Abstract
Despite the success of combination anti-retroviral therapy (cART), around 50% of HIV-infected individuals still display a variety of neuropathological and neurocognitive sequelae known as NeuroHIV. Current research suggests these effects are mediated by long-term changes in CNS function in response to chronic infection and inflammation, and not solely due to active viral replication. In the post-cART era, drug abuse is a major risk-factor for the development of NeuroHIV, and increases extracellular dopamine in the CNS. Our lab has previously shown that dopamine can increase HIV infection of primary human macrophages and increase the production of inflammatory cytokines, suggesting that elevated dopamine could enhance the development of HIV-associated neuropathology. However, the precise mechanism(s) by which elevated dopamine could exacerbate NeuroHIV, particularly in chronically-infected, virally suppressed individuals remain unclear. To determine the connection between dopaminergic alterations and HIV-associated neuroinflammation, we have examined the impact of dopamine exposure on macrophages from healthy and virally suppressed, chronically infected HIV patients. Our data show that dopamine treatment of human macrophages isolated from healthy and cART-treated donors promotes production of inflammatory mediators including IL-1β, IL-6, IL-18, CCL2, CXCL8, CXCL9, and CXCL10. Furthermore, in healthy individuals, dopamine-mediated modulation of specific cytokines is correlated with macrophage expression of dopamine-receptor transcripts, particularly DRD5, the most highly-expressed dopamine-receptor subtype. Overall, these data will provide more understanding of the role of dopamine in the development of NeuroHIV, and may suggest new molecules or pathways that can be useful as therapeutic targets during HIV infection.
Keywords: Dopamine, macrophages, neuroinflammation, HIV, cART
Introduction
Use of combination anti-retroviral treatment (cART) can successfully suppress HIV replication and has transformed HIV infection from a terminal illness to a chronic condition. Antiviral therapy has also dramatically curtailed the incidence of severe neurocognitive complications resulting from HIV infection of the CNS, although milder neurocognitive and neuropathological sequelae remain common (Saylor et al., 2016). Even when viral replication is fully suppressed, using cART with good CNS penetrance, infected individuals still show distinct neuropathology and the collection of neurological symptoms known as HIV-associated neurocognitive disorders (HAND) or NeuroHIV (Schouten et al., 2011; Cross et al., 2013; Cysique et al., 2014; Baker et al., 2015; Gelman, 2015; Boban et al., 2017; Clifford et al., 2017; Sanford et al., 2017a; Underwood et al., 2017; van den Dries et al., 2017). Notably, neurocognitive impairment does not correlate with CNS viral load, indicating development of NeuroHIV and HIV-associated neurological disorders (HAND) does not derive solely from damage associated with actively replicating virus (McArthur, 2004; Sevigny et al., 2004; Tavazzi et al., 2014). In the era of cART, one of the primary drivers of NeuroHIV is thought to be chronic neuroinflammation (Gill and Kolson, 2014; Rao et al., 2014; Hong and Banks, 2015). Studies suggest that chronic HIV-associated neuroinflammation may result from the interplay between host CNS cells and CNS viral reservoir (Finzi et al., 1997; Ruelas and Greene, 2013; Rouzine et al., 2015), which is thought to be comprised mainly of myeloid cells (Aquaro et al., 2002; Churchill and Nath, 2013).
As these cells are the main effectors of the neuroimmune response, factors that modulate myeloid inflammatory processes could have a significant impact on the development of NeuroHIV (Williams and Hickey, 2002; Kaul and Lipton, 2006; Kraft-Terry et al., 2009; Rappaport and Volsky, 2015; Saylor et al., 2016; Soontornniyomkij, 2017). Recent research by our lab and others suggests that one of these factors is dopamine. Dopamine is a catecholamine neurotransmitter that has been researched primarily for its role in modulating cognition, motivation, and movement. However, associations between human behavior and immune function (Basu and Dasgupta, 2000; Dantzer et al., 2008; Patterson, 2009; Muller and Schwarz, 2010; Sarkar et al., 2010; Sampson and Mazmanian, 2015) have been gaining attention in recent years and have implicated dopamine as a novel immunomodulatory agent. Our previous studies have shown that dopamine can both alter the production of inflammatory factors and increase HIV infection in primary human macrophages (Gaskill et al., 2009; Gaskill et al., 2012; Gaskill et al., 2014). These data connect the impact of dopamine on macrophage functions to the disruption in the dopaminergic system.
Dysregulation of dopaminergic neurotransmission has been linked to the development of HIV-associated CNS disease since early in the epidemic (Hriso et al., 1991; Kieburtz et al., 1991; Berger and Arendt, 2000). Early studies found significant changes in dopamine metabolism in HIV-infected individuals (Larsson et al., 1991; Berger et al., 1994; Koutsilieri et al., 1997; di Rocco et al., 2000) and prominent neuropathology in dopamine-rich brain regions including substantia nigra, basal ganglia, and prefrontal cortex. These regions showed volume loss, neuronal atrophy and greater numbers of infected myeloid cells in basal ganglia (BG) and substantia nigra (SN) (Navia et al., 1986; Kure et al., 1990; Reyes et al., 1991; Aylward et al., 1993; Hestad et al., 1993; Aylward et al., 1995; Glass et al., 1995; Itoh et al., 2000; Chan et al., 2016; Clifford et al., 2017; Sanford et al., 2017b). The specific vulnerability of dopaminergic brain regions, even in virally-suppressed individuals, suggests that changes in dopaminergic tone are important to the development of NeuroHIV, potentially through its effects on myeloid cells.
In addition to changes in response to dopaminergic pathology, a number of studies show that dopamine concentrations can be specifically altered by HIV infection. Viral proteins such as Tat can impair the function of the dopamine transporter (DAT), elevating dopaminergic tone by interfering with dopamine uptake from the extracellular environment (Ferris et al., 2009; Zhu et al., 2009; Agrawal et al., 2010; Midde et al., 2012; Liu et al., 2014; Fitting et al., 2015; Zhu et al., 2016; Gaskill et al., 2017). Some data show that patients naive to cART have increased CSF dopamine and demonstrate overactivity in dopaminergic circuits during asymptomatic infection (Scheller et al., 2010; Horn et al., 2013). However, other studies report decreased CSF dopamine during chronic HIV infection (Berger et al., 1994; Lopez et al., 1999; Kumar et al., 2009). As dopamine is sensitive to light and prone to oxidation (Sloviter and Connor, 1977; Shen, 1993; Kankaanpaa et al., 2001; Syslova et al., 2011), differences in CSF or post-mortem analyses may be due to differences in tissue preparations. Together with data that shows neuronal degeneration in dopaminergic brain regions of HIV-infected, cART-naïve patients (Reyes et al., 1991; Lopez et al., 1999; Kumar et al., 2009; Obermann et al., 2009; Kumar et al., 2011), these data may reflect progressive damage due to consistently elevated dopamine during early infection that mediates greater viral replication and inflammation in these areas.
Although these data indicate that the natural progression of HIV infection in the CNS involves the dopaminergic system, today many infected individuals use cART, so it is critical to evaluate the impact of dopamine in the presence of this therapy. With cART, overt dopamine neurodegeneration and many of the behavioral symptoms of dopaminergic dysfunction are still present, although subtler [26, 47-50]. Furthermore, many of the HIV-associated impairments in these patients such as deficits in attention or motivation, reflect specific dysfunction of dopaminergic systems [51-60]. Post-mortem analyses of dopaminergic gene and protein expression demonstrate post-synaptic changes, such as decreased DRD2L expression, that reflect an elevation of dopaminergic tone and were associated with neuroinflammation and neurocognitive impairment (Gelman et al., 2006; Gelman et al., 2012). As dopamine regulates a number of myeloid functions associated with NeuroHIV (Gaskill et al., 2013; Nolan and Gaskill, 2018), the changes in dopamine concentration could initiate or contribute to these effects by disrupting myeloid functions.
It has been shown that exogenous factors that increase extracellular CNS dopamine would substantially accelerate or exacerbate the development of HIV-associated neuroinflammation by increasing the number of CNS myeloid cells exposed to elevated dopamine levels. Around 10% of HIV-infected individuals concurrently abuse drugs (Mathers et al., 2008; Strathdee and Stockman, 2010; UNODC, 2017), all of which mediate their effects by elevating CNS dopamine (Di Chiara and Imperato, 1988; Koob, 1992; Pierce and Kumaresan, 2006). Additionally, common neuropsychiatric co-morbidities of HIV infection, such as depression (Bing et al., 2001; Do et al., 2014), often use dopaminergic therapeutics (D'Aquila et al., 2000; Wood and Reavill, 2007; Cohen et al., 2012; Leggio et al., 2013; Agius and Bonnici, 2017) that could also dysregulate dopaminergic tone in the HIV infected brain. These comorbidities are becoming more common as the HIV-infected population ages (Watkins and Treisman, 2012), potentially increasing the number of infected individuals with altered dopaminergic tone. This suggests that altered dopaminergic tone may be an important factor in the development of HIV-associated neurological disease in the cART era, particularly among vulnerable aging and drug abusing sub-populations.
To better define the impact of dopamine on NeuroHIV, it is critical to determine the precise effects of dopamine on inflammatory processes in human myeloid cells. To do this, we examined the macrophage dopaminergic system and dopaminergic modulation of the HIV-associated inflammatory mediators IL-6, IL-1β, IL-10, TNF-α, CCL2, CXCL8, CXCL9, and CXCL10. The effect of dopamine exposure on the production of these factors was examined in primary human monocyte-derived macrophages isolated from healthy human donors and from virally suppressed, chronically infected HIV patients on cART. The expression of the five subtypes of dopamine receptors on human macrophages varies widely between healthy donors, with DRD5 the most highly expressed in this cell population. Dopamine increased the production of the majority of the inflammatory cytokines and chemokines, and decreased the production of the anti-inflammatory cytokine IL-10 in response to LPS. The dopamine-induced decrease in IL-10 correlated with greater expression of the D1-like dopamine receptors DRD1 and DRD5, while dopamine-induction of IL-6 was negatively correlated with D2-like dopamine receptor expression. Examination of macrophages from healthy versus cART-suppressed donors demonstrated that dopamine modulates cytokine production in a similar manner in healthy and virally-suppressed HIV-infected individuals. Overall, these data provide a greater understanding of the possible impact of dopamine on the development of NeuroHIV, as well as other neuroinflammatory diseases. Furthermore, these findings suggest that targeting dopamine receptors may be a useful therapeutic avenue during HIV infection.
Methods
Reagents
RPMI-1640 medium and penicillin/streptomycin (P/S) were from Invitrogen (Carlsbad, CA, USA). LPS from E. Coli 055:B5, hydroxyethyl piperazineethanesulfonic acid (HEPES), β-mercaptoethanol, Tween 20 and dopamine hydrochloride (DA) were obtained from Sigma-Aldritch (St. Louis, MO, USA). Dopamine was resuspended at 10 mM in dH2O before use. Fetal calf serum (FCS) and human AB serum were from Lonza (Basel, Switzerland). Macrophage colony stimulating factor (M-CSF) was from Peprotech (Rocky Hill, NJ, USA). TaqMan Master Mix, and PCR assay probes for DRD genes and 18s_were purchased from Applied Biosystems (Foster City, CA, USA). Taqman primers were validated for DRD targets in primary human cells using 5 point standard curves to assure optimal amplification efficiency.
Generation of primary human macrophages
Human peripheral blood mononuclear cells (PBMC) were separated from blood obtained from de-identified healthy donors (New York Blood Center, Long Island City, New York; and BioreclamationIVT Collection Center, Miami, Florida) by Ficoll-Paque (GE Healthcare, Piscataway, NJ, USA) gradient centrifugation. After isolation, the percentage of monocytes was calculated by positive selection with CD14+ microbeads (MACS, Miltenyi Biotechnology) and PBMC’s were plated at a density of approximately 1×105 monocytes/cm2 to obtain monocyte-derived macrophages (MDM) via plastic adherence. Monocytes were cultured in RPMI-1640 with 10% FCS, 5% human AB serum, 10 mM HEPES, 1% P/S, and M-CSF (10 ng/mL) for 3 days, washed twice with fresh media to remove non-adherent cells, and cultured for another 3 days in fresh media containing M-CSF. After 6 days in culture cells were washed again and considered to be mature MDM. De-identified demographic information was obtained from the New York Blood Center and BioIVT for every donor to ensure that we were examining a diverse population. Table 1 describes demographic characteristics of individuals from the two cohorts, including age, gender, race, and CMV status. Alcohol and drug use status were not available.
Table 1.
Demographic characteristic of donors (n=47)
| Variable | Statistic |
|---|---|
| Age (years)a | 34.9 (13.8)[17-67] |
| Ethnicity | |
| Caucasian | 31% |
| African-American | 16.7% |
| Latino | 11.9% |
| Not disclosed | 38.1% |
| Gender (% men) | 63.4% |
| CMV status (% +) | 51.4 % |
Mean (standard deviation) [range]
HIV study subjects
12 cART-treated donors were obtained from patients at McGill University Health Centre who participated in a blood banking study of Chronic Viral Illness via Dr. Elias Haddad (Drexel University Division of Infectious Diseases and HIV Medicine). Frozen PBMC Cells from healthy donors were obtained from the Martin Memorial Health Systems in Florida (n = 8) and from the University of Pennsylvania Human Immunology Core (UPenn HIC, n = 4). PBMCs from all cohorts were obtained by leukapheresis between 2014 and 2015 and then frozen to be used for our purposes. All procedures were approved by the Institutional Review Boards at the relevant institutions, and all participants provided signed informed consent. PBMCs were isolated from blood by density-gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare). Cells were subsequently frozen until use for the relevant assays.
Frozen PBMC were thawed rapidly, spun down in 1:1 FBS and RPMI, and treated with Benzonase® Endonuclease (MilliporeSigma, US) before culturing as described. De-identified demographic information was obtained for the cART donors from McGill University, and for healthy donors from UPenn HIC. Table 3 describes demographic and medical characteristics of cART-treated individuals including age, gender, CMV status, years-infected with HIV, and years before cART initiation. Alcohol and drug use status were not available. All cART donors were also HEP C negative, and plasma viral load was <40 copies/ml indicating successful HIV suppression. In addition, to ensure that the cART-treated donors were successfully virally suppressed following MDM isolation and differentiation, we performed a p24 alphaLISA assay on supernatants from MDM cultures derived from these donors. None of the 12 cART suppressed donors showed any viral replication in MDM cultures (data not shown). Demographic information was not available for the healthy donors from VGTI, but all healthy donors were <45 years of age and were negative for HIV and HEP C.
Table 3:
Demographic and medical characteristics of cART-treated donors (cryopreserved McGill cohort, n=12)
| Variable | Statistic |
|---|---|
| Age (years)a | 47 (8.68)[32-59] |
| Gender (% men) | 92.3% |
| Years infecteda | 17.25 (6.31)[8-30] |
| Years without cART | 1.67 (2.81) [0-10] |
Mean (standard deviation) [range]
Quantitative RT-PCR
Once mature, total RNA was extracted from MDM using the RNeasy Mini Plus™ kit (Qiagen) or with TRIzol ™ reagent and chloroform extraction. Purity and concentration of RNA were determined using a NanodropOne spectrophotometer (Nanodrop technologies, Wilmington, DE, USA), and no differences in RNA quality or yield, or PCR amplification were seen for either RNA isolation technique. Synthesis of cDNA was performed on 500 ng/μl of RNA from each donor using the High Capacity cDNA Reverse Transcriptase synthesis kit (Applied Biosystems, Foster City, CA). Specific dopaminergic and housekeeping genes were amplified from cDNA by quantitative PCR (qPCR) from 1 pL of cDNA reaction using gene-specific TaqMan™ primers. Gene expression levels were finally expressed as 2−ΔCt where Δ CT = [CT (sample)- CT (housekeeping gene)]. Human Brain Total RNA was used as a positive control (Life Technologies, Carlsbad, CA) and samples containing no cDNA served as the negative controls. Samples with a CT value above 37 were considered to have no amplification. The expression level of each dopamine receptor for each donor is plotted as the 2−ΔCT in order to demonstrate the relative pattern of expression. In samples in which no amplification occurred ΔCT relative to 18s was set to 32 in order to include those donors in our statistical analysis.
Cytokine Secretion Assay
Primary human monocyte derived macrophages cultured at 1 × 105 cells per cm2 in 48-well plates (BD Falcon) were incubated for 24 hours with different concentrations of dopamine (10−9, 10−8, 10−7, and 10−6 M) in the presence or absence of LPS (1 ng/mL). Treatment with LPS was used as a positive control, and MDM that did not respond to LPS with at least a 1.5-fold increase in the cytokine of interest were excluded from analysis for that cytokine. For cytokines not significantly increased by LPS, the effect of LPS on IL-6 was used as a control for normal macrophage function After 24 hours, supernatants and MDM lysates were collected, aliquoted and stored at −80°C. Supernatants were analyzed for IL-6, IL-10, TNF-α, CCL2, CXCL8, CXCL9, and CXCL10 using alphaLISAs performed according to the manufacturer's protocol (PerkinElmer). For IL-1β and IL-18 cell lysates were analyzed, as these cytokines were not released into the supernatant. The limits of detection for alphaLISAs were IL-6, 1.3 pg/mL; IL-10, 39 pg/ml; IL-1β, 0.6 pg/ml; IL-18, 5 pg/ml; TNF-α, 2.2 pg/mL; CCL2, 3.8 pg/ml; CXCL8, 1.1 pg/mL; CXCL9, 1.6 pg/mL; and CXCL10, 8.6 pg/ml.
Statistical analyses
Statistical analyses were performed using Prism 7.0 (Graphpad, La Jolla, CA, USA). Differences in the expression of dopamine receptor transcripts was determined with a Kruskal-Wallis ANOVA followed by Dunn’s multiple comparisons test. For freshly isolated MDM, the fold-change in the amount of each factor upon dopamine treatment was calculated by normalizing to the cytokine level in the control condition (either untreated MDM or MDM treated with 1 ng/mL LPS) for each individual donor. Extreme data points presumed to be technical outliers were identified via ROUT test (Q = 0.1%) and removed from analysis. Due to the stringency of the outlier test, only the most extreme points were removed from the analysis (2 donors for IL-6 and IL-10; 1 donor for IL-18, and only 1 data point for CXCL8). In order to maintain the ability to perform a matched ANOVA for CXCL8, the missing point was replaced with the mean-value calculated from all the remaining donors for that specific dopamine concentration.
Dopamine-induced changes in cytokine production were then analyzed by one-way analysis of variance (ANOVA); Kruskal-Wallis test for IL-1β, and Friedman test for IL-18, TNF-α, CCL2, CXCL8, CXCL9, and CXCL10 with Dunn’s multiple comparisons test for post-hoc analysis. A significant correlation between dopamine receptor expression (2−ΔCt) and cytokine response to DA (fold change in cytokine production at each DA concentration) was determined using a non-parametric Spearman correlation. For the purpose of correlation analysis, the ΔCT values for DRD1, DRD2, DRD3, and DRD4 were set to a threshold level of 32 in the individual donors that did not express these receptors. These points are indicated by the arrow in figure 1. For the cytokine assays done in the frozen cells, equivalence of dopamine-responsiveness was determined by a repeated measures two-way ANOVA. Parametricity was tested by Kolmogorov-Smirnov test, and cytokine concentration data were determined to be nonparametric in each treatment condition so values were transformed to their log(10) values prior to analysis. Dunnett’s multiple comparisons post-hoc test was then used for pairwise comparison of within-subjects effect of dopamine treatment.
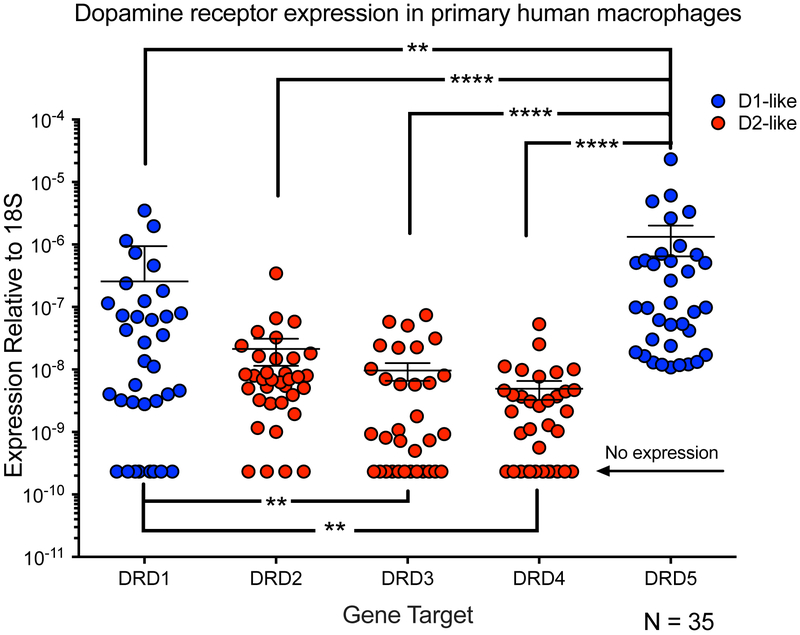
Figure 1:
RNA from human MDM was collected from 35 healthy donors to quantify expression of DRD1, DRD2, DRD3, DRD4, and DRD5. Expression level of each receptor is normalized to the level of the endogenous control 18s (ΔCT), and data is represented as the transformed value 2−ΔCT. Human MDM express significantly greater levels of DRD5 than any other receptor (Friedman test followed by Dunn’s multiple comparisons test, p<.0001****).
Results
Macrophages show a distinct pattern of dopamine receptor expression despite heterogeneity across the population
Our previous data showed that human macrophages express mRNA for all five dopamine receptor subtypes (Gaskill et al., 2009; Gaskill et al., 2012), but did not examine the specific receptors mediating the activity of dopamine in these cells. Therefore, the relative expression of all five dopamine receptor subtypes was quantified in primary human monocyte derived macrophages (MDM) isolated from 35 individual healthy donors (Fig. 1). To do this, mRNA from these cells was isolated and analyzed by qRT-PCR for DRD1, DRD2, DRD3, DRD4, DRD5 and the housekeeping gene 18s, using mRNA from total human brain as a positive control. These analyses showed that MDM express significantly greater levels of DRD5 than any other receptor subtype [Figure 1, n = 35, Kruskal-Wallis test, Kruskal-Wallis statistic 68.6, p < 0.0001, Dunn’s multiple comparisons test, DRD1, 2, 3, 4 vs. DRD5 (p < 0.0001 ****)]. In addition, MDM also express significantly greater DRD1 than DRD3 and DRD4 (Dunn’s multiple comparisons test, DRD3, DRD4 vs DRD1 (p<.01**)] with no significant differences between any of the other receptors. In general, individual donors expressed the same pattern of dopamine receptor subtypes as the overall population (DRD5>DRD1>DRD2>DRD3, DRD4) with minimal exceptions (Supplemental figure 1). While all 35 donors expressed mRNA for the D1-like receptor DRD5 [ΔCT relative to 18s of 22.7 +/− 3.026 (mean +/− SD)] only around 77% of donors expressed mRNA for DRD1 (ΔCT relative to 18s of 26.13 +/− 4.217). For the D2-like receptors, 89% of donors expressed mRNA for DRD2 (ΔCT relative to 18s of 27.34 +/− 2.365), 60% of donors expressed mRNA for DRD3 (ΔCT relative to 18s of 29.27 +/− 2.915) and 66% of donors expressed mRNA for DRD4 (ΔCT relative to 18s of 29.32 +/− 2.33). Every donor expressed mRNA for at least 2 dopamine receptor subtypes, and the expression of DRD1 and DRD5 was positively correlated, as was the expression of DRD2 with DRD3 and DRD4 (Supplemental figure 2). These data show that dopamine receptor expression varies widely between individuals, but transcripts for DRD5 show the most consistent and highest levels of expression among donors. This suggests that D5 may be prominent in mediating the effects of dopamine in primary human macrophages.
Dopamine modulates macrophage cytokine and chemokine secretion
To determine the impact of dopamine on cytokine and chemokine production, MDM isolated from healthy blood donors to the New York Blood center (n = 37) or BioIVT (Miami, FL n =6) were treated with dopamine (10−6, 10−7, 10−8, 10−9M) either alone or with LPS (1 ng/mL) for 24 hours prior to collection of supernatants and lysates for analysis by alphaLISA. The extracellular concentration of dopamine can vary widely due to normal metabolism and/or exogenous stimuli, so a given macrophage is may be exposed to more than a single, precise concentration of dopamine. To accommodate these variations, the concentrations of dopamine used in this study encompass the physiologic range of dopamine levels likely to be encountered in the CNS. LPS treatment was used to model inflammatory conditions and induce production of IL-10 and TNF-α, as macrophages in non-inflammatory conditions produce minimal amounts of IL-10 and TNF-α.
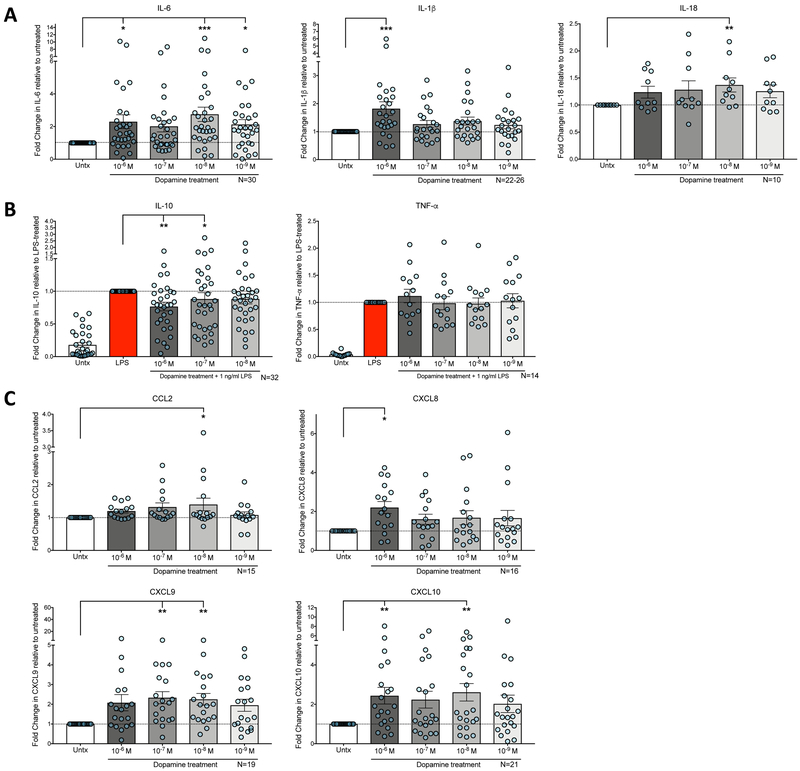
These studies demonstrate that in human MDM, dopamine increases the production of inflammatory cytokines IL-6, IL-1β and IL-18, with the dopamine response to IL-1β and IL-18 seen in cell lysate rather than supernatant [Fig. 2A; IL-6, Friedman test, n = 30, ** p = 0.0041, Friedman statistic 17.71, Dunns multiple comparisons test, 10−6M DA vs. Untx, * p = 0.0359, 10−8M DA vs. Untx, *** p = 0.0002, 10−9M DA vs. Untx, * p = 0.0171; IL-1β, Kruskal-Wallis test, n = 23-26, ** p = 0.0047, Dunn’s multiple comparisons test, 10−6M DA vs. Untx, *** p = 0.0005; IL-18, Friedman test, n = 10, p = 0.1468, Friedman statistic 6.8, Dunn’s multiple comparisons test, 10−8M DA vs. Untx, p = 0.0436]. Changes in IL-1β and IL-18 secretion in MDM supernatants were not examined because IL-1β and IL-18 were not detected in MDM media in any of the treated and untreated conditions (data not shown). Dopamine also inhibits the production of the anti-inflammatory cytokine IL-10 in response to LPS [Fig. 2B, Friedman test, n = 32, ** p = 0.0053, Friedman statistic 12.71, Dunn’s multiple comparisons test, 10−6M DA vs. LPS, ** p = 0.0042, 10−7M DA vs. LPS, * p = 0.011], and increases the production of the chemokines CCL2, CXCL8, CXCL9 and CXCL10 [Figure 2C; CCL2, Friedman test, n = 15, p = 0.0374, Freidman statistic, 10.19, Dunn’s multiple comparisons test, 10−8M DA vs. Untx, * p = 0.0156; CXCL8, Friedman test, n = 16, p = 0.0862, Friedman statistic, 8.15, Dunn’s multiple comparisons test, 10−6M DA vs. Untx,* p = 0.0208; CXCL9, Friedman test, n = 19, ** p = 0.0041, Friedman statistic, 15.28, Dunn’s multiple comparisons test, 10−7M DA vs. Untx, ** p = 0.0028, 10−8M DA vs. Untx, ** p = 0.0028; CXCL10, Friedman test, n = 21,*** p = 0.0211, Friedman statistic, 11.54, Dunns multiple comparisons test, 10−6M DA vs. Untx, * p = 0.0337, 10−8M DA vs. Untx,** p = 0.0072]. Surprisingly, dopamine had no effect on LPS-induced TNF-α production [Figure 2B, Friedman test, n=14, (p > 0.9999), Friedman statistic, 3.686]. The untreated and LPS-stimulated concentration of each factor as well as the fold change induced by each significant concentration of dopamine is reported in Table 2. There are no demographic associations (age, gender, race, or CMV status) with dopamine-mediated changes in cytokine or chemokine production, however baseline IL-10 concentration in untreated cell supernatants was greater in male donors (n= 19, 1,635 pg/mL) than in female donors (n=10, 641 pg/ml) (Supplemental table 1, Mann-Whitney test, Mann-Whitney U statistic, 28, **p.0014). Together, these results indicate that dopamine promotes an inflammatory macrophage phenotype, and that changes in extracellular dopamine concentrations may initiate or exacerbate inflammatory conditions.
Figure 2:
MDM from healthy donors were treated with different concentrations of DA (10−9M, 10−8M −10−7, 10−6M) for 24-hrs, then supernatants were analyzed for IL-6, IL-10, CCL2, CXCL8, CXCL9, and CXCL10 (n=9-28). Lysates were analyzed for IL-1β and IL-18 (n=10-26). Cytokine levels in the DA-treated groups were normalized to the untreated or LPS condition to determine fold change. (A) Compared to the untreated condition, we found a significant increase in the cytokines IL-6, IL-1β, and IL-18 following dopamine exposure. IL-6 was significant at 10−6M, 10−8M, and 10−9M. IL-1β was significant at 10−6M, and IL-18 was significant at 10−8M. (B) Compared to the LPS-alone condition, 10−6M and 10−7M DA inhibits LPS-induced production of the anti-inflammatory cytokine IL-10. Dopamine had no impact on LPS-induced TNF-α production. (C) Compared to the untreated condition, we found a significant increase in the chemokines CCL2, CXCL8, CXCL9, and CXCL10 following dopamine exposure. CCL2 was significant at 10−8M, CXCL8 was significant at 10−6M, CXCL9 was significant at 10−7M and 10−8M, and CXCL10 was significant at 10−6M and 10−8M. Significance was determined using a Kruskall-Wallis test (IL-1β) or a Friedman test (all other factors) followed by Dunn’s multiple comparisons. * p < 0.05, ** p < 0.01, *** p < 0.001.
Table 2.
Cytokine levels in UT and LPS-stimulated macrophages and effect of dopamine on cytokine production
| Cytokine | Effect | Concentration in UT cellsa |
Concentration in LPS- stimulated cellsa |
Dopamine concentration of significant effectb |
p-value |
|---|---|---|---|---|---|
| IL-6 | Inflammatory | 26,872 (+/− 6,625) | 300,390 (+/− 67,051) | 10−6: ↑2.3x (+/− .42) 10−8M: ↑2.8x (+/− .44) 10−9M: ↑2.1x (+/− .30) |
* *** * |
| IL-1β | Inflammatory | 3,057 (+/− 1,418)c | 102,924 (+/−73,646)c | 10−8M: ↑1.8x (+/− .24) | ** |
| IL-18 | Inflammatory | 11,019 (+/− 2,750)c | 19,594 (+/− 3,847)c | 10−8M: ↑1.4x (+/− .13) | * |
| TNF-α | Inflammatory | 408 (+/− 129) | 12,853 (+/− 3,937) | No change | P=.9999 |
| IL-10 | Anti-inflammatory | 914 (+/− 237) | 11,733 (+/− 2,472) | 10−6M: ↓ .766x (+/− .07) 10−7M: ↓ .882x (+/− .10) |
** * |
| CCL2 | Monocyte chemoattractant | 128,563 (+/− 23,695) | 146,365 (+/− 24,859) | 10−8M: ↑ 1.4x (+/− .19) | * |
| CXCL8 | Neutrophil chemoattractant | 312,828 (+/− 84,409) | 1,484,658 (+/− 285,143 | 10−6: ↑ 2.2x (+/− .31) | * |
| CXCL9 | T-cell chemoattractant | 16,547 (+/− 5,413) | 42,011 (+/− 12,421) | 10−7M: ↑ 2.3x (+/− .31) 10−8M: ↑ 2.3x (+/− .30) |
** ** |
| CXCL10 | T-cell chemoattractant | 28,337 (+/− 8,513) | 166,873 (+/− 36,916) | 10−6M: ↑2.4x (+/− .42) 10−7M: ↑2.2x (+/− .43) 10−8M: ↑2.6x (+/− .44) 10−9M: ↑2.0x (+/− .44) |
** |
Mean concentration in pg/mL per 106 cell supernatant (+/−SEM)
Concentration of dopamine: mean fold change from control (+/−SEM)
Mean concentration in pg/ml/ug of protein per 106 cell lysate (+/−SEM)
Dopaminergic changes in cytokine production correlate with dopamine receptor expression
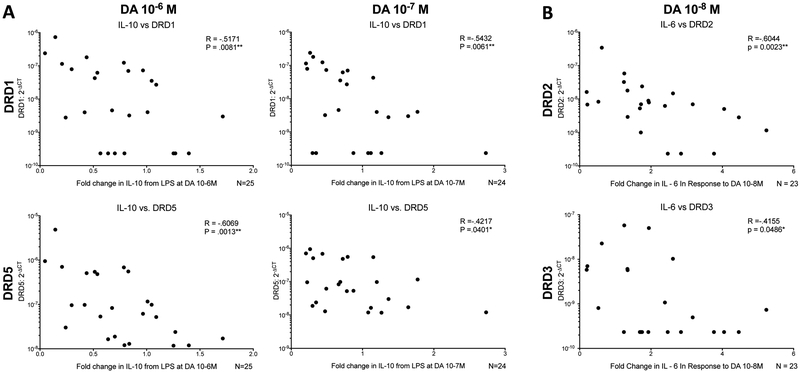
To determine whether the dopamine-mediated changes in cytokine production were associated with expression of specific dopamine receptors, donor-dependent mRNA expression for each dopamine receptor with was correlated with the dopamine-induced fold change in each cytokine. These analyses show that decreases in LPS-induced IL-10 production by dopamine are significantly negatively associated with expression of the D1-like receptors DRD1 and DRD5 [Fig. 3A, DRD1 vs IL-10 fold change at 10−6 M, n = 25, Spearman r, −0.5171, **p = .0081; DRD5 vs IL-10 fold change at 10−6 M, n = 25, Spearman r, −.6069,**p = .0013; DRD1 vs IL-10 fold change at 10−7 M, n = 24, Spearman r, −0.5432, **p = 0.0061; DRD5 vs IL-10 fold change at 10−7 M, n = 24, Spearman r, −0.4217, *p = 0.0401]. The negative correlation indicates that macrophages with higher levels of DRD1 and DRD5 show greater dopamine-mediated reductions in IL-10. Production of IL-6 is negatively associated with the expression of the D2-like dopamine receptors DRD2 and DRD3 at dopamine concentrations of 10−8M, which is the concentration which induced the greatest increase in IL-6 production relative to untreated MDM [Fig. 3B, DRD2 vs IL-6 fold change at 10−8 M, n = 23, Spearman r, −0.6044, **ρ = 0.0023; DRD3 vs IL-6 fold change at 10−8 M, n = 23, Spearman r, −0.4155, *p = 0.0486].
Figure 3:
The expression of each DR subtype was calculated for each donor and plotted against the fold change in cytokine production at 10−6M (IL-10), 10−7M (IL-10), and 10−8M (IL-6) DA. (A) Decreased LPS-induced production of IL-10 by 10−6M and 10−7M dopamine is associated with greater expression of the D1-like receptors DRD1 and DRD5. (B) Decreased production IL-6 at 10−8M is associated with greater expression of the D2-like receptors DRD2 and DRD3. Significance was determined using a Spearman correlation. *p < 0.05, **p<.01, ***p<.001, ****p< 0001.
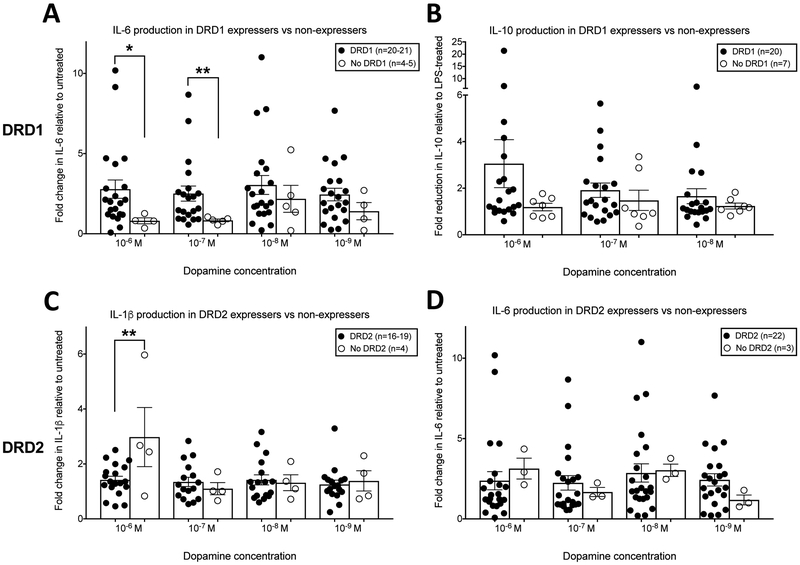
These results suggest that the effect of dopamine on the production of different cytokines may be dependent on both the expression of specific dopamine receptor subtypes and on dopamine concentration. To further examine this hypothesis, cytokine production was examined in MDM lacking DRD1 or DRD2 mRNA. Donors without DRD1 mRNA show a decrease in IL-6 production (n = 4, 10−6 M, 0.81 of untreated condition; 10−7 M, 0.84 of untreated) while donors that do express DRD1 show an increase in IL-6 production (n = 20-21, 10−6 M, 2.8-fold increase from untreated; 10−7 M, 2.5-fold increase from untreated) following exposure to 10−6 and 10−7 M dopamine [Figure 4A, Mann Whitney test, 10−6 M dopamine, Mann-Whitney U statistic, 12, *p = 0.0245; 10−7 M dopamine, Mann-Whitney U statistic, 11, **p = .0056]. Similarly, 10−6 M dopamine inhibited LPS-induced IL-10 production less effectively in donors without DRD1 (n = 7, 1.2 fold reduction from LPS-treated) compared to those expressing DRD1 (n = 20, 3-fold reduction from LPS-treated), an effect that is trending towards significance [Figure 4B, Mann Whitney test, 10−6 M dopamine, Mann-Whitney U statistic, 44, p = 0.1618]. The absence of DRD2 also impacts the dopamine-induced production of IL-1β, and may impact dopamine-induced production of IL-6. In donors without DRD2, dopamine significantly increased IL-1β production (n = 4, 3-fold increase from untreated) more robustly than in donors expressing DRD2 (n= 19, 1.4-fold increase from untreated) [Figure 4C, Unpaired t-test, 10−6 M dopamine, t=2.908, df=21, **p = 0.0084]. In donors without DRD2, dopamine increased IL-6 production (n = 3, 3-fold increase from untreated) more robustly than in donors expressing DRD2 (n= 22, 2.4-fold increase from untreated) [Figure 4D, Mann Whitney test, 10−6 M dopamine, Mann-Whitney U statistic, 14, p = 0.1278]. Altogether, these results suggest that DRD1 expression may be partially responsible for the pro-inflammatory effects of dopamine, and that DRD2 expression may be important for opposing the inflammatory effects of dopamine.
Figure 4:
Donors were separated into two groups based on whether or not they express DRD1 or DRD2. We then compared the fold change in IL-6, IL-10, and IL-1β induced by each concentration of dopamine between the two groups. (A) Donor MDM that do not express DRD1 have significantly lower fold-increase in IL-6 production after exposure to 10−6 and 10−7 M dopamine compared to donors that do express DRD1. (B) Donor MDM that do not express DRD1 (n=7) have less inhibition of IL-10 production after exposure to 10−6 M dopamine, an effect that is trending towards significance at p=.137. (C) Donor MDM that do not express DRD2 have a significantly greater fold-increase in IL-1β production after exposure to 10−6 M dopamine compared to donors that do express DRD2. (D) Donor MDM that do not express DRD2 have greater fold-change in IL-6 after exposure to 10−6 M dopamine, an effect that is trending towards significance at p=.128. Significance was determined via unpaired t-test for IL-1β and Mann-Whitney test for IL-6 and IL-10 (p<.05*, p<.01**).
Macrophage dopamine receptor expression pattern is not altered in MDM from chronically infected, cART-treated individuals
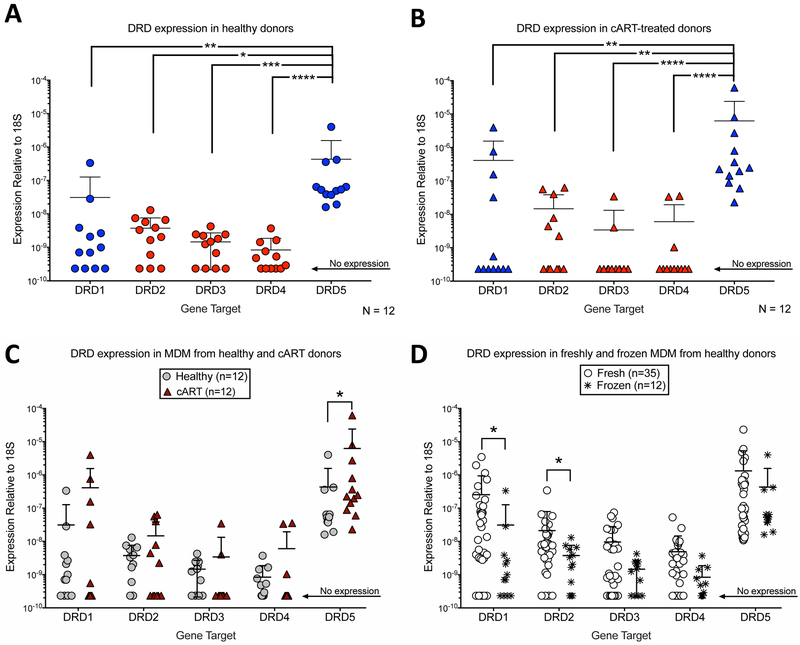
In the current era, the majority of HIV-infected individuals are engaged in cART, therefore dopamine receptor expression was examined in human macrophages isolated from HIV-infected individuals undergoing successful cART treatment (Fig. 5). Frozen PBMC from HIV-infected, cART-treated patients (n = 12) were thawed and differentiated into MDM prior to collection of RNA. This RNA was compared with that collected from frozen PBMC from healthy, uninfected donors (n = 12), which were used as a control. All donors from both the HIV-infected and the healthy cohorts expressed mRNA for DRD5 [mean ΔCT relative to 18s of 23.48 +/− 2.245 in the 12 healthy donors, and 21.07 +/− 3.214 in the 12 cART-treated donors]. For DRD1, 67% of healthy donors and 42% of cART donors expressed mRNA for DRD1 [mean ΔCT of 29.21 +/− 3.207 (healthy) and 28.37 +/− 5.367 (cART)]. For D2-like receptors, 75% of healthy donors and 50% of cART donors expressed mRNA for DRD2 [mean ΔCT of 29.04 +/− 2.119 (healthy) and 29 +/− 3.434 (cART)], 67% of healthy donors and 17% of cART donors expressed mRNA for DRD3 [mean ΔCT of 30.01 +/− 1.596 (healthy) and 31.06 +/− 2.301 (cART)] and 58% of healthy donors and 25% of cART donors expressed mRNA for DRD4 [mean ΔCT of 30.86 +/− 1.372 (healthy) and 30.62 +/− 2.794 (cART)].
Figure 5:
RNA from human MDM isolated from previously frozen PBMC was collected from 12 healthy, and 12 cART-treated donors to quantify expression of DRD1, DRD2, DRD3, DRD4, and DRD5. Expression level of each receptor is normalized to the level of the endogenous control 18s (ΔCT), and data is represented as the transformed value 2−ΔCT. (A, B) Healthy and cART donor MDMs isolated from previously frozen PBMC express significantly greater levels of DRD5 than any other receptor. Significance was determined via Kruskal-Wallis test followed by Dunn’s multiple comparisons test, (*p<0.05, **p<0.01, p<.001 ***, p<0001****). (C) In MDM isolated from previously frozen PBMC, cART donors express significantly greater DRD5 than healthy donors. Significance was determined via individual unpaired Mann-Whitney tests between healthy and cART groups for each dopamine-receptor subtype. There was no significant difference in expression for any other dopamine-receptor subtype (*p<0.05). (D) Across all healthy donors, MDM isolated from previously frozen PBMC express less DRD1 and DRD2 than freshly isolated MDM. Significance was determined via individual unpaired Mann-Whitney tests between fresh and frozen groups for each dopamine-receptor subtype. There was no significant difference in expression for other dopamine-receptor subtype (*p<0.05).
Similar to the freshly isolated macrophages, healthy MDM derived from previously frozen PBMC express significantly greater amounts of DRD5 than any other receptor subtype [Figure 5A, n = 12, Friedman test, Friedman test statistic, 32.53, ****p<.0001, Dunn’s multiple comparisons test, DRD5 vs. DRD1, **p = 0.0023, DRD5 vs. DRD2, *p = 0.0268, DRD5 vs. DRD3, ***p = 0.0006, DRD5 vs. DRD4, ****p <0.0001]. MDM from cART donors demonstrate the same DRD expression pattern [Figure 5B, n = 12, Friedman test, Friedman test statistic, 33.35, ****p<.0001, Dunn’s multiple comparisons test, DRD5 vs. DRD1, **p = 0.0038, DRD5 vs. DRD2, *p = 0.0125, DRD5 vs. DRD3, ***p = 0.0001, DRD5 vs. DRD4, ***p = 0.0002]. These data indicate that the pattern of MDM dopamine-receptor expression is not impacted by chronic, treated HIV-infection. However, comparison of dopamine-receptor expression between the frozen healthy (n=12) and frozen cART (n=12) donors shows that cART donors express significantly more DRD5 mRNA (Figure 5C, Mann-Whitney test, Mann-Whitney U statistic, 35, * p = 0.0284). Of note, while the relative expression pattern of dopamine receptor subtypes remains stable in healthy MDM generated from frozen PBMC (n=12), expression of individual dopamine-receptor subtypes differs compared to freshly isolated MDM (n=35) (Figure 5D, Mann-Whitney test, DRD1, Mann-Whitney U statistic, 117, * p = 0.0211; DRD2, Mann-Whitney U statistic, 118, * p = 0.0239]. This suggests that the freeze-thaw process may affect dopamine-receptor expression in mature MDM. Furthermore, because a lower percentage of cART donor MDM express mRNA for DRD1, DRD2, DRD3, and DRD4, MDM differentiated from cART donors may be more susceptible to changes in DRD expression introduced by the freeze-thaw process.
Dopaminergic modulation of cytokine production is not altered in MDM from chronically infected, cART-treated individuals
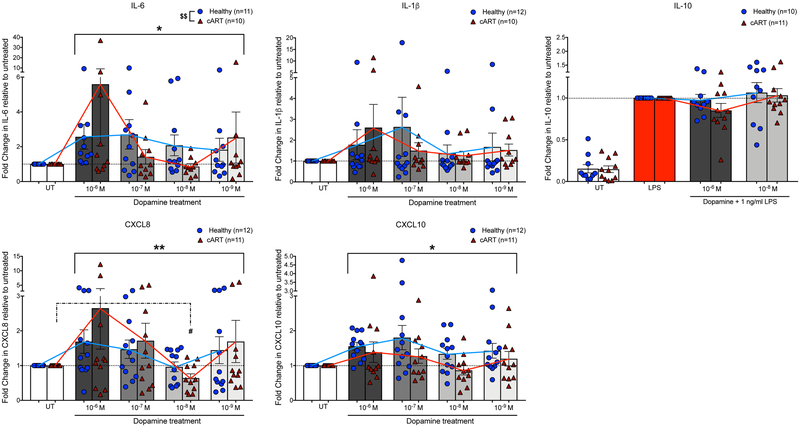
To evaluate the impact of chronic, treated HIV-infection on dopamine-mediated changes in cytokine production, cytokine assays were performed in the previously frozen PBMC isolated from healthy and cART-treated donors. As with the fresh MDM, macrophages from frozen donors were treated with dopamine either alone or with LPS for 24 hours prior to collection of supernatants and lysates for analysis by alphaLISA. Analysis showed dopamine treatment has a similar effect on IL-6, IL-1β, IL-10, CXCL8, and CXCL10 production in MDM from healthy and cART-treated donors, as there is no interaction between dopamine-treatment and donor-group on any cytokine measured [Figure 6, 2way ANOVA, IL-6 interaction, F(4,76)= 1.752, p=.1474; IL-1β interaction, F(4,80)= 0.3049, p=0.8739; IL-10 interaction, F(2,38)= 0.8859, p=0.4207; CXCL8 interaction, F(4,84)= 0.7484, p=0.5618; CXCL10 interaction, F(4,84)= 0.9847, p=0.4204]. There is also a significant main effect of dopamine treatment on IL-6, CXCL8, and CXCL10 [Figure 6, 2way ANOVA, IL-6 dopamine effect, F(4,76)= 3.273, *p=.0157; CXCL8 dopamine effect, F(4,84)= 3.771, **p=.0072; CXCL10 dopamine effect, F(4,84)= 2.912, *p=0.0261], although IL-1β or IL-10 production were not increased [Figure 6, 2way ANOVA, IL-1β dopamine effect, F(4,80)= 1.197, p=0.3187; IL-10 dopamine effect, F(2,38)= 1.645, p=0.2065]. Additionally, with post-hoc analysis of within-group effects, we found a significant decrease in CXCL8 production at 10−8 M dopamine in cART-treated donors (Figure 6, Dunnett’s multiple comparisons test, n=11, *p=0.0473), an effect that we did not previously observe in freshly isolated cells from healthy donors. The effect of dopamine on IL-6 production is trending towards significance in healthy and cART-treated donors at 10−6 M (Dunnett’s multiple comparisons test, Healthy, n=11, p= 0.1066; cART, n=10, p=.0935) and in healthy donors at 10−7 M (Dunnett’s multiple comparisons test, Healthy, n=11, p= .0512). There was a similar trending effect for CXCL0 in healthy donors at 10−6 M and 10−7 M dopamine (Dunnett’s multiple comparisons test, n=12, 10−6 M, p= 0.0609; 10−7 M, p=0.0536). The effect of dopamine on inhibition of IL-10 production was also trending in cART donors at 10−6 M (Dunnett’s multiple comparisons test, n=10, p= 0.1044).
Figure 6:
MDM isolated from healthy and cART-treated donors were treated with different concentrations of DA (10−9M, 10−8M −10−7, 10−6M) for 24-hrs, then supernatants were analyzed for IL-6, IL-10, CXCL8, and CXCL10. Lysates were analyzed for IL-1β. Cytokine levels in the DA-treated groups were normalized to the untreated or LPS condition to show fold change. Raw data was not normally distributed, so statistics were performed on cytokine concentrations after log(10) transformation. Dopamine had a similar effect on MDM cytokine production in healthy and cART-treated donors as there was no interaction between dopamine-treatment and donor-group on cytokine production for IL-6, IL-1β, IL-10, CXCL8, or CXCL10. There was a simple main effect of dopamine treatment for IL-6, CXCL8, and CXCL10 (p<.05*, p<.01**). Within-group pairwise comparison found a significant decrease in CXCL8 production following 10−8M dopamine exposure in cART donors (p<.05#). There was a simple main effect of donor group for IL-6 (p<.05$$). Significance was determined with a repeated measures 2-way ANOVA, and post-hoc analysis with Dunnett’s multiple comparisons test.
For most of the cytokines examined, the effect of dopamine is consistent among MDM derived from healthy, fresh PBMC, healthy, frozen PBMC and cART-treated frozen PBMC (Tables 2 and 5). However, compared to the freshly isolated MDM, the dopamine-induced changes in cytokine production in frozen-derived MDM is much more variable, regardless of donor group (Table 5). This suggests that the process of freezing and thawing PBMC may impact function of mature MDM and introduce greater variability. It is likely that this increased variability within a smaller cohort of donors that resulted in some of the differences in results observed between the studies conducted in freshly isolated and frozen-derived MDM such as the lack of significance in IL-1β production in both groups [Figure 6, Dunnett’s multiple comparisons test, 10−6 M dopamine, healthy, n=12, p.8646; cART, n=10, p = 0.1836]
Table 5.
Cytokine levels and effect of dopamine on cytokine production in MDM isolated from healthy and cART-treated donors
| Cytokine | Effect | Concentration in UT cellsa | Concentration in LPS- stimulated cellsa |
Fold change in response to dopamineb | Simple main effect of dopamine treatment?c |
Interaction between donor group and treatment?c |
Simple main effect of donor group?b |
||
|---|---|---|---|---|---|---|---|---|---|
| IL-6 Health cART |
Inflammatory | 34,260 (+/− 11,560) 20,095 (+/− 10,579) |
689,549 (+/− 306,212) 422,130 (+/− 280,243) |
10−6 2.6x +/− .71 (.1066) 5.59x +/− 3.53 (.094) |
10−7 2.7x +/− .85 (.0512) 1.4x +/− .41 (>.999) |
10−8 2.1x +/− .60 (.424) . .87x +/− .16 (.735) |
Yes, F(4,76)=3.27, p=.0157* |
No, F(4,76)=1.75, p=.1474 |
Yes, F(1,19)=8.6, p=.0085** |
| IL-1β Health cART |
Inflammatory | 1,396 (+/− 198)d 741.8 (+/− 161)d |
34,030(+/− 11.153)d 17,352 (+/− 3,848)d |
1.8x +/− .72 (.865) 2.6x +/− 1.1 (.184) |
2.6x +/− 1.4 (.794) 1.5x +/− .38 (.774) |
1.4x +/− .41 (.998) 1.3x +/− .195 (.913) |
No, F(4,80)=1.2, p=.3187 |
No, F(4,80)=.30, p=.8739 |
Trending, F(1,20)=3.87, p=.0631 |
| IL-10 Health cART |
Anti-inflammatory | 1,256 (+/− 322) 1,107(+/− 251) |
13,620 (+/− 3,235) 26,789 (+/− 12,131) |
.98 +/− .063 (.929) .85x +/− .084 (.104) |
1.1x +/− .12 (.998) 1x +/− .08 (.999) |
No, F(2,38)=1.64, p=.2065 |
No, F(2,38)=.89, p=.4207 |
No, F(1,19)=.04, p=.8347 |
|
| CXCL-8 Health cART |
Neutrophil chemoattractant |
907,692 (+/− 174,774) 1,086,978(+/− 381,335) |
2,932,511 (+/− 599,710) 5,427,559 (+/− 1,895,066) |
1.7x +/− .34 (.610) 2.7 +/–1.2(.908) |
1.5x +/− .27 (.851) 1.7x +/−.5 (.992) |
.96x +/− .14 (.886) .64x +/−.12 (.047)* |
Yes, F(4,84)=3.77, p=.0072** |
No, F(4,84)=.75, p=.5618 |
No, F(1,21)=1.28, p=.2708 |
| CXCL-10 Health cART |
Monocyte chemoattractant |
92,338 (+/− 43,565) 81,249 (+/− 47,756) |
537,267 (+/− 295,600) 216,439 (+/− 131,774) |
1.6x +/– 2.4 (.061) 1.4x +/− .29 (.807) |
1.8x +/− .35 (.054) 1.3x +/− .21 (.893) |
1.3x +/− .16 (.577) .86x +/− .13 (.334) |
Yes, F(4,84)=2.91, p=.0262* |
No, F(4,84)=.98, p=.4204 |
No, F(1,21)=.50, p=.4874 |
Mean concentration in pg/mL per 106 cell supernatant (+/−SEM)
Fold change relative to untreated +/−SEM (p-value, Dunnett’s multiple-comparison test)
F(DFn,DFd), p-value
Mean concentration in pg/mL/μg of protein per 106 cell lysate (+/−SEM)
The statistical analysis also demonstrated a significant main effect of patient group on IL-6 production across all treatment conditions, and a similar trend showing an effect of patient group on IL-1β production [Figure 6, 2way ANOVA, IL-6 donor group effect F(1,19)= 8.605, **p= 0.0085; IL-1β donor group effect F(1,20)= 3.871, p=0.0631]. This is indicative of a slightly lower baseline production of inflammatory mediators in MDM from chronically HIV-infected, cART-treated individuals (Supplemental Figure 3), which agrees with previous studies (Molina et al., 1990; Gordon et al., 2007). Unfortunately, demographic data was not available for healthy donors in the cryopreserved cohort, so we were unable to examine these associations. The cryopreserved cohorts were also too small to reasonably observe correlations between cytokine production and dopamine-receptor expression. All cART donors except 1 were male, and the majority of donors initiated cART within the same year as diagnosis, so we did not examine the effects of gender, or the number of years an infected individual was untreated. However, there was no association between dopamine-induced changes in any cytokine and the donor age or number of years infected with HIV (Spearman correlation, data not shown).
Discussion
Despite the widespread implementation of cART, HIV infection still results in a variety of neuropathologic and neurocognitive sequelae, collectively known as NeuroHIV, for which there a few effective treatments. A major challenge to the development of such adjuvant therapies is that the precise neuropathogenic mechanisms that lead to the development of NeuroHIV have not been identified in chronically infected, virally suppressed individuals. In the cART era, neurocognitive impairment is likely mediated by interactions between host and viral factors that perpetuate immune dysfunction, contributing to chronic inflammation in the CNS. One of these factors is illicit drug use, a history of which is reported by 50 – 80% of HIV-infected individuals (Mathers et al., 2008; Daskalopoulou et al., 2014; Garin et al., 2015) and may contribute to immune dysfunction and inflammation via interactions with the dopaminergic system. These data support this hypothesis, demonstrating that exposure of macrophages to a wide-range of dopamine concentrations promotes an inflammatory environment by modulating the production of numerous cytokines and chemokines. For certain cytokines, the dopamine-induced chances are associated with the expression level of specific macrophage dopamine receptor subtypes, indicating that specific dopamine-receptor subtypes may have differing mechanistic roles in the regulation of the inflammatory environment, and also that an individual’s expression pattern of macrophage dopamine receptors may relate susceptibility to dopamine-induced inflammation. This study also shows that dopamine modulates cytokine production in macrophages derived from chronically infected, virally suppressed individuals, indicating that dopaminergic modulation of myeloid cells could be a source of inflammation in successfully cART-treated individuals. These data indicate that disruption of the dopaminergic system could be an important factor in the development of neuroinflammation and suggests that therapies targeting the myeloid dopamine system may be a viable option for the amelioration of HIV-associated neuroinflammation. Furthermore, the absence of reliable biomarkers for the development of NeuroHIV (Armah et al., 2012; Chan and Brew, 2014; Saylor et al., 2016) suggests that screening dopamine receptor expression to identify of individuals who are more susceptible to myeloid-cell induced inflammation may help clinicians predict poor neurological outcomes and ultimately provide insights into the pathogenic mechanisms of NeuroHIV.
Dopaminergic involvement in NeuroHIV has been studied since the start of the epidemic, when research found that individuals with AIDS were more likely to develop extrapyramidal symptoms in conjunction with the use of neuroleptics and other dopaminergic therapeutics (Hollander et al., 1985; Hriso et al., 1991; Kieburtz et al., 1991). Early studies found HIV-infected individuals showed significant changes in dopamine metabolism (Larsson et al., 1991; Berger et al., 1994; Koutsilieri et al., 1997; di Rocco et al., 2000) and prominent neuropathology, such as volume loss, neuronal atrophy and greater numbers of infected myeloid cells, in dopamine-rich brain regions including substantia nigra (SbN), basal ganglia (BG), and prefrontal cortex (PFC). (Navia et al., 1986; Kure et al., 1990; Reyes et al., 1991; Aylward et al., 1993; Hestad et al., 1993; Aylward et al., 1995; Glass et al., 1995; Itoh et al., 2000). These individuals also displayed relatively higher levels of infection, inflammation and neurological damage in dopaminergic regions (Wiley and Nelson, 1990; Fujimura et al., 1997). Similar, although more subtle, regional neuropathology and inflammation is seen today in cART-treated patients [26, 47-50], and many of the HIV-associated impairments in these patients, such as deficits in attention or motivation reflect specific dysfunction of dopaminergic systems [51-60]. These associations suggest that interactions between the dopaminergic and immune systems during HIV-infection are a key component of the etiology of NeuroHIV.
Dopamine has been shown to regulate the functions of human T-cells, neutrophils and cells of the myeloid lineage such as macrophages, monocytes and microglia (Ricci et al., 1997; Basu and Dasgupta, 2000; Farber et al., 2005; Torres et al., 2005; Devoino et al., 2006; Mastroeni et al., 2009; Nakano et al., 2009a; Strell et al., 2009; Huang et al., 2010; Sarkar et al., 2010; Barnes et al., 2015; Yan et al., 2015; Levite, 2016; Pinoli et al., 2017). A number of these functions are associated with the development of NeuroHIV, including dopaminergic modulation of the production of inflammatory mediators (Gaskill et al., 2012), blood-brain-barrier transmigration and chemotaxis (Li et al., 2003; Watanabe et al., 2006; Mastroeni et al., 2009; Coley et al., 2015; Calderon et al., 2017), and cell differentiation (Nakano et al., 2009b; Prado et al., 2012; Qin et al., 2015). In addition, dopamine can directly influence the spread of infection by increasing HIV replication in both macrophages and T-cells (Rohr et al., 1999; Gaskill et al., 2009; Gaskill et al., 2014). These effects are mediated by both D1-like (DRD1, DRD5) and D2-like subtypes (DRD2, DRD3, DRD4), which are expressed on most human immune cells. While the specific impact of individual dopamine-receptor subtypes in immunomodulation is not well-established, studies in rodent models show activation of D1-like receptors on immune cells including T-cells and dendritic cells (Nakagome et al., 2011; Nakano et al., 2011) promotes inflammation, while activation of D2-like receptors is anti-inflammatory (Laengle et al., 2006; Zhang et al., 2012; Zhang et al., 2015; Zhu et al., 2018), although these studies have not been done in macrophages. Therefore, the specific immunomodulatory role of dopamine receptors on human myeloid cells remains unclear, but these data now demonstrate that primary human MDM express predominantly D1-like dopamine-receptor mRNA, with significantly greater amounts of DRD5 than any other subtype (Fig. 1). This suggests that the impact of dopamine stimulation of macrophages would be predominantly inflammatory, which in the context of HIV may result in exacerbation of neuroinflammation.
Myeloid cell cytokine production plays a major role in the development of inflammation, particularly in the CNS where microglia and macrophages comprise the bulk of the resident immune cell population (Lassmann et al., 1993; Ransohoff and Cardona, 2010; Katsumoto et al., 2014; Prinz and Priller, 2014; Greter et al., 2015). To better evaluate the impact of dopamine on this process, four different dopamine concentrations were used to better model the range of fluctuations in dopaminergic tone to which CNS myeloid cells might be exposed during both normal physiologic conditions, and during HIV-infection and coincident drug use. The lowest concentration, 10−9 M, approximates basal dopamine levels, while 10−8 M represents tonic levels in dopaminergic regions or stimulated release in projection regions by natural rewards. For the higher concentrations, 10−7 M represents levels observed in most cases of drug abuse, and 10−6M represents the highest concentration that myeloid cells could be exposed to during the use of psychostimulants such as cocaine and methamphetamine (Carboni et al., 1989; Koob, 1992; Schiffer et al., 2003; Kimmel et al., 2005; Zachek et al., 2010). These data show significant changes in IL-6 production in response multiple concentrations of dopamine, with both the highest (10−6M) and the lowest (10−9M) concentrations significantly increasing IL-6 2-fold. Significant induction of the chemokines CCL2, CXCL9, and CXCL10 also occurred at multiple concentrations, including the lower concentrations (10−8M and 10−9M), and while not significant, CXCL8 also showed a strong trend toward an increase at these concentrations. This indicates that dopaminergic modulation of these factors could occur in the absence of drug use and may be a function of normal physiology in uninfected individuals. Additionally, during HIV-infection, elevation of IL-6, CCL2, CXCL8, CXCL9, and CXCL10 is believed to play a role in the development of neuropathology (Kaul et al., 2001; Anthony and Bell, 2008; Kraft-Terry et al., 2009; Burdo et al., 2013; Saylor et al., 2016). Thus, our data suggest that even slight elevations in extracellular dopamine may contribute to the development and persistence of chronic, HIV-associated neuroinflammation through cultivation of the myeloid inflammatory milieu.
To better understand this relationship, we examined correlations between the expression of dopamine receptors and the dopamine-mediated change production of individual cytokines. In the healthy, freshly isolated MDM cohort, there is a positive correlation between D1-like receptor expression and inhibition of LPS-induced production of the anti-inflammatory cytokine IL-10. There is also negative correlation between D2-like receptor expression and the production of the inflammatory cytokine IL-6. This supports the hypothesis prevalent in the literature, that D1-like receptors promote an inflammatory macrophage phenotype, while D2-like receptors opposing this effect. We did not observe any correlations for dopamine receptor expression with dopamine-induced production of other cytokines. This implies that both subtypes of dopamine-receptor might be involved in the regulation of individual cytokines. In addition, it is notable that a correlation for IL-6 is only present at 10−8 M dopamine, but not at other concentrations that had a significant effect. This could indicate that distinct dopamine receptor subtypes may be dominant at different dopamine concentrations due to differences in receptor sensitivity. For instance, in certain neuronal populations, DRD1 is preferentially activated at low dopamine concentrations and DRD2 activation is recruited as dopamine concentrations rise, as reflected by the inverted-U shaped dose-response curve for certain dopamine functions (Wang et al., 2004; Kroener et al., 2009; Gjedde et al., 2010; Cools and D'Esposito, 2011; Floresco, 2013). The specific pharmacology of macrophage dopamine receptors remains unclear; while they may be pharmacologically similar to neuronal dopamine receptors, preliminary studies in our group suggest they display their own unique pharmacology (Nickoloff et. al., under review). The associations demonstrated here, as well as the data describing the relative abundance of dopamine receptor transcripts in MDM, will support future studies utilizing dopamine-receptor specific agonists and antagonists to better define dopamine-induced cytokine production. These experiments should also examine how exogenous changes in macrophage inflammation state, such as activation of the inflammasome, regulate dopamine receptor expression and signaling.
As the majority of HIV-infected individuals are currently treated with cART (UNAIDS, 2016), evaluating the effects of dopamine in macrophages from cART treated patients is important to understanding the role of dopamine in the modern pandemic. In these macrophages, dopamine receptor expression is similar to that seen in healthy individuals, with significantly greater DRD5 transcript expression relative to other dopamine receptors for both donor groups. Dopamine-induced changes in cytokine production were also similar in healthy and cART-treated donors, with no significant interaction between donor group and dopamine treatment for any cytokine measured. In this study, dopamine significantly increased production of IL-6, CXCL8, and CXCL10 in macrophages from cART-treated donors, replicating our initial findings but in a smaller cohort (n = 12) of donor macrophages that were isolated from previously frozen PBMC. Notably, we did not observe a significant main effect of dopamine treatment on production of IL-1β or LPS-induced production of IL-10. This may indicate the effect of dopamine on these cytokines is subject to greater interdonor variability and requires a larger cohort size to observe significance. Therefore, additional studies in a larger cohort of HIV-infected individuals are needed to replicate our findings and to resolve differences in effect size between our experiments. Altogether, these results indicate that aberrant dopamine levels during HIV infection due to illicit or therapeutic drug use could promote tissue-damaging inflammation even in individuals on suppressive cART. These results also provide important information for physicians that contraindicates prescription of dopamine-modifying therapeutics to HIV-infected patients. The similar dopamine-responsiveness in healthy and cART-treated donors also indicates that performing future mechanistic experiments in MDM from healthy individuals is a reasonable approach in future studies.
We also observed that MDM isolated from cART-treated patients expressed significantly greater levels of DRD5 mRNA compared to healthy controls isolated in a similar manner (Fig. 6C). This finding could be an artifact of a small sample size, but the prospect that macrophage dopamine receptor expression could be sensitive to an individual’s immune environment is an intriguing one that is worth further inquiry. Increased expression of macrophage DRD5 during chronic HIV-infection could enhance macrophage dopamine responsiveness and contribute to a pro-inflammatory environment. However, unlike in our cohort of freshly isolated PBMC from healthy donors, the macrophages from cART-treated patients did not show any associations between an individual’s dopamine-receptor expression and production of any cytokine. This is likely due to the size of our cART-treated cohort, which may have been too small to elucidate such patterns.
An important caveat to this analysis is the use of previously frozen PBMC from cART-treated patients. The process of freezing, transporting, and thawing PBMC could alter the function of MDM isolated from this population and influence our results. Based on the response to LPS-stimulation in healthy or cART-treated donors, the immune response of frozen cells was intact (Supplemental figure 3, 4). However, the baseline and LPS-stimulated level of cytokines demonstrated greater standard error in MDM derived from frozen PBMC for both healthy and cART treated populations (Tables 2 and 5), indicating that greater variability may have been introduced by the freezing process. Dopamine-responsiveness was also more variable in healthy MDM isolated from previously frozen PBMC than from freshly isolated PBMC (Supplemental figure 4), suggesting that the freezing or thawing process might be a confounding variable. To address this, future analysis should be performed on freshly-isolated MDM from cART-treated patients or by using larger cohorts to offset the impact of cryopreservation variability.
The power of larger cohorts was also observed for MDM obtained from freshly-isolated PBMC. Our prior studies using a primary human macrophages from a smaller cohort of individuals (N = 6-13) showed dopamine modulates production of IL-6, CCL2, TNF-α, CXCL8, and IL-10, but variability between donors was high (Gaskill et al., 2012). Other studies using both human and rodent macrophages also showed dopamine-induced changes in cytokine production, but many of the results are contradictory. Some studies agree with our finding that dopamine-receptor stimulation largely increases macrophage inflammatory cytokine production (Chi et al., 2003; Shavali et al., 2006; Gaskill et al., 2012; Trudler et al., 2014; Qin et al., 2015; Yamamoto et al., 2016), while other studies indicate that dopamine has an inhibitory effect (Hasko et al., 2002; Farber et al., 2005; Capellino et al., 2010; Yan et al., 2015; Yoshioka et al., 2016; Bone et al., 2017). The discontinuity in these findings may be due to differences in experimental design, as like our own initial study, many of these experiments were limited by a small cohort size. Additionally, much of this research focused on a single, non-physiologic concentration of dopamine that could evoke oxidative stress and off-target effect. Other studies used non-specific dopamine receptor agonists and antagonists that may obscure the overall effect of dopamine by preferentially activating individual dopamine receptors to unknown degrees. In addition, there are likely species-specific differences in MDM dopamine responsivity that could account for dissimilarities in the literature. Furthermore, there is high genetic similarity within rodent colonies that may skew results if MDM dopamine-receptor expression is homogenous within experimental groups. This prevents the observation of donor dependent differences in dopamine sensitivity, and the analysis of the association of specific dopamine receptor sub-types with particular cytokines. Therefore, it is difficult to interpret the results of these studies to determine a role for specific macrophage dopamine receptor subtypes in the regulation of particular cytokines. It is also possible that differences in culture conditions between experiments affect baseline cytokine measurements and obscure the impact of dopamine treatment, particularly if the effect of dopamine is subtle.
This experimental protocol is designed to and expand upon prior studies by addressing many of these limitations. To accommodate the high-inter-donor variability inherent to the use of primary cells, MDM were obtained from a large number of individuals recruited from 5 separate cohorts; the New York Blood center (NYBC, n = 35), BioIVT in Miami (n = 6), University of Pennsylvania (n = 4), Martin Memorial Health Systems in Florida (n = 8), and a group of HIV+, cART suppressed patients at McGill University in Montreal (n = 12). Analysis of cytokine production was performed using a sensitive, bead-based AlphaLISA assay with a large dynamic range to more precisely measure changes in cytokine levels. The results of this updated methodology demonstrate that dopamine increases the production of IL-6, IL-1β, IL-18, CCL2, CXCL8, CXCL9, and CXCL10 in non-LPS stimulated macrophages across a range of concentrations (Table 2, Fig. 3). This confirms our previous finding that dopamine increases production of IL-6 and CCL2 in MDM, with a magnitude similar to our previous finding (Gaskill et al., 2012). Contrary to our prior study, in this larger cohort dopamine (10−6 M) increased production of CXCL8 in non-LPS stimulated cells, an effect that was not significant in our prior study (Gaskill), although this difference may be due to the difference in cohort size. Other differences with our previous data, are that dopamine does not change the production of TNF-α in LPS-stimulated macrophages, and that dopamine inhibits rather than increases LPS-induced IL-10 production. As both studies used a significant number of individuals from the NYBC, the differences are unlikely to be due to differences in the cohorts, but the use of a larger number of donors enabled more robust statistical analysis that better accounted for individual variance in dopamine sensitivity. The increased sensitivity of the assays used, the use of LPS as a positive control for capability to produce cytokines, or random variation in the donor population could also have led to these disparities in the results. These differences highlight the value of using a large cohort sourced from several locations to ensure that the heterogeneity of the population can be properly accounted for.
Unfortunately, demographic information available for the Martin Memorial Health (n = 12) and UPenn cohorts (n = 4) was limited, making it impossible to determine whether healthy and cART donors were matched for age, gender, race, or CMV status. The majority of frozen PBMC obtained from these cohorts were from males, so these findings may primarily reflect the response of male donors. However, in the freshly isolated macrophages, there were no group differences between LPS or dopamine response with gender, race, CMV status, and there was no association with age (Supplemental table 1), indicating the influence of these variables on the results may be negligible. However, information on drug abuse history, which could be a major confounding variable, was not available for any of our donor populations. Alcohol or drug use can affect dopamine levels in the periphery (Faraj et al., 1993; Stuerenburg et al., 2002), potentially influencing expression or sensitivity of macrophage dopamine receptors, thereby impacting dopamine-responsiveness. It is possible that some of the inter-donor variability observed could be attributed to unknown differences in substance use. Future analyses of this type will control for this variable and/or specifically investigate the impact of drug abuse history on human macrophage function, MDM dopamine receptor expression, and response to dopamine.
In addition to substances of abuse, many dopaminergic pharmaceuticals are commonly prescribed to treat a range of conditions including ADHD, depression, diabetes, Alzheimer’s, or Parkinson's dementia (Smits et al., 2000; Rogers et al., 2007; Hu et al., 2008; Miller et al., 2009; Garcia-Tornadu et al., 2010; Gate et al., 2010; Via et al., 2010; Kalra et al., 2011; Sun et al., 2012; Kleinridders et al., 2015). Use of these pharmaceuticals in our donor populations is also unknown and should be considered a potential confounding variable. However, the widespread use of dopamine-modifying therapeutics also increases the relevance of our findings, as many of these therapeutics influence extracellular dopamine in the brain by modulating dopamine uptake, release, or metabolism (Tanda et al., 1994; Kaseda et al., 1999; Volkow et al., 2001). These include psychostimulants such as Ritalin (Spanagel et al., 1994; Volkow et al., 2001; Peeters et al., 2003; Zhang et al., 2004) and anti-depressants such as Wellbutrin (dopamine-uptake inhibitor) and Emsam (monoamine oxidase inhibitor) that are widely prescribed in both healthy and HIV-infected populations, often for an extended period of time (Currier et al., 2003; Basu et al., 2005; Huffman and Fricchione, 2005; Cecchelli et al., 2010). Long-term changes in dopaminergic tone due to use of these drugs could affect myeloid cell function and contribute to the development of chronic neuroinflammation. Furthermore, therapeutics acting as dopamine-receptor agonists and antagonists are commonly prescribed for the treatment of depression (Aripiprazole), diabetes (bromocriptine) and certain cancers (Wang et al., 2002; Senogles, 2007; Sarkar et al., 2008). Such drugs can exert their effects in the periphery as well as the brain, yet the effect of these drugs on macrophage function has not been studied. Therefore, understanding the impact of dopamine and dopaminergic agents on macrophage cytokine production has major implications for the promotion of tissue-damaging inflammation in the periphery as well as the brain.
Another area in need of further study is the specific impact of dopamine on microglial function. While related, macrophages and microglia have different developmental origins (Ginhoux et al., 2013; Prinz and Priller, 2014) and may respond differently to dopamine. As resident CNS cells (Prinz et al., 2014), microglia may be exposed to dopamine more consistently and at greater concentrations than peripheral macrophages, as they are often in close proximity to neuronal synapses while regulating synaptic architecture (Wake et al., 2009; Paolicelli et al., 2011; Schafer et al., 2013). Microglial pruning of synaptic dendritic spines is necessary for normal brain development and synaptic plasticity (Paolicelli et al., 2011; Schafer et al., 2012; Wake et al., 2013; Wu et al., 2015), and microglia express dopamine receptors (Mastroeni et al., 2009; Huck et al., 2015; Yoshioka et al., 2016) that could respond to dopamine and modulate microglia activity precisely for the purpose of activity-dependent synaptic pruning. Both IL-1β and IL-6, which this study shows are increased in response to dopamine, may be important mediators of microglial-neuron communication (Monje et al., 2003; Iosif et al., 2006; Yirmiya and Goshen, 2011). Thus, aberrant microglial activation and inflammatory cytokine release mediated by dopamine exposure could adversely impact neuronal structure and function. In one study, induction of IL-1β release in a glial-neuronal co-culture resulted in a loss of dendritic spines in rat cortical neurons, and in HIV-transgenic rats, reduced dendritic spine density is associated with impaired executive function (Festa et al., 2015). Therefore, under conditions of elevated dopamine, modulation of microglial cytokine production could result in inappropriate synaptic pruning or neuronal injury, and in the context of HIV contribute to poor systemic and neurologic outcomes.
The data from this current study show that physiologically relevant dopamine concentrations significantly increase the production of inflammatory cytokines and chemokines in primary human macrophages, suggesting dopamine induces an inflammatory phenotype in these cells. These effects are maintained in the presence of cART, indicating dopamine has the same effects on macrophage-mediated inflammation in chronically infected, virally suppressed individuals as it does in healthy individuals. These inflammatory effects may be linked to the activity of specific dopamine receptors, possibly DR5, as transcripts for this receptor are the most prominently expressed in macrophages despite large interpersonal variability in receptor expression. Overall, these data provide a more substantial foundation for understanding the role of dopamine in the development of NeuroHIV, as well as other inflammatory diseases in which neuroimmune manipulation of myeloid functions plays a significant role. These experiments improve our understanding of the mechanisms by which dopamine exacerbates inflammation, and will inform future studies that may identify signaling pathways that could be targeted therapeutically to prevent or counteract myeloid-cell mediated inflammation during HIV infection.
Supplementary Material
Table 4.
Demographic and characteristics of healthy donors (Cryopreserved UPenn cohort, n=4)
| Donor | Age | Gender | HLA phenotypes | Date of PBMC isolation |
|---|---|---|---|---|
| ND221 | 39 | male | A66,68; B41,35; DR13,8 | 05/26/2014 |
| ND417 | 36 | male | A30, 11; B27.18; DR18, 1 | 04/08/2015 |
| ND436 | 24 | male | A25,2; B18,8; DR17, 16 | 11/19/2015 |
| ND444 | 29 | male | A68,26; B61, 13; DR13,4 | 01/07/2015 |
Abbreviations:
- cART
combination anti-retroviral therapy
- BG
basal ganglia
- DA
dopamine
- DR
dopamine receptor
- HAND
HIV-associated neurocognitive disorder
- M-CSF
macrophage colony stimulating factor
- MDM
monocyte derived-macrophages
- PBMC
peripheral blood mononuclear cells
- SbN
substantia nigra
References
- Agius M, Bonnici H (2017) Antidepressants in use in clinical practice. Psychiatr Danub 29:667–671. [PubMed] [Google Scholar]
- Agrawal L, Louboutin JP, Marusich E, Reyes BA, Van Bockstaele EJ, Strayer DS (2010) Dopaminergic neurotoxicity of HIV-1 gp120: reactive oxygen species as signaling intermediates. Brain research 1306:116–130. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Bell JE (2008) The Neuropathology of HIV/AIDS. Int Rev Psychiatry 20:15–24. [DOI] [PubMed] [Google Scholar]
- Aquaro S, Bagnarelli P, Guenci T, De Luca A, Clementi M, Balestra E, Calio R, Perno CF (2002) Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. Journal of medical virology 68:479–488. [DOI] [PubMed] [Google Scholar]
- Armah KA et al. (2012) HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis 55:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD (1993) Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology 43:2099–2104. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Brettschneider PD, McArthur JC, Harris GJ, Schlaepfer TE, Henderer JD, Barta PE, Tien AY, Pearlson GD (1995) Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. The American journal of psychiatry 152:987–994. [DOI] [PubMed] [Google Scholar]
- Baker LM, Paul RH, Heaps-Woodruff JM, Chang JY, Ortega M, Margolin Z, Usher C, Basco B, Cooley S, Ances BM (2015) The Effect of Central Nervous System Penetration Effectiveness of Highly Active Antiretroviral Therapy on Neuropsychological Performance and Neuroimaging in HIV Infected Individuals. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 10:487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MA, Carson MJ, Nair MG (2015) Non-traditional cytokines: How catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine 72:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS (2000) Dopamine, a neurotransmitter, influences the immune system. Journal of neuroimmunology 102:113–124. [DOI] [PubMed] [Google Scholar]
- Basu S, Chwastiak LA, Bruce RD (2005) Clinical management of depression and anxiety in HIV-infected adults. AIDS 19:2057–2067. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G (2000) HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol 14:214–221. [DOI] [PubMed] [Google Scholar]
- Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B (1994) Cerebrospinal fluid dopamine in HIV-1 infection. AIDS 8:67–71. [DOI] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M (2001) Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of general psychiatry 58:721–728. [DOI] [PubMed] [Google Scholar]
- Boban J, Kozic D, Turkulov V, Ostojic J, Semnic R, Lendak D, Brkic S (2017) HIV-associated neurodegeneration and neuroimmunity: multivoxel MR spectroscopy study in drug-naive and treated patients. Eur Radiol. [DOI] [PubMed] [Google Scholar]
- Bone NB, Liu Z, Pittet JF, Zmijewski JW (2017) Frontline Science: D1 dopaminergic receptor signaling activates the AMPK-bioenergetic pathway in macrophages and alveolar epithelial cells and reduces endotoxin-induced ALI. J Leukoc Biol 101:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Lackner A, Williams KC (2013) Monocyte/macrophages and their role in HIV neuropathogenesis. Immunological reviews 254:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon TM, Williams DW, Lopez L, Eugenin EA, Cheney L, Gaskill PJ, Veenstra M, Anastos K, Morgello S, Berman JW (2017) Dopamine Increases CD14+CD16+ Monocyte Transmigration across the Blood Brain Barrier: Implications for Substance Abuse and HIV Neuropathogenesis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 12:353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellino S, Cosentino M, Wolff C, Schmidt M, Grifka J, Straub RH (2010) Catecholamine-producing cells in the synovial tissue during arthritis: modulation of sympathetic neurotransmitters as new therapeutic target. Annals of the rheumatic diseases 69:1853–1860. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G (1989) Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience 28:653–661. [DOI] [PubMed] [Google Scholar]
- Cecchelli C, Grassi G, Pallanti S (2010) Aripiprazole Improves Depressive Symptoms and Immunological Response to Antiretroviral Therapy in an HIV-Infected Subject with Resistant Depression. Case Rep Med 2010:836214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P, Brew BJ (2014) HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers, and effects of treatment. Curr HIV/AIDS Rep 11:317–324. [DOI] [PubMed] [Google Scholar]
- Chan P, Hellmuth J, Spudich S, Valcour V (2016) Cognitive Impairment and Persistent CNS Injury in Treated HIV. Current HIV/AIDS reports 13:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi DS, Qui M, Krishnaswamy G, Li C, Stone W (2003) Regulation of nitric oxide production from macrophages by lipopolysaccharide and catecholamines. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society 8:127–132. [DOI] [PubMed] [Google Scholar]
- Churchill M, Nath A (2013) Where does HIV hide? A focus on the central nervous system. Current opinion in HIV and AIDS 8:165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford KM, Samboju V, Cobigo Y, Milanini B, Marx GA, Hellmuth JM, Rosen HJ, Kramer JH, Allen IE, Valcour VG (2017) Progressive Brain Atrophy Despite Persistent Viral Suppression in HIV Over Age 60. J Acquir Immune Defic Syndr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LJ, Sclar DA, Culpepper L, Flockhart DA, Hirschfeld RM, Thase ME, VanDenBerg CM (2012) Discussion: a fresh look at monoamine oxidase inhibitors for depression. The Journal of clinical psychiatry 73 Suppl 1:42–45. [DOI] [PubMed] [Google Scholar]
- Coley JS, Calderon TM, Gaskill PJ, Eugenin EA, Berman JW (2015) Dopamine Increases CD14+CD16+ Monocyte Migration and Adhesion in the Context of Substance Abuse and HIV Neuropathogenesis. PloS one 10:e0117450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D'Esposito M (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69:e113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross HM, Combrinck MI, Joska JA (2013) HIV-associated neurocognitive disorders: antiretroviral regimen, central nervous system penetration effectiveness, and cognitive outcomes. S Afr Med J 103:758–762. [DOI] [PubMed] [Google Scholar]
- Currier MB, Molina G, Kato M (2003) A prospective trial of sustained-release bupropion for depression in HIV-seropositive and AIDS patients. Psychosomatics 44:120–125. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Heaton RK, Kamminga J, Lane T, Gates TM, Moore DM, Hubner E, Carr A, Brew BJ (2014) HIV-associated neurocognitive disorder in Australia: a case of a high-functioning and optimally treated cohort and implications for international neuroHIV research. Journal of neurovirology 20:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila PS, Collu M, Gessa GL, Serra G (2000) The role of dopamine in the mechanism of action of antidepressant drugs. European journal of pharmacology 405:365–373. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalopoulou M et al. (2014) Recreational drug use, polydrug use, and sexual behaviour in HIV-diagnosed men who have sex with men in the UK: results from the cross-sectional ASTRA study. Lancet HIV 1:e22–31. [DOI] [PubMed] [Google Scholar]
- Devoino LV, Al'perina EL, Gevorgyan MM, Cheido MA (2006) Interaction between dopamine D1 and D2 receptors in modulation of the immune response. Bull Exp Biol Med 141:553–555. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D (2000) Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clinical neuropharmacology 23:190–194. [DOI] [PubMed] [Google Scholar]
- Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, Fagan JL, Freedman MS, Skarbinski J (2014) Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PloS one 9:e92842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj BA, Camp VM, Davis DC, Kutner M, Cotsonis GA, Holloway T (1993) Elevated concentrations of dopamine sulfate in plasma of cocaine abusers. Biochemical pharmacology 46:1453–1457. [DOI] [PubMed] [Google Scholar]
- Farber K, Pannasch U, Kettenmann H (2005) Dopamine and noradrenaline control distinct functions in rodent microglial cells. Molecular and cellular neurosciences 29:128–138. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM (2009) In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission. Synapse 63:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa L, Gutoskey CJ, Graziano A, Waterhouse BD, Meucci O (2015) Induction of Interleukin-1beta by Human Immunodeficiency Virus-1 Viral Proteins Leads to Increased Levels of Neuronal Ferritin Heavy Chain, Synaptic Injury, and Deficits in Flexible Attention. The Journal of neuroscience : the official journal of the Society for Neuroscience 35:10550–10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF (2015) HIV-1 Proteins, Tat and gp120, Target the Developing Dopamine System. Current HIV research 13:21–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB (2013) Prefrontal dopamine and behavioral flexibility: shifting from an "inverted-U" toward a family of functions. Front Neurosci 7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura RK, Goodkin K, Petito CK, Douyon R, Feaster DJ, Concha M, Shapshak P (1997) HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association 16:146–152. [DOI] [PubMed] [Google Scholar]
- Garcia-Tornadu I, Ornstein AM, Chamson-Reig A, Wheeler MB, Hill DJ, Arany E, Rubinstein M, Becu-Villalobos D (2010) Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology 151:1441–1450. [DOI] [PubMed] [Google Scholar]
- Garin N, Velasco C, De Pourcq JT, Lopez B, Gutierrez Mdel M, Haro JM, Feliu A, Mangues MA, Trilla A (2015) Recreational drug use among individuals living with HIV in Europe: review of the prevalence, comparison with the general population and HIV guidelines recommendations. Front Microbiol 6:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Carvallo L, Eugenin EA, Berman JW (2012) Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. Journal of neuroinflammation 9:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Coley JS, Berman JW (2013) Drug induced increases in CNS dopamine alter monocyte, macrophage and T cell functions: implications for HAND. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 8:621–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW (2014) Dopamine Receptor Activation Increases HIV Entry into Primary Human Macrophages. PloS one 9:e108232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Miller DR, Gamble-George J, Yano H, Khoshbouei H (2017) HIV, Tat and dopamine transmission. Neurobiology of disease 105:51–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW (2009) Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. The American journal of pathology 175:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T (2010) Macrophages in Alzheimer's disease: the blood-borne identity. J Neural Transm (Vienna) 117:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB (2015) Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Current HIV/AIDS reports 12:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Spencer JA, Holzer CE 3rd, Soukup VM (2006) Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 1:410–420. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH Jr., Soukup VM (2012) Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 7:686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill AJ, Kolson DL (2014) Chronic inflammation and the role for cofactors (hepatitis C, drug abuse, antiretroviral drug toxicity, aging) in HAND persistence. Curr HIV/AIDS Rep 11:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Lim S, Hoeffel G, Low D, Huber T (2013) Origin and differentiation of microglia. Front Cell Neurosci 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjedde A, Kumakura Y, Cumming P, Linnet J, Moller A (2010) Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci U S A 107:3870–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC (1995) Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Annals of neurology 38:755–762. [DOI] [PubMed] [Google Scholar]
- Gordon MA, Gordon SB, Musaya L, Zijlstra EE, Molyneux ME, Read RC (2007) Primary macrophages from HIV-infected adults show dysregulated cytokine responses to Salmonella, but normal internalization and killing. AIDS 21:2399–2408. [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Croxford AL (2015) Microglia Versus Myeloid Cell Nomenclature during Brain Inflammation. Front Immunol 6:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Nemeth ZH, Deitch EA (2002) Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a beta-adrenoceptor-mediated mechanism. Journal of neuroimmunology 122:34–39. [DOI] [PubMed] [Google Scholar]
- Hestad K, McArthur JH, Dal Pan GJ, Selnes OA, Nance-Sproson TE, Aylward E, Mathews VP, McArthur JC (1993) Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta neurologica Scandinavica 88:112–118. [DOI] [PubMed] [Google Scholar]
- Hollander H, Golden J, Mendelson T, Cortland D (1985) Extrapyramidal symptoms in AIDS patients given low-dose metoclopramide or chlorpromazine. Lancet 2:1186. [DOI] [PubMed] [Google Scholar]
- Hong S, Banks WA (2015) Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain, behavior, and immunity 45:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Scheller C, du Plessis S, Arendt G, Nolting T, Joska J, Sopper S, Maschke M, Obermann M, Husstedt IW, Hain J, Maponga T, Riederer P, Koutsilieri E, German Competence Network HA (2013) Increases in CSF dopamine in HIV patients are due to the dopamine transporter 10/10-repeat allele which is more frequent in HIV-infected individuals. J Neural Transm (Vienna) 120:1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hriso E, Kuhn T, Masdeu JC, Grundman M (1991) Extrapyramidal symptoms due to dopamine-blocking agents in patients with AIDS encephalopathy. The American journal of psychiatry 148:1558–1561. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, Liu Y, Qian L, Wilson B, Di Monte DA, Ali SF, Zhang J, Block ML, Hong JS (2008) Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Journal of immunology 181:7194–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Qiu AW, Peng YP, Liu Y, Huang HW, Qiu YH (2010) Roles of dopamine receptor subtypes in mediating modulation of T lymphocyte function. Neuro Endocrinol Lett 31:782–791. [PubMed] [Google Scholar]
- Huck JH, Freyer D, Bottcher C, Mladinov M, Muselmann-Genschow C, Thielke M, Gladow N, Bloomquist D, Mergenthaler P, Priller J (2015) De novo expression of dopamine D2 receptors on microglia after stroke. J Cereb Blood Flow Metab 35:1804–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, Fricchione GL (2005) Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol 25:508–510. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O (2006) Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience 26:9703–9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Mehraein P, Weis S (2000) Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta neuropathologica 99:376–384. [DOI] [PubMed] [Google Scholar]
- Kalra S, Kalra B, Agrawal N, Kumar S (2011) Dopamine: the forgotten felon in type 2 diabetes. Recent Pat Endocr Metab Immune Drug Discov 5:61–65. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Ariniemi K, Seppala T (2001) Oxalic acid stabilizes dopamine, serotonin, and their metabolites in automated liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl 753:413–419. [DOI] [PubMed] [Google Scholar]
- Kaseda S, Nomoto M, Iwata S (1999) Effect of selegiline on dopamine concentration in the striatum of a primate. Brain research 815:44–50. [DOI] [PubMed] [Google Scholar]
- Katsumoto A, Lu H, Miranda AS, Ransohoff RM (2014) Ontogeny and functions of central nervous system macrophages. J Immunol 193:2615–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA (2006) Mechanisms of neuronal injury and death in HIV-1 associated dementia. Current HIV research 4:307–318. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988–994. [DOI] [PubMed] [Google Scholar]
- Kieburtz KD, Epstein LG, Gelbard HA, Greenamyre JT (1991) Excitotoxicity and dopaminergic dysfunction in the acquired immunodeficiency syndrome dementia complex. Therapeutic implications. Archives of neurology 48:1281–1284. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Ginsburg BC, Howell LL (2005) Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse 56:129–134. [DOI] [PubMed] [Google Scholar]
- Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, Vienberg SG, Pothos EN, Kahn CR (2015) Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci U S A 112:3463–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (1992) Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends in pharmacological sciences 13:177–184. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Gotz ME, Sopper S, Stahl-Hennig C, Czub M, ter Meulen V, Riederer P (1997) Monoamine metabolite levels in CSF of SIV-infected rhesus monkeys (Macaca mulatta). Neuroreport 8:3833–3836. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE (2009) A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron 64:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Chandler LJ, Phillips PE, Seamans JK (2009) Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PloS one 4:e6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M (2011) Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. Journal of neurovirology 17:26–40. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M (2009) Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. Journal of neurovirology 15:257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure K, Lyman WD, Weidenheim KM, Dickson DW (1990) Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. The American journal of pathology 136:1085–1092. [PMC free article] [PubMed] [Google Scholar]
- Laengle UW, Markstein R, Pralet D, Seewald W, Roman D (2006) Effect of GLC756, a novel mixed dopamine D1 receptor antagonist and dopamine D2 receptor agonist, on TNF-alpha release in vitro from activated rat mast cells. Exp Eye Res 83:1335–1339. [DOI] [PubMed] [Google Scholar]
- Larsson M, Hagberg L, Forsman A, Norkrans G (1991) Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. Journal of neuroscience research 28:406–409. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Schmied M, Vass K, Hickey WF (1993) Bone marrow derived elements and resident microglia in brain inflammation. Glia 7:19–24. [DOI] [PubMed] [Google Scholar]
- Leggio GM, Salomone S, Bucolo C, Platania C, Micale V, Caraci F, Drago F (2013) Dopamine D(3) receptor as a new pharmacological target for the treatment of depression. European journal of pharmacology 719:25–33. [DOI] [PubMed] [Google Scholar]
- Levite M (2016) Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol (Oxf) 216:42–89. [DOI] [PubMed] [Google Scholar]
- Li CY, Tsai CS, Chueh SH, Hsu PC, Wang JY, Wong CS, Ho ST (2003) Dobutamine inhibits monocyte chemoattractant protein-1 production and chemotaxis in human monocytes. Anesth Analg 97:205–209, table of contents. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shi Z, Liu J, Wang Y (2014) HIV transactivator of transcription enhances methamphetamine-induced Parkinson's-like behavior in the rats. Neuroreport. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Smith G, Meltzer CC, Becker JT (1999) Dopamine systems in human immunodeficiency virus-associated dementia. Neuropsychiatry Neuropsychol Behav Neurol 12:184–192. [PubMed] [Google Scholar]
- Mastroeni D, Grover A, Leonard B, Joyce JN, Coleman PD, Kozik B, Bellinger DL, Rogers J (2009) Microglial responses to dopamine in a cell culture model of Parkinson's disease. Neurobiology of aging 30:1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP, Reference Group to the UNoHIV, Injecting Drug U (2008) Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 372:1733–1745. [DOI] [PubMed] [Google Scholar]
- McArthur JC (2004) HIV dementia: an evolving disease. Journal of neuroimmunology 157:3–10. [DOI] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Zhu J (2012) HIV-1 Tat protein decreases dopamine transporter cell surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 7:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JM, Schindler R, Ferriani R, Sakaguchi M, Vannier E, Dinarello CA, Groopman JE (1990) Production of cytokines by peripheral blood monocytes/macrophages infected with human immunodeficiency virus type 1 (HIV-1). The Journal of infectious diseases 161:888–893. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760–1765. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ (2010) Immune System and Schizophrenia. Curr Immunol Rev 6:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagome K, Imamura M, Okada H, Kawahata K, Inoue T, Hashimoto K, Harada H, Higashi T, Takagi R, Nakano K, Hagiwara K, Kanazawa M, Dohi M, Nagata M, Matsushita S (2011) Dopamine D1-like receptor antagonist attenuates Th17-mediated immune response and ovalbumin antigen-induced neutrophilic airway inflammation. Journal of immunology 186:5975–5982. [DOI] [PubMed] [Google Scholar]
- Nakano K, Matsushita S, Saito K, Yamaoka K, Tanaka Y (2009a) [Dopamine as an immune-modulator between dendritic cells and T cells and the role of dopamine in the pathogenesis of rheumatoid arthritis]. Nihon Rinsho Meneki Gakkai Kaishi 32:1–6. [DOI] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Takagi R, Hashimoto K, Tanaka Y, Matsushita S (2009b) Dopamine released by dendritic cells polarizes Th2 differentiation. International immunology 21:645–654. [DOI] [PubMed] [Google Scholar]
- Nakano K, Yamaoka K, Hanami K, Saito K, Sasaguri Y, Yanagihara N, Tanaka S, Katsuki I, Matsushita S, Tanaka Y (2011) Dopamine induces IL-6-dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model. Journal of immunology 186:3745–3752. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW (1986) The AIDS dementia complex: II. Neuropathology. Annals of neurology 19:525–535. [DOI] [PubMed] [Google Scholar]
- Nolan R, Gaskill PJ (2018) The role of Catecholamines in HIV Neuropathogenesis. Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann M, Kuper M, Kastrup O, Yaldizli O, Esser S, Thiermann J, Koutsilieri E, Arendt G, Diener HC, Maschke M, German Competence Network HA (2009) Substantia nigra hyperechogenicity and CSF dopamine depletion in HIV. Journal of neurology 256:948–953. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458. [DOI] [PubMed] [Google Scholar]
- Patterson PH (2009) Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204:313–321. [DOI] [PubMed] [Google Scholar]
- Peeters M, Maloteaux JM, Hermans E (2003) Distinct effects of amantadine and memantine on dopaminergic transmission in the rat striatum. Neurosci Lett 343:205–209. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and biobehavioral reviews 30:215–238. [DOI] [PubMed] [Google Scholar]
- Pinoli M, Marino F, Cosentino M (2017) Dopaminergic Regulation of Innate Immunity: a Review. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 12:602–623. [DOI] [PubMed] [Google Scholar]
- Prado C, Contreras F, Gonzalez H, Diaz P, Elgueta D, Barrientos M, Herrada AA, Lladser A, Bernales S, Pacheco R (2012) Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. Journal of immunology 188:3062–3070. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nature reviews Neuroscience 15:300–312. [DOI] [PubMed] [Google Scholar]
- Prinz M, Tay TL, Wolf Y, Jung S (2014) Microglia: unique and common features with other tissue macrophages. Acta Neuropathol 128:319–331. [DOI] [PubMed] [Google Scholar]
- Qin T, Wang C, Chen X, Duan C, Zhang X, Zhang J, Chai H, Tang T, Chen H, Yue J, Li Y, Yang J (2015) Dopamine induces growth inhibition and vascular normalization through reprogramming M2-polarized macrophages in rat C6 glioma. Toxicol Appl Pharmacol 286:112–123. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE (2010) The myeloid cells of the central nervous system parenchyma. Nature 468:253–262. [DOI] [PubMed] [Google Scholar]
- Rao VR, Ruiz AP, Prasad VR (2014) Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS research and therapy 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport J, Volsky DJ (2015) Role of the macrophage in HIV-associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. Journal of neurovirology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R (1991) Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta neuropathologica 82:39–44. [DOI] [PubMed] [Google Scholar]
- Ricci A, Mariotta S, Greco S, Bisetti A (1997) Expression of dopamine receptors in immune organs and circulating immune cells. Clin Exp Hypertens 19:59–71. [DOI] [PubMed] [Google Scholar]
- Rogers J, Mastroeni D, Leonard B, Joyce J, Grover A (2007) Neuroinflammation in Alzheimer's disease and Parkinson's disease: are microglia pathogenic in either disorder? Int Rev Neurobiol 82:235–246. [DOI] [PubMed] [Google Scholar]
- Rohr O, Sawaya BE, Lecestre D, Aunis D, Schaeffer E (1999) Dopamine stimulates expression of the human immunodeficiency virus type 1 via NF-kappaB in cells of the immune system. Nucleic acids research 27:3291–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzine IM, Weinberger AD, Weinberger LS (2015) An evolutionary role for HIV latency in enhancing viral transmission. Cell 160:1002–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelas DS, Greene WC (2013) An integrated overview of HIV-1 latency. Cell 155:519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Mazmanian SK (2015) Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R, Fellows LK, Ances BM, Collins DL (2017a) Association of Brain Structure Changes and Cognitive Function With Combination Antiretroviral Therapy in HIV-Positive Individuals. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R, Fernandez Cruz AL, Scott SC, Mayo NE, Fellows LK, Ances BM, Collins DL (2017b) Regionally Specific Brain Volumetric and Cortical Thickness Changes in HIV-Infected Patients in the HAART Era. J Acquir Immune Defic Syndr 74:563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S (2008) Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clinical cancer research : an official journal of the American Association for Cancer Research 14:2502–2510. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S (2010) The immunoregulatory role of dopamine: an update. Brain, behavior, and immunity 24:525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nature reviews Neurology 12:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Stevens B (2013) The 'quad-partite' synapse: microglia-synapse interactions in the developing and mature CNS. Glia 61:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller C, Arendt G, Nolting T, Antke C, Sopper S, Maschke M, Obermann M, Angerer A, Husstedt IW, Meisner F, Neuen-Jacob E, Muller HW, Carey P, Ter Meulen V, Riederer P, Koutsilieri E (2010) Increased dopaminergic neurotransmission in therapy-naive asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J Neural Transm (Vienna) 117:699–705. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Marsteller D, Dewey SL (2003) Sub-chronic low dose gamma-vinyl GABA (vigabatrin) inhibits cocaine-induced increases in nucleus accumbens dopamine. Psychopharmacology (Berl) 168:339–343. [DOI] [PubMed] [Google Scholar]
- Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P (2011) HIV-1 infection and cognitive impairment in the cART era: a review. AIDS 25:561–575. [DOI] [PubMed] [Google Scholar]
- Senogles SE (2007) D2 dopamine receptor-mediated antiproliferation in a small cell lung cancer cell line, NCI-H69. Anticancer Drugs 18:801–807. [DOI] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Epstein LG, Marder K (2004) Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology 63:2084–2090. [DOI] [PubMed] [Google Scholar]
- Shavali S, Combs CK, Ebadi M (2006) Reactive macrophages increase oxidative stress and alpha-synuclein nitration during death of dopaminergic neuronal cells in co-culture: relevance to Parkinson's disease. Neurochem Res 31:85–94. [DOI] [PubMed] [Google Scholar]
- Shen YY MY (1993) Determination of the Stability of Dopamine in Aqueous Solutions by High Performance Liquid Chromatography. Journal of Liquid Chromatography 17:1557–1565. [Google Scholar]
- Sloviter RS, Connor JD (1977) Postmortem stability of norepinephrine, dopamine, and serotonin in rat brain. J Neurochem 28:1129–1131. [DOI] [PubMed] [Google Scholar]
- Smits HA, Boven LA, Pereira CF, Verhoef J, Nottet HS (2000) Role of macrophage activation in the pathogenesis of Alzheimer's disease and human immunodeficiency virus type 1-associated dementia. Eur J Clin Invest 30:526–535. [DOI] [PubMed] [Google Scholar]
- Soontornniyomkij V (2017) Neuropathology of HIV-1 Disease In: Global Virology II - HIV and NeuroAIDS (Shapshak P, Levine AJ, Foley BT, Somboonwit C, Singer E, Chiappelli F, Sinnott JT, eds), pp 143–208. New York, NY: Springer New York. [Google Scholar]
- Spanagel R, Eilbacher B, Wilke R (1994) Memantine-induced dopamine release in the prefrontal cortex and striatum of the rat--a pharmacokinetic microdialysis study. Eur J Pharmacol 262:21–26. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Stockman JK (2010) Epidemiology of HIV among injecting and non-injecting drug users: current trends and implications for interventions. Current HIV/AIDS reports 7:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strell C, Sievers A, Bastian P, Lang K, Niggemann B, Zanker KS, Entschladen F (2009) Divergent effects of norepinephrine, dopamine and substance P on the activation, differentiation and effector functions of human cytotoxic T lymphocytes. BMC immunology 10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuerenburg HJ, Petersen K, Baumer T, Rosenkranz M, Buhmann C, Thomasius R (2002) Plasma concentrations of 5-HT, 5-HIAA, norepinephrine, epinephrine and dopamine in ecstasy users. Neuro Endocrinol Lett 23:259–261. [PubMed] [Google Scholar]
- Sun C, Sun L, Ma H, Peng J, Zhen Y, Duan K, Liu G, Ding W, Zhao Y (2012) The phenotype and functional alterations of macrophages in mice with hyperglycemia for long term. J Cell Physiol 227:1670–1679. [DOI] [PubMed] [Google Scholar]
- Syslova K, Rambousek L, Kuzma M, Najmanova V, Bubenikova-Valesova V, Slamberova R, Kacer P (2011) Monitoring of dopamine and its metabolites in brain microdialysates: method combining freeze-drying with liquid chromatography-tandem mass spectrometry. J Chromatogr A 1218:3382–3391. [DOI] [PubMed] [Google Scholar]
- Tanda G, Carboni E, Frau R, Di Chiara G (1994) Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential? Psychopharmacology (Berl) 115:285–288. [DOI] [PubMed] [Google Scholar]
- Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T (2014) Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Current HIV research 12:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres KC, Antonelli LR, Souza AL, Teixeira MM, Dutra WO, Gollob KJ (2005) Norepinephrine, dopamine and dexamethasone modulate discrete leukocyte subpopulations and cytokine profiles from human PBMC. Journal of neuroimmunology 166:144–157. [DOI] [PubMed] [Google Scholar]
- Trudler D, Weinreb O, Mandel SA, Youdim MB, Frenkel D (2014) DJ-1 deficiency triggers microglia sensitivity to dopamine toward a pro-inflammatory phenotype that is attenuated by rasagiline. J Neurochem 129:434–447. [DOI] [PubMed] [Google Scholar]
- UNAIDS (2016) UNAIDS-Global report on HIV infection. [Google Scholar]
- Underwood J, Cole JH, Caan M, De Francesco D, Leech R, van Zoest RA, Su T, Geurtsen GJ, Schmand BA, Portegies P, Prins M, Wit FW, Sabin CA, Majoie C, Reiss P, Winston A, Sharp DJ, Co-morBidity in Relation to Aids C (2017) Grey and white matter abnormalities in treated HIV-disease and their relationship to cognitive function. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC (2017) World Drug Report 2017. In: United Nations. [Google Scholar]
- van den Dries LWJ, Wagener MN, Jiskoot LC, Visser M, Robertson KR, Adriani KS, van Gorp ECM (2017) Neurocognitive Impairment in a Chronically Well-Suppressed HIV-Infected Population: The Dutch TREVI Cohort Study. AIDS patient care and STDs 31:329–334. [DOI] [PubMed] [Google Scholar]
- Via MA, Chandra H, Araki T, Potenza MV, Skamagas M (2010) Bromocriptine approved as the first medication to target dopamine activity to improve glycemic control in patients with type 2 diabetes. Diabetes Metab Syndr Obes 3:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D (2001) Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:RC121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Miyamoto A, Nabekura J (2013) Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci 36:209–217. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience 29:3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS (2004) Selective D2 receptor actions on the functional circuitry of working memory. Science 303:853–856. [DOI] [PubMed] [Google Scholar]
- Wang PS, Walker AM, Tsuang MT, Orav EJ, Glynn RJ, Levin R, Avorn J (2002) Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry 59:1147–1154. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nakayama T, Nagakubo D, Hieshima K, Jin Z, Katou F, Hashimoto K, Yoshie O (2006) Dopamine selectively induces migration and homing of naive CD8+ T cells via dopamine receptor D3. Journal of immunology 176:848–856. [DOI] [PubMed] [Google Scholar]
- Watkins CC, Treisman GJ (2012) Neuropsychiatric complications of aging with HIV. Journal of neurovirology 18:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Nelson JA (1990) Human immunodeficiency virus: infection of the nervous system. Current topics in microbiology and immunology 160:157–172. [DOI] [PubMed] [Google Scholar]
- Williams KC, Hickey WF (2002) Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annual review of neuroscience 25:537–562. [DOI] [PubMed] [Google Scholar]
- Wood M, Reavill C (2007) Aripiprazole acts as a selective dopamine D2 receptor partial agonist. Expert Opin Investig Drugs 16:771–775. [DOI] [PubMed] [Google Scholar]
- Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B (2015) Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol 36:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ohta N, Matsumoto A, Horiguchi Y, Koide M, Fujino Y (2016) Haloperidol Suppresses NF-kappaB to Inhibit Lipopolysaccharide-Induced Pro-Inflammatory Response in RAW 264 Cells. Med Sci Monit 22:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R (2015) Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell 160:62–73. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I (2011) Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, behavior, and immunity 25:181–213. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y, Sugino Y, Tozawa A, Yamamuro A, Kasai A, Ishimaru Y, Maeda S (2016) Dopamine inhibits lipopolysaccharide-induced nitric oxide production through the formation of dopamine quinone in murine microglia BV-2 cells. J Pharmacol Sci 130:51–59. [DOI] [PubMed] [Google Scholar]
- Zachek MK, Takmakov P, Park J, Wightman RM, McCarty GS (2010) Simultaneous monitoring of dopamine concentration at spatially different brain locations in vivo. Biosensors & bioelectronics 25:1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou FM, Dani JA (2004) Cholinergic drugs for Alzheimer's disease enhance in vitro dopamine release. Molecular pharmacology 66:538–544. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Wu J, Manaenko A, Yang P, Tang J, Fu W, Zhang JH (2015) Activation of Dopamine D2 Receptor Suppresses Neuroinflammation Through alphaB-Crystalline by Inhibition of NF-kappaB Nuclear Translocation in Experimental ICH Mice Model. Stroke 46:2637–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, Wang X, Jones JE, Grandy D, Eisner G, Jose PA, Armando I (2012) Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PloS one 7:e38745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM (2009) HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. The Journal of pharmacology and experimental therapeutics 329:1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yuan Y, Midde NM, Gomez AM, Sun WL, Quizon PM, Zhan CG (2016) HIV-1 transgenic rats display an increase in [(3)H]dopamine uptake in the prefrontal cortex and striatum. Journal of neurovirology 22:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Hu Z, Han X, Wang D, Jiang Q, Ding J, Xiao M, Wang C, Lu M, Hu G (2018) Dopamine D2 receptor restricts astrocytic NLRP3 inflammasome activation via enhancing the interaction of beta-arrestin2 and NLRP3. Cell Death Differ. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.