Abstract
This work bridges a gap in the cross-coupling of aliphatic redox-active esters with aryl zinc reagents. Previously limited to primary, secondary and specialized tertiary centers, a new protocol has been devised to enable the coupling of general tertiary systems using Ni-catalysis. The scope of this operationally simple method is broad and can be used to simplify the synthesis of medicinally relevant motifs bearing quaternary centers.
Keywords: quaternary centers, decarboxylative radical coupling, redox-active esters, practical protocols, tertiary acids
Graphical Abstract
Quaternary Center exchange. Fully substituted carbon centers bearing a carboxylate moiety can engage in decarboxylative cross coupling with aryl zinc reagents under Ni catalysis, leaving in their wake an arene group.
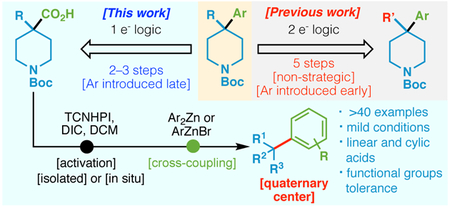
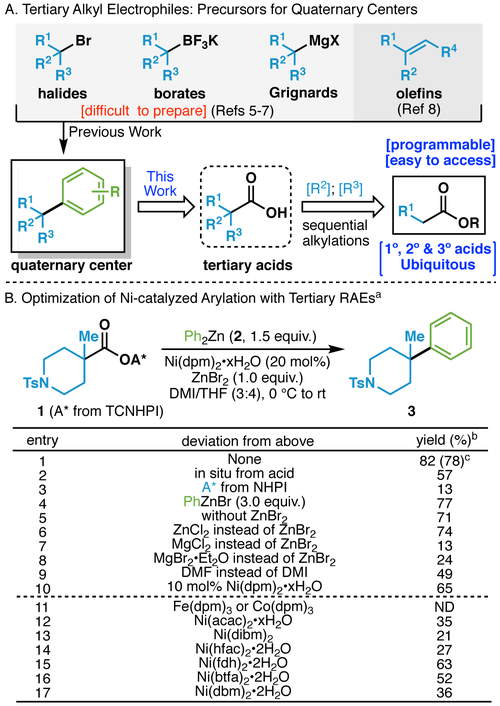
Recent interest in expanding rapid access to unique chemical space through the enablement of new cross-coupling methods has been enormous.1 This, in part, is due to the retrosynthetic logic employed widely in discovery sciences towards medicines, agrochemicals, and materials, placing emphasis on convergent, modular disconnections.2 Such pathways have been further stimulated by the myriad of vendors selling specialized building blocks rendering an almost limitless variety of subunits available to industrial chemists.3 Within this context, we were specifically drawn to the problem of constructing quaternary carbons, specifically those connected to an arene. Such motifs are found throughout the patent literature and are usually accessed through multi-step sequences (Figure 1A).4 Over the past decade, an explosion of interesting new techniques have emerged for achieving direct cross-couplings using alkyl halides,5 boronic acid derivatives,6 Grignard reagents,7 and most recently, olefins.8 While certainly useful, the substituents on such precursors (R1, R2, R3) are not easily programmable in addition to the fact that tertiary halides, borates, and Grignard reagents can be difficult to access. In contrast, a carboxylate coupling partner, of which thousands are available, are inherently diversifiable as R2 and/or R3 substituents can be installed via sequential alkylation chemistry. Here, building on recent findings on the use of redox-active ester (RAE) derivatives for programmed radical cross-coupling,9-11 we present a Ni-catalyzed protocol for the union of tertiary alkyl carboxylic acids with aryl zinc reagents.
Figure 1.
(A) Access to quaternary centers via cross coupling: Prior art. (B) Optimization on Ni-catalyzed cross coupling of tertiary RAEs with arylzinc reagents. a0.1 mmol. bYield determined by 1H NMR with CH2Br2 as an internal standard. cIsolated yield. See Supporting Information for details. DMI = 1,3-dimethyl-2-imidazolidinone, ND = not detected. dpm = 2,2,6,6-tetramethylheptane-3,5-dione, acac = acetylacetone, dibm = iPrCOCH2COiPr, hfac = hexafluoroacetylacetone, fdh = 1,1,1-trifluoro-5,5-dimethyl-2,4-hexanedione, btfa = 3-benzoyl-1,1,-trifluoroacetone, dbm = dibenzoylmethane Ts = tosyl, TCNHPI = N-hydroxytetrachlorophthalimide, NHPI = N-hydroxyphthalimide.
Previous experience in Negishi-couplings of RAEs using bidentate amine ligands under Ni-catalysis showed that only primary and secondary systems were viable. Switching to an Fe-catalyzed system enabled the generation of quaternary centers but only in the context of bridged systems such as cubane, adamantane, and [2.2.2]-bicycles. Unstrained cyclic and acyclic tertiary RAEs remained a glaring limitation of both the Fe- and Ni-systems. Path-pointing studies by Gong5 and Molander6c in their related cross-couplings aided initial feasibility screens suggesting the use of dicarbonyl-based ligands for Ni and Lewis acid as additives. The optimum conditions, shown in Figure 1B for the conversion of tertiary RAE 1 to 3 (entry 1), emerged after extensive investigations that are fully summarized in the Supporting Information. The reaction was amenable to a one-pot procedure involving in situ activation (entry 2). Although the reaction required a TCNHPI- rather than the NHPI-based RAE (entry 3), the aryl zinc species could be either a diaryl or arylzi halide, requiring 1.5 or 3.0 equiv., respectively (entry 4) Consistent with Molander’s observations, the addition of ZnBr2 had a slight but meaningful effect (ca. 10%) on the yield (entries 5 and 6) whereas a lower yield was observed in the presence of Mg-based analogs (entries 7 and 8). A solvent screen identified the DMI/THF mixture as superior to THF alone or DMF in place of DMI (entry 9). Lowering the catalyst loading to 10% decreased the reaction yield to 65% (entry 10). Finally, a screen of metal catalysts and ligands demonstrated that Fe- and Co-catalysts were not interchangeable (entry 11); while Ni(dpm)2•xH2O was optimum (entries 1, 12-17).
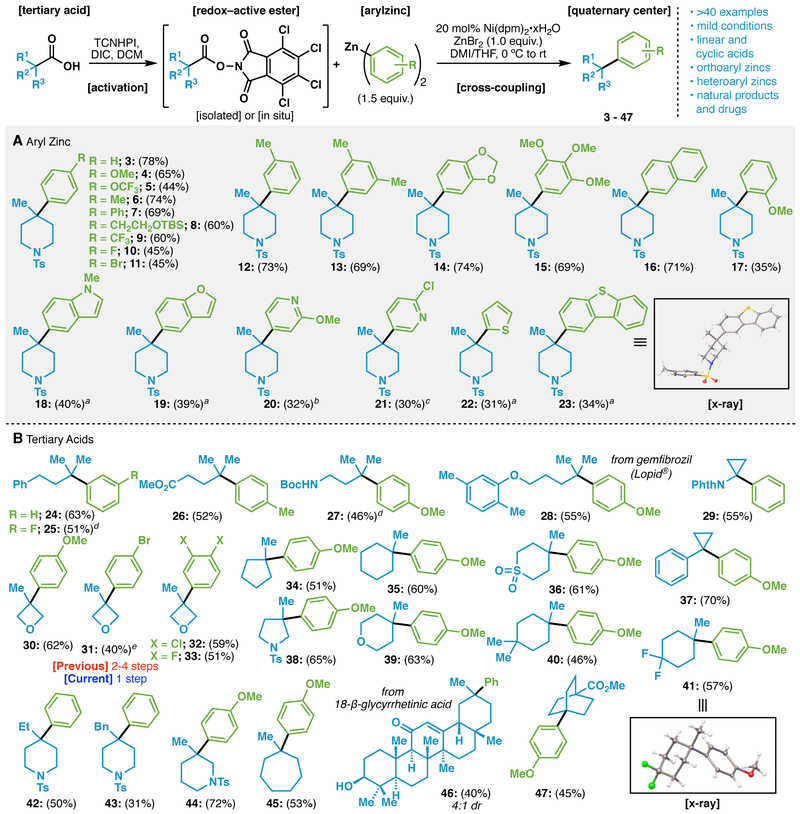
The conditions developed above proved to be general across a range of substrates as shown in Table 1 (>40 examples). The scope with regards to the aryl donor was first evaluated (Scheme 1A) to furnish adducts 3-23. Notable amongst these examples is the functional group tolerance of the reaction with ethers (4, 15, 17), halides (10, 11, 21), benzofurans (19), and pyridines (20, 21). Heterocycles and ortho-substituted arenes (17) diminished the yield somewhat but still provided serviceable quantities of product. Most notably, both electron rich and electron poor substituted arenes are viable coupling partners.
Scheme 1.
Scope of the Ni-catalyzed arylation of tertiary RAEs. Reaction conditions: Redox-active ester (1 equiv), Ni(dpm)2•xH2O (20 mol%), Ar2Zn (1.5 equiv), ZnBr2 (1 equiv) in THF/DMI (4:3) at rt for 12 h. [Isolated] refers to yields from the isolated redox-active ester, and [in situ] refers to yields from the carboxylic acid. a With Ni(dpm)2•xH2O (40 mol%) and Ar2Zn (2.5 equiv). b With Ni(dpm)2•xH2O (40 mol%) and Ar2Zn (2.5 equiv) at 60 °C. c With Ni(dpm)2•xH2O (40 mol%) and Ar2Zn (2.5 equiv) in THF/DMF at 60°C. d With Ni(dpm)2•xH2O (20 mol%) and Ar2Zn (2.5 equiv). e In situ from carboxylic acid (1.0 equiv.), TCNHPI (1.1 equiv.), DIC (1.1 equiv.) and DMAP (0.1 equiv.). Ts: tosyl; TBS: tert-butyldimethylsilyl; Boc: tert-butyloxycarbonyl; Phth: phthalimido; DIC: N/,N’-diisopropylcarbodiimide.
The vast scope of employable tertiary acids is described in Scheme 1B and features acyclic, cyclic, and those originating from natural products and medicines. High chemoselectivity is observed as a multitude of functional groups are compatible such as esters (26, 47), carbamates (27), ethers (28, 30-33, 39), imides (29), sulfonamides (38, 42-44), sulfones (36), enones (46), and even free alcohols (46). Simple acyclic acids as well as 3-, 4-, 5-, 6-, and 7-membered rings can also be enlisted. Of note here are the oxetane-derived products 30-33 that were prepared before in 2-4 steps12 that can now be easily accessed in a single step from the commercial acid. The utility of the transformation was also demonstrated by the synthesis of product 28 derived from Gemfibrozil. Late-stage arylation of the triterpene 18-β-glycyrrhetinic acid furnished product 46 without protection of the A-ring alcohol. The bridged system 47 can be viewed as a saturated mimic of a para-substituted benzene, useful for a variety of medicinal chemistry projects.13 The structure of products 8, 23 and 41 were confirmed by X-Ray crystallographic analysis.
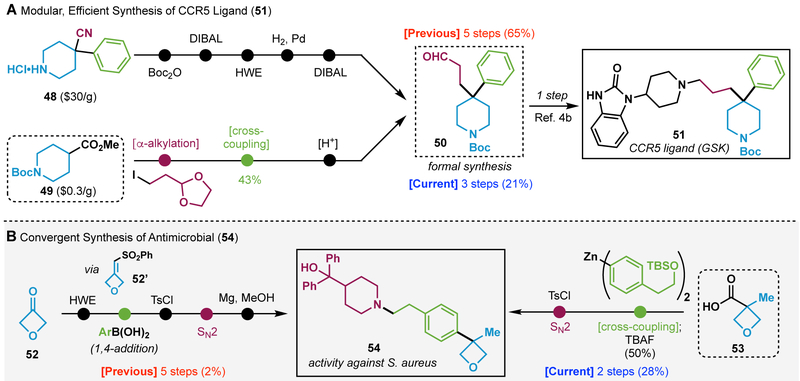
The retrosynthetic simplification enabled by this crosscoupling is further demonstrated with application to two reported medicinally promising scaffolds (Scheme 2). Thus, construction of the CCR5 ligand 51 (Scheme 2A) previously commenced with commercial piperidine 48 and proceeded through a five-step sequence to deliver 50. Of those steps, only one productively made a C─C bond with the remainder dedicated to protecting group chemistry and redox-fluctuations. From a diversity standpoint, the chemists were necessarily wedded to a phenyl group as it originated from the commercial starting material. In contrast, inexpensive isonipecotic acid ester (49) could be processed through 3 simple steps, two of which generate key C─C bonds to deliver key intermediate 50.4a,4b Although the overall yield is not as high as the prior approach, diversity installation is completely modular and could, in principle, permit access to a whole range of substituents at the quaternary carbon by simply changing alkylation and cross-coupling partners. In a similar vein, the anti-microbial compound 54 has previously been accessed using 2-electron retrosynthetic logic commencing from oxetane 52.4c Access to the quaternary center was enabled through a conjugate addition of an aryl boronic acid onto an α,β-unsaturated sulfone (52’). Subsequent deprotection/SN2/desulfonylation delivered 54. Alternatively, radical retrosynthesis11 led to precursor 53 that could be subjected to decarboxylative cross-coupling followed by SN2 to furnish the same product (54) in only two steps with an order of magnitude higher overall yield.
Scheme 2.
Radical retrosynthesis simplifies the synthesis of bioactive compounds from the patent literature.
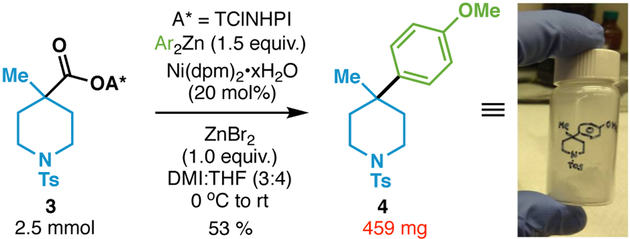
The coupling was also amenable to gram-scale as shown in Scheme 3. Without any modification of the standard conditions, piperidine 4 could be produced in similar yield (53%) to that run on 0.1 mmol scale (65% yield). Despite the advantages outlined herein, the reaction is not without limitations. For example, α-fluorinated and α-trifluoromethylated aliphatic acids were unreactive (only recovery of the starting acid was observed). Tertiary amino acids generally provided low yields (with the exception of 29) and small strained bicyclic systems ([2.1.1] both fully carbon and heteroatom substituted) did not function well. This chemistry is also currently limited to aryl zinc reagents as alkyl variants gave low yield.
Scheme 3.
Gram-scale synthesis of compound 4.
As one of the most ubiquitous functional groups known, carboxylic acids are an ideal starting point for cross-coupling chemistry. When coupled to alkylation or other α-functionalization methods, tertiary acids that are not already commercial are easily prepared in a modular fashion. The simple protocol delineated herein permits simple thermal, scalable access to convert such building blocks to diverse arene-flanked quaternary centers with demonstrable value to society.
Supplementary Material
Acknowledgements
Financial support for this work was provided by NIH (grant number GM-118176). We thank Bristol-Myers Squibb (Jennifer X. Qiao), and Enamine Ltd for providing samples of several tertiary acids. We gratefully thank Shenzhen Haiwei M&E Co. Ltd (fellowship to T.-G.C.), Tsinghua University (fellowship to H.Z.), and the Fulbright foundation (fellowship to P.K.M.). We also thank Dr. Jie Wang and Dr. Julien Vantourout for the help with manuscript preparation; Dr. Joel M. Smith for thoughtful discussions; D.-H. Huang and L. Pasternack (Scripps Research) for assistance with NMR spectroscopy; J. S. Chen and B. Sanchez (Scripps Automated Synthesis Facility); A. Rheigold, M. Gembicky, C. E. Moore (UCSD) for X-ray crystallographic analysis. CCDC 1884000 (8), 1883999 (23), 1884002 (41), 1884001 (54) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Center.
Footnotes
Publisher's Disclaimer: This manuscript has been accepted after peer review and appears as an Accepted Article online prior to editing, proofing, and formal publication of the final Version of Record (VoR). This work is currently citable by using the Digital Object Identifier (DOI) given below. The VoR will be published online in Early View as soon as possible and may be different to this Accepted Article as a result of editing. Readers should obtain the VoR from the journal website shown below when it is published to ensure accuracy of information. The authors are responsible for the content of this Accepted Article.
Contributor Information
Dr. Tie-Gen Chen, Department of Chemistry, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037, United States
Haolin Zhang, Department of Chemistry, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037, United States.
Dr. Pavel K. Mykhailiuk, Department of Chemistry, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037, United States; Enamine Ltd.; Chervonotkatska 78, 02094 Kyiv (Ukraine) and Taras Shevchenko National University of Kyiv; Chemistry Department; Volodymyrska 64, 01601 Kyiv (Ukraine)
Dr. Rohan R. Merchant, Department of Chemistry, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037, United States
Courtney A. Smith, Department of Chemistry, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037, United States
Dr. Tian Qin, Department of Chemistry, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037, United States
Prof. Phil S. Baran, Department of Chemistry, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037, United States
Reference:
- [1].a) For an overview on metal-catalyzed cross-coupling reactions, see: Metal-catalyzed Cross-coupling Reactions; Diederich F, Stang PJ, Eds.; Wiley-VCH: New York, 1998. [Google Scholar]; b) Negishi E, Palladium-Catalyzed Reactions Involving Reductive Elimination In Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi E, Ed.; Wiley-Interscience: New York, 2002; Vol. I, pp 213–1119. [Google Scholar]; c) Transition Metals for Organic Synthesis (Eds.: Beller M, Bolm C), 2nd ed., Wiley-VCH, Weinheim, 2004. [Google Scholar]
- [2].a) Corey EJ, Cheng XM, The Logic of Chemical Synthesis, Wiley, New York, 1989. [Google Scholar]; b) Nicolaou KC, Sorensen EJ, Classics in Total Synthesis, VCH, Weinheim, 1996, chap. 31. [Google Scholar]; c) Zapf A, Beller M in Handbook of Organopalladium Chemistry for Organic Synthesis, Vol. 1 (Ed.: Negishi EI), Wiley, New York, 2002, p. 1209. [Google Scholar]
- [3].Goldberg FW, Kettle JG, Kogej T, Perry MWD, Tomkinson NP, Drug Discov.Today 2015, 18, 659. [DOI] [PubMed] [Google Scholar]
- [4].a) Yang H, Kazmierski WM, Aquino CJ, Patent WO2004055016, 2004.; b) Kazmierski WM, Aquino C, Chauder BA, Deanda F, Ferris R, Jones-Hertzog DK, Kenakin T, Koble CS, Watson C, Wheelan P, Yang H, Youngman M, J. Med. Chem 2008, 51, 6538. [DOI] [PubMed] [Google Scholar]; c) Dunman PM, Krysan DJ, Flaherty DP, Patent WO2014052836A2, 2014.; d) Marson CM, Chem. Soc. Rev 2011, 40, 5514. [DOI] [PubMed] [Google Scholar]
- [5].a) Wang X, Wang S, Xue W, Gong H, J. Am. Chem. Soc 2015, 137, 11562. [DOI] [PubMed] [Google Scholar]; b) Wang X, Ma G, Peng Y, Pitsch CE, Moll BJ, Ly TD, Wang X, Gong H, J. Am. Chem. Soc 2018, 140, 14490. [DOI] [PubMed] [Google Scholar]
- [6].a) Zultanski SL, Fu GC, J. Am. Chem. Soc 2013, 135, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhou Q, Cobb KM, Tan T, Watson MP, J. Am. Chem. Soc 2016, 138, 12057. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Primer DN, Molander GA, J. Am. Chem. Soc 2017, 139, 9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Ni-catalyzed coupling of tertiary R-MgX with alkyl halides: Lohre C, Dröge T, Wang C, Glorius F, Chem. Eur. J 2011, 17, 6052. [DOI] [PubMed] [Google Scholar]; b) Joshi-Pangu A, Wang C-Y, Biscoe MR, J. Am. Chem. Soc 2011, 133, 8478. Cu- and Co-catalyzed coupling of tertiary R-MgX with alkyl halides: [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Terao J, Todo H, Begum SA, Kuniyasu H, Kambe N, Angew. Chem., Int. Ed 2007, 46, 2086. [DOI] [PubMed] [Google Scholar]; (d) Ren P, Stern LA, Hu XL, Angew. Chem., Int. Ed 2012, 51, 9110. [DOI] [PubMed] [Google Scholar]; (e) Yang C-T, Zhang Z-Q, Liang J, Liu J-H, Lu X-Y, Chen H-H, Liu L, J. Am. Chem. Soc 2012, 134, 11124. [DOI] [PubMed] [Google Scholar]; (f) Iwasaki T, Takagawa H, Singh SP, Kuniyasu H, Kambe N, J. Am. Chem. Soc 2013, 135, 9604. [DOI] [PubMed] [Google Scholar]
- [8].a) Green SA, Vásquez-Céspedes S, Shenvi RA, J. Am. Chem. Soc 2018, 140, 11317. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Green SA, Crossley SWM, Matos JLM, Vásquez-Céspedes S, Shevick SL, Shenvi RA, Acc. Chem. Res 2018, 51, 2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Cornella J, Edwards JT, Qin T, Kawamura S, Wang J, Pan C-M, Gianatassio R, Schmidt M, Eastgate MD, Baran PS, J. Am. Chem. Soc 2016, 138, 2174. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS, Science 2016, 352, 801. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang J, Qin T, Chen T-G, Wimmer L, Edwards JT, Cornella J, Vokits B, Shaw SA, Baran PS, Angew. Chem. Int. Ed 2016, 55, 9676. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Toriyama F, Cornella J, Wimmer L, Chen T-G, Dixon DD, Creech G, Baran PS, J. Am. Chem. Soc 2016, 138, 11132. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Qin T, Malins LR, Edwards JT, Merchant RR, Novak AJE, Zhong JZ, Mills RB, Yan M, Yuan C, Eastgate MD, Baran PS, Angew. Chem. Int. Ed 2017, 56, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Sandfort F, O’Neill MJ, Cornella J, Wimmer L, Baran PS, Angew. Chem. Int. Ed 2017, 56, 3319. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Edwards JT, Merchant RR, McClymont KS, Knouse KW, Qin T, Malins LR, Vokits B, Shaw SA, Bao D-H, Wei F-L, Zhou T, Eastgate MD, Baran PS, Nature 2017, 545, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Smith J, Qin T, Merchant RR, Edwards JT, Malins LR, Liu Z, Che G, Shen Z, Shaw SA, Eastgate MD, Baran PS, Angew. Chem. Int. Ed 2017, 56, 11906. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Chen T-G, Barton LM, Lin Y, Tsien J, Kossler D, Bastida I, Asai S, Bi C, Chen JS, Shan M, Fang H, Fang FG, Choi H.-w., Hawkins L, Qin T, Baran PS, Nature 2018, 560, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Li C, Wang J, Barton LM, Yu S, Tian M, Peters DS, Kumar M, Yu AW, Johnson KA, Chatterjee AK, Yan M, Baran PS, Science 2017, 356, eaam7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Huihui KMM, Caputo JA, Melchor Z, Olivares AM, Spiewak AM, Johnson KA, DiBenedetto TA, Kim S, Ackerman LKG, Weix DJ, J. Am. Chem. Soc 2016, 138, 5016. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu X-G, Zhou C-J, Lin E, Han X-L, Zhang S-S, Li Qi., Wang H Angew. Chem. Int. Ed 2018, 57, 13096. [DOI] [PubMed] [Google Scholar]; c) Mao R, Balon J, Hu X Angew. Chem. Int. Ed 2018, 57, 9501. [DOI] [PubMed] [Google Scholar]; d) Mao R, Balon J, Hu X Angew. Chem. Int. Ed 2018, 57, 13624. [DOI] [PubMed] [Google Scholar]; e) Xue W, Oestreich M Angew. Chem. Int. Ed 2017, 56, 11649. [DOI] [PubMed] [Google Scholar]; f) For a seminal use of RAEs of the phthalimide-type, see: Okada K, Okamoto K, Oda M, J. Am. Chem. Soc 1988, 110, 8736. [Google Scholar]
- [11].a) For reviews on radical-based strategies in synthesis, see: Yan M, Lo JC, Edwards JT, Baran PS, J. Am. Chem. Soc 2016, 138, 12692. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Smith JM, Harwood SJ, Baran PS, Acc. Chem. Res 2018, 51, 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Building block 31 was described in at least 2 discovery articles and 9 patents: lona-Minguez S, Höglund A, Ghassemian A, Desroses M, Calderon-Montań̃o JM, Moron EB, Valerie NCK, Wita E, Almlöf I, Koolmeister T, Mateus A, Cazares-Körner C, Sanjiv K, Homan E, Loseva O, Baranczewski P, Darabi A, Mehdizadeh M, Fayezi S, Jemth A-S, Berglund UW, Sigmundsson K, Lundback T, Jensen AJ, Artursson P, Scobie M, Helleday T, J. Med. Chem 2017, 60, 9769.29116786 [Google Scholar]; b) see ref 8a and references therein.
- [13].Locke GM, Bernhard SSR, Senge MO. Chem. Eur. J 2018, in press (DOI: 10.1002/chem.201804225). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.