Abstract
Purpose
Breast conservation therapy (BCT) is standard for T1-T2 tumors, but early trials excluded breast cancers >5 cm. This study was performed to assess patterns and outcomes of BCT for T3 tumors.
Methods
We reviewed the National Cancer Database (NCDB) for noninflammatory breast cancers >5 cm, between 2004–2011 who underwent BCT or mastectomy (Mtx) with nodal evaluation. Patients with skin or chest wall involvement were excluded. Patients having clinical T3 tumors were analyzed to determine outcomes based upon presentation, with those having pathologic T3 tumors, subsequently assessed, irrespective of presentation. Overall survival (OS) was analyzed using multivariable Cox proportional hazards models, with adjusted survival curves estimated using inverse probability weighting.
Results
After exclusions, 37,268 patients remained. Median age and tumor size for BCT vs Mtx was 53 vs 54 years (p<0.001), and 6.0 vs 6.7 cm (p<0.001), respectively. Predictors of BCT included age, race, location, facility type, year of diagnosis, tumor size, grade, histology, nodes examined and positive, and administration of chemotherapy and radiotherapy. OS was similar between Mtx and BCT (p=0.36). This held true when neoadjuvant chemotherapy patients were excluded (p=0.39). BCT percentages declined over time (p<0.001) while tumor sizes remained the same (p=0.77). Median follow up was 51.4 months.
Conclusions
OS for patients with T3 breast cancers is similar whether patients received Mtx or BCT, confirming that tumor size should not be an absolute BCT exclusion. Declining use of BCT for tumors >5 cm in younger patients may be accounted for by recent trends towards mastectomy.
Keywords: Breast conservation, locally advanced breast cancer, mastectomy
Introduction
Breast conservation therapy (BCT) has become a desirable alternative to mastectomy (Mtx) for women with early breast cancer since the 1980s. These procedures have become standard of care [1] and provide equivalent outcomes to mastectomy when accompanied by radiotherapy [2]. Breast conservation surgery is typically a shorter procedure, can be scheduled more quickly for operation [3], and has psychological benefits over mastectomy [4]. These advantages have made this the standard of care, and rates of breast conservation are even now a quality measure for women having early stage breast cancer [5].
Early studies only included tumors up to 5 cm [6–9], out of an abundance of caution because it has long been known that as tumor sizes increase, so do local recurrence rates [7]. Breast conservation also spares the breast, but disfigurement caused by an unfavorable breast-to-tumor ratio when treating larger tumors is felt to obviate any benefit of BCT. Because of this, tumor size greater than 5 cm has remained a relative contraindication to BCT as suggested by NCCN guidelines [1]. There are, however, data to suggest that BCT is feasible and safe for tumors >5 cm.
Previous data has shown that there is no difference in disease-specific survival between BCT and mastectomy for tumors over 5 cm in the Medicare population [10]. That cohort of 5,685 patients was limited to those over 65, however, and we are not aware of any published literature that attempts to reproduce this analysis in a broader population of women. We therefore proceeded with this current study, using a larger, more diverse patient population from the National Cancer Database (NCDB). This investigation was performed to determine whether the overall survival (OS) conferred by BCT was similar to Mtx for noninflammatory T3 breast cancer primaries. This study was also performed to assess trends in the United States for performance of breast conservation in this group where it has traditionally been considered contraindicated.
Materials and Methods
We queried the National Cancer Database (NCDB) for breast cancers >5 cm diagnosed between 2004–2011, to provide adequate follow up, who underwent BCT or Mtx. Male patients were excluded, as BCT is still not considered standard in this patient population. Histology codes were utilized to exclude Paget’s disease and in-situ carcinomas. Patients with unknown tumor size, carcinomas labeled as “diffuse,” inflammatory cancers, and patients with distant metastases were also excluded. Patients who received neoadjuvant chemotherapy were included, although neoadjuvant endocrine therapy or preoperative radiation therapy was excluded.
Clinical T3 (cT3) and pathologic T3 (pT3) tumors were reviewed to assess different objectives. cT3 tumors were used to determine predictors of BCT for tumors >5 cm as clinical stage determines treatment options. pT3 stage was utilized for survival analysis as final pathology is related to outcomes.
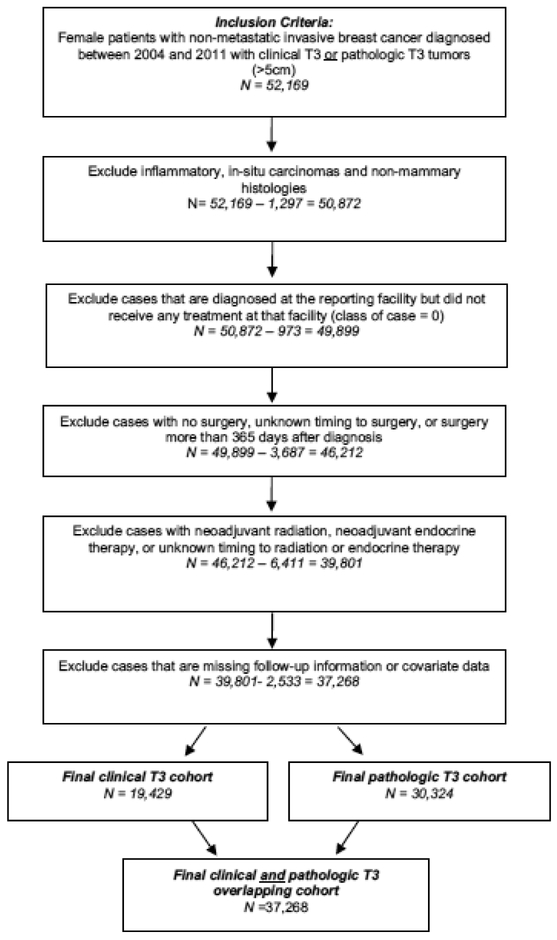
We excluded patients who did not undergo nodal evaluation, or for whom nodal status was not recorded, as this information is critical for survival analysis. Intervals >365 days from the time of diagnosis to surgery, which can affect survival [11], and patients who did not undergo surgery were also excluded. (Figure 1).
Figure 1.
Cohort exclusion diagram
Sensitivity analyses were performed to determine overall survival of BCT vs. Mtx with patients receiving neoadjuvant chemotherapy excluded to eliminate any possible selection bias and confounding on survival. An additional sensitivity analysis was also performed by including pathologic T3 cases that were also clinically T3, to assess clinically accurate T3 lesions.
Age, tumor size, and nodal status were treated as continuous variables, while all others were categorical. Chi-squared and Wilcoxon rank sum tests were used to compare groups. Multivariable logistic regression was used to identify predictors of BCT based on patient, tumor, and treatment characteristics in patients with clinical T3 stage. The Cochran-Armitage test was used to analyze trends in BCT and Mtx over time, and Spearman’s correlation assessed tumor size over time. OS was analyzed using Kaplan Meier methods and multivariable Cox proportional hazards models and adjusted survival curves were estimated using inverse probability weighting.
Results
After all exclusions, there were 37,268 patients fulfilling criteria for analysis (Figure 1). There were a total of 19,429 cT3 patients, among whom 16,502 (85%) underwent mastectomy versus 2,927 (15%) who received BCT. Median age was 54 for mastectomy patients versus 53 for BCT patients (p<0.001). Median tumor size for BCT versus Mtx was 6.0 cm versus 6.7 cm, respectively (p<0.001). Primaries >10 cm comprised only 4.5% of tumors in the BCT group but 8.2% of the Mtx group. Average number of lymph nodes examined was 9.2 for the BCT group and 13.2 for the Mtx group (p<0.001), while the average number of lymph nodes positive was 1.9 and 4.5 for BCT and Mtx, respectively (p<0.001). Fewer patients in the BCT group were >65 (16.3%) as versus <45 years of age (26.7%). Blacks having T3 tumors were more likely to have breast conservation (20.9% vs. 13.9%) than whites. Radiotherapy was administered to 86.9% and 66.5% of the patients having breast conservation and mastectomy, respectively (Table 1).
Table 1.
Characteristics by Surgical Treatment - Clinical T3 patients a n(%)
| BCT (N =2,927) | Mtx (N = 16,502) | Total (N = 19,429) | P-value* | ||
|---|---|---|---|---|---|
| Age at Diagnosis | ≤ 45 | 781 (16.2) | 4048 (83.8) | 4829 | <0.001 |
| 46–55 | 965 (16.9) | 4751 (83.1) | 5716 | ||
| 56–65 | 703 (15.8) | 3742 (84.2) | 4445 | ||
| 65 | 478 (10.8) | 3961 (89.2) | 4439 | ||
| Mean Age at Diagnosis (±SD)b | 53.7 (±12.56) | 55.8 (±14.20) | 55.5 (±13.98) | <0.001 | |
| Race | White | 2093 (13.9) | 13011 (86.1) | 15104 | <0.001 |
| Black | 698 (20.9) | 2635 (79.1) | 3333 | ||
| Other/Unknown | 136 (13.7) | 856 (86.3) | 992 | ||
| Hispanic | No | 2489 (14.9) | 14175 (85.1) | 16664 | 0.456 |
| Yes | 214 (16.0) | 1123 (84.0) | 1337 | ||
| Unknown | 224 (15.7) | 1204 (84.3) | 1428 | ||
| Charlson Comorbidity Score | 0 | 2609 (15.6) | 14123 (84.4) | 16732 | <0.001 |
| 1 | 258 (11.7) | 1955 (88.3) | 2213 | ||
| 2+ | 60 (12.4) | 424 (87.6) | 484 | ||
| Educationc | 21% or more | 546 (15.6) | 2943 (84.4) | 3489 | 0.155 |
| 13% - 20.9% | 750 (15.4) | 4117 (84.6) | 4867 | ||
| 7% - 12.9% | 903 (15.1) | 5063 (84.9) | 5966 | ||
| Less than 7% | 662 (14.0) | 4059 (86.0) | 4721 | ||
| Missing | 66 (17.1) | 320 (82.9) | 386 | ||
| Income | Less than $38,000 | 570 (16.1) | 2965 (83.9) | 3535 | 0.201 |
| $38,000 - $47,999 | 619 (14.4) | 3691 (85.6) | 4310 | ||
| $48,000 - $62,999 | 755 (15.0) | 4291 (85.0) | 5046 | ||
| $63,000 + | 916 (14.9) | 5226 (85.1) | 6142 | ||
| Missing | 67 (16.9) | 329 (83.1) | 396 | ||
| Insurance Status | Medicaid | 374 (17.5) | 1768 (82.5) | 2142 | <0.001 |
| Medicare | 534 (11.2) | 4229 (88.8) | 4763 | ||
| Not Insured | 133 (17.2) | 642 (82.8) | 775 | ||
| Other Government | 35 (16.1) | 182 (83.9) | 217 | ||
| Private Insurance | 1785 (15.9) | 9422 (84.1) | 11207 | ||
| Unknown | 66 (20.3) | 259 (79.7) | 325 | ||
| Urban settingd | Large metropolitan | 1656 (16.0) | 8708 (84.0) | 10364 | <0.001 |
| Small metropolitan | 784 (14.1) | 4789 (85.9) | 5573 | ||
| Suburban | 255 (14.3) | 1530 (85.7) | 1785 | ||
| Rural | 118 (11.8) | 879 (88.2) | 997 | ||
| Unknown | 114 (16.1) | 596 (83.9) | 710 | ||
| Distance to reporting facilitye | ≤10 | 1639 (15.5) | 8907 (84.5) | 10546 | 0.01 |
| 11 – 20 | 626 (14.4) | 3727 (85.6) | 4353 | ||
| 21 – 50 | 447 (15.3) | 2468 (84.7) | 2915 | ||
| >50 | 150 (12.1) | 1087 (87.9) | 1237 | ||
| Unknown | 65 (17.2) | 313 (82.8) | 378 | ||
| Geographic locationf | New England | 188 (18.8) | 810 (81.2) | 998 | <0.001 |
| Middle Atlantic | 396 (16.3) | 2037 (83.7) | 2433 | ||
| South Atlantic | 755 (15.9) | 3979 (84.1) | 4734 | ||
| East North Central | 533 (14.9) | 3050 (85.1) | 3583 | ||
| East South Central | 143 (11.5) | 1100 (88.5) | 1243 | ||
| West North Central | 157 (11.6) | 1195 (88.4) | 1352 | ||
| West South Central | 221 (14.2) | 1336 (85.8) | 1557 | ||
| Mountain | 120 (11.9) | 887 (88.1) | 1007 | ||
| Pacific | 414 (16.4) | 2108 (83.6) | 2522 | ||
| Facility Typeg | Community Cancer Program | ** | 1790 (84.6) | 2117 | <0.001 |
| Comprehensive Community Cancer Program | 1557 (14.0) | 9598 (86.0) | 11155 | ||
| Academic/Research Program | 1038 (16.9) | 5088 (83.1) | 6126 | ||
| Other/Missing | ** | 26 (83.9) | ** | ||
| Year of Diagnosis | 2004 | 248 (17.5) | 1166 (82.5) | 1414 | 0.001 |
| 2005 | 265 (16.7) | 1321 (83.3) | 1586 | ||
| 2006 | 292 (17.2) | 1410 (82.8) | 1702 | ||
| 2007 | 303 (14.9) | 1731 (85.1) | 2034 | ||
| 2008 | 404 (14.0) | 2488 (86.0) | 2892 | ||
| 2009 | 461 (14.9) | 2636 (85.1) | 3097 | ||
| 2010 | 447 (13.5) | 2876 (86.5) | 3323 | ||
| 2011 | 507 (15.0) | 2874 (85.0) | 3381 | ||
| Cancer Sequenceh | 1st | 2761 (15.6 | 14967 (84.4) | 17728 | <0.001 |
| 2+ | 166 (9.8)1 | 1535 (90.2) | 1701 | ||
| Tumor Size | 1 – 7 cm | 2186 (17.6) | 10221 (82.4) | 12407 | <0.001 |
| 7.1 – 10 cm | 609 (11.0) | 4925 (89.0) | 5534 | ||
| >10cm | 132 (8.9) | 1356(91.1) | 1488 | ||
| Mean Tumor Size (±SD)b | 74.1 (±74.25) | 78.8 (±71.16) | 78.1 (±71.65) | <0.001 | |
| No. of Nodes examined | 1 – 5 | 1318 (28.7) | 3274(71.3) | 4592 | <0.001 |
| 6 – 10 | 504 (13.2) | 3313 (86.8) | 3817 | ||
| 11 – 15 | 490 (10.7) | 4094 (89.3) | 4584 | ||
| 16 – 20 | 330 (9.9) | 3011 (90.1) | 3341 | ||
| >20 | 285 (9.2) | 2810 (90.8) | 3095 | ||
| Mean No. of Nodes Examined (±SD)b | 9.2 (±7.94) | 13.2 (±8.33) | 12.6 (±8.39) | <0.001 | |
| No. of Nodes Positive | 0 | 1524 (24.3) | 4748 (75.7) | 6272 | <0.001 |
| 1 – 3 | 919 (14.5) | 5401 (85.5) | 6320 | ||
| 4 – 6 | 212 (8.5) | 2291 (91.5) | 2503 | ||
| 7 – 9 | 112 (7.6) | 1371 (92.4) | 1483 | ||
| ≥10 | 160 (5.6) | 2691 (94.4) | 2851 | ||
| Mean No. of Nodes positive (±SD)b | 1.9 (±3.93) | 4.5 (±6.06) | 4.1 (±5.86) | <0.001 | |
| Grade | 1 | 162 (10.3) | 1410 (89.7) | 1572 | <0.001 |
| 2 | 795 (11.9) | 5875 (88.1) | 6670 | ||
| 3 | 1719 (17.7) | 7971 (82.3) | 9690 | ||
| Undifferentiated | 24 (16.3) | 123 (83.7) | 147 | ||
| Unknown | 227 (16.8) | 1123 (83.2) | 1350 | ||
| Histology | Ductal | 2449 (16.5) | 12359 (83.5) | 14808 | <0.001 |
| Lobular | 306 (8.1) | 3466 (91.9) | 3772 | ||
| Other | 172 (20.3) | 677 (79.7) | 849 | ||
| Radiation | Administered | 2545 (18.8) | 10976 (81.2) | 13521 | <0.001 |
| Not administered | 382 (6.5) | 5526 (93.5) | 5908 | ||
| Chemotherapy | Neoadjuvant | 1934 (19.8) | 7842 (80.2) | 9776 | <0.001 |
| Adjuvant | 664 (10.1) | 5914 (89.9) | 6578 | ||
| None | 316 (10.7) | 2641 (89.3) | 2957 | ||
| Unknown | 13 (11.0) | 105 (89.0) | 118 |
Abbreviations: SD- standard deviation
Chi-square test (categorical variables) or Wilcoxon rank sum test (continuous variables)
Cells have been censored in accordance with NCDB privacy requirements to do so for any cell <11 or for any cell that makes those cells calculable.
Numbers shown as number (row %) unless otherwise indicated.
Age and size analyses are shown here as continuous variables to illustrate cohort compositions.
Education represents the percentage of individuals within a ZIP code from census data having <12 years of education.
Setting definitions: Large metropolitan indicates counties of metropolitan areas of >1,000,000; Small metropolitan, counties in metropolitan areas of <250,000 to 1,000,000; Suburban, urban population of >20,000 adjacent to a metropolitan area or 2500 to 19,999 adjacent to a metropolitan area; rural, completely rural or <2500 or >20,000 urban population nonadjacent to a metropolitan area.
Miles between patient’s residence and the hospital that reported the case
Region groupings: New England (CT, MA, ME, NH, RI, VT), Middle Atlantic (NJ, NY, PA), South Atlantic (DC, DE, FL, GA, MD, NC, SC, VA, WV), East North Central (IL, IN, MI, OH, WI), East South Central (AL, KY, MS, TN), West North Central (IA, KS, MN, MO, ND, NE, SD), West South Central (AR, LA, OK, TX), Mountain (AZ, CO, ID, MT, NM, NV, UT, WY), Pacific (AK, CA, HI, OR, WA).
Community Cancer program: >100 but <500 newly diagnosed cancer cases each year; Comprehensive community cancer program: 500 or more new cases for three consecutive years; Academic/Research program: participates in postgraduate medical education in at least four program areas, and >500 newly diagnosed cancer cases each year.
The number of the cancer that the breast cancer represents, among cancers of any type, if they had >1 cancer of any type during their lifetime.
Overall predictors of BCT based on clinical T stage included age, race, Charlson comorbidity score, geographical location, facility type, year of diagnosis, tumor size, number of lymph nodes examined and positive, nuclear grade, histology, and chemotherapy and radiation therapy administration (Table 2). The largest predictors of BCT use included neoadjuvant chemotherapy administration (odds ratio [OR] 1.687, 95% confidence interval [95% CI], 1.499– 1.898, p<0.001), black race (OR 1.471, 95% CI, 1.310–1.652, p<0.001), and grade III tumors (OR 1.542, 95% CI, 1.275–1.866, p<0.001). Patients less likely to undergo BCT included those who had a second cancer (OR 0.715, 95% CI, 0.600–0.852, p=0.001), and those who did not receive adjuvant radiation (OR 0.259, 95% CI, 0.228–0.294, p<0.001). Age <45, greater number of lymph nodes examined and positive, geographic location outside of New England, lobular histology, year of diagnosis, and treatment at a comprehensive cancer center program were also less likely to receive BCT (Table 2).
Table 2:
Predictors of BCT (cT3 patients)
| OR | 95% CI | p-value | Overall Significance | ||
|---|---|---|---|---|---|
| Age | <0.001 | ||||
| ≤45 | 0.928 | 0.760 | 1.133 | 0.462 | |
| 46 – 55 | 1.145 | 0.954 | 1.376 | 0.147 | |
| 56 – 65 | 1.211 | 1.014 | 1.447 | 0.035 | |
| >65 | Referent | ||||
| Race | <0.001 | ||||
| White | Referent | ||||
| Black | 1.471 | 1.310 | 1.652 | <0.001 | |
| Unknown | 0.766 | 0.616 | 0.953 | 0.017 | |
| Hispanic | 0.297 | ||||
| No | Referent | ||||
| Yes | 1.077 | 0.897 | 1.293 | 0.426 | |
| Unknown | 1.140 | 0.952 | 1.365 | 0.155 | |
| Charlson Comorbidity Score | 0.051 | ||||
| 0 | Referent | ||||
| 1 | 0.836 | 0.716 | 0.975 | 0.022 | |
| 2 | 1.049 | 0.770 | 1.428 | 0.764 | |
| Insurance | 0.060 | ||||
| Private Insurance | Referent | ||||
| Medicaid | 1.086 | 0.944 | 1.250 | 0.249 | |
| Medicare | 0.948 | 0.798 | 1.126 | 0.544 | |
| Not Insured | 1.023 | 0.813 | 1.288 | 0.844 | |
| Other | 1.052 | 0.694 | 1.593 | 0.813 | |
| Unknown | 1.762 | 1.272 | 2.440 | 0.001 | |
| Educationa | 0.318 | ||||
| 21% or more | Referent | ||||
| 13% - 20.9% | 1.139 | 0.983 | 1.319 | 0.084 | |
| 7% - 12.9% | 1.153 | 0.972 | 1.369 | 0.103 | |
| Less than 7% | 1.063 | 0.872 | 1.296 | 0.547 | |
| Missing | 1.912 | 0.055 | 66.780 | 0.721 | |
| Income | 0.755 | ||||
| < $38,000 | Referent | ||||
| $38,000 - $47,999 | 0.907 | 0.783 | 1.050 | 0.190 | |
| $48,000 - $62,999 | 0.921 | 0.786 | 1.078 | 0.306 | |
| $63,000 + | 0.896 | 0.740 | 1.084 | 0.259 | |
| Missing | 0.706 | 0.026 | 19.254 | 0.836 | |
| Urban settingb | 0.330 | ||||
| Large Metropolitan | Referent | ||||
| Small metropolitan | 0.912 | 0.805 | 1.033 | 0.148 | |
| Suburban | 0.832 | 0.677 | 1.022 | 0.079 | |
| Rural | 0.991 | 0.736 | 1.335 | 0.955 | |
| Unknown | 0.964 | 0.719 | 1.291 | 0.804 | |
| Distance to reporting facility (miles)c | 0.236 | ||||
| ≤10 | Referent | ||||
| 11 – 20 | 0.944 | 0.848 | 1.050 | 0.288 | |
| 21 – 50 | 1.108 | 0.964 | 1.274 | 0.148 | |
| >50 | 0.952 | 0.750 | 1.209 | 0.688 | |
| Unknown | 0.857 | 0.226 | 3.242 | 0.820 | |
| Geographic locationd | <0.001 | ||||
| New England | Referent | ||||
| Middle Atlantic | 0.816 | 0.639 | 1.043 | 0.105 | |
| South Atlantic | 0.778 | 0.619 | 0.978 | 0.032 | |
| East North Central | 0.728 | 0.566 | 0.937 | 0.008 | |
| East South Central | 0.558 | 0.413 | 0.755 | 0.000 | |
| West North Central | 0.539 | 0.399 | 0.729 | <0.001 | |
| West South Central | 0.757 | 0.561 | 1.023 | 0.070 | |
| Mountain | 0.641 | 0.4773 | 0.869 | 0.004 | |
| Pacific | 0.925 | 0.724 | 1.183 | 0.536 | |
| Facility Typee | 0.005 | ||||
| Community Program | Referent | ||||
| Comprehensive Community | 0.797 | 0.676 | 0.939 | 0.007 | |
| Academic/Research | 0.963 | 0.801 | 1.158 | 0.687 | |
| Other/Missing | 0.911 | 0.704 | 1.179 | 0.478 | |
| Year of diagnosis | 0.951 | 0.932 | 0.970 | <0.001 | <0.001 |
| Cancer Sequencef | <0.001 | ||||
| First | Referent | ||||
| 2+ | 0.715 | 0.600 | 0.852 | 0.001 | |
| Tumor size | <0.001 | ||||
| 5.1 to 7 cm | Referent | ||||
| 7.1 to 10 cm | 0.576 | 0.521 | 0.637 | <0.001 | |
| >10 cm | 0.457 | 0.367 | 0.568 | <0.001 | |
| No. nodes examined | 0.948 | 0.941 | 0.975 | <0.001 | <0.001 |
| No. nodes positive | 0.928 | 0.914 | 0.943 | <0.001 | <0.001 |
| Grade | <0.001 | ||||
| 1 | Referent | ||||
| 2 | 1.123 | 0.930 | 1.356 | 0.252 | |
| 3 | 1.542 | 1.275 | 1.866 | <0.001 | |
| Undifferentiated | 1.250 | 0.790 | 1.979 | 0.355 | |
| Unknown | 1.416 | 1.114 | 1.801 | 0.005 | |
| Histology | <0.001 | ||||
| Ductal | Referent | ||||
| Lobular | 0.604 | 0.524 | 0.696 | <0.001 | |
| Other | 1.264 | 1.056 | 1.513 | 0.011 | |
| Chemotherapy | <0.001 | ||||
| Adjuvant | Referent | ||||
| Neoadjuvant | 1.687 | 1.499 | 1.898 | <0.001 | |
| None | 1.533 | 1.283 | 1.832 | <0.001 | |
| Unknown | 1.841 | 0.969 | 3.499 | 0.062 | |
| Radiotherapy | <0.001 | ||||
| Administered | Referent | ||||
| Not administered | 0.259 | 0.228 | 0.294 | <0.001 | |
Abbreviations: BCT, breast conservation therapy; cT3, clinical T3 patients
Education represents the percentage of individuals within a ZIP code from census data having <12 years of education.
Setting definitions: Large metropolitan indicates counties of metropolitan areas of >1,000,000; Small metropolitan, counties in metropolitan areas of <250,000 to 1,000,000; Suburban, urban population of >20,000 adjacent to a metropolitan area or 2500 to 19,999 adjacent to a metropolitan area; rural, completely rural or <2500 or >20,000 urban population nonadjacent to a metropolitan area.
Miles between patient’s residence and the hospital that reported the case
Region groupings: New England (CT, MA, ME, NH, RI, VT), Middle Atlantic (NJ, NY, PA), South Atlantic (DC, DE, FL, GA, MD, NC, SC, VA, WV), East North Central (IL, IN, MI, OH, WI), East South Central (AL, KY, MS, TN), West North Central (IA, KS, MN, MO, ND, NE, SD), West South Central (AR, LA, OK, TX), Mountain (AZ, CO, ID, MT, NM, NV, UT, WY), Pacific (AK, CA, HI, OR, WA).
Community Cancer program: >100 but <500 newly diagnosed cancer cases each year; Comprehensive community cancer program: 500 or more new cases for three consecutive years; Academic/Research program: participates in postgraduate medical education in at least four program areas, and >500 newly diagnosed cancer cases each year.
The number of the cancer that the breast cancer represents, among cancers of any type, if they had >1 cancer of any type during their lifetime.
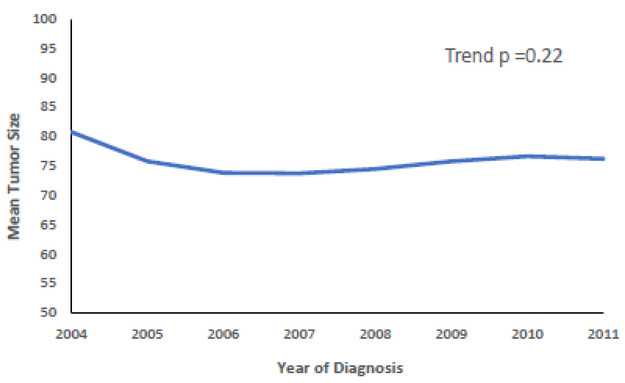
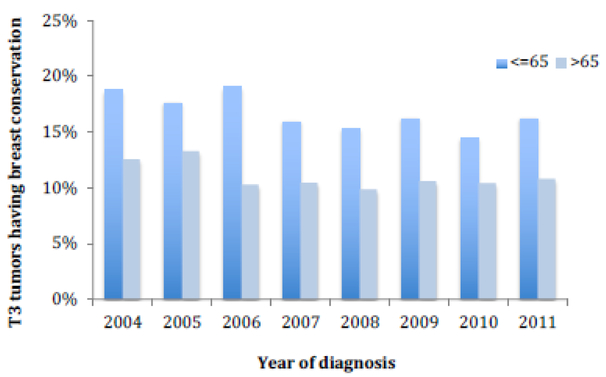
The year of diagnosis predicted whether patients underwent BCT, declining from 17.5% in 2004 to 15% in 2011 (p=0.001) with 2010 having the lowest overall proportion of patients (13.5%) (Supplemental Table 1). However, the mean tumor size did not significantly change from 2004 to 2011 (trend p=0.22) (Figure 2). Over time, the use of BCT decreased in the <65 age group (p<0.001), whereas in the >65 age group, it was not significantly different over time (trend p=0.288) (Figure 3).
Figure 2.
Trend in tumor size over time.
Figure 3.
Unadjusted use of breast conservation over time. P for decreasing trend <0.001 for all ages; p<0.001 for ≤65, p=0.144 for >65.
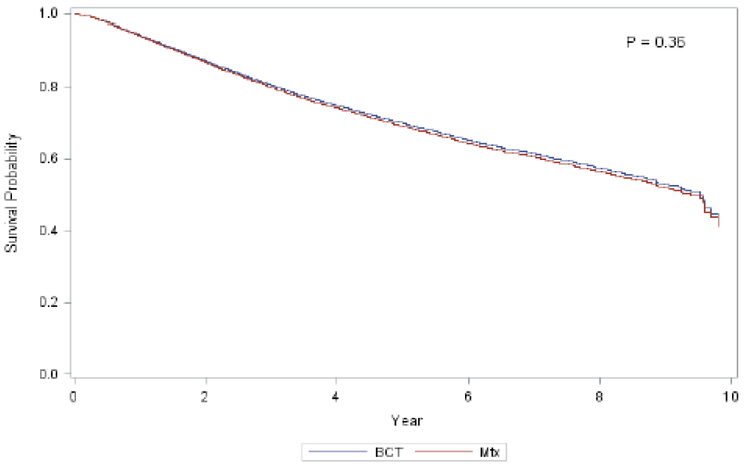
There was no significant difference in OS of pT3 patients when comparing BCT to Mtx (p= 0.163). When adjusting for all of the characteristics listed in Table 2, OS of pT3 patients was still equivalent (HR 0.963, 95% CI, 0.889–1.043, p=0.357) between BCT and Mtx (Supplemental Table 2). The 5-year adjusted OS for BCT was 68% (95% CI 0.652 to 0.709), and 69% for Mtx (95% CI 0.686 to 0.700), p=0.163 (Figure 4).
Figure 4.
Adjusted Overall Survival Breast Conservation vs. Mastectomy
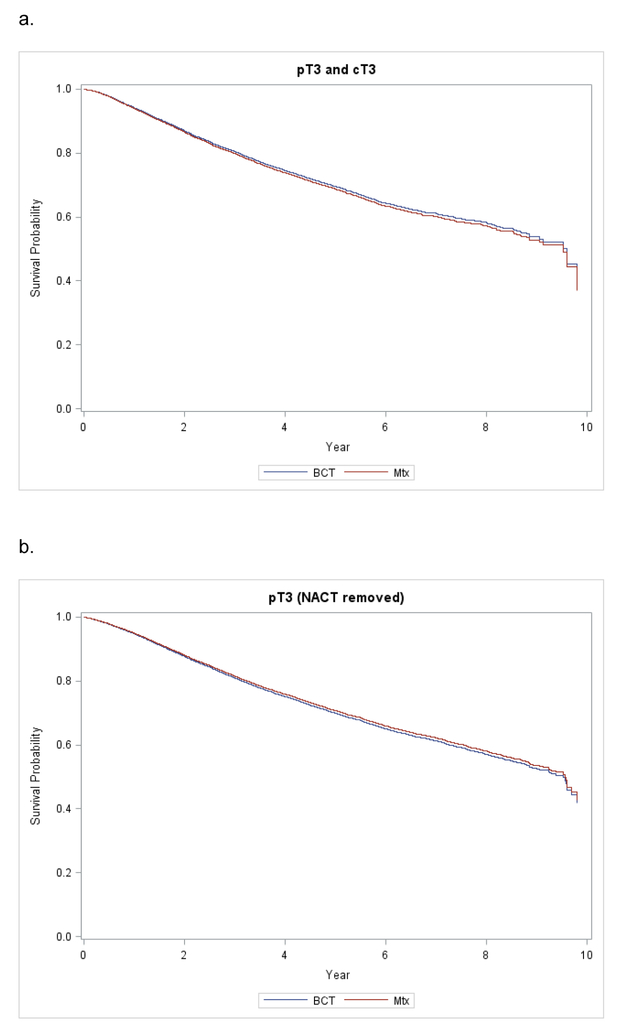
When restricting the analysis to patients who were both clinically and pathologically T3, OS again remained unchanged (HR 0.963, 95% CI, 0.850–1.090, p= 0.551). Finally, when removing patients who received neoadjuvant chemotherapy (a total of 6,424 patients) from the analysis, the survival for pT3 patients who received either Mtx or BCT still did not differ (HR 1.039, 95% CI, 0.951–1.136, p=0.393) (Supplemental Table 2, Figure 5).
Figure 5.
Kaplan-Meier Curves for Adjusted Overall Survival of (A) Combined pT3 and cT3 cohort and (B) pT3 with NACT removed.
Discussion
As the current standard of care for early stage breast cancer, breast conservation has gained wide acceptance since its introduction in the early 1970s [12]. Its application has been cautioned for tumors >5 cm because early trials, while widely varied, only included tumors 5 cm or smaller. Nevertheless, all of these studies found BCT equivalent to mastectomy, such as NSABP B-06, which limited patients to tumors >2 and <4 cm [13], as versus EORTC 10801, which included tumors up to 5 cm [9]. The Danish Cooperative Group included primaries >3 cm in 7% of the cohort, and found an 18% locoregional recurrence rate overall with no difference in OS [14]. Obedian and Haffty did not exclude T3 tumors, but these comprised only 1% of their patients. Still, they also found equivalent relapse-free and OS with negative or close margins [15]. These studies suggested that there is no difference in how larger tumors should be treated, but with none of these analyses powered to evaluate the T3 subgroup, no conclusion can be gleaned from these data. We have now shown, in a large diverse cohort, that BCT confers similar survival to that of Mtx for tumors not previously considered amenable to breast conservation.
Large retrospective series have found that overall survival is inversely proportional to tumor size, which likely impelled exclusion of large tumors in breast conservation trials [16]. We now know that survival is equivalent for smaller tumors treated by BCT versus Mtx, and there is no biological basis for the arbitrary cutoff of 5 cm. Similarly, several small studies have shown the feasibility of BCT for large tumors in terms of overall and disease free survival, with neoadjuvant chemotherapy excluded from those analyses [17,18]. These findings are consistent with ours here, showing equivalent OS between the two treatment approaches, even when controlling for neoadjuvant chemotherapy, although these studies were small retrospective reviews [18,17]. A study by Khanna and colleagues, for example, found actuarial five year OS of 76% and actuarial disease free survival rates of 68% in tumors >4 cm, and no recurrences in patients with negative margins achieved. This coincides with our findings of an adjusted 5 year OS in pT3 patients of 68%.
Although our data show that breast conservation for larger tumors is safe, there was a trend towards mastectomy in younger women over time. Recent studies have similarly found that mastectomy rates in BCT-eligible patients have been increasing since at least 2004 [19–21]. Additionally, rates of bilateral mastectomy for unilateral disease have increased in the absence of factors increasing oncologic risk, with young age as an independent predictor of mastectomy use [22]. This trend may be related to a greater recognition of higher recurrence in younger age groups and its influence on clinical decision-making [23], but it should be noted that survival in young women (<40) is not different when treated via BCT versus Mtx [23]. This is also likely to be driven by increasing patient involvement and changing patient preferences [24].
Although the trend for BCT rates for tumors >5 cm in patients >65 years of age visually appears to decline over time in Figure 2, this was not statistically significant. It remains unknown why this differs from previously published SEER-Medicare data for women ≥65 showing a steady increase in BCT procedures for T3 lesions over time [10]. Meanwhile, women ≤65, who were not previously analyzed, had a significant decrease in BCT procedures for T3 primaries in this study. It is impossible to determine what factors would cause these variations, but both datasets do consistently find that the majority of patients with T3 tumors still undergo mastectomy [10] as expected based upon current guidelines, such as the NCCN [1], and the previous paucity of data supporting its safety.
Our data also suggest a selection bias towards Mtx for more aggressive tumors, shown here by a higher number of lymph nodes examined, greater numbers of positive nodes, and larger tumor sizes in the Mtx group. Although we do not provide benefit when varying the breast procedure based upon nodal status, this may reflect clinicians’ underlying desire to be more “aggressive” in the setting of negative independent predictors of survival [16].
Also consistent with the SEER-Medicare published data on this topic, neoadjuvant chemotherapy use predicted BCT in patients having T3 tumors [10]. While we know that use of neoadjuvant chemotherapy (as versus adjuvant chemotherapy) does not affect overall survival, it does potentially downstage the primary tumor and increase the likelihood of breast conservation [25]. This study indicates that the majority of tumors >5 cm continue to be treated with chemotherapy prior to surgery, with 66% of BCT patients having received chemotherapy in the neoadjuvant setting. Still, our sensitivity analysis removing patients receiving neoadjuvant chemotherapy confirmed the equivalence and safety of BCT versus Mtx.
Although we were unable to evaluate recurrence rates because the NCDB does not include such data, we know from the Early Breast Cancer Trialists’ Collaborative Group meta-analysis that recurrence and survival are linked [26]. The similar OS found here therefore suggests that if any disease-specific survival (DSS) difference exists between BCT and Mtx for T3 tumors, the difference should be small. Moreover, a prior in a different large dataset (SEER-Medicare) has confirmed in that sizable, but smaller national cohort, there is no difference in DSS for T3 tumors undergoing BCT as versus Mtx [10].
Although this was not the focus of this study, a limitation of large datasets, like the NCDB, is the inability to judge cosmetic outcomes. There is some evidence to show that a good cosmetic appearance is feasible in BCT for T3 primaries, and recent oncoplastic techniques may provide more opportunities for cosmetically pleasing local resection of larger tumors. One such study evaluated 540 patients who underwent breast conservation for large tumor-to-breast ratio, and found that oncoplastics provided a “good” cosmetic outcome based on a five point grading scale in 97.7% of patients. The study patients’ disease free survival and OS were also 87.9% and 92.9%, respectively, showing that safety was not compromised for cosmesis [27]. Another small study utilizing oncoplastic surgery with contralateral reduction mammoplasty found that these enabled larger resections, good cosmetic outcomes, and acceptable 5-year survival and local recurrence rates. Oncoplastic techniques have gained refinement and popularity, and may even allow patients having T3 lesions and smaller or borderline breast sizes to undergo breast conservation successfully.
In conclusion, BCT for tumors >5 cm is safe and has an equivalent OS to that of mastectomy. Although this study shows slightly declining use of breast conservation for larger tumors in recent years, practitioners should consider BCT based upon projected cosmetic outcome and patient desires, and no longer consider BCT contraindicated solely based upon an arbitrary size cutoff.
Supplementary Material
ACKNOWLEDGEMENTS:
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Elizabeth Handorf reports funding paid to the institution from Pfizer for unrelated research.
Footnotes
Compliance with Ethical Standards:
Ethical approval: This research was comprised of de-identified database records thus maintaining confidentiality and posing negligible or no risks to the participants within the dataset.
Informed consent: IRB review declared NCDB database review as exempt
Presented, in part, at the Society of Surgical Oncology 2018 Annual Cancer Symposium, March 27–30, 2018 and at the Metropolitan Philadelphia Chapter of the American College of Surgeons Meeting, May 14, 2018 (Poster Presentation Award Winner).
Conflict of Interest: This work was supported by United States Public Health Services grant P30 CA006927 for analysis of the data via support of our biostatistics facility, and by generous private donor support from the Marlyn Fein Chapter of the Fox Chase Cancer Center Board of Associates, for analysis and interpretation of the data.
The authors otherwise have no potential conflicts of interest.
References
- 1.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A, Giordano SH, Goetz MP, Goldstein LJ, Isakoff SJ, Lyons J, Marcom PK, Mayer IA, McCormick B, Moran MS, O’Regan RM, Patel SA, Pierce LJ, Reed EC, Salerno KE, Schwartzberg LS, Sitapati A, Smith KL, Smith ML, Soliman H, Somlo G, Telli ML, Ward JH, Kumar R, Shead DA (2018) Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16 (3):310–320. doi: 10.6004/jnccn.2018.0012 [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative G (1995) Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med 333 (22):1444–1455. doi: 10.1056/NEJM199511303332202 [DOI] [PubMed] [Google Scholar]
- 3.Bleicher RJ, Ruth K, Sigurdson ER, Ross E, Wong YN, Patel SA, Boraas M, Topham NS, Egleston BL (2012) Preoperative delays in the US Medicare population with breast cancer. J Clin Oncol 30 (36):4485–4492. doi: 10.1200/JCO.2012.41.7972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margolis G, Goodman RL, Rubin A (1990) Psychological effects of breast-conserving cancer treatment and mastectomy. Psychosomatics 31 (1):33–39. doi: 10.1016/S0033-3182(90)72214-1 [DOI] [PubMed] [Google Scholar]
- 5.National Accreditation Program for Breast Centers. NAPBC Standards Manual 2018 Edition (2018). In: Surgeons ACo; (ed) [Google Scholar]
- 6.Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ, Menard C, Lippman ME, Lichter AS, Altemus RM (2003) Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer 98 (4):697–702 [DOI] [PubMed] [Google Scholar]
- 7.Consensus statement: treatment of early-stage breast cancer. National Institutes of Health Consensus Development Panel (1992). J Natl Cancer Inst Monogr (11):1–5 [PubMed] [Google Scholar]
- 8.Osborne MP, Ormiston N, Harmer CL, McKinna JA, Baker J, Greening WP (1984) Breast conservation in the treatment of early breast cancer. A 20-year follow-up. Cancer 53 (2):349–355 [DOI] [PubMed] [Google Scholar]
- 9.van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, van der Schueren E, Helle PA, van Zijl K, Bartelink H (2000) Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 92 (14):1143–1150 [DOI] [PubMed] [Google Scholar]
- 10.Bleicher RJ, Ruth K, Sigurdson ER, Daly JM, Boraas M, Anderson PR, Egleston BL (2016) Breast conservation versus mastectomy for patients with T3 primary tumors (>5 cm): A review of 5685 medicare patients. Cancer 122 (1):42–49. doi: 10.1002/cncr.29726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleicher RJ, Ruth K, Sigurdson ER, Beck JR, Ross E, Wong YN, Patel SA, Boraas M, Chang EI, Topham NS, Egleston BL (2016) Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol 2 (3):330–339. doi: 10.1001/jamaoncol.2015.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crile G Jr., Esselstyn CB Jr., Hermann RE, Hoerr SO (1973) Partial mastectomy for carcinoma of the breast. Surg Gynecol Obstet 136 (6):929–933 [PubMed] [Google Scholar]
- 13.Fisher ER, Anderson S, Redmond C, Fisher B (1992) Ipsilateral breast tumor recurrence and survival following lumpectomy and irradiation: pathological findings from NSABP protocol B-06. Semin Surg Oncol 8 (3):161–166 [PubMed] [Google Scholar]
- 14.Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson CK, Andersen KW, Mouridsen HT (1992) Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr (11):19–25 [PubMed] [Google Scholar]
- 15.Obedian E, Haffty BG (2000) Negative margin status improves local control in conservatively managed breast cancer patients. Cancer J Sci Am 6 (1):28–33 [PubMed] [Google Scholar]
- 16.Carter CL, Allen C, Henson DE (1989) Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63 (1):181–187 [DOI] [PubMed] [Google Scholar]
- 17.Khanna MM, Mark RJ, Silverstein MJ, Juillard G, Lewinsky B, Giuliano AE (1992) Breast conservation management of breast tumors 4 cm or larger. Arch Surg 127 (9):1038–1041; discussion 1041–1033 [DOI] [PubMed] [Google Scholar]
- 18.Fitzal F, Riedl O, Wutzl L, Draxler W, Rudas M, Pluschnig U, Handl-Zeller L, Dubsky P, Bachleitner-Hofmann T, Steger G, Jakesz R, Gnant M (2007) Breast-conserving surgery for T3/T4 breast cancer: an analysis of 196 patients. Breast Cancer Res Treat 103 (1):45–52 [DOI] [PubMed] [Google Scholar]
- 19.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA (2015) Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 150 (1):9–16. doi: 10.1001/jamasurg.2014.2895 [DOI] [PubMed] [Google Scholar]
- 20.Albornoz CR, Matros E, Lee CN, Hudis CA, Pusic AL, Elkin E, Bach PB, Cordeiro PG, Morrow M (2015) Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer: The Role of Breast Reconstruction. Plast Reconstr Surg 135 (6):1518–1526. doi: 10.1097/PRS.0000000000001276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuire KP, Santillan AA, Kaur P, Meade T, Parbhoo J, Mathias M, Shamehdi C, Davis M, Ramos D, Cox CE (2009) Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 16 (10):2682–2690 [DOI] [PubMed] [Google Scholar]
- 22.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA (2007) Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 25 (33):5203–5209 [DOI] [PubMed] [Google Scholar]
- 23.Fodor J, Mozsa E, Zaka Z, Polgar C, Major T (2005) [Local relapse in young (< or = 40 years) women with breast cancer after mastectomy or breast conserving surgery: 15-year results]. Magy Onkol 49 (3):203, 205–208. doi:HUON.2005.49.3.0203 [PubMed] [Google Scholar]
- 24.Katz SJ, Lantz PM, Janz NK, Fagerlin A, Schwartz K, Liu L, Deapen D, Salem B, Lakhani I, Morrow M (2005) Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol 23 (24):5526–5533 [DOI] [PubMed] [Google Scholar]
- 25.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr (30):96–102 [DOI] [PubMed] [Google Scholar]
- 26.Early Breast Cancer Trialists’ Collaborative G, Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378 (9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitoussi AD, Berry MG, Fama F, Falcou MC, Curnier A, Couturaud B, Reyal F, Salmon RJ (2010) Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases [outcomes article]. Plast Reconstr Surg 125 (2):454–462. doi: 10.1097/PRS.0b013e3181c82d3e [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.