Abstract
Purpose:
The aim of the study was to use micro-autoradiography to investigate the lung cell types responsible for 2-deoxy-2-[18F]fluoro-D-glucose (FDG) uptake in murine models of acute lung injury (ALI).
Procedures:
C57/BL6 mice were studied in three groups: controls, ventilator-induced lung injury (VILI), and endotoxin. VILI was produced by high tidal volumes and zero end-expiratory pressure and endotoxin ALI, by intranasal administration. Following FDG injection, the lungs were processed and exposed to autoradiographic emulsion. Grain density over cells was used to quantify FDG uptake.
Results:
Neutrophils, macrophages, and type 2 epithelial cells presented higher grain densities during VILI and endotoxin ALI than controls. Remarkably, cell grain density in specific cell types was dependent on the injury mechanism. Whereas macrophages showed high grain densities during endotoxin ALI, similar to those exhibited by neutrophils, type 2 epithelial cells demonstrated the second highest grain density (with neutrophils as the highest) during VILI.
Conclusions:
In murine models of VILI and endotoxin ALI, FDG uptake occurs not only in neutrophils but also in macrophages and type 2 epithelial cells. FDG uptake by individual cell types depends on the mechanism underlying ALI.
Keywords: Micro-autoradiography, 2-Deoxy-2-[18F]fluoro-D-glucose, Positron emission tomography, Ventilator-induced lung injury, Acute lung injury, Endotoxin lung injury, Neutrophil, Macrophage, Type 2 epithelial cell, Lung
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are important causes of morbidity and mortality in critically ill patients [1]. Sepsis is a major etiologic factor for ALI/ARDS [1]. Management of patients usually involves mechanical ventilation, and ventilator-induced lung injury (VILI) is an increasingly recognized condition that can produce and exacerbate lung inflammation and contribute to mortality [2, 3]. Lung inflammation is a key feature of ALI, the degree of which is believed to impact the clinical course of the illness [3, 4]. For these reasons, there is increasing interest in the application of positron emission tomography (PET) with 2-deoxy-2-[18F]fluoro-D-glucose (FDG) to assess the degree of pulmonary inflammation during ALI/ARDS non-invasively, regionally and in vivo [5–9]. Studies in humans and animals suggest that FDG-PET imaging may be a valuable tool to study the mechanisms underlying ALI/ARDS [7–10], predict severe respiratory failure [11], and evaluate the effects of therapeutic interventions [12].
Previous studies speculated that the FDG signal was localized to neutrophils [7, 13, 14]. Thus, in the acutely injured non-tumoral lung, FDG-PET has been interpreted to reflect neutrophilic inflammation. However, studies characterizing the source of the pulmonary FDG signal in the non-tumoral lung were based either on bronchoalveolar lavage samples [7], models of fibrotic lung disease [13], or indirect deductions from PET imaging of humans with pneumonia or bronchiectasis [14]. Consequently, there is no information regarding the pulmonary FDG signal from direct assessment of FDG uptake in lung parenchymal cells, which are likely to contribute to the signal.
Micro-autoradiography is a semi-quantitative method to assess the spatial distribution of radioisotope uptake in tissue [15, 16]. No previous study has addressed the contribution of different pulmonary cell types to FDG uptake during endotoxin exposure and VILI, which are key models of ALI/ ARDS. Furthermore, ALI experiments in mouse and sheep indicated the presence of FDG uptake during significant neutropenia [2, 17], suggesting the contribution of cell types other than neutrophils to the FDG signal. These cell types could play distinct roles in the propagation of ALI. Neutrophils are among the earliest immune cells to be recruited to the injury site, whereas macrophages and type 2 epithelial cells provide important signaling for neutrophil chemoattraction during ALI [18]. Consequently, characterization of the cell types contributing to the FDG uptake signal during ALI/ARDS is essential to accurately understand and interpret experimental and clinical data.
In the present study, we used FDG-based micro-autoradiography in mouse models of acute lung injury due to endotoxin exposure and VILI in order to (1) identify cell types participating in the FDG signal and (2) semiquantitatively assess the contribution of those cell types to the FDG uptake measurement.
Materials and Methods
All experiments were performed under an approved protocol by the Subcommittee on Research Animal Care at the Massachusetts General Hospital, Boston, MA.
Acute Lung Injury Models
Male and female C57/BL6 mice (8–12 weeks old, 20–25 g) were divided into three experimental groups: controls, endotoxin, and VILI. Mice in all groups were fasted for 18 h prior to administration of FDG. For the endotoxin and VILI groups, anesthesia was induced with a combination of intraperitoneal injection of ketamine (120 μg/g) and xylazine (40 μg/g).
In the endotoxin group (n=5), following anesthesia, intranasal endotoxin (250 μg/g in 40 μl, Escherichia coli O55:B5, Sigma-Aldrich) was administered. Mice were then allowed to recover from anesthesia and breathe spontaneously for 18 h before tracer administration (described below).
In the VILI group (n=4), following anesthesia, mice underwent tracheotomy using a 20G angiocatheter. General anesthesia was maintained with additional boluses of ketamine and xylazine, and muscle relaxation was achieved with pancuronium (0.08 μg/kg). VILI was induced by mechanically ventilating the animals with initial peak airway pressures at 22 cmH2O, progressively increased to 30 cmH2O, FIO2 = 1.0, zero end-expiratory pressure, and respiratory rate of 75–115 breaths/min (Harvard Model 687 mechanical ventilator, Boston, MA, USA). During mechanical ventilation, intravascular volume status of the mouse was assessed by evaluation of paw capillary refill, with normal saline boluses (0.2 ml/g) administered for volume replacement as needed. Mice were mechanically ventilated for 3 h; at the end of which, FDG was administered. In the control group (n=3), tracer was administered to spontaneously breathing mice.
Micro-Autoradiography
In all groups, FDG at a dose of 42.2±8.1 MBq was administered intravenously via tail vein. Following tracer administration, the animals were maintained at their pre-administration management mode; i.e., the control and endotoxin groups remained spontane-ously breathing, and the VILI group remained on mechanical ventilation. After a period of 45 min, to allow for tracer circulation and distribution in lung tissue, animals were sacrificed and tissue processed as described below.
Tissue Preparation and Histology
Following animal sacrifice, the lungs were immediately injected with optimal cutting temperature freezing media, extracted, and taken to a −27 °C cryostat for production of fresh frozen sections of 10–12-μm thickness. Tissue was fixed on slides with 70 % ethanol and dried.
In the dark room, slides were coated with RPN41 emulsion (GE/ Amersham, Buckinghamshire, UK) in a dipping chamber and allowed to dry at room temperature for 30 min. An interval of 30–40 min elapsed between animal sacrifice and application of emulsion. The slides were then transferred to a dark box with desiccant, stored at 4 °C for 5 h and then developed under dark room conditions.
Once developed, the slides were stained with Mayer’s hematoxylin (Merck, Darmstadt, Germany) and eosin (Sigma-Aldrich, Steinham, Germany). Using a combined light microscope/camera (80i, Nikon, Melville, NY, USA), tissues were examined for grains, produced as latent images by exposure of the emulsion layer to the photons derived from the positron annihilation from FDG. Here, two closely positioned layers were discernible by slightly different focal points, one displaying the grains produced in the emulsion, with tissue on the background, and the other showing mainly the histological tissue layer beneath the grains. Images of the grain layer were captured for quantification of grain density.
Six to ten tissue sections were obtained per animal, and ten to 12 fields were analyzed per tissue section. Tissue sections and fields not appropriately covered with emulsion were eliminated.
Quality Control Measures
A calibrated well counter was used to confirm the presence of tracer in positive control tissue samples. Metabolically active tissues (heart and liver) were used as positive controls to demonstrate increased levels of FDG uptake. Blood samples were also obtained and analyzed to confirm the systemic circulation of FDG.
Quantification of Grain Density and Cell Counts
We used grain density as our measure of tissue FDG uptake. Grain density has been shown to correlate with signal intensity (hence, cellular metabolic activity) and has been used as a measure of tracer uptake in cells [15, 16].
Grain density was assessed in the four cell types identified by light microscopy with ×400 magnification: neutrophils, macro-phages, and epithelial cells type 1 and type 2. These cell types were chosen based on their relevance to the disease processes and ability to be visualized. Cell types were identified by usual morphological criteria using light microscopy [19].
Cells showing grains were quantified, whereas cells not showing grains were excluded from the analysis. Type 1 epithelial cells always exhibited low tracer uptake with isolated grains, which were directly counted. Neutrophils, macrophages, and type 2 epithelial cells frequently presented overlying grain agglomerates.
In order to quantify FDG grains in these cell types, we used the basic principle that the total number of grains overlying each cell equaled the total area occupied by the grains over each cell divided by the area of a single grain. To compute the total number of grains, slides were digitally captured. On each image, the size of individual grains was measured in pixels, and the average single grain size was calculated. The area of FDG grain agglomerates over each cell type was then quantified in pixels using manual tracing with appropriate software (Image-Pro, version 6.0, Media Cybernetics Inc., Bethesda, MD, USA). The final grain counts on the cell were then obtained (Ngrains, cell). Total cell area in square micrometers was determined using manual tracing with the same software (Areacell). Background grain density was assessed for each examined field by randomly selecting a representative cell-free area on the slide (Areabackground, quantified with Image-Pro) and directly counting the number of grains within that area (Nbackground). This provided a measure of background grain density, which was then subtracted from the cell grain density to correct for background activity. From these measurements, grain density corrected for background (grains per 100 μm2 cell area) was computed, then divided by the quantity of FDG injected (FDGinj, in megabecquerel) to yield the normalized grain density per unit of injected activity (grains/100 μm2/MBq):
Normalized grain density for each cell type was quantified in ten randomly selected fields in each animal and averaged to yield the final results.
In order to provide estimates of percent cell contribution to the FDG signal, we counted the four cell types in the same ten fields where normalized grain density was computed. From these, the average percent number of each cell in the ten fields for each animal was estimated (%Numbercell) and combined with the average percent grain counts for each cell type in the same ten fields (%GrainCountscell) to provide an estimate of the percent cell contributions to FDG uptake in each animal:
where cell=neutrophil, macrophage, type 1 and type 2 epithelial cells. The normalized grain density in the background was on average 0.48±0.52 grains/μm2, i.e., much smaller than that seen in cells of interest.
Micro-Autoradiography with 2-Deoxy-[3H]-D-Glucose
To complement the FDG micro-autoradiography analysis, we performed immunohistochemistry assays in one additional mouse injected with HDG (148 MBq) by tail vein. Tissues were processed as described previously for FDG, with the exception that 4 weeks of exposure time was necessary for slides with grains undergoing development in the emulsion. These samples allowed for use of immunohistochemical methods as described below to qualitatively evaluate HDG uptake in endothelial cells, neutrophils, and type 2 epithelial cells.
Immunohistochemical Staining
2-Deoxy-[3H]-D-glucose-labeled lung sections were subjected to immunohistochemistry to localize endothelial cells, neutrophils, and type 2 epithelial cells, as well as nuclear counter-staining with Hoëchst (Sigma-Aldrich, St. Louis, MO) or 4′,6-diamidino-2-phenylindole (DAPI).
Endothelial cells and neutrophils were identified by staining for P-selectin (also known as CD62P) and Ly-6C/G (also known as Gr-1), respectively, with widely used antibodies: for P-selectin: phycoerythrin (PE)-conjugated anti-human/mouse P-selectin IgG (clone Psel KO2.3, eBioscience, Sandiego, CA); and for Ly-6C/G: Alexa 488-conjugated rat anti-mouse IgG (RB6–8C5, Caltag, Buckingham, UK) [20–22]. These antibodies were diluted to 1:100 with PBS, supplemented with 0.1 % BSA and 0.5 % rat monoclonal anti-mouse CD16/32 antibody (Fc Block, eBioscience, San Diego, CA) and mixed with Hoëchst at 1 μg/ml [23]. Type 2 epithelial cells were identified by staining for surfactant protein-C (SP-C) using an anti-SP-C rabbit polyclonal antibody (SP-C, FL-197, Santa Cruz Biotechnology Inc, CA, USA) diluted 1/40 in 5 % FBS/PBS. Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen, CA, USA) at 1/500 dilution was used as the secondary antibody. DAPI (1 μg/μl) was used for nuclear counter-staining. The mixtures were applied onto tissue sections and incubated in a humid chamber for 1 h at room temperature. After rinsing in PBS, the tissue sections were mounted in an aqueous mounting media (Immu-mount, Shandon, Pittsburg, PA). Images were captured by Nikon (Eclipse E800, Japan) operated by Openlab system (Improvision Inc., Lexington, MA). For SP-C staining, bright field and fluorescence pictures were taken using a fluorescence microscope (Olympus, FSX-100) and merged using ImageJ software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, MA, USA; http://imagej.nih.gov/ij/, 1997–2011).
Statistical Analysis
Results are reported as mean±standard deviation. Differences between groups and cell types were compared using a two-way ANOVA with Bonferroni post hoc analysis (Graphpad Prism, La Jolla, CA, USA). A p value of 0.05 was used as the limit for statistical significance.
Results
Micro-Autoradiography
Histological examination of the endotoxin and VILI groups revealed the presence of inflammatory infiltrates with numerous neutrophils, thickening of the alveolar walls, and alveolar edema. In contrast, no histological signs of ALI were present in the control lungs.
Micro-autoradiography images from both the endotoxin and the VILI groups clearly showed presence of grains over the different studied cell types (Fig. 1a–e). Grains corresponding to FDG uptake were documented over neutrophils (Fig. 1a, b), macrophages (Fig. 1d), type 2 epithelial cells (Fig. 1e), and type 1 epithelial cells (Fig. 1e). In the control group, grains were predominantly localized to alveolar macrophages with minimal grains overlaying other cell types. For both models of ALI, there was an increase in FDG uptake in the lung parenchyma compared with controls. The normalized grain density in the different cell types was significantly affected by both the model of ALI and by the cell type (Fig. 2). In the VILI group, normalized grain density was greater in neutrophils (p<0.001), macrophages (p<0.001), and type 2 epithelial cells (p<0.001) than was observed in type 1 epithelial cells. In the endotoxin group, normalized grain density in neutrophils was higher than that in type 1 (p<0.001) and type 2 (p<0.001) epithelial cells and similar to that of macrophages. Normalized grain density in macrophages was also higher than that in type 1 (p<0.001) and type 2 (p<0.001) epithelial cells.
Fig. 1.
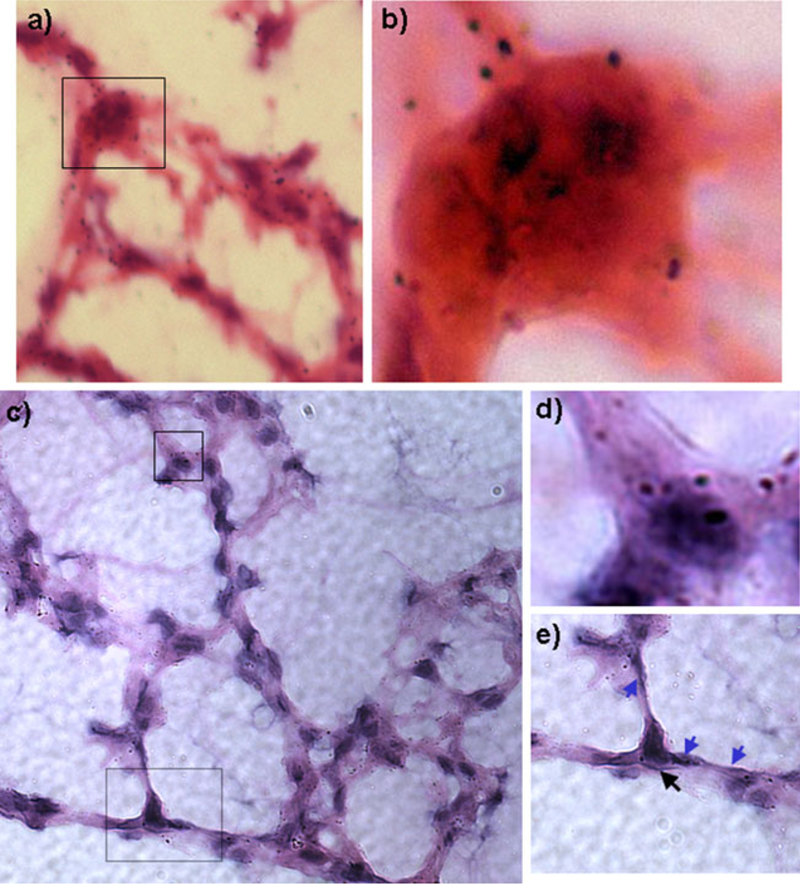
Representative autoradiographic views of different cell types labeled with 2-deoxy-2[18F]fluoro-D-glucose: Top panel: a Hematoxylin–eosin histological sample of lung tissue from instilled endotoxin ALI model, at ×40. Region demarcated by rectangle is amplified in b. b Inset view at ×100 depicting the presence of significant numbers of grains within the neutrophil. Bottom panel: c Lung parenchyma from VILI model at ×40. d Inset view of box from c at ×100 view. Grains are observed within an alveolar macrophage in VILI model. e Inset view of dashed box at ×100 view. Black arrow indicates a type 2 epithelial cell, and blue arrows show type 1 epithelial cells. Overall, these images exemplify the predominance of grains overlying lung parenchyma.
Fig. 2.
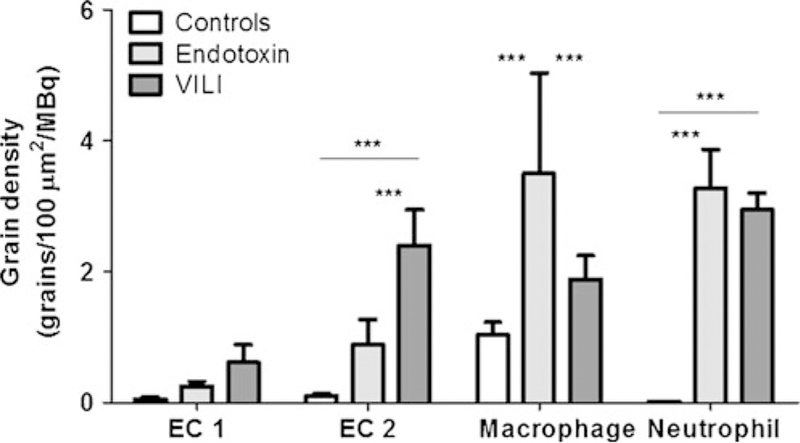
Grain density of 2-deoxy-2-[18F]fluoro-D-glucose normalized by injected FDG in micro-autoradiographic samples. Comparisons among ALI models and controls for epithelial cells type 1 (EC1) and type 2 (EC2), macrophages, and neutrophils. ALI produced an increase in normalized grain density for several cell types as compared to controls. Type 2 epithelial cells demonstrate significantly higher normalized grain density during VILI compared with instilled endotoxin and control conditions. Macrophages demonstrate the highest normalized grain density during endotoxin-induced lung injury. Triple asterisks indicate p<0.001.
Remarkably, the distribution of normalized grain density among cell types was significantly affected by the lung injury model (Fig. 2). Indeed, the grain density over type 2 epithelial cells was substantially different between VILI and endotoxin groups. Whereas, we found type 2 epithelial cells with high normalized grain densities following VILI, significantly lower grain densities in these cells were found in the endotoxin model (p<0.05, Fig. 2). Conversely, macrophages exhibited higher normalized grain densities during endotoxin instillation than was observed during VILI (p<0.01, Fig. 2). As a result, in the endotoxin group, normalized grain densities were higher over neutrophils and macrophages, with less density observed over type 2 epithelial cells. In contrast, in the VILI group, type 2 epithelial cells exhibited a higher normalized grain density than was observed in the endotoxin group (p<0.001).
Analysis of the percent cell contribution to the total FDG activity indicated a significant positive contribution of neutrophils in both ALI models (p<0.001), as well as a significant contribution of macrophages to the FDG activity in controls (p<0.001) and VILI (p<0.005), with a trend observed in endotoxin ALI (p<0.07). Type 2 epithelial cells contributed a significant signal toward total FDG activity in controls (p< 0.005) and in VILI (p<0.05). Neutrophils presented the highest contribution toward FDG activity in both models of ALI (Fig. 3), significantly higher than that of other cell types. Due to lower cell counts and variability in signal observed, type 2 epithelial cells demonstrated a smaller percent contribution than neutrophils toward FDG activity in the VILI group despite the increased activity of type 2 epithelial cells observed in VILI. Macrophages accounted for the largest contribution toward FDG activity in control conditions (Fig. 3), significantly higher than that of other cell types.
Fig. 3.
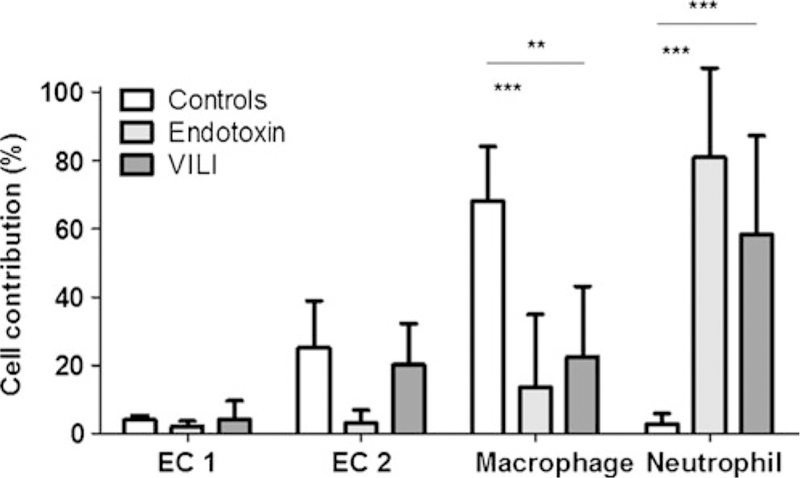
Percent contribution of studied cell types to the total number of 2-deoxy-2-[18F]fluoro-D-glucose grains on cells in micro-autoradiographic samples. Comparisons among ALI models and controls for epithelial cells type 1 (EC1) and type 2 (EC2), macrophages, and neutrophils. Neutrophils demonstrate a higher contribution during ALI than during control conditions. Macrophages, conversely, demonstrate a higher contribution during control conditions than during ALI. Double asterisks indicate p<0.01, and triple asterisks indicate p<0.001.
Immunohistochemistry for Endothelial Cells, Neutrophils, and Type 2 Epithelial Cells
Using tissue from an additional mouse instilled with intranasal endotoxin, examination of immunohistochemistry and HDG sections (Fig. 4) indicated HDG uptake by endothelial cells. P-selectin antibody staining identified endothelial cells (Fig. 4c), and Hoëchst staining confirmed the presence of nucleated cells (Fig. 4d). The merged image clearly demonstrated the overlap of HDG grains and endothelial cells (Fig. 4e).
Fig. 4.
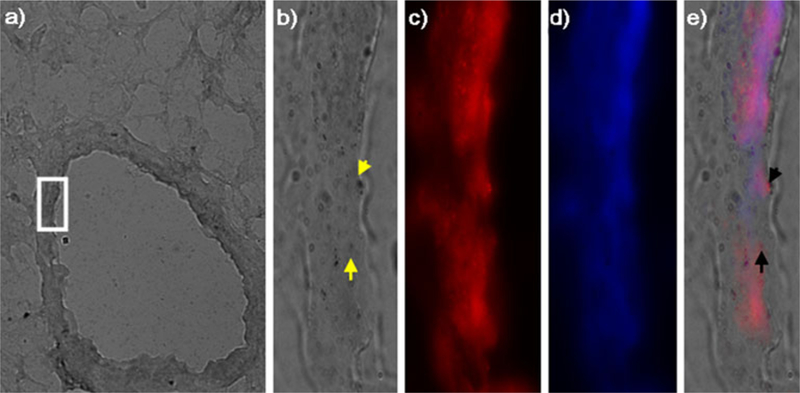
Immunohistochemical staining of endothelial cells and 2-deoxy-[3H]-D-glucose micro-autoradiography from endotoxin-induced lung injury. a Phase contrast image of HDG grains at ×40 magnification. The area in the white rectangle is magnified at ×100 in b–e. b Grains of HDG. Dark granules indicate presence of HDG (yellow arrows). c Endothelial cells identified with P-selectin. d Nuclear counter-staining with Hoëchst. e Merged image of b–d. Note that grains of HDG and endothelial cells (indicated by black arrows) are colocalized.
Examination of immunohistochemistry and HDG sections also indicated HDG uptake by neutrophils (Fig. 5a). Neutrophils were identified by Ly-6C/G staining (Fig. 5b), and DNA Hoëchst staining confirmed the presence of nucleated cells (Fig. 5c). Merged images of the cellular HDG grains, nuclear staining, and Ly-6C/G staining confirmed the speccific uptake of HDG by neutrophils (Fig. 5d). Finally, in a mouse subjected to VILI, we also observed HDG uptake by type 2 epithelial cells (Fig. 6a, b). This was indicated by colocalization of HDG grains and SP-C staining (Fig. 6c).
Fig. 5.
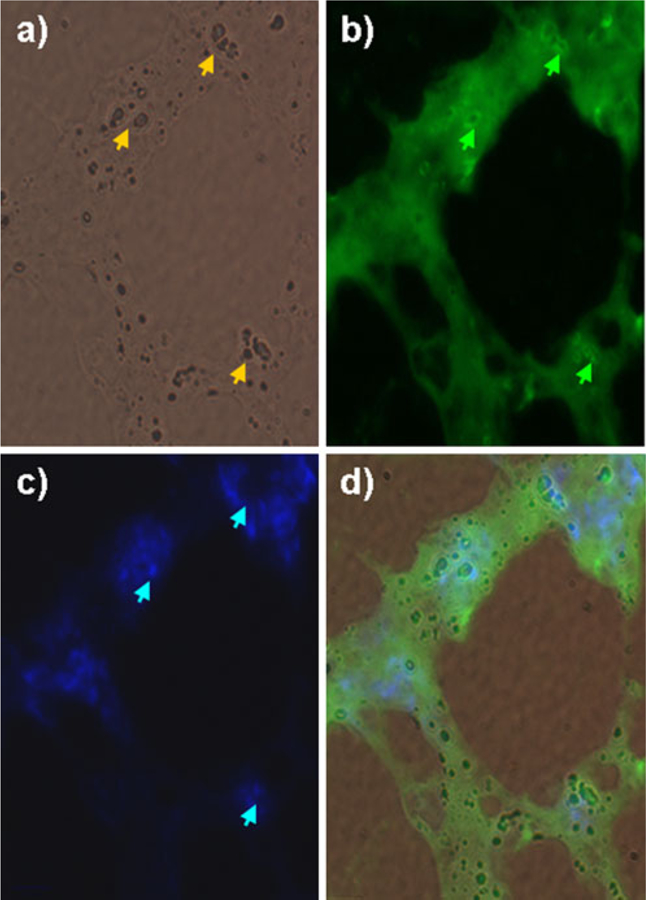
Immunohistochemical staining of neutrophils 2-deoxy-[3H]-D-glucose micro-autoradiography on tissue sections from endotoxin-induced lung injury. a Phase contrast image of HDG grains (yellow arrows) at ×100 magnification. b Neutrophils stained with Alexa-488-conjugated anti-Gr-1 antibody (green arrows). c Nuclear counter-staining with Hoëchst (blue arrows). d Merged image of a–c. Grains of HDG and neutrophils are colocalized.
Fig. 6.
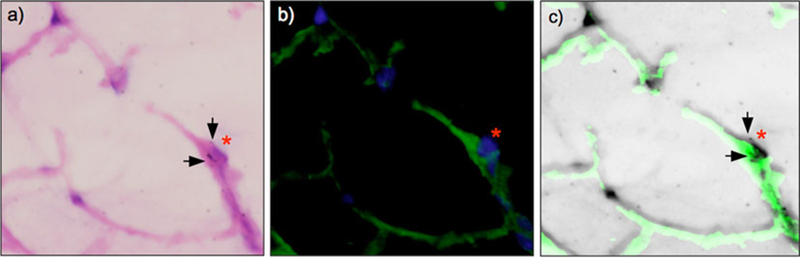
Immunohistochemical staining of type 2 epithelial cells and 2-deoxy-[3H]-D-glucose micro-autoradiography on tissue sections from ventilator-induced lung injury. a Hematoxylin–eosin image with HDG grains (arrows) and type 2 epithelial cells indicated (red asterisk) as confirmed by SP-C immunofluorescence in b (×60 magnification). b Type 2 epithelial cells stained with rabbit anti-SP-C and Alexa Fluor 488 goat anti-rabbit antibody (red asterisk). The nuclei were counter-stained blue with 4′,6-diamidino-2-phenylindole. c Merged image of a–b (and inverted to permit visualization of grains). Grains of HDG (arrows) and type 2 epithelial cells (red asterisk) are colocalized.
Discussion
The main results of our micro-autoradiography study in mouse models of lung injury due to intranasal endotoxin and VILI are as follows: (1) there is an increase in FDG uptake in the lung parenchyma in both models of ALI as compared with that observed in lungs of control mice; (2) increased FDG uptake was observed not only in neutrophils but also in lung macrophages and type 2 epithelial cells; and (3) the normalized grain density in the cell types examined depends upon the lung injury model: in addition to the contribution of neutrophils to FDG activity, FDG uptake is higher in type 2 epithelial cells following VILI than is observed after intranasal endotoxin, whereas FDG uptake is higher in macrophages after intranasal endotoxin than is observed following VILI.
The possibility of non-invasively detecting and quantifying regional inflammation during ALI led to an increased interest in the use of FDG-PET to study the underlying mechanisms and to characterize the severity and prognosis of clinical ALI/ARDS [8–11]. Traditionally, the FDG-PET signal has been interpreted as representing neutrophilic inflammation [7, 13, 14]. However, limited data exist regarding direct measurements of FDG activity in cell types within the pulmonary parenchyma. Thus, it is not currently known if and to what degree cell types, other than neutrophils, contribute to the FDG signal during ALI/ARDS, and therefore, detailed understanding of the source of FDG signal within the lung during ALI is limited.
We observed higher FDG uptake in injured lungs compared with control lungs in both of the ALI models we examined. Our observations in a model of instilled endotoxin are consistent with previous small and large animal models of endotoxin-induced ALI [7, 8]. While we are not aware of any previous studies that examined FDG uptake in mouse models of VILI, our findings indicating increased FDG uptake after murine VILI are compatible with previous findings in sheep [2].
Our examination of specific cell types indicated that neutrophils exhibited high FDG uptake both in the VILI and the endotoxin groups. The contribution by neutrophils to the FDG signal is compatible with the traditional interpretation of the FDG uptake signal as an indicator of neutrophilic inflammation. Such a concept has been supported by the presence of HDG uptake in neutrophils and a correlation between tissue-to-plasma FDG activity ratio and HDG uptake in neutrophils isolated from the bronchoalveolar lavage fluid in models of endotoxin exposure [7, 17]. The contribution of neutrophils to FDG activity was also described in a VILI model in sheep [2], in which the authors reported a correlation between FDG uptake and neutrophil counts in non-neutropenic sheep, with a significant reduction in FDG signal in neutropenic sheep. However, because either bronchoalveolar lavage samples or peripheral blood were used to quantify neutrophils in those studies, a direct statement could not be made about FDG uptake in neutrophils within the pulmonary parenchyma. Yet, such neutrophils might contribute significantly to the FDG signal due to their numbers and metabolic activity during ALI. Our study provides direct evidence of the contribution of parenchymal neutrophils to FDG uptake and substantiates the association of the FDG signal with neutrophilic inflammation in experimental endotoxin-induced ALI and VILI in mice.
A consistent finding in previous ALI studies in neutropenic animals was that a portion of the FDG signal was still present despite significant neutropenia [2, 17]. This observation implies the contribution of cell types other than neutrophils to the FDG signal. Although it is known that macrophages [24], epithelial cells [25], and endothelial cells [26] can exhibit increased metabolic activity during ALI, no direct assessment of their contribution to the FDG uptake signal during ALI has been reported. Our findings of a significant increase in normalized grain density in macrophages and type 2 epithelial cells support the hypothesis that cell types other than neutrophils contribute significantly to the FDG signal during ALI.
Remarkably, the normalized grain densities were differently distributed in the cell types in the two ALI models we examined. In the endotoxin model, the macrophage exhibited the second highest grain density (with the neutrophil exhibiting the highest). In contrast, during VILI, the type 2 epithelial cell exhibited the second highest grain density (with the neutrophil exhibiting the highest). These findings in VILI were corroborated by colocalization of type 2 epithelial staining with HDG grains. To our knowledge, our study is the first to demonstrate uptake of glucose-based tracers by parenchymal lung cell types during ALI. Our results further suggest that metabolic cell activity of different cell types in lung parenchyma depends on the mechanism of lung injury.
The increased grain density in type 2 epithelial cells during VILI could reflect enhanced metabolic activation as a result of exaggerated mechanical forces applied to these cells during mechanical ventilation or as a result of regional stimuli arising from those forces [25]. Indeed, it is known that primary type 2 epithelial cells in cell culture take up 2-deoxy-D-glucose [27]. Furthermore, mechanical lung stretch results in increased metabolic activity of type 2 epithelial cells and production of reactive oxygen species, interleukins, and lactic acid [24, 28, 29]. These cells also show an apical sodium–glucose cotransporter, which is involved in alveolar liquid clearance [30]. This process could be affected during VILI by increases in alveolar permeability [31]. Consequently, our finding of increased FDG uptake by type 2 cells in mouse lungs subjected to VILI might reflect increased metabolic activity associated with production of inflammatory cytokines and reactive oxygen species, as well as with fluid clearance. Use of pancuronium for muscle relaxation in the VILI group is unlikely to have influenced the results since there is no current evidence of an effect of muscle relaxants on the degree of lung injury.
In endotoxin-induced lung injury, the macrophage was the additional cell type that demonstrated significantly increased metabolic activity. This finding is consistent with previous reports describing an endotoxin-stimulated increase in macrophage metabolism [32, 33]. The absence of HDG uptake in alveolar macrophages in previous studies [7, 34] in contrast to our observation of increased FDG uptake by interstitial macrophages, could be due to the nature of the injury mechanism, the different biology of interstitial and alveolar macrophages, the endotoxin doses and routes of administration, the different species, and the temporal and spatial progression of the ensuing lung inflammation.
In our computations, there was a predominance of neutrophil contribution toward the FDG signal in both ALI models. The overall contribution of type 2 epithelial cells and macrophages to the total FDG signal was lower than their relative increase in normalized grain density due to the smaller number of those cells per field, in comparison to neutrophils. Thus, during ALI, the FDG signal is influenced by both the change in individual cell metabolic activity and the relative cell numbers in lung parenchyma; both of these factors can vary in different types of ALI. Such findings would explain previous observations of increased FDG uptake in neutropenic animals [2, 17]. Our finding of different patterns of cellular activation in different lung injury models also implies that the same level of absolute FDG uptake might represent different degrees of neutrophilic infiltration and inflammation, depending on the mechanism of injury. Finally, the contribution of different cell types toward the FDG signal also suggest that those identified types can be used as labeling targets for characterization of specific cellular contributions during ALI.
Methodological Aspects
There is no established method to quantify micro-autoradiographic measurements in the lungs using FDG. Our assessment of grain density in lung tissue was adapted from methods used in solid organs [15, 16]. In line with those studies, we normalized our activity results by the area in which grains were distributed to provide a reproducible measure of the FDG signal. A potential limitation of our approach is that the method to quantify grains was different in type 1 epithelial cells compared with other cell types. Although this limitation might affect the accuracy of our measurements, it does not interfere with our key finding that cell types other than neutrophils contribute to FDG signal in two murine models of ALI. In fact, the computation of coalesced grain area would lead to an underestimation of the number of grains in neutrophils, macrophages, and type 2 epithelial cells.
The method used to compute percent cell contribution to the total FDG uptake based on hematoxylin–eosin slides has significant limitations due to qualitative identification of cell types in hematoxylin–eosin slides and consequent uncertainty in quantifying absolute numbers of each cell type within a given field. Our results regarding those cell contributions toward the total signal should be interpreted as estimates. Future studies should make possible the implementation of more advanced methods for cell identification and counting.
Immunohistochemistry studies, combined with HDG imaging, corroborated our micro-autoradiography results for neutrophils and type 2 epithelial cells. Due to the difficulty of quantifying endothelial cell area by light microscopy in hematoxylin–eosin, we were unable to accurately assess the contribution of those cells to FDG uptake. Nevertheless, we observed that endothelial cells exhibited detectable FDG uptake in the endotoxin model by immunohistochemistry. Hence, our data suggest that these cells can become metabolically activated in response to ALI1 [35]. Our finding is consistent with previous observations of FDG uptake by endothelial cells after stimulation by nitric oxide [26].
Conclusion
FDG uptake, as reflected by normalized grain density, occurs in different cell types within the lung parenchyma during ALI induced by intranasal endotoxin or VILI in mice. Neutrophils show high grain density in both models. In addition, substantial FDG uptake occurs in macrophages and type 2 epithelial cells. Our findings also indicate that the contribution of individual cell types to the total FDG signal during ALI depends on the mechanism of injury. During endotoxin inhalation, neutrophils and macrophages demonstrated the highest FDG uptake, whereas during VILI, type 2 epithelial cells represented an additional important contribution to the FDG uptake signal. In the normal lung, the highest FDG uptake occurred in macrophages. Finally, endothelial cells also demonstrated FDG uptake and, thus, are likely to contribute to the FDG signal during ALI.
Acknowledgments.
This work was partially supported by the National Heart, Lung, and Blood Institute/National Institutes of Health grant HL 5R01HL086827. We would like to express our profound gratitude to Dr. Rosemary Jones and Diane E. Capen for the guidance and criticism on the experiments and manuscript. We would also like to thank Dr. Mauro Tucci for performance of pilot studies, Steve Weise for the technical aspects in manipulation of FDG samples, and Teri Bowman for assistance with methodology for immunohistochemistry.
Footnotes
Conflict of Interest. None of the authors has any conflict of interest associated with this project.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693 [DOI] [PubMed] [Google Scholar]
- 2.Musch G, Venegas JG, Bellani G, Winkler T, Schroeder T, Petersen B, Harris RS, Melo MF (2007) Regional gas exchange and cellular metabolic activity in ventilator-induced lung injury. Anesthesiology 106:723–735 [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282:54–61 [DOI] [PubMed] [Google Scholar]
- 4.Suter PM (2006) Lung inflammation in ARDS—friend or foe? N Engl J Med 354:1739–1742 [DOI] [PubMed] [Google Scholar]
- 5.de Prost N, Tucci MR, Vidal Melo MF (2010) Assessment of lung inflammation with 18F-FDG PET during acute lung injury. Am J Roentgenol 195:292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones HA, Choudhury M, Harris DN (2004) In vivo measurement of circulating leucocyte activation in patients following cardiopulmonary bypass. Nucl Med Biol 31:965–969 [DOI] [PubMed] [Google Scholar]
- 7.Chen DL, Schuster DP (2004) Positron emission tomography with [18F]fluorodeoxyglucose to evaluate neutrophil kinetics during acute lung injury. Am J Physiol Lung Cell Mol Physiol 286:L834–40 [DOI] [PubMed] [Google Scholar]
- 8.Costa EL, Musch G, Winkler T, Schroeder T, Harris RS, Jones HA, Venegas JG, Vidal Melo MF (2010) Mild endotoxemia during mechanical ventilation produces spatially heterogeneous pulmonary neutrophilic inflammation in sheep. Anesthesiology 112:658–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, Messa C, Pesenti A (2011) Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am J Respir Crit Care Med 183:1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Prost N, Costa EL, Wellman T, Musch G, Winkler T, Tucci MR, Harris RS, Venegas JG, Vidal Melo MF (2011) Effects of surfactant depletion on regional pulmonary metabolic activity during mechanical ventilation. J Appl Physiol 111:1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues RS, Miller PR, Bozza FA, Marchiori E, Zimmerman GA, Hoffman JM, Morton KA (2008) FDG-PET in patients at risk for acute respiratory distress syndrome: a preliminary report. Intensive Care Med 34:2273–2278 [DOI] [PubMed] [Google Scholar]
- 12.Chen DL, Bedient TJ, Kozlowski J, Rosenbluth DB, Isakow W, Ferkol TW, Thomas B, Mintun MA, Schuster DP, Walter MJ (2009) [18F] fluorodeoxyglucose positron emission tomography for lung antiinflammatory response evaluation. Am J Respir Crit Care Med 180:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones HA, Clark RJ, Rhodes CG, Schofield JB, Krausz T, Haslett C (1994) In vivo measurement of neutrophil activity in experimental lung inflammation. Am J Respir Crit Care Med 149:1635–1639 [DOI] [PubMed] [Google Scholar]
- 14.Jones HA, Sriskandan S, Peters AM, Pride NB, Krausz T, Boobis AR, Haslett C (1997) Dissociation of neutrophil emigration and metabolic activity in lobar pneumonia and bronchiectasis. Eur Respir J 10:795–803 [PubMed] [Google Scholar]
- 15.Kubota R, Yamada S, Kubota K, Ishiwata K, Ido T (1993) Micro-autoradiographic method to study [18F]FDG uptake in mouse tissue. Nucl Med Biol 20:183–188 [DOI] [PubMed] [Google Scholar]
- 16.Yamada S, Kubota R, Kubota K, Ishiwata K, Ido T (1990) Localization of [18F]fluorodeoxyglucose in mouse brain neurons with micro-autoradiography. Neurosci Lett 120:191–193 [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Kozlowski J, Goodrich AL, Markman N, Chen DL, Schuster DP (2005) Molecular imaging of lung glucose uptake after endotoxin in mice. Am J Physiol Lung Cell Mol Physiol 289:L760–8 [DOI] [PubMed] [Google Scholar]
- 18.Grommes J, Soehnlein O (2011) Contribution of neutrophils to acute lung injury. Mol Med 17:293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mescher A (2009) Junqueira’s basic histology McGraw-Hill Medical, New York [Google Scholar]
- 20.Ochoa CD, Wu S, Stevens T (2010) New developments in lung endothelial heterogeneity: Von Willebrand factor, P-selectin, and the Weibel-Palade body. Semin Thromb Hemost 36:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massaguer A, Engel P, Perez-del-Pulgar S, Bosch J, Pizcueta P (2000) Production and characterization of monoclonal antibodies against conserved epitopes of P-selectin (CD62P). Tissue Antigens 56:117–128 [DOI] [PubMed] [Google Scholar]
- 22.Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR (1991) Characterization and regulation of RB6–8C5 antigen expression on murine bone marrow cells. J Immunol 147:22–28 [PubMed] [Google Scholar]
- 23.Araki M, Hanihara T, Saito T (1988) Histochemical observations on unique rod-like cells in the developing retina of the normal rat. J Neurocytol 17:179–188 [DOI] [PubMed] [Google Scholar]
- 24.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC (1998) Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol 275:L1040–50 [DOI] [PubMed] [Google Scholar]
- 25.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD (1999) Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol 277:L167–73 [DOI] [PubMed] [Google Scholar]
- 26.Paik JY, Lee KH, Ko BH, Choe YS, Choi Y, Kim BT (2005) Nitric oxide stimulates 18F-FDG uptake in human endothelial cells through increased hexokinase activity and GLUT1 expression. J Nucl Med 46:365–370 [PubMed] [Google Scholar]
- 27.Kerr JS, Reicherter J, Fisher AB (1982) 2-Deoxy-D-glucose uptake by rat granular pneumocytes in primary culture. Am J Physiol 243:C14–9 [DOI] [PubMed] [Google Scholar]
- 28.Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM (2005) Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 289:L834–41 [DOI] [PubMed] [Google Scholar]
- 29.Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA (2004) Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology 9:43–53 [DOI] [PubMed] [Google Scholar]
- 30.de Prost N, Saumon G (2007) Glucose transport in the lung and its role in liquid movement. Respir Physiol Neurobiol 159:331–337 [DOI] [PubMed] [Google Scholar]
- 31.Nolop KB, Maxwell DL, Fleming JS, Braude S, Hughes JM, Royston D (1987) A comparison of 99mTc-DTPA and 113mIn-DTPA aerosol clearances in humans. Effects of smoking, hyperinflation, and in vitro oxidation. Am Rev Respir Dis 136:1112–1116 [DOI] [PubMed] [Google Scholar]
- 32.Ryan JL, Glode LM, Rosenstreich DL (1979) Lack of responsiveness of C3H/HeJ macrophages to lipopolysaccharide: the cellular basis of LPS-stimulated metabolism. J Immunol 122:932–935 [PubMed] [Google Scholar]
- 33.Ryan JL, Yohe WB (1981) Lymphocyte mediation of lipopolysaccharide-stimulated macrophage metabolism. J Immunol 127:912–916 [PubMed] [Google Scholar]
- 34.Chen DL, Rosenbluth DB, Mintun MA, Schuster DP (2006) FDG-PET imaging of pulmonary inflammation in healthy volunteers after airway instillation of endotoxin. J Appl Physiol 100:1602–1609 [DOI] [PubMed] [Google Scholar]
- 35.Gando S, Kameue T, Matsuda N, Sawamura A, Hayakawa M, Kato H (2004) Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: role of neutrophil and endothelial activation. Inflammation 28:237–244 [DOI] [PubMed] [Google Scholar]


