Abstract
Numerous approaches have been taken in the hunt for human disease genes. The identification of such genes not only provides a great deal of information about the mechanism of disease development, but also provides potential avenues for better diagnosis and treatment. In this chapter, we review the use of the non-mammalian model organism C. elegans for the identification of human disease genes. Studies utilizing this relatively simple organism offer a good balance between the ability to recapitulate many aspects of human disease, while still offering an abundance of powerful cell biological, genetic, and genomic tools for disease gene discovery. C. elegans and other non-mammalian models have produced, and will continue to produce, key insights into human disease pathogenesis.
Keywords: Caenorhabditis elegans, genetic screens, genomic screens, RNAi, GFP
1. Introduction
The choice of model organism for study is a balance in trade-offs. While humans clearly are best in terms of mimicking human disease, there are practical and ethical limits to investigating disease in people. Other mammals, most notably mice, have proved very useful for modeling and studying human disease, but mice are limited in both how well they recapitulate some diseases and the ability to study them in rapid fashion. With the advent of tools like RNA interference (RNAi) and CRISPR/Cas9 genome editing, as well as more classical biochemical techniques, cell line studies have been very fruitful in identifying signaling pathways, for example, but are limited in that overall organismal physiology is generally not present in cell culture.
Non-mammalian model organisms such as the fruit fly Drosophila melanogaster, the zebrafish Danio rerio, and the nematode Caenorhabditis elegans serve as a happy medium [1–5], allowing for ease of study while still having the physiology present in a whole animal and the ability to recapitulate at least some aspects of human disease. These and other model organisms have played key roles in human disease gene discovery. In the current review, we focus on the use of C. elegans as a non-mammalian model for human disease gene discovery. We first provide a brief introduction to C. elegans biology and the history of C. elegans research. Then we describe the key genetic and genomic techniques that have made C. elegans such a powerful research model. Using this background information, we illustrate two approaches that have been taken to identify human disease genes in C. elegans. In the first set of examples, we discuss how C. elegans disease models have been used for de novo discovery of human disease genes and pathways. In the second set of examples, we show how human disease genes have been engineered into C. elegans to develop models of human disease; these disease models have in turn been used to facilitate discovery of other genes that modulate that same human disease.
2. C. elegans Overview
“You have evolved from worm to man, but much within you is still worm.”
-Friedrich Nietzsche, Thus Spoke Zarathustra
2.1. What Is C. elegans Anyway?
Caenorhabditis elegans is a free living transparent nematode worm [6,7] (Fig. 1). C. elegans starts out as an egg; when these eggs hatch, the nematodes pass through four larval stages before reaching adulthood. The C. elegans life cycle is relatively short, taking about three days for the animals to develop, and with an overall lifespan of about two to three weeks. Adults contain only 959 somatic nuclei and grow to be about a millimeter in length. Despite this small size, C. elegans has many of the organ systems present in more complex organisms, including a digestive system, nervous system, musculature, and reproductive system. These small nematodes also exhibit complex behaviors. C. elegans will move toward things they like and away from things they do not like. The nematodes also eat, excrete, and mate.
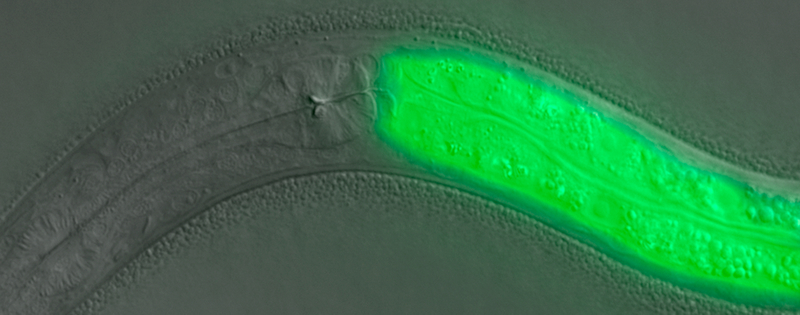
Figure 1.
Depicted is a C. elegans hermaphrodite carrying a lys-7::gfp transgene. In this animal, GFP expression is controlled by the gut-specific lysozyme-7 promoter. The image is an overlay of fluorescence and Nomarski images (images merged using Adobe Photoshop). Image adapted from Fig. 1 in [79]. Copyright © American Society for Microbiology, Molecular and Cellular Biology, 27, 2007, 5544–5553, doi:10.1128/MCB.02070–06.
C. elegans exists as either of two sexes, a hermaphrodite or a male. The existence of self-fertile hermaphrodites has great advantages for the study of development, because mutant stocks that would be unable to mate (such as paralyzed animals) are still able to self-fertilize. Moreover, healthy hermaphrodites produce hundreds of progeny, allowing the generation of large stocks quickly. When males are present, hermaphrodites can cross-fertilize. Thus, the presence of both sexes coupled with the relatively short life cycle allows for rapid genetic crosses.
C. elegans is only three cells in radius, with an outer epidermal layer, a middle muscle layer, and a central intestinal layer, with nervous system, reproductive system, and others tissues in between. The small size, transparent nature, and invariant cell lineage in C. elegans led to an unprecedented view of development in this animal. The full juvenile and adult cell lineages were reported more than 30 years ago [8,9], and more recently, the entire wiring diagram of the nervous system has been determined [10]. In principle, if a cell is moved a few microns or a single neuronal connection is altered by some genetic manipulation, it should be possible to sort that out in C. elegans.
In the wild, C. elegans eats bacteria present in its environment [11]. In the laboratory, C. elegans typically is maintained on small petri dishes seeded with lawns of E. coli [12]. These bacteria are nonpathogenic and serve as a food source. Because of their small size, nematode manipulations are performed using a dissecting microscope. Individual nematodes can be moved from plate to plate using a small platinum wire “pick,” allowing investigators to isolate individual hermaphrodites for self-fertilization and the generation of large populations, or allowing investigators to set up crosses between the sexes. The small size of C. elegans means hundreds or thousands of animals can be maintained inexpensively on an individual dish. When the animals use up all the food, they will starve, and can be maintained as starved populations for months. For long-term storage of stocks, nematodes can be frozen and kept in frozen vials for decades at −80°C or in liquid nitrogen.
In summary, these little animals have many of the organs and exhibit many of the behaviors present in mammals. Moreover, they offer the ability to study diseases in the context of a whole, living, and intact organism, which is not possible in isolated cells. This has been particularly fruitful in the many diseases that affect behavior and the nervous system as described below. Roughly 30–60% of genes in C. elegans have orthologs or strong homologs in mammals [13,14], suggesting that what is discovered about gene function in these small nematodes may be directly applicable to human development and disease.
2.2. Key Discoveries in C. elegans
The modern era of C. elegans research began over 50 years ago when Sydney Brenner first proposed using C. elegans to investigate developmental biology and neurobiology [15,16]. Three of the notable discoveries that earned C. elegans researchers Nobel Prizes included the award to Sydney Brenner, Robert Horvitz, and John Sulston in 2002 for their discoveries related to development and the cell death machinery [17–19]; Andrew Fire and Craig Mello in 2006 for their discovery of RNA interference (RNAi) [20]; and Osamu Shimomura, Martin Chalfie, and Roger Tsien in 2008, for the discovery of Green Fluorescence Protein (GFP) [21,22] and the demonstration that it could be a useful tool in other organisms including C. elegans [23]. Other key discoveries include the identification of microRNAs by Victor Ambros, Gary Ruvkun, and colleagues [24,25]. For a more complete list of key discoveries, see [15].
3. The C. elegans Toolbox
The small size, rapid life cycle, and amazing genetic and genomic tools available have made C. elegans a premier model organism for many purposes. We outline some of these tools here.
3.1. Construction of Transgenic Nematodes
The C. elegans germline initially develops as a multinucleate syncytium prior to membranes forming around each germ cell. Thus, DNA injected into the hermaphrodite gonad can be captured by numerous germ cells, making microinjection much easier than in other systems. DNA captured in this way will form extrachromosomal arrays that are semi-heritable [26,27]. Selectable markers can then be used to maintain stable transgenic lines, and the DNA can be integrated into the genome if desired [28,29]. In addition to direct microinjection, microparticle bombardment coupled with selection methods has been developed to generate stable nematode transgenic lines [30,31]. More recently, sophisticated CRISPR/Cas9-based genome engineering strategies have enabled rapid and precise gene editing, thus facilitating the generation of animals bearing targeted point mutations, deletions, insertions and complex chromosomal rearrangements [32,33].
The ease of C. elegans transgenic construction has served many purposes. Transgenic arrays can be used to restore gene function to “rescue” mutant phenotypes, greatly facilitating the cloning of mutated genes. Another common use for transgenic animals is the construction of GFP reporter strains. Promoter-GFP fusions can be used to determine where in the organism a particular gene is expressed. Protein-GFP fusions can be used for subcellular localization studies, and to quantify protein expression levels in live animals.
3.2. Genetic Tools and Forward Genetics in C. elegans
C. elegans is a diploid organism whose genome contains six chromosomes: five autosomes and one sex chromosome. XX animals are hermaphrodites; XO animals are males. The rapid lifecycle allows for quick genetic screens and crosses. Classical forward genetic screens used mutagens such as ethyl methanesulfonate (EMS) to randomly generate mutations in the nematode germ line [34–36]. F1 hermaphrodite progeny that are heterozygous for these mutations can then be allowed to self-fertilize to isolate F2 homozygous mutants of interest. If the homozygous mutant animals are self-fertile, they can be maintained as a homozygous stock. If the homozygous mutant animals are lethal or sterile, the screen can be engineered to recover heterozygous siblings to maintain the mutant stocks [37].
The ability to visualize C. elegans on a dissecting microscope or in more detail using a compound microscope equipped with differential interference contrast (DIC) optics allows for easy identification of mutant animals. Many classical mutants with visible phenotypes such as Unc (uncoordinated movement) or Dpy (dumpy shaped animals) were isolated by mutagenesis and visual screening for morphological or behavioral phenotypes [38]. More recently, screens have been performed for worms with altered levels or location of GFP expression, altered movement, or altered learning, and almost anything else C. elegans researchers can imagine. There are numerous mapping strategies to determine the identity of the mutant genes ranging from crosses with strains carrying known genetic markers, SNP mapping strains, strains carrying deletion chromosomes, or balancer chromosomes [39,7]. Once the mutation is mapped to a region where a candidate gene is found, the wild type copy of the locus can be injected into animals in an attempt to rescue the mutant phenotype. Alternatively or additionally, RNAi can be delivered to the animals in an attempt to phenocopy the mutant phenotype. The candidate locus also can be sequenced to identify mutations, although more and more frequently whole genome sequencing is being used to identify the causative mutation [40,41]. To simplify mapping and mutation identification, transposon-mediated mutagenesis is also an option in C. elegans [42,35].
In addition to classical forward genetic screens, many researchers have used modifier screens with great success [34–36]. In this case, researchers start with a strain carrying a mutation that induces a phenotype and then mutagenize the animals to isolate mutant animals harboring suppressor or enhancer mutations. For example, one could start with a mildly uncoordinated animal, mutagenize, and screen visually using the dissecting microscope for suppressors that restore normal movement. These modifier mutations can then be genetically separated from the original mutation to determine if the modifier mutation has a phenotype on its own.
The ability to perform rapid genetic crosses also makes C. elegans an excellent system to perform genetic epistasis studies to place novel mutations in known genetic pathways [36].
3.3. Genomic Tools and Reverse Genetics in C. elegans
The discovery of RNAi opened up a whole new world for researchers in all fields including investigators studying C. elegans. Because there is no interferon response in C. elegans, long dsRNAs are not toxic to the nematode. Thus, long dsRNAs rather than siRNAs can be delivered to C. elegans with a concomitant increase in efficiency and specificity of knockdown. C. elegans RNAi screens generally do not suffer from the off-target effects that have plagued mammalian screens. The method of dsRNA delivery in C. elegans is also unique. Andy Fire and colleagues demonstrated the E. coli that are engineered to express dsRNA can be fed to C. elegans, resulting in knockdown of the target gene [43]. Taking advantage of this technique of RNAi feeding, the Ahringer and Vidal labs have generated two genomic RNAi bacterial feeding libraries that cover most of the C. elegans genome [44,45]; each bacterial strain enables the specific RNAi knockdown of a single gene, allowing for rapid and simple genome-wide screening. In these genomic RNAi screens, one simply feeds the bacteria to the nematodes, one bacterial strain at a time, and monitors for the occurrence of the phenotype of interest. Additionally, mutations that enhance RNAi-mediated knockdown have been identified and used to increase the sensitivity of these RNAi screens [46,47].
While RNAi is an invaluable tool, ultimately it is important to be able to monitor the effect of mutation of genes of interest. Unlike RNAi gene knockdowns, mutations allow for less heterogeneous effects. Mutations also can cause unique effects in gene function, such as gain of function or dominant-negative effects. Several labs that make up the C. elegans knockout consortia have been isolating thousands of knockout mutations available to the community of C. elegans researchers [48,49]. Likewise, the Caenorhabditis Genetics Center (CGC) is a stock center that provides ready access to these mutations and the myriad of other mutations that have been isolated and shared by the C. elegans research community. More recent targeted transposon insertion [50], and CRISPR/Cas9 genome editing [32,33] approaches have further enhanced the ability to perform reverse genetics in the nematode by enabling the introduction of almost any change in any gene in the genome.
4. Identifying Novel Human Disease Genes in C. elegans
In the next two sections, we outline several representative examples of human disease gene identification in C. elegans. We apologize to researchers whose work could not be included due to space limitations. Rather than aiming to be comprehensive, our goal is to be illustrative. These specific examples have been chosen to illustrate (1) the advantages of the techniques available in C. elegans to facilitate disease gene discovery and (2) some of the follow-up studies in mammals that have been performed. For de novo disease gene discovery, we outline various genetic and genomic screens for regulators of innate immunity, obesity, and aging (Subheadings 4.1–4.3). For human disease model studies in C. elegans, we outline the investigation of various neurodegenerative diseases (Subheading 5).
4.1. Innate Immunity
Infectious and inflammatory diseases are among the leading causes of death throughout the world. Infectious diseases account for 5 of the top 10 causes of death in the developing world [51]. In developed countries, the top three leading causes of death are heart disease, cancer, and COPD [52]. A key factor common to these three diseases is chronic inflammation [53–56]. This illustrates the importance of proper regulation of innate immunity and inflammation. While a robust innate immune response is essential in our pathogen-rich world, this response must be tightly regulated to prevent inflammatory disease. The identification of genes that regulate innate immunity has led to the identification of numerous genes that affect infectious or inflammatory disease [53,54,57–62].
C. elegans has emerged as a key model system for the discovery of innate immune genes [63–65]. For decades, C. elegans researchers cultured C. elegans on petri dishes containing lawns of nonpathogenic E. coli. However, Ausubel and colleagues discovered that by simply replacing this E. coli lawn with any of a number of human pathogens, the bacteria would infect and kill C. elegans [66–68]. Since then, pathogenesis models have been developed for Gram negative and positive bacteria, fungi, and viruses [69–72]. C. elegans lacks migratory immune cells and does not have an adaptive immune response. The nematode innate immune response is composed of the production of antimicrobial peptides and compounds that fight infection [73]. Importantly, the induction of antimicrobial production in the presence of pathogens is mediated by conserved signaling pathways including MAP kinase cascades [74]. However, there also are differences, most notably the absence of an NFκB homolog in C. elegans. Many investigators have now used C. elegans to study host-pathogen interactions.
Irazoqui and colleagues took a variety of approaches to identify a novel innate immunity regulatory pathway conserved in C. elegans and mammals. They first monitored changes in C. elegans gene expression induced by infection with the Gram positive bacterial pathogen S. aureus [75]. They then used computational analysis of these data to determine that the C. elegans HLH-30 transcription factor (mammalian ortholog TFEB) target DNA sequence was overrepresented in the promoters of the genes whose expression was induced by S. aureus. To test if HLH-30 was involved in this response, they generated HLH-30-GFP transgenic nematodes and found that while HLH-30-GFP was present in both the nucleus and cytoplasm in uninfected worms, all the HLH-30-GFP was present in the nucleus following infection [76]. They then used RNAseq to monitor S. aureus-induced gene expression changes in wild type and hlh-30 mutant animals and discovered that much of the S. aureus-induced gene expression was dependent on the function of HLH-30; moreover, both HLH-30 and its target genes were required for full resistance to S. aureus [76]. This approach illustrates several advantages of the nematode system, including the ease of generating transgenic animals, localization of GFP fusions in the transparent nematode, the availability of a deletion mutant in hlh-30, and the availability of bacteria to deliver hlh-30 dsRNA. Moreover, the identification of HLH-30/TFEB as a key innate immunity regulator was validated in mammalian cells. S. aureus infection in mammalian cell culture leads to redistribution of TFEB into the nucleus, and inhibition of TFEB weakens the S. aureus-induced pro-inflammatory response [75]. Other investigators have independently shown using knockout mice that TFEB affects innate immunity in mammals [77], providing further evidence of the validity of the C. elegans studies.
In a follow-up to these studies, Irazoqui and colleagues used a targeted RNAi screen in which they inhibited most of the kinases and phosphatases in the nematode genome. This targeted RNAi screening approach led to the identification a PLC-PKD-TFEB pathway regulating the nematode innate immune response [78]. They took advantage of the ease of nematode genetics to order the various genes into a pathway, and then went on to show that this signaling pathway functioned similarly in mouse macrophages [78]. This highlights the importance of the C. elegans approach. Similar RNAi screens in mammals would have been significantly more cumbersome and expensive, and it would have been much more complicated to perform the genetic epistasis studies to determine how these genes functioned in an ordered pathway. However, once these details were worked out in C. elegans, the confirmatory cell culture RNAi studies were much more straightforward.
We have used a slightly different strategy with similar results: using C. elegans as a rapid screening tool with follow-up studies in mammalian cells and mice. We used comparative genomics RNAi screens in C. elegans and mouse macrophages to identify innate immunity regulators, subsequently used C. elegans infection models to obtain in vivo validation of these RNAi data, and then used knockout mice to determine the effect of these genes in mammalian disease. We used the ease of generating nematode transgenics to generate 14 different antimicrobial-GFP reporter strains [79]. GFP expression in these lines could be monitored using fluorescence microscopy or by using the COPAS Biosort, a flow cytometer for C. elegans [80]. A key feature of the COPAS Biosort is that it can analyze nematodes in 96-well format, allowing for high-throughput screens. We used bacterial feeding RNAi to inhibit known innate immunity regulators in C. elegans, and found several antimicrobial-GFP reporters whose expression was regulated by these known pathways. This formed the basis for a genomic RNAi screen in which we screened for changes in antimicrobial-GFP levels in the presence of E. coli. To determine if the genes identified could regulate innate immunity in mammals, siRNAs targeting the mouse orthologs of these genes were delivered into mouse macrophage cell lines and the cytokine response induced by lipopolysaccharide (LPS) was monitored. Remarkably, 30–40% of the genes identified in C. elegans had an RNAi-induced defect in the innate immune response in mouse macrophages [81–83]. The ready availability of existing C. elegans knockouts allowed us to rapidly obtain in vivo confirmation that these genes affected host defense. We found that 9 of 10 C. elegans knockouts tested had altered survival in the presence of the nematode and human pathogen P. aeruginosa [81–83]. Armed with the RNAi data in C. elegans and mouse macrophages, and C. elegans knockout data, we then tested four different mouse knockout lines and found that three of the four knockout mice exhibited an altered innate immune response when challenged with LPS ([83,84] and unpublished). Thus, our comparative genomics approach is an efficient method for finding novel innate immunity regulators.
There are several things worth noting about this approach. First, one of the complications of RNAi screens in mammalian cells is the high degree of false-positives due to off-target effects [85]. This is likely not a problem in C. elegans because of the use of long dsRNAs. Moreover, the screens in C. elegans and macrophages involved different methods of dsRNA delivery, different innate immune stimuli, and different immunological readouts. It seems highly unlikely that such different systems would coincidentally report similar results. Plus, the ability to obtain so many nematode mutants relatively rapidly and cheaply for in vivo validation would just not be plausible in mice. By the time these genes had passed all these tests, the efficiency of validating them in vivo in mice was very high. Mammalian follow-up studies focused on genes identified in these screens have led to the investigation of two pathways that regulate the maintenance but not the activation phase of innate immunity [86,84,87].
4.2. Obesity
Obesity has become an epidemic in developed countries; more than 1/3 of adults in the USA are now obese [88]. Obesity is among the leading causes of preventable death and also affects many comorbidities such as Type 2 Diabetes [89]. The excess fat accumulation in obesity is caused by both genetic and environmental factors [90]. The ability to monitor fat accumulation in C. elegans coupled with the ease of RNAi screening in the nematode has led to a number of studies that identified genes that control fat accumulation [91–93]. In one study, McKay et al. [94] demonstrated that RNAi-mediated inhibition of genes known to affect fat accumulation in mammals, including SREBP and C/EBP homologs, led to arrested C. elegans development. Moreover, these animals did not accumulate fat [94], as assayed using Sudan Black or Nile Red staining. The authors reasoned that inhibition of other genes that affect fat production would likewise arrest larval development and would be lethal. The investigators used RNAi to inhibit 80 genes known to be larval-lethal when inhibited, and discovered that 10 gene inhibitions affected fat accumulation. They then used RNAi to verify that these genes affected mammalian cells as well [94]. Ashrafi et al. [95] used genome-wide RNAi screens followed by Nile Red staining to identify the full complement of genes that alter fat accumulation in C. elegans; these investigators identified 305 gene inactivations that reduced fat accumulation and 112 gene inactivations that increased fat accumulation. In another approach, a GFP reporter that localized to fat droplets was used as a screening tool to identify RNAi treatments that altered fat accumulation [96]. All these studies, and many others, demonstrate the ease of RNAi screening in C. elegans coupled with the effective readout tools available to study different diseases in a transparent organism.
4.3. Aging
The study of aging in C. elegans is unusual in that prior to these investigations, most researchers would not have even considered aging a disease that could be investigated and manipulated genetically. Thus, not only have C. elegans studies of aging been fruitful for finding potential human disease genes, but these studies also established that aging was a phenomenon that could be studied genetically in the first place.
As we grow older, we become increasingly frail and eventually die. Age is a major risk factor for a wide variety of diseases. These include almost all of the major neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, as well as cardiovascular disease, metabolic disease, and many cancers. Until recently, aging was not considered a genetically tractable phenomenon and instead was thought to result from the unregulated accumulation of all sorts of errors that together lead to the decay in function and death of the organism. As a result, our understanding of the mechanisms of aging was very poor. Over the last 25 years, however, our understanding of aging has been transformed by pioneering studies in C. elegans. Powerful genetics coupled with a relatively short lifespan of 20 days make C. elegans an excellent system to study aging. Its short lifespan makes it possible to conduct experiments that just are not practical in mice (mean life span of 2 years) or humans (mean lifespan of 80 years). In addition, its simple and inexpensive ease of manipulation makes it possible to assay the lifespan of hundreds or even thousands of worms. These studies have shown that aging is a regulated phenomenon that can be studied with the tools of molecular biology and genetics, and that many of the genes that regulate aging in nematodes also regulate aging in other organisms, including Drosophila, mice, and possibly humans.
The first forward-genetic screen for long-lived C. elegans mutants was conducted in the 1980s by Michael Klass [97]. This elegant genetic screen surmounted several technical challenges specific to C. elegans aging studies. Nematodes produce hundreds of progeny, and thus, parents will rapidly be lost among their progeny as they grow on small petri dishes. To measure the lifespan of a population of worms, one has to separate each worm from its progeny, typically by daily transfer to new petri plates until reproduction ends. This is a very cumbersome process. Moreover, once a mutant worm is deemed long-lived, one needs to obtain progeny to maintain a mutant line that can be studied; however, old worms are no longer fertile. Klass overcame these two challenges using a known temperature-sensitive spermatogenesis mutation. After mutagenesis, F2 animals were each transferred singly to new “master” plates where they reproduced at the lower permissive temperature. Some of the F3 progeny were grown at a high “restrictive” temperature, where they developed into animals that could not self-fertilize. Klass determined the lifespan of thousands of such cohorts to identify eight long-lived mutants. He re-isolated these mutants from their respective master plates that were maintained at the permissive temperature, since their siblings had the same mutations. Three of these mutations were subsequently mapped and shown to be in the same genetic locus, named age-1 [98,99]. Remarkably, age-1 mutant animals lived more than twice as long as wild-type control animals. These studies showed that mutations in a single gene could have a dramatic effect on the lifespan of a multicellular organism.
A few years later, Cynthia Kenyon’s laboratory discovered that mutations in another gene, daf-2, could more than double C. elegans lifespan; moreover, the aged daf-2 mutant animals remained youthful in appearance and mobility, even when all wild-type control animals had died [100]. The daf-2 mutation was previously known to also affect the developmental decision to form dauer larvae [101]. Under unfavorable growth conditions of high-temperature, low food, and high population density, C. elegans develops into developmentally-arrested, stress-resistant, non-feeding dauer larvae; dauers can resume development into fertile adults once they encounter a more favorable environment [102]. daf-2 mutant animals were known to inappropriately form dauer larvae at high temperature, but in an otherwise favorable growth environment. Kenyon and colleagues showed that at a low temperature where these mutant animals did not form dauers, they instead developed into fertile adults that were long-lived. A few years earlier, the Riddle lab [103,104,101] had performed several genetic screens and assembled a genetic pathway for the regulation of dauer formation. Kenyon and colleagues took advantage of this knowledge and asked whether a similar regulatory pathway existed for lifespan. They found that daf-16, a gene required for daf-2 mutant animals to form dauer larvae, is also necessary for the increased lifespan of daf-2 mutant adults [100]. Taken together, these finding demonstrated that aging is subject to regulation.
Subsequent studies have shown that daf-2, age-1, and daf-16 are all part of a conserved insulin/IGF1 signaling pathway: daf-2 encodes the worm’s only ortholog of the human insulin and IGF1 receptor tyrosine kinases; age-1 encodes a phosphoinositide 3-kinase (PI-3 kinase); and daf-16 encodes a FOXO transcription factor that is negatively regulated by the age-1 effector kinases AKT-1 and AKT-2 [105,106]. These genes are part of a well-conserved signaling pathway, raising the question of whether insulin/IGF1 signaling likewise regulated lifespan in other organisms. Subsequent studies in the fruit fly Drosophila melanogaster [106–110] and mice [111,112] showed that manipulation of the insulin/IGF1 signaling pathway can increase lifespan in fruit flies and mice. While these follow-up mouse studies were critical to demonstrate that these pathways were conserved in mammals, these studies highlight the practicality of forward genetic screens for lifespan in C. elegans, which would be a much more challenging in mice.
These remarkable studies prompted the question of whether similar mechanisms may regulate aging in humans [113,114]. Several candidate-based and unbiased association studies have since identified variants in the daf-16 ortholog FOXO3A that are associated with exceptional longevity in humans from multiple ethnic origins [115–124]. In addition, mutations in the IGF1 receptor gene that cause diminished IGF1 signaling were found to be more prevalent in a cohort of Ashkenazi Jewish centenarians, compared to control individuals that do not exhibit exceptional longevity [125,126]. Taken together, these findings suggest that differences in human lifespan may result, at least in part, from the normal variation in signaling by the IGF1 receptor and its transcriptional effector FOXO3A.
Since the discovery of the regulation of lifespan by insulin/IGF-1 signaling, the study of aging in C. elegans has exploded, leading to the discovery of hundreds of genes that affect lifespan. These lifespan-determining genes have been identified by a combination of forward and reverse-genetic approaches. One of the most fruitful approaches has been to determine the effect of each gene on lifespan by systematically knocking down each gene in the genome using RNAi. To date, three such genome-wide RNAi screens have been completed [127–129]. In addition, a genome-wide RNAi screen was performed to identify genes whose knockdown shortens lifespan in daf-2 mutant animals [130], as well as numerous more targeted screens [131,132]. It likely will take many years until all these discoveries are replicated in mammalian systems, but investigators are already tackling the question of whether aging may be “druggable,” potentially leading to an extension of “healthspan” and lifespan, and a delay in the onset of many age-related diseases [133,134].
5. Modeling Human Diseases in C. elegans
In contrast to the above approaches which involve screening de novo in C. elegans for genes that alter a phenotype that is involved in human disease, an alternate approach has been to artificially engineer the human disease into C. elegans, typically by expressing the human disease gene in the nematode. Animals engineered to exhibit the human disease are then used as tools to screen for suppressers or enhancers of the disease phenotype with the goal of finding additional gene targets that affect the disease in humans. While there are many examples of this approach, they are perhaps best exemplified by the study of neurodegenerative disorders in C. elegans, as outlined below.
5.1. Poly-Glutamine Repeat Diseases.
Trinucleotide repeat diseases are typically neurodegenerative or neuromuscular disorders caused by inheritance of a trinucleotide repeat (often greater than 30 repeats in length) in particular genes [135–139]. These trinucleotide repeats are formed by the expansion of unstable shorter triplet repeats present in the genome [135–139]. Some of the most studied triplet repeat disorders are caused by expansion of CAG repeats. These are the poly-Glutamine (polyQ) repeat diseases, which include Huntington’s disease, spinocerebellar ataxias, and many others. Key questions about such disorders include how these unstable repeats expand in the genome, why there is apparently a threshold length for the repeat beyond which disease occurs, and how to develop possible treatments.
Expression of polyQ repeat proteins in C. elegans muscle [140] or neurons [141,142] recapitulates some aspects of human polyQ disease. In particular, some of these authors and others have found a similar threshold for the number of repeats that cause disease. Expression of roughly 35–40 repeats of polyQ-YFP were required to induce polyQ-protein aggregation and resulting muscle or neuronal dysfunction. The ability to monitor YFP-tagged polyQ-protein aggregation in this transparent organism allowed for straightforward modifier screens to monitor polyQ-induced aggregation or dysfunction. For example, Nollen et al. [143] used a genomic RNAi screen to identify 186 genes whose inhibition led to increased or earlier onset aggregation of Q35-YFP (polyQ protein with 35 Q repeats). These genes fell into five broad functional categories, including regulation of RNA metabolism, protein synthesis, protein folding, protein degradation, and protein trafficking. Similarly, candidate based-approaches have been used to identify modifiers of polyQ aggregation in C. elegans. For example, overexpression of the C. elegans homolog of the torsin gene suppressed polyQ aggregation [144]. Likewise, overexpression of ubiquitin suppressed polyQ-induced toxicity in C. elegans and mammalian cells while inhibition of ubiquitin expression induced the opposite effect. [145]. The ease of such genetic and genomic studies in C. elegans coupled with the ability to monitor fluorescently tagged polyQ proteins in this transparent organism has made such studies very straightforward and powerful.
5.2. Alzheimer’s Disease.
Alzheimer’s disease is the sixth leading cause of death in the USA, affecting more than 5 million people in the USA and more than 35 million people worldwide [146,147]. As is the case for most age-dependent diseases, the incidence of Alzheimer’s disease is expected to increase in the future. Despite intensive study, much about Alzheimer’s disease remains a mystery, and no effective treatments have been developed. Much of the research focus centers on trying to understand the aggregation of proteins such as Tau or beta amyloid and the resulting effects on neurological function [146,147].
Several investigators have used overexpression of wild type or mutant Tau as a model for tauopathy in C. elegans [148,149]. Kraemer and colleagues [150] expressed wild type or mutant Tau in all nematode neurons; they observed that Tau aggregated in these animals and that Tau overexpression led to a moderate uncoordinated phenotype. They used this model as the basis for a genome-wide RNAi screen for enhancers of this uncoordinated phenotype [151]. The genes and pathways identified in this screen as potential modifiers of Tau-induced pathology were very similar to those identified in Drosophila screens, suggesting that they may be conserved regulators that might play a role in tauopathies and Alzheimer’s disease [152]. In addition to their genomic RNAi screen, the investigators performed a forward genetic screen to identify mutations that suppress the Tau-induced uncoordinated phenotype. In this genetic screen, they identified mutations in sut-2, which suppressed the Tau aggregation, uncoordinated, and neurodegenerative phenotypes induced by Tau overexpression in C. elegans [153]. Moreover, overexpression of sut-2 in nematodes exacerbated Tau-induced neurotoxicity, the opposite of the RNAi-induced phenotype [154]. The role of SUT-2 was not unique to C. elegans. Follow-up studies in mammalian cells demonstrated that (1) Tau overexpression increased expression of the mammalian homolog MSUT2, (2) MSUT2 RNAi in mammalian cells diminished aggregation of insoluble Tau, and (3) there is less MSUT2 present in the brain in autopsy samples from Alzheimer’s disease patients [154]. Thus, these genomic and genetic modifier screens in C. elegans successfully identified key genes to investigate in the human disease.
Studies in C. elegans relevant to Alzheimer’s disease are not limited to the investigation of Tau. For example, beta-amyloid-expressing models of disease have also been engineered in C. elegans [155–160]. Likewise, investigation of the nematode homologs of Presinilin 1 and 2, mutations in which cause early-onset familial Alzheimer’s disease [161–163], led to the discovery that nematode and human Presinilin 1 regulates Notch signaling [164–166]. As an illustration of the power of genetic screens in C. elegans, our lab conducted a sensitized forward genetic screen in C. elegans to identify genes that function with the Presinilins. In this screen, we identified mutations in two novel genes (aph-1and pen-2) that enhanced the phenotype induced by mutation of sel-12 (Presenilin) [167]. aph-1 also was identified in a C. elegans genetic screen for enhancers of Notch signaling [168]. These genes were later shown to be part of the evolutionarily-conserved γ-secretase protease complex, where they regulate the maturation of Presenilin [169], the catalytic component of this complex. This complex is involved in the proteolytic maturation or degradation of many transmembrane proteins, including the Amyloid Precursor Protein (APP), which is important in Alzheimer’s disease pathogenesis, and the Notch receptor.
5.3. Parkinson’s Disease
Parkinson’s disease is second only to Alzheimer’s disease as the most common neurodegenerative disease. Like Alzheimer’s disease, Parkinson’s disease usually, but not exclusively, is an age-dependent disease, with an incidence of roughly 1% in people over 65 rising to an incidence of 5% by age 85 [170–172]. The primary cause of Parkinson’s disease is a loss of dopaminergic neurons in the substantia nigra region of the brain. This results in the neurological symptoms that are a hallmark of the disease, including tremor of the hands, legs, limbs, and jaw, muscle rigidity of the limbs and trunk, bradykinesia, and postural instability. A key histological feature of patients with Parkinson’s disease is the accumulation of Lewy Bodies in the brain.
A number of genomic and candidate gene-based RNAi screens have been performed in C. elegans models of Parkinson’s disease. These models have focused on overexpression of α-Synuclein, a key candidate Parkinson’s disease protein. α-Synuclein is the main component of Lewy Bodies. It is overexpressed and often mis-expressed in the brain of Parkinson’s disease patients, and mutations in α-Synuclein have been identified in some patients [173,174]. α-Synuclein is not present in C. elegans. Nematode researchers have taken advantage of this to overexpress α-Synuclein and screen for genes that affect α-Synuclein aggregation or cell function [175]. In two studies, YFP or GFP-tagged human α-Synuclein was expressed in nematode muscle using cell-type specific promoters. Aggregated α-Synuclein was monitored by the appearance of punctate fluorescent structures, and either a genomic RNAi screen [176] or an RNAi screen of 900 priority candidate genes (based on various bioinformatics approaches) [177] led to the discovery of numerous genes that affect α-Synuclein aggregation. Many of these genes, in turn, were found to serve a neuroprotective function.
RNAi screens focusing on neurons in C. elegans are more challenging because nematode neurons are somewhat resistant to RNAi. Thus, to study the effects of α-Synuclein expressed in neurons, Kuwahara et al. [178] took advantage of a mutation, eri-1, that enhances RNAi in C. elegans. They expressed human α-Synuclein in all nematode neurons in a strain carrying this eri-1 mutation. Under these conditions, there was little gross effect on the animals. They then performed an enhancer RNAi screen targeting 1,673 prioritized candidate genes (genes known to affect the nervous system) to identify RNAi treatments that induced a visible phenotype such as uncoordinated movement or growth retardation. Ten candidate genes passed their screening criteria; four of these genes functioned in the endocytic machinery, implicating endocytosis in the pathogenesis of α-Synuclein.
6. Conclusion
The choice of models to investigate human disease is often a trade-off between how well the model mimics the human condition and how easy it is to manipulate the system. Invertebrate models such as C. elegans and D. melanogaster have been invaluable for the study of development, signaling pathways, and many other aspects of biology. In this chapter, we have outlined several examples that illustrate the ease of such C. elegans studies. Some of the features that have rendered C. elegans such a powerful research organism include the ease of genetics (forward genetic screening, transgenic animal construction, mutation mapping), cell biology (using GFP in a transparent organism with a fully-described and invariant cell lineage), genomics (RNAi and other techniques), modifier screens (enhancement and suppression), and the ability to mimic many human diseases. We also have highlighted how more and more frequently, follow-up studies in mammals have validated these nematode findings. The tools and ease-of-use of C. elegans and other “simple” model organisms continues to make them invaluable for research, and these organisms will continue to play an important role in our understanding of human disease and human disease gene discovery in the future.
Acknowledgments
This work was supported by National Institutes of Health Grant 1R01ES025161 to S.A.
REFERENCES
- 1.Aitman TJ, Boone C, Churchill GA, Hengartner MO, Mackay TF, Stemple DL (2011) The future of model organisms in human disease research. Nature reviews Genetics 12 (8):575–582. [DOI] [PubMed] [Google Scholar]
- 2.Lieschke GJ, Currie PD (2007) Animal models of human disease: zebrafish swim into view. Nature reviews Genetics 8 (5):353–367. [DOI] [PubMed] [Google Scholar]
- 3.Lopez Hernandez Y, Yero D, Pinos-Rodriguez JM, Gibert I (2015) Animals devoid of pulmonary system as infection models in the study of lung bacterial pathogens. Frontiers in microbiology 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markaki M, Tavernarakis N (2010) Modeling human diseases in Caenorhabditis elegans. Biotechnology journal 5 (12):1261–1276. [DOI] [PubMed] [Google Scholar]
- 5.Pandey UB, Nichols CD (2011) Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacological reviews 63 (2):411–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(1997). In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds) elegans C II. 2nd edn., Cold Spring Harbor (NY: ), [Google Scholar]
- 7.Wood WB (1988) The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, New York [Google Scholar]
- 8.Sulston JE, Horvitz HR (1977) Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Developmental biology 56 (1):110–156. [DOI] [PubMed] [Google Scholar]
- 9.Sulston JE, Schierenberg E, White JG, Thomson JN (1983) The embryonic cell lineage of the nematode Caenorhabditis elegans. Developmental biology 100 (1):64–119. [DOI] [PubMed] [Google Scholar]
- 10.Varshney LR, Chen BL, Paniagua E, Hall DH, Chklovskii DB (2011) Structural properties of the Caenorhabditis elegans neuronal network. PLoS computational biology 7 (2):e1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuel BS, Rowedder H, Braendle C, Felix MA, Ruvkun G (2016) Caenorhabditis elegans responses to bacteria from its natural habitats. Proceedings of the National Academy of Sciences of the United States of America 113 (27):E3941–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis JA, Fleming JT (1995) Basic culture methods. Methods in cell biology 48:3–29. [PubMed] [Google Scholar]
- 13.Shaye DD, Greenwald I (2011) OrthoList: a compendium of C. elegans genes with human orthologs. PloS one 6 (5):e20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonnhammer EL, Durbin R (1997) Analysis of protein domain families in Caenorhabditis elegans. Genomics 46 (2):200–216. [DOI] [PubMed] [Google Scholar]
- 15.Corsi AK, Wightman B, Chalfie M (2015) A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics 200 (2):387–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strange K (2006) An overview of C. elegans biology. Methods in molecular biology 351:1–11. [DOI] [PubMed] [Google Scholar]
- 17.Ellis HM, Horvitz HR (1986) Genetic control of programmed cell death in the nematode C. elegans. Cell 44 (6):817–829. [DOI] [PubMed] [Google Scholar]
- 18.Check E (2002) Worm cast in starring role for Nobel prize. Nature 419 (6907):548–549. [DOI] [PubMed] [Google Scholar]
- 19.Marx J (2002) Nobel Prize in Physiology or Medicine. Tiny worm takes a star turn. Science 298 (5593):526. [DOI] [PubMed] [Google Scholar]
- 20.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 (6669):806–811. [DOI] [PubMed] [Google Scholar]
- 21.Roda A (2010) Discovery and development of the green fluorescent protein, GFP: the 2008 Nobel Prize. Analytical and bioanalytical chemistry 396 (5):1619–1622. [DOI] [PubMed] [Google Scholar]
- 22.Kricka LJ, Stanley PE (2009) Scientists awarded Nobel Prize for work with GFP. Luminescence : the journal of biological and chemical luminescence 24 (1):1. [DOI] [PubMed] [Google Scholar]
- 23.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as a marker for gene expression. Science 263 (5148):802–805. [DOI] [PubMed] [Google Scholar]
- 24.Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75 (5):843–854. [DOI] [PubMed] [Google Scholar]
- 25.Wightman B, Ha I, Ruvkun G (1993) Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75 (5):855–862. [DOI] [PubMed] [Google Scholar]
- 26.Mello C, Fire A (1995) DNA transformation. Methods in cell biology 48:451–482. [PubMed] [Google Scholar]
- 27.Stinchcomb DT, Shaw JE, Carr SH, Hirsh D (1985) Extrachromosomal DNA transformation of Caenorhabditis elegans. Molecular and cellular biology 5 (12):3484–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. The EMBO journal 10 (12):3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fire A (1986) Integrative transformation of Caenorhabditis elegans. The EMBO journal 5 (10):2673–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Praitis V, Casey E, Collar D, Austin J (2001) Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157 (3):1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweinsberg PJ, Grant BD (2013) C. elegans gene transformation by microparticle bombardment. WormBook : the online review of C elegans biology:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickinson DJ, Goldstein B (2016) CRISPR-Based Methods for Caenorhabditis elegans Genome Engineering. Genetics 202 (3):885–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HM, Colaiacovo MP (2016) CRISPR-Cas9-Guided Genome Engineering in C. elegans. Current protocols in molecular biology / edited by Frederick M Ausubel [et al] 115:31 37 31–31 37 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson P (1995) Mutagenesis. Methods in cell biology 48:31–58. [PubMed] [Google Scholar]
- 35.Kutscher LM, Shaham S (2014) Forward and reverse mutagenesis in C. elegans. WormBook : the online review of C elegans biology:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Sherwood DR (2011) Dissection of genetic pathways in C. elegans. Methods in cell biology 106:113–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fay DS (2013) Classical genetic methods. WormBook : the online review of C elegans biology:1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77 (1):71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams BD (1995) Genetic mapping with polymorphic sequence-tagged sites. Methods in cell biology 48:81–96. [DOI] [PubMed] [Google Scholar]
- 40.Hu PJ (2014) Whole genome sequencing and the transformation of C. elegans forward genetics. Methods 68 (3):437–440. [DOI] [PubMed] [Google Scholar]
- 41.Hobert O (2010) The impact of whole genome sequencing on model system genetics: get ready for the ride. Genetics 184 (2):317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bessereau JL (2006) Transposons in C. elegans. WormBook : the online review of C elegans biology:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timmons L, Fire A (1998) Specific interference by ingested dsRNA. Nature 395 (6705):854. [DOI] [PubMed] [Google Scholar]
- 44.Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408 (6810):325–330. [DOI] [PubMed] [Google Scholar]
- 45.Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, Vidal M (2004) Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome research 14 (10B):2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kennedy S, Wang D, Ruvkun G (2004) A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature 427 (6975):645–649. [DOI] [PubMed] [Google Scholar]
- 47.Simmer F, Tijsterman M, Parrish S, Koushika SP, Nonet ML, Fire A, Ahringer J, Plasterk RH (2002) Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Current biology : CB 12 (15):1317–1319. [DOI] [PubMed] [Google Scholar]
- 48.Consortium CeDM (2012) large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3 2 (11):1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitani S (2009) Nematode, an experimental animal in the national BioResource project. Experimental animals / Japanese Association for Laboratory Animal Science 58 (4):351–356. [DOI] [PubMed] [Google Scholar]
- 50.Frokjaer-Jensen C (2015) Transposon-Assisted Genetic Engineering with Mos1-Mediated Single-Copy Insertion (MosSCI). Methods in molecular biology 1327:49–58. [DOI] [PubMed] [Google Scholar]
- 51.World Health Statistics 2014 (2015).
- 52.Detailed Tables for the National Vital Statistics Report (NVSR) “Deaths: Final Data for 2013” (2015). vol 64. [PubMed] [Google Scholar]
- 53.Chaudhuri N, Dower SK, Whyte MK, Sabroe I (2005) Toll-like receptors and chronic lung disease. Clinical science 109 (2):125–133. [DOI] [PubMed] [Google Scholar]
- 54.Cook DN, Pisetsky DS, Schwartz DA (2004) Toll-like receptors in the pathogenesis of human disease. Nature immunology 5 (10):975–979. [DOI] [PubMed] [Google Scholar]
- 55.Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140 (6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda K, Akira S (2005) Toll-like receptors in innate immunity. International immunology 17 (1):1–14. [DOI] [PubMed] [Google Scholar]
- 57.Kovach MA, Standiford TJ (2011) Toll like receptors in diseases of the lung. International immunopharmacology 11 (10):1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medvedev AE (2013) Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 33 (9):467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Misch EA, Hawn TR (2008) Toll-like receptor polymorphisms and susceptibility to human disease. Clinical science 114 (5):347–360. [DOI] [PubMed] [Google Scholar]
- 60.Netea MG, Wijmenga C, O’Neill LA (2012) Genetic variation in Toll-like receptors and disease susceptibility. Nature immunology 13 (6):535–542. [DOI] [PubMed] [Google Scholar]
- 61.O’Neill LA (2003) Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr Opin Pharmacol 3 (4):396–403. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz DA, Cook DN (2005) Polymorphisms of the Toll-like receptors and human disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 41 Suppl 7 (29):S403–407. [DOI] [PubMed] [Google Scholar]
- 63.Ermolaeva MA, Schumacher B (2014) Insights from the worm: the C. elegans model for innate immunity. Seminars in immunology 26 (4):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irazoqui J, Ausubel F (2010) 99th Dahlem Conference on infection, inflammation, and chronic inflammatory disorders: Caenorhabditis elegans as a model to study tissues involved in host immunity and microbial pathogenesis. Clinical and experimental immunology 160:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pukkila-Worley R, Ausubel FM (2012) Immune defense mechanisms in the Caenorhabditis elegans intestinal epithelium. Current opinion in immunology 24 (1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahajan-Miklos S, Tan MW, Rahme LG, Ausubel FM (1999) Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96 (1):47–56. [DOI] [PubMed] [Google Scholar]
- 67.Tan MW, Mahajan-Miklos S, Ausubel FM (1999) Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proceedings of the National Academy of Sciences of the United States of America 96 (2):715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM (1999) Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proceedings of the National Academy of Sciences of the United States of America 96 (5):2408–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen LB, Troemel ER (2015) Microbial pathogenesis and host defense in the nematode C. elegans. Current opinion in microbiology 23:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diogo J, Bratanich A (2014) The nematode Caenorhabditis elegans as a model to study viruses. Archives of virology 159 (11):2843–2851. [DOI] [PubMed] [Google Scholar]
- 71.Arvanitis M, Glavis-Bloom J, Mylonakis E (2013) Invertebrate models of fungal infection. Biochimica et biophysica acta 1832 (9):1378–1383. [DOI] [PubMed] [Google Scholar]
- 72.Darby C (2005) Interactions with microbial pathogens. WormBook : the online review of C elegans biology:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dierking K, Yang W, Schulenburg H (2016) Antimicrobial effectors in the nematode Caenorhabditis elegans: an outgroup to the Arthropoda. Philosophical transactions of the Royal Society of London Series B, Biological sciences 371 (1695). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim DH, Ewbank JJ (2015) Signaling in the innate immune response. WormBook : the online review of C elegans biology:1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Irazoqui JE, Troemel ER, Feinbaum RL, Luhachack LG, Cezairliyan BO, Ausubel FM (2010) Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS pathogens 6:e1000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Visvikis O, Ihuegbu N, Labed SA, Luhachack LG, Alves AM, Wollenberg AC, Stuart LM, Stormo GD, Irazoqui JE (2014) Innate host defense requires TFEB-mediated transcription of cytoprotective and antimicrobial genes. Immunity 40 (6):896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pastore N, Brady OA, Diab HI, Martina JA, Sun L, Huynh T, Lim JA, Zare H, Raben N, Ballabio A, Puertollano R (2016) TFEB and TFE3 cooperate in the regulation of the innate immune response in activated macrophages. Autophagy:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Najibi M, Labed SA, Visvikis O, Irazoqui JE (2016) An Evolutionarily Conserved PLC-PKD-TFEB Pathway for Host Defense. Cell reports 15 (8):1728–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alper S, McBride SJ, Lackford B, Freedman JH, Schwartz DA (2007) Specificity and complexity of the C. elegans innate immune response. Molecular and cellular biology 27 (15):5544–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pulak R (2006) Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods in molecular biology 351:275–286. [DOI] [PubMed] [Google Scholar]
- 81.Alper S, Laws R, Lackford B, Boyd WA, Dunlap P, Freedman JH, Schwartz DA (2008) Identification of innate immunity genes and pathways using a comparative genomics approach. ProcNatl Acad Sci USA 105 (19):7016–7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Arras L, Laws R, Leach SM, Pontis K, Freedman JH, Schwartz DA, Alper S (2014) Comparative genomics RNAi screen identifies Eftud2 as a novel regulator of innate immunity. Genetics 197 (2):485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Arras L, Seng A, Lackford B, Keikhaee MR, Bowerman B, Freedman JH, Schwartz DA, Alper S (2013) An evolutionarily conserved innate immunity protein interaction network. The Journal of biological chemistry 288 (3):1967–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Arras L, Yang IV, Lackford B, Riches DW, Prekeris R, Freedman JH, Schwartz DA, Alper S (2012) Spatiotemporal Inhibition of Innate Immunity Signaling by the Tbc1d23 RAB-GAP. Journal of immunology 188 (6):2905–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Editorial (2003) Whither RNAi? Nature cell biology 5 (6):489–490. [DOI] [PubMed] [Google Scholar]
- 86.De Arras L, Alper S (2013) Limiting of the innate immune response by SF3A-dependent control of MyD88 alternative mRNA splicing. PLoS Genet 9 (10):e1003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Connor BP, Danhorn T, De Arras L, Flatley BR, Marcus RA, Farias-Hesson E, Leach SM, Alper S (2015) Regulation of toll-like receptor signaling by the SF3a mRNA splicing complex. PLoS Genet 11 (2):e1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama 307 (5):491–497. [DOI] [PubMed] [Google Scholar]
- 89.Haslam DW, James WP (2005) Obesity. Lancet 366 (9492):1197–1209. [DOI] [PubMed] [Google Scholar]
- 90.Bleich S, Cutler D, Murray C, Adams A (2008) Why is the developed world obese? Annual review of public health 29:273–295. [DOI] [PubMed] [Google Scholar]
- 91.Ashrafi K (2007) Obesity and the regulation of fat metabolism. WormBook : the online review of C elegans biology:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones KT, Ashrafi K (2009) Caenorhabditis elegans as an emerging model for studying the basic biology of obesity. Disease models & mechanisms 2 (5–6):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng J, Greenway FL (2012) Caenorhabditis elegans as a model for obesity research. International journal of obesity 36 (2):186–194. [DOI] [PubMed] [Google Scholar]
- 94.McKay RM, McKay JP, Avery L, Graff JM (2003) C elegans: a model for exploring the genetics of fat storage. Developmental cell 4 (1):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G (2003) Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421 (6920):268–272. [DOI] [PubMed] [Google Scholar]
- 96.Liu Z, Li X, Ge Q, Ding M, Huang X (2014) A lipid droplet-associated GFP reporter-based screen identifies new fat storage regulators in C. elegans. Journal of genetics and genomics = Yi chuan xue bao 41 (5):305–313. [DOI] [PubMed] [Google Scholar]
- 97.Klass MR (1983) A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev 22 (3–4):279–286. [DOI] [PubMed] [Google Scholar]
- 98.Friedman DB, Johnson TE (1988) A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118 (1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Friedman DB, Johnson TE (1988) Three mutants that extend both mean and maximum life span of the nematode, Caenorhabditis elegans, define the age-1 gene. Journal of gerontology 43 (4):B102–109. [DOI] [PubMed] [Google Scholar]
- 100.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366 (6454):461–464.101. [DOI] [PubMed] [Google Scholar]
- 101.Riddle DL, Swanson MM, Albert PS (1981) Interacting genes in nematode dauer larva formation. Nature 290 (5808):668–671. [DOI] [PubMed] [Google Scholar]
- 102.Hu PJ (2007) Dauer. WormBook : the online review of C elegans biology:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Albert PS, Riddle DL (1988) Mutants of Caenorhabditis elegans that form dauer-like larvae. Developmental biology 126 (2):270–293. [DOI] [PubMed] [Google Scholar]
- 104.Golden JW, Riddle DL (1984) A pheromone-induced developmental switch in Caenorhabditis elegans: Temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proceedings of the National Academy of Sciences of the United States of America 81 (3):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murphy CT, Hu PJ (2013) Insulin/insulin-like growth factor signaling in C. elegans. WormBook : the online review of C elegans biology:1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Altintas O, Park S, Lee SJ (2016) The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB reports 49 (2):81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giannakou ME, Partridge L (2007) Role of insulin-like signalling in Drosophila lifespan. Trends in biochemical sciences 32 (4):180–188. [DOI] [PubMed] [Google Scholar]
- 108.Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M (2004) Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429 (6991):562–566. [DOI] [PubMed] [Google Scholar]
- 109.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS (2001) A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292 (5514):107–110. [DOI] [PubMed] [Google Scholar]
- 110.Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L (2001) Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292 (5514):104–106. [DOI] [PubMed] [Google Scholar]
- 111.Kappeler L, De Magalhaes Filho C, Dupont J, Leneuve P, Cervera P, Perin L, Loudes C, Blaise A, Klein R, Epelbaum J, Le Bouc Y, Holzenberger M (2008) Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS biology 6 (10):e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421 (6919):182–187. [DOI] [PubMed] [Google Scholar]
- 113.Brooks-Wilson AR (2013) Genetics of healthy aging and longevity. Human genetics 132 (12):1323–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung WH, Dao RL, Chen LK, Hung SI (2010) The role of genetic variants in human longevity. Ageing research reviews 9 Suppl 1:S67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anselmi CV, Malovini A, Roncarati R, Novelli V, Villa F, Condorelli G, Bellazzi R, Puca AA (2009) Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation research 12 (2):95–104. [DOI] [PubMed] [Google Scholar]
- 116.Daumer C, Flachsbart F, Caliebe A, Schreiber S, Nebel A, Krawczak M (2014) Adjustment for smoking does not alter the FOXO3A association with longevity. Age 36 (2):911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A (2009) Association of FOXO3A variation with human longevity confirmed in German centenarians. Proceedings of the National Academy of Sciences of the United States of America 106 (8):2700–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuningas M, Magi R, Westendorp RG, Slagboom PE, Remm M, van Heemst D (2007) Haplotypes in the human Foxo1a and Foxo3a genes; impact on disease and mortality at old age. European journal of human genetics : EJHG 15 (3):294–301. [DOI] [PubMed] [Google Scholar]
- 119.Li Y, Wang WJ, Cao H, Lu J, Wu C, Hu FY, Guo J, Zhao L, Yang F, Zhang YX, Li W, Zheng GY, Cui H, Chen X, Zhu Z, He H, Dong B, Mo X, Zeng Y, Tian XL (2009) Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Human molecular genetics 18 (24):4897–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lunetta KL, D’Agostino RB Sr., Karasik D, Benjamin EJ, Guo CY, Govindaraju R, Kiel DP, Kelly-Hayes M, Massaro JM, Pencina MJ, Seshadri S, Murabito JM (2007) Genetic correlates of longevity and selected age-related phenotypes: a genome-wide association study in the Framingham Study. BMC medical genetics 8 Suppl 1:S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E, Study of Osteoporotic F (2009) Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging cell 8 (4):460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soerensen M, Dato S, Christensen K, McGue M, Stevnsner T, Bohr VA, Christiansen L (2010) Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging cell 9 (6):1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Soerensen M, Nygaard M, Dato S, Stevnsner T, Bohr VA, Christensen K, Christiansen L (2015) Association study of FOXO3A SNPs and aging phenotypes in Danish oldest-old individuals. Aging cell 14 (1):60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD (2008) FOXO3A genotype is strongly associated with human longevity. Proceedings of the National Academy of Sciences of the United States of America 105 (37):13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P (2008) Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proceedings of the National Academy of Sciences of the United States of America 105 (9):3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tazearslan C, Huang J, Barzilai N, Suh Y (2011) Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles. Aging cell 10 (3):551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Curran SP, Ruvkun G (2007) Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet 3 (4):e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G, Lee SS (2005) A systematic RNAi screen for longevity genes in C. elegans. Genes & development 19 (13):1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hansen M, Hsu AL, Dillin A, Kenyon C (2005) New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet 1 (1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Samuelson AV, Carr CE, Ruvkun G (2007) Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes & development 21 (22):2976–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ni Z, Lee SS (2010) RNAi screens to identify components of gene networks that modulate aging in Caenorhabditis elegans. Briefings in functional genomics 9 (1):53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yanos ME, Bennett CF, Kaeberlein M (2012) Genome-Wide RNAi Longevity Screens in Caenorhabditis elegans. Current genomics 13 (7):508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L (2015) Interventions to Slow Aging in Humans: Are We Ready? Aging cell 14 (4):497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Riera CE, Dillin A (2015) Can aging be ‘drugged’? Nature medicine 21 (12):1400–1405. [DOI] [PubMed] [Google Scholar]
- 135.Weber JJ, Sowa AS, Binder T, Hubener J (2014) From pathways to targets: understanding the mechanisms behind polyglutamine disease. BioMed research international 2014:701758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao XN, Usdin K (2015) The Repeat Expansion Diseases: The dark side of DNA repair. DNA repair 32:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Olejniczak M, Urbanek MO, Krzyzosiak WJ (2015) The role of the immune system in triplet repeat expansion diseases. Mediators of inflammation 2015:873860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee DY, McMurray CT (2014) Trinucleotide expansion in disease: why is there a length threshold? Current opinion in genetics & development 26:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iyer RR, Pluciennik A, Napierala M, Wells RD (2015) DNA triplet repeat expansion and mismatch repair. Annual review of biochemistry 84:199–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morley JF, Brignull HR, Weyers JJ, Morimoto RI (2002) The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 99 (16):10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brignull HR, Moore FE, Tang SJ, Morimoto RI (2006) Polyglutamine proteins at the pathogenic threshold display neuron-specific aggregation in a pan-neuronal Caenorhabditis elegans model. The Journal of neuroscience : the official journal of the Society for Neuroscience 26 (29):7597–7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Faber PW, Alter JR, MacDonald ME, Hart AC (1999) Polyglutamine-mediated dysfunction and apoptotic death of a Caenorhabditis elegans sensory neuron. Proceedings of the National Academy of Sciences of the United States of America 96 (1):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nollen EA, Garcia SM, van Haaften G, Kim S, Chavez A, Morimoto RI, Plasterk RH (2004) Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proceedings of the National Academy of Sciences of the United States of America 101 (17):6403–6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Caldwell GA, Cao S, Sexton EG, Gelwix CC, Bevel JP, Caldwell KA (2003) Suppression of polyglutamine-induced protein aggregation in Caenorhabditis elegans by torsin proteins. Human molecular genetics 12 (3):307–319. [DOI] [PubMed] [Google Scholar]
- 145.Wang H, Lim PJ, Yin C, Rieckher M, Vogel BE, Monteiro MJ (2006) Suppression of polyglutamine-induced toxicity in cell and animal models of Huntington’s disease by ubiquilin. Human molecular genetics 15 (6):1025–1041. [DOI] [PubMed] [Google Scholar]
- 146.Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. The New England journal of medicine 362 (4):329–344. [DOI] [PubMed] [Google Scholar]
- 147.Burns A, Iliffe S (2009) Alzheimer’s disease. Bmj 338:b158. [DOI] [PubMed] [Google Scholar]
- 148.Dujardin S, Colin M, Buee L (2015) Invited review: Animal models of tauopathies and their implications for research/translation into the clinic. Neuropathology and applied neurobiology 41 (1):59–80. [DOI] [PubMed] [Google Scholar]
- 149.Wentzell J, Kretzschmar D (2010) Alzheimer’s disease and tauopathy studies in flies and worms. Neurobiology of disease 40 (1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski JQ, Schellenberg GD (2003) Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proceedings of the National Academy of Sciences of the United States of America 100 (17):9980–9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kraemer BC, Burgess JK, Chen JH, Thomas JH, Schellenberg GD (2006) Molecular pathways that influence human tau-induced pathology in Caenorhabditis elegans. Human molecular genetics 15 (9):1483–1496. [DOI] [PubMed] [Google Scholar]
- 152.Hannan SB, Drager NM, Rasse TM, Voigt A, Jahn TR (2016) Cellular and molecular modifier pathways in tauopathies: the big picture from screening invertebrate models. Journal of neurochemistry 137 (1):12–25. [DOI] [PubMed] [Google Scholar]
- 153.Guthrie CR, Schellenberg GD, Kraemer BC (2009) SUT-2 potentiates tau-induced neurotoxicity in Caenorhabditis elegans. Human molecular genetics 18 (10):1825–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guthrie CR, Greenup L, Leverenz JB, Kraemer BC (2011) MSUT2 is a determinant of susceptibility to tau neurotoxicity. Human molecular genetics 20 (10):1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alexander AG, Marfil V, Li C (2014) Use of Caenorhabditis elegans as a model to study Alzheimer’s disease and other neurodegenerative diseases. Frontiers in genetics 5:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ewald CY, Li C (2010) Understanding the molecular basis of Alzheimer’s disease using a Caenorhabditis elegans model system. Brain structure & function 214 (2–3):263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hassan WM, Dostal V, Huemann BN, Yerg JE, Link CD (2015) Identifying Abeta-specific pathogenic mechanisms using a nematode model of Alzheimer’s disease. Neurobiology of aging 36 (2):857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hassan WM, Merin DA, Fonte V, Link CD (2009) AIP-1 ameliorates beta-amyloid peptide toxicity in a Caenorhabditis elegans Alzheimer’s disease model. Human molecular genetics 18 (15):2739–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Link CD (2006) C. elegans models of age-associated neurodegenerative diseases: lessons from transgenic worm models of Alzheimer’s disease. Experimental gerontology 41 (10):1007–1013. [DOI] [PubMed] [Google Scholar]
- 160.Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y (2006) Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. The Journal of neuroscience : the official journal of the Society for Neuroscience 26 (50):13102–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. (1995) Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269 (5226):973–977. [DOI] [PubMed] [Google Scholar]
- 162.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, et al. (1995) Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376 (6543):775–778. [DOI] [PubMed] [Google Scholar]
- 163.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375 (6534):754–760. [DOI] [PubMed] [Google Scholar]
- 164.Levitan D, Doyle TG, Brousseau D, Lee MK, Thinakaran G, Slunt HH, Sisodia SS, Greenwald I (1996) Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 93 (25):14940–14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Levitan D, Greenwald I (1995) Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer’s disease gene. Nature 377 (6547):351–354. [DOI] [PubMed] [Google Scholar]
- 166.Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LH, Sisodia SS (1997) Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 387 (6630):288–292. [DOI] [PubMed] [Google Scholar]
- 167.Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D (2002) aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Developmental cell 3 (1):85–97. [DOI] [PubMed] [Google Scholar]
- 168.Goutte C, Tsunozaki M, Hale VA, Priess JR (2002) APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proceedings of the National Academy of Sciences of the United States of America 99 (2):775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Luo WJ, Wang H, Li H, Kim BS, Shah S, Lee HJ, Thinakaran G, Kim TW, Yu G, Xu H (2003) PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. The Journal of biological chemistry 278 (10):7850–7854. [DOI] [PubMed] [Google Scholar]
- 170.Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363 (9423):1783–1793. [DOI] [PubMed] [Google Scholar]
- 171.Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annual review of pathology 6:193–222. [DOI] [PubMed] [Google Scholar]
- 172.Davie CA (2008) A review of Parkinson’s disease. British medical bulletin 86:109–127. [DOI] [PubMed] [Google Scholar]
- 173.Atik A, Stewart T, Zhang J (2016) Alpha-Synuclein as a Biomarker for Parkinson’s Disease. Brain pathology 26 (3):410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schulz-Schaeffer WJ (2010) The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta neuropathologica 120 (2):131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chege PM, McColl G (2014) Caenorhabditis elegans: a model to investigate oxidative stress and metal dyshomeostasis in Parkinson’s disease. Frontiers in aging neuroscience 6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA (2008) C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet 4 (3):e1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hamamichi S, Rivas RN, Knight AL, Cao S, Caldwell KA, Caldwell GA (2008) Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson’s disease model. Proceedings of the National Academy of Sciences of the United States of America 105 (2):728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kuwahara T, Koyama A, Koyama S, Yoshina S, Ren CH, Kato T, Mitani S, Iwatsubo T (2008) A systematic RNAi screen reveals involvement of endocytic pathway in neuronal dysfunction in alpha-synuclein transgenic C. elegans. Human molecular genetics 17 (19):2997–3009. [DOI] [PubMed] [Google Scholar]