Abstract
The BCRP/ABCG2 efflux transporter is expressed on the membrane of placental syncytiotrophoblasts and protects the fetus from toxicant exposure. Syncytiotrophoblasts arise from the fusion of cytotrophoblasts, a process negatively regulated by the endocannabinoid, anandamide (AEA). It is unknown whether AEA can influence fetal concentrations of xenobiotics by modulating the expression of transporters in syncytiotrophoblasts. Here, we sought to characterize and identify the mechanism(s) responsible for AEA-mediated down-regulation of the BCRP transporter in human placental explants and BeWo trophoblasts. Treatment of human placental explants with AEA (1 μM, 24 h) reduced hCGα, syncytin-1, and BCRP mRNAs by ~30%. Similarly, treatment of BeWo trophoblasts with AEA (0-10 μM, 3-24 h) coordinately down-regulated mRNAs for hCGβ, syncytin-2, and BCRP. In turn, AEA increased the sensitivity of trophoblasts to the cytotoxicity of mitoxantrone, a known BCRP substrate, and environmental and dietary contaminants including mycoestrogens and perfluorinated chemicals. AEA-treated trophoblasts also demonstrated reduced BCRP transport of the mycoestrogen zearalenone and the diabetes drug glyburide, labeled with BODIPY. The AEA-mediated reduction of BCRP mRNA was abrogated when placental cells were co-treated with AM630, a CB2 receptor inhibitor, or 8-Br-cAMP, a cAMP analog. AEA reduced intracellular cAMP levels in trophoblasts by 75% at 1 hr, and completely inhibited forskolin-induced phosphorylation of the cAMP response element binding protein (CREB). AEA also decreased p-CREB binding to the BCRP promoter. Taken together, our data indicate that AEA down-regulates placental transporter expression and activity via CB2-cAMP signaling. This novel mechanism may explain the repression of placental BCRP expression observed during diseases of pregnancy.
Keywords: Endocannabinoid, BCRP, placenta, transporter, ABCG2, anandamide, cAMP
1. Introduction
Fetal exposure to environmental toxicants can result in developmental disorders, congenital malformations, and preterm birth (reviewed in Gluckman and Hanson, 2004). The placenta aids in protecting the fetus from these exposures by utilizing an array of efflux xenobiotic transporters that prevent the transplacental trafficking of toxicants. The breast cancer resistance protein (BCRP/ABCG2) is one such efflux transporter that, in the placenta, limits exposure of the fetus to certain toxicants (reviewed in Mao, 2008). BCRP was simultaneously discovered by three groups, one of which identified it in the placenta and originally named it the placental ABC transporter (ABCP) (Allikmets et al., 1998). In the placenta, BCRP is highly expressed on the apical surface of syncytiotrophoblasts where it plays a key role protecting the fetus from exposure to xenobiotics present in the maternal circulation (Hahnova-Cygalova et al., 2011). It is well known that BCRP transports a wide array of toxicants, including zearalenone, an estrogenic mycotoxin commonly found on cereal crops, glyburide, a prescription drug used for gestational diabetes, as well as the synthetic fluorosurfactant, perfluorooctanoic acid (PFOA) (Dankers et al., 2013; Gedeon et al., 2008; Jonker et al., 2002; Xiao et al., 2015).
Placental BCRP expression is down-regulated in a number of gestational diseases including preeclampsia, fetal growth restriction, and HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome (Evseenko et al., 2007; Gormley et al., 2017; Jebbink et al., 2015; Mason et al., 2011; Nishizawa et al., 2011). Clinically, disruption of BCRP activity has the potential to increase fetal exposure to toxicants (reviewed in Mao, 2008). While the exact mechanism(s) responsible for altering placental BCRP expression in gestational disorders are unknown, multiple regulatory factors have been shown to modulate BCRP levels in trophoblasts, including hormones, hypoxia, prostaglandins, membrane cholesterol, and dietary constituents (Bircsak et al., 2016; Francois et al., 2017; Mason et al., 2014; Szilagyi et al., 2017; Wang et al., 2008; Wang et al., 2006).
One approach to identifying critical mediators involved in placental BCRP dysregulation is to interrogate pathways known to be disrupted during pregnancy disorders. The endocannabinoid system has an established role in regulating appetite, memory, pain, and mood. More recently, however, the endocannabinoid system was identified as a regulator of placentation and has therefore been suggested as a potential therapeutic target for diseases of pregnancy such as preeclampsia (reviewed in Costa, 2016). The endocannabinoid receptors, cannabinoid receptors 1 and 2 (CB1 and CB2), as well as the catabolic enzyme fatty acid amide hydrolase (FAAH) are expressed in cytotrophoblasts, syncytiotrophoblasts, and the mesenchymal core throughout gestation (Habayeb et al., 2008; Park et al., 2003; Trabucco et al., 2009). The signaling molecule anandamide (AEA), an endogenous ligand for CB1 and CB2, is produced by the endometrium throughout pregnancy (Liu et al., 2002). In general, biochemical responses elicited by endocannabinoids exhibit biphasic responses; high or low levels of AEA can be harmful to placental development and function (Cella et al., 2008; Liu, et al., 2002; Wang et al., 1999; Xie et al., 2012). Assessment of placentas from preeclamptic women, for instance, has shown increased CB1 and decreased FAAH protein expression, both of which enhance AEA signaling (Aban et al., 2013; Fugedi et al., 2014). Key roles for AEA have been shown during trophoblast syncytialization, invasion, and apoptosis (Costa, 2016). Likewise, fetal endothelial vascular tone and nitric oxide synthase activity are modulated by AEA signaling (Cella, et al., 2008). However, the precise role of endocannabinoid dysregulation in the etiology of gestational diseases has yet to be clarified.
The function and localization of BCRP within the placenta provides protection of the fetus from exposure to toxicants. It is therefore critical to understand the underlying cellular mechanisms that drive BCRP expression. Recognizing that BCRP expression is down-regulated in gestational disorders associated with perturbed endocannabinoid signaling, we hypothesized that AEA would decrease the placental expression and activity of BCRP. Additional studies were designed to characterize the underlying molecular mechanism(s) involved.
2. Materials and Methods
2.1. Chemicals
Unless stated otherwise, all chemicals were from Sigma-Aldrich (St. Louis, MO), and all chemicals and supplies were obtained from within the USA.
2.2. Patient Selection, Tissue Processing and Placental Explant Culture
Placentas were obtained from healthy women between the ages of 18–45 years following term delivery by scheduled Cesarean section (Supplemental Table 1). Placentas from patients with pregnancy complications such as diabetes, preeclampsia, infection, hypertension, autoimmune disease, and fetal growth restriction were excluded. The study was approved by the Institutional Review Board of Rutgers University Robert Wood Johnson Medical School (protocol #20150001445).
Within 2 h of delivery, placentas were collected and processed. Villous tissue to be used for immunohistochemical staining was prepared using the PAXgene Tissue System (Qiagen, Germany), embedded in paraffin, and sectioned. Explants were collected and cultured as previously described (Bircsak, et al., 2016). Placentas were examined for discernible irregularities. The maternal decidua and the chorionic plate along with the overlying membranes were removed, and sections of villous tissue from the central maternal surface were collected. Sections were then washed in sterile PBS to remove maternal blood before dissection into individual 8 mm3 pieces. In a 24-well tissue culture plate, explants were cultured and maintained in a humidified incubator at 37°C and 5% CO2/95% air environment in 1 mL of a phenol red-free DMEM and F12 1:1 mixture (Life Technologies, Carlsbad, CA), with 10% charcoal-stripped, dextran-treated fetal bovine serum (Atlanta Biologicals, Miami, FL) and 1% penicillin-streptomycin (Life Technologies). Cell culture medium was replaced every 24 h. AEA was dissolved in ethanol with a final ethanol concentration of 0.05% (v/v) in treatment media. Circulatory AEA has a short half-life in vivo (< 5 min), and homogenates of rat placenta have been shown to hydrolyze AEA (~200-500 pmol/min/mg protein) (Fonseca et al., 2014; Willoughby et al., 1997). Due to the short half-life of AEA, explant cultures that were incubated with AEA (0.1-10 μM) for a total of 24 h, were replenished with fresh AEA once after 12 hrs. Following the 24-hour treatment, explants were removed from culture plates, washed in PBS, snap frozen, and stored at −80°C until analysis.
2.3. BeWo Cell Culture
BeWo human choriocarcinoma cells (B30 subclone) were generously provided by Dr. Nicholas lllsley (Hackensack University Medical Center, Hackensack, NJ) and maintained at 37°C in a 5% CO2/95% air humidified environment in a DMEM and F12 1:1 mixture (Life Technologies, Carlsbad, CA), supplemented with 10% fetal bovine serum (Atlantic Biologicals) and 1% penicillin-streptomycin (Life Technologies) (Baumann et al., 2014). This cell line recapitulates features of trophoblasts because of their ability to undergo syncytialization, secrete human choriogonadotropin (hCG), and express transporters such as BCRP (Ceckova et al., 2006; Kudo et al., 2003; Pattillo and Gey, 1968). For all experiments, cells (between passages 24 and 45) were grown to 70-80% confluence before treatment with AEA in combination with rimonabant (100 nM), a CB1 inverse agonist, AM630 (100 nM), a CB2 antagonist, forskolin (50 μM), a stimulator of adenylate cyclase, or 8-Br-cAMP (1 mM), a stable, cell-permeable cAMP analog. AEA was dissolved in ethanol, and all other chemicals were dissolved in DMSO prior to use. The final concentration of solvent in culture medium did not exceed 0.1% (v/v). Treatment medium differed from culture medium which consisted of phenol red free DMEM:F12 (Life Technologies) supplemented with 10% charcoal-stripped, dextran-treated fetal bovine serum (Atlanta Biologicals) and 1% penicillin-streptomycin (Life Technologies).
2.4. Western Blotting
Explants were thawed on ice and extracted in 200 μL of homogenization buffer (250 mM sucrose, 10 mM Tris-HCI buffer (pH 7.4)) containing protease inhibitor cocktail (Sigma P8340, 1%, v/v), and lysed using a bead homogenizer (Tissue-Lyser LT, Qiagen) for 3 min at 40 Hz. BeWo cell lysates for Western blotting were collected in buffer containing 20 mM Tris-HCI, 150 mM NaCI, 5 mM ethylenediaminetetraacetic acid, 1 % Triton X-100 and the protease inhibitor cocktail. Lysates were centrifuged at 1000 × g for 10 min to remove cellular debris before use. Unless otherwise stated, all Western blotting was performed using equipment from BioRad as previously described (Zheng et al., 2013). Proteins were resolved through Tris-HCI polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were incubated overnight in primary antibodies used to detect BCRP (BXP-53, 1:5000; Enzo Life Sciences, Farmingdale, NY), β-Actin (ab8227, 1:2000, Abeam, Cambridge, MA), CREB (9197, 1:1000, Cell Signaling Technologies, Danvers, MA), p-CREB (9198, 1:1000 Cell Signaling Technologies), CB1 (10006590, 1:1000, Cayman Chemical, Ann Arbor, Ml), or CB2 (10006590, 1:1000, Cayman Chemical) followed by incubation with either HRP-linked rabbit or HRP-linked rat secondary antibodies (1:1000, 2 h, Cell Signaling Technologies). After the addition of a Luminata Forte Western HRP substrate (Millipore, Billerica, MA), chemiluminescent protein-antibody complexes were visualized using a Fluorchem Imager (ProteinSimple, Santa Clara, CA). Semi-quantitative analysis of protein band intensity was performed using AlphaView Software (ProteinSimple).
2.5. RNA Isolation and Analysis Using Quantitative Polymerase Chain Reaction (qPCR)
Explants to be used for mRNA expression analysis were thawed on ice and placed in 500 μL of RNAzol and homogenized using a bead homogenizer (Tissue-Lyser LT, Qiagen) for 4 min at 40 Hz. BeWo cells were scraped into RNAzol and homogenized. Nuclease-free water (200 μL) was added to lysates, which were then centrifuged at 13,000 × g for 15 min to precipitate DNA, lipids, and proteins. RNA was precipitated from the supernatant by adding 500 μL isopropanol and centrifuging at 13,000 × g for 15 min. The resulting pellet was washed twice with 500 μL ethanol, allowed to dry, and resuspended in 50 μL nuclease-free water. RNA content and purity were determined by measuring absorbance at 260 and 280 nm using a NanoDrop Spectrophotometer (Fisher Scientific). RNA was converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher) and a MultiGene OptiMax Thermal Cycler (Labnet International Inc., Edison, NJ). RNA (1000 ng) was combined with RT buffer, RT random primers, 4 mM dNTP mix, 2.5 U MultiScribe Reverse transcriptase, and 20 U RNase inhibitor in nuclease free water to a final volume of 20 μL. Samples were incubated in the thermocycler using the following steps: 10 min at 25°C, 120 min at 37°C, 5 min at 85°C, and held at 4°C until samples were removed. Quantitative PCR was performed with specific forward and reverse primers (see Supplemental Table 2, Integrated DNA Technologies, Inc., Coralville, IA), cDNA, Sybr Green dye (Life Technologies), and a ViiA7 RT-PCR System (Life Technologies). Ct values were converted to ΔΔCt values by comparison with ribosomal protein 13A (RPL13A) as the reference gene and normalized to the control group for each respective experiment.
2.6. Cell Viability Assay
Cell viability was assessed as an indirect approach to determine the effect of AEA on BCRP efflux activity. BeWo cells were seeded at 10,000 cells/well in 96-well plates and allowed to adhere overnight. BeWo cells were incubated for 24 h with either AEA (10 μM) or Ko143 (1 μM) in combination with mitoxantrone (0.6-600 μM), zearalenone (0.5-500 μM), α-zearalanol (0.5-500 μM), perfluorooctanoic acid (2-2000 μM), or perfluorooctanesulfonic acid (2-2000 μM). At the end of treatment, cell viability was assessed using the Alamar Blue assay (Invitrogen) (Page et al., 1993). Cells were incubated in 100 μι HBSS containing Alamar Blue (0.1 mg/mL resazurin) for 4 h. Fluorescence was then measured using Ex/Em: 535/580 nm. These values were normalized to the vehicle control group and used to calculate LC50 values for each substrate in the presence of AEA, Ko143, or vehicle control.
2.7. Hoechst 33342 Transport
For Hoechst 33342 transport studies, BeWo cells were incubated in the presence or absence of AEA (10 μM) and 8-Br-cAMP (1 mM) for 24 h, detached from the culture plates with trypsin and plated in a 96-well round bottom plates at 100,000 cells/well. Plates were centrifuged (500 × g, 5 min, 5°C) to remove media and cells were washed using ice cold HBSS. Cells were re-suspended in 100 μL of treatment medium containing Hoechst 33342 (5 μM), an established fluorescent BCRP substrate, in the presence or absence of the BCRP-specific inhibitor, Ko143 (1 μM) (Bircsak et al, 2013). Plates were incubated at 37°C for 30 min for uptake of Hoechst 33342. Cells were then centrifuged and washed with ice cold HBSS. At this point, cells used to measure relative fluorescence units (RFU)UPTAKE were resuspended in 50 μL HBSS and set aside on ice for analysis. The remaining cells were re-suspended in Hoechst 33342-free treatment medium with or without Ko143 and incubated for 1 h at 37°C during which Hoechst 33342 was actively transported from cells. Cells were then washed and re-suspended in 50 μL HBSS and placed on ice for measurement of RFUEFFLUX. Quantification of intracellular fluorescence was performed using a Cellometer Vision automated cell counter (Nexcelom Bioscience, Lawrence, MA) fitted with a VB-450-302 filter (excitation/emission = 375/450 nm). The total number of cells analyzed for each sample ranged from 100 to 1000. Fluorescence was normalized to cell size. Percent efflux was calculated using: %efflux = ((RFUUPTAKE − RFUEFFLUX)/(RFUUPTAKE))*100%.
2.8. Zearalenone Transport and HPLC Measurement
After experimental treatments, BeWo cells were detached from culture plates with trypsin, washed, resuspended in medium, and added to 2 × 96-well round bottom plates at 200,000 cells/well. Plates were centrifuged (500 × g, 5 min, 5°C) to remove media and cells were washed using cold HBSS. Cells were then re-suspended in 100 μL of growth medium containing zearalenone (50 μM) in the presence or absence of the BCRP-specific inhibitor, Ko143 (1 μM) (Bircsak, et al., 2013). Plates were incubated at 37°C for 60 min. Plates were centrifuged and washed with ice cold HBSS. One plate was re-suspended with 50 μL HBSS per well of cells and set on ice. The remaining plate of cells was re-suspended in substrate-free growth medium with or without Ko143 and incubated an additional 30 min at 37°C. The cells were then washed and re-suspended in 50 μL HBSS. For both plates, cells from 3 wells were pooled and combined with ethanol (50% final concentration). Cells were sheared through a 26-gauge needle 10× and then incubated for 1 h at −20°C. Samples were centrifuged at 1000 × g for 10 min and the resulting supernatants were analyzed forzearalenone content. Zearalenone was quantified by HPLC using a method adapted from a previous publication (De Baere et al., 2012). A Jasco (Easton, MD) HPLC system equipped with a PU-4185 binary pump, UV 4075 detector (254 nm), AS-2055 autosampler, Zorbax Eclipse 3 mm × 15 cm C18 column (Agilent, Santa Clara, CA), and Spectra-Physics (Santa Clara, CA) FL2000 fluorescent detector (Ex/Em: 275/440) was used. Peak areas were quantified using ChromNav V2 and compared to a standard curve. Zearalenone (ZLN) values were normalized to protein concentration before being used to calculate %efflux using: %efflux = ((ZLNUPTAKE − ZLNEFFLUX)/(ZLNUPTAKE))*100%. The mobile phase used was H2O:acetonitrile at a ratio of 60:40 which was titrated to pH = 3.0 using formic acid. The retention time of zearalenone was 13-14 min.
2.9. BODIPY-Glyburide Transwell Transport
BeWo cells were seeded on collagen-coated 24-well multiwall inserts (Cat# 351181, 1.0 μM pore, high density PET membrane, Corning, Tewksbury, MA) at a density of 200,000 cells per insert. Medium was changed daily, and cell monolayers were assessed for transepithelial electric resistance (TEER). On day 2 post-seeding, culture medium was replaced with treatment medium that contained AEA (10 μM, 24 h) or solvent. Transport assays were performed on day 3 post seeding on those cell monolayers with a TEER value of 80-160 Ω*cm2 (Li et al., 2013). Monolayer integrity was confirmed by measuring the permeability of Lucifer yellow (20 μM) (Hidalgo et al., 1989). The percent of Lucifer yellow rejection for these experiments was 95.3 ± 3.7% as calculated using % rejection = (1 − (Cr/(Cr + CD))) * 100, where Cris the final concentration in the receiver compartment and Cd is the initial concentration in the donor compartment (Nkabinde et al., 2012). AEA pre-treatment did not affect TEER values or Lucifer Yellow transport. On the day of the experiment, both compartments were washed with HBSS. Cells were then incubated in HBSS with 1 μM BODIPY-glyburide (Thermo-Fisher) in the basolateral compartment. Ko143 (1 μM) was included during the assay as a control for BCRP inhibition. One hundred μL samples were collected from the apical compartments at 30 min intervals and fluorescence was measured using Ex/Em: 428/540 nm. Data were corrected for pmol of BODIPY-glyburide removed at each time point. TEER was measured in each well immediately prior to and after the experiment to ensure membrane integrity throughout the assay. Permeability coefficients were calculated using Papp (cm/s) = (Q/t)/(A*C0), where Q is the initial nmol of BODIPY-glyburide transported to the receiver compartment at time t (s), A is the cell surface area (cm2) and C0 is the initial concentration of BODIPY-glyburide (μM) (Li, et al., 2013).
2.10. 3H-Anandamide Transport
AEA transport was assessed in BeWo cells following modifications to a published protocol (Maccarrone et al., 2003). BeWo cells were added to a 96-well round bottom plate at 100,000 cells/well. Plates were centrifuged (500 × g, 5 min, 5°C) to remove media. Cells were then resuspended in 100 μL of serum- and phenol red-free treatment medium containing 100 nM 3H-AEA (Movarek Biochemicals, Brea, CA) in the presence or absence of the BCRP-specific inhibitor, Ko143 (1 μM) (Bircsak, et al., 2013), or MM-22, a biotinylated AEA analog that blocks AEA uptake (Fezza et al., 2008). Plates were incubated at 37°C or 4°C (inhibiting active transport) for 30 min. Cells were then centrifuged and washed with ice cold HBSS with containing 1% bovine serum albumin. Cells were then re-suspended in substrate-free medium with or without inhibitors and incubated an additional 15 min at 37°C or 4°C. After the 15 min incubation, cells were centrifuged, washed, lysed using 1M NaOH, neutralized using 1M HCI, and added to 4 mL ScintiSafe Econo 1 liquid scintillation fluid (Fisher Scientific, Waltham,MA). Radioactivity was detected using a TriCarb 2100TR Liquid Scintillation Analyzer (PerkinElmer-Packard, Waltham, MA). AEA concentrations were quantified using a standard curve and normalized to protein amounts as determined using the detergent-compatible assay (BioRad).
2.11. Immunohistochemistry
Tissue sections (5 μm) were deparaffinized, and blocked with 25% normal goat serum (Invitrogen, Grand Island, NY) and 1 % bovine serum albumin at room temperature for 2 h. Sections were then incubated overnight at 4°C with antibodies used to detect CB1 (1:250, Cayman Chemical) and CB2 (1:250, Cayman Chemical). Blocking peptides against CB1 and CB2 (1:25, Cayman Chemical) were incubated with the primary antibody mixture for 1 h at room temperature prior to use. Sections were than washed and incubated at room temperature for 30 min with biotinylated goat anti-rabbit secondary antibody (Vector Labs, Burlingame, CA). Antibody-protein binding was visualized using a DAB Peroxidase Substrate Kit (Vector Labs) and counterstained with hematoxylin. Slides were imaged by light microscopy on an Olympus BX51 microscope (Waltham, MA) fitted with a ProgRes C14+ camera (Jenoptik, Jena, Germany), and images were white-balanced in Adobe Photoshop CS2 (Adobe Systems, San Jose, CA).
2.12. Measurement of Intracellular cAMP
BeWo cells grown to 70-80% confluence on 100 mM plates were treated with AEA (10 μM) and/or the adenylyl cyclase activator forskolin (FOR, 50 μM) in treatment medium. After 1 h at 37°C, the cells were then washed with HBSS and scraped into 200 μL 0.1M HCI. Extracts were vortexed for 10 sec and incubated at room temperature for 20 min. cAMP concentrations in the extracts was determined using a cAMP enzyme-linked immunosorbent assay kit (ELISA, Cayman Chemical). Data were normalized to protein concentrations in the HCI extracts as determined using a detergent-compatible assay (BioRad).
2.13. Chromatin Immunoprecipitation (ChIP)
ChIP was performed on BeWo cells using the SimpleChIP Enzymatic Chromatin IP Kit (Agarose Beads) (Cell Signaling Technologies). Cells were grown on 150 mm plates to 80% confluence in growth medium, washed, and then incubated in the presence or absence of AEA (10 μM) and forskolin (50 μM) in treatment medium for 60 min. Cells were then crosslinked with 1% formaldehyde and ChIP was performed according to the manufacturer’s protocol. Immunoprecipitation of crosslinked, digested DNA-protein complexes was accomplished using a rabbit monoclonal antibody to p-CREB (9198, 1:200, Cell Signaling Technologies) or rabbit IgG as a negative control. Purified DNA from ChIP preparations and corresponding 2% input samples was quantified by qPCR using SYBR Green and primers for NR4A3 (4829, Cell Signaling Technologies), a positive control for p-CREB ChIP, α-satellite repeats (4486, Cell Signaling Technologies), a negative control for p-CREB ChIP, and the BCRP cAMP response element (CRE) (Xie et al., 2015). Primer sequences for the BCRP CRE can be found in Supplemental Table 2. Percent input was calculated using PI = 2% × 2^(Ct2% input − Ctsample).
2.14. Statistical Analysis
Data are presented as mean ± SE and analyzed using GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA). According to the number of comparisons and variables, either one-way analysis of variance with Newman-Keuls post-test or a two-tailed student’s t test was used to assess statistical significance (p < 0.05). LC50 values were generated by plotting log (inhibitor) vs. response using a variable slope (Hill slope = −4.69 to −0.28) and robust fitting method.
3. Results
3.1. AEA Down-Regulates the Expression of Syncytialization Markers and the BCRP Transporter in Human Placental Explants
To recapitulate human placenta AEA signaling in situ, we cultured explants from healthy, term placentas and exposed them to AEA ex vivo. Because it was the purpose of this study to examine the effects of AEA on both BCRP expression and syncytialization, treatment of explants with AEA occurred during days 2-3 post-collection, which falls within the window of syncytium regeneration (Miller et al., 2005). Transcripts of syncytins and hCG are well-established markers of trophoblast syncytialization in both placental explants and BeWo cells and as a result were quantified to confirm the repressive effects of AEA on syncytialization (Gupta et al, 2016). While BCRP protein is expressed primarily in the syncytium (Szilagyi et al., 2017), it is not known whether BCRP is coordinately regulated with markers of syncytialization. Exposure of human placental explants to AEA (1 μM) for 24 h reduced hCGα, syncytin-1, and BCRP mRNA expression by 48%, 25%, and 46%, respectively (Fig 1A). By comparison, syncytin-2 mRNA expression was unchanged by AEA treatment (data not shown) and little to no hCGβ transcripts were detected in any of the placentas (Ct > 30 cycles, data not shown). Similar to its mRNA expression, BCRP protein was down-regulated by 30% in placental explants treated with AEA (1 μM) for 24 h (Fig 1B). Anandamide treatment did not induce any visible changes to the morphology of the explants (data not shown).
Fig. 1. Expression of syncytialization-related genes and the BCRP transporter in human placental explants treated with anandamide.
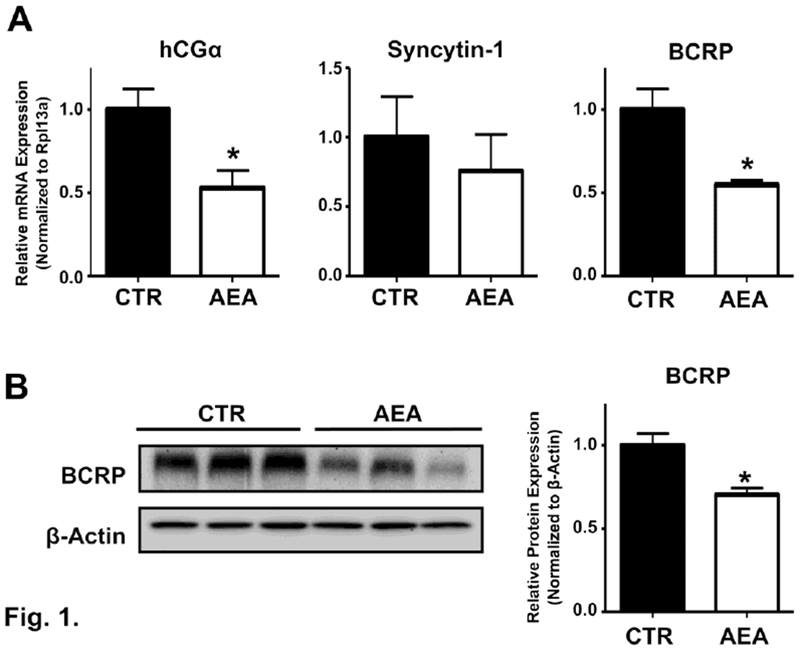
A. mRNA expression of BCRP and syncytialization-related genes. qPCR was performed on total RNAs extracted from term placental explants treated with AEA (1 μM) for 24 h as described in the Materials and Methods. Data represent the mean of 3 placentas (3-6 samples from each placenta) ± SE. B. Left. Representative image of protein expression of BCRP in AEA-treated explants. Protein homogenates were prepared from control and AEA (1 μM, 24 h) treated term placental explants and analyzed for expression of BCRP (72 kDa) by Western blotting. β-Actin (42 kDa) was used as a loading control. Right. Densitometry analysis of BCRP Western blots from control and AEA (1 μM, 24 h) treated explants. Data represent the mean of 3 placentas (3-5 samples from each placenta) ± SE. Asterisks (*) represents statistically significant differences (p < 0.05) compared to control explants.
3.2. Time- and Concentration-Dependent Down-Regulation of Syncytialization Markers and the BCRP Transporter by AEA in Human Trophoblasts
In subsequent studies, immortalized BeWo cells were used to interrogate trophoblast signaling and identify the mechanism by which AEA down-regulates BCRP. Similar to a prior report (Costa et al., 2015a), we demonstrated that AEA inhibits BeWo syncytialization using time- and concentration-dependent studies. AEA (10 μM) decreased the mRNA expression of hCGβ and syncytin-2 by 40-50% at 18 and 24 h (Fig 2A). Both transcripts returned to control levels by 48 h, likely due to the hydrolysis of AEA by FAAH, which has been previously detected in BeWo cells (Habayeb, et al., 2008). Down-regulation of syncytialization markers was also dependent upon the concentration of AEA (24 h). AEA significantly lowered the mRNA expression of hCGβ at 0.01 μM (71%) and syncytin-2 at 1 μM (40%), with both transcripts similarly repressed at higher concentrations (Fig 2B). AEA did not alter the expression of syncytin-1 or hCGα mRNAs in BeWo cells (data not shown).
Fig. 2. Expression of syncytialization-related genes and the BCRP transporter in human BeWo trophoblast cells treated with anandamide.
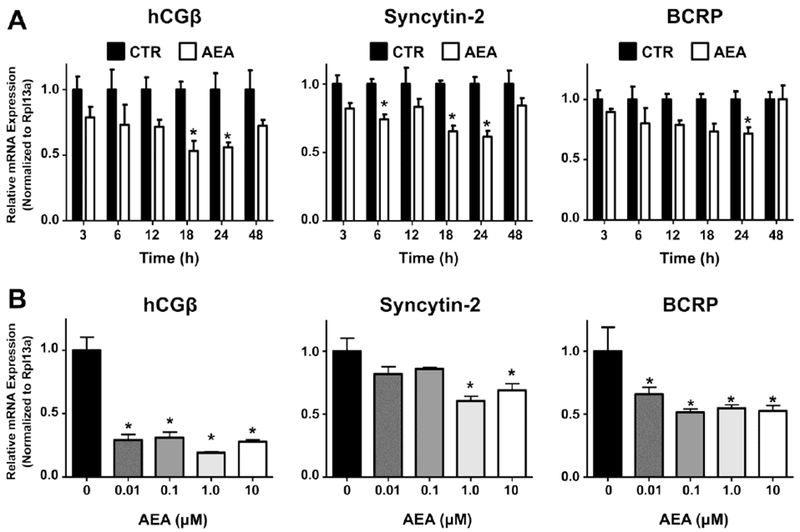
A. Time-dependent mRNA expression of syncytialization markers and BCRP. qPCR was performed as described in the Materials and Methods in BeWo cells treated with AEA (10 μM) at various time points. Black bars represent vehicle-treated cells and white bars represent AEA-treated cells. Data are presented as mean ± SE (n = 4). Asterisks (*) represent statistically significant differences (p < 0.05) compared to time-matched control cells. B. Concentration-dependent mRNA expression of syncytialization–related genes and BCRP. qPCR was performed in BeWo cells treated with AEA (0.01-10 μM) for 24 h. Data are presented as mean ± SE (n = 4). Asterisks (*) represent statistically significant differences (p < 0.05) compared to control cells.
Similar to syncytin-2 and hCGβ, BCRP expression was also down-regulated by AEA. In the presence of AEA (10 μM), there was a maximal reduction of BCRP mRNA at 24 h with a return to control levels by 48 h (Fig 2A). Evaluation of AEA concentrations between 0.01 μM and 10 μM revealed that the expression of BCRP mRNA in BeWo cells was decreased 30-50% at all concentrations of AEA tested (Fig 2B).
3.3. AEA Reduces the Protein Expression and Transport Activity of BCRP in Human Trophoblasts
Similar to the regulation of BCRP mRNA, treatment of BeWo cells with AEA (10 μM) for 24 h reduced protein expression (Fig 3A). Using Hoechst 33342 (5 μM) and Ko143 (1 μM), a well-established fluorescent substrate and inhibitor of BCRP, respectively, we quantified the ability of AEA to alter BCRP activity. Vehicle-treated BeWo cells were able to efflux 35% of the Hoechst 33342 dye that was taken up (Fig. 3B, CTR). By comparison, exposure to AEA (10 μM) for 24 h decreased BCRP efflux activity to 15% which was similar to the functional inhibitor Ko143 (8%). The cellular retention of zearalenone, a known BCRP substrate and developmental toxicant, was also quantified in BeWo cells treated with AEA (Xiao, et al., 2015; Zhao et al., 2013). In vehicle-treated cells loaded with zearalenone, BeWo cells were able to efflux 71% of accumulated zearalenone. By comparison, AEA (10 μM, 24h) and Ko143 (1 μM) reduced the efflux of zearalenone to 33% and 49%, respectively (Fig. 3C).
Fig. 3. BCRP protein expression and efflux activity in human BeWo trophoblast cells treated with anandamide.
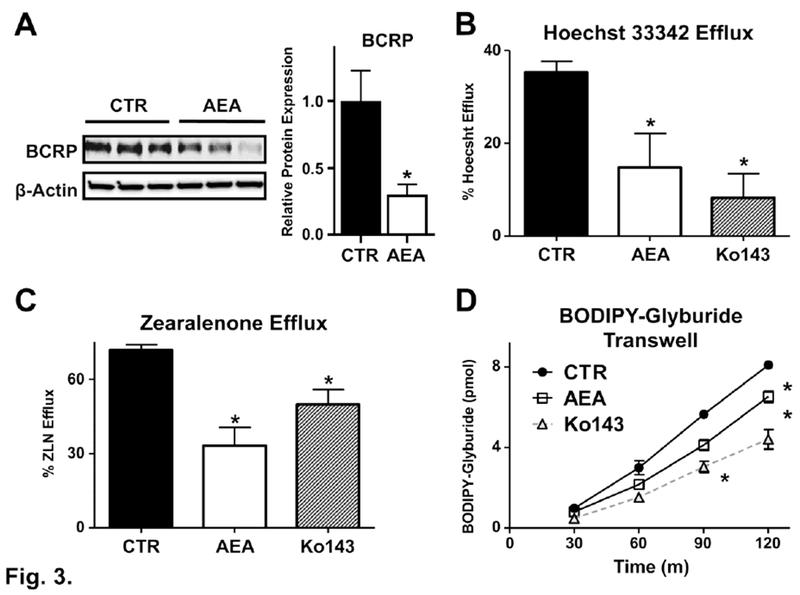
A. Protein expression of BCRP in AEA-treated cells and corresponding densitometry analysis. Lysates were prepared from control and AEA (10 μM, 24 h) treated BeWo cells and analyzed for expression of BCRP protein (72 kDa) by Western blotting. β-Actin (42 kDa) was used as a loading control. B and C. Inhibition of BCRP Hoechst 33342 and zearalenone efflux activity by AEA. Control and AEA (10 μM, 24 h) treated BeWo cells were trypsinized and subjected to either the Hoechst 33342 (5 μM, B) or zearalenone (50 μM, C) cell accumulation assay as described in the Materials and Methods. Data are presented as mean ± SE (n = 3-5). D. Inhibition of BODIPY-glyburide transport across BeWo monolayers. Control and AEA (10 μM, 24 h) treated BeWo cells in transwell inserts were assessed for basolateral-to-apical translocation of BODIPY-glyburide (1 μM) over 2 h as described in the Materials and Methods. Data represent the mean ± SE (n = 3 independent experiments). One group of cells in each experiment were incubated in the presence Ko143 (1 μM) during the assay to provide a positive control for BCRP inhibition. Asterisks (*) represent statistically significant differences (p < 0.05) compared to control cells.
In order to better understand how AEA may affect transplacental drug trafficking during pregnancy, we quantified the basolateral-to-apical transport of the BCRP substrate, BODIPY-glyburide, in polarized BeWo trophoblasts grown on Transwell inserts (Bircsak, et al., 2016). The appearance of BODIPY-glyburide in the apical compartment increased over time up to 7.9 pmol by 2 h (Fig 3D). AEA (10 μM, 24h) and Ko143 (1 μM, present during assay) reduced the transepithelial transport of BODIPY-glyburide by 20% and 32%, respectively, after 2 h (Fig 3D). The apparent permeability (Papp) of BODIPY-glyburide in this experiment was 3.3*10−3 cm/s under control conditions, which was reduced to 2.7*10−3 cm/s and 2.2*10−3 cm/s by AEA and Ko143, respectively. Reductions in the basolateral-to-apical transport of BODIPY-glyburide by AEA could potentially increase placental and/or fetal exposure to this and other structurally similar chemicals.
The ability of BCRP to transport AEA is unknown. Using 3H-AEA (100 nM) and MM-22 (50 μM), a known inhibitor of AEA uptake, the intracellular retention of AEA was reduced 27% (Fig 4A). By comparison, exposure to the BCRP inhibitor Ko143 (1 μM) had no impact on AEA retention (Fig 4A). A separate group of cells were incubated at 4°C to inhibit active transport, which resulted in a 44% reduction in the intracellular concentration of AEA (Fig 4A). Additionally, the presence of AEA (10 μM) did not impact the ability of BeWo cells to efflux Hoechst 33342. Together, these data suggest that AEA is not a substrate or inhibitor of BCRP (Fig 4B).
Fig. 4. AEA is not a substrate or inhibitor of BCRP.
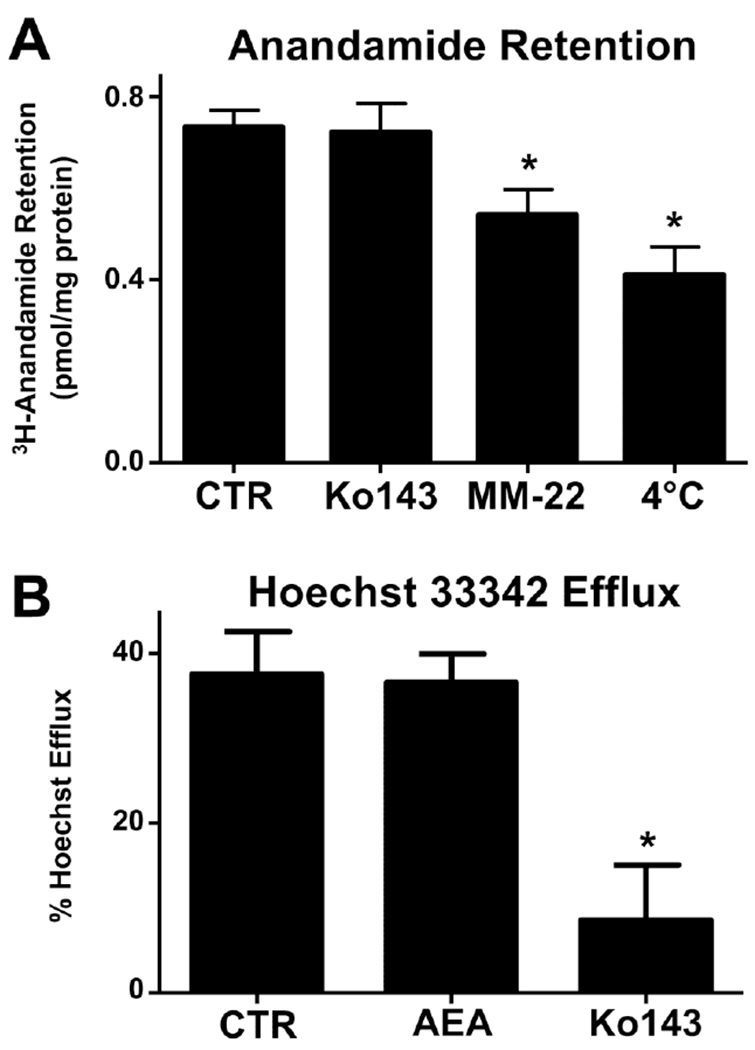
A. 3H-Anandamide accumulation in human BeWo trophoblast cells. Untreated BeWo cells were trypsinized and subjected to the 3H-AEA (100 nM) transport assay as described in Materials and Methods. During the uptake and efflux phase, cells were incubated in the presence of Ko143 (1 μM), MM-22 (25 μM), or DMSO. One set of cells was incubated at 4°C for the duration of the assay. Data are presented as mean ± SE (n = 6). B. Competitive inhibition of Hoechst 33342 efflux in BeWo cells. Untreated BeWo cells were trypsinized and subjected to either the Hoechst 33342 (5 μM) cell accumulation assay as described in the Materials and Methods in the presence of vehicle, AEA (10 μM), or Ko143 (1 μM) during the uptake and efflux phase. Data are presented as mean ± SE (n = 3). Asterisks (*) represent statistically significant differences (p < 0.05) compared to control cells.
3.4. Down-Regulation of BCRP Expression by AEA Heightens the Sensitivity of Human Trophoblasts to Chemical-Induced Cytotoxicity
We measured BeWo cell viability using Alamar Blue to screen the ability of AEA to alter placental toxicity by reducing BCRP expression. BeWo cells were incubated (24 h) in the presence of vehicle or AEA (10 μM) along with the environmental contaminants zearalenone (0.5-500 μM), α-zearalanol (0.5-500 μM), perfluorooctanoic acid (2-2000 μM), and perfluorooctanesulfonic acid (2-2000 μM). It has been shown that these chemicals are potential substrates or inhibitors of BCRP (Dankers, et al., 2013). Mitoxantrone (0.6-600 μM), a commonly used cytotoxic substrate of BCRP, was used as a positive control for this experiment, and Ko143 (1 μM) was used as a known pharmacological inhibitor of BCRP for comparison. LC50 values for 50% cytotoxicity in BeWo cells are presented in Table 1. Neither Ko143 nor AEA alone affected BeWo cell viability (data not shown). The LC50 value of mitoxantrone for BeWo cells was 10.7 μM. In the presence of Ko143 and AEA, BeWo cells were more sensitive to mitoxantrone (LC50 = 3.1 and 3.2 μM for Ko143 and AEA, respectively). In the absence of AEA, BeWo cells were more sensitive to the cytotoxicity of α-zearalanol and least sensitive to the cytotoxicity of perfluorooctanesulfonic acid. Following exposure to either Ko143 or AEA for 24h, BeWo cells were 2- to 3-fold more sensitive to all tested environmental chemicals. These data suggest that the down-regulation of BCRP by AEA inhibits xenobiotic efflux and sensitizes placental cells to chemical toxicity.
Table 1.
Effect of AEA on cell viability in the presence of BCRP substrates and environmental contaminants.
| LC50 values (μM) |
|||
|---|---|---|---|
| Control | Ko143a | AEA | |
| MTXb | 10.7 ± 1.1 | 3.1 ± 1.8* | 3.2 ± 1.3* |
| ZLN | 182.9 ± 3.7 | 84.8 ± 19.3* | 73.9 ± 33.3* |
| α-ZRN | 77.4 ± 6.4 | 36.0 ± 10.1* | 27.4 ± 9.1* |
| PFOA | 672.9 ± 58.2 | 450.0 ± 163.9 | 326.3 ± 47.3 |
| PFOS | 724.7 ± 56.8 | 438.3 ± 87.7* | 277.5 ± 74.6* |
LC50 values are presented as the mean of three experiments +/− SEM.
Statistically significant differences (p < 0.05) compared to control cells as determined by one-way ANOVA with Newman-Keuis post-test.
BeWo cells were incubated in the presence of Ko143 (1 μM), AEA (10 μM), or vehicle (rows) and various concentrations of known or potential BCRP substrates (columns)
Abbreviations: MTX, mitoxantrone; ZLN, zearalenone; α-ZRN, α-zearalanol; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid
3.5. Down-Regulation of BCRP Expression by AEA in Human Trophoblasts Involves CB2 Receptor Signaling
In order to delineate the mechanism by which AEA reduces BCRP expression, we assessed the role of the CB1 and CB2 cannabinoid receptors. Consistent with previous publications (Habayeb, et al., 2008; Park, et al., 2003; Trabucco, et al., 2009), the staining of CB1 was localized mainly to syncytiotrophoblasts and fetal endothelium in term placentas from healthy pregnancies (Fig 5A, top panel). CB2 showed similar staining, but was additionally expressed on the surface of fetal erythrocytes (Fig 5A, bottom panel). Consistent with these findings, CB1 and CB2 proteins were also expressed in lysates of naive BeWo cells as determined by Western blot (Fig 5B).
Fig. 5. Expression and function of cannabinoid receptors in human term placentas and human BeWo trophoblast cells.
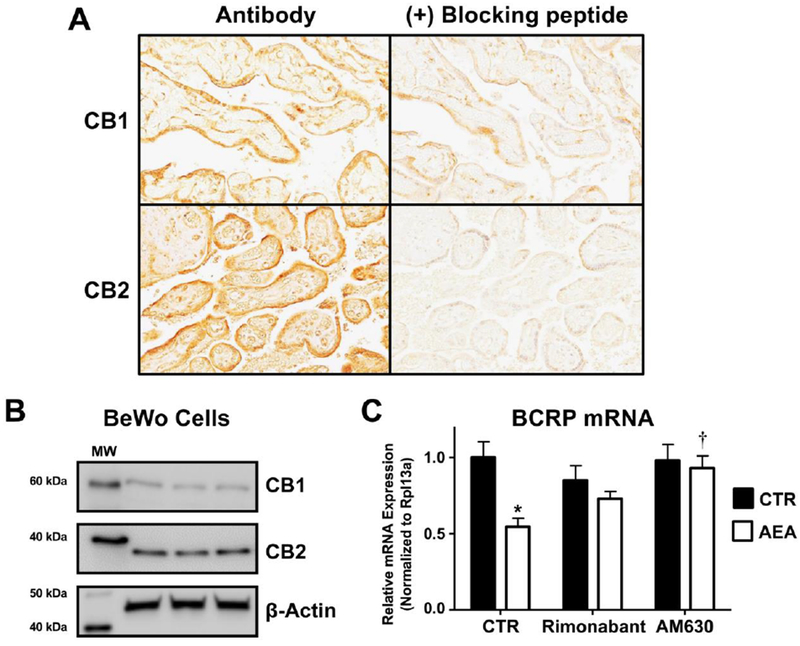
A. Immunohistochemical staining of the CB1 and CB2 receptors in term human placentas. Sections of paraffin-embedded healthy term placentas (5 μm) were prepared and stained with antibodies against CB1 and CB2 as well as respective blocking peptides and visualized using a Vectastain DAB kit (brown staining) as described in the Materials and Methods. Magnification 20×. B. Protein expression of the CB1 and CB2 receptors in BeWo cells. Lysates were prepared from naïve BeWo cells and analyzed for expression of CB1 (60 kDa) and CB2 (38 kDa) by Western blotting. β-Actin (42 kDa) was used as a loading control. C. CB-receptor dependence of AEA down-regulation of BCRP. qPCR was performed on cells treated with vehicle control (CTR), rimonabant (0.1 μM), or AM630 (0.1 μM) with or without AEA (10 μM) for 24 h. Black bars represent vehicle-treated cells and white bars represent AEA-treated cells. Data are presented as mean ± SE (n = 4). Asterisks (*) represent statistically significant differences (p < 0.05) compared to control cells. Daggers (†) represent statistically significant differences (p < 0.05) compared to AEA-treated cells.
To test the involvement of CB1 and CB2 receptors in the regulation of BCRP by AEA, pharmacological modulators of each receptor were employed. Notably, the down-regulation of BCRP mRNA by AEA (10 μM, 24 h) in BeWo cells was completely inhibited by AM630 (0.1 μM, co-treatment with AEA), a CB2-selective inhibitor, but only partially inhibited by rimonabant (0.1 μM, co-treatment with AEA), a CB1 inverse agonist (Fig 5C).
3.6. Regulation of BCRP by AEA is Mediated by cAMP
The signal transduction pathways of CB2 involve a number of well-known intracellular messengers, including cAMP, ERK/JNK, ceramides, PI3K/AKT/mTOR, and β-catenin (reviewed in Pisanti et al., 2013). In order to identify the downstream AEA-CB receptor signals involved in regulating BCRP, inhibitors of TRPV1 (SB366791, 1 μM), PPARα (GW6471, 5 μM), PPARγ (GW9662, 5 μM), and ERK (PD98059) were tested though none of these chemicals reversed the effects of AEA (data not shown). Capsaicin (1 μM, 24 h), a well-known agonist of TRPV1, also had no effect on BCRP expression in BeWo cells (data not shown). AEA has also been shown to inhibit NF-κB, but SN-50, a peptide inhibitor of NF-κB, did not alter BCRP expression (data not shown).
Interestingly, AEA (10 μM, 1 h) decreased basal intracellular levels of cAMP in BeWo cells from 34.8 to 12.4 pmol/mg protein (Fig 6A). Forskolin (50 μM, 1 h), an activator of adenylyl cyclase, increased intracellular levels of cAMP to 12,800 pmol/mg protein which was also reduced by AEA to 8,250 pmol/mg protein (Fig 6A). These data suggested that cAMP-mediated signaling may be involved in the repression of BCRP by AEA. Interestingly, 8-Br-cAMP (1 mM, 24 h), a stable analog of cAMP, completely attenuated the down-regulation of BCRP expression and efflux activity by AEA (10 μM, 24 h) in BeWo cells (Fig 6B). In measuring the BCRP-mediated Hoechst 33342 efflux by BeWo cells, control cells transported an average of 33% of the Hoechst 33342 dye that was taken up (Fig 6C). 8-Br-cAMP itself had no effect on Hoechst 33342 transport, but it completely prevented the ability of AEA (10 μM) to lower Hoechst 33342 efflux from BeWo cells (Fig 6C). Forskolin (50 μM, 24h) partially inhibited the AEA-mediated reduction of BCRP transcription but had no effect on the AEA-mediated reduction of Hoechst 33342 efflux (data not shown).
Fig. 6. cAMP regulation of BCRP expression and activity in human BeWo trophoblast cells treated with anandamide.
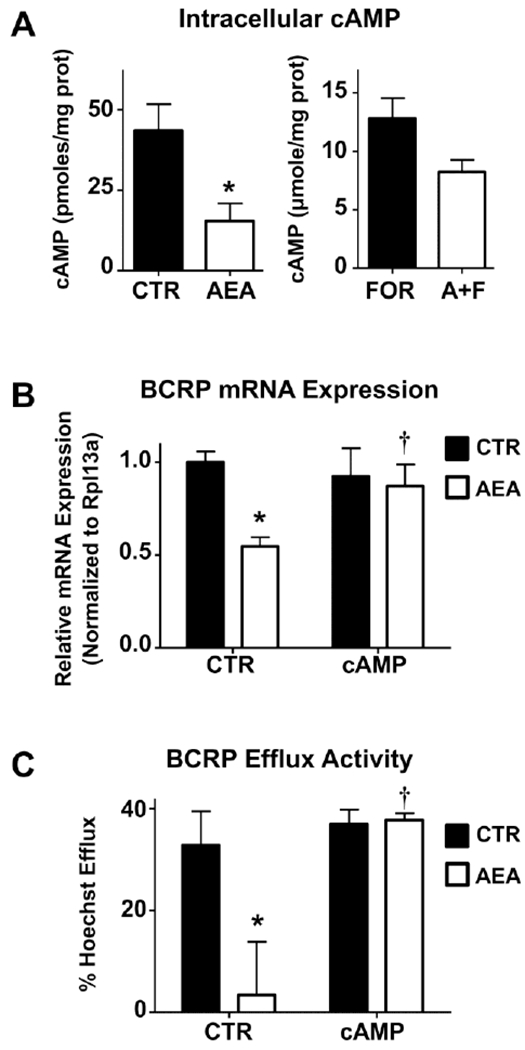
A. Effects of AEA on intracellular cAMP levels. BeWo cells were treated (± 10 μM AEA; ± 50 μM forskolin, FOR) for 1 h and collected in 0.1 M HCI. Intracellular concentrations of cAMP were determined by ELISA and normalized to protein concentration. Data are presented as mean ± SE (n = 4). B. 8-Br-cAMP abrogates AEA effects on BCRP mRNA. qPCR was performed on BeWo cells treated with AEA (10 μM), 8-Br-cAMP (1 mM), or both for 24 h. Black and white bars represented samples treated in the absence or presence of AEA, respectively. Data are presented as mean ± SE (n = 4). D. Inhibition of BCRP efflux activity by AEA is cAMP-dependent. BeWo cells were treated with either AEA (10 μM), 8-Br-cAMP (1 mM), or both for 24 h. Cells were then trypsinized and subjected to the Hoechst 33342 transport assay as described in Materials and Methods. Data represent the mean ± SE (n = 3-5). Asterisks (*) represent statistically significant differences (p < 0.05) compared to control cells. Daggers (†) represent statistically significant differences (p < 0.05) compared to AEA-treated cells.
Signal transduction by intracellular cAMP involves phosphorylation of CREB (cAMP response element binding protein), which then binds to promoter sequences to increase transcription of target genes (Montminy and Bilezikjian, 1987). CREB phosphorylation in BeWo cells after 1 h treatment with AEA (10 μM), forskolin (50 μM), or both was examined by Western blot. As determined by densitometry, control cells had varying levels of p-CREB relative to total CREB protein, but no p-CREB was detected in cells treated with AEA (10 μM, 1 h) (Fig 7A). Exposure to forskolin (50 μM, 1 h) consistently increased CREB phosphorylation, which was again abolished when cells were co-treated with AEA (Fig 7A). ChIP was used to assess p-CREB binding to the BCRP promoter region (base pairs −504 to −403) (Xie, et al., 2015). Samples precipitated with a p-CREB antibodies yielded a significantly higher signal compared to an IgG control for the BCRP cAMP response element and NR4A3, a positive control for p-CREB ChIP, but not α-satellites, a negative control for p-CREB ChIP (Fig 7B, left). Importantly, treatment of trophoblasts with AEA (10 μM, 1 h) decreased p-CREB binding to the BCRP promoter by 68% (Fig 7B, right). Co-treatment of cells with AEA and forskolin (50 μM, 1 h) partially reversed the effects of AEA and resulted in only a 12% reduction in p-CREB binding.
Fig. 7. AEA inhibits p-CREB signaling and BCRP promoter binding in BeWo cells.
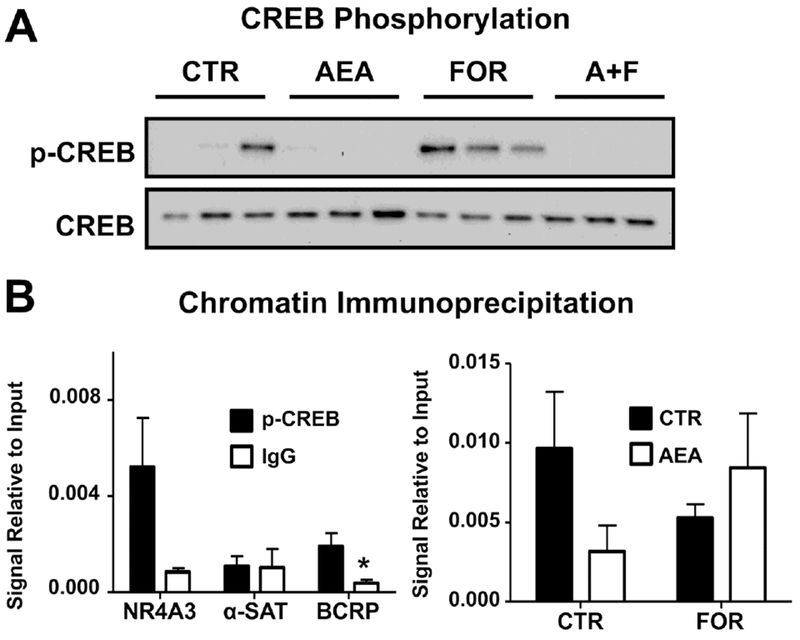
A. Western blot of p-CREB signaling in AEA- and FOR-treated cells. Lysates were prepared from BeWo cells treated for 1 h with AEA (10 μM), FOR (50 μM), or both, and analyzed for p-CREB and CREB (37 kDa) by Western blot. B. Validation of chromatin immunoprecipitation. ChIP was performed on untreated cells using a p-CREB antibody (black bars) or an IgG control (white bars), and samples were analyzed by qPCR for NR4A3, a positive control for p-CREB ChIP, α-satellites, a negative control, and the unique BCRP cAMP response element sequence. Data are presented as mean ± SE (n = 3-4). C. ChIP analysis of p-CREB binding to the BCRP promoter region. BeWo cells treated for 1 h with AEA (10 μM), FOR (50 μM), or both. ChIP was performed using a p-CREB antibody and samples were analyzed by qPCR for the unique BCRP cAMP response element sequence. Data are presented as mean ± SE (n = 3-5). Ct values for each sample were compared against respective 2% input controls. Asterisks (*) represent statistically significant differences (p < 0.05) compared to p-CREB precipitated samples.
4. Discussion
Because of its enrichment on the apical surface of syncytiotrophoblasts and its ability to efflux a wide range of xenobiotics, BCRP protects the fetus from chemical toxicities during development. Recognizing this critical role in the placental barrier, it is necessary to elucidate the biochemical pathways that regulate BCRP expression and activity under healthy and pathological conditions. While it has been shown that phytocannabinoids such as cannabidiol and Δ9-tetrahydrocannabinol, both found in Cannabis sativa, can decrease BCRP expression and activity, there is no research demonstrating this same relationship with endogenous cannabinoids (Feinshtein et al, 2013; Holland et al, 2007; Spiro et al, 2012). Our studies demonstrate for the first time that AEA down-regulates BCRP mRNA and protein expression in both human BeWo trophoblast cells and human term placental explants. This repression was observed at concentrations as low as 10 nM AEA in BeWo cells. Notably, AEA is an agonist for both the CB1 and CB2 receptors, and previous studies have demonstrated a potential role for both isoforms in regulating trophoblast differentiation (Costa, et al., 2015a; Habayeb, et al., 2008; Sun et al, 2010). In a primary culture of human cytotrophoblasts, AEA was found to decrease alkaline phosphatase (ALP) activity and hCG release through both receptors (Costa, et al., 2015a). However, AEA decreased intracellular cAMP in cytotrophoblasts through CB2 activation, but not CB1 activation (Costa, et al., 2015a). Using previously published CB1 and CB2 signaling modulators (Barth and Rinaldi-Carmona, 1999; Ross et al., 1999), we have similarly shown that only inhibition of CB2 fully reversed the AEA-induced down-regulation of BCRP, although the potential role of CB1 in vivo cannot be ruled out. Recently the BCRP promoter was shown to possess a functional cAMP response element (BP −504 to −403), which may further explain the selectivity of this mechanism toward CB2 in comparison to CB1 (Xie, et al., 2015). We have also demonstrated that AEA decreases intracellular cAMP concentrations in BeWo cells and that 8-Br-cAMP can reverse the repressive effects of AEA on BCRP expression and activity. Further, we have shown that AEA inhibits both CREB phosphorylation and reduces p-CREB promoter binding and the subsequent transcription of BCRP. Collectively, these data point to AEA’s ability to repress BCRP expression in the placenta via CB2-mediated inhibition of cAMP-p-CREB signaling (Fig. 8).
Fig. 8. Proposed mechanism of AEA-mediated down-regulation of BCRP and syncytialization-related genes.
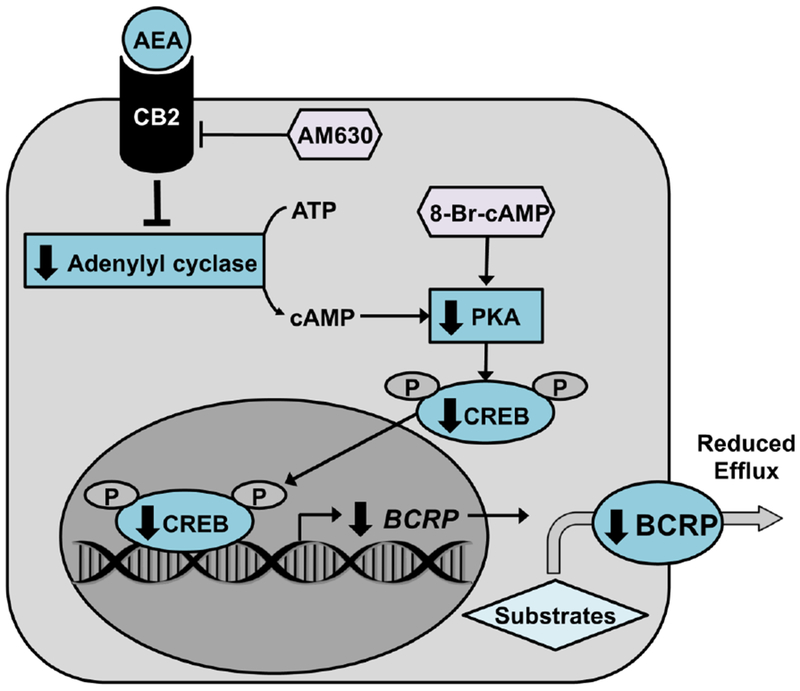
AEA binds to and activates the CB2 receptor, which in turn inhibits adenylyl cyclase. The corresponding decrease in cAMP production reduces basal protein kinase A (PKA) activation and, therefore, CREB phosphorylation. The DNA sequence for BCRP, along with genes involved in trophoblast syncytialization, contain a cAMP response element (BP −504 to −403) to which p-CREB would normally bind. Decreased p-CREB signaling would therefore reduce BCRP transcription and efflux activity.
The effects of AEA treatment on trophoblast syncytialization in placental explants and BeWo trophoblast cells largely mirrored observations from prior studies where AEA reduced syncytialization as indicated by a reduction in hCG secretion and intracellular cAMP (Costa, et al., 2015a; Gupta, et al., 2016; Wang, et al., 1999). However, in the current study, the specific genes that were sensitive to AEA treatment varied between explants (hCGα, syncytin-1) and BeWo cells (hCGβ, syncytin-2). This inconsistency may be due to the fact that BeWo cells reflect the signaling of first trimester trophoblasts (Pattillo et al., 1968), whereas explants were isolated from term placentas, which express little or no hCGβ (Lin et al., 1995). Conversely, the discrepancy in target syncytialization genes may also be due to the influence of other cell types (i.e. Hofbauer and endothelial cells) in explants that are not present in BeWo cell cultures.
There is increasing attention being placed on identifying environmental and dietary chemicals whose transplacental transfer as well as placental toxicity may be reduced by BCRP efflux from syncytiotrophoblasts. We have demonstrated indirectly that a cell viability assay can be used to screen environmental contaminants as potential substrates. While this experiment is useful, the concentrations required for cytotoxicity in vitro far exceed those expected in human exposures. Nonetheless, this approach allows us to identify potentially novel BCRP substrates (i.e., zeranol and perfluorooctanesulfonic acid) using Ko143 and demonstrate the ability of AEA to repress BCRP transport of those substrates. We further confirmed the ability of AEA to lower the functional activity of BCRP in BeWo cells using cell accumulation and Transwell assays. Notably, AEA increased the cellular retention of BCRP substrates Hoechst 33342 and zearalenone and decreased the basolateral-to-apical transport of BODIPY-glyburide. We have also demonstrated that AEA retention in BeWo cells was not altered by Ko143, indicating that AEA is not a substrate of BCRP. Therefore, we can conclude that AEA reduces BCRP activity by inhibiting its transcription rather than through competitive or functional inhibition. However, these data do not rule out the potential role of post-translational modifications to the BCRP protein underlying the mechanism of AEA’s repression, which should be considered in future studies. As demonstrated here, the consequence of aberrant AEA signaling, as seen in pathological pregnancies, may be increased fetal exposure to environmental contaminants and drugs.
There is substantial, albeit varying, evidence that dysregulation of the endocannabinoid system may be a driving factor behind diseases of pregnancy (reviewed in Costa, 2016). AEA regulates trophoblast differentiation, apoptosis and invasion, and disruption of these tightly coordinated processes is generally associated with placental disorders leading to poor neonatal outcomes (Costa, et al., 2015a; Costa et al., 2015b; Gupta, et al., 2016; Roland et al., 2016; Sitras et al., 2009; Sun, et al., 2010; Zhang et al., 2016). In one study, preeclamptic placentas showed higher expression of n-arachidonyl phosphatidyl ethanolamine phospholipase D an enzyme essential for AEA synthesis, and lower FAAH expression but no change in CB1 or CB2 expression (Aban, et al., 2013). Another study published that CB1 expression was higher in placentas from pregnancies complicated by preeclampsia compared to controls, with no change in CB2 receptor levels (Fugedi, et al., 2014). Contradictory to these data is one study that demonstrated circulating levels of AEA are lower in preeclampsia (Molvarec et al., 2015). Blood AEA concentration, however, is not necessarily an indicator of local AEA concentration or AEA signaling within the placenta and may be under the control of feedback mechanisms. Regardless, the endocannabinoid system is also an emerging regulator of the hypoxic response, which is a known pathologic factor in preeclampsia due to restrictive blood flow in the placenta (Aban et al., 2016). Hypoxia inducible factor-1α (HIF1α), for instance, regulates FAAH expression (Aban, et al., 2016). Further cobalt chloride-induced apoptosis in placental explants, an ex vivo model of hypoxia, was abrogated by inhibition of CB1 receptor signaling using AM251 (Aban, et al., 2016). Hypoxia also down-regulates BCRP expression in BeWo cells by a HIF-1α related mechanism, and it has been shown that BCRP levels are reduced in the placentas of preeclamptic patients (Francois, et al., 2017; Gormley, et al., 2017; Jebbink, et al., 2015; Nishizawa, et al., 2011). It is possible that hypoxic conditions in the placenta during gestational disorders may be responsible for the reduction in BCRP expression, and this could result via aberrant endocannabinoid signaling. Ectopic pregnancies and spontaneous miscarriage have also been linked to aberrant endocannabinoid signaling, but no studies have yet been performed to assess placental BCRP expression in these gestational disorders (Gebeh et al., 2013; Gebeh et al., 2012; Trabucco, et al., 2009).
Placentas from pregnancies complicated by fetal growth restriction exhibit decreased BCRP mRNA expression (Evseenko, et al., 2007; Ruebner et al., 2012). While a specific role for AEA has not been identified, it is now commonly recognized that fetal growth restriction is a complication of cannabis exposure during pregnancy (Davitian et al., 2006; El Marroun et al., 2009; Warner et al., 2014). Unfortunately, cannabis has been misleadingly suggested as a safe remedy for nausea and emesis during pregnancy and 34-60% of cannabis users continue use during pregnancy (Jaques et al., 2014; Moore et al., 2010; Passey et al., 2014; Schempf and Strobino, 2008). Chronic cannabis exposure has also been shown to affect endocannabinoid signaling up to 6 months after complete cessation, highlighting the importance of further understanding how the endocannabinoid system regulates placentation and the placental barrier (Muhl et al., 2014).
Endocannabinoids can dysregulate placentation and potentially neonatal outcomes. Taken together, our data suggest that the expression and activity of BCRP may also be regulated by endocannabinoid signaling. This novel observation may point to a potential explanation for the altered placental expression of BCRP previously observed in gestational diseases. With reduced BCRP expression, there is potential for altered xenobiotic disposition in the placenta, higher fetal xenobiotic exposures, and increased risk of adverse outcomes in newborns.
Supplementary Material
6.
Funding
This work was supported by the National Institute of Environmental Health Sciences [Grants ES029275, ES020522, ES005022, ES007148] and the National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant AR055073], components of the National Institutes of Health.
Abbreviations
- ABC
ATP-binding cassette
- AEA
anandamide
- AKT
protein kinase B
- ALP
alkaline phosphatase
- BCRP
breast cancer resistance protein
- cAMP
cyclic adenosine monophosphate
- CB
cannabinoid receptor
- CREB
cAMP response element binding protein
- DAB
3,3′-diaminobenzidine
- ERK
extracellular signalαregulated kinases
- JNK
c-Jun N-terminal kinase
- FAAH
fatty acid amide hydrolase
- hCG
human choriogonadotropin
- HELLP
hemolysis, elevated liver enzymes, low platelet count
- HIF1α
hypoxia inducible factor 1α
- H33342
Hoechst 33342
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PFOS
perfluorooctanesulfonic acid
- PFOA
perfluorooctanoic acid
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PKA
protein kinase A
- PPAR
peroxisome proliferator-activated receptors
- RPL13A
ribosomal protein 13A
- TEER
transepithelial electrical resistance
- TRPV1
transient receptor potential cation channel subfamily V member 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Aban C, Leguizamon GF, Cella M, Damiano A, Franchi AM, and Farina MG (2013). Differential expression of endocannabinoid system in normal and preeclamptic placentas: Effects on nitric oxide synthesis. Placenta 34, 67–74. [DOI] [PubMed] [Google Scholar]
- Aban C, Martinez N, Carou C, Albamonte I, Toro A, Seyahian A, Franchi A, Leguizamon G, Trigubo D, Damiano A, and Farina M (2016). Endocannabinoids participate in placental apoptosis induced by hypoxia inducible factor-1. Apoptosis 21, 1094–1105. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, and Dean M (1998). A human placenta-specific atp-binding cassette gene (abcp) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 58, 5337–5339. [PubMed] [Google Scholar]
- Barth F, and Rinaldi-Carmona M (1999). The development of cannabinoid antagonists. Curr Med Chem 6, 745–755. [PubMed] [Google Scholar]
- Baumann MU, Schneider H, Malek A, Palta V, Surbek DV, Sager R, Zamudio S, and Illsley NP (2014). Regulation of human trophoblast glut1 glucose transporter by insulin-like growth factor i (igf-i). PLoS One 9, e106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bircsak KM, Gibson CJ, Robey RW, and Aleksunes LM (2013). Assessment of drug transporter function using fluorescent cell imaging. Curr Protoc Toxicol 57, Unit 23.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bircsak KM, Gupta V, Yuen PY, Gorczyca L, Weinberger BI, Vetrano AM, and Aleksunes LM (2016). Genetic and dietary regulation of glyburide efflux by the human placental breast cancer resistance protein transporter. J Pharmacol Exp Ther 357, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceckova M, Libra A, Pavek P, Nachtigal P, Brabec M, Fuchs R, and Staud F (2006). Expression and functional activity of breast cancer resistance protein (bcrp, abcg2) transporter in the human choriocarcinoma cell line bewo. Clin Exp Pharmacol Physiol 33, 58–65. [DOI] [PubMed] [Google Scholar]
- Cella M, Leguizamon GF, Sordelli MS, Cervini M, Guadagnoli T, Ribeiro ML, Franchi AM, and Farina MG (2008). Dual effect of anandamide on rat placenta nitric oxide synthesis. Placenta 29, 699–707. [DOI] [PubMed] [Google Scholar]
- Costa MA (2016). The endocannabinoid system: A novel player in human placentation. Reprod Toxicol 61, 58–67. [DOI] [PubMed] [Google Scholar]
- Costa MA, Fonseca BM, Mendes A, Braga J, Teixeira NA, and Correia da Silva G (2015a). The endocannabinoid anandamide affects the synthesis of human syncytiotrophoblast-related proteins. Cell Tissue Res 362, 441–446. [DOI] [PubMed] [Google Scholar]
- Costa MA, Fonseca BM, Teixeira NA, and Correia-da-Silva G (2015b). The endocannabinoid anandamide induces apoptosis in cytotrophoblast cells: Involvement of both mitochondrial and death receptor pathways. Placenta 36, 69–76. [DOI] [PubMed] [Google Scholar]
- Dankers AC, Roelofs MJ, Piersma AH, Sweep FC, Russel FG, van den Berg M, van Duursen MB, and Masereeuw R (2013). Endocrine disruptors differentially target atp-binding cassette transporters in the blood-testis barrier and affect leydig cell testosterone secretion in vitro. Toxicol Sci 136, 382–391. [DOI] [PubMed] [Google Scholar]
- Davitian C, Uzan M, Tigaizin A, Ducarme G, Dauphin H, and Poncelet C (2006). Maternal cannabis use and intra-uterine growth restriction. Gynecol Obstet Fertil 34, 632–637. [DOI] [PubMed] [Google Scholar]
- De Baere S, Osselaere A, Devreese M, Vanhaecke L, De Backer P, and Croubels S (2012). Development of a liquid-chromatography tandem mass spectrometry and ultra-high-performance liquid chromatography high-resolution mass spectrometry method for the quantitative determination of zearalenone and its major metabolites in chicken and pig plasma. Anal Chim Acta 756, 37–48. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Steegers EA, Jaddoe VW, Hofman A, Verhulst FC, van den Brink W, and Huizink AC (2009). Intrauterine cannabis exposure affects fetal growth trajectories: The generation r study. J Am Acad Child Adolesc Psychiatry 48, 1173–1181. [DOI] [PubMed] [Google Scholar]
- Evseenko DA, Murthi P, Paxton JW, Reid G, Emerald BS, Mohankumar KM, Lobie PE, Brennecke SP, Kalionis B, and Keelan JA (2007). The abc transporter bcrp/abcg2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. Faseb j 21, 3592–3605. [DOI] [PubMed] [Google Scholar]
- Feinshtein V, Erez O, Ben-Zvi Z, Eshkoli T, Sheizaf B, Sheiner E, and Holcberg G (2013). Cannabidiol enhances xenobiotic permeability through the human placental barrier by direct inhibition of breast cancer resistance protein: An ex vivo study. Am J Obstet Gynecol 209, 573.e571–573.e515. [DOI] [PubMed] [Google Scholar]
- Fezza F, Oddi S, Di Tommaso M, De Simone C, Rapino C, Pasquariello N, Dainese E, Finazzi-Agro A, and Maccarrone M (2008). Characterization of biotin-anandamide, a novel tool for the visualization of anandamide accumulation. J Lipid Res 49, 1216–1223. [DOI] [PubMed] [Google Scholar]
- Fonseca BM, Battista N, Correia-da-Silva G, Rapino C, Maccarrone M, and Teixeira NA (2014). Activity of anandamide (aea) metabolic enzymes in rat placental bed. Reprod Toxicol 49, 74–77. [DOI] [PubMed] [Google Scholar]
- Francois LN, Gorczyca L, Du J, Bircsak KM, Yen E, Wen X, Tu MJ, Yu AM, Illsley NP, Zamudio S, and Aleksunes LM (2017). Down-regulation of the placental bcrp/abcg2 transporter in response to hypoxia signaling. Placenta 51, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugedi G, Molnar M, Rigo J Jr., Schonleber J, Kovalszky I, and Molvarec A (2014). Increased placental expression of cannabinoid receptor 1 in preeclampsia: An observational study. BMC Pregnancy Childbirth 14, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebeh AK, Willets JM, Bari M, Hirst RA, Marczylo TH, Taylor AH, Maccarrone M, and Konje JC (2013). Elevated anandamide and related n-acylethanolamine levels occur in the peripheral blood of women with ectopic pregnancy and are mirrored by changes in peripheral fatty acid amide hydrolase activity. J Clin Endocrinol Metab 98, 1226–1234. [DOI] [PubMed] [Google Scholar]
- Gebeh AK, Willets JM, Marczylo EL, Taylor AH, and Konje JC (2012). Ectopic pregnancy is associated with high anandamide levels and aberrant expression of faah and cb1 in fallopian tubes. J Clin Endocrinol Metab 97, 2827–2835. [DOI] [PubMed] [Google Scholar]
- Gedeon C, Anger G, Piquette-Miller M, and Koren G (2008). Breast cancer resistance protein: Mediating the trans-placental transfer of glyburide across the human placenta. Placenta 29, 39–43. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, and Hanson MA (2004). Living with the past: Evolution, development, and patterns of disease. Science 305, 1733–1736. [DOI] [PubMed] [Google Scholar]
- Gormley M, Ona K, Kapidzic M, Garrido-Gomez T, Zdravkovic T, and Fisher SJ (2017). Preeclampsia: Novel insights from global rna profiling of trophoblast subpopulations. Am J Obstet Gynecol 217, 200 e201–200 e217. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Malhotra SS, Malik A, Verma S, and Chaudhary P (2016). Cell signaling pathways involved during invasion and syncytialization of trophoblast cells. Am J Reprod Immunol 75, 361–371. [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Taylor AH, Bell SC, Taylor DJ, and Konje JC (2008). Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology 149, 5052–5060. [DOI] [PubMed] [Google Scholar]
- Hahnova-Cygalova L, Ceckova M, and Staud F. (2011). Fetoprotective activity of breast cancer resistance protein (bcrp, abcg2): Expression and function throughout pregnancy. Drug Metab Rev 43, 53–68. [DOI] [PubMed] [Google Scholar]
- Hidalgo IJ, Raub TJ, and Borchardt RT (1989). Characterization of the human colon carcinoma cell line (caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96, 736–749. [PubMed] [Google Scholar]
- Holland ML, Lau DT, Allen JD, and Arnold JC (2007). The multidrug transporter abcg2 (bcrp) is inhibited by plant-derived cannabinoids. Br J Pharmacol 152, 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaques SC, Kingsbury A, Henshcke P, Chomchai C, Clews S, Falconer J, Abdel-Latif ME, Feller JM, and Oei JL (2014). Cannabis, the pregnant woman and her child: Weeding out the myths. J Perinatol 34, 417–424. [DOI] [PubMed] [Google Scholar]
- Jebbink J, Veenboer G, Boussata S, Keijser R, Kremer AE, Elferink RO, van der Post J, Afink G, and Ris-Stalpers C (2015). Total bile acids in the maternal and fetal compartment in relation to placental abcg2 expression in preeclamptic pregnancies complicated by hellp syndrome. Biochim Biophys Acta 1852, 131–136. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH, and Schinkel AH (2002). The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A 99, 15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA, Millo J, Sargent IL, and Redman CW (2003). Manipulation of cd98 expression affects both trophoblast cell fusion and amino acid transport activity during syncytialization of human placental bewo cells. J Physiol 550, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, van Ravenzwaay B, Rietjens IM, and Louisse J (2013). Assessment of an in vitro transport model using bewo b30 cells to predict placental transfer of compounds. Arch Toxicol 87, 1661–1669. [DOI] [PubMed] [Google Scholar]
- Lin LS, Roberts VJ, and Yen SS (1995). Expression of human gonadotropin-releasing hormone receptor gene in the placenta and its functional relationship to human chorionic gonadotropin secretion. J Clin Endocrinol Metab 80, 580–585. [DOI] [PubMed] [Google Scholar]
- Liu WM, Duan EK, and Cao YJ (2002). Effects of anandamide on embryo implantation in the mouse. Life Sci 71, 1623–1632. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Di Rienzo M, Battista N, Gasperi V, Guerrieri P, Rossi A, and Finazzi-Agro A (2003). The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through cb1 receptor-dependent inhibition of protein kinase c, activation protein-1, and transglutaminase. J Biol Chem 278, 33896–33903. [DOI] [PubMed] [Google Scholar]
- Mao Q (2008). Bcrp/abcg2 in the placenta: Expression, function and regulation. Pharm Res 25, 1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CW, Buhimschi IA, Buhimschi CS, Dong Y, Weiner CP, and Swaan PW (2011). Atp-binding cassette transporter expression in human placenta as a function of pregnancy condition. Drug Metabolism and Disposition 39, 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CW, Lee GT, Dong Y, Zhou H, He L, and Weiner CP (2014). Effect of prostaglandin e2 on multidrug resistance transporters in human placental cells. Drug Metab Dispos 42, 2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, and Huppertz B (2005). Human placental explants in culture: Approaches and assessments. Placenta 26, 439–448. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Fugedi G, Szabo E, Stenczer B, Walentin S, and Rigo J Jr. (2015). Decreased circulating anandamide levels in preeclampsia. Hypertens Res 38, 413–418. [DOI] [PubMed] [Google Scholar]
- Montminy MR, and Bilezikjian LM (1987). Binding of a nuclear protein to the cyclic-amp response element of the somatostatin gene. Nature 328, 175–178. [DOI] [PubMed] [Google Scholar]
- Moore DG, Turner JD, Parrott AC, Goodwin JE, Fulton SE, Min MO, Fox HC, Braddick FM, Axelsson EL, Lynch S, Ribeiro H, Frostick CJ, and Singer LT (2010). During pregnancy, recreational drug-using women stop taking ecstasy (3,4-methylenedioxy-n-methylamphetamine) and reduce alcohol consumption, but continue to smoke tobacco and cannabis: Initial findings from the development and infancy study. J Psychopharmacol 24, 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhl D, Kathmann M, Hoyer C, Kranaster L, Hellmich M, Gerth CW, Faulhaber J, Schlicker E, and Leweke FM (2014). Increased cb2 mRNA and anandamide in human blood after cessation of cannabis abuse. Naunyn-Schmiedeberg’s Archives of Pharmacology 387, 691–695. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, Ota S, Suzuki M, Kato T, Sekiya T, Kurahashi H, and Udagawa Y (2011). Comparative gene expression profiling of placentas from patients with severe pre-eclampsia and unexplained fetal growth restriction. Reprod Biol Endocrinol 9, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkabinde LA, Shoba-Zikhali LN, Semete-Makokotlela B, Kalombo L, Swai HS, Hayeshi R, Naicker B, Hillie TK, and Hamman JH (2012). Permeation of plga nanoparticles across different in vitro models. Curr Drug Deliv 9, 617–627. [DOI] [PubMed] [Google Scholar]
- Page B, Page M, and Noel C (1993). A new fluorometric assay for cytotoxicity measurements in-vitro. IntJ Oncol 3, 473–476. [PubMed] [Google Scholar]
- Park B, Gibbons ΗM, Mitchell MD, and Glassa M (2003). Identification of the cb1 cannabinoid receptor and fatty acid amide hydrolase (faah) in the human placenta. Placenta 24, 473–478. [DOI] [PubMed] [Google Scholar]
- Passey ME, Sanson-Fisher RW, D’Este CA, and Stirling JM (2014). Tobacco, alcohol and cannabis use during pregnancy: Clustering of risks. Drug Alcohol Depend 134, 44–50. [DOI] [PubMed] [Google Scholar]
- Pattillo RA, and Gey GO (1968). The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Research 28, 1231–1236. [PubMed] [Google Scholar]
- Pattillo RA, Gey GO, Delfs E, and Mattingly RF (1968). Human hormone production in vitro. Science 159, 1467–1469. [DOI] [PubMed] [Google Scholar]
- Pisanti S, Picardi P, D’Alessandro A, Laezza C, and Bifulco M (2013). The endocannabinoid signaling system in cancer. Trends Pharmacol Sci 34, 273–282. [DOI] [PubMed] [Google Scholar]
- Roland CS, Hu J, Ren CE, Chen H, Li J, Varvoutis MS, Leaphart LW, Byck DB, Zhu X, and Jiang SW (2016). Morphological changes of placental syncytium and their implications for the pathogenesis of preeclampsia. Cell Mol Life Sci 73, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, and Pertwee RG (1999). Agonist-in verse agonist characterization at cb1 and cb2 cannabinoid receptors of I759633, I759656, and am630. Br J Pharmacol 126, 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruebner M, Langbein M, Strissel PL, Henke C, Schmidt D, Goecke TW, Faschingbauer F, Schild RL, Beckmann MW, and Strick R (2012). Regulation of the human endogenous retroviral syncytin-1 and cell-cell fusion by the nuclear hormone receptors ppary/rxra in placentogenesis. Journal of Cellular Biochemistry 113, 2383–2396. [DOI] [PubMed] [Google Scholar]
- Schempf AH, and Strobino DM (2008). Illicit drug use and adverse birth outcomes: Is it drugs or context? J Urban Health 85, 858–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitras V, Paulssen RH, Gronaas H, Leirvik J, Hanssen TA, Vartun A, and Acharya G (2009). Differential placental gene expression in severe preeclampsia. Placenta 30, 424–433. [DOI] [PubMed] [Google Scholar]
- Spiro AS, Wong A, Boucher AA, and Arnold JC (2012). Enhanced brain disposition and effects of delta9-tetrahydrocannabinol in p-glycoprotein and breast cancer resistance protein knockout mice. PLoS One 7, e35937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Xie H, Yang J, Wang H, Bradshaw ΗB, and Dey SK (2010). Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc Natl Acad Sci USA 107, 16887–16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi JT, Vetrano AM, Laskin JD, and Aleksunes LM (2017). Localization of the placental bcrp/abcg2 transporter to lipid rafts: Role for cholesterol in mediating efflux activity. Placenta 55, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucco E, Acone G, Marenna A, Pierantoni R, Cacciola G, Chioccarelli T, Mackie K, Fasano S, Colacurci N, Meccariello R, Cobellis G, and Cobellis L (2009). Endocannabinoid system in first trimester placenta: Low faah and high cb1 expression characterize spontaneous miscarriage. Placenta 30, 516–522. [DOI] [PubMed] [Google Scholar]
- Wang H, Unadkat JD, and Mao Q (2008). Hormonal regulation of bcrp expression in human placental bewo cells. Pharm Res 25, 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou L, Gupta A, Vethanayagam RR, Zhang Y, Unadkat JD, and Mao Q (2006). Regulation of bcrp/abcg2 expression by progesterone and 17beta-estradiol in human placental bewo cells. Am J Physiol Endocrinol Metab 290, E798–807. [DOI] [PubMed] [Google Scholar]
- Wang J, Paria BC, Dey SK, and Armant DR (1999). Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol Reprod 60, 839–844. [DOI] [PubMed] [Google Scholar]
- Warner TD, Roussos-Ross D, and Behnke M (2014). It’s not your mother’s marijuana: Effects on maternal-fetal health and the developing child. Clin Perinatol 41, 877–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Moore SF, Martin BR, and Ellis EF (1997). The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther 282, 243–247. [PubMed] [Google Scholar]
- Xiao J, Wang Q, Bircsak KM, Wen X, and Aleksunes LM (2015). Screening of environmental chemicals identifies zearalenone as a novel substrate of the placental bcrp/transporter. Toxicol Res (Camb) 4, 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Sun X, Piao Y, Jegga AG, Handwerger S, Ko MS, and Dey SK (2012). Silencing or amplification of endocannabinoid signaling in blastocysts via cb1 compromises trophoblast cell migration. J Biol Chem 287, 32288–32297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Nakanishi T, Natarajan K, Safren L, Hamburger AW, Hussain A, and Ross DD (2015). Functional cyclic amp response element in the breast cancer resistance protein (bcrp/abcg2) promoter modulates epidermal growth factor receptor pathway- or androgen withdrawal-mediated bcrp/abcg2 transcription in human cancer cells. Biochimica et biophysica acta 1849, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hu X, Gao G, Wang Y, Chen P, and Ye Y (2016). Autophagy protects against oxidized low density lipoprotein-mediated inflammation associated with preeclampsia. Placenta 48, 136–143. [DOI] [PubMed] [Google Scholar]
- Zhao F, Li R, Xiao S, Diao H, Viveiros MM, Song X, and Ye X (2013). Postweaning exposure to dietary zearalenone, a mycotoxin, promotes premature onset of puberty and disrupts early pregnancy events in female mice. Toxicological Sciences 132, 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Po I, Mishin V, Black AT, Heck DE, Laskin DL, Sinko PJ, Gerecke DR, Gordon MK, and Laskin JD (2013). The generation of 4-hydroxynonenal, an electrophilic lipid peroxidation end product, in rabbit cornea organ cultures treated with uvb light and nitrogen mustard. Toxicol Appl Pharmacol 272, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


