Abstract
IMPORTANCE
Perimenopause is accompanied by an increased risk of new and recurrent depression. The coincidence of declining ovarian function with the onset of depression led to the inference that “withdrawal” from physiologic estradiol levels underpinned depression in perimenopause. To our knowledge, this is the first controlled systematic study to directly test the estrogen withdrawal theory of perimenopausal depression (PMD).
OBJECTIVE
To examine the role of estradiol withdrawal in PMD.
DESIGN, SETTING, AND PARTICIPANTS
Initial open-label treatment with estradiol followed by randomized, double-blind, placebo-controlled, parallel-design evaluation of continued estradiol treatment was evaluated at an outpatient research facility at the National Institutes of Health Clinical Center. An intent-to-treat analysis was performed between October 2003 and July 2012. Participants included asymptomatic postmenopausal women with past PMD responsive to hormone therapy (n = 26) and asymptomatic postmenopausal women with no history of depression (n = 30) matched for age, body mass index, and reproductive status who served as controls. Data were analyzed between November 2012 and October 2013 by repeated-measures analysis of variance.
INTERVENTIONS
After 3 weeks of open-label administration of transdermal estradiol (100 μg/d), participants were randomized to a parallel design to receive either estradiol (100 μg/d; 27 participants) or matched placebo skin patches (29 participants) for 3 additional weeks under double-blind conditions.
MAIN OUTCOMES AND MEASURES
Center for Epidemiologic Studies-Depression Scale and 17-item Hamilton Depression Rating Scale (completed by raters blind to diagnosis and randomization status), self-administered visual analog symptom ratings, and blood hormone levels obtained at weekly clinic visits.
RESULTS
None of the women reported depressive symptoms during open-label use of estradiol. Women with past PMD who were crossed over from estradiol to placebo experienced a significant increase in depression symptom severity demonstrated using the Center for Epidemiologic Studies-Depression Scale and 17-item Hamilton Depression Rating Scale, with mean (SD) scores increasing from estradiol (ie, 2.4 [2.0] and 3.0 [2.5]) to placebo (8.8 [4.9] and 6.6 [4.5], respectively [P = .0004 for both]). Women with past PMD who continued estradiol therapy and all women in the control group remained asymptomatic. Women in both groups had similar hot-flush severity and plasma estradiol levels during use of placebo.
CONCLUSIONS AND RELEVANCE
In women with past PMD that was previously responsive to hormone therapy, the recurrence of depressive symptoms during blinded hormone withdrawal suggests that normal changes in ovarian estradiol secretion can trigger an abnormal behavioral state in these susceptible women. Women with a history of PMD should be alert to the risk of recurrent depression when discontinuing hormone therapy.
Depression risk increases in perimenopause, and depression is cited1–3 as a primary reason for resuming menopausal hormone therapy (HT). Community-based epidemiologic studies4–8 have documented a 1.5- to 3-fold greater risk of first onset of depression and recurrent depression in women in perimenopause compared with those who are premenopausal or several years postmenopausal. Observational studies9–11 have reported the emergence of depressive symptoms after the discontinuation of HT in 5% to 10% of women.
The role of estradiol-either declining levels or low levels-in the precipitation of perimenopausal depression (PMD) is unknown, largely owing to the associational and indirect nature of the evidence linking ovarian function and depression. Correlative studies7,12–15 with plasma follicle-stimulating hormone (FSH) or response to HT provided indirect evidence of the association between changes in reproductive hormones and mood disturbances. However, even prospective epidemiologic studies cannot test the estradiol withdrawal hypothesis since perimenopausal changes in the secretion of several hormonal and metabolic factors could confound the effects of estradiol withdrawal. In other studies16,17 of the role of ovarian steroids in affective disturbance, changes in ovarian steroids (in the context of otherwise normal levels) have been shown to directly trigger depression, but only in a subset of women with histories of mood disorders linked to reproductive function. In the present study, we directly examined the hypothesis that declining ovarian function (estradiol withdrawal) underpins depression occurring in perimenopause. We further sought to examine whether the response to estradiol withdrawal differs in women with past PMD compared with those without past depression.
Methods
Participant Selection
Between October 2003 and July 2012, women were self-referred in response to newspaper advertisements or were referred by a physician. All women were aged 45 to 65 years, postmenopausal, and in good health; none had clinically significant mood symptoms. We recruited 2 groups of women. The first group comprised women with past perimenopausal depression who reported a history of major or minor depression (confirmed by the Structured Clinical Interview for DSM-IV18) at midlife in association with menstrual cycle irregularity (ie, skipped menstrual periods or prolonged periods of amenorrhea), with remission (also confirmed by the Structured Clinical Interview retrospectively) of this depression after HT (past PMD). In addition, many women reported the experience of distressing vasomotor symptoms and/or vaginal dryness proximate to the episode of depression. The second group included matched asymptomatic women who were either currently receiving HT or had previously received HT and who had no past depression (controls) (eMethods in Supplement 1). The trial protocol was approved by the National Institute of Mental Health Intramural Research Board and appears in Supplement 2, and all women provided oral and written informed consent. All participants received financial compensation according to the guidelines of the National Institutes of Health Healthy Volunteer Office.
Study Design
During a 3-week baseline phase, all women received open- label transdermal estradiol (Vivelle-Dot) therapy (100 μg/d) and completed symptom ratings to confirm the absence of mood symptoms before entry into the double-blind study (Figure 1). After determination that mood symptoms remained absent during open-label use of estradiol, women were randomized to a parallel design in which they received either estradiol (at the same dose given during the open-label period) or matched placebo skin patches for 3 additional weeks. At the end of the trial, all women received 1 week of oral medroxyprogesterone acetate (5 mg/d) to induce progestin-withdrawal menstrual bleeding. All women attended weekly outpatient visits in which outcome measures were obtained, adverse effects were monitored, and blood samples were drawn.
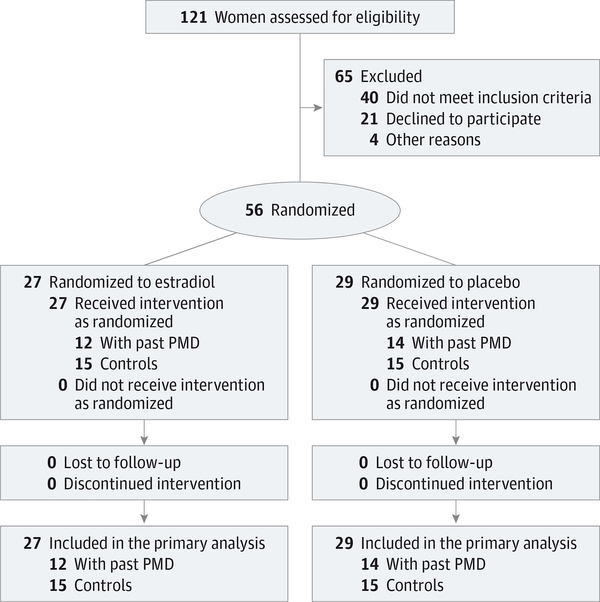
Figure 1. CONSORT Diagram.
All women were postmenopausal; therefore, endogenous estradiol production did not contribute to the changes in reproductive hormones that were observed. Twenty-three women with past perimenopausal depression (PMD) and 16 control women were receiving hormone therapy before starting the study.
Assessment of Symptoms
Depressive symptoms were monitored with rating scales completed during weekly clinic visits, including the self-rated Center for Epidemiologic Studies-Depression Scale (CES-D)19 and the 17-item Hamilton Depression Rating Scale (HDRS)20 (completed by blinded raters). As secondary measures, women completed the Beck Depression Inventory21 and a visual analog scale measuring the severity of 11 mood and behavioral symptoms. Finally, to monitor the presence and severity of vasomotor symptoms, women completed a daily rating form.22
Statistical Analysis
An intent-to-treat analysis was performed between November 2012 and October 2013. We first tested for significant differences in mood symptoms developing during the double-blind conditions compared with the open-label (baseline) conditions in each of the 4 groups (ie, women with and without past PMD randomized to double-blind administration of estradiol or placebo). Second, we compared symptom ratings during double-blind treatment conditions across all 4 randomization groups (ie, women with and those without past PMD who were randomized to either estradiol or placebo).
We obtained 6 repeated measures on the same woman during the baseline (3 weekly measures) and double-blind (3 weekly measures) phases. Analyses were done with SAS, version 9.2 (SAS Institute Inc), repeated-measures analysis of variance using PROC MIXED (for mixed models). For each symptom rating and blood hormone level, the predictor variables of interest-double-blind treatment (placebo or estradiol) and history of PMD (yes or no)-were modeled as fixed effects. The covariance pattern across time was structured as first-order autoregressive. We used the Kenward-Roger method for computing the degrees of freedom for tests of fixed effects.
For each of the 16 symptom outcomes, we performed 2 types of analyses to explore the effect of a history of PMD in the 2 treatment groups. We first compared mood symptom values during the double-blind conditions vs during the open-label (baseline) conditions separately in each of the 4 groups (ie, women with and without past PMD randomized to estradiol or placebo). Second, we compared symptom ratings during double-blind treatment conditions in 4 ways (ie, women with vs without past PMD in the estradiol group; similarly for women in the placebo group). In the first analysis, we determined whether symptoms changed from baseline (ie, open-label estradiol, weeks 1–3 of the trial) to the double-blind treatment phase (ie, weeks 4–6 of the trial) in any of the 4 randomization groups (as described above). For the second analysis, we included the mean of the baseline symptom measure for each symptom as a covariate (since symptom rating scores were higher in the women with past PMD compared with control women despite receiving open-label estradiol) and compared differences in symptom ratings across each of the 4 randomization groups to test for the effects of past PMD and double-blind treatment. Plasma levels of estradiol, estrone, and FSH during double-blind treatment also were analyzed across randomization groups. For all analyses, the value of the estimator (least square means) and associated SE of the estimator and P values are reported. For each symptom measure, the P values reported are the Bonferroni value adjusted for multiple comparisons within that symptom, and 2-sided P < .05 was considered statistically significant.
Clinical characteristics in women with past PMD and control women were compared to ensure similarity across groups and included the following measures: age, body mass index, menstrual bleeding, weekly mean hot-flush severity scores of 2 or more (minimal severity), and CES-D score. The CES-D cutoff scores are typically used as a screen to identify clinically significant depression; a cutoff score of greater than 16 has been shown4,23,24 to correlate with clinically significant depression. In addition, a score between 8 and 15 has been used to define subsyndromal depression.25–27 We used the Fisher exact test for categorical variables and t tests for continuous variables.
Finally, the possible relationships between the magnitude of the change in plasma estradiol and FSH levels and mood symptoms in women with past PMD in whom estradiol therapy was withdrawn were examined using Spearman correlation coefficients as follows. The difference between the mean plasma estradiol or FSH levels during open-label administration of estradiol and those during administration of placebo were correlated with both the corresponding differences in CES-D symptom scores and the maximum CES-D score (ie, most symptomatic) during the placebo phase (withdrawal).
Results
Participant Characteristics
Sixty women entered the trial: 30 women had past PMD and 30 had no history of depression. Four women with past PMD were excluded from the trial after the open-label phase: 1 was not adherent to treatment, and 3 experienced distressing life events that affected their participation (Figure 1 and Table 1).
Table 1.
Characteristics of Participants Prior to Study Entry
| Group |
|||||
|---|---|---|---|---|---|
| Past PMD |
Control |
||||
| Characteristic | Estradiol (n = 12) | Placebo (n = 14) | Estradiol (n = 15) | Placebo (n = 15) | P Valuea |
| Age, mean (SD), y | 54.8 (5.2) | 56.1 (3.8) | 56.0 (3.1) | 56.7 (4.3) | .45 |
| BMI, mean (SD) | 25.3 (4.3) | 26.7 (6.5) | 25.6 (5.5) | 26.1 (3.6) | .68 |
| Years since LMP, mean (SD) | 5.8 (3.7) | 7.1 (4.2) | 5.6 (3.1) | 6.2 (4.0) | .45 |
| Reported PMS, No. (%) | 6 (50) | 8 (57) | 1 (7) | 0 | NA |
| Past MDE, No. (%) | 3 (25) | 1 (7) | 0 | 0 | NA |
| Past PPD (non-SCID), No. (%)b | 2 (17) | 1 (7) | 0 | 0 | NA |
| STRAW Stage, No. (%)c | |||||
| Stage +1 | 6 (50) | 5 (36) | 8 (53) | 6 (4) | NA |
| Stage +2 | 6 (50) | 9 (64) | 7 (47) | 9 (60) | NA |
| Current smoker, No. (%) | 2 (17) | 1 (7) | 0 | 0 | NA |
| Pregnancy history, No. (%) | |||||
| Never pregnant, No. (%) | 1 (8) | 3 (21) | 4 (27) | 2 (13) | NA |
| Nulliparity | 3 (25) | 3 (21) | 4 (27) | 3 (20) | NA |
| Prestudysymptom scores, mean (SD) | |||||
| CES-D | 7.9 (8.3) | 6.1 (6.1) | 1.9 (2.8) | 1.2 (3.4) | NA |
| HDRS | 4.6 (7.1) | 4.5 (4.6) | 0.3 (0.7) | 1.2 (2.4) | NA |
| BDI | 3.1 (3.9) | 3.4 (5.5) | 0.0 (0.0) | 0.7 (1.8) | NA |
Abbreviations: BDI, Beck Depression Inventory; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CES-D, Center for Epidemiologic Studies-Depression Scale; HDRS, Hamilton Depression Rating Scale; LMP, last menstrual period; MDE, major depressive episode; NA, not applicable; PMD, perimenopausal depression; PMS, premenstrual syndrome; PPD, postpartum depression; SCID, Structured Clinical Interview for DSM-IV; STRAW, Stages of Reproductive Aging Workshop Criteria.
The t test was used to compare age, BMI, and years since LMP between women with past PMD and control women. Statistical comparisons were not performed for clinical characteristics that were not relevant to outcomes and are provided for descriptive purposes only (including prestudy mood ratings).
Diagnoses of PPD did not meet the SCID criterion requiring onset of depression to occur within 4 weeks of delivery.
Stage +1, within 4years of LMP; stage+2, after 4 years since LMP.28
Women with past PMD and control women did not differ significantly in the following measures: mean (SD) age, 55.5 (4.4) and 56.3 (3.7) years, respectively; body mass index, 26.1 (4.9) and 26.4 (5.3) (calculated as weight in kilograms divided by height in meters squared), respectively; and time since the last menstrual period, 6.7 (3.9) and 5.9 (3.5) years, respectively (t test, P > .05 for all comparisons). Eighteen of the 26 women with PMD had a past major depression in perimenopause and 8 had a past minor depression in perimenopause (ie, did not meet the full 5 criteria required for major depression). Four of the 26 women with past PMD reported additional episodes of major depression that were premenopausal (ie, unrelated to the menopause transition), 3 of whom were randomized to estradiol and 1 to placebo. One of these 4 women with a past major depression reported symptoms of premenstrual irritability in the past, but none of these women reported episodes of prenatal or postpartum depression.
Symptom Ratings
Comparisons Between Open-Label and Double-blind Conditions
Women with past PMD who were randomized to placebo experienced a significant increase in depression severity ratings (ie, CES-D, HDRS [P < .001, both comparisons] and Beck Depression Inventory scores [the last at a trend of P = .05]) as well as in several of the visual analog scale symptom scores (depression, social isolation, early morning wakening, and feeling unmotivated) compared with their baseline estradiol treatment (Table 2, Figure 2). The other 3 groups remained asymptomatic and did not experience a significant change in depression symptom severity during the double-blind conditions compared with open-label estradiol administration. Neither of the placebo groups experienced a significant increase in the severity of day and nighttime hot flushes.
Table 2.
Comparisons of Symptom Rating Scores During Open-Label and Double-blind Treatment
| Meana |
|||||
|---|---|---|---|---|---|
| Measure | Past PMD | Phase | Least Squares (SE) | Arithmetic (SD) | P Valueb |
| CES-D score | Yes | OL Estradiol | 2.4 (1.0) | 2.4 (2.0) | .0004 |
| Placebo | 9.1 (1.0) | 8.8 (4.9) | |||
| Yes | OL Estradiol | 3.1 (0.5) | 3.1 (1.6) | >.99 | |
| DB Estradiol | 2.8 (0.5) | 2.8 (1.5) | |||
| No | OL Estradiol | 0.9 (0.4) | 0.9 (1.5) | >.99 | |
| Placebo | 1.1 (0.4) | 1.1 (1.8) | |||
| No | OL Estradiol | 1.4 (0.5) | 1.4 (2.2) | .13 | |
| DB Estradiol | 0.8 (0.5) | 0.8 (1.4) | |||
| HDRS score | Yes | OL Estradiol | 3.0 (0.9) | 3.0 (2.5) | .0004 |
| Placebo | 7.0 (0.9) | 6.6 (4.5) | |||
| Yes | OL Estradiol | 3.5 (0.7) | 3.6 (1.8) | >.99 | |
| DB Estradiol | 3.1 (0.7) | 3.1 (2.6) | |||
| No | OL Estradiol | 0.9 (0.2) | 0.9 (0.8) | >.99 | |
| Placebo | 0.9 (0.2) | 0.9 (0.9) | |||
| No | OL Estradiol | 0.8 (0.3) | 0.8 (1.1) | >.99 | |
| DB Estradiol | 0.7 (0.3) | 0.7 (1.1) | |||
| BDI score | Yes | OL Estradiol | 1.7 (0.8) | 1.7 (2.3) | .05 |
| Placebo | 4.4 (0.9) | 4.3 (3.7) | |||
| Yes | OL Estradiol | 1.7 (0.5) | 1.7 (1.8) | .42 | |
| DB Estradiol | 1.1 (0.5) | 1.1 (1.5) | |||
| No | OL Estradiol | 0.2 (0.1) | 0.2 (0.3) | .84 | |
| Placebo | 0.2 (0.1) | 0.2 (0.4) | |||
| No | OL Estradiol | 0.2 (0.1) | 0.2 (0.4) | .36 | |
| DB Estradiol | 0.04 (0.1) | 0.04 (0.2) | |||
| Visual analog scale | |||||
| Depression | Yes | OL Estradiol | 5.5 (2.3) | 5.5 (6.1) | .01 |
| Placebo | 14.1 (2.3) | 14.0 (10.2) | |||
| Yes | OL Estradiol | 5.3 (1.7) | 5.5 (3.5) | >.99 | |
| DB Estradiol | 6.7 (1.7) | 6.8 (7.4) | |||
| No | OL Estradiol | 2.8 (0.4) | 2.8 (1.7) | >.99 | |
| Placebo | 2.6 (0.4) | 2.5 (1.8) | |||
| No | OL Estradiol | 3.0 (0.5) | 2.9 (2.2) | .45 | |
| DB Estradiol | 2.4 (0.5) | 2.4 (1.1) | |||
| Anxiety | Yes | OL Estradiol | 8.6 (3.9) | 8.6 (10.4) | .07 |
| Placebo | 18.5 (3.9) | 18.2 (17.6) | |||
| Yes | OL Estradiol | 11.2 (2.7) | 11.5 (9.7) | >.99 | |
| DB Estradiol | 9.7 (2.6) | 9.6 (9.0) | |||
| No | OL Estradiol | 3.0 (0.6) | 3.0 (2.3) | >.99 | |
| Placebo | 2.8 (0.6) | 2.9 (2.3) | |||
| No | OL Estradiol | 4.1 (1.8) | 4.3 (5.7) | >.99 | |
| DB Estradiol | 5.6 (1.8) | 5.3 (8.4) | |||
| Irritability | Yes | OL Estradiol | 10.1 (3.8) | 10.1 (10.4) | .11 |
| Placebo | 20.4 (3.8) | 20.4 (17.5) | |||
| Yes | OL Estradiol | 11.0 (2.3) | 11.1 (8.9) | .11 | |
| DB Estradiol | 7.6 (2.3) | 7.7 (7.1) | |||
| No | OL Estradiol | 2.7 (0.5) | 2.8 (1.6) | .83 | |
| Placebo | 2.8 (0.5) | 2.8 (2.3) | |||
| No | OL Estradiol | 4.5 (1.2) | 4.6 (5.8) | .43 | |
| DB Estradiol | 2.3 (1.2) | 2.3 (1.1) | |||
| Avoid socializing | Yes | OL Estradiol | 5.8 (2.3) | 5.8 (6.3) | .03 |
| Placebo | 12.4 (2.3) | 12.4 (10.5) | |||
| Yes | OL Estradiol | 6.5 (1.7) | 7.0 (8.9) | >.99 | |
| DB Estradiol | 5.0 (1.7) | 4.9 (3.7) | |||
| No | OL Estradiol | 2.3 (0.4) | 2.3 (1.5) | >.99 | |
| Placebo | 2.5 (0.4) | 2.4 (1.8) | |||
| No | OL Estradiol | 2.6 (0.3) | 2.5 (1.4) | >.99 | |
| DB Estradiol | 2.5 (0.3) | 2.5 (1.0) | |||
| Early morning wakening | Yes | OL Estradiol | 9.6 (3.3) | 9.5 (10.0) | .04 |
| Placebo | 15.6 (3.3) | 15.8 (14.4) | |||
| Yes | OL Estradiol | 13.9 (2.7) | 13.7 (7.1) | >.99 | |
| DB Estradiol | 13.1 (2.6) | 13.5 (9.5) | |||
| No | OL Estradiol | 4.0 (1.0) | 4.1 (5.0) | >.99 | |
| Placebo | 3.3 (1.0) | 3.3 (3.2) | |||
| No | OL Estradiol | 3.5 (0.7) | 3.3 (1.7) | >.99 | |
| DB Estradiol | 3.5 (0.7) | 3.3 (3.6) | |||
| Anhedonia | Yes | OL Estradiol | 5.6 (2.3) | 5.6 (5.5) | .07 |
| Placebo | 12.4 (2.3) | 12.4 (11.3) | |||
| Yes | OL Estradiol | 7.3 (2.2) | 8.0 (8.8) | >.99 | |
| DBEstradiol | 6.1 (2.1) | 5.2 (3.7) | |||
| No | OL Estradiol | 2.1 (0.4) | 2.1 (1.3) | >.99 | |
| Placebo | 2.3 (0.4) | 2.3 (1.7) | |||
| No | OL Estradiol | 2.4 (0.3) | 2.4 (1.2) | >.99 | |
| DB Estradiol | 2.3 (0.3) | 2.4 (1.0) | |||
| Decreased energy | Yes | OL Estradiol | 10.9 (3.2) | 10.9 (9.3) | .18 |
| Placebo | 18.9 (3.2) | 18.9 (14.0) | |||
| Yes | OL Estradiol | 12.4 (3.2) | 12.7 (14.7) | >.99 | |
| DB Estradiol | 9.5 (3.1) | 9.6 (7.8) | |||
| No | OL Estradiol | 3.1 (0.6) | 3.1 (2.2) | >.99 | |
| Placebo | 2.9 (0.6) | 2.9 (2.0) | |||
| No | OL Estradiol | 3.2 (0.5) | 3.2 (1.7) | >.99 | |
| DB Estradiol | 3.0 (0.5) | 3.0 (2.0) | |||
| Disturbed sleep | Yes | OL Estradiol | 13.5 (3.8) | 13.5 (11.3) | .09 |
| Placebo | 22.8 (3.9) | 22.7 (17.7) | |||
| Yes | OL Estradiol | 21.6 (4.1) | 22.2 (14.9) | .20 | |
| DB Estradiol | 15.5 (4.1) | 15.6 (13.2) | |||
| No | OL Estradiol | 4.7 (1.7) | 4.8 (7.2) | >.99 | |
| Placebo | 4.3 (1.7) | 4.2 (5.1) | |||
| No | OL Estradiol | 3.6 (0.7) | 3.4 (1.9) | >.99 | |
| DB Estradiol | 3.9 (0.7) | 3.8 (4.2) | |||
| Unmotivated | Yes | OL Estradiol | 6.6 (2.7) | 6.6 (6.5) | .02 |
| Placebo | 14.9 (2.7) | 14.0 (12.8) | |||
| Yes | OL Estradiol | 5.8 (1.6) | 5.6 (4.7) | >.99 | |
| DB Estradiol | 6.7 (1.6) | 7.0 (7.4) | |||
| No | OL Estradiol | 3.3 (0.8) | 4.3 (7.8) | >.99 | |
| Placebo | 3.1 (0.8) | 2.7 (1.6) | |||
| No | OL Estradiol | 2.8 (0.4) | 3.4 (2.0) | >.99 | |
| DB Estradiol | 2.6 (0.4) | 2.6 (1.1) | |||
| Excess worry | Yes | OL Estradiol | 5.4 (3.0) | 5.4 (5.0) | .07 |
| Placebo | 14.2 (3.0) | 14.0 (16.4) | |||
| Yes | OL Estradiol | 7.9 (1.6) | 8.4 (7.6) | .55 | |
| DB Estradiol | 5.0 (1.5) | 5.1 (4.4) | |||
| No | OL Estradiol | 3.4 (0.9) | 3.5 (3.5) | >.99 | |
| Placebo | 3.5 (0.9) | 3.4 (3.9) | |||
| No | OL Estradiol | 2.7 (0.4) | 2.7 (1.6) | >.99 | |
| DB Estradiol | 2.7 (0.4) | 2.8 (1.8) | |||
| Decreased concentration | Yes | OL Estradiol | 9.1 (3.6) | 9.1 (8.2) | .18 |
| Placebo | 17.9 (3.6) | 17.9 (17.6) | |||
| Yes | OL Estradiol | 9.5 (2.5) | 9.9 (11.9) | .82 | |
| DB Estradiol | 7.6 (2.5) | 7.6 (6.4) | |||
| No | OL Estradiol | 3.5 (0.8) | 3.5 (3.4) | .82 | |
| Placebo | 2.9 (0.8) | 2.8 (2.4) | |||
| No | OL Estradiol | 2.7 (0.3) | 2.6 (1.6) | .82 | |
| DB Estradiol | 2.3 (0.3) | 2.3 (1.1) | |||
| Nighttime hot flushes | Yes | OL Estradiol | 1.2 (0.1) | 1.1 (0.3) | .43 |
| Placebo | 1.5 (0.1) | 1.4 (0.5) | |||
| Yes | OL Estradiol | 1.4 (0.2) | 1.4 (0.8) | .51 | |
| DB Estradiol | 1.3 (0.2) | 1.1 (0.3) | |||
| No | OL Estradiol | 1.1 (0.1) | 1.1 (0.2) | .43 | |
| Placebo | 1.2 (0.1) | 1.2 (0.3) | |||
| No | OL Estradiol | 1.1 (0.1) | 1.1 (0.3) | .20 | |
| DB Estradiol | 1.1 (0.1) | 1.0 (0.1) | |||
| Daytime hot flushes | Yes | OL Estradiol | 1.2 (0.1) | 1.2 (0.3) | .26 |
| Placebo | 1.5 (0.1) | 1.5 (0.5) | |||
| Yes | OL Estradiol | 1.2 (0.1) | 1.2 (0.3) | >.99 | |
| DB Estradiol | 1.1 (0.1) | 1.1 (0.3) | |||
| No | OL Estradiol | 1.2 (0.1) | 1.2 (0.2) | >.99 | |
| Placebo | 1.2 (0.1) | 1.2 (0.3) | |||
| No | OL Estradiol | 1.1 (0.03) | 1.1 (0.2) | .38 | |
| DB Estradiol | 1.0 (0.03) | 1.0 (0.1) | |||
Abbreviations: BDI, Beck Depression Inventory; CES-D, Center for Epidemiologic Studies-Depression Scale; DB, double-blind; HDRS, Hamilton Depression Rating Scale; OL, open-label; PMD, perimenopausal depression.
Least squares means were adjusted by PROC MIXED (SAS Institute Inc) using residual maximum likelihood methods, whereas arithmetic means are not adjusted.
P values represent post hoc Bonferroni comparisons of least squares means.
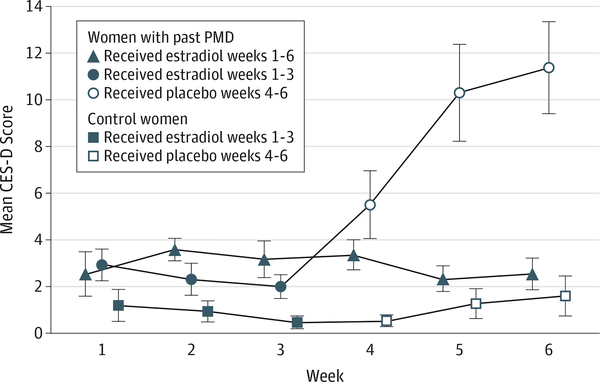
Figure 2. Estradiol Withdrawal Precipitation of Depressive Symptoms.
Women with past perimenopausal depression (PMD) randomized to placebo (but not those randomized to estradiol and control women randomized to either estradiol or placebo) had significantly increased (ie, more symptomatic) Center for Epidemiologic Studies-Depression Scale (CES-D) scores compared with scores during the open-label administration of estradiol. In addition, CES-D scores in women with PMD during use of placebo were significantly increased compared with the scores of both control women randomized to placebo and those with past PMD randomized to estradiol. The dosage of estradiol used throughout the study was 100 μg/d. Data markers indicate the arithmetic mean; error bars, SE.
Comparisons Across Groups During Double-blind Conditions
While receiving placebo, women with past PMD had significantly increased depression scores compared with both the women with past PMD who were randomized to estradiol (ie, continuation) and control women who received placebo (Table 3, Figure 2). This pattern of significant effects of estradiol withdrawal on mood in the women with past PMD was observed in depression severity measures (ie, CES-D [compared with controls receiving placebo, P < .001; compared with women with past PMD receiving estradiol, P < .001], HDRS [compared with controls receiving placebo, P < .001; compared with women with past PMD receiving estradiol, P = .002], and Beck Depression Inventory [compared with controls receiving placebo, P = .02; compared with women with past PMD receiving estradiol, P = .03]) as well as in several visual analog scale symptom scores. In addition, there were no significant differences in any symptom score measured in women with no past history of depression (control women) who received double-blind estradiol compared with control women who received placebo (withdrawal); P values were nonsignificant for all comparisons. Nor were there significant differences in the CES-D, Beck Depression Inventory, HDRS, or any of the visual analog scale symptom scores between women with a past PMD during double-blind estradiol administration compared with the control women. No significant differences in either daytime or nighttime hot-flush severity were observed between women with past PMD and control women who received placebo (P = .23 and .82, respectively). There also were no significant differences in hot-flush severity between placebo and estradiol use during the double-blind phase in either the women with past PMD or the control women (Table 3; P values ranged from .18 to .51).
Table 3.
Comparisons of Symptom Rating Scores During Double-blind Treatment
| Meana |
|||||
|---|---|---|---|---|---|
| Measure | Group | Subgroup | Least Squares (SE) | Arithmetic (SD) | P Valueb |
| CES-D score | Placebo | Past PMD | 8.4 (1.0) | 8.8 (4.9) | .0004 |
| Control | 1.8 (0.9) | 1.1 (1.8) | |||
| Estradiol | Past PMD | 2.4 (0.4) | 2.8 (1.5) | .07 | |
| Control | 1.1 (0.3) | 0.8 (1.4) | |||
| Past PMD | Placebo | 9.3 (1.0) | 8.8 (4.9) | .0004 | |
| Estradiol | 2.6 (1.1) | 2.8 (1.5) | |||
| Control | Placebo | 1.3 (0.3) | 1.1 (1.8) | .73 | |
| Estradiol | 0.6 (0.3) | 0.8 (1.4) | |||
| HDRS score | Placebo | Past PMD | 5.6 (0.6) | 6.6 (4.5) | .0008 |
| Control | 2.0 (0.5) | 0.9 (0.9) | |||
| Estradiol | Past PMD | 2.2 (0.7) | 3.1 (2.6) | >.99 | |
| Control | 1.3 (0.6) | 0.7 (1.1) | |||
| Past PMD | Placebo | 7.1 (0.8) | 6.6 (4.5) | .002 | |
| Estradiol | 2.7 (0.8) | 3.1 (2.6) | |||
| Control | Placebo | 0.8 (0.2) | 0.9 (0.9) | >.99 | |
| Estradiol | 0.7 (0.2) | 0.7 (1.1) | |||
| BDI scale score | Placebo | Past PMD | 3.8 (0.7) | 4.3 (3.7) | .02 |
| Control | 0.6 (0.7) | 0.2 (0.4) | |||
| Estradiol | Past PMD | 0.8 (0.2) | 1.1 (1.5) | .68 | |
| Control | 0.3 (0.2) | 0.04 (0.2) | |||
| Past PMD | Placebo | 4.3 (0.7) | 4.3 (3.7) | .03 | |
| Estradiol | 1.1 (0.8) | 1.1 (1.5) | |||
| Control | Placebo | 0.2 (0.1) | 0.2 (0.4) | >.99 | |
| Estradiol | 0.05 (0.1) | 0.04 (0.2) | |||
| Visual analog scale | |||||
| Depression | Placebo | Past PMD | 12.7 (1.7) | 14.0 (10.2) | .004 |
| Control | 3.7 (1.7) | 2.5 (1.8) | |||
| Estradiol | Past PMD | 5.9 (1.6) | 6.8 (7.4) | .82 | |
| Control | 2.9 (1.4) | 2.4 (1.1) | |||
| Past PMD | Placebo | 14.0 (2.2) | 14.0 (10.2) | .11 | |
| Estradiol | 6.6 (2.4) | 6.8 (7.4) | |||
| Control | Placebo | 2.6 (0.3) | 2.5 (1.8) | >.99 | |
| Estradiol | 2.3 (0.3) | 2.4 (1.1) | |||
| Anxiety | Placebo | Past PMD | 15.3 (2.4) | 18.2 (17.6) | .04 |
| Control | 5.7 (2.3) | 2.9 (2.3) | |||
| Estradiol | Past PMD | 8.4 (2.8) | 9.6 (9.0) | >.99 | |
| Control | 6.5 (2.5) | 5.3 (8.4) | |||
| Past PMD | Placebo | 19.3 (3.0) | 18.2 (17.6) | .08 | |
| Estradiol | 8.2 (3.3) | 9.6 (9.0) | |||
| Control | Placebo | 2.9 (1.6) | 2.9 (2.3) | >.99 | |
| Estradiol | 5.6 (1.7) | 5.3 (8.4) | |||
| Irritability | Placebo | Past PMD | 18.1 (3.2) | 20.4 (17.5) | .03 |
| Control | 5.0 (3.1) | 2.8 (2.3) | |||
| Estradiol | Past PMD | 5.7 (1.0) | 7.7 (7.1) | .67 | |
| Control | 3.7 (0.9) | 2.3 (1.1) | |||
| Past PMD | Placebo | 20.6 (3.2) | 20.4 (17.5) | .03 | |
| Estradiol | 7.1 (3.5) | 7.7 (7.1) | |||
| Control | Placebo | 3.0 (0.4) | 2.8 (2.3) | .37 | |
| Estradiol | 2.1 (0.4) | 2.3 (1.1) | |||
| Avoid socializing | Placebo | Past PMD | 10.3 (1.5) | 12.4 (10.5) | .04 |
| Control | 4.3 (1.5) | 2.4 (1.8) | |||
| Estradiol | Past PMD | 4.5 (0.7) | 4.9 (3.7) | .41 | |
| Control | 2.8 (0.6) | 2.5 (1.0) | |||
| Past PMD | Placebo | 12.5 (1.9) | 12.4 (10.5) | .03 | |
| Estradiol | 4.4 (2.1) | 4.9 (3.7) | |||
| Control | Placebo | 2.5 (0.2) | 2.4 (1.8) | >.99 | |
| Estradiol | 2.4 (0.2) | 2.5 (1.0) | |||
| Early morning wakening | Placebo | Past PMD | 12.8 (1.7) | 15.8 (14.4) | .03 |
| Control | 5.9 (1.6) | 3.3 (3.2) | |||
| Estradiol | Past PMD | 7.6 (1.8) | 13.5 (9.5) | >.99 | |
| Control | 8.2 (1.5) | 3.3 (3.6) | |||
| Past PMD | Placebo | 17.9 (1.9) | 15.8 (14.4) | .06 | |
| Estradiol | 10.3 (2.2) | 13.5 (9.5) | |||
| Control | Placebo | 3.1 (0.7) | 3.3 (3.2) | >.99 | |
| Estradiol | 3.7 (0.7) | 3.3 (3.6) | |||
| Anhedonia | Placebo | Past PMD | 10.7 (1.9) | 12.4 (11.3) | .07 |
| Control | 3.8 (1.9) | 2.3 (1.7) | |||
| Estradiol | Past PMD | 4.8 (0.8) | 5.2 (3.7) | .25 | |
| Control | 2.5 (0.7) | 2.4 (1.0) | |||
| Past PMD | Placebo | 12.6 (2.2) | 12.4 (11.3) | .08 | |
| Estradiol | 4.6 (2.4) | 5.2 (3.7) | |||
| Control | Placebo | 2.4 (0.2) | 2.3 (1.7) | >.99 | |
| Estradiol | 2.2 (0.2) | 2.4 (1.0) | |||
| Decreased energy | Placebo | Past PMD | 16.9 (2.7) | 18.9 (14.0) | .02 |
| Control | 4.8 (2.6) | 2.9 (2.0) | |||
| Estradiol | Past PMD | 7.6 (1.4) | 9.6 (7.8) | .35 | |
| Control | 4.1 (1.2) | 3.0 (2.0) | |||
| Past PMD | Placebo | 19.0 (2.9) | 18.9 (14.0) | .10 | |
| Estradiol | 8.9 (3.1) | 9.6 (7.8) | |||
| Control | Placebo | 2.9 (0.3) | 2.9 (2.0) | >.99 | |
| Estradiol | 2.9 (0.3) | 3.0 (2.0) | |||
| Disturbed sleep | Placebo | Past PMD | 19.7 (2.9) | 22.7 (17.7) | .02 |
| Control | 7.2 (2.8) | 4.2 (5.1) | |||
| Estradiol | Past PMD | 9.3 (2.4) | 15.6 (13.2) | >.99 | |
| Control | 9.0 (2.1) | 3.8 (4.2) | |||
| Past PMD | Placebo | 25.4 (3.4) | 22.7 (17.7) | .07 | |
| Estradiol | 12.3 (3.7) | 15.6 (13.2) | |||
| Control | Placebo | 3.9 (0.8) | 4.2 (5.1) | >.99 | |
| Estradiol | 4.4 (0.8) | 3.8 (4.2) | |||
| Unmotivated | Placebo | Past PMD | 14.1 (2.1) | 14.8 (12.8) | .01 |
| Control | 3.8 (2.0) | 3.1 (2.8) | |||
| Estradiol | Past PMD | 4.6 (0.8) | 7.0 (7.4) | >.99 | |
| Control | 4.0 (0.7) | 2.6 (1.1) | |||
| Past PMD | Placebo | 14.1 (2.2) | 14.0 (12.8) | .15 | |
| Estradiol | 7.2 (2.3) | 7.0 (7.4) | |||
| Control | Placebo | 2.9 (0.4) | 3.1 (2.8) | >.99 | |
| Estradiol | 2.8 (0.5) | 2.6 (1.1) | |||
| Excessive worry | Placebo | Past PMD | 12.6 (2.3) | 14.0 (16.4) | .09 |
| Control | 4.9 (2.2) | 3.4 (3.9) | |||
| Estradiol | Past PMD | 5.1 (1.1) | 5.1 (4.4) | .51 | |
| Control | 2.7 (0.9) | 2.8 (1.8) | |||
| Past PMD | Placebo | 14.9 (2.9) | 14.1 (16.4) | .07 | |
| Estradiol | 3.8 (3.2) | 5.1 (4.4) | |||
| Control | Placebo | 3.2 (0.5) | 3.4 (3.9) | >.99 | |
| Estradiol | 3.1 (0.6) | 2.8 (1.8) | |||
| Decreased concentration | Placebo | Past PMD | 15.2 (3.0) | 17.9 (17.6) | .12 |
| Control | 5.2 (2.9) | 2.8 (2.4) | |||
| Estradiol | Past PMD | 6.2 (1.2) | 7.6 (6.4) | .33 | |
| Control | 3.1 (1.1) | 2.3 (1.1) | |||
| Past PMD | Placebo | 17.8 (3.3) | 17.9 (17.6) | .14 | |
| Estradiol | 7.0 (3.6) | 7.6 (6.4) | |||
| Control | Placebo | 2.6 (0.4) | 2.8 (2.4) | >.99 | |
| Estradiol | 2.5 (0.4) | 2.3 (1.1) | |||
| Nighttime hot flushes | Placebo | Past PMD | 1.5 (0.1) | 1.4 (0.5) | .82 |
| Control | 1.2 (0.1) | 1.2 (0.3) | |||
| Estradiol | Past PMD | 1.1 (0.1) | 1.1 (0.3) | >.99 | |
| Control | 1.0 (0.1) | 1.0 (0.1) | |||
| Past PMD | Placebo | 1.5 (0.1) | 1.4 (0.5) | .51 | |
| Estradiol | 1.1 (0.1) | 1.1 (0.3) | |||
| Control | Placebo | 1.2 (0.1) | 1.2 (0.3) | .21 | |
| Estradiol | 1.0 (0.1) | 1.0 (0.1) | |||
| Daytime hot flushes | Placebo | Past PMD | 1.5 (0.1) | 1.5 (0.5) | .23 |
| Control | 1.2 (0.1) | 1.2 (0.3) | |||
| Estradiol | Past PMD | 1.1 (0.1) | 1.1 (0.3) | >.99 | |
| Control | 1.0 (0.05) | 1.0 (0.1) | |||
| Past PMD | Placebo | 1.5 (0.1) | 1.5 (0.5) | .18 | |
| Estradiol | 1.1 (0.1) | 1.1 (0.3) | |||
| Control | Placebo | 1.2 (0.04) | 1.2 (0.3) | .27 | |
| Estradiol | 1.0 (0.04) | 1.0 (0.1) | |||
Abbreviations: BDI, Beck Depression Inventory; CES-D, Center for Epidemiologic Studies-Depression scale; DB, double-blind; HDRS, Hamilton Depression Rating Scale; PMD, perimenopausal depression.
Least squares means were adjusted for symptom rating scores during open-label estradiol administration, whereas arithmetic means were not adjusted.
P values represent post hoc Bonferroni comparisons of least squares means.
Plasma Hormone Levels
Plasma hormone levels (Table 4) did not differ significantly between the women with PMD and control women during the double-blind conditions. Otherwise, plasma hormone levels showed expected differences, predicted by the study design, between double-blind administration of estradiol and placebo in both women with PMD and control women.
Table 4.
Comparison of Plasma Hormone Levels During Double-blind Treatment
| Meanb |
|||||
|---|---|---|---|---|---|
| Measurea | Group | Subgroup | Least Squares (SE) | Arithmetic (SD) | P Valuec |
| Estradiol, pg/mL | Placebo | Past PMD | 10.5 (2.5) | 10.4 (10.7) | >.99 |
| Control | 8.5 (2.3) | 8.4 (7.3) | |||
| Estradiol | Past PMD | 159.5 (18.1) | 196.5 (112.4) | >.99 | |
| Control | 153.2 (14.9) | 126.5 (92.8) | |||
| Past PMD | Placebo | 9.5 (18.4) | 10.4 (10.7) | .0004 | |
| Estradiol | 195.2 (20.9) | 196.5 (112.4) | |||
| Control | Placebo | −4.5 (13.2) | 8.4 (7.3) | .0004 | |
| Estradiol | 140.7 (13.2) | 126.5 (92.8) | |||
| Estrone, pg/mL | Placebo | Past PMD | 17.4 (2.1) | 17.8 (6.4) | .73 |
| Control | 21.5 (2.0) | 21.0 (10.2) | |||
| Estradiol | Past PMD | 77.3 (7.5) | 93.1 (55.1) | >.99 | |
| Control | 66.9 (6.1) | 56.6 (31.1) | |||
| Past PMD | Placebo | 19.2 (8.5) | 17.8 (6.4) | .0004 | |
| Estradiol | 91.3 (9.7) | 93.1 (55.1) | |||
| Control | Placebo | 17.6 (4.1) | 21.0 (10.2) | .0004 | |
| Estradiol | 60.1 (4.2) | 56.6 (31.1) | |||
| FSH, mlU/mL | Placebo | Past PMD | 84.3 (4.4) | 73.7 (25.9) | >.99 |
| Control | 82.9 (4.0) | 91.3 (26.4) | |||
| Estradiol | Past PMD | 39.3 (3.2) | 31.8 (29.5) | >.99 | |
| Control | 38.1 (2.7) | 44.8 (16.9) | |||
| Past PMD | Placebo | 75.7 (3.9) | 73.7 (25.9) | .0004 | |
| Estradiol | 28.5 (4.3) | 31.8 (29.5) | |||
| Control | Placebo | 94.1 (3.3) | 91.3 (26.4) | .0004 | |
| Estradiol | 41.8 (3.3) | 44.8 (16.9) | |||
Abbreviations: FSH, follicle-stimulating hormone; PMD, perimenopausal depression.
SI conversion factor: To convert estradiol and estrone to picomoles per liter, multiply by 3.671 and 3.699, respectively.
Blood samples were centrifuged, aliquoted, and stored at −70°C until the time ofassay. Blood samples were assayedforestradiol and estrone by liquid chromatography-tandem mass spectrometry techniques at NMS Laboratories.
Plasma FSH levels were determined at the National Institutes of Health Clinical Center laboratory by microparticle enzyme immunoassay (AxSYM System, Abbott Diagnostics).
Least squares means were adjusted for individual plasma hormone levels during open-label estradiol administration (baseline), whereas arithmetic means are not adjusted.
P values represent post hoc Bonferroni comparisons of least squares means.
Additional Comparisons
During placebo administration, 5 women with past PMD (36%) and none of the control women had clinically significant depression based on self-rating depression scores (CES-D scores) of 16 or higher. During double-blind estradiol therapy, no woman had CES-D scores at that level (Fisher exact test, P < .001). Including women who met our criterion scores for subsyndromal depression (ie, CES-D scores >8), depressive symptoms were reported in a total of 11 women (79%) with past PMD during placebo, 1 woman (8%) with past PMD receiving double-blind estradiol, and 1 woman (7%) from each control group who were randomized to either placebo or estradiol (Fisher exact test, P < .001 for all comparisons). No significant differences were observed between women with past PMD and control women in the numbers reporting either hot-flush severity of 2 (minimal) or more or menstrual bleeding during administration of placebo (Fisher exact text, P > .99 for all comparisons). In the double-blind phase of the trial, the numbers of participants with past PMD and control women who reported hot-flush severity of 2 or more during placebo use did not differ significantly from those receiving estradiol (Fisher exact test, P = .17 and P = .22 for past PMD and control women, respectively). Seven-day mean scores of hot-flush severity of 2 (minimal) or more during the 3 weeks of placebo administration in women with past PMD were 5 women (36%) for both daytime and nighttime; in control women, the numbers were 3 women (20%) for both daytime and nighttime. One woman with past PMD reported severity scores of 2 or more during double-blind estradiol administration. There were no significant differences between women with past PMD and control women during use of placebo in the number reporting menstrual bleeding (1 and 2 women, respectively) (Fisher exact test, P values ranged from .20 to >.99). Finally, the numbers of women reporting vaginal bleeding did not differ significantly either during the open-label and double-blind conditions or between the randomized placebo and estradiol groups (Fisher exact test, P > .99 for all comparisons). Neither the change in CES-D scores from open-label estradiol to placebo nor the maximum CES-D score during placebo use correlated with the absolute change (ie, difference between open-label estradiol and placebo) in the plasma levels of either estradiol or FSH.
Discussion
During perimenopause, the incidence of depression increases and predicts increased all-cause and cardiovascular mortality.29 The possible role of ovarian steroids in the onset of PMD is only indirectly suggested by the temporal coincidence of changes in reproductive function and mood symptoms as well as by reports13–15 of the short-term efficacy of estradiol in this condition. In the present study, we attempted to determine whether sudden, blinded withdrawal of estradiol would precipitate depressive symptoms and whether the withdrawal would do so differentially in women with a history of PMD. Our findings support both predictions. Estradiol withdrawal precipitated depressive symptoms in women with a history of PMD (although the mean depressive symptom scores remained within the normal ranges), but no depressive symptoms were seen after estradiol withdrawal in women with no history of PMD. Furthermore, depressive symptoms did not emerge in women with past PMD who continued to receive estradiol. The recurrence of depressive symptoms in women with past PMD occurred in the absence of differences in several measures that could influence mood including baseline clinical characteristics (other than PMD), the severity of daytime/nighttime hot flushes, and plasma hormone levels after estradiol withdrawal. The lack of depressive symptoms in the control women despite identical hormone manipulation (and similar levels of hot flushes and plasma estradiol levels) demonstrates that estradiol withdrawal differentially affects central nervous system function in some women so as to render them susceptible to depression. This finding suggests that perimenopausal changes in estradiol levels can trigger depression, but only in the susceptible subgroup.
Our experiment failed to model distinct elements of the natural course of menopause, including the marked variability and higher peak levels of estradiol secretion.30,31 Nonetheless, our study demonstrated the ability of withdrawal of estradiol, even from relatively lower levels (ie, relative to the high plasma levels reported30–33 in some perimenopausal women), to precipitate depressive symptoms in women with a history of PMD. Previous clinic-based studies34,35 found no beneficial effects of differing rates of tapering of HT on the emergence of withdrawal symptoms, suggesting that the rate of estradiol withdrawal would not influence the onset of estradiol withdrawal-induced symptoms.
In addition to not fully recapitulating estradiol levels in perimenopause, this study had several limitations. First, the relatively short duration of estradiol withdrawal could have reduced the likelihood of precipitating and detecting distressing hot flushes and, possibly, mood disturbances. However, both epidemiologic and clinic-based studies15,36,37 suggest that vasomotor symptoms are not uniform accompaniments of depression in perimenopause. Moreover, the duration of withdrawal was sufficient to produce depressive symptoms (but not hot flushes) in women with past PMD. Second, blinding could have been compromised by the onset of vaginal bleeding; however, this occurred infrequently and was as commonly reported during estradiol use (both open-label and double-blind) as it was during estradiol withdrawal and occurred in both women with past PMD and control women. Third, since most of the women in our sample experienced depression only in the context of perimenopause (and had depression responsive to HT), the findings do not necessarily represent women with PMD with a history of depression unrelated to perimenopause. Nonetheless, a large proportion of women with PMD have first-onset depression,7,38 and small therapeutic trials13–15 suggest that there are no differences in the treatment response to estradiol in women with first-onset vs recurrent depression in perimenopause. However, the role of estradiol withdrawal in PMD cannot be generalized beyond the present sample, that is, to either women who experience depression independent of the menopause transition or, obviously, those with depression that does not respond to HT. Fourth, given the retrospective nature of the diagnosis used in this study, it is possible that recall bias may have led some women to falsely conclude that the onset of depression was linked to the menopausal transition. Fifth, women with past PMD were more symptomatic than were control women at baseline, and they remained more symptomatic compared with control women during the open-label administration of estradiol. We addressed this potential problem by including scores during the open-label phase as a covariate in our analysis. Nevertheless, it is possible that the increase in baseline ratings may have contributed to the degree of symptom severity experienced during estradiol withdrawal but cannot account for the significant differences that we observed. Finally, it is possible that as yet unknown factors, the effects of which we could not control, could contribute to the differential response to estradiol withdrawal in women with PMD.
There are several mechanisms by which changes in estradiol levels might mediate the observed effects on mood. First, estradiol modulates the activity of virtually every system implicated in the pathogenesis of depression including regulation of neurotransmitter synthesis and metabolism, stress axis activation, neuroplasticity (including regulation of brain-derived neurotrophic factor), epigenesis, and immune system activation.39 Second, estradiol signaling through estrogen receptor β reverses depressive- and anxiety-like behaviors in animal studies.40–42 Third, reward responsiveness, which is disturbed in depression,43 is modulated by estradiol in both rodents and humans.44,45 Finally, discontinuation of long-term estradiol therapy in postmenopausal women is accompanied by decreases in medial frontal and temporo-occipital metabolism.46 Thus, through local signaling or network-level dysfunction, particularly in frontolimbic regions, estradiol withdrawal could precipitate affective dysregulation.
What remains unclear is the reason for differential susceptibility to the mood-destabilizing effects of estradiol withdrawal. Of interest in this regard is the recent identification of increased sensitivity to estrogen regulation among transcripts that were differentially expressed in women who developed postpartum depression.47 Alternatively, basic science studies48 have reported the de novo production of estradiol from androgens in brain regions involved in mood regulation (eg, hippocampus). Thus, it is possible that women who did not develop depressive symptoms after estradiol withdrawal have sufficient central nervous system aromatase activity to prevent the development of depressive symptom during declining estradiol secretion.
Conclusions
Although women with past PMD had a recurrence of depressive symptoms during estradiol withdrawal, control women had no perturbations of mood during the same hormonal manipulation. These observations, in the context of similar plasma reproductive hormone levels, suggest that normal changes in ovarian estradiol secretion can trigger an abnormal behavioral state in susceptible women.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health (NIH). Novartis Pharmaceuticals supplied estradiol skin patches (Vivelle-Dot) and matched placebos for this study.
Role of the Funder/Sponsor: The NIMH,NIH, and Novartis Pharmaceuticals had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclaimer: The views expressed in this article do not necessarily represent the views of the NIMH, NIH, Department of Health and Human Services, or the US government.
Conflict of Interest Disclosures: This work was written as part of the official duties of Peter J. Schmidt, MD, asa government employee.
Contributor Information
Peter J. Schmidt, Section on Behavioral Endocrinology, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
Rivka Ben Dor, Section on Behavioral Endocrinology, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
Pedro E. Martinez, Section on Behavioral Endocrinology, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
Gioia M. Guerrieri, Section on Behavioral Endocrinology, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
Veronica L. Harsh, Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, Charlottesville.
Karla Thompson, Section on Behavioral Endocrinology, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
Deloris E. Koziol, Biostatistics and Clinical Epidemiology Service, Clinical Center, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
Lynnette K. Nieman, Intramural Research Program on Reproductive and Adult Endocrinology, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
David R. Rubinow, Department of Psychiatry, University of North Carolina, Chapel Hill.
REFERENCES
- 1.McIntyre RS, Konarski JZ, Grigoriadis S, et al. Hormone replacement therapy and antidepressant prescription patterns: a reciprocal relationship. CMAJ. 2005;172(1):57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Citarella A, Andersen M, Sundström A, Bardage C, Hultman CM, Kieler H. Initiating therapy with antidepressants after discontinuation of hormone therapy. Menopause. 2013;20(2):146–151. [DOI] [PubMed] [Google Scholar]
- 3.Joffe H Antidepressant use after discontinuation of hormone therapy: what can one infer about post-hormone therapy depression? Menopause. 2013;20(2):123–125. [DOI] [PubMed] [Google Scholar]
- 4.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61(1):62–70. [DOI] [PubMed] [Google Scholar]
- 5.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63 (4):385–390. [DOI] [PubMed] [Google Scholar]
- 6.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4): 375–382. [DOI] [PubMed] [Google Scholar]
- 7.Freeman EW, Sammel MD, Boorman DW, Zhang R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry. 2014; 71(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN). J Affect Disord. 2007;103(1–3): 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294(2):183–193. [DOI] [PubMed] [Google Scholar]
- 10.Grady D, Ettinger B, Tosteson ANA, Pressman A, Macer JL. Predictors of difficulty when discontinuing postmenopausal hormone therapy. Obstet Gynecol. 2003;102(6):1233–1239. [DOI] [PubMed] [Google Scholar]
- 11.Ness J, Aronow WS, Beck G. Menopausal symptoms after cessation of hormone replacement therapy. Maturitas. 2006;53(3):356–361. [DOI] [PubMed] [Google Scholar]
- 12.Daly RC, Danaceau MA, Rubinow DR, Schmidt PJ. Concordant restoration of ovarian function and mood in perimenopausal depression. Am J Psychiatry. 2003;160(10):1842–1846. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183(2):414–420. [DOI] [PubMed] [Google Scholar]
- 14.Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women:a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58(6):529–534. [DOI] [PubMed] [Google Scholar]
- 15.Joffe H, Petrillo LF, Koukopoulos A, et al. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011;96(7):E1044–E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209–216. [DOI] [PubMed] [Google Scholar]
- 17.Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–930. [DOI] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 19.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 20.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 22.Endicott J, Nee J, Cohen J, Halbreich U. Premenstrual changes: patterns and correlates of daily ratings. J Affect Disord. 1986;10(2):127–135. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RE, Vernon SW. The Center for Epidemiologic Studies Depression Scale: its use in a community sample. Am J Psychiatry. 1983;140(1): 41–46. [DOI] [PubMed] [Google Scholar]
- 24.Beekman ATF, Geerlings SW, Deeg DJH, et al. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry. 2002;59(7):605–611. [DOI] [PubMed] [Google Scholar]
- 25.Cohen CI, Magai C, Yaffee R, Walcott-Brown L . Racial differences in syndromal and subsyndromal depression in an older urban population. Psychiatr Serv. 2005;56(12):1556–1563. [DOI] [PubMed] [Google Scholar]
- 26.Cohen CI, Goh KH, Gustave M. A prospective study of outcome and predictors of subclinical and clinical depression in an older biracial sample of psychiatric outpatients. J Affect Disord. 2010;121(3): 204–211. [DOI] [PubMed] [Google Scholar]
- 27.Vahia IV, Meeks TW, Thompson WK, et al. Subthreshold depression and successful aging in older women. Am J Geriatr Psychiatry. 2010;18(3): 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril. 2001;76(5):874–878. [DOI] [PubMed] [Google Scholar]
- 29.Wassertheil-Smoller S, Shumaker S, Ockene J, et al. ; Women’s Health Initiative (WHI). Depression and cardiovascular sequelae in postmenopausal women. Arch Intern Med. 2004;164(3):289–298. [DOI] [PubMed] [Google Scholar]
- 30.Tepper PG, Randolph JF Jr, McConnell DS, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). J Clin Endocrinol Metab. 2012;97(8):2872–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81(4):1495–1501. [DOI] [PubMed] [Google Scholar]
- 32.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16(1):50–59. [DOI] [PubMed] [Google Scholar]
- 33.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92(8):3060–3067. [DOI] [PubMed] [Google Scholar]
- 34.Lindh-Astrand L, Bixo M, Hirschberg AL, Sundström-Poromaa I, Hammar M. A randomized controlled study of taper-down or abrupt discontinuation of hormone therapy in women treated for vasomotor symptoms. Menopause. 2010;17(1):72–79. [DOI] [PubMed] [Google Scholar]
- 35.Haimov-Kochman R, Barak-Glantz E, Arbel R, et al. Gradual discontinuation of hormone therapy does not prevent the reappearance of climacteric symptoms: a randomized prospective study. Menopause. 2006;13(3):370–376. [DOI] [PubMed] [Google Scholar]
- 36.Bromberger JT, Kravitz HM, Matthews K, Youk A, Brown C, Feng W. Predictors of first lifetime episodes of major depression in midlife women. Psychol Med. 2009;39(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman EW, Sammel MD, Lin H. Temporal associations of hot flashes and depression in the transition to menopause. Menopause. 2009;16(4): 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg EM, Rubinow DR, Bartko JJ, et al. A cross-sectional evaluation of perimenopausal depression. J Clin Psychiatry. 2008;69(6):973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubinow DR, Schmidt PJ, Craft CD. Gonadal steroids and mood disorders In: Charney D, Buxbaum J, Sklar P, Nestler E, eds. Neurobiology of Mental Illness. 4thedNew York, NY: Oxford University Press; 2013:483–495. [Google Scholar]
- 40.Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 β-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-β knockout (BERKO) mice. Psychopharmacology (Berl). 2005;179(3): 637–643. [DOI] [PubMed] [Google Scholar]
- 41.Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERβ-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78(3):523–529. [DOI] [PubMed] [Google Scholar]
- 42.Lund TD, Rovis T, Chung WCJ, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146(2):797–807. [DOI] [PubMed] [Google Scholar]
- 43.Nestler EJ, Carlezon WA Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. [DOI] [PubMed] [Google Scholar]
- 44.Dreher J-C, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA. 2007;104(7): 2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23(2):693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasgon NL, Geist CL, Kenna HA, Wroolie TE, Williams KE, Silverman DHS. Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia. PLoS One. 2014;9(3):e89095. doi: 10.1371/journal.pone.0089095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta D, Newport DJ, Frishman G, et al. Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychol Med. 2014;44(11):2309–2322. [DOI] [PubMed] [Google Scholar]
- 48.Fester L, Prange-Kiel J, Jarry H, Rune GM. Estrogen synthesis in the hippocampus. Cell Tissue Res. 2011;345(3):285–294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.