Abstract
Objective:
To evaluate the association between early (within 10d) pRBC transfusion and the development of severe ROP.
Study Design and Methods:
This was a single-center retrospective study. Inclusion criteria were preterm infants born ≤ 32 weeks gestation or weighing ≤ 1500g. Severe ROP was defined as infants requiring retinal laser ablation or bevacizumab injection. Logistic regression was used to identify the association between transfusions and severe ROP.
Results:
A total of 1635 infants were included in the final analysis. The severe ROP incidence was 8% (126/1635). Ninety-one percent (115/126) of infants who developed severe ROP received a pRBC transfusion in the first 10d. Early transfusion was associated with severe ROP; adjusted odds ratio of 3.8 (95% CI: 1.8-8.1).
Conclusion:
pRBC transfusions in the first 10 days of life are associated with an almost four-fold increased risk of severe ROP, independent of gestational age at birth or bronchopulmonary dysplasia (BPD) status.
Keywords: Neonatology, ophthalmology, laser therapy, transfusion
Introduction
Retinopathy of prematurity (ROP) is a leading cause of childhood blindness in the United States and is increasingly prevalent in the developing world, particularly as the overall mortality associated with prematurity declines.(1) ROP was first described in the 1940s after the use of supplemental oxygen became widespread and within a decade had blinded more than 10,000 infants.(2) Excessive oxygen exposure is unequivocally recognized as a mediator of ROP.(3) However, the mechanism of ROP development is a complex multiphase phenomenon that likely has many contributory factors.
In the late 1970s, several studies began reporting cases of ROP, then known as retrolental fibroplasia (RLF), in infants without excessive oxygen exposure. Early studies showed that infants receiving exchange transfusions or multiple simple transfusions were at higher risk of developing RLF, and recent studies have also suggested a link between blood transfusions and the development of ROP.(4-9) However, many of these studies are limited by small sample size, lack of detail regarding timing of transfusions or failure to stratify by severity of retinopathy. Controlling for the clinical illness of infants is complex and many of the patients receiving transfusions were already considered high-risk for development of ROP. In addition, early stage ROP has a high chance of spontaneous regression.(10) Thus, it is important to use a clinically relevant outcome such as ROP that is severe enough to warrant therapeutic intervention.
The objective of our study was to investigate the link between transfusion timing and the development of severe ROP (defined as intervention by either laser ablative surgery or bevacizumab) while controlling for illness severity with regression modeling.
Methods
Patient Selection
The inclusion criteria for this retrospective cohort were all infants admitted to our level IV NICU (St. Louis Children’s Hospital) born ≤ 32 weeks completed gestational age (EGA) or weighing ≤ 1500 grams at birth from 2008-2015. We characterized infants as either inborn, immediately transferred (transferred <24 hours after birth) or as outborn (transferred >24 hours of life). Outborn infants were included in the study if they had appropriate documentation of transfusion status and our collected outcome data. Infants with incomplete records from referring hospitals were excluded in the final analysis.
Patient characteristics (EGA, birth weight, sex, race, antenatal steroid status, mode of delivery, Apgar scores), clinical data (chorioamnionitis, culture positive sepsis, necrotizing enterocolitis, inotrope use, postnatal steroid use) and outcomes (bronchopulmonary dysplasia (BPD), highest stage of ROP, laser therapy and/or bevacizumab for ROP) were collected. BPD was defined as need for supplemental oxygen at 36 weeks post-menstrual age.(11) Chorioamnionitis diagnosis was based on histologic examination of the placenta. NEC was defined as Bell Stage 2a or greater.(12) This project was approved as per 45 CFR 46.111 by the Human Research Protection Office at Washington University in St Louis.
Transfusion data
The timing and number of pRBC transfusions was extracted from the blood bank database and verified with physician or nursing documentation in the patients’ chart by a single investigator (CL). For patients who were transferred to the NICU later in their course, their transfusion records were extracted from the referral center paper chart. We anticipated a large amount of transfusion data. As such, prior to collection of the full dataset, we investigated a cohort (n=222) of premature infants <28 weeks who were recruited into an independent cerebral monitoring observational study. The median age of transfusion in this subset was 10 days of life. We therefore used the DOL#10 as an a piori cut-off to define early versus late transfusion. We defined “early transfusions” as those occurring in the first 10 days of life. All transfusions beyond DOL#10 were considered “late transfusions”. Infants receiving any number of early transfusions were labeled “Early Transfused.” Infants that did not receive early transfusions but did receive late transfusions were labeled “Late Transfused.” Infants that received no transfusions at any time were combined with Late Transfused infants to serve as a “Control” group for univariate analysis to assist with identifying covariates to control for in the final logistic regression model. All infants in our initial subset were included in the final cohort.
All transfusions were performed at the discretion of the clinical team. Our institutional guidelines recommend a transfusion for a hemoglobin ≤ 10 g/dL in critically ill infants and ≤ 8 g/dL in stable but symptomatic infants. Transfusions were generally 15ml/kg of cytomegalovirus (CMV) negative, irradiated and leukoreduced packed red blood cells. Whenever possible the same donor was used if patients require multiple transfusions. During the study period, erythropoietin use was not widely used and 2-3mg/kg of oral iron supplementation was initiated at 21-28 days of life.
Retinopathy of Prematurity
Severe ROP was defined as ROP that required laser ablative surgery and/or treatment with bevacizumab. Infants at our institution underwent standardized screening beginning in the fourth postnatal week or 31 weeks post-menstrual age, whichever is later, if they had an estimated gestational age of less than 30 completed weeks or weighed less than 1500 grams at birth. Infants greater than 30 weeks but less than 32 completed weeks were screened at the fourth postnatal week if they were deemed to have a concerning course by the care team, generally characterized by significant exposure to high oxygen concentrations.(13) Infants received treatment for ROP if they had threshold disease (Stage 3 ROP and plus disease in zone I or II) or high-risk pre-threshold disease (any stage ROP with plus disease in zone I, Stage 3 with or without plus disease in zone I, or stage 2 or 3 with plus disease in zone II) as determined by the attending ophthalmologist caring for the patient.(13, 14)
Statistical analysis
Univariate analysis between infants with and without severe ROP were performed using the Mann-Whitney U test for continuous variables and a two-sided Pearson Chi-square for categorical variables. This analysis was also performed between the Early Transfusion group and those infants who were not transfused in the first 10 days of life.
Binary logistic regression was used to assess the association between transfusions and severe ROP and to adjust for the differences between patients who received early transfusions and those that did not. Important covariates used in the final model included known risk factors for ROP (EGA, birth weight, BPD) as well as illness severity characteristics that correlated with ROP on univariate analysis and increased the predictive power of the model containing only EGA, birth weight and BPD (postnatal steroid use, inotrope need, chorioamnionitis, PDA ligation). Early Transfusion, number of transfusions, and Late Transfusion were assessed with binary logistic regression using the aforementioned covariates entered in block in a single step. Collinearity was evaluated by assessing the variance inflation factor (VIF). Only variables with VIF < 3 were included in regression analysis to avoid multi-collinearity. Post-hoc sensitivity analysis was performed by replacing other day of life thresholds in the regression model and assessing odds ratio, confidence interval and Nagelkerke R2. Statistical analysis was performed using IBM SPSS Statistics Version 24.0 (IBM corporation, Armonk, NY).
Results
Patient Characteristics
A total of 1896 infants that met inclusion criteria were identified and 4464 packed red blood cell transfusions (pRBC) were given. In the overall cohort, 31% (1405/4464) occurred in the first 10 days of life, meeting the a priori definition of “early transfusion”, followed by a sharp drop of in transfusion rate over the following days (Figure 1). Only the 1636 infants with complete charts, including clinical outcomes and transfusion histories were included in the final analysis (Figure 2). Of the included patients; 1238 were inborn, 376 were outborn but immediately transferred after birth, and 22 were later transfers but had complete records available. The sample characteristics are described in Table 1. The mean (SD) gestational age was 28 (2.8) weeks; birthweight was 1172 (435) grams; and severe ROP incidence was 8% (126/1656).
Figure 1.
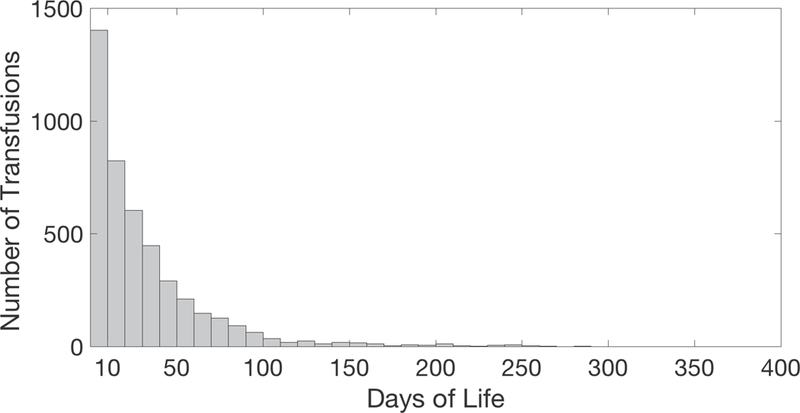
Histogram displaying the number of transfusions given on each respective postnatal day. Note that 31% of all transfusions occurred on or before postnatal day 10
Figure 2.

Patient flow char diagram
Table 1.
Sample Characteristics
| N= 1636 | |
|---|---|
| Gestational age at birth, mean ± SD, weeks | 28 ± 2.8 |
| Birth weight, mean ± SD, grams | 1172 ± 435 |
| Birth weight percentile, mean ± SD | 46 ± 28 |
| Intrauterine growth restriction, n (%) | 172 (11) |
| Male sex, n (%) | 866 (53) |
| African-American race, n (%) | 726 (44) |
| Antenatal steroids, n (%) | 1353 (83) |
| Apgar 5 min, mean ± SD | 6.1 ± 2.2 |
| Received inotropes, n (%) | 540 (33) |
| Chorioamnionitisa, n (%) | 419 (26) |
| PDA requiring surgery, n (%) | 156(10) |
| NECb, n (%) | 111 (7) |
| BPDc, n (%) | 512 (31) |
| Postnatal steroids, n (%) | 220 (13) |
| Culture positive sepsis, n (%) | 127(8) |
| Died prior to discharge, n (%) | 221 (13) |
| Early Transfusion, n (%) | 602(37) |
| Late Transfusion, n (%) | 249 (15) |
| Initial Hgb, mean ± SD | 14.2 ± 3.0 |
| Number of transfusions, median (interquartile range) | 1.0 (3) |
| Severe ROP | 126 (8) |
Footnotes:
Based on histologic examination of the placenta,
Defined as Bell Stage 2a or greater,
Defined as need for supplemental oxygen at 36 weeks PMA.
Statistical analysis
The results of univariate analysis are summarized in Tables 2 and 3. Comparing early transfused and control infants: infants who received early transfusions were of younger gestational age and birthweight and were more likely to be African-American males with higher rates of chorioamnionitis, postnatal steroid use, inotrope use, NEC, BPD and severe ROP. Infants who developed severe ROP were also more likely to have a lower gestational age and birth weight as well as more postnatal steroid use, inotrope use, chorioamnionitis, PDA ligations and BPD. They also had a higher rate of early transfusion and increased number of transfusions. There was a statistical but clinically insignificant difference in initial hemoglobin (14.3 vs 13.0 g/dL, p<0.001).
Table 2.
Comparison of Early Transfusion vs Control
| Control N =1034 |
Early Transfusion N= 602 |
p-Value | |
|---|---|---|---|
| Gestational age at birth, mean ± SD, weeks | 29.4 ± 2.2 | 25.8 ± 2.3 | <0.001 |
| Birth weight, mean ± SD, grams | 1336 ± 388 | 889 ± 360 | <0.001 |
| Birth weight percentile, mean ± SD | 46 ± 28 | 47 ± 28 | 0.222 |
| Intrauterine growth restriction, n (%) | 112 (11) | 60 (10) | 0.582 |
| Male sex, n (%) | 521 (50) | 345 (57) | 0.007 |
| African-American race, n (%) | 438 (42) | 288 (48) | 0.031 |
| Antenatal steroids, n (%) | 875 (85) | 478 (79) | 0.007 |
| Apgar 5 min, mean ± SD | 6.7 ± 1.9 | 5.0 ± 2.4 | <0.001 |
| Received inotropes, n (%) | 140 (14) | 400 (66) | <0.001 |
| Chorioamnionitisa, n (%) | 217 (21) | 202 (34) | <0.001 |
| PDA requiring surgery, n (%) | 28 (3) | 128 (21) | <0.001 |
| NECb, n (%) | 55 (5) | 56 (9) | 0.002 |
| BPDc, n (%) | 194 (18) | 318 (53) | <0.001 |
| Postnatal steroids, n (%) | 37 (4) | 183 (30) | <0.001 |
| Culture positive sepsis, n (%) | 43 (4) | 84 (14) | <0.001 |
| Died prior to discharge, n (%) | 59 (6) | 162 (27) | <0.001 |
| Severe ROP, n (%) | 11 (1) | 115 (19) | <0.001 |
| Initial Hgb, mean ± SD | 15.1 ± 2.8 | 12.6 ± 2.8 | <0.001 |
| Number of transfusions, median (interquartile range) | 0 (0) | 5 (6) | <0.001 |
| ROP by stage, n |
<0.001 |
||
| - Stage 0 | 515 | 125 | |
| - Stage 1 | 130 | 89 | |
| - Stage 2 | 44 | 91 | |
| - Stage 3 | 23 | 134 | |
| - Stage 4 | 0 | 6 | |
| - Stage 5 | 0 | 1 |
Footnotes:
Based on histologic examination of the placenta,
Defined as Bell Stage 2a or greater,
Defined as need for supplemental oxygen at 36 weeks PMA.
Table 3.
Comparison of Severe ROP Status
| No Severe ROP N =1510 |
Severe ROP N= 126 |
p-Value | |
|---|---|---|---|
| Gestational age at birth, mean ± SD, weeks | 28.3 ± 2.8 | 25 ± 1.6 | <0.001 |
| Birth weight, mean ± SD, grams | 1207 ± 432 | 754 ± 194 | <0.001 |
| Birth weight percentile, mean ± SD | 46 ± 28 | 44 ± 26 | 0.301 |
| Intrauterine growth restriction, n (%) | 159 (11) | 13 (10) | 0.94 |
| Male sex, n (%) | 795 (53) | 71 (56) | 0.424 |
| African-American race, n (%) | 671 (44) | 55 (44) | 0.864 |
| Antenatal steroids, n (%) | 1249 (83) | 104 (83) | .960 |
| Apgar 5 min, mean ± SD | 6.2 ± 2.2 | 4.9 ± 2.4 | <0.001 |
| Received inotropes, n (%) | 447 (30) | 93 (74) | <0.001 |
| Chorioamnionitisa, n (%) | 375 (25) | 44 (34) | 0.013 |
| PDA requiring surgery, n (%) | 109 (7) | 47 (37) | <0.001 |
| NECb, n (%) | 99 (7) | 12 (10) | 0.203 |
| BPDc, n (%) | 409 (27) | 103 (82) | <0.001 |
| Postnatal steroids, n (%) | 142 (9) | 78 (62) | <0.001 |
| Culture positive sepsis, n (%) | 104 (7) | 23 (18) | <0.001 |
| Died prior to discharge, n (%) | 214 (14) | 7 (6) | 0.007 |
| Early Transfusion, n (%) | 487 (32) | 115 (91) | <0.001 |
| Late Transfusion, n (%) | 241 (16) | 8 (6) | 0.003 |
| Initial Hgb, mean ± SD | 14.3 ± 3.0 | 13.0 ± 2.8 | <0.001 |
| Number of transfusion, median (interquartile range) | 0 (3) | 8 (6) | <0.001 |
Footnotes:
Based on histologic examination of the placenta,
Defined as Bell Stage 2a or greater,
Defined as need for supplemental oxygen at 36 weeks PMA.
One hundred fifteen out of 602 Early Transfused infants developed severe ROP compared to 11 out of 1034 remaining infants (Odds Ratio 22.0, 95% CI: 11.7-41.1, p<0.001). To adjust for clinical illness, a binary regression model (R-squared = .419, p<0.001) was created which included known risk factors for ROP and clinical factors of illness severity (EGA, BPD, birth weight, postnatal steroid use, inotrope need, chorioamnionitis, PDA ligation) that were closely linked to severe ROP. Apgar at 5 minutes and culture positive sepsis were also correlated with severe ROP with p-values <0.001 but they did not increase the R-squared when added to the regression model and were not included in the final model. Tables 4,5 and 6 describe regression models for Early Transfusion, Late transfusion and total number of transfusions while maintaining the same covariates. The adjusted odds ratio for Early Transfusion was 3.84 (95% CI: 1.819-8.121). The total number of transfusions was also associated with severe ROP when using the same regression model (R-squared = .415, p<0.001) with an adjusted odds ratio of 1.09 per transfusion (95%CI: 1.041-1.148). Late Transfusion (R-squared = .404, p<0.001) was not associated with the development of severe ROP (adjusted odds ratio 0.539 95%CI: 0.244-1.187). Sensitivity analysis is displayed in Table 7. The day of life 10 threshold improved the overall fit of the model with the highest R-squared of .419.
Table 4.
Logistic regression model for early transfusion and severe ROP.
| 95% C.I.for EXP(B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | Lower | Upper | |
| Early transfusion | 1.346 | .382 | 12.444 | 1 | .000 | 3.843 | 1.819 | 8.121 |
| EGA | −.196 | .088 | 4.933 | 1 | .026 | .822 | .692 | .977 |
| Birth weight | −.001 | .001 | 1.636 | 1 | .201 | .999 | .998 | 1.000 |
| BPDa | 1.166 | .281 | 17.178 | 1 | .000 | 3.209 | 1.849 | 5.571 |
| Postnatal steroids | 1.080 | .243 | 19.804 | 1 | .000 | 2.943 | 1.830 | 4.735 |
| PDA ligation | .443 | .249 | 3.165 | 1 | .075 | 1.557 | .956 | 2.536 |
| Chorioamnionitisb | −.284 | .239 | 1.411 | 1 | .235 | .753 | .472 | 1.202 |
| Inotrope use | .190 | .262 | .526 | 1 | .468 | 1.209 | .724 | 2.019 |
| Constant | 1.441 | 2.105 | .469 | 1 | .494 | 4.224 | ||
Nagelkerke R Square .419, P<0.001.
Defined as need for supplemental oxygen at 36 weeks postmenstrual age,
based on histologic examination of placenta.
Table 5.
Logistic regression model for transfusion number and severe ROP.
| 95% C.I.for EXP(B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | Lower | Upper | |
| Transfusion number | .089 | .025 | 12.727 | 1 | .000 | 1.093 | 1.041 | 1.148 |
| EGA | −.259 | .085 | 9.250 | 1 | .002 | .772 | .653 | .912 |
| Birth weight | −.001 | .001 | 1.604 | 1 | .205 | .999 | .998 | 1.000 |
| BPDa | 1.094 | .283 | 14.969 | 1 | .000 | 2.985 | 1.715 | 5.195 |
| Postnatal steroids | .986 | .249 | 15.705 | 1 | .000 | 2.680 | 1.646 | 4.365 |
| PDA ligation | .363 | .254 | 2.034 | 1 | .154 | 1.437 | .873 | 2.365 |
| Chorioamnionitisb | −.322 | .242 | 1.769 | 1 | .184 | .724 | .451 | 1.165 |
| Inotrope use | .095 | .278 | .118 | 1 | .731 | 1.100 | .638 | 1.895 |
| Constant | 3.746 | 1.927 | 3.780 | 1 | .052 | 42.366 | ||
Nagelkerke R Square .415, P<0.001.
Defined as need for supplemental oxygen at 36 weeks postmenstrual age,
based on histologic examination of placenta.
Table 6.
Logistic regression model for late transfusion and severe ROP.
| 95% C.I.for EXP(B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | Lower | Upper | |
| Late transfusion | −.619 | .403 | 2.356 | 1 | .125 | .539 | .244 | 1.187 |
| EGA | −.256 | .087 | 8.639 | 1 | .003 | .774 | .653 | .918 |
| Birth weight | −.001 | .001 | 2.929 | 1 | .087 | .999 | .998 | 1.000 |
| BPDa | 1.270 | .281 | 20.354 | 1 | .000 | 3.561 | 2.051 | 6.182 |
| Postnatal steroids | 1.133 | .243 | 21.780 | 1 | .000 | 3.106 | 1.930 | 5.000 |
| PDA | .470 | .251 | 3.520 | 1 | .061 | 1.600 | .979 | 2.614 |
| Chorioamnionitisb | −.297 | .239 | 1.548 | 1 | .213 | .743 | .465 | 1.187 |
| Inotrope use | .373 | .260 | 2.070 | 1 | .150 | 1.453 | .874 | 2.416 |
| Constant | 4.135 | 1.966 | 4.423 | 1 | .035 | 62.518 | ||
Nagelkerke R Square .404, P<0.001.
Defined as need for supplemental oxygen at 36 weeks postmenstrual age,
based on histologic examination of placenta.
Table 7.
Sensitivity analysis of regression model using same covariates as in Table 4, replacing Early Transfusion with thresholds at 7, 10, 14, 21 and at any point during their stay.
| Early Transfusion Threshold in DOL |
Odds ratio | 95% C.I. | Nagelkerke R2 |
p-Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| 7 | 2.073 | 1.133 | 3.792 | .408 | 0.018 |
| 10 | 3.843 | 1.819 | 8.121 | .419 | <0.001 |
| 14 | 2.863 | 1.297 | 6.321 | .410 | 0.009 |
| 21 | 4.698 | 1.707 | 12.924 | .415 | 0.003 |
| Any transfusion | 5.137 | 1.491 | 17.693 | .413 | 0.009 |
Discussion
In this large retrospective cohort, early pRBC transfusions were strongly correlated with the development of severe ROP even when controlling for gestational age, BPD and clinical illness.
Blood transfusions have been implicated with ROP but studies have been limited by the difficulty of studying the timing and number of transfusions in large numbers of infants, and the difficulty in defining clinically significant ROP. ROP of at least stage 1 is common during a preterm infant’s course and even higher stage ROP will often regress spontaneously. In addition, not all stage 3 ROP will require treatment as the extent of disease, velocity of progression and presence of plus disease will impact treatment decisions.(10, 14) This makes the correlation to any-stage specific ROP difficult to interpret. Hence, a functional definition of severe ROP is useful. Slidsborg et al. used a large retrospective cohort of Danish national birth registries to demonstrate a strong link between transfusion and need for treatment of ROP.(9) This study was limited by inclusion of infants born prior to 2008 and limited investigation into the timing or number of transfusions. Similarly, Akkoyun et al did find that the volume of blood transfusions received was linked to the severity of ROP but only performed limited regression analysis and did not investigate the timing of transfusions.(15) Dani et al demonstrated that transfusion in the first 7 days and at 2 months were independently linked to ROP but no patients in their study had severe enough ROP to warrant treatment or developed blindness.(16) We built upon these prior studies by evaluating the effect of early versus late transfusions in a modern cohort, using a pragmatic clinical definition of severe ROP and controlling for clinical illness severity. In addition, being at a single center had the advantage of having the same ophthalmologists conducting ROP screens with a consistent approach to treatment thresholds for ROP.
Prospective trials assessing transfusions and ROP have shown results conflicting with retrospective analysis. A prospective trial comparing a strict transfusion protocol to a more liberalized protocol failed to show a difference in the severity of ROP among the study groups. This study was performed after day of life 29 and may have missed the window of ROP vulnerability.(17) Two other randomized trials comparing high and low hematocrit targets failed to show a difference in ROP.(18, 19) These trials had significant number of protocol violations leading to a loss of differential transfusion exposure, underscoring the difficulty in conducting randomized trials to assess the association of early, potentially life-saving interventions and long term negative outcomes. In addition, these studies were powered to detect differences in a composite outcome or simply transfusion number. Often it requires large observational studies to identify a link between an exposure and a distant outcome.
In the premature retina, vessel growth that would occur in utero slows or ceases after birth and exposure to the relatively hyperoxic environment. As the infant matures and retinal metabolism increases, the local tissue hypoxia leads to abnormal vessel growth and the development of retinopathy.(1) We hypothesize that the association between transfusions and ROP could be related in part to the rapid increase in adult hemoglobin (HbA) following a 15ml/kg pRBC transfusion resulting in a shift of the oxygen disassociation curve to the right and increased retinal tissue oxygen delivery at a time when it is susceptible to hyperoxia. In fact, a study by Stutchfield et al lends credence to this hypothesis by showing that infants with lower percent of fetal hemoglobin (HbF) were more prone to development of higher stage ROP.(20) There is also evidence to suggest that early transfusions in preterm infants drastically changes the HbF percentage and shifts the hemoglobin oxygen dissociation curve to the right.(21) In our study, Late Transfused infants were not at higher risk of severe ROP, which is consistent with the biphasic nature of the disease and the physiologic increase in HbA. Limiting early transfusions may be possible by instituting delayed cord clamping, conscientiously limiting phlebotomy and adopting restrictive early transfusion guidelines.(22, 23) While we chose to highlight 10 days of life as a cut-off and it had the biggest impact on retinopathy in our cohort, being transfused at any time was associated with an increased risk of severe ROP and the exact window of highest risk has yet to be identified.
This study benefited from a large sample size, and with nearly all of the infants treated in one institution, we benefited from a consistent clinical practice model and screening and treatment program for ROP. While transfusions are most often used to improve hemoglobin/hematocrit and oxygen carrying capacity in a critically sick neonate, an additional consideration is that transfusions are sometimes used as means of volume expansion. However, several studies suggest that in the critically ill neonate, the extent of hypovolemia may be overestimated and that pRBC transfusions do not have benefit over other strategies to maintain perfusion.(24-28) In order to limit the transfusion exposure when premature infants are most at risk, a combination of limiting blood loss through phlebotomy and utilizing alternative methods (i.e. crystalloid expansion or earlier initiation of dopamine) to control hypotension could be considered.
Limitations
One of the limitations is the retrospective nature of our study, making it possible that the transfusions themselves are not risk factors for ROP, but merely indicators of some other unstudied phenomenon. We attempted to correct for this by collecting numerous indicators of clinical illness and other outcome data. The patients that received early transfusions were indeed sicker and younger overall. However, the sample size allows reliable regression modeling to control for the level of illness severity. Unfortunately, our study design did not allow for accurately capturing supplemental oxygen administration or FiO2 measurements in sufficient detail to address in our analysis. In fact, even accurately deducing the number and timing of ventilator days is nearly impossible with such a large retrospective cohort. To overcome this limitation, we used BPD diagnosis as a proxy for oxygen exposure and severity of lung disease.
This study spanned 8 years of patient care, and there may have been variations in clinical practice. However, our transfusion practice guidelines and rate of severe ROP have remained consistent over the course of this study. We were also unable to address the indications for transfusion or the immediate pre and post transfusion hematocrit. Future studies will be needed to investigate if the anemia itself accounts for or potentiates the effect of transfusions as has been suggested in NEC.(29) Future studies should also consider the elapsed time between collection and transfusion of the red cells. Although there is some evidence to suggest that older blood is associated with worse outcomes, generally neonates are transfused with fresher blood from as few donors as possible.(30, 31)
It is important to note that our cohort consists of infants born before we adopted universal delayed cord clamping for infants born < 32 weeks EGA. It is possible that the emergence of delayed cord clamping will lead to higher initial HbF percent and less early transfusions overall or attenuate the effect of transfusions, thus decreasing the risk of severe ROP. Decreasing the use of early pRBC transfusions may potentially decrease the development of severe ROP.
Conclusions
Packed red blood cell transfusions in the first 10 days of life are associated with an almost four-fold increased risk of severe retinopathy of prematurity. This relationship holds true even when correcting for gestational age, lung disease and clinical illness. Each individual transfusion increased the risk of severe ROP by 9% but later transfusions did not carry the same risk. Future studies are warranted to evaluate the impact of delayed cord clamping and restrictive transfusion policies on rates of ROP.
Acknowledgments
Funding Source: This work was supported by the following grants:
1. Washington University Institute of Clinical and Translational Sciences KL2 Training Program (NIH/NCATS KL2 TR000450 [to ZV])
2. The Barnes-Jewish Hospital Foundation and the Washington University Institute of Clinical and Translational Sciences Clinical and Translational Funding Program (NIH/NCATS UL1 TR000448)
3. Washington University in St. Louis Center for Biomedical Informatics, Clinical Investigation Data Exploration Repository (NIH/NCATS UL1 TR000448)
Abbreviations:
- BPD
bronchopulmonary dysplasia
- DOL
days of life
- EGA
estimated gestational age
- HbF
fetal hemoglobin
- PDA
patent ductus arteriosus
- pRBC
packed red blood cell
- ROP
retinopathy of prematurity
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10(2):133–40. [DOI] [PubMed] [Google Scholar]
- 2.Sears JE, Pietz J, Sonnie C, Dolcini D, Hoppe G. A change in oxygen supplementation can decrease the incidence of retinopathy of prematurity. Ophthalmology. 2009;116(3):513–8. [DOI] [PubMed] [Google Scholar]
- 3.Saugstad OD, Sejersted Y, Solberg R, Wollen EJ, Bjoras M. Oxygenation of the newborn: a molecular approach. Neonatology. 2012;101(4):315–25. [DOI] [PubMed] [Google Scholar]
- 4.Lucey JF, Dangman B. A reexamination of the role of oxygen in retrolental fibroplasia. Pediatrics. 1984;73(1):82–96. [PubMed] [Google Scholar]
- 5.Gunn TR, Easdown J, Outerbridge EW, Aranda JV. Risk factors in retrolental fibroplasia. Pediatrics. 1980;65(6):1096–100. [PubMed] [Google Scholar]
- 6.Sacks LM, Schaffer DB, Anday EK, Peckham GJ, Delivoria-Papadopoulos M. Retrolental fibroplasia and blood transfusion in very low-birth-weight infants. Pediatrics. 1981;68(6):770–4. [PubMed] [Google Scholar]
- 7.Shohat M, Reisner SH, Krikler R, Nissenkorn I, Yassur Y, Ben-Sira I. Retinopathy of prematurity: incidence and risk factors. Pediatrics. 1983;72(2):159–63. [PubMed] [Google Scholar]
- 8.Seiberth V, Linderkamp O. Risk factors in retinopathy of prematurity. a multivariate statistical analysis. Ophthalmologica. 2000;214(2):131–5. [DOI] [PubMed] [Google Scholar]
- 9.Slidsborg C, Jensen A, Forman JL, Rasmussen S, Bangsgaard R, Fledelius HC, et al. Neonatal Risk Factors for Treatment-Demanding Retinopathy of Prematurity: A Danish National Study. Ophthalmology. 2016;123(4):796–803. [DOI] [PubMed] [Google Scholar]
- 10.Good WV, Early Treatment for Retinopathy of Prematurity Cooperative G. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004;102:233–48; discussion 48–50. [PMC free article] [PubMed] [Google Scholar]
- 11.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82(4):527–32. [PubMed] [Google Scholar]
- 12.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fierson WM, American Academy of Pediatrics Section on O, American Academy of O, American Association for Pediatric O, Strabismus, American Association of Certified O. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189–95. [DOI] [PubMed] [Google Scholar]
- 14.International Committee for the Classification of Retinopathy of P. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991–9. [DOI] [PubMed] [Google Scholar]
- 15.Akkoyun I, Oto S, Yilmaz G, Gurakan B, Tarcan A, Anuk D, et al. Risk factors in the development of mild and severe retinopathy of prematurity. J AAPOS. 2006;10(5):449–53. [DOI] [PubMed] [Google Scholar]
- 16.Dani C, Reali MF, Bertini G, Martelli E, Pezzati M, Rubaltelli FF. The role of blood transfusions and iron intake on retinopathy of prematurity. Early Hum Dev. 2001;62(1):57–63. [DOI] [PubMed] [Google Scholar]
- 17.Brooks SE, Marcus DM, Gillis D, Pirie E, Johnson MH, Bhatia J. The effect of blood transfusion protocol on retinopathy of prematurity: A prospective, randomized study. Pediatrics. 1999;104(3 Pt 1):514–8. [DOI] [PubMed] [Google Scholar]
- 18.Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301–7. [DOI] [PubMed] [Google Scholar]
- 19.Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115(6):1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stutchfield CJ, Jain A, Odd D, Williams C, Markham R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: a pilot prospective cohort study. Eye (Lond). 2017;31(10):1451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Halleux V, Truttmann A, Gagnon C, Bard H. The effect of blood transfusion on the hemoglobin oxygen dissociation curve of very early preterm infants during the first week of life. Semin Perinatol. 2002;26(6):411–5. [DOI] [PubMed] [Google Scholar]
- 22.Venancio JP, Santos AM, Guinsburg R, Peres Cde A, Shinzato AR, Lora MI. Strict guideline reduces the need for RBC transfusions in premature infants. J Trop Pediatr. 2007;53(2):78–82. [DOI] [PubMed] [Google Scholar]
- 23.Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005;115(5):1299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill AB, Weindling AM. Randomised controlled trial of plasma protein fraction versus dopamine in hypotensive very low birthweight infants. Arch Dis Child. 1993;69(3 Spec No):284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeschke MG, Chinkes DL, Finnerty CC, Przkora R, Pereira CT, Herndon DN. Blood transfusions are associated with increased risk for development of sepsis in severely burned pediatric patients. Crit Care Med. 2007;35(2):579–83. [DOI] [PubMed] [Google Scholar]
- 26.Lacroix J, Hebert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–19. [DOI] [PubMed] [Google Scholar]
- 27.Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care (Reprint). Pediatrics. 2015;136 Suppl 2:S196–218. [DOI] [PubMed] [Google Scholar]
- 28.Wyckoff MH, Perlman JM, Laptook AR. Use of volume expansion during delivery room resuscitation in near-term and term infants. Pediatrics. 2005;115(4):950–5. [DOI] [PubMed] [Google Scholar]
- 29.Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants. JAMA. 2016;315(9):889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girelli G, Antoncecchi S, Casadei AM, Del Vecchio A, Isernia P, Motta M, et al. Recommendations for transfusion therapy in neonatology. Blood Transfus. 2015;13(3):484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52(6):1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


