Abstract
Acute lung injury (ALI) is characterized by endothelial barrier disruption resulting in increased vascular permeability. As focal adhesion kinase (FAK), a non-receptor protein tyrosine kinase, is involved in endothelial cell (EC) barrier regulation, we hypothesized that FAK inhibition could attenuate agonist-induced EC barrier disruption relevant to ALI. Human lung EC were pretreated with one of three pharmacologic FAK inhibitors, PF-573,228 (PF-228, 10 μM), PF-562,271 (PF-271, 5 μM) or NVP-TAE226 (TAE226, 5 μM) for 30 min prior to treatment with thrombin (1 U/ml, 30 min). Western blotting confirmed attenuated thrombin-induced FAK phosphorylation associated with all three inhibitors. Subsequently, EC were pretreated with either PF-228 (10 μM), TAE226 (5 μM) or PF-271 (5 μM) for 30 min prior to thrombin stimulation (1 U/ml) followed by measurements of barrier integrity by transendothelial electrical resistance (TER). Separately, EC grown in transwell inserts prior to thrombin (1 U/ml) with measurements of FITC-dextran flux after 30 min confirmed a significant attenuation of thrombin-induced EC barrier disruption by PF-228 alone. Finally, in a murine ALI model induced by LPS (1.25 mg/ml, IT), rescue treatment with PF-228 was associated with significantly reduced lung injury. Our findings PF-228, currently being studied in clinical trials, may serve as a novel and effective therapeutic agent for ALI.
Keywords: endothelial permeability, acute lung injury, FAK
1.0. INTRODUCTION
Acute lung injury (ALI) is a challenging clinical problem associated with significant morbidity and mortality (1). However, effective targeted therapeutics for patients with ALI remain non-existent. As a prominent feature of ALI is increased lung vascular permeability associated with endothelial cell (EC) barrier disruption, strategies aimed at mitigating EC barrier dysfunction may ultimately yield novel and effective ALI treatments (2). One potential target in this regard is focal adhesion kinase (FAK), a 120 kD non-receptor tyrosine kinase that serves a central role in EC barrier regulation via diverse effects on cell signaling and function (3–5). While the role of FAK on EC barrier regulation is complex, the recent reports of novel pharmacologic FAK inhibitors in other clinical contexts led us to hypothesize that these agents would have beneficial effects in ALI.
FAK is commonly found associated with focal adhesion complexes at the EC membrane, with linkages to integrins and actin cytoskeleton serving as anchor points for actin stress fibers, and is a mediator of cell-matrix attachments, adherens junctional formation, and lamellipodial dynamics (3, 6). While abundant evidence has implicated FAK as an important mediator of EC barrier function and vascular permeability variable experimental contexts described in the literature have implicated both increased and decreased barrier integrity associated with FAK activity. The potential role of FAK in ALI in particular can be gleaned from evidence of increased lung vascular permeability in mice deficient in EC FAK (4). In contrast, lung knockdown of FAK by siRNA is associated with an attenuation of LPS-induced lung injury in rats (7).
The role of FAK in cancer biology has been more thoroughly characterized as increased FAK expression and activation has been reported in a variety of solid tumors (8). This observation has led to intense interest in FAK inhibition as a potential cancer treatment. Indeed, to date a number of pharmacologic inhibitors of FAK have been identified, several of which are actively being studied in pre-clinical and early phase cancer trials (9, 10). We investigated the potential role of three such inhibitors as novel therapeutic agents for ALI, including PF-573,228 (PF-228), PF-562,271 (PF-271) and NVP-TAE226 (TAE226), all ATP-competitive small molecule inhibitors of FAK activation.
2.0. MATERIALS AND METHODS
2.1. Antibodies and reagents.
The small-molecule compounds PF-562,271 (PF-271), PF-573,228 (PF-228) and NVP-TAE226 (TAE226) were purchased from Selleck Chemicals (Houston, TX). The primary phospho-FAK antibodies (Tyr-397, Tyr-576 and Tyr-925) were purchased from Cell Signaling (Danvers, MA); total FAK antibody was obtained from Santa Cruz (Dallas, Texas). Enhanced chemiluminescence Western blot detection reagents were purchased from Thermo Fisher Scientific Inc. (Rockford, IL). Alexa Fluor 488 goat anti-rabbit IgG and Texas Red-X Phalloidin were purchased from Invitrogen (Cambridge, MA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Cell culture and Western blotting.
Human pulmonary artery EC were purchased from Clonetics (San Diego, CA) and were cultured in EGM-2 supplemented with 2% FBS, hydrocortisone, hFGF, VEGF, ascorbic acid, hEGF, GA-1000, heparin, R3-IGF-1 (Clonetics). The cells were incubated in 75 cm2 flask and cultured at 37°C in 5%CO2 and 95% air. All cells were used at passages 4–8. For Western blotting, samples were harvested with RIPA buffer containing proteinase inhibitors and phosphatase inhibitors as per standard protocols. After sonication and centrifugation, the supernatant was collected, Laemmli sample buffer added, and was then boiled and subsequently analyzed by SDS–PAGE. After transfer to a nitrocellulose membrane (Bio-Rad, Inc., Hercules, CA), Western blotting was performed using appropriate primary antibodies and horseradish peroxidase-conjugated secondary antibodies prior to visualization via chemiluminescence (Amersham Biosciences, Piscataway, NJ). Blot density was determined by Alpha Imager software (Alpha Innotech, San Leandro, CA).
2.3. Measurements of transendothelial electrical resistance (TER).
EC were grown to confluence over evaporated gold microelectrodes connected to a phase-sensitive lock-in amplifier as previously described (11). TER was measured in response to specific agonists using an electrical cell-substrate impedance sensing system (Applied BioPhysics Inc., Troy, NY).
2.4. Measurements of FITC-dextran transwell flux.
A commercially available kit (Millipore, Billerica, CA) was used to measure EC monolayer permeability to high and low molecular weight proteins based on the Transwell model our laboratory previously described (11). In separate experiments, 100 μl FITC-dextran (2,000 kDa) or 2 μg/ml sodium fluroescein (376 Da) was added to cells and incubated for 1 h. The transwell insert was then removed and 100 μl medium collected. Fluorescent density was analyzed on a Titertek Fluoroskan II Microplate Fluorometer (Diversified Equipment, Lorton, VA) at excitation and emission wavelengths of 485 nm and 530 nm, respectively.
2.5. Immunofluorescence imaging.
Confluent EC grown on coverslips were exposed to experimental conditions, fixed with 3.7% formaldehyde, and permeabilized with 0.25% Triton X-100. After blocking with 2% bovine serum albumin, cells were exposed to primary antibodies for 60 min. Fluorescently-tagged secondary antibodies were applied for 60 min. Cells were imaged using a Nikon video imaging system. To quantify Texas Red stained actin stress fibers, images were analyzed with respect to the ratio of stress fiber area relative to whole cells using MetaVue 4.6 (Universal Imaging, Downington, PA) and statistically processed using Sigma Plot 7.1 (SPSS Science, Chicago).
2.6. Murine ALI model.
All experiments and animal care procedures were approved by the University of Illinois at Chicago (UIC) Animal Care and Use Committee and were performed in accordance with Guide for the Care and Use of Laboratory Animals, Eighth Edition published by the Institute for Laboratory Animal Research. The animals were housed in the UIC animal facility in the Biologic Resources Laboratory (BRL), a centralized animal facility. The staff of the BRL oversaw the procurement, care, and maintenance of animals used in the research. Procedure rooms for the proposed animal experiments are adjacent to the animal housing facilities.
Murine ALI experiments were conducted as we have previously reported (12). Briefly, female C57Bl/6 (20–25 g) mice 8–10 weeks old were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were anesthetized with intraperitoneal mix of ketamine (150 mg/kg) and xylazine (15 mg/kg). For the group of animals exposed to LPS, Escherichia coli 127-B8 endotoxin solution (2.5 mg/kg) or sterile saline was instilled intratracheally via a 20-gauge intravenous catheter. PF-228 or vehicle was injected intraperitoneally (IP) 1 h after the administration of LPS. The animals were allowed to recover and observed for 18 h after which lung injury was assessed. Predetermined primary outcomes included bronchoalveolar (BAL) lavage total protein and cell counts as well evaluations of lung histology as detailed below.
All mice were observed daily for signs of pain, distress or moribund features such as marked lethargy, hunched posture with rough coat or significant respiratory distress with acryocyanosis, mouth breathing, or tachypnea. No animals required additional anesthesia or met criteria for euthanasia prior to achieving the experimental endpoints.
2.7. BAL protein and cell counts.
BAL was performed by flushing the lungs with 1 ml of cold Hanks’ balanced salt solution (HBSS; Invitrogen, Grand Island, NY) through the tracheal cannula, as previously described (13). The recovered lavage fluid (∼0.8 ml) was centrifuged (500 × g for 20 min), red blood cells from the pellet were lysed using ACK lysing buffer and samples were processed and suspended in HBSS for cell count. Total and differential cell counts were counted as we have previously described (12) with an automated cell counter (TC-20, Biorad). The supernatant from BAL fluid was centrifuged again (15,000 × g for 10 min), and the supernatant was stored at −80°C for further protein analysis. The protein concentration in BAL was determined with the use of Pierce™ BCA protein assay kit (Thermo Scientific, Waltham, MA).
2.8. Lung histology.
To characterize histological alterations, select lungs from each experimental group were inflated to 30 cm H2O with 10% formalin for histological evaluation by hematoxylin and eosin (H&E) staining. Representative images were selected for each experimental condition. To quantify relative inflammatory cell infiltration, H&E stained lung sections (40x magnification, n=3 per condition) were assessed by an individual experienced in lung pathology and blinded to the experiments. The number of neutrophils visible in either the alveolar spaces or interstitium were counted and averaged from five randomly selected fields for each lung section.
2.9. Statistical analysis.
Student’s t-test was used to compare the means of data from two experimental groups while significant differences (p < 0.05) amongst multiple group comparisons were confirmed by two-way ANOVA. Results are expressed as means ± SE.
3.0. RESULTS
3.1. Attenuation of thrombin-induced FAK phosphorylation by pharmacologic FAK inhibitors.
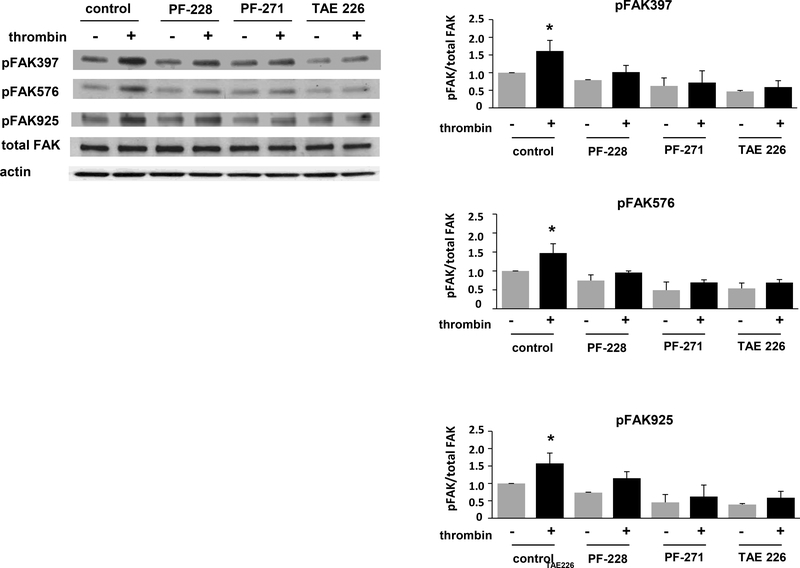
Initially, to confirm efficacy of FAK inhibition, human pulmonary artery EC were pretreated with either PF-228 (10 μM), PF-271 (5 μM) or TAE226 (5 μM) for 30 min prior to thrombin stimulation (1 U/ml). Cell lysates were collected 30 min after thrombin and subjected to Western blotting for phosphorylated FAK. Compared to vehicle-treated control cells, all three inhibitors affected a significant attenuation of thrombin-induced phosphorylation of FAK at Tyr397, Tyr576, and Tyr925 (Figure 1). Of note, while the inhibitors are characterized by similar IC50 concentrations, these studies were preceded by a series of dose-response experiments which identified the dosing above for each inhibitor as the lowest dose associated with significant inhibition of FAK phosphorylation in response to thrombin (data not shown).
Figure 1. Effect of PF-228, PF-271, and TAE226 on FAK phosphorylation by thrombin.
Human pulmonary artery EC were pretreated with PF-228 (10 μM), PF-271 (5 μM) or TAE226 (5 μM) for 30 min prior to thrombin treatment (1 U/ml, 30 min). Lysates were used for Western blotting with antibodies specific for phosphorylated FAK (Tyr397, Tyr576, or Tyr925). (A) Representative blots shown. (B-D) Densitometry was performed and effects of PF-228 relative to PF-271 and TAE226 were quantified. (n = 3/condition, *p < 0.05)
3.2. PF-228 alone attenuates thrombin-induced EC barrier disruption.
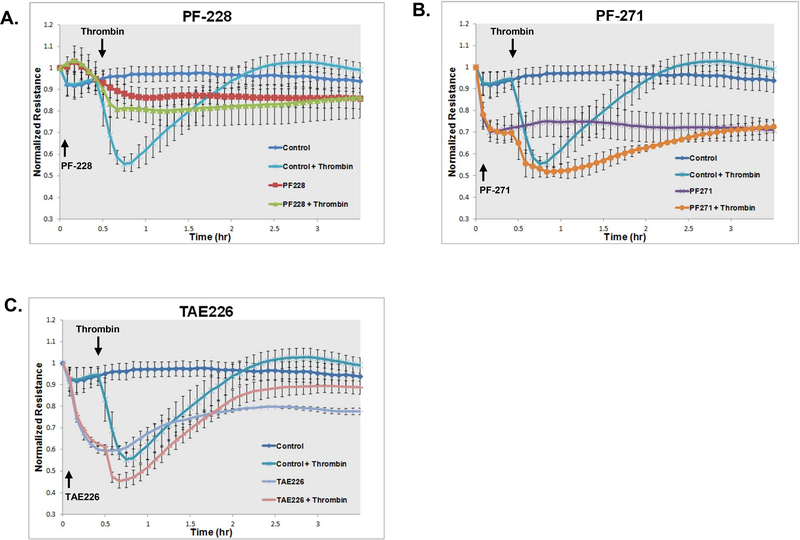
To assess the effects of pharmacologic FAK inhibitors of EC barrier function, human pulmonary artery EC were grown to confluence overlying gold-plated microelectrodes prior to treatment with either PF-228 (10 μM), PF-271 (5 μM) or TAE226 (5 μM) for 30 min followed by thrombin stimulation (1 U/ml) and real-time measurements of TER (Figure 2). Notably, while thrombin treatment alone resulted in a marked drop in resistance consistent with disruption of the EC monolayer with a nadir reached at 15 min followed by recovery, pretreatment with PF-228 significantly attenuated thrombin-induced reductions in TER (60% of the reduction in TER compared to thrombin alone, p < 0.05) consistent with barrier protection. In contrast, however, both PF-271 and TAE226 treatment resulted in significant decreases in basal resistances compared to PF-228 and did not attenuate thrombin effects. Indeed, treatment with PF-271 in particular appeared to augment thrombin effects in these experiments.
Figure 2. Differential effects of pharmacologic FAK inhibition on EC barrier function as measured by transendothelial electrical resistance (TER).
Human pulmonary artery EC were grown to confluence overlying gold-plated microelectrodes prior to pretreatment with PF-228 (10 μM), PF-271 (5 μM), TAE226 (5 μM) or vehicle for 30 min followed by stimulation with thrombin (1 U/ml). TER measurements recorded over time and normalized to baseline resistances are shown. (n = 3/condition)
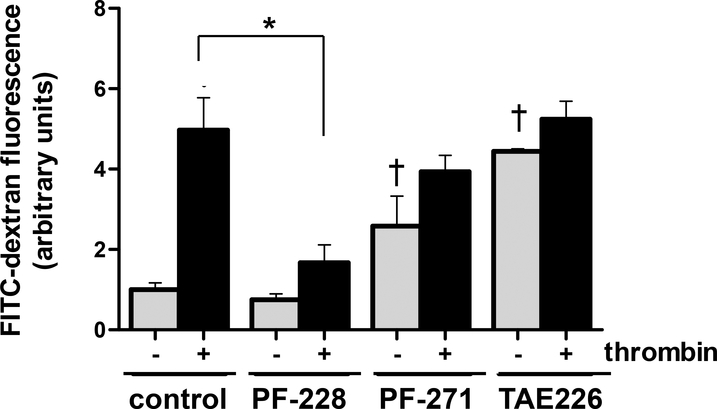
Subsequently, in experiments aimed at further characterizing the effects of FAK inhibitors on EC barrier function, human pulmonary artery EC were grown to confluence in transwell inserts prior to treatment with either PF-228 (10 μM), PF-271 (5 μM) or TAE226 (5 μM) for 30 min followed by thrombin stimulation (1 U/ml) and measurements of FITC-dextran monolayer flux (Figure 3). Consistent with measurements of TER, PF-228 alone was found to significantly reduce thrombin-induced monolayer disruption as measured by FITC-dextran flux (77% reduction in measured FITC-dextran compared to thrombin alone, p < 0.05). Moreover, while neither PF-271 nor TAE226 attenuated thrombin effects in these experiments, both were also associated with increased basal FITC-dextran flux consistent with increased monolayer permeability.
Figure 3. Differential effects of pharmacologic FAK inhibition on EC barrier function as measured by FITC-dextran transwell flux.
Human pulmonary artery EC were grown to confluence in transwell inserts prior to pretreatment with PF-228 (10 μM), PF-271 (5 μM), TAE226 (5 μM) or vehicle for 30 min followed by stimulation with thrombin (1 U/ml). FITC-dextran flux was then measured 30 min after thrombin. (n = 3/condition, *p < 0.05, †p < 0.05 compared to untreated controls)
3.3. Effect of PF-228 on thrombin-induced EC actin stress fiber formation and FAK localization.
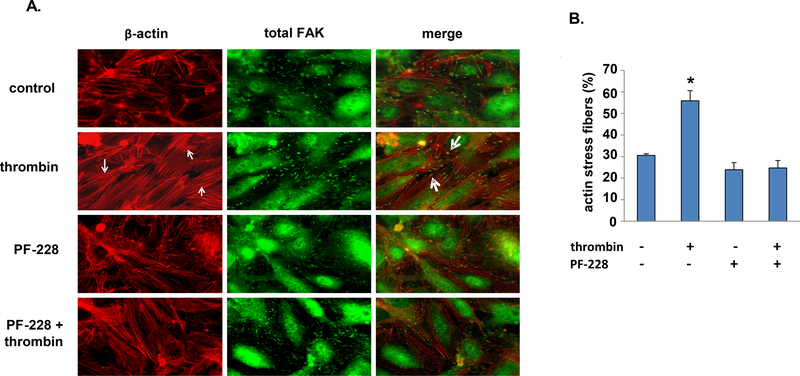
As only PF-228 was found to be protective in the setting of thrombin-induced EC barrier disruption we performed immunofluorescence imaging studies to characterize its effects on both actin stress fiber formation and FAK localization after thrombin (Figure 4). Cells treated with thrombin (1 U/ml, 5 min) were found to have increased actin stress fiber formation with increased FAK localization at the ends of stress fibers corresponding to sites of focal adhesion complexes. In contrast, cells treated with PF-228 had both significantly decreased actin stress fibers in response to thrombin and, in association with this, more diffuse staining for FAK throughout the cells.
Figure 4. Effect of PF-228 on EC actin stress fiber formation and FAK localization after thrombin.
Confluent human pulmonary artery EC were pretreated with PF-228 (10 μM, 30 min) or vehicle and then stimulated with thrombin (1 U/ml, 5 min) or vehicle. (A) Immunofluorescent imaging of cells stained for F-actin (red) and FAK (green) was performed and representative images are shown. Increased actin stress fiber formation after thrombin with co-localization of FAK at the ends of stress fibers are noted (small and large arrows, respectively). (B) Quantification of actin stress fibers was performed separately and is expressed as a percentage of the total area imaged. (n = 3 representative images from separate experiments for each experimental condition, *p < 0.05 compared to each other condition)
3.4. PF-228 post-treatment attenuates LPS-induced murine ALI.
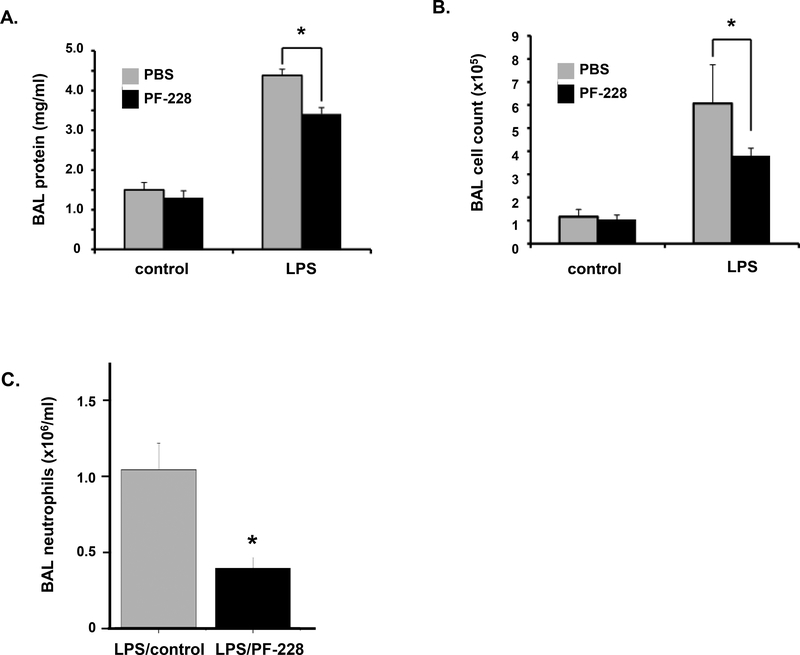
To extend out in vitro findings we investigated the effects of PF-228 as rescue treatment in a murine model of ALI induced by LPS. Mice were administered LPS (1.25 mg/kg, intratracheally) followed by PF-228 (80 μg/g, intraperitoneal injection) or vehicle 1 h later. BAL fluid was then collected 18 h after LPS and analyzed for total protein and cell counts (Figure 5). Consistent with evidence of vascular protection in vitro, PF-228 administered after LPS was associated with a significant decrease in both total protein and total cell counts measured in the BAL fluid of mice administered LPS (35% and 17% reductions, respectively, compared to LPS alone; p < 0.05).
Figure 5. PF-228 attenuates LPS-induced murine ALI: BAL protein and cell counts.
Mice were treated with PF-228 (80 μg/g, intraperitoneal injection) or vehicle 1 h after the administration of LPS (1.25 mg/kg, intratracheal injection). Animals were sacrificed 18 h after LPS and BAL fluid was collected for measurements of (A) total protein and (B) total cell counts. (*p < 0.05, n = 3 animals/control group and n = 5 animals/LPS-treated group). (C) A separate series of experiments were performed with the same experimental conditions and BAL neutrophils were calculated in LPS-treated animals with and without PF-228 pre-treatment. (n = 3/condition, *p < 0.05)
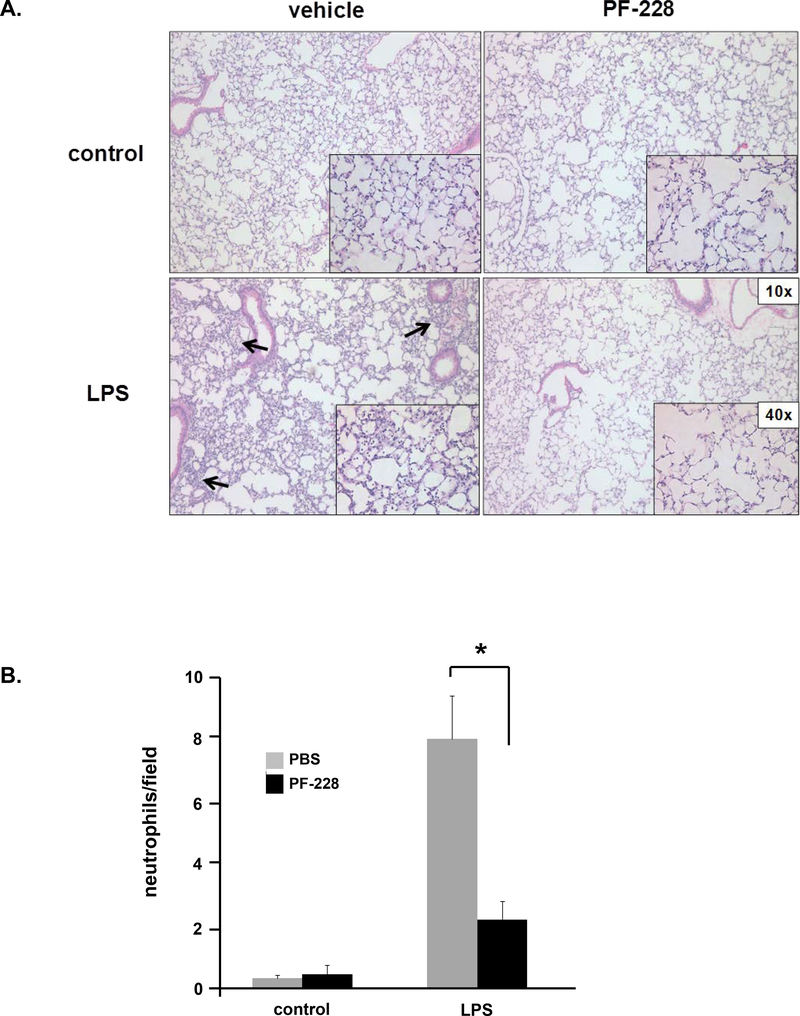
To corroborate the BAL fluid analyses, lungs from select animals were harvested and used for histologic evaluation. H&E staining demonstrated increases in interstitial edema and inflammatory cell extravasation after LPS that was noticeably attenuated in animals that were also treated with PF-228 1 h after LPS (Figure 6). Collectively, these data are consistent with significant beneficial effects of PF-228 as rescue treatment in murine ALI.
Figure 6. PF-228 attenuates LPS-induced murine ALI: lung histology.
Mice were treated with PF-228 (80 μg/g) or vehicle 1 h after the administration of LPS (1.25 mg/kg, intratracheal injection). Animals were sacrificed 18 h after LPS and lungs were harvested for histological evaluation by hematoxylin and eosin staining. (A) Areas of increased infiltration of inflammatory cells in animals administered LPS alone are denoted (arrows). Representative images are shown. (B) Quantification of neutrophils in both the alveolar space and the interstitium was performed as described and is expressed as neutrophils/field, calculated from the average of five randomly selected fields, for each condition. (n = 3/condition, *p < 0.05)
4.0. DISCUSSION
Abundant literature has firmly implicated FAK as an important mediator of EC responses in ALI. While the precise role of FAK in EC barrier function and signaling is complex, our results confirm protective effects of PF-228, an ATP-competitive small molecule FAK inhibitor, both on EC barrier function in vitro and in vivo as rescue treatment in a murine model of LPS-induced ALI. Moreover, these effects are associated with demonstrable inhibition of agonist-induced FAK phosphorylation and intracellular redistribution to actin stress fiber anchorage sites associated with focal adhesion complexes.
The signaling events associated with EC barrier regulation mediated by FAK in response to mechanical and inflammatory stimuli relevant to ALI are an area of ongoing investigation (14, 15). For example, FAK phosphorylation of paxillin, a focal adhesion protein, is a key regulatory mechanism of the small GTPases Rho and Rac which themselves regulate actin cytoskeletal rearrangement and EC barrier integrity (16). Consistent with this, both VEGF (vascular endothelial growth factor) and hepatocyte growth factor mediate EC FAK activity and, notably, expression levels of both have been reported to be in ALI (17, 18). In addition, increased formation of focal adhesion complexes, which include FAK, with subsequent phosphorylation of paxillin is induced by excessive mechanical stretch of EC, a pathogenic stimuli of ventilator-induced lung injury (19).
Separately, increased expression of reactive oxygen species (ROS) induced by NADPH activation has also been identified as a pathogenic mechanism of ALI that is mediated by FAK (20–22). Evidence for this is provided by increases in both total and phosphorylated FAK associated with increased EC permeability after treatment with the ROS, hydrogen peroxide (H2O2) (23) (20). In contrast, EC expressing a FAK-related non-kinase demonstrate decreased EC permeability after H2O2 treatment as well as attenuated downstream FAK signaling including paxillin phosphorylation (20). More recently, EC-specific FAK activity has been implicated as an important mediator of vascular permeability in mice injected subcutaneously with B16F0 melanoma cells (24). Collectively, these reports suggest that specific targeting of FAK inhibition could attenuate vascular permeability associated with ALI potentially yielding clinical benefits in patients. Still, it should be noted that the literature is somewhat mixed when it comes to defining the role of FAK in mediating EC barrier function as reports also suggest increases in vascular permeability associated with reduced levels of FAK both in vitro and in vivo (4, 25).
Several ATP-competitive small molecule inhibitors of FAK have been identified including the bis-amino pyrimidine inhibitors, PF-228 and PF-271 and the bis-anilino pyrimidine inhibitor, TAE226 (26, 27). To date, these agents have been studied largely for their potential role in cancer treatment. For example, PF-228 has been found to inhibit angiogenesis and promote apoptosis in mouse endothelioma cells (28). TAE226 has now been investigated in several preclinical studies and was found to attenuate tumor angiogenesis (29–32). These studies also confirmed robust FAK inhibition in normal EC. Similarly, PF-271 has also demonstrated potent FAK inhibition in vivo (26). Notably, PF-271 has been studied in a phase I clinical trial and was found to be both safe and to have some degree of clinical efficacy in patients with advance solid tumors although it was also found to have nonlinear pharmacokinetic properties (33).
The limitations of the current study need to be acknowledged including the recognized translational value of murine studies in general and the use of only female mice in our studies. Nonetheless, it is notable that we have identified significant differential effects of pharmacologic FAK inhibitors on EC barrier function and ALI. Still, it is unclear why qualitative differences were observed with respect to PF-228, which we found to be EC barrier-protective, and both PF-271 and TAE226, which were not only not protective but were also associated with decreased basal EC barrier function, despite dosing of each based on minimum concentrations effecting robust inhibition of FAK phosphorylation. One potential explanation is that there may be off-target effects of these inhibitors that contribute to their effects in these models (34). This is an important area of ongoing investigation that is not addressed in the current study and represents a limitation in our ability to definitively interpret our results. Nonetheless, these compounds have demonstrated high selectivity for FAK affecting a stable helical formation that requires a glycine residue next to the kinase activation loop ‘DFG’ motif and towards the N-terminal (27). Moreover, it is notable that to some degree both PF-271 and TAE226 also target proline-rich tyrosine kinase 2 (PYK2), a FAK ortholog with significant sequence homology to FAK that also mediates EC barrier function (35), while PF-228 does not (8). Further studies characterizing the effects of each inhibitor on PYK2 in our models are now underway.
It is possible that FAK inhibition results in complex and variable cellular responses dependent on the degree of inhibition, the specific context and/or the precise timing of these events. For example, PF-271 was previously reported to attenuate VEGF-induced permeability in murine EC (36). Additionally, this possibility is fully consistent with what appears to be somewhat contradictory literature describing both beneficial and detrimental effects of FAK on EC barrier regulation. Indeed, another recent report described effects of PF-228 in direct contrast to our findings. Specifically, PF-228 was found to inhibit FAK phosphorylation (Y397) in rat lung microvascular EC, as we report here in human pulmonary artery EC, but was also noted to lower basal TER consistent with decreased monolayer integrity (37). Critical differences between the prior report and our findings, however, include the dosing employed (0.1 μM vs. 10 μM, respectively) as well as the cell type studied given known qualitative differences in EC barrier responses by rat pulmonary artery and pulmonary microvascular EC (38). Finally, it should be noted that these effects of PF-228 were previously studied only in relevant in vitro models.
Additional studies are now needed to fully characterize the potential effects of FAK inhibition in general in ALI and the possible therapeutic benefits of PF-228 in particular in this context. Certainly, if our findings can be firmly correlated with effects of PF-228 specifically on FAK activity this would support further investigation of PF-228, as well as alternative strategies aimed at the modulating FAK activity, to treat patients with or at risk for ALI. Nonetheless, if further investigation should ultimately indicate that EC protection by PF-228 is not fully attributable to FAK inhibition alone this would support the need for studies to identify the off target effects of PF-228 that could in turn yield novel targets for further exploration as potential ALI therapies.
Acknowledgments
FUNDING
This work was funded in part by P01 HL 126609 (YE, JJ).
ABBREVIATIONS
- ALI
acute lung injury
- BAL
bronchoalveolar lavage
- EC
endothelial cell
- FAK
focal adhesion kinase
- IP
intraperitoneally
- IT
intratracheally
- LPS
lipopolysaccharide
- PF-228
PF-573,228
- PF-271
PF-562,271
- ROS
reactive oxygen species
- TAE226
NVP-TAE226
- TER
transendothelial electrical resistance
Footnotes
CONFLICTING INTERESTS
The authors have no conflicting interests to report.
REFERENCES
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Investigators LS, Group ET. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016; 315: 788–800. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 2011; 6: 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol 2006; 172: 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt TT, Tauseef M, Yue L, Bonini MG, Gothert J, Shen TL, Guan JL, Predescu S, Sadikot R, Mehta D. Conditional deletion of FAK in mice endothelium disrupts lung vascular barrier function due to destabilization of RhoA and Rac1 activities. Am J Physiol Lung Cell Mol Physiol 2013; 305: L291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quadri SK, Bhattacharya J. Resealing of endothelial junctions by focal adhesion kinase. Am J Physiol Lung Cell Mol Physiol 2007; 292: L334–342. [DOI] [PubMed] [Google Scholar]
- 6.Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol 2007; 9: 1046–1056. [DOI] [PubMed] [Google Scholar]
- 7.Petroni RC, Teodoro WR, Guido MC, Barbeiro HV, Abatepaulo F, Theobaldo MC, Biselli PC, Soriano FG. Role of focal adhesion kinase in lung remodeling of endotoxemic rats. Shock 2012; 37: 524–530. [DOI] [PubMed] [Google Scholar]
- 8.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014; 14: 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrita MA, Jones LM, Quizi JL, Sabourin LA, McKay BC, Addison CL. Focal adhesion kinase inhibitors are potent anti-angiogenic agents. Mol Oncol 2011; 5: 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn KB, Heffler M, Golubovskaya VM. Evolving therapies and FAK inhibitors for the treatment of cancer. Anticancer Agents Med Chem 2010; 10: 722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Sharma R, Rizzo AN, Siegler JH, Garcia JG, Jacobson JR. Role of claudin-5 in the attenuation of murine acute lung injury by simvastatin. Am J Respir Cell Mol Biol 2014; 50: 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 2005; 288: L1026–1032. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Sammani S, Mitra S, Ma SF, Garcia JG, Jacobson JR. Critical role for integrin-beta4 in the attenuation of murine acute lung injury by simvastatin. Am J Physiol Lung Cell Mol Physiol 2012; 303: L279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JG. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J 2003; 17: 2240–2249. [DOI] [PubMed] [Google Scholar]
- 15.Shikata Y, Birukov KG, Garcia JG. S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J Appl Physiol (1985) 2003; 94: 1193–1203. [DOI] [PubMed] [Google Scholar]
- 16.Birukova AA, Cokic I, Moldobaeva N, Birukov KG. Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF. Am J Respir Cell Mol Biol 2009; 40: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol (1985) 2004; 97: 1605–1617. [DOI] [PubMed] [Google Scholar]
- 18.Yanagita K, Matsumoto K, Sekiguchi K, Ishibashi H, Niho Y, Nakamura T. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem 1993; 268: 21212–21217. [PubMed] [Google Scholar]
- 19.Bhattacharya S, Sen N, Yiming MT, Patel R, Parthasarathi K, Quadri S, Issekutz AC, Bhattacharya J. High tidal volume ventilation induces proinflammatory signaling in rat lung endothelium. Am J Respir Cell Mol Biol 2003; 28: 218–224. [DOI] [PubMed] [Google Scholar]
- 20.Usatyuk PV, Natarajan V. Regulation of reactive oxygen species-induced endothelial cell-cell and cell-matrix contacts by focal adhesion kinase and adherens junction proteins. Am J Physiol Lung Cell Mol Physiol 2005; 289: L999–1010. [DOI] [PubMed] [Google Scholar]
- 21.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J Immunol 2002; 168: 3974–3982. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J 2002; 16: 1713–1720. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Yip R, Polena S, Sharma M, Rao S, Griciene P, Gintautas J, Jerome H. Reactive oxygen species increased focal adhesion kinase production in pulmonary microvascular endothelial cells. Proc West Pharmacol Soc 2004; 47: 54–56. [PubMed] [Google Scholar]
- 24.Alexopoulou AN, Lees DM, Bodrug N, Lechertier T, Fernandez I, D’Amico G, Dukinfield M, Batista S, Tavora B, Serrels B, Hodivala-Dilke K. Focal Adhesion Kinase (FAK) tyrosine 397E mutation restores the vascular leakage defect in endothelium-specific FAK-kinase dead mice. J Pathol 2017; 242: 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem 2006; 281: 2296–2305. [DOI] [PubMed] [Google Scholar]
- 26.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, Coleman K, Yao L, Martinez-Alsina L, Lorenzen M, Berliner M, Luzzio M, Patel N, Schmitt E, LaGreca S, Jani J, Wessel M, Marr E, Griffor M, Vajdos F. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res 2008; 68: 1935–1944. [DOI] [PubMed] [Google Scholar]
- 27.Lietha D, Eck MJ. Crystal structures of the FAK kinase in complex with TAE226 and related bis-anilino pyrimidine inhibitors reveal a helical DFG conformation. PLoS One 2008; 3: e3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mabeta P PF573,228 inhibits vascular tumor cell growth, migration as well as angiogenesis, induces apoptosis and abrogates PRAS40 and S6RP phosphorylation. Acta Pharm 2016; 66: 399–410. [DOI] [PubMed] [Google Scholar]
- 29.Schultze A, Decker S, Otten J, Horst AK, Vohwinkel G, Schuch G, Bokemeyer C, Loges S, Fiedler W. TAE226-mediated inhibition of focal adhesion kinase interferes with tumor angiogenesis and vasculogenesis. Invest New Drugs 2010; 28: 825–833. [DOI] [PubMed] [Google Scholar]
- 30.Sakurama K, Noma K, Takaoka M, Tomono Y, Watanabe N, Hatakeyama S, Ohmori O, Hirota S, Motoki T, Shirakawa Y, Yamatsuji T, Haisa M, Matsuoka J, Tanaka N, Naomoto Y. Inhibition of focal adhesion kinase as a potential therapeutic strategy for imatinib-resistant gastrointestinal stromal tumor. Mol Cancer Ther 2009; 8: 127–134. [DOI] [PubMed] [Google Scholar]
- 31.Kurio N, Shimo T, Fukazawa T, Okui T, Hassan NM, Honami T, Horikiri Y, Hatakeyama S, Takaoka M, Naomoto Y, Sasaki A. Anti-tumor effect of a novel FAK inhibitor TAE226 against human oral squamous cell carcinoma. Oral Oncol 2012; 48: 1159–1170. [DOI] [PubMed] [Google Scholar]
- 32.Hao HF, Takaoka M, Bao XH, Wang ZG, Tomono Y, Sakurama K, Ohara T, Fukazawa T, Yamatsuji T, Fujiwara T, Naomoto Y. Oral administration of FAK inhibitor TAE226 inhibits the progression of peritoneal dissemination of colorectal cancer. Biochem Biophys Res Commun 2012; 423: 744–749. [DOI] [PubMed] [Google Scholar]
- 33.Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, Fingert H, Pierce KJ, Xu H, Roberts WG, Shreeve SM, Burris HA, Siu LL. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol 2012; 30: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 34.Roh ME, Cosgrove M, Gorski K, Hitchcock IS. Off-targets effects underlie the inhibitory effect of FAK inhibitors on platelet activation: studies using Fak-deficient mice. J Thromb Haemost 2013; 11: 1776–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Buul JD, Anthony EC, Fernandez-Borja M, Burridge K, Hordijk PL. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating beta-catenin tyrosine phosphorylation. J Biol Chem 2005; 280: 21129–21136. [DOI] [PubMed] [Google Scholar]
- 36.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD. VEGF-induced vascular permeability is mediated by FAK. Dev Cell 2012; 22: 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chichger H, Braza J, Duong H, Harrington EO. SH2 domain-containing protein tyrosine phosphatase 2 and focal adhesion kinase protein interactions regulate pulmonary endothelium barrier function. Am J Respir Cell Mol Biol 2015; 52: 695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Troyanovsky B, Alvarez DF, King JA, Schaphorst KL. Thrombin enhances the barrier function of rat microvascular endothelium in a PAR-1-dependent manner. Am J Physiol Lung Cell Mol Physiol 2008; 294: L266–275. [DOI] [PubMed] [Google Scholar]