A study of adults with knee or hip OA who completed a standardized cognitive task during a lab visit suggests that cognitive task demands may be an important contributor to fatigue and pain for people with OA.
MeSH TERMS: activities of daily living, cognition, fatigue, osteoarthritis, pain, task performance and analysis
Abstract
OBJECTIVE. Adults with osteoarthritis (OA) experience fatigue in daily life that is negatively related to physical activity; however, it is unclear how task demands affect fatigue and occupational performance. We examined effects of a cognitive task on subsequent symptoms and activity.
METHOD. Adults with knee or hip OA completed a standardized cognitive task during a lab visit. Objective physical activity and symptoms were tracked during two home-monitoring periods (i.e., 4-day period before and 5-day period after the lab visit). Multilevel modeling was used to compare pretask with posttask fatigue, pain, and activity levels.
RESULTS. Fatigue increased and pain decreased for 2 days after performing the lab task. The authors found no pretask to posttask changes in activity levels. At posttask, daily fatigue and activity patterns changed relative to baseline.
CONCLUSION. For adults with symptomatic OA, cognitive task demands may be an important contributor to fatigue and pain.
Fatigue is prevalent among older adults and is associated with adverse health outcomes (Avlund, Damsgaard, Sakari-Rantala, Laukkanen, & Schroll, 2002; Eldadah, 2010; Hardy & Studenski, 2008). Fatigue remains difficult to understand and treat because it may result from many factors. It is a symptom in specific diseases (e.g., cancer, congestive heart failure; Alexander et al., 2010), and it is associated with other conditions such as sleep problems and pain (Eyigor, Eyigor, & Uslu, 2010). Additionally, the fatigue experience is further complicated by the aging process. For example, tiredness in daily activities is a strong predictor of mobility problems (Avlund, Pedersen, & Schroll, 2003) and dependence in daily activities among older adults (Avlund et al., 2002).
Measurement is another issue that contributes to difficulty in understanding fatigue. Self-reported fatigue is measured in various ways (e.g., tiredness, interference with activities) over various time referents (e.g., week, month) across studies (Eldadah, 2010). Moreover, recall-based instruments, which are biased to peak and recent experiences (Stone, Broderick, Schwartz, & Schwarz, 2008), are most often used. Contributing to contradictory findings is that fatigue is not often studied in the context of activity. For instance, in studies examining how fatigue changes with advancing age, evidence suggests both that fatigue increases (Beute, Wiltink, Schwarz, Weidner, & Brähler, 2002) and that it decreases (Aggarwal, McBeth, Zakrzewska, Lunt, & Macfarlane, 2006; Stone et al., 2008). These disparate findings may partially reflect declining frequency or intensity of activity with age, such that decreased fatigue with age may be a consequence of engaging in less activity.
Given these issues, emerging research on fatigue has begun to examine fatigue within the context of performing activities to better understand the complex nature of fatigue and develop interventions (Alexander et al., 2010). Contextualizing fatigue within activity is referred to as fatigability, defined as the association or ratio of the frequency, duration, or intensity of activity to perceived fatigue (Eldadah, 2010). Research on fatigability in healthy persons and in specific medical conditions is limited.
Our research group became interested in fatigue among adults with knee or hip osteoarthritis (OA) after we conducted a study in which we measured pain and fatigue severity several times per day concurrently with physical activity. In that study, fatigue was more severe and more strongly related to decreased physical activity than was pain, the cardinal OA symptom (Murphy, Smith, Clauw, & Alexander, 2008). We used data from that study to determine whether periods of high activity were associated with increased fatigue (a measure of fatigability) and found that women with OA were 4 times more likely to have increased fatigue following a high bout of physical activity than were age-matched controls (Murphy & Smith, 2010).
Studying fatigability is relevant to occupational therapy practice because practitioners are uniquely suited to address how fatigue affects occupational performance. According to the Occupational Therapy Practice Framework: Domain and Process (2nd ed.; American Occupational Therapy Association, 2008), task demands (whether physical, mental, or emotional) can affect occupational performance; however, the effects of such task demands on fatigability are not well understood. Existing research that shows an association between cognitive or physical effort and fatigue uses measures of exercise, endurance, or strength as indicators of physical activity. These measures of a person’s capacity may not reflect actual activity in daily life and, thus, limit the generalizability of the findings (Blackwood, MacHale, Power, Goodwin, & Lawrie, 1998; Krupp & Elkins, 2000; Marcora, Staiano, & Manning, 2009).
To better understand how task demands affect fatigability and address the gaps in the literature, our group examined how standardized lab-based physical tasks resulted in changes in fatigue, pain, and activity in older adults with OA (Schepens, Kratz, & Murphy, 2012). We used ecological momentary assessment (EMA), in which self-reported fatigue and pain and objective physical activity were assessed throughout the day across 4 days at baseline (pretask) and 5 days posttask. We expected to find that, relative to baseline, fatigue and pain would increase and activity would decrease following the physically demanding lab task. Results showed that participation in lab-based physical tasks led to short-term increases in fatigue and decreases in physical activity but had no effects on pain. Physical activity levels were lower only on the task day, and changes in within-day patterns returned to baseline by the day after the task. Fatigue levels were higher on the day of task performance but lower than baseline on the subsequent 3 days, returning to baseline levels by Day 4 (Schepens et al., 2012).
This study extends our previous examination of fatigability subsequent to a physically demanding task to investigate the effects of cognitive effort on subsequent daily physical symptoms and activity. We addressed three research questions: Compared with baseline,
Are fatigue and pain higher and activity lower in a 5-day period after the cognitive task (i.e., are there carryover effects)?
Are there changes in daily patterns of fatigue, pain, and activity after the cognitive task?
Does the association between perceived fatigue and objective activity level (i.e., fatigability) change from baseline after the cognitive task?
On the basis of previous findings for the fatiguing effects of lab-based physical tasks (Schepens et al., 2012) and findings supporting the relationship between cognitive effort, physical performance, and fatigue (Krupp & Elkins, 2000; Marcora et al., 2009), we hypothesized that there would be increased fatigue and decreased activity posttask lasting no longer than the task day. We also hypothesized that daily patterns of fatigue and activity would be different from baseline on the day of the cognitive task and would return to baseline patterns on the subsequent day.
Method
Research Design
The University of Michigan Institutional Review Board approved this research. After a baseline visit, participants completed two lab visits. One lab visit involved participating in tasks designed to be physically fatiguing. Physical tasks included sweeping, placing groceries on a shelf, navigating a door while holding a bag of groceries, and walking (Schepens et al., 2012). The other lab visit, the focus of this article, involved participating in tasks designed to be mentally fatiguing. The order of these two visits was randomized to minimize misinterpretation of results due to carryover effects from either lab visit. At baseline, participants completed questionnaires and physical performance tests, were instructed in using an accelerometer, and were measured for body mass index (BMI). A 4-day home-monitoring period followed the baseline visit, and a 5-day period followed both lab visits. This study used data from baseline and cognitive lab visits and associated home-monitoring periods.
Sample
Community-living older adults (aged 65 and older) with knee or hip OA were screened between July 2009 and December 2010. Eligibility criteria included experiencing pain and fatigue 3 days/wk that interfered with functioning; having adequate cognition, operationalized as ≥5 on the six-item screener (Callahan, Unverzagt, Hui, Perkins, & Hendrie, 2002); and speaking English. Exclusion criteria included history of medical conditions that interfere with functioning or cause pain or fatigue, knee or hip surgery in the past 6 mo, current rehabilitation for OA, nonambulatory status, and inability to operate the accelerometer.
Cognitive Task
The cognitive task required performance of 10-min computer tasks separated by a 5-min simple response time task (15-min circuits). Tasks were discontinued when participants were too fatigued to continue or reached the 2.5-hr time limit. The protocol was designed using open source versions of computerized cognitive tests available from the Psychological Experiment Building Language (PEBL, Version 0.09) test battery (Mueller, 2008). Cognitive tests were chosen to elicit a variety of cognitive functions such as memory and problem solving, with the intention of eliciting fatigue in the participants. The cognitive tests included in the lab task were as follows (in order of administration):
Berg’s Card Sorting Test (Berg, 1948): Participants sorted cards with various shapes and colors into piles according to an unknown, ever-changing rule.
Digit span: Participants were presented with a series of digits and were instructed to recall the list by typing the numbers into a keyboard.
Four Choice Response Time (Wilkinson & Houghton, 1975): Participants were required to strike a key depending on the quadrant of the screen in which a visual stimulus appeared.
Implicit Association Test (Greenwald, McGhee, & Schwartz, 1998): Participants reported whether an image depicted something manufactured (e.g., airplane) or natural (e.g., flower).
Lexical Decision (Meyer & Schvaneveldt, 1971): Participants indicated whether text presented was a word or nonword.
PEBL Perceptual Vigilance Task (Wilkinson & Houghton, 1982): Participants pressed the space bar as quickly as possible in response to a series of visual stimuli, separated by a time delay of 2–12 s.
Spatial Cueing (Posner, 1976): Participants responded to an intermittent stimulus as fast as possible, indicating where they thought the next stimulus would appear given a probabilistic cue.
Stroop Task (Stroop, 1935): Participants responded to either the color or name of a stimulus word by pressing a key; the task alternated between series of color and name stimuli.
Tower of London (Shallice, 1982): Participants reproduced a pattern of colored disks by rearranging a prestacked pile in as few moves as possible.
Simple response time: Participants were asked to press a key as fast as they could on the appearance of an X for 5 min.
Measures
Baseline.
Participants completed self-report measures and the Timed Up and Go Test (TUG), a measure with excellent criterion validity and test–retest reliability in older adult samples (r = .97; Podsiadlo & Richardson, 1991). The Multidimensional Fatigue Inventory (MFI; Smets, Garssen, Bonke, & De Haes, 1995) was used to assess levels of general, physical, and mental fatigue. Each subscale consists of four items scored on a 5-point scale, ranging from 1 (yes, that is true) to 5 (no, that is not true), and has a possible range of 4–20. The MFI demonstrated good internal consistency (intraclass correlation coefficient [ICC] >.80) when tested in a variety of samples (Smets et al., 1995). The Center for Epidemiologic Studies Depression scale (CES–D; Radloff, 1977) was used to measure depressive symptoms; it has 20 items that are rated on a 4-point scale ranging from 0 (rarely or none of the time) to 3 (most or all of the time), for a possible range of 0–60. The CES–D demonstrated good internal consistency in a healthy sample (ICC = .85) and patient sample (ICC = .90).
Physical Activity.
A wrist-worn accelerometer (Actiwatch-Score, Phillips Respironics, Mini-Mitter, Bend, OR) was used to measure activity levels and patterns during home-monitoring periods. Accelerometers measure acceleration recorded as activity counts. The devices can be worn at different body sites (e.g., wrist, hip) and are most sensitive at collecting data at the site on which it is worn (Murphy, 2009). Although wrist-worn accelerometers are not recommended to approximate energy expenditure, the Actiwatch-Score is a valid and reliable assessment of physical activity levels. Specifically, the Actiwatch-Score has demonstrated criterion validity, with strong correlations between activity counts and movement from a motion analysis system (r = .88), and it has excellent interunit reliability (r = .98; Gironda, Lloyd, Clark, & Walker, 2007; Murphy, 2009). We defined activity as the average activity counts per minute over a specified interval. The intervals occurred between symptom-reporting periods during each day of the home-monitoring periods.
OA Symptoms.
During the cognitive lab visit, participants rated current fatigue and pain levels immediately following the simple response time task, before beginning the next circuit. During home-monitoring periods, participants entered their fatigue and pain into the Actiwatch-Score at wake up, 11 a.m., 3 p.m., 7 p.m., and 11 p.m. (or bedtime). Momentary symptoms were assessed by using a scale ranging from 0 (no fatigue–pain at all) to 10 (fatigue–pain as bad as I can imagine) that was adapted from the Brief Fatigue Inventory (Mendoza et al., 1999).
Data Analysis
Descriptive statistics were calculated for the study variables. To characterize a typical baseline day, fatigue, pain, and activity were averaged for each of the five time points (e.g., wake up, 11 a.m.) over the 4 days of the baseline home-monitoring period. These typical baseline days for activity, pain, and fatigue served as comparisons for fatigue, pain, and activity on the days after the cognitive task.
Because the EMA physical symptoms and activity counts data have a hierarchical structure, with hourly observations nested within each day and days nested within individual participants, linear regression analyses, which assume that observations are independent, could not be used. Instead, these data were analyzed with multilevel modeling (MLM) using SAS v. 9.2 PROC MIXED (SAS Institute, Cary, NC), which is a special kind of regression that models within- (Level 1 hourly, Level 2 daily) and between-person (Level 3) variance and accounts for the fact that multiple observations within a person or day are not independent. Variables were centered so that Level 1 and Level 2 variables were person centered and Level 3 variables (i.e., average symptoms and activity) were sample centered (Enders & Tofighi, 2007).
Sample- and person-centered fatigue, pain, or activity were tested as covariates and retained if significant; sample-centered covariates controlled for whether a person was high or low on that variable relative to the group (e.g., a person with relatively high pain), and person-centered covariates controlled for concurrent symptoms and activity (e.g., predicting momentary pain while controlling for concurrent pain). Additional Level 3 demographic (i.e., age, gender, BMI), psychological (i.e., CES–D), and physical functioning (i.e., TUG) covariates were entered into the MLM and were eliminated if not significant (p < .05 to retain), one by one, starting with the weakest predictor. Ultimately, none of these demographic, psychological, or physical functioning variables (e.g., TUG) were retained in the final model.
A set of five dummy codes for the day variable was developed (Cohen, Cohen, West, & Aiken, 2003) to compare levels of fatigue, pain, and activity across time on Days 1–5 (Day 1 = task day) to baseline (Day 0). To test daily effects, we created interaction terms representing linear (Time), quadratic (Time2), and cubic (Time3) effects of time to use as predictors of symptoms and activity. Interaction terms including the appropriate time effects and dummy variables (e.g., Dummy × Time2) were developed to test differences in daily patterns of symptoms and activity on Days 1–5 compared with baseline (Cohen et al., 2003). Finally, to test whether the association between fatigue and activity changed pretask to posttask, we entered interaction terms, including the dummy-coded day variable and fatigue (e.g., Dummy × Fatigue) into an equation predicting activity. Although our sample size was relatively small, our research questions pertained to within-day processes that have effective sample sizes represented by the number of momentary observations (Snijders, 2005), which were n = 643 for activity, n = 876 for pain, and n = 878 for fatigue.
Because home-monitoring data on the cognitive task day included only time points between 3 p.m. and 11 p.m., post hoc analyses were conducted to examine whether analyses that used only afternoon–evening time points were different from the all-time-points analyses. Results are reported only where significant differences for the afternoon–evening analyses (compared with all-time-points analyses) were found.
Results
Sample Characteristics
Eighty-seven people were screened, and 35 were enrolled in the primary study. Reasons for exclusion are reported elsewhere (Schepens et al., 2012). Four participants who did not complete the cognitive lab task, corresponding home-monitoring period, or both were excluded from the original pool of 35 participants. The final sample (N = 31) consisted of 19 women (61.3%); 24 participants (77.4%) were White, and 2 (6.5%) were African-American. Five participants (16.1%) declined to report their ethnicity or race (Table 1). CES–D scores indicated that the sample reported normal mood. On average, fatigue levels were moderate, with the exception of mild mental fatigue; TUG scores were slower than age-based norms recorded for healthy older adults (9.2 s; Bohannon, 2006); and BMI fell into the overweight category (BMI ≥ 30). Age was positively associated with physical fatigue and uncorrelated with average EMA activity. The EMA fatigue score did not correlate with MFI general or physical fatigue but showed a significant correlation with MFI mental fatigue.
Table 1.
Zero-Order Correlations and Distribution Characteristics of Demographic and Key Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1. Age | — | |||||||||
| 2. Timed Up and Go | −.05 | — | ||||||||
| 3. Body mass index | −.10 | −.12 | — | |||||||
| 4. CES–D Depression | −.31† | .08 | .20 | — | ||||||
| 5. EMA Fatigue | −.18 | .11 | .20 | .24 | — | |||||
| 6. EMA Activity | −.12 | −.30 | −.38* | −.11 | — | |||||
| 7. EMA Pain | −.25 | .22 | .22 | .35* | .61** | .08 | — | |||
| 8. MFI General Fatigue | .16 | −.32† | .43* | .04 | .27 | .01 | −.06 | — | ||
| 9. MFI Physical Fatigue | .35* | −.10 | −.27 | −.16 | −.16 | −.20 | −.19 | 64** | — | |
| 10. MFI Mental Fatigue | −.18 | −.12 | −.03 | .09 | .48** | .26 | .38* | .25 | −.06 | — |
| M (SD) | 72.7 (6.2) | 12.8 (4.5) | 30.2 (4.7) | 12.2 (9.2) | 4.0 (1.7) | 281.9 (109.7) | 2.7 (1.6) | 12.7 (3.6) | 12.2 (3.6) | 8.5 (3.6) |
| Minimum–maximum | 65–87 | 8.1–34.3 | 23.0–41.7 | 0–37 | 0.3–7.0 | 130.6–637.9 | 0.2–6.6 | 4–19 | 4–20 | 4–17 |
Note. Ecological momentary assessment (EMA) Fatigue, Activity, and Pain are aggregated values across home data collection periods. CES–D = Center for Epidemiologic Studies Depression Scale; M = mean; MFI = Multidimensional Fatigue Inventory; SD = standard deviation.
†p < .10. *p < .05. **p < .01.
Lab-Based Cognitive Task Characteristics
Time spent in the cognitive tasks ranged from 22 to 178 min (mean [M] = 103 ± 43.84). Fatigue increased significantly from pretask (M = 3.00 ± 0.41) to posttask (M = 5.66 ± 0.45), F(1, 28) = 35.22, p < .001, as did pain (pretask M = 1.17 ± 0.32; posttask M = 2.28 ± 0.46), F(1, 28) = 9.44, p < .01. Time spent participating in the lab tasks was not significantly correlated with pretask to posttask changes in fatigue (r = .18, p = .36) or pain (r = .24, p = .22).
Fatigue
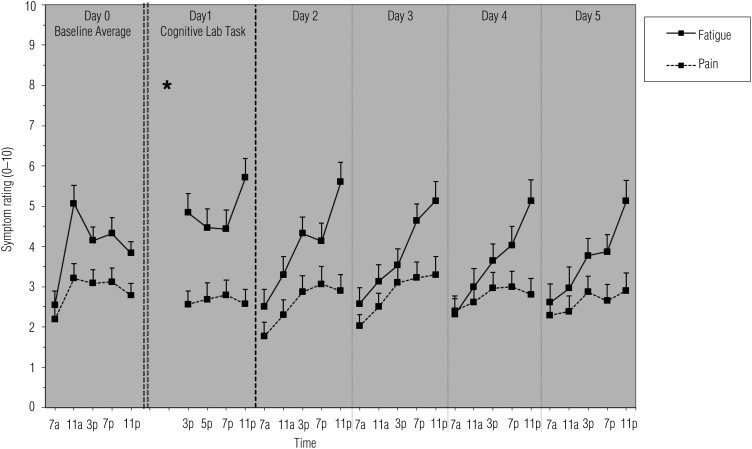
Compared with baseline fatigue, participants had significantly higher fatigue on Day 1 (β = 1.10, standard error [SE] = 0.21, p < .001); Days 2–5 were not significantly different from baseline (β range = −0.13–0.24, SE range = 0.20–0.21, all ps > .23). When only afternoon–evening values were considered, both Day 1 (β = 0.98, SE = 0.22, p < .001) and Day 2 (β = 0.58, SE = 0.24, p = .01) fatigue were significantly higher than baseline.
During the pretask baseline period, fatigue showed a daily pattern that significantly increased from morning to night (β = 0.44, SE = 0.12, p < .002). We found relatively higher levels midday compared with nighttime levels (negative quadratic effect; β = −0.01, SE = 0.004, p < .004). All days (Days 1–5) had a significantly different daily pattern from baseline (Figure 1); specifically, there was a significantly more positive linear effect of time (β range = 0.08–0.12, SE range = 0.03–0.06, p range < .001–.03). Compared with baseline, where fatigue levels peaked at midday, posttask fatigue increased from morning to evening with peak fatigue just before bedtime.
Figure 1.
Means and standard errors of fatigue and pain across the study period.
Note. * = time of cognitive lab visit.
Pain
Figure 1 depicts mean pain across days. Pain levels on Day 1 (β = −0.51, SE = 0.15, p < .001) and Day 2 (β = −0.37, SE = 0.15, p = .01) were significantly lower compared with baseline; pain levels were similar to baseline for Days 3–5 (β range = −0.02–0.21; SE range = 0.14–0.15; all ps > .15).
Across the assessment period, the overall daily pattern of pain showed a significant positive linear (β = 0.19, SE = 0.06, p = .001) and negative quadratic (β= −0.005, SE = 0.002, p = .01) effect of time. In general, lowest pain was reported in the morning, and peak pain was reported midday. Pain patterns did not change from pretask to posttask (β range = −0.03–0.0001; SE range = 0.005–0.01; all ps > .06).
Activity
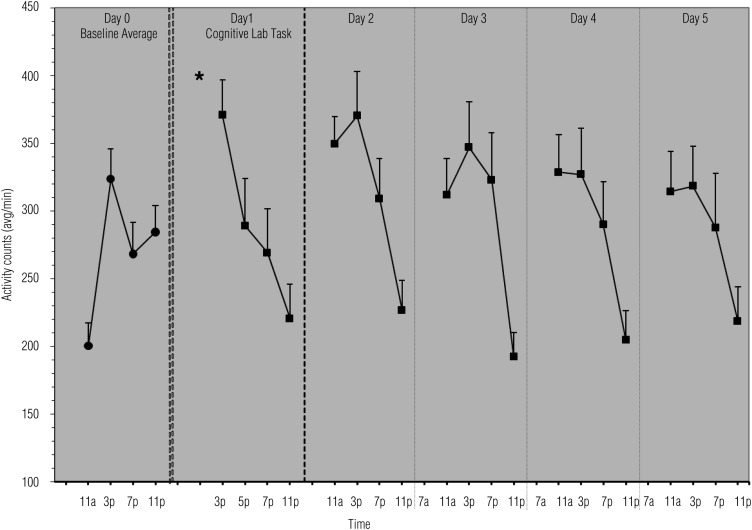
Physical activity levels were not different on Days 1–5 compared with baseline (β range = 1.46–28.34; SE range = 16.50–16.94; all ps > .09; Figure 2).
Figure 2.
Means and standard errors of physical activity across the study period.
Note. * = time of cognitive lab visit.
For the baseline period, there were significant positive linear (β = 370.08, SE = 66.41, p < .001), negative quadratic (β = −20.80, SE = 4.14, p < .001), and positive cubic (β = 0.38, SE = 0.08, p < .001) effects of time on activity. The positive cubic effect at baseline (Column 1, Figure 2) shows a “checkmark” pattern, with an increase in activity from morning to night, interrupted by a dip around 7 p.m. This cubic effect, however, is lost posttask, when activity shows a significantly more negative linear effect of time, indicated by a relatively steady decline in activity after the lab on Day 1 (β = −24.67, SE = 4.17, p < .001) and from morning to evening on Days 2–5 (β range = −17.55–14.91, SE range = 3.10–3.14, all ps < .001). The cubic within-day pattern of baseline activity was not reestablished by the end of the posttask home period.
Association of Fatigue and Activity: Fatigability
Across the study period, controlling for mean activity and momentary pain, fatigue was negatively associated with activity (β = −5.60, SE = 2.61, p = .03), and pain was positively associated with activity (β = −11.92, SE = 4.04, p = .003). There were no significant changes from pretask to posttask in the association between fatigue and activity (i.e., fatigability) on any of the posttask days (β range = −14.16 to −5.37, SE range = 9.32–9.46, all ps > .10).
Discussion
We examined the effect of a standardized lab-based cognitive task on subsequent levels and patterns of fatigue, pain, and activity in older adults with OA. Unlike studies that examine the association of cognitive effort and physical performance measured by exercise or strength (Blackwood et al., 1998; Krupp & Elkins, 2000; Marcora et al., 2009), we used accelerometry to measure physical activity in daily life. Consequently, these data offer a glimpse into how a cognitively fatiguing event may affect subsequent occupational performance in the lives of older adults with OA.
Our results indicate that participation in lab-based cognitive tasks had significant and, in some cases, lasting effects on symptoms and activity levels. Pain was lower and fatigue was higher on the task day and the following day. The positive carryover effect on pain is consistent with literature showing that in people with arthritis, self-reported pain was lower after a stressful day, possibly because of redoubling of coping efforts to address initially higher pain (Affleck, Tennen, Urrows, & Higgins, 1994). Alternatively, it is well established that the experience of pain is highly dependent on attention to pain sensations (Eccleston & Crombez, 1999), and our unexpected finding may be accounted for by the reallocation of attention to the cognitive task, resulting changes in fatigue, or both. Compared with our first study (Schepens et al., 2012), which looked at the effects of physically fatiguing lab tasks on subsequent pain, fatigue, and activity, the cognitive lab visit resulted in similarly higher levels of fatigue on Day 1. In the first study, however, the physical task resulted in significantly lower fatigue on Days 2–4, whereas the cognitive task in this study showed no such positive carryover effect on fatigue.
Daily pattern of fatigue changed significantly after the cognitive tasks. Rather than building from morning to midday and subsiding toward evening, as is seen in the baseline period, for 4 days posttask fatigue showed a steady within-day increase. In contrast with the first study, where fatigue pattern changed only on the day of the task and returned to baseline by the next day, changes in fatigue persisted through study cessation. Correlation results suggest that EMA fatigue may be capturing mental rather than physical fatigue. If this is the case, it is not surprising that participants would demonstrate less cognitive endurance and greater cognitive fatigability throughout the day and in the days after the cognitive task. Unlike fatigue, daily pain patterns did not change following the cognitive tasks.
In terms of physical activity, we found in the first study (Schepens et al., 2012) that on the day of the physical lab task only, activity levels declined significantly and showed a pattern where activity steadily declined toward bedtime (a more linear pattern than baseline). In contrast, the cognitive task resulted in no changes in activity level. However, daily patterns of activity were significantly altered through the end of the study period after the cognitive task. Much like the task day pattern found in the first study, every day subsequent to the cognitive task showed a steady decline in activity from morning to evening. Notably, the aforementioned change in fatigue pattern is overlaid by this more linear posttask change in activity.
The patterns and levels of activity in this study are similar to those found in previous OA samples (Murphy et al., 2008; Schepens et al., 2012). This is the first study to examine and discover changes in daily activity following a cognitively fatiguing task in OA, and we have no theoretical, clinical, or empirical evidence to indicate why cognitive effort alters later physical activity patterns. Therefore, future studies that include a larger and more diverse sample and mixed methods that incorporate qualitative data should seek to replicate and further investigate the causes and implications of the changes in daily patterns resulting from fatiguing tasks.
Implications for Occupational Therapy Practice
The results of this study suggest several important implications for occupational therapy practice:
Cognitive task demands may play an important role in the physical activity patterns and experience of fatigue and pain in older adults with hip or knee OA.
A cognitively demanding task may result in sustained changes in daily activity patterns and reports of symptoms.
Occupational therapists should expand their focus beyond physical activity and consider incorporating measures of cognitive activity and cognitive fatigue into clinical assessment and treatment planning for clients for whom fatigue is problematic because cognitive effort and fatigue may be affecting their occupational performance and experience of symptoms.
Strengths and Limitations
A main strength of this study is the use of EMA and concurrent physical activity assessment that allowed us to examine daily processes, thereby providing insight into the ways in which symptoms and activity change within and across days as well as the dynamic associations between symptoms and activity. Some limitations should also be noted. Sample characteristics, mostly White women with low baseline levels of pain and fatigue, limit generalizability. This was secondary analysis, and efforts to replicate and extend these findings are necessary to fully interpret the results. The ecological validity of the lab-based cognitive task (i.e., a neuropsychological test battery done through computer) is not known. Although the task was advantageous to include for our ability to standardize task demand across participants, it is not directly generalizable as a typical daily cognitive task. Incorporation of real-life cognitive tasks such as navigating computer Web sites, making grocery lists, balancing a checkbook, or drafting letters may allow better translation of research findings into clinical practice.
Additionally, although our results indicate that the cognitive task resulted in increased fatigue, we cannot be certain that people concluded the tasks when they were legitimately fatigued. Participants may have stopped because of boredom, increased pain, or other reasons. Given our small sample size, we were unable to examine subgroup differences in symptom and activity levels and patterns after the lab-based task.
Conclusion
This is the first study to examine effects of a cognitively fatiguing lab task on subsequent symptoms and physical activity in older adults with OA. When compared with previous findings in which we examined the same response to a physically fatiguing lab task, the cognitive task had larger effects on symptoms and activity (Schepens et al., 2012). This study supports the idea that treatment and interventions that target OA symptoms may benefit from closer consideration of the cognitive demands of daily tasks. Addressing fatigue (in particular, cognitive fatigue) in addition to pain appears to be important, even though it is not commonly addressed in OA. Understanding changes in activity patterns after cognitively demanding tasks offers occupational therapy practitioners insight into potential timing of interventions targeting fatigue or pain management in persons with OA. These findings may also shed light on the timing of interventions that aim to improve activity levels in this rather sedentary population. The role of cognitive effort appears to be an important and understudied factor that relates to symptom experience and physical activity for older adults.
Acknowledgments
Susan Murphy conceptualized the study concept and design. Stacey Schepens was responsible for acquisition of participants and data as well as for data preparation. Anna Kratz, Stacey Schepens, and Susan Murphy were responsible for data analysis and interpretation and manuscript preparation.
Contributor Information
Anna L. Kratz, Anna L. Kratz, PhD, is Assistant Professor, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor
Stacey L. Schepens, Stacey L. Schepens, PhD, OTR, is Postdoctoral Fellow, Division of Occupational Science and Occupational Therapy, University of Southern California, Los Angeles
Susan L. Murphy, Susan L. Murphy, ScD, OTR/L, is Associate Professor, Department of Physical Medicine and Rehabilitation, University of Michigan, 300 North Ingalls Building, 9th Floor, Ann Arbor, MI, 48109, and Research Health Science Specialist, Geriatric Research, Education and Clinical Center, Veterans Affairs Ann Arbor Health Care System; sumurphy@umich.edu
References
- Affleck G., Tennen H., Urrows S., & Higgins P. (1994). Person and contextual features of daily stress reactivity: Individual differences in relations of undesirable daily events with mood disturbance and chronic pain intensity. Journal of Personality and Social Psychology, 66, 329–340. 10.1037/0022-3514.66.2.329 [DOI] [PubMed] [Google Scholar]
- Aggarwal V. R., McBeth J., Zakrzewska J. M., Lunt M., & Macfarlane G. J. (2006). The epidemiology of chronic syndromes that are frequently unexplained: Do they have common associated factors. International Journal of Epidemiology, 35, 468–476. 10.1093/ije/dyi265 [DOI] [PubMed] [Google Scholar]
- Alexander N. B., Taffet G. E., Horne F. M., Eldadah B. A., Ferrucci L., Nayfield S., & Studenski S. (2010). Bedside-to-Bench conference: Research agenda for idiopathic fatigue and aging. Journal of the American Geriatrics Society, 58, 967–975. 10.1111/j.1532-5415.2010.02811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Occupational Therapy Association. (2008). Occupational therapy practice framework: Domain and process (2nd ed.). American Journal of Occupational Therapy, 62, 625–683. 10.5014/ajot.62.6.625 [DOI] [PubMed] [Google Scholar]
- Avlund K., Damsgaard M. T., Sakari-Rantala R., Laukkanen P., & Schroll M. (2002). Tiredness in daily activities among nondisabled old people as determinant of onset of disability. Journal of Clinical Epidemiology, 55, 965–973. 10.1016/S0895-4356(02)00463-8 [DOI] [PubMed] [Google Scholar]
- Avlund K., Pedersen A. N., & Schroll M. (2003). Functional decline from age 80 to 85: Influence of preceding changes in tiredness in daily activities. Psychosomatic Medicine, 65, 771–777. 10.1097/01.PSY.0000082640.61645.BF [DOI] [PubMed] [Google Scholar]
- Berg E. A. (1948). A simple objective technique for measuring flexibility in thinking. Journal of General Psychology, 39, 15–22. [DOI] [PubMed] [Google Scholar]
- Beute M. E., Wiltink J., Schwarz R., Weidner W., & Brähler E. (2002). Complaints of the ageing male based on a representative community study. European Urology, 41, 85–92, discussion 92–93. 10.1016/S0302-2838(01)00003-3 [DOI] [PubMed] [Google Scholar]
- Blackwood S. K., MacHale S. M., Power M. J., Goodwin G. M., & Lawrie S. M. (1998). Effects of exercise on cognitive and motor function in chronic fatigue syndrome and depression. Journal of Neurology, Neurosurgery, and Psychiatry, 65, 541–546. 10.1136/jnnp.65.4.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon R. W. (2006). Reference values for the Timed Up and Go Test: A descriptive meta-analysis. Journal of Geriatric Physical Therapy, 29, 64–68. 10.1519/00139143-200608000-00004 [DOI] [PubMed] [Google Scholar]
- Callahan C. M., Unverzagt F. W., Hui S. L., Perkins A. J., & Hendrie H. C. (2002). Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care, 40, 771–781. 10.1097/00005650-200209000-00007 [DOI] [PubMed] [Google Scholar]
- Cohen J., Cohen P., West S., & Aiken L. (2003). Applied multiple regression/correlation analysis for the behavioral sciences. Mahwah, NJ: Erlbaum. [Google Scholar]
- Eccleston C., & Crombez G. (1999). Pain demands attention: A cognitive–affective model of the interruptive function of pain. Psychological Bulletin, 125, 356–366. 10.1037/0033-2909.125.3.356 [DOI] [PubMed] [Google Scholar]
- Eldadah B. A. (2010). Fatigue and fatigability in older adults. PM&R: Journal of Injury, Function, and Rehabilitation, 2, 406–413. 10.1016/j.pmrj.2010.03.022 [DOI] [PubMed] [Google Scholar]
- Enders C. K., & Tofighi D. (2007). Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychological Methods, 12, 121–138. 10.1037/1082-989X.12.2.121 [DOI] [PubMed] [Google Scholar]
- Eyigor S., Eyigor C., & Uslu R. (2010). Assessment of pain, fatigue, sleep and quality of life (QoL) in elderly hospitalized cancer patients. Archives of Gerontology and Geriatrics, 51, e57–e61. 10.1016/j.archger.2009.11.018 [DOI] [PubMed] [Google Scholar]
- Gironda R. J., Lloyd J., Clark M. E., & Walker R. L. (2007). Preliminary evaluation of reliability and criterion validity of Actiwatch-Score. Journal of Rehabilitation Research and Development, 44, 223–230. 10.1682/JRRD.2006.06.0058 [DOI] [PubMed] [Google Scholar]
- Greenwald A. G., McGhee D. E., & Schwartz J. K. L. (1998). Measuring individual differences in implicit cognition: The Implicit Association Test. Journal of Personality and Social Psychology, 74, 1464–1480. [DOI] [PubMed] [Google Scholar]
- Hardy S. E., & Studenski S. A. (2008). Fatigue and function over 3 years among older adults. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 63, 1389–1392. 10.1093/gerona/63.12.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp L. B., & Elkins L. E. (2000). Fatigue and declines in cognitive functioning in multiple sclerosis. Neurology, 55, 934–939. 10.1212/WNL.55.7.934 [DOI] [PubMed] [Google Scholar]
- Marcora S. M., Staiano W., & Manning V. (2009). Mental fatigue impairs physical performance in humans. Journal of Applied Physiology, 106, 857–864. 10.1152/japplphysiol.91324.2008 [DOI] [PubMed] [Google Scholar]
- Mendoza T. R., Wang X. S., Cleeland C. S., Morrissey M., Johnson B. A., Wendt J. K., & Huber S. L. (1999). The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer, 85, 1186–1196. [DOI] [PubMed] [Google Scholar]
- Meyer D. E., & Schvaneveldt R. W. (1971). Facilitation in recognizing pairs of words: Evidence of a dependence between retrieval operations. Journal of Experimental Psychology, 90, 227–234. 10.1037/h0031564 [DOI] [PubMed] [Google Scholar]
- Mueller S. T. (2008). PEBL: The Psychology Experiment Building Language (Version 0.09). Retrieved from http://pebl.sourceforge.net
- Murphy S. L. (2009). Review of physical activity measurement using accelerometers in older adults: Considerations for research design and conduct. Preventive Medicine, 48, 108–114. 10.1016/j.ypmed.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. L., & Smith D. M. (2010). Ecological measurement of fatigue and fatigability in older adults with osteoarthritis. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 65, 184–189. 10.1093/gerona/glp137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. L., Smith D. M., Clauw D. J., & Alexander N. B. (2008). The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Care and Research, 59, 849–856. 10.1002/art.23710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlo D., & Richardson S. (1991). The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39, 142–148. [DOI] [PubMed] [Google Scholar]
- Posner M. I. (1976). Chronometric explorations of mind. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Radloff L. S. (1977). The CES–D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Schepens S. L., Kratz A. L., & Murphy S. L. (2012). Fatigability in osteoarthritis: Effects of an activity bout on subsequent symptoms and activity. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 67, 1114–1120. 10.1093/gerona/gls076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. (1982). Specific impairments of planning. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 298(1089), 199–209. 10.1098/rstb.1982.0082 [DOI] [PubMed] [Google Scholar]
- Smets E. M., Garssen B., Bonke B., & De Haes J. C. (1995). The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research, 39, 315–325. 10.1016/0022-3999(94)00125-O [DOI] [PubMed] [Google Scholar]
- Snijders T. A. B. (2005). Power and sample size in multilevel linear models. In Everitt B. S. & Howell D. C. (Eds.), Encyclopedia of statistics in behavioral science (Vol. 3, pp. 1570–1573). Chichester, England: Wiley. [Google Scholar]
- Stone A. A., Broderick J. E., Schwartz J. E., & Schwarz N. (2008). Context effects in survey ratings of health, symptoms, and satisfaction. Medical Care, 46, 662–667. 10.1097/MLR.0b013e3181789387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662. [Google Scholar]
- Wilkinson R. T., & Houghton D. (1975). Portable four-choice reaction time test with magnetic tape memory. Behavior Research Methods and Instrumentation, 7, 441–446. [Google Scholar]
- Wilkinson R. T., & Houghton D. (1982). Field Test of Arousal: A portable reaction timer with data storage. Human Factors, 24, 487–493. [DOI] [PubMed] [Google Scholar]