBD youth. Building on this approach, Brotman et al. (Brotman et al., 2007) showed that a family-based preventive intervention for children at risk for antisocial behavior led to increases in the cortisol response in anticipation of a peer social challenge.
Current Study
Based on previous studies supporting the effect of a treatment regimen on DBD, we examined the impact of treatment for DBD on both gonadal (testosterone) and adrenal (cortisol) hormones. The underlying theory was that if treatment assisted in regulating behavior and psychological change, then similar effects should be reflected in hormone regulation. The intervention included multimodal therapy in which participants were randomized to one of two treatment arms: Community (COMM) and Clinic (CLIN). The aims of the study were to examine: (1) whether treatment of DBD influences rate of change in cortisol and testosterone across the follow-up period when compared to a non-DBD healthy comparison condition and (2) whether treatment effects differences in the rate of change of cortisol and testosterone across time were driven largely by sex. Differences were examined by sex as fewer studies of DBDs are available in girls and sex differences are noted in various types of antisocial behavior, their onset and the like (Moffitt, Caspi, Rutter, & Silva, 2001).
Methods
Participants
Participants were referred regardless of health care network, insurance coverage, or referral source. An initial screen was conducted to obtain information regarding the child’s psychiatric diagnosis, behavioral problems, and treatment needs followed by a second screen for diagnostic assessment to determine intellectual functioning (A. S. Kaufman & Kaufman, 1990) as well as the presence of ODD or CD using the Kiddie SADS (J. Kaufman, Birmaher, Brent, Rao, & Ryan, 1996). Inclusion criteria were: (1) age 6–11 years, (2) residence with at least one parent/guardian, (3) intellectual level no more than two standard deviations below age norms, (4) parent consent and child assent as approved by the university’s Institutional Review Board, and (5) diagnosis of ODD and/or CD from the referring provider. Children were excluded for: (1) concurrent individual or family participation in a treatment program for DBD, (2) current psychosis, bipolar disorder, major depressive disorder marked by significant vegetative signs, substance abuse, or eating disorder, or (3) suicidality with a plan or homicidality.
We included 84 youth from a unique larger, randomized clinical trial (RCT) for treatment of early-onset DBD (Kolko, Dorn, Bukstein, & Burke, 2008). Participants were selected for this report from the RCT based on availability of salivary samples collected as part of regularly scheduled assessments. The total number of participants assigned to either the COMM or CLIN treatment conditions was 139. The first 52 participants were excluded from the current analysis because the RCT began prior to securing funding for saliva hormone analyses. Three additional participants declined to provide saliva samples and were omitted from this analysis, resulting in the COMM (n = 42) and CLIN (n = 42) conditions included in the current analysis. A Healthy Comparison (HC) group (n = 69) was included to serve as a non-DBD comparison condition. HCs were recruited through advertisements in newspapers and flyers posted in children’s centers. They did not meet criteria for a current or past DSM-IV disorder (J. Kaufman et al., 1996). They were chosen to reduce the likelihood of developing the hierarchically related disorders of ODD and CD over the course of long-term follow-up. HC cases were matched to a randomly selected subset of the total sample of clinically referred children in the parent study. The match was based on age (± 9 months), gender, race, and total socioeconomic score (SES) (Table 1). Attrition across the follow-up was very low for all participants and ranged from 2.6%−5.9%.
Table 1.
Raw Means and Standard Deviations for Mean Cortisol, Diurnal Cortisol Change, and Testosterone
| Pre | Post | 6-Month Follow-up | 1-Year Follow-up | 2-Year Follow-up | 3-Year Follow-up | |
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Mean Cortisol (μg/dL) | ||||||
| DBD | 14 (.08) | .15 (.09) | .13 (.07) | .15 (.08) | .14 (.09) | .15 (.10) |
| HC | .16 (.08) | -- | -- | .15 (.08) | .16 (.10) | .16 (.08) |
| Diurnal Cortisol Change (μg/dL) | ||||||
| DBD | .39 (.36) | .36 (.33) | .39 (.35) | .24 (.35) | .24 (.29) | .20 (.28) |
| HC | .30 (.41) | -- | -- | .29 (.34) | .30 (.31) | .32 (.34) |
| Testosterone (pg/mL) | ||||||
| DBD | 38.38 (19.73) | 36.74 (19.96) | 48.91 (26.53) | 56.54 (34.20) | 61.48 (38.15) | 76.51 (43.09) |
| HC | 41.24 (24.16) | -- | -- | 55.47 (28.48) | 65.50 (36.53) | 83.26 (44.01) |
Note: DBD = Combined Community and Clinic treatment groups for disruptive behavior disorder; HC = Healthy Comparisons; -- = no visit.
Procedures
Screening.
The Schedule for Affective Disorders and Schizophrenia for School-Aged Children (KSADS) for DSM-IV Present and Lifetime (J. Kaufman et al., 1996) was used to determine psychiatric diagnoses in the clinical participants and lack of diagnoses in the HC group. Master’s-level interviewers were trained by clinicians who originally received training by the authors who developed the revised KSADS. Regular review was conducted of inter-rater reliabilities with project diagnosticians and group training that included reliability assessments with other research diagnosticians. Kappa coefficients were high (CD [κ = .76]; ODD [κ = .70]; ADHD [κ = .77]) in the parent study.
Treatment.
The COMM and CLIN treatments were similar in the content and techniques used to achieve a family’s individualized goals for reducing DBD. Both used cognitive-behavioral skills training, psychiatric consultation as needed, parent management training, family therapy, school consultation, and peer interventions to encourage affiliation with prosocial peers. Treatments differed in the ecological contexts in which they were delivered. Children randomized to the COMM condition received treatment in the family’s home, at school, and/or in community settings. Adaptations unique to the COMM condition included home-based visits to modify family routines/activities, school visits/teacher consultations, visits to local agencies, and structured peer play activities coordinated by the therapist and/or parent. Case management involved establishing linkages between children/family members and agencies, and case/crisis management followed the above tasks and included weekly clinician visits. The CLIN condition received outpatient treatment in a traditional clinic setting with phone consultation given to teachers and parents to address disruptive behaviors. Routine calls were made to parents to monitor the resolution of any serious behavioral concerns and maintain ongoing case contact. Caregivers and children occasionally met with the study psychiatrist to discuss medication if indicated. Medications were given on a case-by-case basis. Clinicians offered suggestions to promote participation in local programs or conducted enrichment programming based on their familiarity with each program and were available by phone to address emergent conflicts or crises.
Assessment and Follow-Up.
All participants were seen in the clinic for research assessments. Master’s-level clinicians completed treatment evaluations. Each child and parent informant was given $10 per assessment along with the benefit of treatment at no cost. Participants in the treatment conditions provided saliva samples as part of scheduled evaluations at: pre-treatment (Pre), post-treatment (Post), and six-month (6-month FU), one-year (12-month FU), two-year (24-month FU), and three-year follow-up (36-month FU). Saliva samples for the HC condition were collected at the pre-treatment, one-year, two-year, and three-year follow-up assessments. Timing of saliva collection had to fit into existing parent study procedures because the study already had begun before saliva collection was funded. Pair-matched HC participants were seen within two hours of their match. Start time of collection in the lab ranged from 7:15 a.m. to 7:35 p.m. (mean 1:06 p.m. ± 2.90 hours; median 1:10 p.m.). Sample 1 was collected 15–20 minutes following arrival to the clinic assessment but before the diagnostic interview. Sample 2 was collected immediately following the diagnostic interview (M = 52 minutes, SD = 21 minutes). Participants also collected a Sample 3 at home before bed on the same night as their assessment and Sample 4 the next morning immediately on awakening. Samples 3 and 4 reflected diurnal variation of hormone concentrations using a difference score. Participants were instructed not to brush their teeth or eat for two hours before collections and to swish their mouth with water prior to passively drooling into collection tubes. Trident® sugarless gum was used to stimulate saliva as is consistent with previous studies (Granger, Schwartz, Booth, & Arentz, 1999). All participants received an additional $10 at each assessment for the saliva collections.
Use of steroid medication (inhaled, oral, and topical) within the past two weeks was also assessed at each evaluation in order to control for the effects of steroids on concentrations of cortisol. Participants reporting steroid medication use at the time of the treatment evaluation had their hormone data for that particular evaluation omitted from analysis. Steroid use ranged from four (3-year FU) to eleven (Pre) participants across time points.
Median number of treatment sessions for COMM and CLIN was 21 and 16, respectively. Evaluation of treatment was conducted on multiple measures and is reported elsewhere (Kolko et al., 2009).
Hormones.
Saliva for cortisol and testosterone was stored at −80° C until assayed in duplicate using a highly sensitive enzyme immunoassay at Salimetrics (State College, PA) (Table 1). The cortisol assay has a lower limit of sensitivity of .003 μg/dL (range .003 – 1.8 μg/dL), and average intra- and inter-assay coefficients of variation are 4.8% and 8.8%, respectively. Mean cortisol was derived from Samples 1 and 2 to enhance the reliability of a single estimate of cortisol that was based on repeated sampling. The difference between morning and evening values of cortisol (Sample 4 – Sample 3) was determined to approximate a diurnal profile of cortisol activity from a relative peak concentration in the morning to a relative low concentration before sleep. The assay for testosterone has a lower limit of sensitivity of 1.5 pg/mL (range 3.7 – 360 pg/mL), and average intra- and inter-assay coefficients of variation are 5.0% and 7.35%, respectively. Samples 1 and 2 were pooled and subsequently assayed because there are no minute-to-minute fluctuations similar to cortisol.
Statistical Analyses
Hierarchical linear modeling (Bryk & Raudenbush, 1992) was conducted to assess changes in hormone concentrations throughout the three-year follow-up. Trend parameters (e.g., linear, quadratic) assessing average within-subject change over time were first estimated in unconditional models for each hormone. Linear trends provided the best fit for each hormone and thus were retained in subsequent conditional models. Conditional models were then conducted to compare differences in each hormone concentration over time between DBD and HC conditions (Aim 1) and between DBD and HC conditions using a boys-only sample to assess whether treatment effects could be replicated in a sex-specific analysis (Aim 2). Missing data were addressed through maximum-likelihood estimation under the assumption of missing at random. Effect size estimates (ES) were obtained for significant group by time interactions (Raudenbush & Liu, 2001).
Results
Descriptive statistics for hormone concentrations appear in Table 1. Outlying values for hormones were winsorized to ± 3 standard deviations of respective means in order to minimize bias resulting from extreme values. COMM, CLIN, and HC conditions were compared on age, sex (boy = 0, girl = 1), SES, and minority status (Caucasian = 0, minority = 1) at pre-treatment. Chi-square tests did not reveal any significant differences between conditions on sex or minority status (p’s = .36-.82). Similarly, a multivariate analysis of variance indicated that there were no significant differences between the three conditions on age or SES (p’s = .35-.44). Co-morbid attention-deficit/hyperactivity disorder (ADHD) across treatment groups was assessed, as was the relationship between ADHD diagnosis and hormone concentrations at pretreatment. Although rates of co-morbid ADHD were common across the treatment groups (COMM = 28; CLIN = 29), they did not differ significantly between groups nor did ADHD diagnosis have a significant relationship with hormone concentrations (p’s = .16-.82). Psychotropic medication use at pre-treatment and post-treatment was evaluated to assess its relationship with hormone concentrations. Use of psychotropic medications, specifically antidepressants (e.g., selective serotonin reuptake inhibitors [SSRIs], tricyclics), antipsychotics, and stimulants, was not significantly related to hormone concentrations at pre- or post-treatment (p’s = .12-.97). Thus, demographic variables, ADHD diagnosis, or psychotropic medication use were not included as covariates in subsequent models (see Table 2).
Table 2.
Demographics for Community, Clinic, and Healthy Comparison Conditions
| Pre | Post | 6-Month Follow-up | 1-Year Follow-up | 2-Year Follow-up | 3-Year Follow-up | |
|---|---|---|---|---|---|---|
| M(SD) or % | M(SD) or % | M(SD) or % | M(SD) or % | M(SD) or % | M(SD) or % | |
| (N = 153) | (N = 84) | (N = 81) | (N = 149) | (N = 148) | (N = 144) | |
| Age (years) | ||||||
| DBD | 8.89 (1.73) | 9.45 (1.74) | 9.99 (1.77) | 10.49 (1.76) | 11.48 (1.77) | 12.54 (1.80) |
| HC | 9.21 (1.64) | -- | -- | 10.75 (1.66) | 11.75 (1.65) | 12.76 (1.65) |
| SES1 | ||||||
| DBD | 36.39 (11.42) | 36.00 (11.80) | 35.77 (11.16) | 37.38 (11.24) | 36.19 (11.00) | 35.45 (11.05) |
| HC | 38.01 (10.97) | -- | -- | 38.03 (11.16) | 37.92 (11.35) | 38.10 (11.83) |
| Gender2 | ||||||
| DBD | 21% | 21% | 21% | 22% | 22% | 23% |
| HC | 17% | -- | -- | 18% | 16% | 16% |
| Minority3 | ||||||
| DBD | 54% | 54% | 53% | 52% | 54% | 56% |
| HC | 42% | -- | -- | 40% | 43% | 43% |
Note: Note: DBD = Combined Community and Clinic treatment groups for disruptive behavior disorder; HC = Healthy Comparisons; -- = no visit. Comparisons not significantly different across conditions. -- = no visit.
=Socioeconomic status using Hollingshead criteria
= Percent female
=Percent minority.
Comparisons Between Treatment Conditions and Healthy Comparisons – Full Sample
Previous research indicated that the COMM and CLIN conditions did not differ significantly on clinical and behavioral outcomes, following treatment and throughout the same follow-up period (Kolko et al., 2009), and we found no hormone differences on mean diurnal cortisol change or testosterone when analyzing via the RCT design. Therefore, these two treatment groups were combined into a single group termed “DBD” and compared to the HC group (DBD = 1, HC = 0) to determine whether treatment produced changes in hormone trajectories for a DBD sample when compared to a non-DBD group.
Mean Cortisol
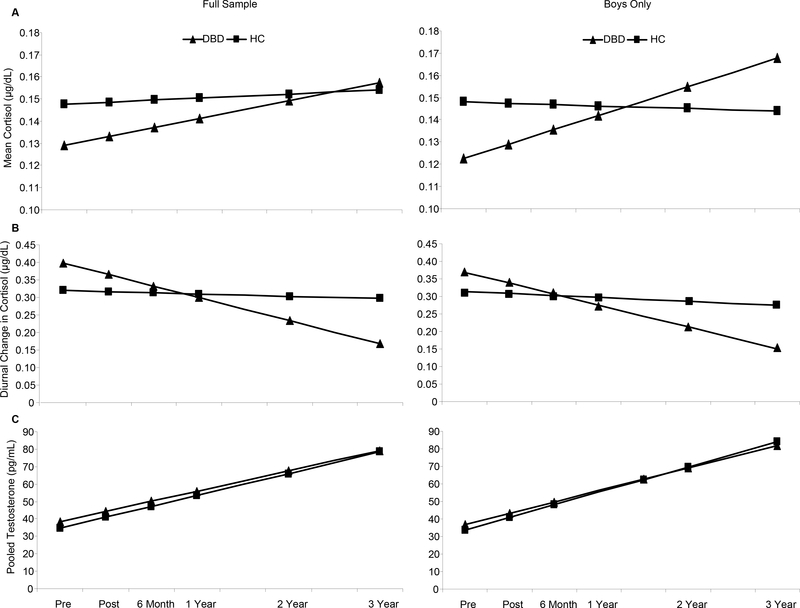
The time at which saliva samples were collected was added to the conditional model for mean cortisol to account for variation in the time of day that Samples 1 and 2 were collected. After controlling for the effects of sampling time (b = −.01, p < .001), none of the other parameters included in the model achieved statistical significance. Thus, the results suggest that treatment did not influence trajectories of mean cortisol concentrations relative to a non-DBD sample (Figure 1a).
Figure 1.
Predicted Growth Curves by Treatment Condition Throughout Three-Year Follow-Up for: (a) Mean Cortisol: No treatment effects noted for the Full-Sample analysis. For boys only, the DBD group had significantly (b = −.03, p < .05) lower mean cortisol at the intercept with significantly increased mean cortisol concentrations over time than the HC group (b = .01, p < .01, ES = 1.00). (b) Diurnal Cortisol Change: There was a significant group by time interaction (b = −0.06, p < .01, ES = −2.35) for the Full Sample, indicating that the mean trajectory of diurnal change for the DBD group as a whole was significantly different when compared to the mean trajectory of the HC condition. This same group by time interaction remained significant in the Boys-Only analysis, (b = −0.05, p < .05, ES = −1.73). (c) Testosterone. There were no significant group differences at intercept or in the group by time interactions in either the Full-Sample or Boys-Only analyses. Note: No HC data were collected at Post and 6-month FU. Raw means for hormones are presented in Table 1.
Diurnal Cortisol Change
Significant effects were noted, however, for diurnal cortisol change when the treatment groups were compared to the non-clinical sample. Results revealed a significant group by time interaction (b = −0.06, p < .01, ES = −2.35) indicating that the average trend of diurnal cortisol change for the DBD group was significantly different when compared to the average trend of the HC condition. As seen in Figure 1b, participants in the HC condition experienced relatively little diurnal changes in cortisol from morning to evening assessments throughout follow-up. However, the trend for the DBD condition illustrates a significant reduction in the diurnal change of cortisol values obtained from morning and evening assessments over time. That is, the difference between morning and evening cortisol concentrations for the DBD condition became smaller over time, suggesting a flattening of the diurnal profile (see also Table 1). There was no significant group by intercept effect noted in this model.
Testosterone
On average, there was a significant linear increase in concentrations of testosterone for the DBD participants (b = 11.67, p <. 001), a finding consistent with normal biological maturation processes for the age range included in the sample. However, there were no significant group differences at intercept or in the group by time interaction (Figure 1c). Results suggest that treatment did not significantly change concentrations of testosterone over time when compared to a non-clinical sample.
Comparisons Between Treatment Conditions and Healthy Comparisons – Boys-only Mean Cortisol
After controlling for the effects of sampling time (b = −.01, p < .001), the group by intercept term was significant (b = −.03, p < .05), indicating that DBD boys had significantly lower concentrations of mean cortisol when compared to HC boys prior to treatment. The group by time interaction was also significant (b = .01, p < .01, ES = 1.00), indicating that trajectories for DBD boys increased significantly over time when compared to HC boys. This analysis suggests that, at least for boys, treatment increased mean concentrations of cortisol across follow-up.
Diurnal Cortisol Change
Similar to the full-sample analysis, there was a significant group by time interaction (b = −0.05, p < .05, ES = −1.73) showing that DBD boys experienced significantly less change between morning and evening cortisol samples throughout the follow-up period when compared to HC boys. The direction of change was the same as the full-sample analysis and again suggests a flattening of the diurnal profile for DBD boys. There was no significant group by intercept effect noted in this model.
Testosterone
Results comparing testosterone trajectories for DBD and HC boys were similar to the full-sample model. Across the follow-up, there was a significant linear increase in concentrations of testosterone for DBD boys (b = 12.84, p < .001). However, there were no significant group differences at intercept or in the group by time interaction, suggesting that treatment did not significantly change concentrations of testosterone for boys.
Discussion
In analyses comparing the HC to the DBD treatment groups, mean cortisol was no different in the total DBD group than in the HC group, but it was significantly lower in the DBD boys at pre-treatment. These findings are consistent with other studies that reported lower cortisol in DBD youth (Lahey et al., 1995; McBurnett et al., 2000; Pajer et al., 2001; van Goozen & Fairchild, 2006; van Goozen et al., 1998). Controlling for time of day of sampling, DBD boys also had a significantly steeper rise in mean cortisol across time and following treatment. These results show that the HPA axis may be sensitive to treatment with respect to diurnal changes.
In other analyses (Shenk, Dorn, Kolko, Susman, & Noll, 2009), greater diurnal cortisol change marginally predicted a poorer treatment outcome. As such, we included diurnal cortisol in the analysis so as to determine whether treatment and its accompanied behavioral and psychological changes could alter diurnal cortisol over time. We found that treatment significantly reduced values of diurnal cortisol change over time, a finding consistent with earlier results and corresponding with behavioral improvements in the two treatment groups (Shenk et al., 2009). We are cautious in the interpretation of change related to treatment because there is a dearth of literature to compare with our findings on diurnal cortisol change. One study of foster children indicated that compared to a special treatment group, those in the usual-care group had morning-to-evening cortisol activity that became increasingly flattened across the year (Fisher et al., 2007), indicating a change in the HPA axis with treatment. Further research is needed to illuminate the mechanism for such a difference in diurnal cortisol. Knowing why this difference occurs may assist in considering methods of altering diurnal cortisol because diurnal rhythms of cortisol are important for the functioning of multiple physiologic systems. Alternatively, these results should be replicated in order to determine whether some third variable besides treatment may have altered the diurnal change.
Additional differences might have been more likely to emerge if a measure of cortisol reactivity were available, given that previous studies did find differences in reactivity to a stressor depending on levels of disruptive behavior (Susman et al., 1997) and differences in treatment outcome depending on the initial cortisol reactivity profile (van de Wiel et al., 2004). Because the hormone component of the study merged with the existing clinical trial, we were unable to add a measure of cortisol reactivity. Alternatively, lower cortisol may be a marker of antisocial behavior that is stable for longer periods of time than 36 months. The HPA axis also may be more resistant to change; that is, a new set point for the axis does not develop within a short time frame. To our knowledge, there are no studies that can provide information on return of a more “normative” cortisol concentration post-treatment (e.g., a concentration that approaches the values represented by the HC group).
No differences were noted in testosterone when comparing DBD groups to the HC group. Previous literature has indicated that higher testosterone is associated with higher aggressive behaviors and that there are mean group differences in testosterone for children and adolescents with and without DBD (Olweus, 1986; Popma, Vermeiren et al., 2007; Scerbo & Kolko, 1994; van Bokhoven et al., 2004). Our analyses with the HC group revealed that change in testosterone across the follow-up was not different from the DBD group and that the increase in testosterone over time was consistent with the developmental literature. Interestingly, other analyses indicate that participants with a DBD diagnosis and with higher testosterone at pre-treatment were more likely to be in a low treatment response trajectory; that is, to have a poorer outcome with respect to DBD (Shenk et al., 2009). These combined results suggest that higher pre-treatment testosterone may indeed increase risk for a poorer treatment response in clinical samples and that treatment may not significantly affect concentrations of testosterone over time. These results add considerably to the existing literature. Specifically, in the clinical sample, higher pre-treatment concentrations of testosterone were a significant correlate of treatment outcome. However, when DBD groups are compared to a non-DBD sample, groups did not differ significantly in concentrations of testosterone at either pre-treatment or over time. Thus, one implication is that testosterone is an important correlate of DBD symptoms and treatment outcomes, especially in clinical samples, but that it is not mechanistically related to such outcomes. Indeed, rates of testosterone increased over time in these groups whereas reductions in DBD symptoms have been observed in this same sample (Shenk et al., 2009). The increase in testosterone also may be a result of normal maturational changes that occur during this age. Specifically, pubertal status was not measured in the parent study, which could impact our findings. Age is a strong correlate of puberty but cannot be substituted for a measure of pubertal status.
Other specific limitations should be addressed in the study. First, the total sample of the RCT could not be included in the current analyses. However, we determined that there were no differences in the subsample of 84 COMM and CLIN participants for whom we collected saliva compared to the full RCT sample for age, race, SES, and diagnoses of ODD and CD. A second limitation lies with the varied sampling time for hormones. This issue is particularly relevant for mean cortisol but unlikely for diurnal cortisol change. Although the variability of cortisol across sampling time is well known, we were unable to change the procedures and timing of the parent study. The data were carefully examined for outliers, and we statistically controlled for sampling time with mean cortisol. Third, the subsample of girls is small, and therefore we are unable to examine the treatment groups for sex differences. Sex may have a greater impact when examining testosterone because more distinct sex differences are evident in this hormone, particularly in puberty and beyond. For example, Pajer and colleagues reported that a higher free testosterone index was noted in post-pubertal girls who had symptoms of aggressive CD (Pajer et al., 2006). Although Pajer and colleagues used a different measure of testosterone, it is worth noting the potential limitation regarding testosterone in the study at hand.
Implications for Research, Policy, and Practice
In conclusion, when the active treatments for the total DBD group were compared to the HC group, there were no significant changes for mean cortisol or testosterone concentrations. There was evidence for boys with DBD to have lower mean cortisol at baseline, suggesting that attenuation of cortisol concentrations may be a trait-like characteristic of DBD boys. We cannot rule out similar findings in girls as our subsample of girls was limited. When comparing the total DBD with the HC group, differences were noted in diurnal cortisol change, indicating that using this measure to study the HPA axis may offer advantages over mean cortisol. In a family intervention, Fisher and colleagues (2007) also reported changes in diurnal cortisol across time, although diurnal cortisol was measured in a different way. Thus, future research may need to focus not only on diurnal cortisol changes but also highlight hormone reactivity at pre- and post-treatment, potentially obtainable by using a challenge paradigm that was not included in this study.
The causative factors of DBD are no doubt complex, and treatment strategies parallel these challenges. Examining hormone trajectories in response to treatment in this study offers one of the first opportunities to determine whether treatment for DBD can alter hormone concentrations across a three-year period. Inclusion of biological processes in prevention or intervention research with youth is rare, yet such processes are vital to enhance our understanding of development and change in psychopathology (Adam, Sutton, Doane, & Mineka, 2008; Beauchaine, Neuhaus, Brenner, & Gatzke-Kopp, 2008; Cicchetti & Gunnar, 2008) and specifically in the study of antisocial behavior (van Goozen & Fairchild, 2008). Importantly, the hormone concentrations reported here indicate that all concentrations likely fall within a normal range; that is, none were so high (or low) as to indicate clinical abnormality. Further study of hormones in DBD within a larger sample with a more balanced gender representation potentially offers a method of identifying subtypes of children with DBD who have differential responses to treatment or modalities of treatment.
Table 3.
Rates of Oppositional Defiant and Conduct Disorders Throughout Follow-up for Community and Clinic Groups
| Pre | Post | 1-Year Follow-up | 2-Year Follow-up | 3-Year Follow-up | |
|---|---|---|---|---|---|
| COMM | |||||
| Sample Size | 42 | 42 | 41 | 40 | 38 |
| ODD | 81% | 50% | 56% | 45% | 40% |
| CD | 19% | 7% | 15% | 15% | 24% |
| CLIN | |||||
| Sample Size | 42 | 41 | 41 | 41 | 39 |
| ODD | 83% | 63% | 56% | 36% | 36% |
| CD | 17% | 7% | 7% | 7% | 13% |
Note: Diagnostic interviews not conducted at the 6-month Follow-up assessment. No significant differences on rates of ODD or CD at any time point between groups.
References
- Adam EK, Sutton JM, Doane LD, & Mineka S (2008). Incorporating hypothalamic-pituitary-adrenal axis measures into preventive interventions for adolescent depression: Are we there yet? Development and Psychopathology, 20(3), 975–1001. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (Fourth ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Archer J (1991). The influence of testosterone on human aggression. British Journal of Psychology, 82(1), 1–28. [DOI] [PubMed] [Google Scholar]
- Archer J (2006). Testosterone and human aggression: An evaluation of the challenge hypothesis. Neuroscience and Biobehavioral Reviews, 30(3), 319–345. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, & Gatzke-Kopp L (2008). Ten good reasons to consider biological processes in prevention and intervention research. Development and Psychopathology, 20(3), 745–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Book AS, Starzyk KB, & Quinsey VL (2001). The relationship between testosterone and aggression: A meta-analysis. Aggression and Violent Behavior, 6(6), 579–599. [Google Scholar]
- Brotman LM, Gouley KK, Huang K-Y, Kamboukos D, Fratto C, & Pine DS (2007). Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Archives of General Psychiatry, 64(10), 1172–1179. [DOI] [PubMed] [Google Scholar]
- Bryk AS, & Raudenbush SW (1992). Hierarchical linear models: Applications and data analysis methods (2nd ed.). Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- Cicchetti D, & Gunnar MR (2008). Integrating biological measures into the design and evaluation of preventive interventions. Development and Psychopathology, 20(3), 737–743. [DOI] [PubMed] [Google Scholar]
- Dabbs JM Jr., Campbell BC, Gladue BA, Midgley AR, Navarro MA, Read GF, et al. (1995). Reliability of salivary testosterone measurements: A multicenter evaluation. Clinical Chemistry, 41(11), 1581–1584. [PubMed] [Google Scholar]
- Ehrenkranz J, Bliss E, & Sheard MH (1974). Plasma testosterone: Correlation with aggressive behavior and social dominance in man. Psychosomatic Medicine, 36(6), 469–475. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, et al. (2008). Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biological Psychiatry, 64(7), 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington DP (1991). Childhood aggression and adult violence: Early precursors and later life outcomes In Pepler DJ & Rubin KH (Eds.), The development and treatment of childhood aggression (pp. 5–29). Hillsdale, NJ: Psychology Press. [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, & Burraston BO (2007). Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology, 32(8–10), 892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Schwartz EB, Booth A, & Arentz M (1999). Salivary testosterone determination in studies of child health and development. Hormones and Behavior, 35(1), 18–27. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, & Hastings P (2003). Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Development and Psychopathology, 15(2), 431–449. [PubMed] [Google Scholar]
- Inoff-Germain G, Arnold GS, Nottelmann ED, Susman EJ, Cutler GB Jr., & Chrousos GP (1988). Relations between hormone levels and observational measures of aggressive behavior of young adolescents in family interactions. Developmental Psychology, 24(1), 129–139. [Google Scholar]
- Kaufman AS, & Kaufman NL (1990). Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service, Inc. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, & Ryan N (1996). Kiddie-Sads-Present and Lifetime Version (K-SADS- PL): Diagnostic Interview. University of Pittsburgh Medical Center: Instrument developed at Western Psychiatric Institute and Clinic. [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, & Zahn-Waxler C (2001). Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology, 13(3), 695–719. [DOI] [PubMed] [Google Scholar]
- Kolko DJ, Dorn LD, Bukstein O, & Burke JD (2008). Clinically referred ODD children with or without CD and healthy controls: Comparison across contextual domains. Journal of Child and Family Studies, 17(5), 714–734. [Google Scholar]
- Kolko DJ, Dorn LD, Bukstein OG, Pardini D, Holden EA, & Hart J (2009). Community vs. clinic-based modular treatment of children with early-onset ODD or CD: A clinical trial with 3-year follow-up. Journal of Abnormal Child Psychology, 37(5), 591–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Hart EL, Frick PJ, Applegate B, Zhang Q, et al. (1995). Four-year longitudinal study of conduct disorder in boys: Patterns and predictors of persistence. Journal of Abnormal Psychology, 104(1), 83–93. [DOI] [PubMed] [Google Scholar]
- Mazur A, & Booth A (1998). Testosterone and dominance in men. Behavioral and Brain Sciences, 21(3), 353–397. [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, & Loeber R (2000). Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry, 57(1), 38–43. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M, & Silva PA (2001). Sex differences in antisocial behaviour: Conduct disorder, delinquency, and violence in the Dunedin Longitudinal Study. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Olweus D (1986). Aggression and hormones: Behavioral relationships with testosterone and adrenaline In Olweus D, Block J & Radke-Yarrow M (Eds.), Development of antisocial and prosocial behavior: Research, theories, and issues (pp. 51–72). Orlando, FL: Academic Press. [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, & Neal S (2001). Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry, 58(3), 297–302. [DOI] [PubMed] [Google Scholar]
- Pajer K, Tabbah R, Gardner W, Rubin RT, Czambel RK, & Wang Y (2006). Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology, 31(10), 1245–1256. [DOI] [PubMed] [Google Scholar]
- Popma A, Doreleijers TA, Jansen LM, van Goozen SH, van Engeland H, & Vermeiren R (2007). The diurnal cortisol cycle in delinquent male adolescents and normal controls. Neuropsychopharmacology, 32(7), 1622–1628. [DOI] [PubMed] [Google Scholar]
- Popma A, Vermeiren R, Geluk CA, Rinne T, van den Brink W, Knol DL, et al. (2007). Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biological Psychiatry, 61(3), 405–411. [DOI] [PubMed] [Google Scholar]
- Raine A (2002). Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology, 30(4), 311–326. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, & Liu X-F (2001). Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychological Methods, 6(4), 387–401. [PubMed] [Google Scholar]
- Scerbo AS, & Kolko DJ (1994). Salivary testosterone and cortisol in disruptive children: Relationship to aggressive, hyperactive, and internalizing behaviors. Journal of the American Academy of Child and Adolescent Psychiatry, 33(8), 1174–1184. [DOI] [PubMed] [Google Scholar]
- Schaal B, Tremblay RE, Soussignan R, & Susman EJ (1996). Male testosterone linked to high social dominance but low physical aggression in early adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 35(number10), 1322–1330. [DOI] [PubMed] [Google Scholar]
- Shenk CE, Dorn LD, Kolko DJ, Susman EJ, & Noll JG (2009). Influence of adrenal and gonadal hormones on treatment response for children with disruptive behavior disorders. Poster presented at the 2009 Biennial Meeting of the Society for Research in Child Development, Denver, CO. [Google Scholar]
- Shirtcliff EA, & Essex MJ (2008). Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology, 50(7), 690–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, & Johnson D (2005). Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology, 17(1), 167–184. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, & Kirillova GP (2003). Salivary cortisol, personality, and aggressive behavior in adolescent boys: a 5-year longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry, 42(9), 1101–1107. [DOI] [PubMed] [Google Scholar]
- Sondeijker FE, Ferdinand RF, Oldehinkel AJ, Tiemeier H, Ormel J, & Verhulst FC (2008). HPA-axis activity as a predictor of future disruptive behaviors in young adolescents. Psychophysiology, 45(3), 398–404. [DOI] [PubMed] [Google Scholar]
- Susman EJ (2006). Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews, 30(3), 376–389. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Inoff-Germain G, Nottelmann ED, & Chrousos GP (1997). Cortisol reactivity, distress behavior, and behavioral and psychological problems in young adolescents: A longitudinal perspective. Journal of Research on Adolescence, 7(1), 81–105. [Google Scholar]
- Susman EJ, Inoff-Germain G, Nottelmann ED, Loriaux DL, Cutler GB Jr., & Chrousos GP (1987). Hormones, emotional dispositions, and aggressive attributes in young adolescents. Child Development, 58(4), 1114–1134. [PubMed] [Google Scholar]
- van Bokhoven I, Van Goozen SH, van Engeland H, Schaal B, Arsenault L, Seguin JR, et al. (2004). Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. Journal of Neural Transmission, 112, 1083–1096. [DOI] [PubMed] [Google Scholar]
- van Bokhoven I, van Goozen SH, van Engeland H, Schaal B, Arseneault L, Séguin JR, et al. (2006). Salivary testosterone and aggression, delinquency, and social dominance in a population-based longitudinal study of adolescent males. Hormones and Behavior, 50(1), 118–125. [DOI] [PubMed] [Google Scholar]
- van de Wiel NM, van Goozen SH, Matthys W, Snoek H, & van Engeland H (2004). Cortisol and treatment effect in children with disruptive behavior disorders: A preliminary study. Journal of the American Academy of Child and Adolescent Psychiatry, 43(8), 1011–1018. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, & Fairchild G (2006). Neuroendocrine and neurotransmitter correlates in children with antisocial behavior. Hormones and Behavior, 50(4), 647–654. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, & Fairchild G (2008). How can the study of biological processes help design new interventions for children with severe antisocial behavior? Development and Psychopathology, 20(3), 941–973. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Fairchild G, Snoek H, & Harold GT (2007). The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin, 133(1), 149–182. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, & Van Engeland H (1998). Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry, 43(7), 531–539. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, van den Ban E, Matthys W, Cohen-Kettenis PT, Thijssen JH, & van Engeland H (2000). Increased adrenal androgen functioning in children with oppositional defiant disorder: A comparison with psychiatric and normal controls. Journal of the American Academy of Child and Adolescent Psychiatry, 39(11), 1446–1451. [DOI] [PubMed] [Google Scholar]