Abstract
Rodent lesion studies have revealed the existence of two causally dissociable spatial memory systems, localized to the hippocampus and striatum, that are preferentially sensitive to environmental boundaries and landmark objects, respectively. Here we test whether these two memory systems are causally dissociable in humans by examining boundary- and landmark-based memory in typical and atypical development. Adults with Williams syndrome (WS) – a developmental disorder with known hippocampal abnormalities – and typical children and adults, performed a navigation task that involved learning locations relative to a boundary or a landmark object. We found that boundary-based memory was severely impaired in WS compared to typically-developing mental-age matched (MA) children and chronological-age matched (CA) adults, whereas landmark-based memory was similar in all groups. Furthermore, landmark-based memory matured earlier in typical development than boundary-based memory, consistent with the idea that the WS cognitive phenotype arises from developmental arrest of late maturing cognitive systems. Together, these findings provide causal and developmental evidence for dissociable spatial memory systems in humans.
Keywords: Navigation, Williams-Beuren syndrome, Boundary, Landmark, Hippocampus
Introduction
Some of the strongest evidence for the idea that the mammalian brain possesses multiple memory systems comes from rodent lesion studies (Nadel, 1994; Squire, 2004). Damage to the rodent hippocampal formation impairs the ability to remember locations defined by distances and directions to environmental boundaries, but spares the ability to remember locations defined by distances and directions to landmark objects (Pearce, Roberts, & Good, 1998). By contrast, damage to the rodent dorsal striatum results in the converse impairment: spatial memory relative to landmark objects is impaired, but not spatial memory relative to environmental boundaries (Kosaki, Poulter, Austen, & McGregor, 2015). These results suggest a double dissociation in rodents between a hippocampal boundary-based memory system and a striatal landmark-based memory system (see also McDonald & White, 1993).
Consistent with this rodent literature, neuroimaging studies in humans have found that fMRI activation in the hippocampus is associated with recall of boundary-related locations, whereas fMRI activity in the striatum is associated with recall of landmark object-related locations (Doeller, King, & Burgess, 2008). Moreover, the use of gross navigational strategies (e.g., place-based vs. response-based) that are thought to be mediated by the boundary- and landmark-based memory systems have been found to differentially activate the human hippocampus and striatum, respectively (Hartley, Maguire, Spiers, & Burgess, 2003; Iaria, Petrides, Dagher, Pike, & Bohbot, 2003; Iglói, Doeller, Berthoz, Rondi-Reig, & Burgess, 2010; Marchette, Bakker, & Shelton, 2011). Despite this neuroimaging evidence for the existence of these two systems however, whether boundary- and landmark-based memory systems are causally dissociable in humans in the same way that they are in rodents remains unknown.
Here we directly address this question by examining boundary- and landmark-based memory in adults with Williams syndrome (WS) – a rare genetic developmental disorder that results in anatomical and functional abnormalities of the hippocampus (Meyer-Lindenberg, Mervis, & Berman, 2006; Meyer-Lindenberg et al., 2005). Hippocampal abnormalities have also been found in mice with a genetic deletion similar to that of WS (Meng et al., 2002). Given the known hippocampal abnormalities in WS, we predicted that adults with WS would exhibit impaired boundary-based memory, but unimpaired landmark-based memory, relative to mental age-matched (MA) children, which would provide causal evidence for the existence of dissociable spatial memory systems in humans.
In addition to examining whether boundary- and landmark-based memory are causally dissociable in humans, we also tested a recent theory regarding the developmental origins of the WS cognitive phenotype according to which the WS cognitive profile arises from developmental arrest of specific cognitive systems during childhood (Landau & Ferrara, 2013; Landau & Hoffman, 2012). A key prediction from this theory is that the cognitive systems that are typically fully mature early in development will be unimpaired in WS adults compared to typical chronological age-matched (CA) adults, but those systems that typically have a long developmental trajectory will be arrested at an early functional level, with little to no change thereafter. To test this prediction, we also examined the typical developmental profile of the boundary- and landmark-systems by comparing a group of typical adults to the typical children, and assessed whether and how this typical developmental profile relates to the spatial memory profile observed in WS.
Are boundary- and landmark-based memory causally dissociable in humans?
We first tested the hypothesis that WS adults have an impairment to boundary-based memory, but not landmark-based memory. To do so, we used a virtual navigation paradigm based on a task previously designed to dissociate these spatial memory systems (Doeller et al., 2008; Pearce et al., 1998). In this paradigm, participants learned the locations of test objects inside a virtual-reality arena comprising a landmark object, a circular boundary wall, and distal cues for orientation. On each trial, participants indicated the remembered location of a hidden test object by navigating to that location from a random starting point. The locations of the test objects were tethered at a constant distance and direction from either the landmark or boundary, and the relative position of the landmark and boundary was intermittently changed across trials. This design thus allowed us to independently evaluate boundary- and landmark-based spatial memory.
Methods
Participants.
Eighteen adults with WS and 18 typically developing (TD) children participated in the study. Participant characteristics are presented in Table 1. WS participants (9 female) were recruited through the Williams Syndrome Association, and gave informed consent prior to participating in compliance with the Emory Institutional Review Board (IRB). All WS participants had been positively diagnosed by a geneticist and also received the FISH test which checks for a microdeletion on the long arm of chromosome 7. Typical children (9 female) were recruited in Atlanta, GA, USA, and parents gave consent for their child’s participation in the study in compliance with the Emory IRB. All participants had normal or corrected to normal vision. The children were chosen so as to be individually non-verbal mental age-matched (MA) and sex-matched to the WS participants. To assess intelligence, participants were tested on a standardized intelligence test, the Kaufman Brief Intelligence Test (KBIT) (Kaufman & Kaufman, 1990). This test yields scores for two components of IQ: Verbal and Non-verbal (Matrices) (Table 1). Participants were matched on the non-verbal component of the IQ scores specifically because non-verbal IQ is particularly susceptible to impairment in WS (Jarrold, Baddeley, & Hewes, 1998). The Matrices subtest consists of a set of simple to complex visuospatial matrix problems with a forced-choice response paradigm. Matching of the raw non-verbal scores between the WS and MA groups was done as closely as possible (t(34)=0.0, p=1.0), with a mode of 0 points difference (maximum difference = 2, N=1). Given the known relative strength of language abilities in WS compared to non-verbal abilities, the WS group unsurprisingly had significantly higher raw verbal scores than the MA children (t(34)=2.13, p=0.041).
Table 1.
WS and MA group participant characteristics.
| WS Adults (n=18) | MA Children (n=18) | |||
|---|---|---|---|---|
| M (±1 SEM) | Range | M (±1 SEM) | Range | |
|
Chronological Age (years) |
26.60 | 18-50 | 8.69 | 6.0-10.33 |
|
Verbal KBIT
(raw score) |
65.44 (2.92) | 37-82 | 55.78 (3.48) | 21-80 |
|
Matrices KBIT (raw score) |
25.67 (1.86) | 11-38 | 25.67 (1.93) | 12-39 |
Virtual-reality Environment and Design.
We used Source SDK Hammer Editor to construct a virtual reality environment that was rendered and displayed from the first person-perspective using the commercial game software Portal (www.valvesoftware.com, Valve Software, Bellevue, WA). The arena was limited by a circular boundary wall and contained a rotationally-symmetric landmark object (a trashcan); it was also surrounded by distal cues (mountains and sky), rendered at infinity. Thus, the distal cues could be used to determine heading, but locations within the arena could only be defined based on distances to the bounding wall or the landmark object. The boundary wall was 130 virtual units (vu) in diameter and 10 vu in height relative to a simulated eye-level of 4 vu.
Participants could navigate through the arena by using their right hand to operate keys to move forward or backwards and turn left or right. They were familiarized with the keyboard controls through 1–2 minutes of free exploration of an unrelated virtual arena before starting the main experiment. Once participants were comfortable with the keyboard controls, the main experiment began, which involved learning the locations of two test objects (cake, radiator) within the arena. Following initial familiarization with the test object locations at the beginning of the experiment (using methods described in the next paragraph), each trial began with the display of a word denoting one of the test objects, which was read aloud to each participant. Participants then navigated to the remembered location of that object from a random starting point (the “replace” phase; Fig. 1A). When they reached their goal, they indicated as such verbally, and the experimenter made a button press response. Participants were then teleported to a pseudorandom arena position and that trial’s object appeared in its correct location and was collected by walking into it (the “feedback” phase). Participants were teleported to a new position prior to each feedback phase to encourage them to explore the arena, and to learn test object locations from multiple viewpoints. A set of 8 trials (four per experimental object) composed a block, and there were three blocks in the experiment. Crucially, the landmark object was moved relative to the boundary between blocks 1 and 2 and again between blocks 2 and 3. One test object maintained its location relative to the boundary after these moves and the other test object maintained its location relative to the landmark object (Figure 1B), making it possible to independently assess learning of the test object locations relative to the landmark and boundary cues, respectively.
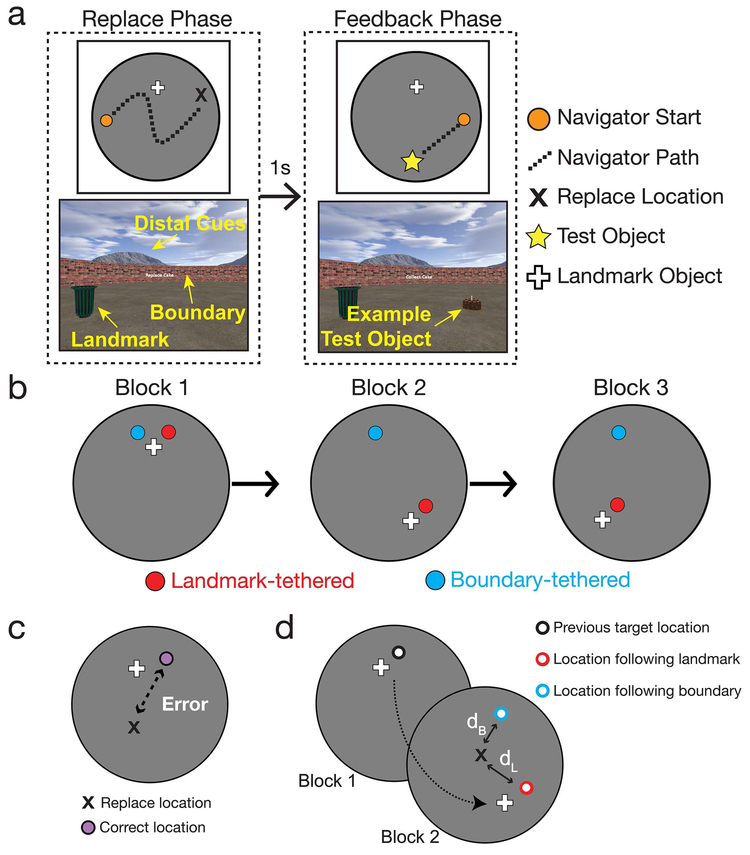
Figure 1. Behavioral paradigm.
A) Trial structure (after initial learning of object locations in block 1, see Methods). On each trial, participants navigated to the remembered location of the test object (“replace” phase) and, after a short delay with a black screen, received feedback (“feedback” phase). Top shows a map of the virtual trajectory taken by the participant on each phase of a typical trial, and bottom shows example views of the virtual environment from the participant’s perspective. The name of the test object remained on the center of the screen during the entire trial. B) Participants learned two object locations over three blocks. The landmark was moved relative to the boundary at the start of block 2, and again at the start of block 3. One object was tethered to the landmark (red dot) and one object was tethered the boundary (blue dots). C) Performance was measured in terms of distance error, which is the distance between the correct test object location (purple dot) and recalled test object location for each trial in virtual units (VU). D) The relative influence of the landmark on memory for test object locations was calculated as dB/(dL+dB), where dL is the distance of the response from the test object location previously associated with the landmark and dB is the distance of the response from the test object location previously associated with the boundary.
Prior to the start of the first replace phase during block 1, but not blocks 2–3, participants collected each test object in pseudorandom order twice (i.e., performed the feedback phase twice per test object) in order to initially learn the locations of the objects. Within a block, memory for the test object locations were assessed in pseudorandom order, with the constraint that the same test object could not be tested more than twice in a row. The positions of the test objects in the arena were the same for each participant.
Analysis.
Performance was assessed in terms of the distance between each object’s replaced location and the correct location (i.e., Distance Error) (Figure 1C). To determine if performance was better than would be expected by chance, we computed the average performance that would occur if participants replaced the objects inside the arena by choosing a random location on each trial, and then compared the observed performance to random performance using two-tailed one-sample t-tests. On average, random behavior would result in an average distance error of 43.5 vu for the landmark-tethered object and 41 vu for the boundary-tethered object.
We also examined the relative influence of the landmark on memory for the test object locations (Figure 1D). This measure reflects the overall relative bias to rely on one or the other spatial memory systems. The relative influence of the landmark was calculated as dB/(dL+dB), where dL is the distance of the response from the test object location previously associated with the landmark and dB is the distance of the response from the test object location previously associated with the boundary. This measure ranges from 0 to 1, where 0 is complete influence of the boundary and 1 is complete influence of the landmark. For block 3, two locations were associated with the boundary for the landmark-tethered object, one from block 1 and the other from block 2, and so we used the location associated with the lowest dB.
Results
Boundary-based memory is selectively impaired in Williams syndrome.
Data from block 1 were analyzed separately from the data from blocks 2–3, as the critical distinction between boundary-tethered and landmark-tethered objects is not made until the later blocks. During block 1, both the MA and WS groups performed the task better than would be expected by chance (MA and WS mean distance error ±1 SEM: 14.03±1.42, 14.84±0.85, respectively; both planned t-tests, 2-tailed: t(17)s>19.91, ps<0.001), and there was no significant difference in error between groups (t(34)=0.50, p=0.618). Thus, both groups understood the task, and could perform the task equally well when the landmark and the boundary provided redundant spatial information.
We then turned to our main prediction that adults with WS will exhibit impaired boundary-based, but not landmark-based, spatial memory compared to MA children. During blocks 2–3, the WS adults performed differently than MA children (Figure 2A). To quantify this difference, we analyzed performance error using a 2×2 ANOVA with factors for group (WS vs. MA) and object type (landmark-tethered vs. boundary-tethered). There were significant main effects of both factors, with greater error in the WS than MA group (F(1,34)=8.22, p=0.007, ηp2 =0.20), and greater error for the boundary- than landmark-tethered object (F(1,34)=326.00, p<0.001, ηp2 =0.91). Critically, there was also a significant interaction between group and object type (F(1,34)=15.10, p<0.001, ηp2 =0.31): the WS and MA groups performed similarly on the landmark-tethered object (t(34)=1.10, p=0.277), but the WS participants showed significantly greater error than the MA children on the boundary-tethered object (t(34)=4.41, p<0.001). Note that these results are qualitatively identical if we exclude the first trial of block 2, during which participants do not yet know which entity (landmark or boundary) is the correct reference for each test object (interaction between object type and group: F(1,34)=11.16, p=0.002, ηp2 =0.21). Thus, consistent with our prediction, these results demonstrate that boundary-based but not landmark-based memory is impaired in WS participants compared to MA children, providing causal evidence for dissociation between these two spatial memory systems in humans.
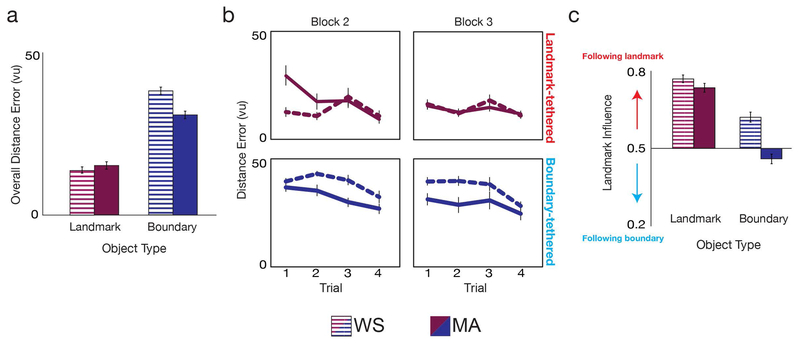
Figure 2. Boundary- and landmark-based memory systems are causally dissociable and competitively interact in humans.
A) Overall distance error (average over all trials and blocks 2–3) for the landmark-tethered object (red) and boundary-tethered object (blue), for the WS (striped colors) and MA (dark colors) groups. Compared to the MA group, WS participants were significantly impaired at replacing the boundary-tethered objects but not the landmark-tethered object B) Distance error for the landmark-tethered object (top row, red) and boundary-tethered object (bottom row, blue) for all trials during blocks 2–3 of the replace phase, for the WS (dashed lines) and MA (solid lines) groups. C) Relative influence of the landmark on the landmark-tethered and boundary-tethered object replace locations, for the WS and MA groups. Compared to the MA group, WS participants were more influenced by the landmark for the boundary-tethered object, but not the landmark-tethered object. All error bars indicate ±1 SEM.
The impaired boundary-based memory found in the WS group was not due to the WS adults being unable to perform the task, or performing the task in a qualitatively different way than MA children (Karmiloff-Smith, 1998). Despite impaired performance for the boundary-tethered object, the WS participants (as well as MA children) performed better than chance (WS: t(17)=2.41, p=0.028; MA: t(17)=8.52, p<0.001). Moreover, the detailed pattern of error on the boundary-tethered object (i.e., by block and trial) demonstrated that the WS participants and the MA children performed the task similarly across blocks and trials (Fig. 2B): a 2×2×4 ANOVA of boundary-tethered object error with factors for group, block (2–3), and trial (1–4) found no significant group by block (F(1,34)=0.11, p=0.737), group by trial (F(1,34)=0.62, p=0.552), or group by block by trial (F(1,34)=0.793, p=0.380) interactions. (We report the results of an analogous ANOVA of landmark-tethered object error in the next section.) Finally, the impaired performance for the boundary-tethered object in WS was not caused by a speed-accuracy tradeoff, as there was no significant interaction between group and object type in response latency (F(1,34)=1.61, p=0.214; Figure S1A). Together, these results show that despite their overall impairment on the boundary-tethered object, the WS adults understood the task, and performed the task in a qualitatively similar way to the MA children.
Although the WS and MA groups were by design matched on nonverbal IQ, the WS participants had higher verbal IQ than MA children. Thus, it is possible that the WS individuals do in fact have impaired landmark-based spatial memory, but compensated for this deficit by using a verbal navigational strategy. For example, WS participants may have learned the test object locations by verbally encoding their positions which they then used as a retrieval cue (e.g., “the cake is next to the trashcan”) (Farran, Courbois, Van Herwegen, Cruickshank, & Blades, 2012). However, we think this is unlikely for three reasons. First, such a verbal encoding strategy could work equally well for both the landmark- and boundary-tethered objects. Second, there was no significant correlation between verbal IQ and performance on the landmark-tethered object in either the WS or MA groups (both r2s<0.010, ps>0.698; Figure S1B). Third, to ensure that verbal IQ could not explain our effects, we reran the above analyses using a set of older TD children individually matched to the WS participants on verbal IQ (Supplemental Methods; Table S1). Results of this comparison of performance between the WS and verbal IQ matched groups were qualitatively identical to the comparison between the WS and MA groups, thus ruling out verbal IQ as a confounding factor (Figure S2).
The boundary- and landmark-based memory systems interact competitively.
Rodent lesion studies have found that the boundary- and landmark-based memory systems interact competitively. One consequence of this competition is that damage to one system can result in a bias to rely on the other system (Kosaki et al., 2015; Pearce et al., 1998; see also, Poldrack & Packard, 2003; Lee, Duman, & Pittenger, 2008). Does the WS boundary-specific memory deficit bias WS individuals to rely more on the landmark-based memory system? We addressed this question in two ways.
First, if WS individuals have a landmark-based memory bias, then we would expect these individuals to be more likely than the MA children to recall the test objects in the location predicted by the landmark. To test this idea, we computed the relative influence of the landmark on the replace locations during all trials of blocks 2–3 (Figure 2C). An analysis of landmark influence using a 2×2 ANOVA with factors for group and object type found a significant main effect of group (F(1,34)=30.41, p<0.001, ηp2 =0.47), with greater landmark influence in the WS than MA group. There was also a significant interaction (F(1,34)=11.86, p=0.002, ηp2 =0.26): the landmark had similar influence on memory for the landmark-tethered object location in both groups (t(34)=1.38, p=0.177), but had more influence on memory for the boundary-tethered object location in the WS than in the MA group (t(34)=5.922, p<0.001). In fact, for the boundary-tethered object, 15 WS participants (83%) were more influenced by the landmark than the boundary, compared to only 4 MA children (22%) (Yates’ χ2(1)=11.15, p<0.001). (For completeness, note that for the landmark-tethered object, all WS participants (100%) and MA children (100%) were more influenced by the landmark than the boundary.) These results demonstrate that the landmark had more influence over WS spatial memory than the boundary, providing evidence of competitive interaction between dissociable systems for landmark- and boundary-based memory in WS.
Second, if WS individuals have a landmark-based memory bias, then we would expect that on the first trial of block 2, when they do not yet know the appropriate spatial references for the landmark-tethered and boundary-tethered objects, they should be more likely than MA children to recall the landmark-tethered object in its correct location. A 2×2×4 ANOVA of landmark-tethered object distance error with factors for group, block (2–3), and trial (1–4) revealed significant group by block (F(1,34)=6.07, p=0.019, ηp2 =0.152) and group by block by trial (F(1,34)=7.51, p=0.010, ηp2 =0.181) interactions, driven by the WS group performing better than MA children on trial 1 of block 2 (t(34)=3.16, p=0.003), but not significantly differently on any other trials during either block 2 or 3 (all t(34)s<1.69, all ps>0.1) (Figure 2B). These results demonstrate that although the WS and MA groups have qualitatively similar landmark-based memory, when WS individuals did not know whether the landmark or the boundary was the correct spatial reference, they were more likely than MA children to use the landmark. Moreover, the better performance for the landmark-tethered object in the WS group on the first trial of block 2 was not due to a boundary-based memory bias in the MA children, as the WS and MA groups performed similarly for the boundary-tethered object on this trial (t(34)=1.48, p=0.148) (interaction between object type and group for block 2 trial 1: F(1,34)=11.43, p=0.002, ηp2 =0.25). These results thus provide further support for competitive interaction between dissociable landmark- and boundary-based memory systems.
Landmark-based memory does not reflect a beaconing strategy.
There are two possible ways in which participants may have used the landmark as a point of reference for recalling the landmark-tethered object location. First, if participants were using landmark-based spatial memory, as predicted here, they would have learned that the landmark-tethered test object was in a given distance and direction from the landmark, with direction being derived from the distal cues. Second, they might have used the landmark as a beacon, moving towards it and then choosing a random location proximal to it. The beaconing strategy requires participants to encode an association between the test object and the landmark, but not the spatial relationship between them. Both strategies could result in low distance error, but only the latter strategy would result in low angular error. To assess these two alternatives, we computed the unsigned angular error for the landmark-tethered object during blocks 2–3, and compared this error to the angular error that would be expected by chance. If participants recalled the objects at a random orientation around the landmark, then the unsigned angular errors would occur on a possible range of 0°−180°, and thus the angular error expected by chance would be 90° on average. Critically, angular error was lower than would be expected by chance (i.e., 90°) in both the WS and MA groups (both t(17)s>11.56, ps<0.001), and there was no significant difference in angular error between the WS and MA groups (t(34)=0.90, p=0.373) (Figure S3). Therefore, the WS participants (as well as MA children) recalled the landmark-tethered object as being a fixed distance and direction from the landmark, rather than simply beaconing to the landmark and choosing a random location nearby.
How does spatial memory develop in the typical population and in WS?
We next characterized the typical developmental profile of the boundary- and landmark-based memory systems, and determined whether and how this developmental profile relates to the spatial memory profile observed in WS.
Methods
Eighteen typical adults performed the same virtual navigation task used to assess spatial memory in the WS and MA groups. The typical adults were chronological age (CA) matched individually to the WS participants, and sex-matched to both the WS and MA groups. Age matching was done as closely as possible, with a modal difference of one year (maximum difference = 4, N=1). All CA adults were recruited in Philadelphia, PA, USA, gave informed consent prior to participating in compliance with the University of Pennsylvania IRB, and had normal or corrected to normal vision. All details of the task were the same as described above, except that CA adults indicated the recalled test object location on each trial by pressing a key themselves, rather than by making a verbal indication. To examine the development of boundary- and landmark-based memory systems in typical and atypical development, we compared the spatial memory profile of CA adults to that observed in MA children and WS adults.
Results
Boundary-based memory matures later than landmark-based memory in typical development.
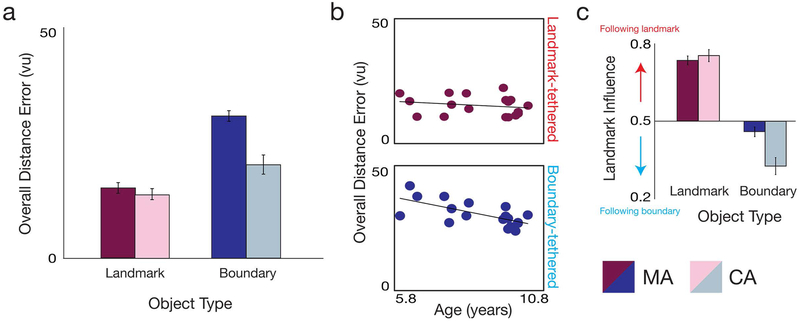
To characterize the typical developmental profile of landmark- and boundary-based memory, we first compared error between CA adults (average age = 26.60 years old) and MA children (average age = 8.69 years old) during blocks 2–3 (Figure 3A; see also Figure S4 for performance breakdown by block and trial). We conducted a 2×2 ANOVA with factors for object type (landmark vs. boundary) and group (CA vs. MA). There were significant main effects of both factors, with better performance in the typical adults than children (F(1,34)=15.44, p<0.001, ηp2 =0.31), and greater error for the boundary- than landmark-tethered object (F(1,34)=38.35, p<0.001, ηp2 =0.70). Crucially, there was also a significant interaction between object type and group (F(1,34)=13.60, p=0.001, ηp2 = 0.29): error for the landmark-tethered object was similar between the children and adults (t(34)=0.98, p=0.335), whereas error for the boundary-tethered object was greater in children (t(34)=4.50, p<0.001). These results are qualitatively identical if we exclude the first trial of block 2 (interaction between object type and group: F(1,34)=16.85, p<0.001, ηp2 =0.33). Children thus showed disproportionately worse performance for the boundary-tethered object than the landmark-tethered object, indicating that boundary-based memory matures later than landmark-based memory in typical development.
Figure 3. Boundary-based memory matures later than landmark-based memory in typical development.
A) Overall distance error (average over all trials and blocks 2–3) for the landmark-tethered object (red) and boundary-tethered object (blue), for the MA children (dark colors) and CA adults (lights colors). Distance error for the MA group is re-plotted from Figure 2A. Compared to the CA adults, MA children were significantly worse at replacing the boundary-tethered objects but not the landmark-tethered object. B) Overall distance error (average over all trials and blocks 2–3) for the landmark-tethered (top row, red) and boundary-tethered object (bottom row, blue), as a function of age in the MA children. There was improvement in performance with age for the boundary-tethered object, but not the landmark tethered object. C) Relative influence of the landmark on landmark-tethered and boundary-tethered object replace locations during blocks 2–3, for the MA children and CA adults. Landmark influence for the MA group is re-plotted from Figure 2C. Compared to CA adults, MA children were more influenced by the landmark for the boundary-tethered object, but not the landmark-tethered object. All error bars indicate ±1 SEM.
To further assess the typical developmental trajectory for the boundary- and landmark-based memory systems, we also tested whether age was related to boundary- and landmark-based memory performance within the MA group itself, which included children ranging from 6 to 10 years old (Figure 3B). There was significant decrease in overall error during blocks 2–3 with age for the boundary-tethered object (r2=0.44, p=0.003), but not for the landmark-tethered object (r2=0.04, p=0.406), and boundary-based memory improved marginally faster than landmark-based memory with age (comparison of Fischer z-transformed correlation coefficients between age and distance error for the boundary- vs. landmark-tethered objects, controlling for correlation between boundary- and landmark-tethered object error: z=1.53, p=0.063). Moreover, comparison of spatial memory between the CA adults and the group of older verbal IQ matched TD children (n = 18; mean age: 9.94 years; range: 9.5–10.5; Supplemental Methods; Table S1) revealed that boundary-based memory is not adultlike even by 10 years old (Figure S5). Therefore, the boundary-based system develops from 6–10 years old and beyond, whereas the landmark-based system is adultlike by at least the youngest age we tested (6 years old).
Does the less mature boundary-based memory system in children result in a bias to rely on the adult-like landmark-based system instead, indicating a competitive interaction even in typical development? To address this question, we examined the relative influence of the landmark on the replace locations during blocks 2–3 (Figure 3C). An analysis of landmark influence using a 2×2 ANOVA with factors for object type and group revealed a main effect of object type (F(1,34)=174.95, p<0.001, ηp2 =0.84), with greater relative influence of the landmark on memory for the landmark-tethered object location than the boundary-tethered object location. Indeed, both the MA and CA groups were more influenced by the landmark than the boundary when replacing the landmark-tethered object (both t(17)s>32.57, ps<0.001), and more influenced by the boundary when replacing the boundary-tethered object (both t(17)s>10.64, ps<0.001). Most importantly, there was also a main effect of group (F(1,34)=7.18, p=0.011, ηp2 =0.17), such that the landmark had less influence on memory for the test object locations in typical adults than the children. This developmental decrease in landmark influence was found for the boundary-tethered object (t(34)=3.44, p=0.002), but not the landmark-tethered object (t(34)=0.57, p=0.572) (interaction between group and object type: F(1,34)=7.49, p=0.010, ηp2 =0.18). The longer development of boundary-based memory compared to landmark-based memory was thus due (at least in part) to a decreasing reliance on the landmark-based system over development. However, this developmental landmark bias only impacted memory for the boundary-tethered object location overall, as it was not sufficiently strong that MA children had better performance than CA adults on the landmark-tethered object on the first trial of block 2 (t(34)=1.01, p=0.319; Figure S4).
Landmark-based memory is spared in Williams syndrome.
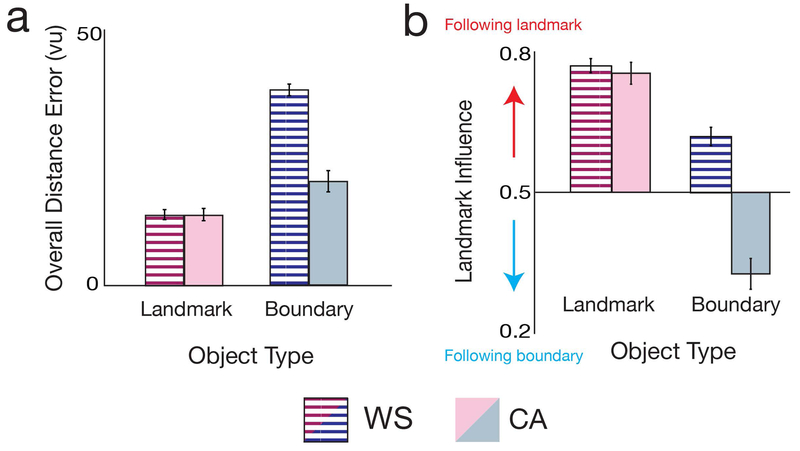
The impaired boundary-based memory found here in WS, coupled with the protracted typical development of boundary-based memory, suggests that the later developing boundary-based memory system is arrested in WS. This result partially supports the hypothesis that the WS cognitive profile arises from developmental arrest of the same cognitive systems found in typical development. However, a further prediction of this hypothesis is that the earlier maturing landmark-based system is completely spared in WS, developing typically in WS into adulthood. To test this idea, we compared the WS spatial memory profile to that of the CA adults. To do so, we compared overall performance for the boundary- and landmark-tethered objects during blocks 2–3 between the WS and CA adults (Figure 4A). A 2×2 ANOVA of distance error with factors for group (WS vs. CA) and object type revealed significant main effects of both factors, with greater error in the WS than CA group (F(1,34)=34.03, p<0.001, ηp2=0.50), and greater error for the boundary- than landmark-tethered (F(1,34)=143.74, p<0.001, ηp2=0.81). Compared to CA adults, WS individuals exhibited impaired memory for the boundary-tethered object (t(34)=7.54, p<0.001), but crucially not the landmark-tethered object (t(34)=0.01, p=0.996) (interaction: F(1,34)=48.08, p<0.001, ηp2=0.59). These results are qualitatively identical if we exclude the first trial of block 2 (interaction between object type and group: F(1,34)=43.88, p<0.001, ηp2=0.56). Thus, consistent with our prediction, the earlier developing landmark-based memory system is spared in WS.
Figure 4. Landmark-based memory is spared in Williams syndrome.
A) Overall distance error (average over all trials and blocks 2–3) for the landmark-tethered object (red) and boundary-tethered object (blue), for the CA (light colors) and WS (striped colors) groups. Distance error for the CA and WS groups is re-plotted from Figure 3A and Figure 2A, respectively. Compared to the CA group, WS participants were significantly impaired at replacing the boundary-tethered objects but not the landmark-tethered object. B) Relative influence of the landmark on landmark-tethered and boundary-tethered object replace locations, for the CA and WS groups. Landmark influence for the CA and WS groups is re-plotted from Figure 3C and Figure 2C, respectively. Compared to CA adults, WS participants were more influenced by the landmark for the boundary-tethered object, but not the landmark-tethered object. All error bars indicate ±1 SEM.
As expected, the impaired boundary-based memory in WS also resulted in a stronger landmark-based memory bias compared to CA adults. Examination of the relative landmark influence during blocks 2–3 in a 2×2 ANOVA with factors for group (WS vs. CA) and object type revealed significant main effects of both factors, with greater overall landmark influence on the replace locations in the WS than CA group (F(1,34)=48.22, p<0.001, ηp2 =0.59), and greater landmark influence for the landmark- than boundary-tethered object (F(1,34)=129.92, p<0.001, ηp2 =0.79) (Figure 4B). There was also a significant interaction (F(1,34)=30.17, p<0.001, ηp2=0.47): the landmark had a similar influence on the replace locations for the landmark-tethered object in both groups (t(34)=0.59, p=0.561), but WS were more biased to recall the boundary-tethered object in the location predicted by the landmark than the CA adults (t(34)=7.66, p<0.001). Furthermore, on the first trial of block 2, WS performed better than CA adults for the landmark-tethered object (t(34)=4.21, p<0.001), but similarly for the boundary-tethered object (t(34)=1.74, p=0.09) (interaction between object type and group: F(1,34)=9.99, p=0.003, ηp2 =0.23). These results suggest that arrest of the boundary-based memory system in WS may have caused greater reliance on the intact landmark-based memory system instead.
General Discussion
There were two primary goals of this study. First, we sought to determine if boundary- and landmark-based memory are causally dissociable systems in humans. We found evidence for such a dissociation in the performance profile of the WS group: compared to both typical mental-age matched children (MA group) and chronological-age matched adults (CA group), WS adults showed impaired boundary-based memory but intact landmark-based memory. WS adults also displayed a bias to rely more heavily on landmark objects than on boundaries, even when the boundaries were the more reliable spatial reference. This latter observation is consistent with theories that propose that spatial learning is mediated by multiple competitively interacting memory systems (Poldrack & Packard, 2003), the outputs of which are weighted during retrieval according to their previously experienced reliability and usefulness (Xu, Regier, & Newcombe, 2017). Second, we sought to understand the development of boundary- and landmark-based memory in typical participants, and how this development relates to the WS cognitive profile. We found that boundary-based memory matures later than landmark-based memory in typical development. Not only does this finding provide additional developmental evidence for dissociable spatial memory systems in humans, it also supports the idea that the WS profile arises from developmental arrest of cognitive systems that typically mature later. Although it has yet to be established precisely when boundary-based memory is arrested in WS individuals, previous work in other cognitive domains suggests that it may be around 4 years old (Bellugi, Bihrle, Neville, Doherty, & Jernigan, 1992; Dilks, Hoffman, & Landau, 2008; Landau & Ferrara, 2013; Landau & Hoffman, 2012).
Our results dovetail with previous research showing that WS individuals have difficulties navigating in large-scale spaces and often get lost in unfamiliar environments (Farran, Blades, Boucher, & Tranter, 2010; Farran et al., 2010; Foti et al., 2011). Previous work has shown that WS individuals are especially impaired at place-based spatial memory tasks (Mandolesi et al., 2009), but exhibit relatively intact response- or route-based spatial learning (Purser et al., 2015; but see Farran et al., 2015), particularly with repeated environmental exposures (Farran et al., 2010; Farran, Courbois, Van Herwegen, & Blades, 2012). Indeed, when WS and TD individuals were tested on both place-based and response-based memory tasks, the WS individuals were comparatively impaired on the place-based tasks (Bostelmann et al., 2017).
Given that both boundary-related spatial coding (O’Keefe & Burgess, 1998; Bird, Capponi, King, Doeller, Burgess, 2010; Doeller, King, & Burgess, 2008) and place-based memory (O’Keefe & Nadel, 1978; Hartley, Maguire, Spiers, & Burgess, 2003; Iaria, Petrides, Dagher, Pike, & Bohbot, 2003; Iglói, Doeller, Berthoz, Rondi-Reig, & Burgess, 2010; Marchette, Bakker, & Shelton, 2011) have been strongly associated with hippocampal function, and hippocampal development is abnormal in WS (Meyer-Lindenberg, Mervis, & Berman, 2006; Meyer-Lindenberg et al., 2005), it is possible that hippocampal abnormalities account for the boundary-related deficit observed here. Supporting this view, previous work has shown that hippocampal damage in TD adults results in boundary-based memory deficits (Astur, Taylor, Mamelak, Philpott, & Sutherland, 2002; Goodrich-Hunsaker, Livingstone, Skelton, & Hopkins, 2010; Guderian et al., 2015). Yet, an earlier study using a similar task as that employed here found that TD patients with hippocampal lesions were impaired at learning locations relative to both boundaries and landmark objects (Guderian et al., 2015), a pattern that might argue against a hippocampal locus for the boundary vs. landmark memory dissociation observed here. It is worth noting, however, that patients in this study were not able to learn the task when both the boundary- and landmark-tethered object provided redundant information, making it difficult to interpret “impairments” in learning either the boundary- or landmark-tethered object alone. A second possible neural locus for the boundary-related deficit is the parietal lobe. Supporting this view, we recently found that the Occipital Place Area (Dilks, Julian, Paunov, & Kanwisher, 2013), a scene-responsive region located near a parieto-occipital region known to be atypical in WS (Meyer-Lindenberg et al., 2004), is causally involved in boundary-based localization in typical adults (Julian, Ryan, Hamilton, & Epstein, 2016). Thus abnormalities in this region, or the parietal regions that receive its immediate outputs, might account for the boundary-related deficit, either in themselves, or in concert with abnormalities to the hippocampus.
Our results also build on previous work showing that WS individuals are impaired at using boundaries to recover their orientation following disorientation (Lakusta, Dessalegn, & Landau, 2010; but see Ferrara & Landau, 2015). In these experiments, a disoriented navigator is trained to locate a reward in one corner of a rectangular room that does not contain any orienting cues besides the boundaries. Following training, memory for the reward location is tested. Because there are only four possible reward locations, each of which is equidistant to the boundaries, the reorientation task may interrogate a navigator’s ability to recall locations on the basis of her orientation relative to boundaries (e.g., the corner to the left or right of the short wall) to a greater extent than her ability to recall locations based on distance to boundaries per se (as in the spatial memory task used here). The relationship between the WS orientation memory deficits revealed by the reorientation task and the location memory impairments discovered here has yet to be determined, but there are two possibilities. First, the WS boundary-based location and orientation memory deficits may have dissociable etiologies, because memory for location and orientation rely on different neural circuits, with representations of location in the hippocampus (O’keefe & Nadel, 1978) and representations of orientation in extra-hippocampal structures (Winter & Taube, 2014). Consistent with this idea, the ability to use boundaries for reorientation typically develops before the use of landmarks (e.g., a colored wall or landmark object) (Hermer & Spelke 1994; Lee, Shusterman, & Spelke, 2006), whereas here we found that the ability to use boundaries for location memory develops after the use of landmarks, together suggesting that orientation and location memory may be mediated by dissociable systems with different developmental trajectories. However, if the WS phenotype arises from impaired late-developing cognitive systems (Landau & Ferrara, 2013; Landau & Hoffman, 2012), this would suggest that boundary-based orientation abilities should be relatively spared in WS, which they are not. Thus, a second alternative is that the WS boundary-based orientation and location memory deficits both relate to the same brain abnormalities within the hippocampus and parietal lobe. Indeed, the hippocampus is also involved in recall of rewarded locations during reorientation (Sutton, Joanisse, & Newcombe, 2010; Keinath et al., 2017), in addition to hippocampal inputs mediating representations of orientation (Weiss et al., 2017). Future studies that directly compare boundary-based location and orientation memory in WS are needed to dissociate these two alternatives.
Our finding that boundary-based memory develops later in typically-developing participants is consistent with previous research showing that hippocampal-dependent navigational strategies emerge later in typical human development than striatal-dependent ones (Akers & Hamilton, 2007; Lehnung et al., 1998; Herzog & Ferstl, 1998; Leplow et al., 2003; Herzog, Ferstl & Mehdorn, 2003; Bullens, Iglói, Berthoz, Postma, Rondi-Reig, 2010; Overman, Pate, Moore, Peuster, 1996; but see Bohbot et al., 2012; Bostelmann et al., 2017). Consistent with this observation, the hippocampus is known to have a comparatively long developmental trajectory (Gómez & Edgin, 2016). Notably, one earlier study using a real-world version of the same behavioral paradigm as in the present work also found that boundary-based memory is not adultlike by 7 years old (Bullens et al., 2010). Yet, the developmental trajectory of landmark-based memory could not be determined based on this prior study because adults in this study were strongly biased to rely on the boundary rather than the landmark to recall test object locations. In contrast, TD adults in the present study were able to use the landmark object to recall test object locations, thus allowing us to detect any potential development of landmark-based memory from childhood to adulthood. Our results suggest that landmark-based memory is adultlike by at least 6 years old, whereas boundary-based memory continues to develop beyond 10 years of age.
Together, these results help to elucidate the ontogeny of boundary- and landmark-based memory systems. WS is caused by a well-defined 1.6-Mb heterozygous deletion of approximately 25 genes on chromosome band 7q11.23 (Francke, 1999). Given that visuospatial deficits in WS are present across the whole lifespan (Landau & Hoffman, 2012), it is possible that the selective impairment of boundary-based memory in WS found here arises from genetic dysfunction early in brain development. Resolving this issue is critical because multiple memory systems may be adaptive specializations shaped by natural selection to solve different problems posed by the environment (Sherry & Schacter, 1987). However, the precise genetic contribution to boundary- and landmark-based memory remains unknown. The present work suggests that examining spatial memory in animal models of WS (Osborne, 2010) could be particularly fruitful for establishing the neurogenetic origin of multiple memory systems.
In sum, we have demonstrated that boundary-based memory is impaired in WS, whereas landmark-based memory is spared, and that boundary-based memory matures later than landmark-based memory in typical development. These findings provide causal and developmental evidence for dissociable human spatial memory systems, and demonstrate that the WS spatial memory phenotype arises from developmental arrest of late maturing cognitive systems. These findings help to close the gap between rodent and human studies of the memory systems that causally guide spatial behavior, and reinforce the importance of using insights from typical development to elucidate cases of atypical development.
Supplementary Material
Research highlights.
The current study used an established spatial memory paradigm to examine boundary- and landmark-based spatial memory in typical and atypical development.
Boundary-based memory was impaired in adults with Williams syndrome compared to typically-developing children and adults, whereas landmark-based memory was similar in all groups.
Landmark-based memory matured earlier in typical development than boundary-based memory.
These findings together provide causal and developmental evidence for dissociable spatial memory systems in humans.
Acknowledgements
We express our sincere thanks to the individuals who participated in this study and their families. We would also like to thank the Emory Child Study Center and the Williams Syndrome Association for assistance in recruiting participants. The work was supported by NSF IGERT grant 0966142 to J.B.J, NSF Graduate Research Fellowship to J.B.J., NSF grant SBE-1041707 to R.A.E., NIH grant EY022350 to R.A.E., NICHD grant T32HD071845 to F.S.K., NEI grant T32EY007092 to F.S.K., and Emory College (D.D.D).
References
- Akers KG, & Hamilton DA (2007). Comparison of developmental trajectories for place and cued navigation in the Morris water task. Developmental psychobiology, 49(6), 553–564. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, & Sutherland RJ (2002). Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behavioural brain research, 132(1), 77–84. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Bihrle AM, Neville HJ, Doherty S, & Jernigan TL (1992). Language, cognition, and brain organization in a neurodevelopmental disorder In Gunner M & Nelson C (Eds.), Developmental behavioral neuroscience: The Minnesota symposia on child psychology (pp. 201–232). Hillsdale, NJ: Erlbaum Press. [Google Scholar]
- Bird CM, Capponi C, King JA, Doeller CF, Burgess N (2010). Establishing the boundaries: the hippocampal contribution to imagining scenes. Journal of Neuroscience, 30(35), 11688–11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, McKenzie S, Konishi K, Fouquet C, Kurdi V, Schachar R, et al. (2012). Virtual navigation strategies from childhood to senescence: evidence for changes across the life span. Frontiers in aging neuroscience, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostelmann M, Fragnière E, Costanzo F, Di Vara S, Menghini D, Vicari S, et al. (2017). Dissociation of spatial memory systems in Williams syndrome. Hippocampus. [DOI] [PubMed] [Google Scholar]
- Bullens J, Iglói K, Berthoz A, Postma A, & Rondi-Reig L (2010). Developmental time course of the acquisition of sequential egocentric and allocentric navigation strategies. Journal of experimental child psychology,107(3), 337–350. [DOI] [PubMed] [Google Scholar]
- Bullens J, Nardini M, Doeller CF, Braddick O, Postma A, & Burgess N (2010). The role of landmarks and boundaries in the development of spatial memory. Developmental Science, 13(1), 170–180. [DOI] [PubMed] [Google Scholar]
- Dilks DD, Hoffman JE, & Landau B (2008). Vision for perception and vision for action: Normal and unusual development. Developmental Science, 11, 474–486. [DOI] [PubMed] [Google Scholar]
- Dilks DD, Julian JB, Paunov AM, & Kanwisher N (2013). The occipital place area is causally and selectively involved in scene perception. Journal of neuroscience, 33(4), 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, King JA, & Burgess N (2008). Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proceedings of the National Academy of Sciences, 105(15), 5915–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farran EK, Blades M, Boucher J, & Tranter LJ (2010). How do individuals with Williams syndrome learn a route in a real-world environment? Developmental Science, 13(3), 454–468. [DOI] [PubMed] [Google Scholar]
- Farran EK, Courbois Y, Van Herwegen J, & Blades M (2012). How useful are landmarks when learning a route in a virtual environment? Evidence from typical development and Williams syndrome. Journal of Experimental Child Psychology, 111(4), 571–586. [DOI] [PubMed] [Google Scholar]
- Farran EK, Courbois Y, Van Herwegen J, Cruickshank AG, & Blades M (2012). Colour as an environmental cue when learning a route in a virtual environment: typical and atypical development. Research in Developmental Disabilities, 33(3), 900–908. [DOI] [PubMed] [Google Scholar]
- Farran EK, Purser HR, Courbois Y, Ballé M, Sockeel P, Mellier D, & Blades M (2015). Route knowledge and configural knowledge in typical and atypical development: a comparison of sparse and rich environments. Journal of Neurodevelopmental Disorders, 7(1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara K, & Landau B (2015). Geometric and featural systems, separable and combined: Evidence from reorientation in people with Williams syndrome. Cognition, 144, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti F, Petrosini L, Cutuli D, Menghini D, Chiarotti F, Vicari S, & Mandolesi L (2011). Explorative function in Williams syndrome analyzed through a large-scale task with multiple rewards. Research in Developmental Disabilities, 32(3), 972–985. [DOI] [PubMed] [Google Scholar]
- Francke U (1999). Williams-Beuren syndrome: genes and mechanisms. Human Molecular Genetics, 8(10), 1947–1954. [DOI] [PubMed] [Google Scholar]
- Gómez RL, & Edgin JO (2016). The extended trajectory of hippocampal development: Implications for early memory development and disorder. Developmental Cognitive Neuroscience, 18, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, & Hopkins RO (2010). Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus, 20(4), 481–491. [DOI] [PubMed] [Google Scholar]
- Guderian S, Dzieciol AM, Gadian DG, Jentschke S, Doeller CF, Burgess N, et al. (2015). Hippocampal volume reduction in humans predicts impaired allocentric spatial memory in virtual-reality navigation. Journal of neuroscience, 35(42), 14123–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Maguire EA, Spiers HJ, & Burgess N (2003). The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron, 37(5), 877–888. [DOI] [PubMed] [Google Scholar]
- Hermer L, & Spelke ES (1994). A geometric process for spatial reorientation in young children. Nature, 370, 57–59. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, & Bohbot V r. D. (2003). Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. Journal of neuroscience, 23(13), 5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglói K, Doeller CF, Berthoz A, Rondi-Reig L, & Burgess N (2010). Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proceedings of the National Academy of Sciences, 107(32), 14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrold C, Baddeley AD, & Hewes AK (1998). Verbal and nonverbal abilities in the Williams syndrome phenotype: Evidence for diverging developmental trajectories. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 39(4), 511–523. [PubMed] [Google Scholar]
- Julian JB, Ryan J, Hamilton RH, & Epstein RA (2016). The occipital place area is causally involved in representing environmental boundaries during navigation. Current Biology, 26(8), 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A (1998). Development itself is the key to understanding developmental disorders. Trends in cognitive sciences, 2(10), 389–398. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (1990). K-BIT: Kaufman brief intelligence test: American Guidance Service. [Google Scholar]
- Keinath AT, Julian JB, Epstein RA, & Muzzio IA (2017). Environmental Geometry Aligns the Hippocampal Map during Spatial Reorientation. Current Biology, 27, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaki Y, Poulter SL, Austen JM, & McGregor A (2015). Dorsolateral striatal lesions impair navigation based on landmark-goal vectors but facilitate spatial learning based on a “cognitive map”. Learning & Memory, 22(3), 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakusta L, Dessalegn B, & Landau B (2010). Impaired geometric reorientation caused by genetic defect. Proceedings of the National Academy of Sciences, 107(7), 2813–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, & Ferrara K (2013). Space and language in Williams syndrome: Insights from typical development. Wiley Interdisciplinary Reviews: Cognitive Science, 4(6), 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, & Hoffman JE (2012). Spatial representation: From gene to mind: Oxford University Press. [Google Scholar]
- Lee AS, Duman RS, & Pittenger C (2008). A double dissociation revealing bidirectional competition between striatum and hippocampus during learning. Proceedings of the National Academy of Sciences, 105(44), 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Shusterman A, & Spelke ES (2006). Reorientation and landmark-guided search by young children evidence for two systems. Psychological Science, 17, 577–582. [DOI] [PubMed] [Google Scholar]
- Lehnung M, Leplow B, Friege L, Herzog A, Ferstl R, & Mehdorn M (1998). Development of spatial memory and spatial orientation in preschoolers and primary school children. British Journal of Psychology, 89(3), 463–480. [DOI] [PubMed] [Google Scholar]
- Leplow B, Lehnung M, Pohl J, Herzog A, Ferstl R, & Mehdorn M (2003). Navigational place learning in children and young adults as assessed with a standardized locomotor search task. British Journal of Psychology, 94(3), 299–317. [DOI] [PubMed] [Google Scholar]
- Marchette SA, Bakker A, & Shelton AL (2011). Cognitive mappers to creatures of habit: differential engagement of place and response learning mechanisms predicts human navigational behavior. Journal of neuroscience, 31(43), 15264–15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, & White NM (1993). A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behavioral neuroscience, 107(1), 3. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, & White NM (1994). Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behavioral and neural biology, 61(3), 260–270. [DOI] [PubMed] [Google Scholar]
- Mandolesi L, Addona F, Foti F, Menghini D, Petrosini L, & Vicari S (2009). Spatial competences in Williams syndrome: a radial arm maze study. International Journal of Developmental Neuroscience, 27(3), 205–213. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, et al. (2002). Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron, 35(1), 121–133. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen RK, Morris CA, et al. (2004). Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron, 43(5), 623–631. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, & Berman KF (2006). Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nature Reviews Neuroscience, 7(5), 380–393. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Sarpal D, Koch P, Steele S, Kohn P, et al. (2005). Functional, structural, and metabolic abnormalities of the hippocampal formation in Williams syndrome. Journal of Clinical Investigation, 115(7), 1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L (1994). Multiple memory systems: What and why, an update. Memory systems, 1994, 39–63. [Google Scholar]
- O’Keefe J, Burgess N (1996). Geometric determinants of the place fields of hippocampal neurons. Nature, 381(6581), 381. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L (1978). The hippocampus as a cognitive map: Oxford: Clarendon Press. [Google Scholar]
- Osborne LR (2010). Animal models of Williams syndrome. Paper presented at the American Journal of Medical Genetics Part C: Seminars in Medical Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH, Pate BJ, Moore K, & Peuster A (1996). Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behavioral neuroscience, 110(6), 1205. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Roberts AD, & Good M (1998). Hippocampal lesions disrupt navigation based on cognitive maps but not heading vectors. Nature, 396(6706), 75–77. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, & Packard MG (2003). Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia, 41(3), 245–251. [DOI] [PubMed] [Google Scholar]
- Purser HR, Farran EK, Courbois Y, Lemahieu A, Sockeel P, Mellier D, & Blades M (2015). The development of route learning in Down syndrome, Williams syndrome and typical development: investigations with virtual environments. Developmental Science, 18(4), 599–613. [DOI] [PubMed] [Google Scholar]
- Sherry DF, & Schacter DL (1987). The evolution of multiple memory systems. Psychological review, 94(4), 439. [Google Scholar]
- Squire LR (2004). Memory systems of the brain: a brief history and current perspective. Neurobiology of learning and memory, 82(3), 171–177. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Joanisse MF, & Newcombe NS (2010). Spinning in the scanner: Neural correlates of virtual reorientation. Journal of Experimental Psychology: Learning, Memory, and Cognition, 36(5), 1097. [DOI] [PubMed] [Google Scholar]
- Weiss S, Talhami G, Gofman-Regev X, Rapoport S, Eilam D, & Derdikman D (2017). Consistency of Spatial Representations in Rat Entorhinal Cortex Predicts Performance in a Reorientation Task. Current Biology, 27(23), 3658–3665. [DOI] [PubMed] [Google Scholar]
- Winter SS, & Taube JS (2014). Head direction cells: from generation to integration In Space, time and memory in the hippocampal formation (pp. 83–106): Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.