Abstract
Importance:
Dyslipidemia in young adults in the United States during their childbearing years is common and the consequences for the next generation are poorly understood. Further understanding of the harmful consequences of elevated low-density lipoprotein cholesterol (LDL-C) in young adults may help inform population screening and management strategies.
Objective:
To examine whether adult levels of serum LDL-C are associated with maternal pre-pregnancy LDL-C beyond that attributable to inherited genetic sequence polymorphisms, diet, physical activity, and body mass index.
Design:
The Framingham Heart Study, a population-based inception cohort initiated in 1948.
Setting:
A multi-generational study of families in Framingham, Massachusetts.
Participants:
The analyses included 538 parent-offspring pairs with parental LDL-C measured in the study prior to the offspring’s birth (241 mother-offspring, 297 father-offspring pairs, mean offspring age 26 [SD 3] years). Parental pre-birth, parental concurrent, and adult offspring assessments occurred in 1971–1983, 1998–2001 and 2002–2005, respectively.
Exposure:
Maternal pre-pregnancy LDL-C with comparison to paternal pre-pregnancy LDL-C in relation to concurrent parental-offspring LDL-C.
Main outcomes and measures:
Adult offspring LDL-C examined as both a continuous and dichotomous outcome (using a threshold of 130 mg/dL).
Results:
Adult offspring LDL-C was associated with maternal pre-pregnancy LDL-C after adjustment for family relatedness and offspring lifestyle, anthropometric, and inherited genetic factors (β=0.32 [SE 0.05] mg/dL, p<0.0001). After multivariable adjustment, adults exposed to elevated maternal pre-pregnancy LDL-C were at a 3.8 (95% CI 1.5, 9.8) times higher odds of having elevated LDL-C (p=0.004) and had an adjusted LDL-C 18 (95% CI 9, 27) mg/dL higher than those unexposed. Maternal pre-pregnancy LDL-C explained 13% of the variation in adult offspring LDL-C beyond common genetic variants and classic risk factors for elevated LDL-C.
Conclusions and Relevance:
Adult offspring dyslipidemia is associated with maternal pre-pregnancy dyslipidemia in excess of measured lifestyle, anthropometric, and inherited genetic factors. The findings support the possibility of a maternal epigenetic contribution to cardiovascular disease risk in the general population. Further research is warranted to determine if ongoing public health efforts to identify and reduce dyslipidemia in young adults prior to their childbearing years may have additional potential health benefits for the subsequent generation.
Keywords: maternal exposure, dyslipidemia, epigenetics
INTRODUCTION
Maternal health and in utero exposures are important determinants of long-term cardiometabolic outcomes among adult offspring, with maternal adiposity and hyperglycemia comprising the bulk of the studied effects.1–3 Independent of adiposity and hyperglycemia, elevated low-density lipoprotein cholesterol (LDL-C) is a well-established causal risk factor for atherosclerotic cardiovascular disease (CVD).4 However, the impact of maternal lipoprotein abnormalities on offspring cardiovascular health in the general population has been underexplored despite the frequent occurrence of dyslipidemia among women of childbearing age.5 In the United States, a quarter of women of childbearing age had an elevated LDL-C (>130 mg/dL) in the 2007–2008 U.S. National Health and Nutrition Examination Survey (NHANES).6
Maternal hypercholesterolemia has been linked to abnormal offspring cholesterol regulation in a fetal pathology case series,7 a pediatric observational cohort8 and among adults with Familial Hypercholesterolemia (FH).9–11 For example, individuals with FH, an autosomal dominant disorder of lipoprotein metabolism, have significantly elevated LDL-C throughout life. Adult offspring with a maternal history of heterozygous FH, and therefore higher in utero exposure to LDL-C, were found in some studies to have higher LDL-C as compared to those with paternal inheritance of FH.9,10 Subsequently, a higher mortality rate was observed among offspring with maternal as compared to paternal inheritance of the same FH genetic variant.12 Similarly, a higher burden of offspring atherosclerosis is also seen in animal models of maternal hypercholesterolemia.13–15 It is unknown whether higher pre-pregnancy maternal LDL-C is associated with increased offspring dyslipidemia and CVD risk in the general population. Exposure to elevated maternal LDL-C may explain an additional component of inter-individual variation in LDL-C beyond that attributable to lifestyle factors and inherited genetic sequence variation.16
The paucity of evidence on the effect of maternal dyslipidemia on adult offspring outcomes is likely due to the lack of cholesterol screening or routine measurement of cholesterol levels in young healthy women prior to pregnancy in previous generations. The Framingham Heart Study (FHS), a multigenerational population-based cohort, provides a unique opportunity to study lipoprotein levels in adulthood among a subset of participants whose parents were assessed in the study with LDL-C levels prior to their birth.
METHODS
Study design
We conducted an analysis of prospectively collected clinical and laboratory data from the Offspring and Third Generation cohorts of the FHS, a community-based multigenerational cohort of families in Framingham, MA, USA. The design of the FHS has been previously described, but briefly, in 1971, 5124 children and spouses of children of the Original cohort were enrolled in the FHS Offspring cohort.17 In 2002, 4095 Third Generation participants, who had at least one parent in the Offspring cohort, were enrolled and underwent standard clinical examinations.18 The current study analyses drew upon participant data from the Offspring cohort examination cycles 1–2 (1971–1983), Offspring cohort examination cycle 7 (1998–2001) and Third Generation cohort examination cycle 1 (2002–2005). Details of the FHS examinations and protocols are available at http://www.framinghamheartstudy.org/researchers/.
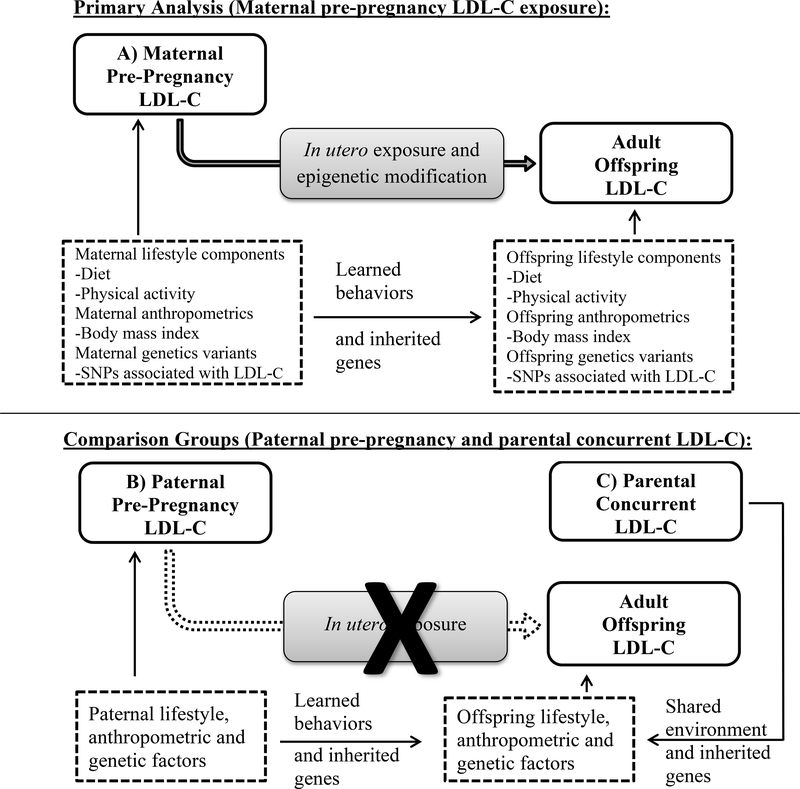
The current study examined the association between elevated maternal pre-pregnancy LDL-C (participants from the FHS Offspring Cohort) and adult offspring LDL-C (participants from the FHS Third Generation Cohort) with successive adjustments for potential confounders (body mass index [BMI], smoking, diet, physical activity, and inherited genetic sequence variants known to be associated with LDL-C levels). Potential confounders were identified based on established mechanisms in published literature and depicted in Figure 1.19 Paternal pre-pregnancy LDL-C levels were examined as a comparison of a parental association lacking the in utero exposure but similar contribution of inherited genetic sequence variation and shared early life environment. Parental (maternal and paternal) LDL-C measured concurrent with the adult offspring’s LDL-C were examined as an additional comparison to demonstrate the contribution of shared adulthood environmental factors and genetic components. The purpose of the multiple adjustments and comparisons was to determine if there is a residual association of maternal pre-pregnancy and adult offspring LDL-C in excess of shared early and later life environment, learned behaviors (diet and physical activity), anthropometric features (BMI) and inherited genetic sequence variation -- a contribution that may be attributable to maternal intergenerational epigenetic transmission. The hypothesis that maternal pre-pregnancy dyslipidemia may impart an effect on adult offspring dyslipidemia beyond that of measured confounders and genetic factors and of a greater magnitude compared to paternal pre-pregnancy dyslipidemia was pre-specified prior to the start of the analyses.
Figure 1.
Schematic diagram depicting the potential pathway from maternal pre-pregnancy LDL-C to adult offspring LDL-C via in utero exposure and epigenetic transmission with confounding by shared lifestyle factors, anthropometrics and inherited genetic variants. Paternal pre-pregnancy LDL-C (B) exhibits similar pathways except for the in utero exposures and subsequent epigenetic modifications. Concurrent parental LDL-C (C) shares an adulthood environment and genetic variants. Neither comparison group would be expected to demonstrate an association between parental and offspring LDL-C due to in utero LDL-C exposure after adjusting for the confounding pathways.
Study Sample
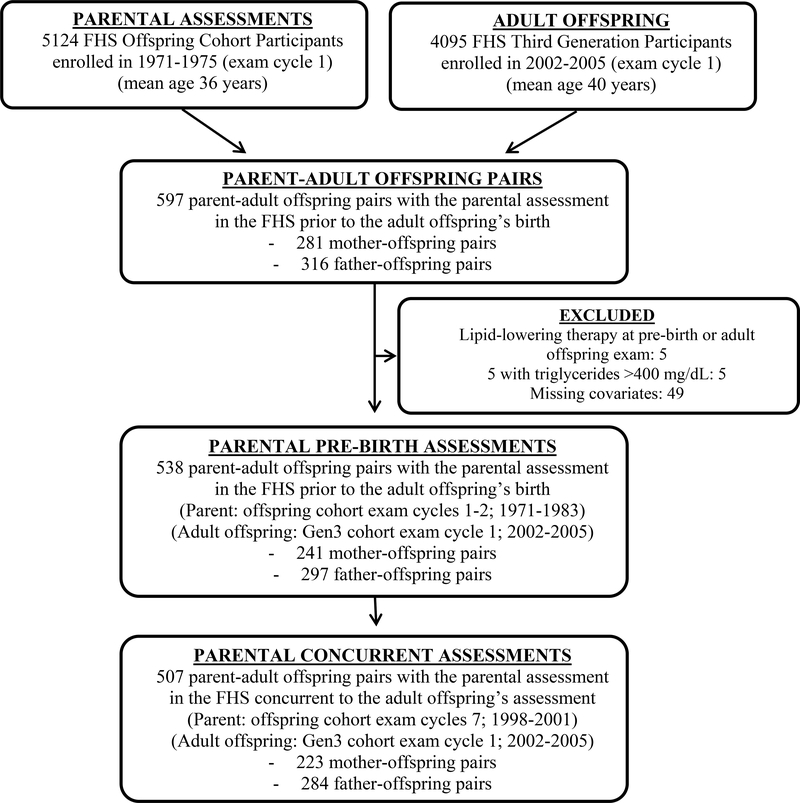
For the present analysis, we studied participants in the Third Generation cohort (comprising the ‘adult offspring’ in the current study) who: 1) attended the first examination cycle (2002 to 2005), 2) had at least one parent in the preceding generation cohort who was examined prior to their birth (Offspring cohort exam cycles 1–2; 1971–1983), and 3) had serum LDL-C measurements available for both the parental pre-birth assessment and adult offspring at enrollment. We did not include parents in the Original cohort as LDL-C was not available in the early examination cycles (1950s). Biological parent-offspring pairs were identified using self-reported relationships and confirmed with genetic pedigree data to avoid issues of non-paternity. There were 597 parent-adult offspring pairs with parental pre-birth and adult offspring examinations (281 mother-offspring and 316 father-offspring). We excluded pairs with an individual on lipid-lowering therapy at either the parental pre-birth assessment or adult offspring assessment (n=5; 1 father and 4 adult offspring), triglyceride level >400 mg/dL, as LDL-C could not be accurately calculated (n=5), and those with missing parental or offspring lipid measurements (n=49). After exclusions, there were 538 biological parent-offspring pairs (241 mother-offspring and 297 father-offspring pairs), of which 116 adult children were in both mother-offspring and father-offspring pairs (Figure 2). Parental LDL-C measured concurrently with the adult offspring LDL-C (parental LDL-C from the Offspring Cohort exam cycle 7; 1998–2001) was available for 507 parent-offspring pairs (223 mother-offspring and 284 father-offspring pairs).
Figure 2.
Flowchart of study sample inclusions for analyses.
The Boston University Medical Center Institutional Review Board approved the main study protocols and all participants signed written informed consent.
Lifestyle, Clinical, Laboratory, and Genetic Assessments
At each study visit, participants underwent a medical history interview and routine physical examination, including measurement of height, weight, and blood pressure using standardized approaches. Details of the covariate measurements are described in the Supplemental Methods. In summary, dietary intake was ascertained in the adult offspring (Third Generation cohort) via a 126-item semi-quantitative self-reported food frequency questionnaire. A physical activity index (PAI), expressed in metabolic equivalents (METs), was calculated by assigning each self-reported activity category a MET value based on the oxygen consumption required to perform activities in the category and deriving a weighted average of the MET values based on the proportion of time spent on activities in each category.20. Plasma total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and triglycerides (TRIG) were measured on morning samples obtained after an 8-hour fast. LDL-C was calculated according to the Friedewald equation.21 To convert TC, HDL-C, or LDL-C from mg/dL to mmol/L, multiply by 0.02586. To convert TRIG from mg/dL to mmol/L, multiply by 0.01129. The LDL-C genetic risk score (GRS) for each participant included 37 known LDL-C related SNPs reported in 2010 by the Global Lipids Genetic Consortium16 weighted by summation of genotypes (coded additively for the risk allele) multiplied by the reported effect-size estimates (eTable 1).
Statistical Analysis
We generated summary statistics with counts, frequencies, means and standard deviations for the demographic and clinical characteristics of the sample. Generalized estimating equations (GEE) with a linear (‘identity’) link for continuous outcomes and logit link for binary outcomes were used to account for familial correlations between measurements (PROC GENMOD in SAS; REPEATED statement). A compound symmetry correlation matrix was specified with robust variance estimators. For continuous LDL-C exposure and outcomes, GEE models were run with successive adjustment for: model 1 (M1) parental age and adult offspring age and sex, M2) previous covariates plus parental and adult offspring BMI and parental smoking status, M3) previous covariates plus adult offspring dietary and physical activity measures, and M4) previous covariates plus the adult offspring’s LDL-C GRS. There were no statistically significant interactions for offspring sex and parental LDL-C in any models (p>0.1) so sex-pooled analyses are presented.
LDL-C levels were dichotomized at 130 mg/dL for both parent and offspring in agreement with published guidelines as elevated.22 For dichotomous exposures and outcomes, GEE models were conducted with elevated LDL-C in the adult offspring as the outcome, elevated LDL-C in the parent as the predictor of interest, and successive adjustments for the same covariates as described above. For dichotomous exposures and continuous outcomes, the adjusted mean difference in adult offspring LDL-C (M4) for those exposed to elevated parental pre-pregnancy LDL-C compared to unexposed was generated. For continuous exposures and dichotomous outcomes, restricted penalized cubic splines were used to illustrate the relation between the continuous parental pre-pregnancy LDL-C levels and the fully adjusted (M4) odds ratio (OR) for elevated adult offspring LDL-C.23
The additional association of the maternal pre-pregnancy LDL-C as compared to paternal pre-pregnancy LDL-C was contrasted by including both parental pre-pregnancy LDL-C values in the same regression model for a subset of participants with LDL-C data on both parents. In addition, we utilized measures of model improvement to contrast the parental models, namely c-statistic, net reclassification index (NRI), and integrated discrimination index (IDI). Details are provided in the Supplemental Methods. The dichotomized elevated parental pre-pregnancy LDL-C, as opposed to the continuous measure, was focused on as it represents a clinically relevant measure to exert a pathologic effect above a homeostatic normal range, is more easily elicited in a clinical medical history interview and is a current threshold to promote healthy lifestyle changes in young adults.
Additional analyses were conducted on the other lipid panel components (HDL-C and TG). There were insufficient maternal pre-pregnancy hypertriglyceridemia (> 150mg/dL) cases or parental post-pregnancy low HDL-C (< 40 mg/dL in men and < 50 mg/dL in women) cases to include in dichotomous models (n=9 and 18, respectively), so only models examining continuous exposure/outcomes are presented.
Several sensitivity analyses were conducted. First, parents who were started on lipid-lowering therapy after their initial assessment and were on lipid-lowering therapy at the concurrent assessment with adult offspring LDL-C (n=15) were excluded from the successive GEE models, as described above. We conducted an additional sensitivity analysis to account for statin therapy with a second approach. For participants on lipid lowering therapy (at either the pre-birth parental examination [n=1], concurrent parental examination [n=15] or adult offspring examination [n=4]), we multiplied the treated LDL-C by 1.35 to account for the average lowering effect of statin therapy in order to estimate the untreated LDL-C.24 For the dichotomous models, treated individuals were included in the elevated LDL-C group. Successive GEE models that included participants with the estimated untreated LDL-C levels were then conducted. A third sensitivity analysis excluded maternal examinations taken within 9 months prior to the offspring’s birth (n=21; 14/21 [67%] within the first trimester) to account for any measurements taken during the pregnancy. Ordinary least squares linear and logistic regression models (not adjusted for family structure) were conducted, with successive covariate adjustment as described above, resulting in minimal difference in estimates as compared to the GEE models. A bias factor was calculated to determine the magnitude of unmeasured residual confounding that would have to be present to explain away any significant associations between maternal pre-pregnancy LDL-C and adult offspring LDL-C.25
All analyses were conducted using SAS 9.3 (Cary, NC). A two-sided p-value of < 0.05 was considered statistically significant.
RESULTS
Study Sample Characteristics
There were a total of 538 parent-offspring pairs (241 mother-offspring and 297 father-offspring). The characteristics of the study sample with parental characteristics at the pre-pregnancy examination are outlined in Table 1 and parental characteristics at the concurrent examination with the offspring’s LDL-C are reported in eTable 2. The fathers were on average slightly older than the mothers (29 vs. 27 years old) with higher BMIs (26.0 vs. 22.3 kg/m2). Smoking was common in the pre-pregnancy parental examinations – 46% of mothers and 40% of fathers. Parental concurrent examinations were unavailable for 17 (7%) mothers and 14 (5%) fathers. As expected, the current study sample differs from the overall FHS sample because the study sample was selected for having a pre-pregnancy parental examination and is therefore a younger subset (eTable 3).
Table 1:
Characteristics of Mother-Offspring and Father-Offspring Pairs with Characteristics of Parents at the Pre-Birth Examination
| Mother - Offspring Pairs | Father - Offspring Pairs | |||
|---|---|---|---|---|
| Mothers | Offspring | Fathers | Offspring | |
| n | 241 | 241 | 297 | 297 |
| Age (years) | 27 (4) | 26 (3) | 29 (5) | 26 (3) |
| Time between assessment and offspring birth (years) | 3.3 (2.3) | - | 3.1 (2.2) | - |
| Physical activity index (METs) | - | 37.5 (8.4) | - | 37.4 (7.7) |
| Total energy intake (kcal/d) | - | 2193 (999) | - | 2257 (952) |
| Trans fat intake (g/day) | - | 2.7 (1.6) | - | 2.7 (1.5) |
| Saturated fat intake (g/day) | - | 27.6 (14.9) | - | 27.8 (14.6) |
| Current smokers | 110 (46%) | 38 (16%) | 120 (40%) | 47 (16%) |
| BMI (kg/m2) | 22.3 (3.1) | 25.5 (5.2) | 26.0 (3.2) | 25.0 (5.0) |
| LDL-C (mg/dL) | 109 (28) | 100 (30) | 127 (36) | 97 (31) |
| LDL-C > 130 mg/dL, n (%) | n (%) | n (%) | n (%) | n (%) |
| LDL-C GRS (sum of alleles of 37 LDL-C SNPs * effect size estimate) | - | 79 (7.4) | - | 79 (7.1) |
Values are mean (standard deviation) and proportions presented as n (%), unless otherwise specified.
BMI = body mass index, LDL-C = low-density lipoprotein cholesterol, GRS = Genetic Risk Score. To convert LDL-C from mg/dL to mmol/L, multiply by 0.02586. Mother-offspring and father-offspring pairs are not mutually exclusive. Pre-pregnancy LDL-C are available for both parents of 116 adult offspring for which the offspring are represented in both offspring groups.
Association of Maternal Pre-Pregnancy LDL-C with Adult Offspring LDL-C
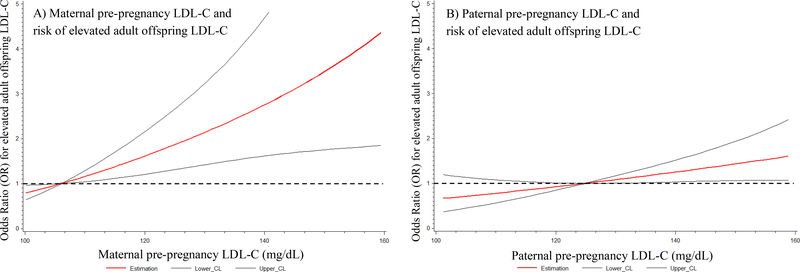
Maternal pre-pregnancy LDL-C was significantly correlated to adult offspring LDL-C (β=0.38 [SE=0.06] mg/dL, p<0.0001) in the age and sex adjusted models (M1; scatterplot in eFigure 1). After successive adjustments for anthropometric, lifestyle, and inherited genetic factors (Table 2a), the association between maternal pre-pregnancy LDL-C and adult offspring LDL-C was attenuated but remained significantly correlated (M4; β=0.32 [SE=0.05] mg/dL, p<0.0001). In excess of those confounding factors, maternal pre-birth LDL-C explained 13% of the variation in adult offspring LDL-C (partial r2=0.13; M4). In the fully adjusted models (Table 2b; M4), the odds of elevated LDL-C (>130 mg/dL) among adult offspring was 3.8 (95% CI 1.5, 9.8) times higher for those exposed to elevated maternal pre-pregnancy LDL-C compared to unexposed (p=0.005). The mean adult offspring LDL-C was 18 (95% CI 9, 27) mg/dL greater among those exposed to elevated maternal pre-birth LDL-C as compared to unexposed. The relationship between maternal pre-pregnancy LDL-C and the OR for elevated adult offspring LDL-C is presented in Figure 3a. The full regression model output for all covariates is reported in eTable 4 and eTable 5.
Table 2:
Association of Adult Offspring LDL-C with Parental LDL-C at Pre-Pregnancy Examinations Analyzed with a Continuous (a) and Dichotomous (b) Approach
| OUTCOME: | PRE-PREGNANCY EXAM LEVEL: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Maternal LDL-C (mg/dL) | Paternal LDL-C (mg/dL) | |||||||
| a) Adult Offspring LDL-C (mg/dL) | N | β | (95% CI) | p-value | N | β | (95% CI) | p-value |
| M1: adjusted for parental age, offspring age and sex | 241 | 0.38 | (0.27, 0.49) | <0.001 | 297 | 0.24 | (0.12, 0.35) | <0.001 |
| M2: M1 + adjusted for parental BMI and smoking, offspring BMI | 241 | 0.35 | (0.25, 0.46) | <0.001 | 297 | 0.25 | (0.13, 0.38) | <0.001 |
| M3: M2 + adjusted for offspring diet and physical activity | 216 | 0.38 | (0.27, 0.48) | <0.001 | 271 | 0.24 | (0.10, 0.37) | <0.001 |
| M4: M3 + adjusted for offspring LDL-C genetic risk score | 209 | 0.32 | (0.22, 0.42) | <0.001 | 263 | 0.13 | (0.02, 0.25) | 0.02 |
| Elevated Maternal LDL-C (>130 mg/dL) | Elevated Paternal LDL-C (>130 mg/dL) | |||||||
| b) Elevated Adult Offspring LDL-C (>130 mg/dL) | N | OR | (95% CI) | p-value | N | OR | (95% CI) | p-value |
| M1: adjusted for parental age, offspring age and sex | 241 | 5.0 | (2.2–11.2) | <0.001 | 297 | 1.9 | (1.0–3.9) | 0.06 |
| M2: M1 + adjusted for parental BMI and smoking, offspring BMI | 241 | 4.6 | (2.0–10.6) | <0.001 | 297 | 2.0 | (1.0–4.2) | 0.07 |
| M3: M2 + adjusted for offspring diet and physical activity | 216 | 4.7 | (1.8–12.1) | 0.001 | 271 | 1.8 | (0.8–4.0) | 0.15 |
| M4: M3 + adjusted for offspring LDL-C genetic risk score | 209 | 3.8 | (1.5–9.8) | 0.005 | 263 | 1.8 | (0.8–4.2) | 0.18 |
LDL-C = low-density lipoprotein cholesterol, BMI = body mass index, β = linear model regression coefficient, SE = standard error, M# = model #.
Figure 3:
Odds ratio (OR) for elevated adult offspring LDL-C (red line) and 95% CI (black lines) across a range of maternal (A) and paternal (B) pre-pregnancy LDL-C levels estimated with penalized restricted cubic splines in fully adjusted models for anthropometric, lifestyle and genetic factors (model 4). Dashed line depicts no elevated risk (OR = 1).
Comparison of Maternal vs. Paternal Pre-Pregnancy Contribution
The association between paternal pre-pregnancy LDL-C and adult offspring LDL-C was attenuated after adjustment for anthropometrics, lifestyle, and genetic factors (continuous exposure/outcome in Table 2a and dichotomous exposure/outcome in Table 2b; scatterplot in eFigure 2). The regression beta coefficient for the association of parental pre-pregnancy LDL-C with adult offspring LDL-C were approximately twice as great for maternal as compared to paternal pre-pregnancy LDL-C (Table 2; M4).
In a second approach to contrast the association between maternal and paternal pre-pregnancy LDL-C, the subset of adults adult offspring among whom pre-pregnancy lipids were available for both parents were examined (n=116). When both maternal and paternal pre-pregnancy LDL-C were added to the same regression model, the maternal pre-pregnancy LDL-C remained associated with adult offspring LDL-C while the paternal pre-pregnancy LDL-C did not (OR=6.2 [95% CI 1.6, 24], p=0.009 and OR=0.6 [95% CI 0.2, 2.3], p=0.5 for mothers and fathers, respectively). Results from the analyses comparing model c-statistic, NRI and IDI, are presented in the Supplemental Results. The full regression model output for all covariates is reported in eTable 6 and eTable 7.
Comparison of Parental Pre-Pregnancy vs. Concurrent LDL-C Assessments
There were 507 parent-offspring pairs (223 mother-offspring and 284 father-offspring pairs) in the concurrent parental LDL-C examination with the adult offspring LDL-C measure. There were no parental losses between pre-birth and concurrent assessments due to known coronary heart disease-related deaths. Concurrent parental measurements were not associated with adult offspring LDL-C in the fully adjusted models (Table 3; M4).
Table 3:
Association of Adult Offspring LDL-C with Parental LDL-C at Concurrent Examinations Analyzed with a Continuous (a) and Dichotomous (b) Approach
| OUTCOME: | CONCURRENT EXAM LEVEL: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Maternal LDL-C (mg/dL) | Paternal LDL-C (mg/dL) | |||||||
| a) Adult Offspring LDL-C (mg/dL) | N | β | (95% CI) | p-value | N | β | (95% CI) | p-value |
| M1: adjusted for parental age, offspring age and sex | 224 | 0.27 | (0.14, 0.41) | <0.001 | 283 | 0.07 | (−0.04, 0.18) | 0.23 |
| M2: M1 + adjusted for parental BMI and smoking, offspring BMI | 224 | 0.25 | (0.13, 0.38) | <0.001 | 283 | 0.07 | (−0.04, 0.18) | 0.21 |
| M3: M2 + adjusted for offspring diet and physical activity | 201 | 0.23 | (0.11, 0.35) | 0.002 | 259 | 0.05 | (−0.06, 0.17) | 0.37 |
| M4: M3 + adjusted for offspring LDL-C genetic risk score | 196 | 0.14 | (0.02, 0.27) | 0.02 | 253 | 0.04 | (−0.08, 0.17) | 0.48 |
| Elevated Maternal LDL-C (>130 mg/dL) | Elevated Paternal LDL-C (>130 mg/dL) | |||||||
| b) Elevated Adult Offspring LDL-C (>130 mg/dL) | N | OR | (95% CI) | p-value | N | OR | (95% CI) | p-value |
| M1: adjusted for parental age, offspring age and sex | 224 | 2.6 | (1.2–5.7) | 0.01 | 283 | 2.3 | (1.1–4.7) | 0.03 |
| M2: M1 + adjusted for parental BMI and smoking, offspring BMI | 224 | 2.3 | (1.1–4.8) | 0.03 | 283 | 2.2 | (1.0–4.7) | 0.05 |
| M3: M2 + adjusted for offspring diet and physical activity | 201 | 2.5 | (1.1–5.5) | 0.02 | 259 | 2.0 | (0.8–4.8) | 0.1 |
| M4: M3 + adjusted for offspring LDL-C genetic risk score | 196 | 1.9 | (0.8–4.4) | 0.1 | 253 | 1.7 | (0.7–4.3) | 0.3 |
LDL-C = low-density lipoprotein cholesterol, BMI = body mass index, OR = Odds ratio, CI = confidence interval, M# = model #.
Analyses for Parental-Offspring HDL-C and TG
Additional models for HDL-C and log-transformed TG (study sample distributions available in eTable 8) are presented in eTable 9 and eTable 10. A differential association for maternal over paternal pre-pregnancy and pre-pregnancy vs. concurrent examinations was not observed for HDL-C and logTG.
Sensitivity Analyses
The OR for elevated LDL-C in an adult offspring was lower in the sensitivity analysis after excluding parents taking lipid-lowering therapy at the parental concurrent assessment (OR for elevated adult offspring LDL-C 1.3 [95% CI 0.5, 3.5], p=0.6 and OR 2.1 [95% CI 0.7, 6.2], p=0.2, due to elevated concurrent maternal and paternal LDL-C, respectively). There were no substantial changes in the results after utilizing a correction factor (1.35) to estimate untreated LDL-C (eTable 11). The sensitivity analysis excluding maternal assessment within 9 months prior to the birth of a offspring had little effect on the OR for maternal pre-birth assessment on the adult offspring’s elevated LDL-C (OR 3.5 [95% CI 1.2, 10.1], p=0.02).
Bias factor for unmeasured residual confounding
The bias analyses indicated that a large amount of unmeasured confounding would have been required to account for the association between elevated maternal pre-pregnancy LDL-C and elevated adult offspring LDL-C. For example, the unmeasured confounder(s) would be required to result in an over 3-fold increase in elevated adult offspring LDL-C and be present in 70% of exposed vs. 30% unexposed (see eTable 12 for a range a bias scenarios).
DISCUSSION
We demonstrated that elevated LDL-C in mothers prior to the birth of a child was associated with an increased risk of elevated LDL-C in their adult offspring. The association persisted after adjustment for maternal BMI, smoking, and adult offspring lifestyle factors, BMI, and inherited genetic variants known to be associated with LDL-C. To our knowledge, this is the first study demonstrating a link in the general population between elevated maternal pre-pregnancy LDL-C and adult offspring LDL-C -- a major contributor to atherosclerotic CVD. The explanation for an enduring risk associated with elevated maternal pre-pregnancy LDL-C beyond genetic variants and lifestyle factors is likely multifactorial and may be mediated by direct effects on the developing fetal organ systems and through epigenetic modifications transferred via gametes or introduced in utero due to the nutrient milieu.
LDL-C is taken up by the maternal aspect of the placenta and cholesterol is used as a nutrient source for the developing fetus.26 In humans, low maternal serum cholesterol is associated with preterm birth and lower birthweights;27 replicating similar results from experimental studies in mice.28 In contrast, fetal over-nutrition with excess cholesterol due to increased LDL-C uptake likely has pathological effects on the developing fetus. In humans, fetuses of hypercholesterolemic mothers have an increase of aortic fatty streaks;7 also replicating findings from experimental studies in animals.29 Intrauterine exposure to elevated LDL-C may leave lasting effects on organ systems or epigenetic metabolism dysregulation that ultimately influences the ability of the offspring to regulate LDL-C in later life, as observed in this study, with important implications for risk of a higher future burden of atherosclerosis and CVD.
In the FHS, adult offspring without maternal pre-birth elevated LDL-C exposure were observed to have a lower LDL-C of a clinically relevant degree (18 mg/dL). A meta-analysis of randomized trials of statin treatment from the Cholesterol Treatment Trialists’ Collaboration revealed that a comparable decrease in LDL-C resulted in a 20% decrease in ischemic heart disease events.30 The elevated LDL-C outcomes observed in the adult offspring occurred at a relatively young age (mean 26 years). Available evidence demonstrates that early and prolonged exposure to elevated LDL-C carries significant adverse CVD consequences beyond that of developing dyslipidemia later in life.31 It was previously shown in the FHS that an increasing length of exposure to elevated LDL-C levels was associated with increased subclinical atherosclerosis, as measured by coronary artery calcium burden, 32 and higher CVD mortality.33 The early development of LDL-C elevation among adult offspring exposed to maternal pre-pregnancy elevated LDL-C levels may carry further consequences for the subsequent generation. Early development of dyslipidemia may accelerate a transgenerational cycle as an even greater proportion of young adult offspring go on to develop dyslipidemia in their childbearing years.
The association observed between concurrent parental LDL-C and adult offspring LDL-C in the unadjusted model is largely attenuated in the fully adjusted model. This suggests that the relationship is largely accounted for by measurable inherited genetic and lifestyle factors and only when the pre-birth level is considered is the larger maternal effect observable. In the fully adjusted model, maternal pre-birth LDL-C explained 13% of the inter-individual variation in adult offspring LDL-C. Further study of the underlying mechanisms linking maternal pre-birth LDL-C and adult offspring LDL-C, may reveal novel causal pathways resulting in dyslipidemia that are independent of inherited genetic sequence variants.
The current study has limitations. This study was limited to a subset of participants in the FHS because it required participants’ parents to have been enrolled and assessed prior to their birth. Second, we used pre-pregnancy elevated LDL-C as a proxy for intrauterine exposure and, lipids are known to increase during pregnancy. It has been previously demonstrated that maternal pre-pregnancy lipids predict peri-pregnancy lipids34 and that those entering pregnancy higher remain higher throughout. Further studies with direct measures of LDL-C during pregnancy would further support our findings. However, misclassification of mothers who did not have elevated pre-pregnancy LDL-C but went on to develop abnormal LDL-C during pregnancy would attenuate and not inflate the observed effect. Third, we only had data on cross-sectional adult offspring diet and physical activity and were not able to include any consideration of childhood diet and physical activity or parental pre-pregnancy diet and physical activity. Similarly, we had no data on offspring LDL-C during childhood. Residual confounding from a larger maternal environmental contribution to offspring LDL-C may exist. For example, mothers may play a larger role than fathers in establishing lifelong eating behaviors that contribute to adult offspring LDL-C levels. Fourth, birth outcomes data (such as birthweight) were not available for the adult offspring in our study and therefore whether the associations we observed are independent of birthweight and other birth outcomes cannot be assessed in this study. Fifth, we are unable to confirm co-habitation of offspring with one or both parents during childhood and development, which may falsely attenuate the concurrent exam analysis results. Sixth, our findings are limited to a population of European-descent and may not be generalizable to other ethnicities. Seventh, intergenerational epigenetic transmission is a hypothesized mechanism underlying our findings, and these phenomena (e.g. DNA methylation, histone modifications, etc.) were not examined directly in this study. Recent evidence of altered offspring DNA methylation in relation to maternal diet and metabolic intrauterine environment supports this premise.35–39 Aside from epigenetic mechanisms, the association between maternal pre-pregnancy and adult offspring LDL-C after adjustments may be due to unmeasured residual confounding (e.g. from shared environment, learned behaviors, inheritance of maternal mitochondrial DNA or unmeasured rare genetic variants). Rare genetic variants are likely not a major source of unmeasured confounding as recent studies show that rare variants explain very little additional inter-individual variation in lipid levels in the general population beyond the common variants included here.40,41 The bias analysis demonstrates that any unmeasured confounding would need a large effect size and be relatively frequent suggesting that the maternal pre-birth LDL-C association results are fairly robust to unmeasured confounding. We did observe a statistically significant association of continuous paternal pre-birth LDL-C with adult offspring LDL-C with a 2.5-times larger regression coefficient for maternal vs paternal associations in the fully adjusted model (0.32 vs 0.13 mg/dL increase in adult offspring LDL-C for each 1 mg/dL increase in parental pre-birth LDL-C). The smaller paternal pre-birth association is less robust to residual confounding and may be due to the inability to completely account for inherited lifestyle and genetic factors. However, transgenerational epigenetic inheritance from the paternal line transmitted via sperm is described in animal models,42,43 and we may be observing this effect; albeit smaller than for the maternal LDL-C association.
The strength of the study is that the mothers had lipid measurements obtained at a relatively young age prior to the birth of their children and the testing was not based on any indication (e.g. obesity or family history of heart disease), which was not standard practice at the time. This allowed us to assess outcomes in adult offspring with no parental testing bias. In addition, father-offspring pairs were confirmed using genetic data excluding any possibility that non-paternity issues would artificially reduce the father-offspring associations.
An improved understanding of the CVD risk associated with the intrauterine environment may inform population-based lifestyle strategies for women in their childbearing years to identify those at risk and to direct lipid-specific nutritional and lifestyle interventions or therapeutics. Further research is needed to determine if the knowledge of the risk associated with maternal pre-pregnancy dyslipidemia may motivate behavior change in young women of childbearing age driven by considerations of their child’s health beyond the consideration of their own health. Regardless, if the association is driven by epigenetic mechanisms or shared environment and learned behaviors, early lifestyle interventions would have the potential to affect either mechanism. For example, behavioral counselling for young adults with elevated LDL-C to increase physical activity and replace saturated and trans fats with polyunsaturated fats is recommended in the American Heart Association / American College of Cardiology guidelines.44 Although further research is required, our results support the possibility of benefits for the subsequent generation as well from these interventions in young adults. Additional studies with larger sample sizes would be needed to support our findings and improve the precision and confidence intervals of the effect estimates. Lastly, incorporating intrauterine exposures into CVD risk prediction models may improve discriminative properties, although this remains to be formally addressed.
In conclusion, maternal pre-pregnancy LDL-C levels are associated with adult offspring LDL-C levels beyond that attributable to measured lifestyle, anthropometric, and inherited genetic factors. Intergenerational maternal epigenetic transmission mechanisms, currently poorly understood, may mediate this effect. We postulate that identifying young women of childbearing age with elevated LDL-C and initiating lipid-specific interventions may further reduce the transgenerational cycle of dyslipidemia and CVD risk.
Supplementary Material
ACKNOWLEDGEMNTS:
All authors made substantial contributions to the conception and design of the current study. AL performed the analyses and all authors contributed to interpretation of the data. MM drafted the manuscript and all authors provided critical review and gave final approval of the published version. We acknowledge the immense contribution of the participants and staff of the Framingham Heart Study.
Authors MM and AL had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. The lead author (MM) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted.
SOURCES OF FUNDING: The Framingham Heart Study is administered by Boston University and is supported by the U.S. National Heart, Lung, and Blood Institute (contract N01-HC-25195 and HHSN268201500001I). MM is supported by a research fellowship from Boston University and the Tommy Kaplan Fund, Department of Cardiology, Boston Children’s Hospital, Boston, MA, USA. The funding sources had no role in the current study design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
CONFLICTS OF INTEREST: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) no authors have support from industry for the submitted work; (2) no authors have relationships with companies that might have an interest in the submitted work in the previous 3 years; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) all authors have no non-financial interests that may be relevant to the submitted work.
DATA SHARING: Participant level phenotype and genotype data from the Framingham Heart Study are accessible from the U.S. National Center for Biotechnology Information (NCBI) database of Genotypes and Phenotypes (dbGaP)45 at https://dbgap.ncbi.nlm.nih.gov/ to approved scientific investigators pursuing research questions that are consistent with the informed consent agreements provided by individual research participants.
REFERENCES
- 1.Hochner H, Friedlander Y, Calderon-Margalit R, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation. 2012;125(11):1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palinski W, Nicolaides E, Liguori A, Napoli C. Influence of maternal dysmetabolic conditions during pregnancy on cardiovascular disease. J Cardiovasc Transl Res. 2009;2(3):277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estampador AC, Franks PW. Genetic and epigenetic catalysts in early-life programming of adult cardiometabolic disorders. Diabetes, metabolic syndrome and obesity : targets and therapy. 2014;7:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham I, Cooney MT, Bradley D, Dudina A, Reiner Z. Dyslipidemias in the prevention of cardiovascular disease: risks and causality. Current cardiology reports. 2012;14(6):709–720. [DOI] [PubMed] [Google Scholar]
- 5.Palinski W Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease. Circulation. 2014;129(20):2066–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laz TH, Rahman M, Berenson AB. Trends in Serum Lipids and Hypertension Prevalence Among Non-Pregnant Reproductive-Age Women: United States National Health and Nutrition Examination Survey 1999–2008. Matern Child Health J. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Napoli C, D’Armiento FP, Mancini FP, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100(11):2680–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daraki V, Georgiou V, Papavasiliou S, et al. Metabolic profile in early pregnancy is associated with offspring adiposity at 4 years of age: the Rhea pregnancy cohort Crete, Greece. PLoS One. 2015;10(5):e0126327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Graaf A, Vissers MN, Gaudet D, et al. Dyslipidemia of mothers with familial hypercholesterolemia deteriorates lipids in adult offspring. Arterioscler Thromb Vasc Biol. 2010;30(12):2673–2677. [DOI] [PubMed] [Google Scholar]
- 10.Kusters DM, Avis HJ, Braamskamp MJ, et al. Inheritance pattern of familial hypercholesterolemia and markers of cardiovascular risk. Journal of lipid research. 2013;54(9):2543–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napoli C, Palinski W. Maternal hypercholesterolemia during pregnancy influences the later development of atherosclerosis: clinical and pathogenic implications. Eur Heart J. 2001;22(1):4–9. [DOI] [PubMed] [Google Scholar]
- 12.Versmissen J, Botden IP, Huijgen R, et al. Maternal inheritance of familial hypercholesterolemia caused by the V408M low-density lipoprotein receptor mutation increases mortality. Atherosclerosis. 2011;219(2):690–693. [DOI] [PubMed] [Google Scholar]
- 13.Napoli C, Witztum JL, Calara F, de Nigris F, Palinski W. Maternal hypercholesterolemia enhances atherogenesis in normocholesterolemic rabbits, which is inhibited by antioxidant or lipid-lowering intervention during pregnancy: an experimental model of atherogenic mechanisms in human fetuses. Circ Res. 2000;87(10):946–952. [DOI] [PubMed] [Google Scholar]
- 14.Napoli C, de Nigris F, Welch JS, et al. Maternal hypercholesterolemia during pregnancy promotes early atherogenesis in LDL receptor-deficient mice and alters aortic gene expression determined by microarray. Circulation. 2002;105(11):1360–1367. [DOI] [PubMed] [Google Scholar]
- 15.Alkemade FE, Gittenberger-de Groot AC, Schiel AE, et al. Intrauterine exposure to maternal atherosclerotic risk factors increases the susceptibility to atherosclerosis in adult life. Arterioscler Thromb Vasc Biol. 2007;27(10):2228–2235. [DOI] [PubMed] [Google Scholar]
- 16.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–290. [DOI] [PubMed] [Google Scholar]
- 18.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165(11):1328–1335. [DOI] [PubMed] [Google Scholar]
- 19.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 20.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Archives of internal medicine. 1979;139(8):857–861. [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 22.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. [DOI] [PubMed] [Google Scholar]
- 23.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–1057. [DOI] [PubMed] [Google Scholar]
- 24.Cholesterol Treatment Trialists C, Fulcher J, O’Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–1405. [DOI] [PubMed] [Google Scholar]
- 25.Vanderweele TJ, Mukherjee B, Chen J. Sensitivity analysis for interactions under unmeasured confounding. Stat Med. 2012;31(22):2552–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woollett LA. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. Am J Clin Nutr. 2005;82(6):1155–1161. [DOI] [PubMed] [Google Scholar]
- 27.Edison RJ, Berg K, Remaley A, et al. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics. 2007;120(4):723–733. [DOI] [PubMed] [Google Scholar]
- 28.Woollett LA. Review: Transport of maternal cholesterol to the fetal circulation. Placenta. 2011;32 Suppl 2:S218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palinski W, D’Armiento FP, Witztum JL, et al. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Circ Res. 2001;89(11):991–996. [DOI] [PubMed] [Google Scholar]
- 30.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326(7404):1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ference BA, Yoo W, Alesh I, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60(25):2631–2639. [DOI] [PubMed] [Google Scholar]
- 32.Tsao CW, Preis SR, Peloso GM, et al. Relations of long-term and contemporary lipid levels and lipid genetic risk scores with coronary artery calcium in the framingham heart study. J Am Coll Cardiol. 2012;60(23):2364–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navar-Boggan AM, Peterson ED, D’Agostino RB Sr., Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation. 2015;131(5):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vahratian A, Misra VK, Trudeau S, Misra DP. Prepregnancy body mass index and gestational age-dependent changes in lipid levels during pregnancy. Obstet Gynecol. 2010;116(1):107–113. [DOI] [PubMed] [Google Scholar]
- 35.Petropoulos S, Guillemin C, Ergaz Z, et al. Gestational Diabetes Alters Offspring DNA Methylation Profiles in Human and Rat: Identification of Key Pathways involved in Endocrine System Disorders, Insulin Signaling, Diabetes Signaling and IL-K Signaling. Endocrinology. 2014:en20141643. [DOI] [PubMed] [Google Scholar]
- 36.Teh AL, Pan H, Chen L, et al. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome research. 2014;24(7):1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morales E, Groom A, Lawlor DA, Relton CL . DNA methylation signatures in cord blood associated with maternal gestational weight gain: results from the ALSPAC cohort. BMC research notes. 2014;7:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soubry A, Murphy SK, Wang F, et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int J Obes (Lond). 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobi EW, Goeman JJ, Monajemi R, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nature communications. 2014;5:5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peloso GM, Auer PL, Bis JC, et al. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. American journal of human genetics. 2014;94(2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lange LA, Hu Y, Zhang H, et al. Whole-exome sequencing identifies rare and low-frequency coding variants associated with LDL cholesterol. American journal of human genetics. 2014;94(2):233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siklenka K, Erkek S, Godmann M, et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science. 2015. [DOI] [PubMed] [Google Scholar]
- 44.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2960–2984. [DOI] [PubMed] [Google Scholar]
- 45. Mailman MD, Feolo M, Jin Y, et al. The NCBI dbGaP database of genotypes and phenotypes. Nat Genet. 2007;39(10):1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.