Abstract
Purpose
We previously created a self-assembled cartilage-like complex in vitro from only three cartilage components, hyaluronic acid (HA), aggrecan (AG) and type II collagen, without other materials such as cross-linking agents. Based on this self-organized AG/HA/collagen complex, we have created three novel types of biphasic cartilage and bone-like scaffolds combined with hydroxyapatite (HAP) for osteochondral tissue engineering. These scaffolds have been developed from self-assembled cartilage component molecules and HAP at the nanometer scale by manipulating the intermolecular relations.
Patients and methods
The surface structure of each self-organized biphasic cartilage and bone-like scaffold was evaluated by scanning electron microscopy, whereas the viscoelasticity was also analyzed in vitro. Three types of artificial cartilage–HAP conjugates were implanted into an osteochondral defect in rat knee joints, and bone and cartilage tissues of the implanted site were examined 4 and 8 weeks after implantation. The tissues were examined histopathologically to evaluate the effects of the implantation on the articular cartilage and subchondral bone tissues.
Results
Our in vitro and in vivo data reveal that the self-organized biphasic cartilage and bone-like scaffold conjugated with HAP are superior to the scaffold with no HAP in both cartilage regeneration and subchondral bone regeneration.
Conclusion
Our present study indicates that the self-organized biphasic cartilage and bone-like scaffold, which is conjugated with an HAP layer, may have potential not only to repair articular cartilage defects but also to ameliorate the degeneration of subchondral bone in the diseases with osteochondral defect.
Keywords: cartilage tissue engineering, subchondral bone, articular cartilage, osteoarthritis, self assembly, hydroxyapatite
Introduction
New technologies such as tissue engineering are now attracting attention for the reconstruction of joint tissues in cases of advanced stage joint degeneration and destruction, such as in traumatic injury and arthritis, including rheumatoid arthritis and osteoarthritis (OA).1–4 For bone tissue engineering, scaffolds made from the biomaterial hydroxyapatite (HAP) have already been so well devised that they have sufficiently high affinity and enough rigidity similar to bone to be usable for the repair of bone defects.5–7 It is well known that autologous and allograft bones show good osteoconductive power and biomechanical properties for the treatment of healing bone defect due to stable structure, little immunogenicity and osteogenic capacity. Also, it has been demonstrated that application of scaffolds, such as HAP, shows a good ability to facilitate bone repair and an osteogenic potential.7,8
In contrast to bone tissue, a suitable biomaterial for cartilage repair still remains to be developed. Such a material requires perfect cartilage-specific tissue qualities represented by properties such as high elasticity and high lubrication.9–11 Articular cartilage is a highly organized tissue composed primarily of proteoglycan (aggrecan [AG]), type II collagen and hyaluronic acid (HA) with a small amount of other proteins including elastin, type IX and type X collagen, which has poor spontaneous self-healing capacity.10,11 Cartilage tissue engineering using autologous chondrocyte implantation, scaffolds, growth factors or their combinations has been tried as a therapeutic strategy for cartilage repair.12–14 However, there are still several problems to be addressed in developing a strategy for cartilage regeneration.
The first problem to be overcome is the cell source limitation. Symptomatic full-thickness cartilage defects have been treated with autologous chondrocyte implantation.15,16 This strategy requires a two-stage process whereby the patient’s chondrocytes are culture-expanded in vitro to obtain a sufficient number and then implanted into the defect.17 In general, for autologous chondrocyte implantation, a patient’s chondrocytes are cultured in a collagen or agarose gel to form a three-dimensional biomaterial. To obtain enough chondrocytes, continuous cell culture over several passages is required.14,17 During passages in culture, chondrocytes are known to dedifferentiate and to lose chondrocyte-specific properties.2 To improve the availability of chondrocyte resources for cartilage tissue engineering, it has recently been demonstrated that differentiated chondrocytes can be induced from mesenchymal stem cells or induced pluripotent stem (iPS) cells.18,19 However, the risk of oncogenesis, such as teratoma, still remains during the differentiation of human somatic cells, including chondrocytes, from mesenchymal stem cells or iPS cells.20–22 Moreover, it takes several months for the tissue-engineered cartilage-like tissue to grow and become organized into mature cartilage even when grafted in the defect site.18,19 Further studies are needed to clarify whether mesenchymal stem cells and iPS cells are suitable as a cell source for cartilage tissue engineering.
The next problem to be considered is how to prevent the leakage of inoculated chondrocytes from the cartilage defect site. To contain cells at the defect site, it is necessary to patch over the defect with a membrane.15–17 Generally, autologous periosteum has been used as a covering membrane. However, periosteum may be inappropriate for this use, since it has the osteogenic capacity to promote ossification/calcification of covering membranes.23 To create an articular cartilage-like tissue applicable to clinical use in cartilage repair while overcoming the problems of cell supply and covering membranes, various kinds of biomaterials have been created as a scaffold for chondrocyte growth and cartilage regeneration.11,14
The third problem of cartilage tissue engineering is the regeneration of subchondral bone in OA. OA is nowadays one of the most frequent chronic diseases and, with the increase in life expectancy, both its prevalence and incidence are expected to rise. This condition is progressive and leads to functional decline and loss in quality of life, with important health care and society costs. Thus, it is important to develop the novel therapeutic strategy for osteoarthritic lesions.
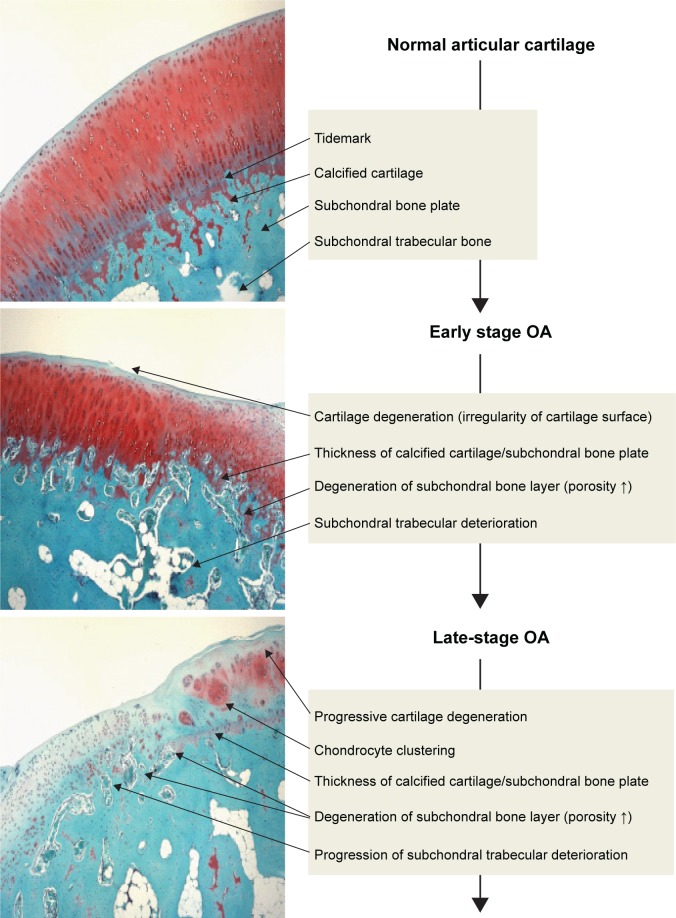
OA has been considered to primarily involve degeneration of articular cartilage. However, recently, subchondral bone deterioration has been widely recognized as a hallmark of OA.24–26 OA subchondral bone is known to be hypomineralized and to show abnormal bone metabolism, resulting in histopathological degeneration of subchondral bone (Figure 1).27,28 A functional joint unit comprising articular cartilage and subchondral bone may regulate the homeostasis and maintenance potential of articular cartilage against the progression of OA.29 Thus, histopathological changes of subchondral bone may be involved in the pathogenesis and pathophysiology of cartilage degeneration. Consequently, for cartilage repair in OA, regeneration of subchondral bone as well as articular cartilage is necessary. Although numerous reports have already demonstrated that novel technology, involving autologous chondrocyte implantation, tissue engineering using mesenchymal stem cells or iPS cells and biomaterials, may have therapeutic potential for cartilage repair, it still remains unclear whether implantation of autologous chondrocytes or recently developed biomaterials can repair the histopathological changes of subchondral bone in OA. If the biomaterial may have the potential to repair the osteochondral defect (chondrogenic and osteogenic potential), it may be a useful tool for severe OA with subchondral bone degeneration as well as cartilage degeneration.
Figure 1.
Osteochondral damage during the progression of OA.
Notes: OA subchondral bone is known to be hypomineralized and to show abnormal bone metabolism, resulting in histopathological degeneration of subchondral bone as well as cartilage damage. During progression of OA, the subchondral bone is also damaged as well as the articular cartilage layer. Injuries of either tissue adversely affect the mechanical environment as well as the homeostatic balance of the entire joint, resulting in the development of OA.
Abbreviation: OA, osteoarthritis.
We have recently created three types of self-organized biphasic cartilage and bone-like scaffolds combined with HAP, for use in osteochondral tissue engineering, which were developed through self-assembled cartilage component molecules and HAP at the nanometer scale by manipulating intermolecular relations. In the current study, we demonstrate that it is possible to create a novel self-organized biphasic cartilage and bone-like scaffold combined with an HAP layer for osteochondral tissue repair, with high performance as well as microstructures comparable to hyaline articular cartilage and subchondral bone in vitro and in an in vivo rat model.
Patients and methods
Scaffolds for osteochondral tissue engineering
In the present study, we prepared three types of self-assembled artificial cartilage scaffolds: 1) self-organized cartilage-like scaffold; 2) self-organized biphasic cartilage and bone-like scaffold combined with an HAP bone block; and 3) self-organized biphasic cartilage and bone-like scaffold coated with HAP powder.
Self-organized cartilage-like scaffold formed from HA, proteoglycan and type II collagen
Solutions were generated by mixing proteoglycan (AG; Sigma-Aldrich Co., St Louis, MO, USA) at a final concentration of 1.0 mg/mL and HA (molecular weight: 280 × 106 Da; Chugai Pharmaceutical, Inc., Tokyo, Japan) at a final concentration of 15% in distilled–deionized water (DDW) at room temperature. Human type II collagen (Collagen Research Center, Inc., Tokyo, Japan) was dissolved in DDW at a final concentration of 0.6 mg/mL at room temperature. The AG + HA solution and the collagen solution were adjusted to pH 9.0. Then, equal volumes of the AG + HA and the collagen solutions were mixed at pH 9.0 and incubated at 37°C in a humidified 95% air and 5% CO2 atmosphere for 5 hours. After incubation, the mixture was centrifuged for 30 minutes at 50,000 × g and then specimens of the self-organized AG/ HA/collagen complex were prepared for each experiment.
Formation of the self-organized biphasic cartilage and bone-like scaffold combined with an HAP bone block
We have newly created the self-organized biphasic cartilage and bone-like scaffold combined with an HAP bone block. The self-organized AG/HA/collagen complex was formed by the method described earlier, and then the complex was placed onto an HAP block (homogeneous HAP, porous disk-like block: pore size 100–300 µm, diameter 4 mm × height 2 mm; HOYA Technosurgical, Corp., Tokyo, Japan) and centrifuged at 50,000 × g for 30 minutes. Specimens of the self-organized AG/HA/collagen complex combined with the HAP block were then prepared for each experiment.
Formation of the self-organized biphasic cartilage and bone-like scaffold coated with HAP powder
We prepared two types of self-organized biphasic cartilage and bone-like scaffold coated with HAP powder (particle size: 40 nm and 5 µm). It has been reported that material components with hydroxyl groups connect to HAP powders by dehydration condensation between each hydroxyl group.30,31 HAP powders were kindly donated by SofSera Co. Ltd., Osaka, Japan) and suspended in DDW (1.0 mg/mL). The self-organized AG/HA/collagen complex was formed by the method described earlier, then carefully put into a 1.0 mg/mL suspension of one of the HAP powders and incubated at 4°C overnight. Only the bottom surface of the complex was coated with the HAP solution. Specimens of the self-organized AG/HA/collagen complex coated with HAP powder were then prepared for each experiment.
After the formation of each self-assembled complex, the complex was adjusted by PBS for the adaptation of the in vitro biomaterial to the in vivo microenvironment. The complex was incubated at 37°C in a humidified 95% air and 5% CO2 atmosphere, to adapt itself to in vivo condition.
Viscoelasticity of the self-organized biphasic cartilage and bone-like scaffold
The viscoelasticity (compression modulus) of the self-assembled scaffolds described earlier was measured using an elasticity measuring device (Reogel E1500; UBM Ltd., Kyoto, Japan; experimental conditions: temperature 37°C [35°C–40°C], 10 Hz, equipment configuration [parallel plate]). The scaffold was air-dried overnight using a drying chamber. After complete drying of the complex, the viscoelasticity (compression modulus) of the dried complex was also evaluated using an elasticity measuring device. The dried scaffold was rehydrated with sterilized saline. Then, the viscoelasticity (compression modulus) of the rehydrated complex was again analyzed in the same manner.
Chondrocyte culture in the self-organized scaffold
Human articular cartilage tissue samples were obtained from the knee joint of a patient with OA during arthroplastic knee surgery after obtaining informed consent (male, 79 years). The study protocol was reviewed and approved by the local ethics committee of St Marianna University School of Medicine (permission number: 1315). The procedures followed were in accordance with the ethical standards of the ethics committee and with the Declaration of Helsinki, 1975, as revised in 2000. The patient gave written informed consent before participating in the study. The severity of knee OA was evaluated by the Kellgren and Lawrence classification and was classified as grade 4.32,33 Articular cartilage explants were cut into small pieces, washed with PBS and digested with 1.5 mg/mL collagenase B (Sigma-Aldrich Co.) in DMEM (Dako Denmark A/S, Glostrup, Denmark) overnight on a shaking platform at 37°C. Isolated chondrocytes were collected following centrifugation, washed three times with PBS, resuspended and cultured in DMEM supplemented with 10% heat-inactivated fetal calf serum, 2 mM L -glutamine, 25 mM 2[4-(2-hydroxyethyl)–1-piperazinyl] ethane sulfonic acid (HEPES) and 100 U/mL penicillin and streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2 as previously reported.2
The self-organized AG/HA/collagen complex was formed by the method described earlier. Each complex was washed three times with PBS and then the complex was transferred into DMEM. Human chondrocytes were suspended in the DMEM with each self-organized complex at a cell density of 5×104/mL followed by slight shaking, and the complex was incubated at 37°C in a humidified 95% air and 5% CO2 atmosphere. After incubation for 1 or 2 weeks, some of the complexes were fixed for 2 days in 4% paraformaldehyde solution. Specimens of self-assembled complexes conjugated with chondrocytes were prepared for scanning electron microscopy (SEM) study.
Scanning electron microscopy
SEM analysis was performed to verify the surface structure of scaffolds. After serial fixations of scaffold samples with 2% glutaraldehyde, 1% osmium tetroxide and 1% tannic acid aqueous solution, the surface morphology of the scaffolds was observed under a scanning electron microscope (S-4800; Hitachi Ltd., Tokyo, Japan).
Implantation of the self-organized scaffolds for cartilage repair
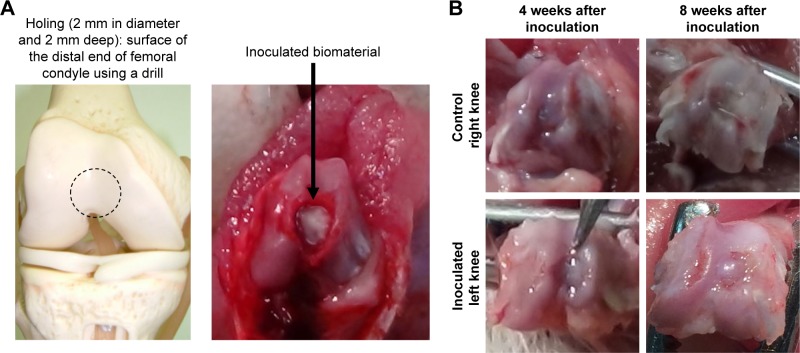
The animal study protocol was reviewed and approved by the Animal Care and Use Committee of St Marianna University School of Medicine (permission number: 1708019). The procedures followed were in accordance with the fundamental guidelines for proper conduct of animal experiment and related activities in academic research institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology in Japan. Healthy, 9–11-week old male Sprague Dawley (SD) rats (n=30) were used (Clea Japan Inc., Tokyo, Japan). Hair on the knee joints of bilateral hind limbs was shaved under anesthesia, and the skin surface was disinfected with 70% ethanol (Wako Pure Chemical Industries, Ltd., Osaka, Japan). After disinfection, a skin incision was made in the surgical site over the knee joint. The articular capsule was incised using an external juxta-articular approach (medial approach), and the knee joint was exposed by everting the patella. A hole (2 mm in diameter and 2 mm deep) was made in the surface of the distal end of the left femoral condyle using a 2-mm diameter drill, and a piece of the test scaffold was implanted in the operated animals of 4- and 8-week observation groups (the self-organized AG/HA/collagen complex alone-inoculated group: n=3, each self-organized AG/HA/collagen complex-HAP group: n=4). After implantation, the surgical site was rinsed with physiological saline (Otsuka Pharmaceutical Factory Inc., Tokyo, Japan), and the incised skin was sutured. In each animal, the contralateral side was also subjected to the same surgical manipulation, but no test material was implanted as a control.
The general condition of all rats was observed once a day during the experimental period. Rats were sacrificed by exsanguination from bilateral femoral arteries under anesthesia in a painless manner at 4 or 8 weeks after surgery. After euthanasia, the tissues including the implanted material were sampled and macroscopically observed. Tissues were fixed with 10% neutral-buffered formalin, then decalcified with EDTA, embedded in paraffin by a routine procedure and cut into 6 µm sections. The sections were stained with H&E for histological assessment of the general morphology of the repair tissue, or Safranin O/fast green (SO/FG) and toluidine blue, to indicate glycosaminoglycan content as per standard protocols.34
Histological assessment
Sections were analyzed under light microscopy to examine collagen fiber orientation and to distinguish between hyaline cartilage and fibrocartilage. The histological quality of the repair tissue was assessed and scored using the International Cartilage Repair Society (ICRS) II VAS (each of 14 parameters scored 0%–100%),35,36 assessing the parameters listed in Table 1. It has been demonstrated that ICRS II may be most adequate for analysis of tissue-engineered and repaired cartilage and that ICRS II represents an improvement over the current histological cartilage repair scoring system, although various histologic scoring systems exist for tissue-engineered, repaired and osteoarthritic cartilage.36
Table 1.
International Cartilage Repair Society II scoring system for cartilage repair
| Histological parameters | Scores |
|---|---|
| 1. Tissue morphology | 0%: full-thickness collagen fibers, 100%: normal cartilage |
| 2. Matrix staining (metachromasia) | 0%: no staining, 100%: full metachromasia |
| 3. Cell morphology | 0%: no round/oval cells, 100%: mostly round/oval cells |
| 4. Chondrocyte clustering (four or more grouped cells) | 0%: present, 100%: absent |
| 5. Surface architecture | 0%: delamination, or major irregularity, 100%: smooth surface |
| 6. Basal integration | No integration, 100%: complete integration |
| 7. Formation of a tidemark | 0%: no calcification front, 100%: tidemark |
| 8. Subchondral bone abnormalities/marrow fibrosis | 0%: abnormal, 100%: normal marrow |
| 9. Inflammation | 0%: present, 100%: absent |
| 10. Abnormal calcification/ossification | Present, 100%: absent |
| 11. Vascularization (within the repaired tissue) | Present, 100%: absent |
| 12. Surface/superficial assessment | 0%: total loss or complete disruption, 100%: resembles intact articular cartilage |
| 13. Mid/deep zone assessment | 0%: fibrous tissue, 100%: normal hyaline cartilage |
| 14. Overall assessment | 0%: bad (fibrous tissue), 100%: good (hyaline cartilage) |
Results
A self-organized cartilage-like complex formed from HA, proteoglycan (AG) and type II collagen
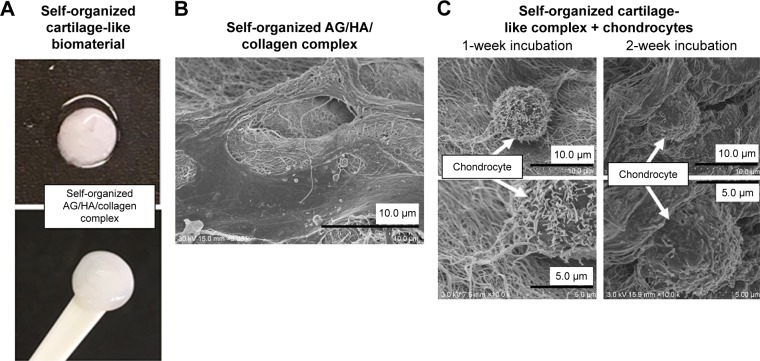
Our previous study of the self-organized AG/HA/collagen complex revealed that it has a nanocomposite structure in which the fibrous collagen formed is bound to AG linking to HA, resembling cartilage tissue, although the original collagen gel was merely a gel form of collagen branches having no solid tissue structure.2,37 In a similar manner, we created a self-assembled cartilage-like complex from only three cartilage components, HA, AG and type II collagen in vitro, without any other materials such as cross-linking agents (Figure 2A). Analysis with SEM revealed a fibrous and three-dimensional reticulate structure (Figure 2B).
Figure 2.
Self-organized cartilage-like scaffold formed from HA, AG and type II collagen for articular cartilage tissue engineering.
Notes: (A) Macroscopic image of self-organized cartilage-like scaffold. (B) The structure of the self-organized AG/HA/collagen complex was observed by SEM. The topological structure of the complex had a fibrous appearance. (C) After 1- and 2-week incubation of chondrocytes with the self-organized AG/HA/collagen complex, chondrocytes were present on the scaffold of fibers forming the complex.
Abbreviations: AG, aggrecan; HA, hyaluronic acid; SEM, scanning electron microscopy.
Observation of the self-organized complex implanted with chondrocytes
After incubation of the self-organized AG/HA/collagen complex with chondrocytes for 1 or 2 weeks, the complex remained dense and the chondrocytes were homogeneously present on the scaffold of fibers forming the complex. Cytoplasmatic extensions denominated the phylopodia, on the surface of the self-organized scaffolds, indicating that chondrocytes were alive within the tissue after the 2-week incubation (Figure 2C).
Self-organized biphasic cartilage and bone-like scaffold combined with an HAP bone block
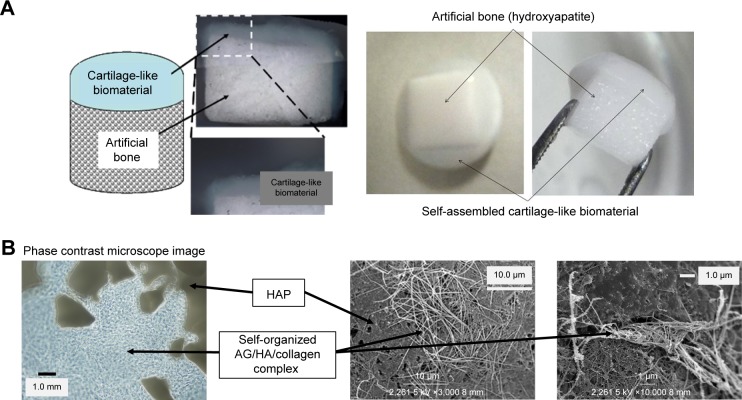
A self-organized biphasic cartilage and bone-like scaffold combined with an HAP bone block was obtained by self-organization of the AG/HA/collagen complex on the HAP block in vitro (Figure 3). The self-organized AG/HA/ collagen complex bound to the HAP bone block strongly. SEM analysis revealed that fibers of the AG/HA/collagen complex penetrated the interior of multiple pores of the HAP block.
Figure 3.
Self-organized biphasic cartilage and bone-like scaffold combined with an HAP bone block.
Notes: (A) Macroscopic image of our self-organized cartilage-like scaffold combined with an HAP block. (B) The structure of the self-organized cartilage-like scaffold combined with an HAP bone block was observed by SEM.
Abbreviations: HAP, hydroxyapatite; SEM, scanning electron microscopy.
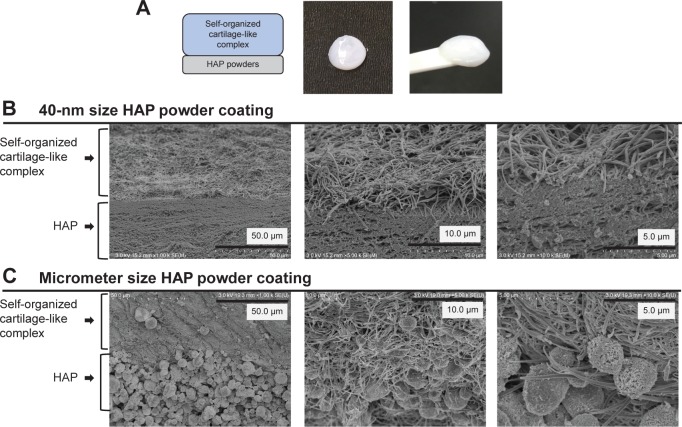
Self-assembled artificial cartilage–HAP powder conjugate
One side of the self-organized AG/HA/collagen complex was coated with an HAP powder of either 40 nm or 5 µm particle size (Figure 4A). We confirmed by SEM that both sizes of HAP powder were regularly bound to self-organized cartilage-like complexes in the form of laminae (Figure 4B: 40-nm powder size coating group, Figure 4C: 5-µm powder size coating group).
Figure 4.
Self-assembled artificial cartilage–HAP powder conjugate.
Notes: (A) Diagram illustrating the formation of a self-assembled artificial cartilage–HAP powder conjugate and its macroscopic image. (B) The structure of the 40-nm size HAP powder coating complex was observed by SEM. (C) The structure of the 5-µm size HAP powder coating complex was observed by SEM.
Abbreviations: HAP, hydroxyapatite; SEM, scanning electron microscopy.
Viscoelasticity of self-organized biphasic cartilage and bone-like scaffolds
Nondried scaffolds
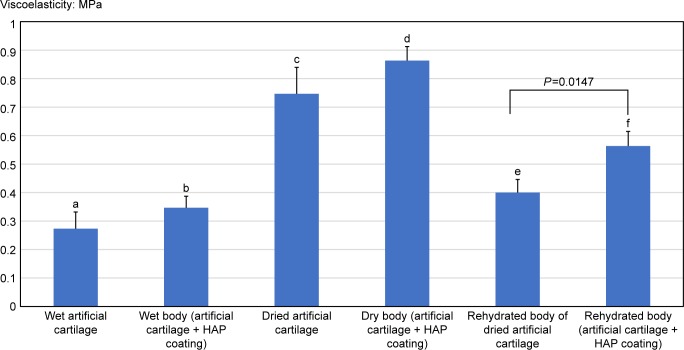
Viscoelasticity (compression modulus) of the nondried (wet) self-organized AG/HA/collagen complex and the nondried self-assembled complex–HAP powder conjugate waŝ0.273 and 0.347 MPa, respectively. Although no significant difference in the viscoelasticity was observed between the self-organized AG/HA/collagen complex and the self-assembled complex–HAP powder conjugate, the mean viscoelasticity (compression modulus) was higher in the self-assembled complex–HAP powder conjugate (Figure 5, b: 0.347 MPa) than in the self-organized AG/HA/collagen complex (Figure 5, a: 0.273 MPa).
Figure 5.
Viscoelasticity of self-organized biphasic cartilage and bone-like scaffold.
Notes: Only the artificial cartilage and the HAP-coated cartilage were measured and compared in terms of viscoelasticity by dividing it into three groups of wet, nondried and rehydrated. A significant difference between the two scaffolds was observed after rehydration. There were significant differences in the viscoelasticity between two groups as following: a vs c: P<0.01, b vs d: P<0.01, a vs e: P<0.01, and b vs f: P<0.01.
Abbreviation: HAP, hydroxyapatite.
Dried scaffolds
The viscoelasticity (compression modulus) of both dried scaffolds (dried self-organized AG/HA/collagen complex and dried self-assembled complex–HAP powder conjugate) was significantly higher than that of their respective nondried original scaffolds (nondried self-organized AG/HA/collagen complex and nondried self-assembled complex–HAP powder conjugate) (Figure 5, a vs c: P<0.01, b vs d: P<0.01). Although no significant difference in viscoelasticity was observed between the dried self-organized AG/HA/collagen complex and the dried self-assembled complex–HAP powder conjugate, the viscoelasticity of the dried self-assembled complex–HAP powder conjugate tended to increase (Figure 5, d: 0.863 MPa) in comparison with the dried self-organized AG/HA/collagen complex (Figure 5, c: 0.747 MPa).
Rehydrated scaffolds
The mean viscoelasticity (compression modulus) of the rehydrated self-assembled complex–HAP powder conjugate (Figure 5, e: 0.563 MPa) was significantly higher than that of the rehydrated self-organized AG/HA/collagen complex (Figure 5, f: 0.4 MPa) (P=0.0147). Interestingly, viscoelasticity of both rehydrated scaffolds (rehydrated self-organized AG/HA/collagen complex and rehydrated self-assembled complex–HAP powder conjugate) was significantly higher than that of the respective nondried original scaffolds (nondried self-organized AG/HA/collagen complex and nondried self-assembled complex–HAP powder conjugate) (Figure 5, a vs e: P<0.01, b vs f: P<0.01).
Scaffold implantation into knee cartilage defects
In the preliminary experiments for the present study, we recognized the chondrogenic potential of our self-organized cartilage-like scaffolds that were inoculated into the osteochondral defect of animals. The findings of SO-positive cytoplasmic staining in cells with rounded/ spheroidal morphology and typical cartilage lacuna formation indicated the chondrogenic activity of the scaffold in vivo (supplemental preliminary data). Based on these preliminary findings, we have designed the present in vivo study to evaluate the repair of osteochondral defect by the self-organized scaffold.
Life span of rats used for in vivo experiments is about 2–4 years. If converted to human life span, 1 year of rats’ life span would be equivalent to 20–40 years in human. Therefore, it is thought that 4 and 8 weeks of rats may be equivalent to about 1.6–3 years and 4–6 years in human, respectively. From the point of view of clinical and physiological relevance, to evaluate whether the scaffold may have a useful tool for osteochondral tissue engineering, we think that 4 and 8 weeks as time points for the in vivo experiment are suitable for period of observation.
In control right knee joints, defects were filled with scar tissue and there were clefts between the scar tissue and the healthy surrounding cartilage tissue in both the 4- and 8-week observation groups. In contrast, in the left knee joints, the defect was filled with repair tissue that was similar to the surrounding cartilage tissue.
Sham-operated right knee joints (without implantation of scaffold)
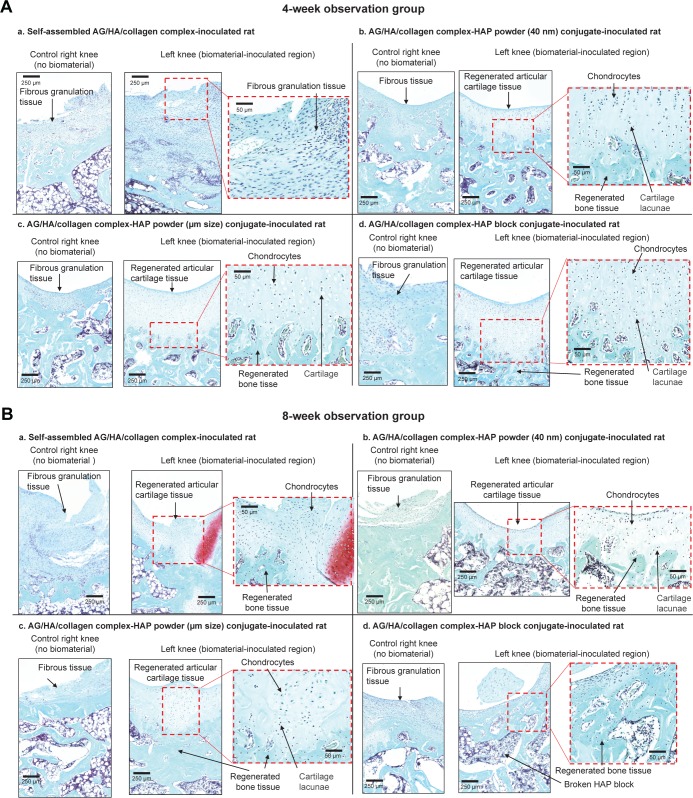
In most sham-operated right knee joints, only fibrous scar tissue with hyperplasia of spindle-shaped fibroblastic cells was observed in the defect, with no regeneration of the cartilage tissue in either the 4- or the 8-week observation group (Figure 6A and B).
Figure 6.
Safranin staining cross-sections of repaired articular cartilage and subchondral bone tissues in knee joins at 4 and 8 weeks after scaffold implantation.
Notes: (A) Representative images of control right knee joints and scaffold-implanted left knee joints at 4 weeks after scaffold implantation (control groups: n=3, each scaffold inoculated group: n=4). (B) Representative images of control right knee joints and scaffold-implanted left knee joints at 8 weeks after scaffold implantation (control groups: n=3, each scaffold-inoculated group: n=4).
Abbreviations: AG, aggrecan; HA, hyaluronic acid; HAP, hydroxyapatite.
Scaffold-implanted left knee joints (4-week observation groups)
As shown in the representative images in Figure 6A, in the 4-week observation group, a stratiform cartilage-like structure with formation of cartilage cavities and the growth of chondrocytes was observed in the scaffold implantation hole, indicating initiation of cartilage regeneration induced by the implanted scaffold. In some implantation sites, there was a depression of the surface layer in the center of the drilled hole. Around this depressed surface layer, poor differentiation of chondrocytes and a low level of SO-stained tissue, indicating the low level of proteoglycan production by chondrocytes, were observed.
The success rates of osteochondral tissue regeneration were 33% (1 of 3 knees) in the self-organized AG/HA/ collagen complex-inoculated group, 50% (2 of 4 knees) in the self-assembled AG/HA/collagen complex–HAP powder (40 nm size) conjugate-inoculated group, 75% (3 of 4 knees) in the self-assembled AG/HA/collagen complex–HAP powder (5 µm size) conjugate-inoculated group and 100% (3 of 3 knees, 1 sample was broken) in the self-assembled AG/HA/ collagen complex–HAP block conjugate-implanted group.
Scaffold-implanted left knee joints (8-week observation groups)
As shown in the representative images in Figure 6B, in the 8-week observation group, the growth of chondrocytes, the formation of cartilage cavities and a stratiform cartilage-like structure were observed in the scaffold implantation hole, indicating initiation of osteochondral tissue regeneration induced by the implanted biomaterial.
The success rates of osteochondral tissue regeneration were 33% (1 of 3 knees) in the self-organized AG/HA/ collagen complex-inoculated group, 75% (3 of 4 knees) in the self-assembled AG/HA/collagen complex–HAP powder (40 nm size) conjugate-inoculated group and 75% (3 of 4 knees) in the self-assembled AG/HA/collagen complex–HAP powder (5 µm size) conjugate-inoculated group. Unfortunately, when the tissue was cut into 6 µm sections, most of the HAP block parts were broken in the self-assembled AG/HA/collagen complex–HAP block conjugate-implanted group.
Histological assessment of osteochondral tissue regeneration
The histological quality of osteochondral tissue regeneration by scaffolds was evaluated by the ICRS II scoring system (Table 1). The ICRS II scoring system has the potential to evaluate both levels of ossification/classification and subchondral abnormality as well as articular cartilage abnormality. A higher score in the ICRS II scoring system represents tissue better resembling healthy articular cartilage.
The 4-week observation groups
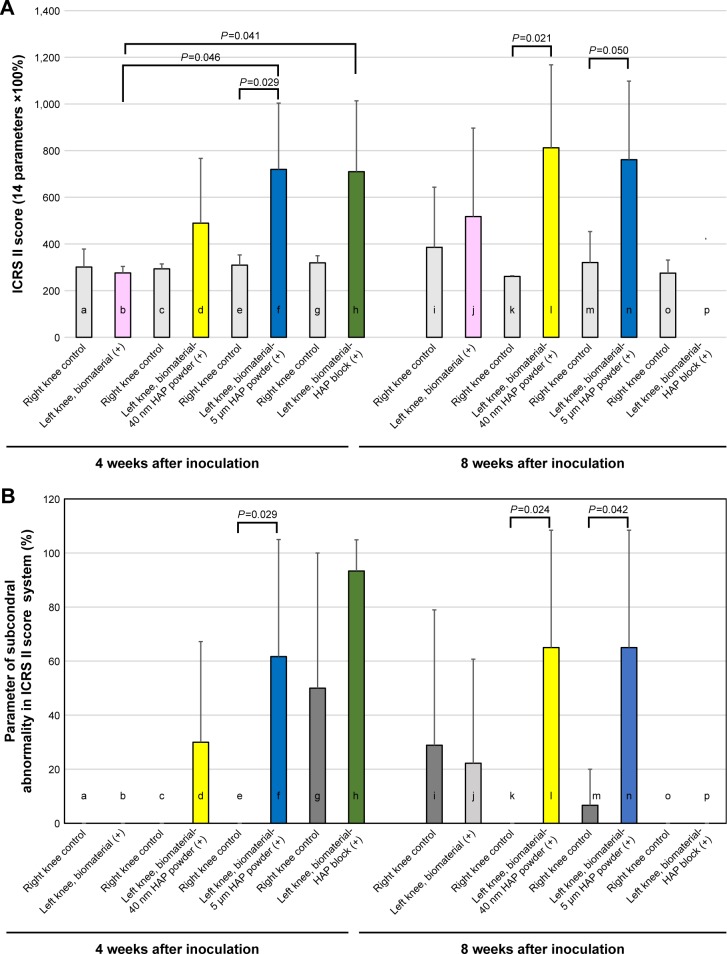
There was no significant difference in the ICRS II score between the control right knee group and the self-organized AG/HA/collagen complex-inoculated group (Figure 7A, a vs b: P=0.621). In contrast, the score of the self-assembled AG/HA/collagen complex–HAP powder (5 µm size) conjugate-inoculated group was significantly higher than that of the control right knee group (Figure 7A, e vs f: P=0.029). There was a tendency to increase the mean IRCS II score of the self-assembled AG/HA/collagen complex–HAP powder (40 nm size) conjugate-inoculated group and the self-assembled AG/HA/collagen complex–HAP block conjugate-inoculated group in comparison with each control, although no significant difference was found in the score between the scaffold-inoculated group and the control.
Figure 7.
Histological assessment of osteochondral tissue regeneration by ICRS II scoring system.
Notes: (A) The histological quality of cartilage regeneration by scaffolds was evaluated by the ICRS II scoring system (Table 1). A higher score in the ICRS II scoring system represents tissue resembling healthy articular cartilage (control groups: n=3, each scaffold-inoculated group: n=4). (B) Histological assessment of subchondral bone regeneration was evaluated using parameters for subchondral abnormality in the ICRS II scoring system (control groups: n=3, each scaffold-inoculated group: n=4).
Abbreviations: HAP, hydroxyapatite; ICRS, International Cartilage Repair Society.
Among the four types of our scaffolds, the self-assembled AG/HA/collagen complex–HAP powder (5 µm size) conjugate-inoculated group and the self-assembled AG/HA/ collagen complex–HAP block conjugate-inoculated group were superior to the only self-assembled AG/HA/collagen complex-inoculated group in terms of the ICRS II score (Figure 7A, b vs f: P=0.0046, b vs h: P=0.041). The score of the self-assembled complex–HAP powder (40 nm size) conjugate-inoculated group was higher than that of the only self-assembled complex-inoculated group, although no significant difference was observed between the two groups (Figure 7A, b vs d: P=0.252).
The 8-week observation groups
There were significant differences in ICRS II score between the control right knee group and the self-organized AG/HA/ collagen complex–HAP powder (40 nm size) conjugate-inoculated group (Figure 7A, k vs l: P=0.021). There was a tendency to increase the mean ICRS II score of the self-assembled AG/HA/collagen complex–HAP powder (5 µm size) conjugate-inoculated group in comparison with their respective controls (Figure 7A, m vs n: P=0.05). In contrast, no significant difference was observed in the score between the self-assembled AG/HA/collagen complex-inoculated group and the control (Figure 7A, i vs j: P=0.645). Regarding the self-organized AG/HA/collagen complex–HAP block, most of the HAP block parts were broken, when the scaffold inoculated tissue was cut in 6 µm sections. Therefore, histological analysis by ICRSII score was not impossible in the self-organized AG/HA/collagen complex–HAP block (Figure 7B, p).
Among the three types of scaffolds (except for the self-organized AG/HA/collagen complex–HAP block), the mean ICRS II score of both self-assembled AG/HA/ collagen complex–HAP powder conjugate-inoculated groups (40 nm and 5 µm powder sizes) was superior to the only self-assembled AG/HA/collagen complex-inoculated group, although no significant differences were observed between each scaffold-inoculated group and their respective control group (Figure 7A, j vs l: P=0.339, j vs n: P=0.408).
Histological assessment of subchondral bone regeneration (using the parameter for subchondral abnormality in the ICRS II scoring system)
The histological quality of subchondral bone abnormality/ bone marrow fibrosis in tissue regenerated by scaffolds was assessed using the parameter for evaluation of subchondral bone abnormalities in the ICRS II scoring system (Table 1, histological parameter number 8). A higher score in this system indicates tissue resembling healthy subchondral bone.
In both the 4- and 8-week observation groups, most sham-operated right knee joints had a very low ICRS II mean score (Figure 7B, a, c, e, k and o: mean score 0%; g, i and m: mean score <30%; h: mean score 50%), indicating that the subchondral bone was not regenerated in control knees with no scaffolds.
Most of the knees implanted with the self-assembled AG/HA/collagen complex alone showed no regeneration of subchondral bone at either 4 or 8 weeks (Figure 7B, b and j). In contrast, the groups implanted with both self-organized AG/HA/collagen complex–HAP powder conjugates (both 40 nm and 5 µm powder size) showed significantly higher ICRS II scores in comparison with their respective controls at both 4 and 8 weeks (Figure 7B, e vs f: P=0.029, k vs l: 0.024, m vs n: P=0.042, but not significant, c vs d: P=0.158).
The mean score of the self-assembled AG/HA/collagen complex–HAP block conjugate-inoculated group did show a tendency to increase in comparison with their respective controls, although no significant differences were observed between the self-assembled AG/HA/collagen complex–HAP block-implanted group and the control (Figure 7B, g vs h: P=0.217). Histological analysis by ICRSII score was not impossible in the self-organized AG/HA/collagen complex– HAP block, because of the breaking down of the inoculated tissue (Figure 7B, p).
Among the four types of scaffolds, both the self-assembled AG/HA/collagen complex–HAP powder conjugates (40 nm and 5 µm powder sizes) were superior to the self-assembled AG/HA/collagen complex alone in the mean score of parameter for subchondral abnormality at both 4 and 8 weeks (Figure 7B).
Discussion
OA is a common age-related disease with complex pathophysiology, in which the articular cartilage, the underlying bone and other joint tissues are altered.38,39 Recently, the role of subchondral bone has attracted increasing interest for the pathogenic processes of osteochondral joint damage in OA.25–29,40 Subchondral bone is a complex structure with a subchondral plate and underlying trabeculae, which is biomechanically and biochemically connected to overlying cartilage.26,27,40 The functional conditions of articular cartilage and subchondral bone are tightly connected.29,41,42 During progression of OA, the subchondral bone is also damaged as well as the articular cartilage layer. Injuries of either tissue adversely affect the mechanical environment as well as the homeostatic balance of the entire joint, resulting in the development of OA (Figure 1). Strong evidence has demonstrated that subchondral bone degeneration is associated with damage of articular cartilage in OA.29,42 Therefore, tissue engineering approaches for articular cartilage repair should be accompanied by an adequate restoration of the underlying subchondral bone.
In the present study, to clarify the chondrogenic and osteogenic potential of our scaffolds, we studied it using the osteochondral defect model of rat knee joints. Indeed, this animal model is not representative of osteoarthritic lesions (degenerations of articular cartilage and subchondral bone tissues). Further studies are needed to clarify whether our scaffolds may have the potential to treat the osteoarthritic lesion. Based on our results in the present study, we would like to expand the study for the potential application of the self-assembled scaffold for OA with subchondral bone degeneration as well as cartilage degeneration. If the scaffold may have the potential to repair the osteochondral defect (chondrogenic and osteogenic potentials), it might be a useful tool for osteochondral defect lesions including severe OA with subchondral bone degeneration in addition to cartilage degeneration (supplemental preliminary data). We will study the potential of our scaffold using the OA animal model. In the present study, we demonstrate for the first time that it is possible to create a self-organized biphasic cartilage and bone-like scaffold combined with HAP to repair both the articular cartilage and the subchondral bone, with high performance as well as microstructures comparable to hyaline articular cartilage and subchondral bone.
Previously, we observed that the HA–AG/type II collagen self-organized complex consisted of 10–500 µm long (2–50 nm diameter) collagen fibers at pH 6–10.2,34 HA retains AG molecule in the matrix through specific protein–HA interactions. It is thought that HA forms a scaffold for binding large chondroitin sulfate proteoglycans (AG) in the articular cartilage tissue. HA with superior water-holding ability (2–6 L at 1 g HA) keeps water in the articular cartilage (60%–70% water/wet weight of cartilage tissue).10,11 Thus, in our study, we used HA as a component for the self-organized cartilage-like complex as well as AG and type II collagen. Our previous in vivo data revealed that the self-assembled AG/HA/collagen complex was able to induce regeneration of articular cartilage tissue by acting as a scaffold to maintain chondrocyte characteristics in the cartilage defect model.2
In the present study, we inoculated the self-organized complex with no human cells (chondrocytes). The aim of our in vivo study was to clarify whether the self-organized complex without cell source can repair the degeneration of articular cartilage and subchondral bone (osteochondral defect). The results indicated that our self-organized biphasic cartilage and bone-like scaffold can induce cartilage and subchondral tissue regeneration in an in vivo osteochondral defect rat model (Figures 6–8). Of course, in the in vitro study, we analyzed whether chondrocytes can survive and maintain into the complex or not. As shown in Figure 2C, after incubation of our self-organized AG/HA/collagen complex with chondrocytes for 2 weeks, the chondrocytes, with cytoplasmatic extensions denominated the phylopodia, were homogeneously present on the scaffold of fibers forming the complex. The result from in vitro experiment showed that the complex was environment suitable for growth and survival of chondrocytes.
Figure 8.
Implantation of self-organized scaffold for cartilage and subchondral bone tissue repair.
Notes: (A) Representative images: creation of the defect (2 mm in diameter and 2 mm deep) in the surface of the distal end of the femoral condyle using a drill. Implantation of the scaffold for osteochondral tissue repair. (B) Macroscopic images of the defect 4 and 8 weeks after implantation were compared between control and implanted groups.
Numerous reports have already demonstrated that cartilage tissue engineering, which involves optimized combination of novel scaffolds, cell sources (mesenchymal stem cells, iPS cells) and growth factors, is a suitable therapeutic strategy for cartilage repair.10–14 However, it remains unclear whether these self-organized scaffolds for cartilage repair have the potential to repair the degenerated subchondral bone in OA. In addition, our previous study did not test whether our previous scaffolds were able to induce regeneration of the subchondral bone layer in OA.2
In the current study, we have created three types of cartilage-like scaffolds combined with HAP, for osteochondral tissue engineering, which have been developed by combining self-assembled cartilage component molecules (AG, HA and type II collagen) and HAPs (40 nm powder size HAP [Figure 4B], 5 µm powder size HAP [Figure 4C] or an HAP block [Figure 3]) at the nanometer scale by manipulating intermolecular relations. As shown in Figure 3, both HAP powders with 40 nm and 5 µm diameter size were regularly bound to the self-organized AG/HA/collagen complex in the form of laminae. We postulated that layered HAP on the self-organized scaffolds may induce not only cartilage tissue regeneration but also regeneration of the subchondral bone layer in a degenerated osteochondral lesion in the diseases with osteochondral defect. In the present study, histological evaluation using a cartilage repair scoring system (ICRS II) indicates that the self-organized cartilage-like scaffolds conjugated with an HAP layer are superior to the self-organized cartilage-like scaffolds with no HAP in terms of cartilage regeneration, with new cartilage tissue resembling normal cartilage morphology with a healthy surface and mid/ deep architecture and healthy chondrocyte morphology. In addition, evaluation of parameters of subchondral abnormality using the ICRS II scoring system revealed that the self-organized cartilage-like scaffolds conjugated to an HAP layer, but not the self-organized cartilage-like scaffolds without HAP, can induce regeneration of the subchondral bone layer comparable to normal subchondral bone tissue.
Our present study clearly provides evidence that the cartilage-like scaffold, without conjugated HAP, is not enough to regenerate damaged subchondral bone tissue, which is functionally connected with articular cartilage. As mentioned earlier, our cartilage-like scaffold without HAP may have only weak potential to induce complete osteochondral tissue regeneration. Indeed, although the self-organized cartilage-like AG/HA/collagen complex alone (without HAP) showed a tendency to induce articular cartilage regeneration in vivo, even at 8 weeks after implantation, no significant difference in the ICRS II score for cartilage repair was observed between the group implanted with the self-organized AG/HA/collagen complex and its control (Figure 7A). In contrast, the self-organized AG/HA/collagen complexes with an HAP layer were superior to the self-organized AG/HA/collagen complex alone without an HAP layer in terms of articular cartilage regeneration as well as subchondral bone regeneration (Figure 7A and B). Unfortunately, when the tissue was cut into 6 µm sections, most of the HAP block parts were broken in the self-assembled AG/HA/collagen complex–HAP block conjugate-implanted group. Histological analysis by ICRSII score was not possible in the self-organized AG/HA/collagen complex–HAP block. Therefore, it still remains unknown which is superior in the scaffold with HAP powders and the scaffold with HAP block. Also, we have studied the level of ossification/calcification (parameter no 10 in Table 1) of the scaffold-inoculated knee joints and the control knee joints. The level of the ossification/calcification was significantly lower in the scaffold-inoculated group than in each control (data not shown). Numerous reports and reviews have already demonstrated that biomaterials for cartilage repair are very usable for the intervention against cartilage defects.10–19,37,42 However, there are few reports concerning whether biomaterials can induce regeneration following subchondral bone degeneration or not. Further studies are needed to verify the exact mechanism and process of cartilage regeneration by biomaterials. We would like to conclude that an HAP layer may be necessary for scaffolds to repair the degeneration of articular cartilage and subchondral bone tissues in OA.
The viscoelasticity of mature articular cartilage is known to be 1.0–3.0 MPa.43 Regarding the viscoelasticity of our self-organized scaffold, the viscoelasticity of the complete dried scaffold (about 0.8–0.9 MPa) was higher than that of the original (~0.3 MPa). Interestingly, the viscoelasticity of rehydrated scaffold (~0.5–0.6 MPa) was significantly higher than that of the original, but its viscoelasticity was lower than that of the dried scaffold. We assumed a dried scaffold to be control of a wet scaffold. At a clinical place, it may be easy to sterilize a dried material in comparison with a wet material. We supposed that we could use the rehydrated complex from the sterilized and dried complex in clinic. Therefore, we analyzed three types of the self-organized complex (wet, dried and rehydrated complex) to obtain basic data of scaffolds. Although further studies are needed to clarify the usefulness of the rehydrated complex from dried one, a rehydrated complex may be useful as a scaffold for tissue engineering.
Since our self-organized scaffolds showed lower viscoelasticity in comparison with mature articular cartilage, further studies are needed to improve the viscoelasticity of the self-organized cartilage-like scaffold. In the present study, viscoelasticity of the rehydrated self-assembled complex–HAP powder conjugate was significantly higher than that of the original self-organized AG/HA/collagen complex. As shown in Figure 4, the viscoelasticity of the HAP-coated scaffold tended to increase in comparison with the original (HAP-uncoated) scaffold. This suggests that conjugation of the HAP with the self-organized cartilage-like material may induce improvements of its viscoelasticity. We have been investigating the development of scaffolds for cartilage repair, which can induce perfectly cartilage-specific tissue qualities represented by properties of high elasticity and high lubrication.
Conclusion
In the present study, we have newly created three types of self-organized biphasic cartilage and bone-like scaffolds combined with HAP, for cartilage tissue engineering, which have been developed through self-assembly of cartilage component molecules (HA, AG and type II collagen) and HAP alone, without other materials, such as cross-linking agents, at the nanometer scale by manipulating the intermolecular relations. Our in vitro and in vivo data reveal that self-organized biphasic cartilage and bone-like scaffolds conjugated to HAP are superior to the self-organized cartilage-like scaffold with no HAP in both cartilage regeneration and subchondral bone defects. Our present study indicates that the self-organized cartilage-like biomaterial, which is conjugated with an HAP layer, may have the potential to repair not only articular cartilage defects but also the degenerated subchondral bone in diseases with osteochondral defect.
Supplementary Materials
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number 26462323 from the Japan Society for the Promotion of Science (JSPS). We would like to thank M Suzuki, S Mogi, M Tamaki, J Tamate and J Niimi R Karasawa for excellent technical assistance.
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yudoh K, Karasawa R. A novel biomaterial for cartilage repair generated by self-assembly: creation of a self-organized articular cartilage-like tissue. J Biomater Nanobiotechnol. 2012;3(2):125–129. [Google Scholar]
- 3.Liu-Bryan R. Inflammation and intracellular metabolism: new targets in oa. Osteoarthritis Cartilage. 2015;23(11):1835–1842. doi: 10.1016/j.joca.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu N, Liao J, Lin S, et al. PCL-PEG-PCL film promotes cartilage regeneration in vivo. Cell Prolif. 2016;49(6):729–739. doi: 10.1111/cpr.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith IO, McCabe LR, Baumann MJ. MC3T3-E1 osteoblast attachment and proliferation on porous hydroxyapatite scaffolds fabricated with nanophase powder. Int J Nanomedicine. 2006;1(2):189–194. doi: 10.2147/nano.2006.1.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang PF, Li G, Wang JF, Zheng QJ, Wang Y. Development, characterization, and validation of porous carbonated hydroxyapatite bone cement. J Biomed Mater Res B Appl Biomater. 2009;90B(2):886–893. doi: 10.1002/jbm.b.31360. [DOI] [PubMed] [Google Scholar]
- 7.Dutta SR, Passi D, Singh P, Bhuibhar A. Ceramic and non-ceramic hydroxyapatite as a bone graft material: a brief review. Ir J Med Sci. 2015;184(1):101–106. doi: 10.1007/s11845-014-1199-8. [DOI] [PubMed] [Google Scholar]
- 8.Fouad H, Alfotawi R, Alothman O, et al. Porous polyethylene coated with functionalized hydroxyapatite particles as a bone reconstruction material. Materials. 2018;11(4):521. doi: 10.3390/ma11040521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman AP. Articular cartilage repair. Am J Sports Med. 1998;26(2):309–324. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- 10.Gaut C, Sugaya K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen Med. 2015;10(5):665–679. doi: 10.2217/rme.15.31. [DOI] [PubMed] [Google Scholar]
- 11.Correia CR, Reis RL, Mano JF. Multiphasic, Multistructured and hierarchical strategies for cartilage regeneration. Adv Exp Med Biol. 2015;881:143–160. doi: 10.1007/978-3-319-22345-2_9. [DOI] [PubMed] [Google Scholar]
- 12.Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Knutsen G, Drogset JO, Engebretsen L, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am. 2016;98(16):1332–1339. doi: 10.2106/JBJS.15.01208. [DOI] [PubMed] [Google Scholar]
- 14.Rai V, Dilisio MF, Dietz NE, Agrawal DK. Recent strategies in cartilage repair: a systemic review of the scaffold development and tissue engineering. J Biomed Mater Res A. 2017;105(8):2343–2354. doi: 10.1002/jbm.a.36087. [DOI] [PubMed] [Google Scholar]
- 15.Grande DA, Pitman MI, Peterson L, Menche D, Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7(2):208–218. doi: 10.1002/jor.1100070208. [DOI] [PubMed] [Google Scholar]
- 16.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 17.Katsube K, Ochi M, Uchio Y, et al. Repair of articular cartilage defects with cultured chondrocytes in atelocollagen gel. Comparison with cultured chondrocytes in suspension. Arch Orthop Trauma Surg. 2000;120(3–4):121–127. doi: 10.1007/pl00021232. [DOI] [PubMed] [Google Scholar]
- 18.Seo S, Na K. Mesenchymal stem cell-based tissue engineering for chondrogenesis. J Biomed Biotechnol. 2011;2011(6):1–8. doi: 10.1155/2011/806891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reppel L, Schiavi J, Charif N, et al. Chondrogenic induction of mesenchymal stromal/stem cells from Wharton’s jelly embedded in alginate hydrogel and without added growth factor: an alternative stem cell source for cartilage tissue engineering. Stem Cell Res Ther. 2015;6(1):260. doi: 10.1186/s13287-015-0263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8(5):409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 21.Gropp M, Shilo V, Vainer G, et al. Standardization of the teratoma assay for analysis of pluripotency of human ES cells and biosafety of their differentiated progeny. PLoS One. 2012;7(9):e45532. doi: 10.1371/journal.pone.0045532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osada N, Kikuchi J, Umehara T, et al. Lysine-specific demethylase 1 inhibitors prevent teratoma development from human induced pluripotent stem cells. Oncotarget. 2018;9(5):6450–6462. doi: 10.18632/oncotarget.24030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colnot C, Zhang X, Knothe Tate ML, Tate MLK. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res. 2012;30(12):1869–1878. doi: 10.1002/jor.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirazi R, Shirazi-Adl A. Computational biomechanics of articular cartilage of human knee joint: effect of osteochondral defects. J Biomech. 2009;42(15):2458–2465. doi: 10.1016/j.jbiomech.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Gomoll AH, Madry H, Knutsen G, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434–447. doi: 10.1007/s00167-010-1072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Yin J, Gao J, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grynpas MD, Alpert B, Katz I, Lieberman I, Pritzker KP. Subchondral bone in osteoarthritis. Calcif Tissue Int. 1991;49(1):20–26. doi: 10.1007/BF02555898. [DOI] [PubMed] [Google Scholar]
- 28.Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012;51(2):204–211. doi: 10.1016/j.bone.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Castañeda S, Roman-Blas JA, Largo R, Herrero-Beaumont G. Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol. 2012;83(3):315–323. doi: 10.1016/j.bcp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Hatakeyama W, Taira M, Chosa N, Kihara H, Ishisaki A, Kondo H. Effects of apatite particle size in two apatite/collagen composites on the osteogenic differentiation profile of osteoblastic cells. Int J Mol Med. 2013;32(6):1255–1261. doi: 10.3892/ijmm.2013.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda K, Taira M, Yokota J, Hattori M, Ishisaki A, Kondo H. Effects of addition of nano-hydroxyapatite to highly-pressed collagen on osteogenic differentiation in osteoblastic SaOS-2 cells. Nano Biomed. 2016;8(2):91–100. [Google Scholar]
- 32.Lawrence JS. Osteoarthrosis. In: Lawrence JS, editor. Rheumatism in Populations. London, UK: William Heinemann Medical Books; 1977. pp. 98–155. [Google Scholar]
- 33.Brandt KD, Fife RS, Braunstein EM, Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 1991;34(11):1381–1386. doi: 10.1002/art.1780341106. [DOI] [PubMed] [Google Scholar]
- 34.Yui N, Yoshioka H, Fujiya H, et al. The DNA repair enzyme apurinic/apyrimidinic endonuclease (APEX nuclease) 2 has the potential to protect against down-regulation of chondrocyte activity in osteoarthritis. Int J Mol Sci. 2014;15(9):14921–14934. doi: 10.3390/ijms150914921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mainil-Varlet P, van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38(5):880–890. doi: 10.1177/0363546509359068. [DOI] [PubMed] [Google Scholar]
- 36.Rutgers M, van Pelt MJ, Dhert WJ, Creemers LB, Saris DB. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18(1):12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Yudoh K, Kumai T, Yui N, Karasawa R. Cartilage tissue engineering-a novel biomaterial for cartilage repair generated by self-assembly: creation of a self-organized articular cartilage-like tissue. Nov Tech Arthritis Bone Res. 2017;1(2):555557. [Google Scholar]
- 38.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Golightly YM, Allen KD, Jordan JM. Defining the burden of osteoarthritis in population-based surveys. Arthritis Care Res. 2016;68(5):571–573. doi: 10.1002/acr.22716. [DOI] [PubMed] [Google Scholar]
- 40.Burr DB. Gallant MA: bone remodeling in osteoarthritis. Nat Rev Rheumatol. 2012;8:665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- 41.Henrotin Y, Pesesse L, Sanchez C. Subchondral bone and osteoarthritis: biological and cellular aspects. Osteoporos Int. 2012;23(S8):847–851. doi: 10.1007/s00198-012-2162-z. [DOI] [PubMed] [Google Scholar]
- 42.Sharma AR, Jagga S, Lee SS, Nam JS. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci. 2013;14(10):19805–19830. doi: 10.3390/ijms141019805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaharwar AK, Schexnailder PJ, Schmidt G. Nanocomposite polymer biomaterials for tissue repair of bone and cartilage: a material science perspective. In: Sitharaman Balaji., editor. Nanobiomaterials Handbook. 20. Vol. 24. Boca Raton, FL: CRC Press, LLC; 2011. pp. 1–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.