Abstract
The neuropathological hallmarks of Parkinson’s disease (PD) are the degeneration and death of dopamine-producing neurons in the ventral midbrain, the widespread intraneuronal aggregation of alpha-synuclein (a-syn) in Lewy bodies and neurites, neuroinflammation, and gliosis. Signs of microglia activation in the PD brain postmortem as well as during disease development revealed by neuroimaging, implicate immune responses in the pathophysiology of the disease. Intensive research during the last two decades has advanced our understanding of the role of these responses in the disease process, yet many questions remain unanswered. A transformative finding in the field has been the confirmation that in vivo microglia are able to respond directly to pathological a-syn aggregates but also to neuronal dysfunction due to intraneuronal a-syn toxicity well in advance of neuronal death. In addition, clinical research and disease models have revealed the involvement of both the innate and adaptive immune systems. Indeed, the data suggest that PD leads not only to a microglia response, but also to a cellular and humoral peripheral immune response. Together, these findings compel us to consider a more holistic view of the immunological processes associated with the disease. Central and peripheral immune responses aimed at maintaining neuronal health will ultimately have consequences on neuronal survival. We will review here the most significant findings that have contributed to the current understanding of the immune response in PD, which is proposed to occur early, involve peripheral and brain immune cells, evolve as neuronal dysfunction progresses, and is likely to influence disease progression.
Keywords: alpha-synuclein, neuroinflammation, monocytes, microglia, T-cells, cytokines, toll-like-receptors, immunomodulation, neurodegeneration, MHC-II, auto-antibodies
GRAPHICAL ABSTRACT
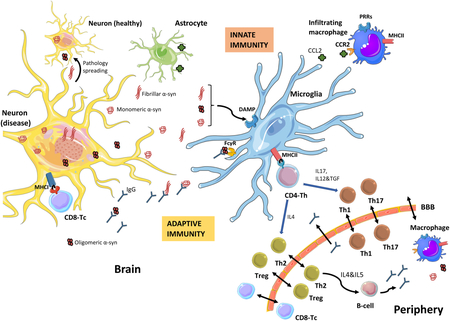
Clinical and basic research have revealed the involvement of both the innate and adaptive immune systems in Parkinson’s disease (PD). PD leads not only to a microglia response in brain, but also to a cellular and humoral peripheral immune response. This has resulted into the current understanding of the immune response in PD, which is proposed to occur early, involve peripheral and brain immune cells, evolve as neuronal dysfunction progresses, and is likely to influence disease progression.
INTRODUCTION
Parkinson’s Disease (PD) is a neurodegenerative disorder characterized by the progressive dysfunction and loss of neurons in the brain, in particular the dopaminergic population in ventral tier of the substantia nigra (SN) pars compacta in the midbrain; as well as by the presence of intraneuronal fibrillar forms of a-synuclein (a-syn) in Lewy bodies and neurites. Besides the neuronal loss, and the Lewy body pathology, PD is characterized by significant gliosis. Although this overt gliosis was first reported in the beginning of the 20th century by Charles de Foix (Foix & Nicolesco, 1925), the contemporary description of microglia activation in human PD brains was reported by McGeer and colleagues in 1988 (McGeer et al., 1988) when they described increased human leukocyte antigen DR (HLA-DR) expression in the SN of PD patients. Their study in human brains together with consecutive reports from other laboratories, showing Tumor Necrosis Factor (TNF) expression in SN from patients (Boka et al., 1994), and the presence of elevated pro-inflammatory cytokines in brain and cerebrospinal fluid (CSF) (Mogi et al., 1994a; Mogi et al., 1994b; Mogi et al., 1995) prompted the “inflammation hypothesis of neurodegeneration” in PD. Recent epidemiological studies in humans have reported that chronic consumption of certain non-steroidal anti-inflammatory drugs (NSAIDs) in mid-life was associated with decreased incidence of PD (Gagne & Power, 2010; Gao et al., 2011b), suggesting an active role for inflammatory responses in PD pathogenesis.
Although the majority of diagnosed PD does not arise from genetic mutations, there is growing appreciation that specific genetic variants in various genes expressed in immune cells can modify risk for development of PD. In particular, mutations in the gene encoding Leucine-rich repeat kinase-2 (LRRK2) are the most common cause of familial PD and makes up about 1% of sporadic PD (Hernandez et al., 2016). Although its function in cells is not completely understood, it is highly expressed in immune cells (Hakimi et al., 2011) and we found it to be increased in immune cells in the peripheral blood of PD patients compared to age-matched healthy controls (Cook et al., 2017); raising the possibility that it may play a role in PD pathogenesis due to its proposed function in the immune system (Dzamko et al., 2015; Cook et al., 2017). Genetic variants in the LRRK2 gene have not only been associated with increased risk for PD, but also for Crohn’s disease (Hui et al., 2018). Highlighting the relevance of the immune system, GWAS analyses have suggested common genetic pathways between PD and autoimmune disease (Witoelar et al., 2017). Furthermore, specific single nucleotide polymorphisms (SNPs) in the TNF gene have been associated with increased risk of PD (Chu et al., 2012), as have SNPs in the HLA genetic loci (Hamza et al., 2010), which encodes part of the major histocompatibility complex (MHC) class II, and that functionally translate to augmented antigen presentation capacity in peripheral immune cells of PD patients who carry the high-risk SNP (Kannarkat et al., 2015). In summary, multiple genetic studies support a role for the immune system in PD pathogenesis.
The resident immune cells of the brain, the microglia, are cells of myeloid linage but they are distinctly different from bone marrow derived peripheral macrophages (Li & Barres, 2018). The microglia are influenced by brain signals, but also respond to peripheral signals during development and during adult life (Matcovitch-Natan et al., 2016). They are essential for neuronal health and they are involved in neuronal plasticity (Weinhard et al., 2018). Based on postmortem analysis of PD brains by the McGeers, the extensive microgliosis was believed to be the consequence of neuronal death that could potentially have a secondary role in neurodegeneration. Thus, most early studies of inflammation and immune responses in PD were focused on the nigrostriatal system in models where neuronal death was present and immune responses prior to degeneration were largely ignored. However, several important findings during the last two decades have modified our understanding of the immune response associated with PD. First, the acknowledgement that a-syn aggregation occurs in multiple brain areas (Braak et al., 2003), and that this aggregation can lead to neuronal dysfunction without cell death (Phan et al., 2017) led researchers to investigate areas where a-syn pathology is present beyond the nigrostriatal pathway. Second, the realization that a-syn neuronal pathology is associated with microglia activation even in the absence of neuronal loss (Sanchez-Guajardo et al., 2010; Barkholt et al., 2012; Watson et al., 2012) suggests that microgliosis is not a mere response to neuronal death. Accordingly, microglia activation in PD patients has been described not only in SN where cell death is significant (Ouchi et al., 2005), but also in other areas of the CNS, such as hippocampus, and cortex (Imamura et al., 2003; Ouchi et al., 2005; Iannaccone et al., 2013; Doorn et al., 2014), areas associated with a-syn pathology and symptoms of the disease, thereby suggesting a relationship between microgliosis, a-syn and neuronal dysfunction that does not require neuronal death.
Another significant change in the field stems from the novel Braak hypothesis, which proposes that a-syn pathology starts somewhere outside the CNS, in the peripheral nervous system (gut or nasal epithelia) (Braak et al., 2003); therefore, advocating for a more holistic view of the disease and suggesting that the peripheral immune cells and not microglia might be the first ones to encounter misfolded a-syn. In that regard, mounting evidence now supports the involvement of peripheral immune cells in PD (reviewed herein). Moreover, it has been suggested that peripheral inflammation via the gastrointestinal system may contribute to disease pathogenesis or that infections can contribute somehow to the risk of developing PD (Deleidi & Isacson, 2012; Houser & Tansey, 2017).
Finally, the discovery of three additional important events related to a-syn have further contributed to this paradigm shift in the field: first, the active release of a-syn by neurons in culture (Emmanouilidou et al., 2010); secondly, that immune cells, primarily microglia and macrophages, are the main cell types clearing extracellular a-syn (Lee et al., 2008); and lastly, the description and acceptance of a-syn as an inflammagen capable of initiating a sterile immune response (reviewed below). Altogether these findings have contributed to the current understanding of the immune response in PD that is now proposed to i) occur early, ii) involve peripheral and brain immune cells, iii) evolve as neuronal dysfunction progresses, and iv) is likely to contribute to disease progression.
The microglia response happens early due to changes in neuronal health and correlates with a-synuclein pathology
Several labs have confirmed the initial observations by the McGeers regarding increased MHCII expression on cells in the brain of PD patients post mortem (McGeer et al., 1988; Croisier et al., 2005; Orr et al., 2005; Miklossy et al., 2006). In addition, multiple animal models of PD have corroborated this microgliosis seen in patients (see (Sanchez-Guajardo et al., 2013b). As mentioned, this microgliosis is not a mere consequence of neuronal death, and we and others have shown that microglia respond early to the effects of a-syn toxicity in neurons, well in advance of cell death while still being modulated by it (Theodore et al., 2008; Sanchez-Guajardo et al., 2010; Barkholt et al., 2012). Moreover, microgliosis in the absence of or prior to cell death has been reported in multiple a-syn based PD models, corroborating that cell death is not necessary to elicit a microglial response (Gomez-Isla et al., 2003; Tofaris et al., 2006; Su et al., 2008; Emmer et al., 2011; Watson et al., 2012). Taken together, these data strongly suggest that cell death is not required for an adaptive microglial response.
A significant observation in PD post-mortem brains was the correlation of MHCII expression in microglia to a-syn pathology (Croisier et al., 2005), but not necessarily to disease duration or motor symptoms (Croisier et al., 2005; Orr et al., 2005); while the expression of the lysosomal protein CD68 was (indirectly) correlated to disease duration (Croisier et al., 2005). These findings argue for an early, long lasting but dynamic response of microglia that changes progressively as disease advances, as a-syn accumulates pathologically and cell death occurs. Indeed, we have shown that in the viral-vector-a-syn rat PD model the microglia early (but steady) response is associated with MHCII expression and that in contrast CD68 is associated with the microglia response during or after cell death (Sanchez-Guajardo et al., 2010; Barkholt et al., 2012). Since the early microglia activation response is related to a-syn aggregation and degeneration and not cell death per se, this would be expected to occur in multiple CNS areas, consistent with post-mortem analyses of PD patient brains (Hunot et al., 1996; Knott et al., 2000; Imamura et al., 2003; Croisier et al., 2005; Halliday & Stevens, 2011; Doorn et al., 2014). The microglia response should be expected to change and evolve with time as the disease progresses and this response will include changes in microglia number (proliferation), cytoskeletal changes (morphological changes), and protein expression (Sanchez-Guajardo et al., 2010; Watson et al., 2012).
The early and progressive microglia activation described above is supported by in vivo imaging human studies; PET binding of the peripheral benzodiazepine receptor (PBR/TSPO) ligand [11C]-(R) PK11195, suggest microglial activation in midbrain of patients with idiopathic REM sleep behavioral disorders (RBD, considered a prodromal form of PD) (Stokholm et al., 2017). The microglia response seems therefore to occur early although is not only restricted to midbrain. Indeed, in fully diagnosed PD patients the microgliosis is not only found in midbrain, but also in other areas such as striatum, pons, cortex and hippocampus (Gerhard et al., 2006; Iannaccone et al., 2013). This widespread microglia activation has also been confirmed using another TSPO ligand, [11C]DPA713, (Terada et al., 2016). Interestingly, this prevalent microglia activation was found at early stages, (PD patients Hoehn& Yahr stages1–2), but it increased with time in particular in cortical areas, suggesting a progressive increase of the immune response as the disease develops and the a-syn pathology and neurodegeneration evolves (Terada et al., 2016). Accordingly, levels of microglia activation (i.e. binding of PK11195 PET) correlates with disease severity as evaluated by the degree of motor defects using the UPRDS assessment (Ouchi et al., 2005). Furthermore, the PK11195 PET signal, increased as the [11C]-CFT (a dopamine transporter ligand, i.e. a marker of dopaminergic terminals) decreased in the striatum in the patients (Ouchi et al., 2009).
This microgliosis, seems to have consequences on neuronal function as cortical microgliosis, PK11195 PET signal, correlates with decreased glucose metabolism and inversely correlates to cognitive scores (MMSE) in PD patients with dementia (Edison et al., 2013). This microglia response, can eventually contribute to neuronal death if a pro-inflammatory event is sustained. Indeed, increased oxidative enzymes COX1 and iNOS (Hunot et al., 1996; Knott et al., 2000) and pro-inflammatory cytokines have been reported to be increased in human PD SN (Boka et al., 1994) and striatum (Nagatsu et al., 2000) and if persistent, this pro-inflammatory response may hasten cell death. Accordingly, in rodents a robust immune activation via intracerebral injection of the bacterial membrane molecule: lipopolysaccharide (LPS) results in nigral dopaminergic cell death (Machado et al., 2011; Hoban et al., 2013).
Peripheral monocytes are modified in Parkinson’s disease
It is suggested that during neurodegeneration peripheral monocytes/macrophages (as mentioned, different myeloid cells than microglia) infiltrate the CNS. Accordingly, proteins that are highly expressed in monocytes/macrophages but not in brain-resident microglia, such as the scavenger receptor CD163 (Polfliet et al., 2006); or the chemokine receptor CCR2 (Mizutani et al., 2012), are present in cells that infiltrate the brain of animal models of PD-like degeneration (Tentillier et al., 2016; Harms et al., 2017; Harms et al., 2018) and in PD and Alzheimer’s patients’ brains post mortem (Pey et al., 2014). Furthermore, analyses of blood cells in patients revealed changes in the immune compartment during active disease. PD patients display increased monocyte precursors in blood and enriched classical monocytes, while the alternatively activated (CD16+, including intermediate and non-classical) monocytes were decreased (Grozdanov et al., 2014). Another study suggested that classical monocytes showed increased levels of CCR2 expression, although the total number of CCR2+ cells were reduced (Funk et al., 2013). The receptor CCR2 has been shown to be critical for recruitment of macrophages and controls the migration of peripheral cells into the CNS (Gliem et al., 2016) where mainly astrocytes produce the respective ligand: the chemokine CCL2 (Farina et al., 2007). The relevance of the infiltrating peripheral monocytes in the degenerative event in PD has been confirmed in rodent PD models of dopaminergic degeneration. The anti-inflammatory targeted modification of the CD163+ monocytes in the 6-OHDA rat model using dexamethasone (Tentillier et al., 2016) or the deletion of the CCR2 (thus avoiding macrophage recruitment) in the viral-vector-a-syn PD mouse model (Harms et al., 2018), both resulted in dopaminergic neuronal protection in SN. CCR2 deletion was however not protective for the SN neurons in the MPTP mouse model (Kalkonde et al., 2007; Parillaud et al., 2017), suggesting that the relevance and number of infiltrating monocytes may be timing and/or insult-dependent. The toxic models, 6-OHDA and MPTP lead to neuronal loss, but not to relevant a-syn pathology (only certain chronic MPTP protocol (not widely used) has been associated to a-syn pathology in mice (Fornai et al., 2005)). While 6-OHDA is intracerebrally administered, MPTP is injected repeatedly in the periphery, and that could account for the differences observed. It should also be noted that MPTP has been reported to directly affect blood lymphocytes and spleen T-cells (Chi et al., 1992), which could also account for the different immune responses. However, an excess of CCL2 production in brain leads to increased infiltration of macrophages that could enhance MPTP-induced dopaminergic death in mice, further suggesting a deleterious role for the infiltrating cells (Parillaud et al., 2017). The authors suggest that it is the microglia that exert a tight regulation on the CCL2-CCR2 axis, and that if microglia are dysfunctional (for example by knocking out CX3CR1), this could lead to neurodegeneration (Parillaud et al., 2017). Thus, healthy microglia may be critical to limit the extent of infiltration of peripheral monocytes into the CNS.
Indeed, microglia health and homeostasis and the CX3CR1-CX3CL1 axis have been shown to be essential for protection of neurons against inflammatory stress and toxicity, both local or peripheral (Cardona et al., 2006). While the CX3CR1 is expressed in microglia and monocytes/macrophages, in brain, neurons produce a membrane-anchored CX3CL1 form that can be cleaved into a soluble circulating form. Several studies have demonstrated that soluble fractalkine (CX3CL1) can protect dopaminergic neurons in PD rodent models. Specifically, treatment with CX3CL1 was neuroprotective in the 6-OHDA rat model (Pabon et al., 2011) and also in the MPTP mouse model (Morganti et al., 2012). Accordingly, the lack of CX3CR1 expression in microglia lead to increase of cell death in the MPTP model (Cardona et al., 2006). However, data from a-syn-based models are thus far contradictory. While one study showed that viral vector-mediated a-syn overexpression in the SN of CX3CR1 knock-out mice resulted in a reduced immune response (compared to wildtype mice) and decreased dopaminergic neuron death (Thome et al., 2015); another lab using the same a-syn model reported that overexpression of soluble fractalkine protected dopaminergic nigral neurons against the a-syn induced toxicity (Nash et al., 2015).
Another important regulatory axis between immune cells and brain is the CD200-CD200R pair. CD200 is expressed in multiple cells including neurons, while the receptor is expressed only in myeloid cells including monocytes/macrophages and microglia, where it serves to limit inflammatory activation (Wright et al., 2000; Wright et al., 2003). Monocyte-derived macrophages from PD patients showed a decreased ability to upregulate CD200R expression upon inflammatory signals, and even when increased, this CD200R was unable to dampen the pro-inflammatory response (Luo et al., 2010). These findings suggest a possible failure in the signal that controls macrophage activation in subjects with PD. In the MPTP model, CD200 has been shown to modulate microglia activation (Ren et al., 2016); and blocking of the CD200R using an antibody leads to increased dopaminergic toxicity by rotenone and iron, further supporting the importance of the CD220-CD200R axis in PD pathophysiology (Wang et al., 2011). Interestingly, in postmortem analysis of dementia with Lewy bodies (DLB), microglia activation and CD200 expression were associated with tau and amyloid pathology rather than with a-syn pathology (Walker et al., 2017). These findings suggest a regional specificity of the immune response in human brain undergoing neurodegeneration.
Monocytes in CSF from PD patients display higher HLA-DR (MHCII) expression compared to those in blood (Fiszer et al., 1994) and interestingly, monocytes infiltrating the brain in a rodent viral-vector-a-syn PD model also express high levels of MHCII (Harms et al., 2017). PD patient monocytes also display elevated TLR2 and TLR4 expression (Drouin-Ouellet et al., 2015), two receptors reported to interact with a-syn (Fellner et al., 2013; Kim et al., 2013). In addition, the monocytes in PD patients seems to have an abnormal response to defined immune stimuli that some have described as exaggerated (Reale et al., 2009; Grozdanov et al., 2014), while others describe them as impaired (Bessler et al., 1999; Hasegawa et al., 2000). Additionally, phagocytic capacities in PD patient monocytes are impaired (Grozdanov et al., 2014; Bliederhaeuser et al., 2016) and they may have reduced capacity to clear a-syn (Gardai et al., 2013). Thus, the peripheral monocyte population in PD is anomalous, it infiltrates brain and could eventually contribute to disease progression by virtue of being dysfunctional and hyper-inflamed.
The Adaptive immune system is also involved in Parkinson’s disease
The expression of MHCII, a protein involved in antigen presentation, by microglia found in the vicinity of a-syn neuronal deposition (Croisier et al., 2005) infers the involvement of the adaptive immune system, with the microglia acting as antigen presenting cells to T-cells. Indeed T-cell infiltration has been observed in the brain of PD patients (Brochard et al., 2009) and in animal models of PD-like degeneration (Theodore et al., 2008; Brochard et al., 2009; Sanchez-Guajardo et al., 2010; Harms et al., 2017). The presence and infiltration of T-cells may be related to a decrease in the phagocytic activity of microglia and an increase in the pro-inflammatory phenotype of the myeloid compartment in the brain (Sommer et al., 2016). The specific relevance of the CD4 T-cell population has been confirmed by the dopaminergic neuroprotection observed when: deleting CD4 cells using the MPTP mouse model (Brochard et al., 2009), or when using a MHC-II global knockout mouse (that also has arrested CD4 population) (Harms et al., 2013) or by enhancing CD4 regulatory T-cells through a-syn vaccination in rats (Sanchez-Guajardo et al., 2013a). Notably, neuronal MHC-I expression, and thus signaling to CD8 T-cells (T-cytotoxic, Tc), has also been implicated in PD-like neurodegeneration and specifically to the selective vulnerability of catecholaminergic neurons in rodents (Cebrian et al., 2014).
Analysis of the lymphocytes in peripheral blood of PD patients suggests an impairment in the CD4 T-helper (Th) cells (Bas et al., 2001; Gruden et al., 2011; Stevens et al., 2012) and alterations in the CD4:CD8 ratio (Bas et al., 2001; Hisanaga et al., 2001; Baba et al., 2005). Moreover, examination of the CD4-Th subpopulations suggests an abnormal distribution with the effector memory (antigen-experienced) fraction overrepresented in detriment to the naïve T-cells (Fiszer et al., 1994; Bas et al., 2001; Saunders et al., 2012; Stevens et al., 2012). A recent study reported that the reduction in CD4-Th cells in PD patients is due to a decrease in Th2, Th17 and regulatory T-cell populations; furthermore the authors reported that CD4-Th cells from PD patients show a Th1-biased immune response with increased IFN-gamma and TNF production, strongly suggesting involvement of CD4 cells in the pathophysiology of the disease (Kustrimovic et al., 2018).
With regards to which antigens are being processed by immune cells and presented via MHC-II to the T-cells, a-syn constitutes an obvious candidate. Indeed, a recent study reported that PD patient-derived T-cells respond to a-syn-derived peptides in ways that T-cells from healthy controls do not (Sulzer et al., 2017). The authors implicate mainly a CD4-Th2 (IL5) response, normally associated with a protective response that could lead to B-cell activation and a protective humoral response (see below). We could speculate that the decrease in the Th2 population observed in patients (Kustrimovic et al., 2018), leads to a loss of this putative protective immune response (for further reading see (Mosley & Gendelman, 2017)). Accordingly, a humoral response in PD patients has been described, which seems to decrease with time, as antibodies recognizing monomeric and fibrillar a-syn are found early in PD but decrease with time (5 vs. 10 years into disease duration) (Gruden et al., 2011; Yanamandra et al., 2011). These findings suggest an early protective role for auto-IgG against-a-syn that is lost with time. Indeed, anti-a-syn antibodies were decreased in PD serum compared to controls in a cohort where disease duration was 10 years (Besong-Agbo et al., 2013). A recent study highlights the importance of the affinity of the auto-anti-a-syn antibody and reports decreased levels of high-affinity autoantibodies against a-syn in PD patients compared to healthy controls (Brudek et al., 2017). The protective role of the auto-IgG could be related to their ability to enhance clearance of a-syn, which seems more efficient via IgG through FcGamma receptors (FcγR) (see below). The reason for the decrease in IgG is unclear, but PD patients show a decrease in the number of B cells (Gruden et al., 2011; Stevens et al., 2012), and they actually produce less IgG (Marttila et al., 1985). But as mentioned above, it could be related to the decrease in the Th2 CD4 cells that might result in decreased B cell activation (Kustrimovic et al., 2018). Interestingly, the IgG deposition in neurons correlated negatively with the degree of cell death in PD brains, thereby suggesting a time-dependent response or possible a positive role in neuron survival (Orr et al., 2005). More in-depth investigations are needed to clarify the role of B-cells in PD pathogenesis and pathophysiology. In summary, the immune response in PD involves both the innate and adaptive immune systems and not only microglia but also peripheral immune cells. Moreover, the data suggest that while some aspects of the immune response seem to drive pathogenesis, others may be aimed at neuroprotection.
Soluble immune biomarkers may help stage disease and correlate with clinical symptoms in Parkinson’s disease
Besides the mentioned changes in autoantibodies, multiple labs have reported abnormal cytokine profiles in blood and CSF from patients. A recent metanalysis reports that pro-inflammatory cytokines such as IL-1b, IL-6 and TNF, but also others such as IL-10 (known for its anti-inflammatory actions) and RANTES (known for its chemotactic properties) are increased in PD, suggesting a complex ongoing regulation of the immune response (Qin et al., 2016). Interestingly, these soluble markers were correlated to non-motor symptoms, which are proposed to precede motor symptomatology (Pessoa Rocha et al., 2014; Kohler et al., 2016). In particular, depression in PD patients has been associated with increased of several cytokines, although there is no clean consensus of which factors are up or down in PD patients relative to healthy controls. Correlations have been reported for: IL-2 (Katsarou et al., 2007; Lindqvist et al., 2012), TNF (Menza et al., 2010; Lindqvist et al., 2012) and IL-6 (Selikhova et al., 2002). However, IL-6 has also been correlated to other markers of disease such as UPDRS and activities of daily living (Muller et al., 1998; Hofmann et al., 2009; Delgado-Alvarado et al., 2017). In summary, the immune system appears to be activated very early in PD and various aspects of the immune response evolve as the disease progresses. However, changes in cytokine levels are not specific to PD as they also found in other neurodegenerative or psychiatric diseases, suggesting common pathogenic inflammatory mechanisms across neurological disease, and supporting the relevance of the immune system in neuronal health and normal brain function. Therefore, no single inflammatory marker represents an ideal PD biomarker, but rather combinations of them or combinations with neuronal-related biomarkers will most likely give a better opportunity to help in future patient selection, diagnosis and prognosis. Specifically, biomarker panels using cytokines/chemokines (Brockmann et al., 2017) or combining neuronal and inflammatory markers (Delgado-Alvarado et al., 2017; Eidson et al., 2017) are being proposed by us an others, as valuable tools to stage disease and potentially to distinguish clinical subtypes, and to monitor disease progression. For example chemokines IL-8, CCL2 (MCP-1) and CCL-4 (MIP-1beta) and the brain derived neurotrophic factor (BDNF) seem to be good markers to discriminate subtypes in PD-LRRK2 patients because these markers are highest in patients with diffuse/malignant PD as compared to those with mainly pure motor disease. (Brockmann et al., 2017). These immune markers, IL-8, CCL2, CCL4, were also correlated to more severe motor defects PD (Reale et al., 2009), and they were also higher in glucocerebrosidase (GBA)-PD (Chahine et al., 2013). Interestingly, in GBD-PD patients IL-8 was correlated with higher cognitive deficits (Chahine et al., 2013), suggesting that an immune component is more readily manifested in cases where the course of PD progression is aggressive and associated with cognitive impairment.
The role of a-synuclein in the microglia response in Parkinson’s disease
As mentioned, one of the key factors influencing the immune response is a-syn pathology. The description of a-syn as a danger associated molecular pattern (DAMP) able of inducing sterile inflammation has deeply influenced the study of the immune response in the disease. The pro-inflammagen property of a-syn in microglia and macrophages has been corroborated multiple times (Kannarkat et al., 2013; Sanchez-Guajardo et al., 2013b; Allen Reish & Standaert, 2015). It was Zhang et al. who first reported the ability of oligomeric a-syn to induce microglia activation that could lead to oxidative stress and neuronal death (Zhang et al., 2005). Moreover, this microglia-driven oxidative stress will lead to oxidation of a-syn in nearby cells which will further feed the neuro-toxic process (Shavali et al., 2006). The a-syn-induced oxidative stress has been related to CD11b and resultant PHOX NADPH oxidase activation (Zhang et al., 2005; Jin et al., 2007; Zhang et al., 2007; Hou et al., 2018). Multiple labs have shown now that a-syn can initiate a pro-inflammatory response in microglia inducing production of IL-6, IL-1β and TNF (Klegeris et al., 2008; Su et al., 2008; Lee et al., 2009; Lee et al., 2010; Roodveldt et al., 2010; Couch et al., 2011). This induced inflammatory profile changes dependent on solubility and a-syn modification (such as truncation) (Fellner et al., 2013). It has been suggested that the pro-inflammatory capacity increases as solubility of a-syn decreases, thus it is likely to be the fibrillar form of a-syn that induces the highest production of TNF and IL-1β (Hoffmann et al., 2016). Therefore, as disease progresses and a-syn aggregates into toxic forms, the proinflammatory effect would also be expected to escalate. In addition, a-syn-induced microglia activation results in release of soluble secondary molecules that can act as chemoattractants (Kim et al., 2009; Wang et al., 2015), thereby leading to the recruitment of immune cells in the vicinity of areas with high levels of pathogenic a-syn. This could therefore explain the described correlation between activated microglia and neurons with a-syn pathology (Croisier et al., 2005).
We should note that microglia have different roles with respect to a-syn and PD: while their pro-inflammatory response can contribute to pathogenesis, other effector functions of microglia such as phagocytosis can contribute to the clearance of extracellular a-syn, which would be predicted to slow disease progression. The activation of cytokine secretion and clearance by phagocytosis are therefore processes that could occur in parallel, although most believe they do not and microglia that are highly secretory are not simultaneously highly phagocytic. Specifically, when in culture, microglia are the cells that most efficiently take up and clear a-syn, and this is dependent on the activation stage of microglia decreasing when a pro-inflammatory profile is acquired (Lee et al., 2008). Accordingly, this phagocytic clearance might be compromised when microglia are exposed to aggregated a-syn due to its pro-inflammagen properties (Park et al., 2008; Choi et al., 2015; York et al., 2017). The solubility of a-syn also influences the uptake of a-syn, increasing with fibrilization (Hoffmann et al., 2016). For example the microglia TLR2 uptake of oligomeric a-syn seems to be conformation specific (Kim et al., 2013), while, the TLR-4 uptake includes all types of a-syn, independent of its solubility (Stefanova et al., 2011). Also FcγR seem to mediate a-syn internalization and clearance in microglia, as well as their activation (Cao et al., 2012). FcγR are IgG receptors that are involved in processing and uptake of opsonized IgG-antigen complexes (Guilliams et al., 2014). FcγR can be inhibitory or activating and it is highly expressed in monocytes, macrophages and dendritic cells (Guilliams et al., 2014). In brain, microglia is the main cell that can express all the different FcγRs, but they are also expressed in neurons and astrocytes (Fuller et al., 2014). Interestingly, the uptake of a-syn by glia FcγR is more efficient if a-syn is complexed with IgG (Bae et al., 2012; Gustafsson et al., 2017). Therefore, as disease progresses the type or amount of a-syn found and/or released by neurons might differ, thus these diverse a-syn forms would be taken up or presented to microglia differently, resulting in distinctive immune response that will evolve as the disease advances.
Besides being responsible for a-syn uptake, TLR 2 and 4 are also able to induce an immune response to a-syn (Fellner et al., 2013; Kim et al., 2013). This interaction signals through the adaptor protein Myeloid Differentiation primary response gene-88 (Myd-88), which leads to NF-κB nuclear translocation (Daniele et al., 2015). The NF-κB transcription factor is consistently associated with a-syn microglia activation (Klegeris et al., 2008; Reynolds et al., 2008; Wilms et al., 2009; Lee et al., 2010; Couch et al., 2011; Hoenen et al., 2016). As mentioned TLR4 not only mediates a-syn inflammatory activation, but also phagocytosis (Fellner et al., 2013). In fact, the absence of TLR4 increases a-syn-induced neurotoxicity and results in elevated a-syn levels and pro-inflammatory markers in an a-syn multiple system atrophy (MSA) mouse model (Stefanova et al., 2011). This paradoxical observation suggests that the decrease of a-syn uptake by the absence of TLR4 results in elevated extracellular a-syn that can further promote a pro-inflammatory response possibly through other receptors (such as CD11b or TLR2). On the other hand, treatment with a selective TLR4 agonist that promoted a-syn clearance without inducing pro-inflammatory activation, resulted in neuroprotection in vivo in the a-syn MSA mouse model (Venezia et al., 2017). In parallel, as mentioned TLR2 has been associated with microglia activation induced by a specific a-syn oligomeric confirmation associated with neuron-released a-syn (Kim et al., 2013). Distinct from TLR4, the absence of TLR2 reduced microglia activation and dopaminergic neuronal cell death associated with a-syn in vivo, possibly suggesting a less relevant role of this receptor in the clearance process (Kim et al., 2013). Additional studies are needed to explore the role of TLR4 in PD. Nevertheless, taken together these data suggest that not one, but several proteins can mediate the effect of a-syn on microglia, thus resulting in a complex event of parallel signaling (for a review please see (Ferreira & Romero-Ramos, 2018)).
Finally, it should be obvious that if Braak’s hypothesis regarding the periphery (gut) being the initiation site of a-syn pathology is correct, the immune system would be expected to play a critical role in limiting the propagation of aggregated a-syn between cells. Specifically, the role of microglia and gut macrophages would be essential, as they should be the cells clearing the misfolded protein and thus avoiding spreading to other healthy neurons. Interestingly, membrane-associated proteins initially linked to the immune system have been proposed as putative a-syn receptors responsible for the spread and propagation of a-syn between neurons. For example, interaction of aggregated a-syn with FcγRIIB (an inhibitory FcγR) in neurons via endocytosis leads to the phosphorylation of the FcγRIIB immunoreceptor tyrosine-based inhibitory motif. This phosphorylation through a downstream mechanism involving SH-containing tyrosine phosphatase 1/2, (SHP 1/2), resulted in enhancement of a-syn uptake (Choi et al., 2018). One study reported that the immune receptor lymphocyte activation gene 3 (LAG3) (Mao et al., 2016) is necessary to mediate the uptake of a-syn in neurons, although expression of LAG3 in neurons has not been confirmed by other studies and LAG3 may be more relevant for uptake of a-syn by immune cells. Finally, TLR2 has been implicated in a-syn uptake not only by microglia, as discussed above, but also by neurons (Dzamko et al., 2017). Therefore, interactions of a-syn with specific membrane proteins on two distinct cell populations, microglia and neurons, has been shown to initiate a cascade of events in neuronal and/or immune cells that could contribute to disease progression.
Is Parkinson’s disease a consequence of changes in the immune system function?
A-syn is expressed ubiquitously in the brain, but the protein is also found in immune cells: microglia, monocytes, lymphocytes and others (Shin et al., 2000). Few labs have addressed the role of a-syn in immune cells, and most work has been done using an a-syn knockout mice. This line showed a defective B cell development and IgG production (Xiao et al., 2014), and a reduction in T cells as well as a defective Th2 phenotype in CD4+ cells (Shameli et al., 2016), which is consistent with the observations in PD patients discussed above. In vitro, the absence of a-syn in microglia lead to a hyper-responsive microglia phenotype with defective phagocytosis (Austin et al., 2006). On the other hand, expression of a-syn in neurons, has also been associated with an immune response to viruses in the brain, in particular to neuron-to-neuron viral transmission (Beatman et al., 2015). Furthermore downregulation of a-syn in dopaminergic neurons leads to a robust immune response, via MHC-I that results in cell death (Benskey et al., 2018), underscoring the relevance of the MHC-I signaling in this neuronal population (Cebrian et al., 2014).
Other PD-associated gene products, such as LRRK2 and GBA are expressed in immune cells and mutations in these genes will most likely result in functional alterations in immune cells. We will shortly highlight recent findings regarding LRRK2, however, a detailed discussion of these proteins is beyond the scope of this review and we refer the reader elsewhere (Dzamko et al., 2015; Cook & Tansey, 2017). Studies regarding the function of LRRK2 on immune cells are numerous. Data from labs using knockouts or knock-down of LRRK2 or the LRRK2 G2019S mutation support a role for LRRK2 in microglia and macrophage motility and their inflammatory response (see review (Lee et al., 2017)); but the extent to which in vivo inflammatory phenotypes are a result of cell autonomous effects of LRRK2 in neurons versus immune cells has yet to be clearly delineated. For example, transgenic LRRK2 G2019S mice injected with LPS display altered peripheral immune responses (rather than alterations in microglia activation) that promoted dopaminergic neurodegeneration via soluble mediators (Kozina et al., 2018). LRRK2 has been associated with phagocytic activity of macrophages and microglia via phosphorylation of Scar (orthologous to human Wiskott-Aldrich syndrome protein-family verproline homologous 2 (Wave2)) that when compromised leads to dopaminergic cell death (Kim et al., 2018). Moreover, the LRRK2 (G2019S) mutation has been associated with expansion of myeloid cells with a bigger suppressive activity on CD4-T cells that could not support a Th17 response (Park et al., 2017). Nevertheless, as mentioned above we have shown that in sporadic PD, LRRK2 protein levels were increased in peripheral blood immune cells in patients compared to age-matched healthy controls (Cook et al., 2017). Together, these studies support a plausible role for LRRK2 in the central and peripheral immune system response in PD that needs to be further investigated at the mechanism level.
PINK1 and parkin have also been associated with immune responses and neuronal dysfunction and death (review in (Dzamko et al., 2015). Lack of parkin was shown by our group to result in nigral dopaminergic degeneration after chronic peripheral inflammation induced by intraperitoneal injections of LPS (Frank-Cannon et al., 2008). We further showed that lack of parkin results in a NFkB-dependent increased production of TNF and IL-6 upon stimulation of macrophages as compared to wildtype cells (Tran et al., 2011). More recent studies demonstrate that PINK and parkin suppress mitochondria antigen presentation through mitochondrial-derived vesicles (Matheoud et al., 2016); specifically, a loss of function of these proteins leads to enhancement of immune signaling through MHCI. Moreover, another paper suggests that failure of PINK and or parkin function can lead to defective mitophagy during mitochondrial damage, which results in stimulator of interferon genes (STING)-mediated inflammatory activation, followed by dopaminergic neurodegeneration in the SN of mice (Sliter et al., 2018). Mitochondrial damage and failure have long been proposed as central pathological events not only in genetic PD but also in idiopathic PD and in relation to a-syn pathology (Grunewald et al., 2018). Together, all of these studies support a role for parkin in immunomodulation and additional investigations into this area is merited.
Infections as putative players in the risk of developing Parkinson’s disease
The possibility that immune cells, in the brain or in the periphery, might play a role in the disease pathogenesis and/or progression, also raise the interesting possibility that early and unrelated inflammatory events in a person’s life, like those induced by infections, might also be associated to the etiology of PD. The importance of peripheral bacterial-related inflammation is supported by the synergistic effect observed in studies using peripheral LPS injections in a-syn based rodent models. A single peripheral injection of LPS in a-syn transgenic mice resulted in persistent inflammation, increase oxidative stress, which induced nitration and aggregation of a-syn resulting in dopaminergic neurodegeneration (Gao et al., 2011a). A key player in this synergy is microglia NOX2 activation (Zhang et al., 2018). Therefore, this raises the possibility that diseases with peripheral inflammatory components could promote a-syn pathology (Houser & Tansey, 2017).
With regards to peripheral organs, several groups have reported alterations in the gut microbiome of PD patients (Hasegawa et al., 2015; Keshavarzian et al., 2015; Scheperjans et al., 2015; Unger et al., 2016; Hill-Burns et al., 2017; Petrov et al., 2017), which may be related to the effects of diets, infections, or genetic variants in immune-related genes that have not been investigated in depth. In association with dysbiosis in one PD cohort (Hill-Burns et al., 2017), our group has been able to detect increased levels of NFkB-regulated inflammatory factors in the stool of patients with sporadic PD relative to household controls (Houser et al., 2018), raising the possibility that gut inflammation may create a permissive environment for non-motor gastrointestinal symptoms and perhaps exacerbation of motor symptoms. For instance, a recent meta-analysis suggest that prevalence of infections with Helicobacter pylori is higher among PD patients; and that those PD patients infected with H. pylori showed more severe motor symptoms, suggesting that the infections can aggravate the disease (Dardiotis et al., 2018). For further consideration of these hypotheses, we refer the reader to a review on how intestinal inflammation could potentially contribute to a-syn upregulation and aggregation in peripheral organs such as the gut that are connected anatomically to the gut in various ways and therefore potentially involved in any propagation of a-syn from the periphery to the CNS (Houser & Tansey, 2017). Finally, although the effects of the gut microbiome on the immune environment of the human brain is likely to be much more complex than in rodents, the relevance of the gut microbiome has also been demonstrated in mouse studies. Gut microbiota has been shown to influence a-syn-induced neurodegeneration, and in fact gut microbiota from PD patients has been reported to enhance the a-syn toxicity, while antibiotics decrease it (Sampson et al., 2016).
Regarding viral infection, several suggestions have been put forward in the literature, related to viruses with certain brain tropism (see review (Olsen et al., 2018). Virus-induced models of PD have been reported following infection with influenza A virus (Takahashi et al., 1995) and Japanese encephalitis virus (Ogata et al., 1997). Influenza virus H5N1 leads to microgliosis and a-syn pathology (phosphorylation) in mouse (Jang et al., 2009). A recent report suggests that inflammation-associated serine-threonine kinase, PKR (EIF2AK2), which is involved in protection against viral infection, leads to phosphorylation of Ser129 in a-syn, which is suggested to play a role in the disease phenotype (Reimer et al., 2018). Therefore, certain viral infections that lead to a-syn pathology could contribute to the onset or progression of neurodegenerative disease. Accordingly, an increase in the incidence of PD has been associated with herpes simplex virus (HSV) and influenza virus infections (Harris et al., 2012; Vlajinac et al., 2013). Interestingly, cross-reactivity between a-syn and an HSV-1 epitope has been detected in PD patients, suggesting that HSV-1 could promote an auto-immune response against a-syn (Caggiu et al., 2016). The same group also reported that homologous HSV1 and a-syn peptide can both stimulate T-cell responses (with TNF production) in PD patients, further suggesting that HSV-1 can contribute to disease phenotype (Caggiu et al., 2017). Additional research is needed to clarify the role of viral infections, if any, in PD pathogenesis and progression.
How is knowledge of the role of the immune system and inflammation changing Parkinson’s disease therapeutic approaches?
The data suggesting a putative neuroprotective role of IgG and the relevance of T-cells have aided in the design of immunotherapy approaches and both active and passive vaccination strategies have been used in PD models and are currently in human trials by Prothena (Jankovic et al., 2018) Biogen (Brys et al., 2018) and Affiris (http://www.affiris.com/news/affiris-announces-encouraging-long-term-data-from-a-series-of-first-in-human-studies-using-affitope-pd01a-targeting-oligomeric-alpha-synuclein-in-early-parkinsons-disease-patients/), among others. The overarching hypothesis underlying the passive immunization approach is that the administration of IgG will enhance a-syn clearance via FcγR. This approach has now been shown to be neuroprotective in vivo in multiple studies (Bae et al., 2012; Games et al., 2014; Mandler et al., 2015; Spencer et al., 2017). The antibodies prevented the spread of a-syn between neurons and astrocytes in a mouse a-syn transgenic, improved motor behavior, and decreased neurodegeneration (Bae et al., 2012). Similarly, passive immunization also decreased axonal a-syn pathology and memory deficits induced by a-syn pathology in the hippocampus (Spencer et al., 2017). In an a-syn MSA mouse model, active a-syn immunization lead to decreased a-syn-induced demyelinization and neurodegeneration, and to reduce a-syn spreading to astrocytes (Mandler et al., 2015). These findings suggest that this approach could potentially exert neuroprotection in different brain areas, cell types and a-syn-related diseases, and result in diminished disease phenotypes.
The passive approach requires carefully selected high-affinity antibodies, as suggested by the findings in PD patients, and the epitope recognized seems to be critically important. Indeed, it has been suggested that anti-a-syn antibodies targeting the N-terminal of human a-syn might be the most efficient in limiting a-syn pathology (Shahaduzzaman et al., 2015). However, other studies report C-terminal-targeted antibodies may be superior (Games et al., 2014). Epitope selection and deletion of the binding domain for FcγR are also required to avoid maladaptive T-cell activation, and other modifications can be also done to enhance brain penetrance (Spencer et al., 2016).
Active immunization with a-syn epitopes however, involves both IgG and cellular responses, and this will also require a careful selection of the epitope as well as of the adjuvant. For example, nitrated a-syn enhanced the effector CD4 T-cell response and induced neurotoxicity in the mouse MPTP model (Reynolds et al., 2010), underscoring the relevance of the protein/peptide chosen. Consistent with this, we have demonstrated that after a-syn immunization the T-cell compartment recognizes and responds to different a-syn modifications (fibrillar and nitrated) in vivo (Olesen et al., 2018). Such responses are essential for the fate of neurons, and the increase or adoptive transfer of regulatory T-cells (CD4+CD25+Foxp3) has been shown to be neuroprotective (Reynolds et al., 2007; Reynolds et al., 2010). Our group has also shown that active a-syn vaccination that leads to an increase in CD4 T-cells and regulatory T-cells in the brain and the heightened humoral response, reduces a-syn pathology in nigrostriatal neurons (Sanchez-Guajardo et al., 2013a). This vaccination strategy was associated with modifications of the peripheral T-cell compartment, hence supporting the role of the peripheral immune system in PD-like degeneration (Christiansen et al., 2016). Therefore, a careful combination of cellular and humoral responses seems like a valid and promising approach, which has been recently corroborated in an a-syn transgenic mouse (Rockenstein et al., 2018). Another novel active vaccination strategy combines a-syn with the chaperone Glucose-related protein-94 (Grp94), which has been shown to result in specific cellular immune responses (Labrador-Garrido et al., 2016) that could ameliorate the microglia activation response in the MPTP mouse model of dopaminergic degeneration, both directly and also by adoptive transfer of the resulting splenocytes (Villadiego et al., 2018). Therefore multiple novel therapeutic approaches are being developed with the intent of modulating the immune system to achieve a neuroprotective profile rather than silencing or suppressing the immune system.
CONCLUSIONS
A wealth of epidemiological, immunohistological, biofluid, and genetic evidence supports a role for the innate and adaptive immune system in the pathophysiology of PD. Although microglia activation was initially thought to be present in post-mortem PD brains as a result of neuronal death, it is now clear that microglia activation occurs early in the disease likely in response to neuronal dysfunction caused by a-syn aggregation and toxicity (Fig. 1). While microglia are likely to be critical in clearing aggregated a-syn, other effector functions such as production of reactive oxygen and nitrogen species and cytokines may bombard neurons with apoptotic signals that compromise their survival and contribute to their demise. Production of chemokines by microglia may also have critical roles in triggering infiltration of peripheral innate and adaptive immune cells into the CNS and importantly the consequences of this infiltration are still being delineated. However, growing evidences support an active role for these peripheral immune cells in the disease progression and the neuronal health. A more in-depth understanding of the immune response and how it evolves as the disease progresses is likely to lead to development of novel and more effective immunomodulatory therapies that can restore the immune response to homeostatic levels, rather than immunosuppress the elderly individual.
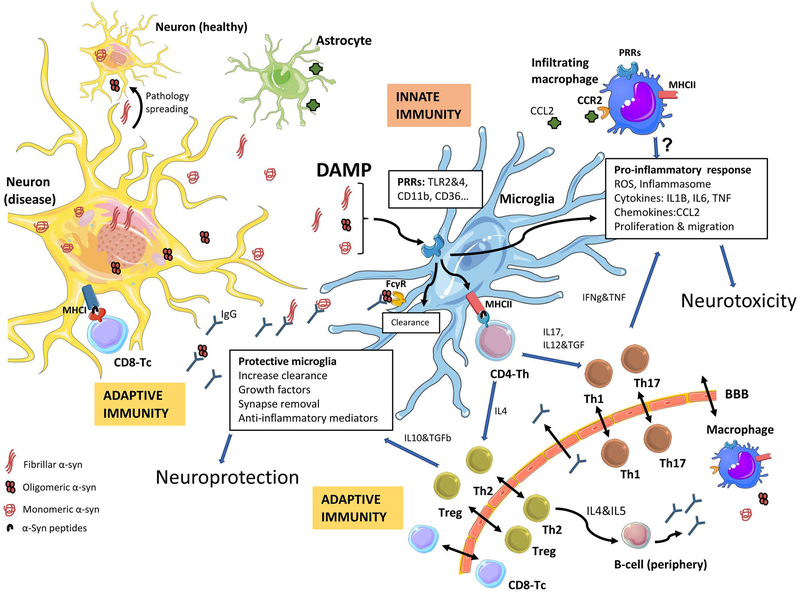
Figure 1. Immune response to alpha-synuclein induced neurodegeneration.
In Parkinson’s disease, α-synuclein (α-syn) undergoes modifications (phosphorylation, nitration, truncation…) and forms toxic oligomers that progressively form aggregates in the nervous system. The neurons, through exocytosis, will release all types of α-syn (monomeric, oligomeric or fibrillar) to the extracellular media, where it will be taken up and cleared by microglia and/or macrophages, but also by astrocytes. If this process fails, the α-syn can be taken up by neighboring neurons, promoting the spread of pathological forms of α-syn. Microglia will also encounter α-syn when phagocyting (trogocytosis) synapses (not shown). α-Syn can act as a damage-associated molecular pattern (DAMP) and via diverse immune receptors, pattern recognition-receptors (PRRs), will initiate a sterile inflammatory response, which will result in a pro-inflammatory immune milieu that will contribute to neuronal dysfunction and neurodegeneration. The α-syn-induced microglia activation also leads to proliferation and migration of microglia and to the infiltration of peripheral monocytes/macrophages, likely through a CCL2-CCR2 process. In parallel, α-syn peptides will be presented by neurons, through MHCI, and by microglia (or by infiltrating monocytes/macrophages from the periphery) through MHCII. This will initiate an adaptive immune response through CD8 (T-cytotoxic cells, Tc) or CD4 (T-helper cells, Th), respectively. Depending on the cytokines produced the CD4-Th cells will undergo differentiation/maturation to various helper subsets, including differentiation into Th1 or Th17 T-cells, which typically potentiate pro-inflammatory events; whereas differentiation into Th2 or regulatory (Treg) T-cells typically resolve the inflammation and lead to B-cell activation (in the periphery) with production of antibodies that may cross the BBB into the CNS. The clearance of α-syn will be aided by the presence of IgG, to mitigate the pro-inflammatory process and the spread of pathologic α-syn to other healthy neurons. In parallel to this event, the α-syn-induced neurodegenerative process will change the expression of proteins involved in neuron-glia interactions, such as CD200 and CX3CL1 (not shown), which will also trigger a microglia response (Image partially constructed using Servier Medical Art).
ACKNOWLEDGEMENTS
Funding support was provided from the Michael J. Fox Foundation (MGT and MR-R), the National Institutes of Health (1RF1AG057247 and 5RF1AG057247, and 3RF1AG051514–01S1 (MGT)), the Bjarne Saxhof Fund administered through the Danish Parkinson’s Foundation (MR-R) and the AUFF AU IDEAS center NEURODIN (MR-R).
ABREVIATIONS
- a-syn
alpha-synuclein
- BDNF
brain derived neurotrophic factor
- CCR2
C-C chemokine receptor type 2
- Th
CD4 T-helper
- CSF
cerebrospinal fluid
- CCL2
chemokine (C-C motif) ligand 2 = monocyte chemoattractant protein 1 (MCP1)
- CCL4
chemokine (C-C motif) ligand 4 = macrophage inflammatory protein 1 beta (MIP-1B)
- CX3CL1
chemokine (C-X3-C motif) ligand 1 = fractalkine
- Cx3CR1
Cx3C chemokine receptor 1
- DAMP
damage-associated molecular pattern
- FcγR
FcGamma receptors
- GBA
glucocerebrosidase
- GWAS
genome-wide association study
- HSV
herpes simplex virus
- MHC
histocompatibility complex
- HLA-DR
human leukocyte antigen DR
- 6-OHDA
6-hydroxydopamine
- IL
Interleukin
- LRRK2
Leucine-rich repeat kinase-2
- LPS
lipopolysaccharide
- LAG-3
lymphocyte activation gene 3
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MSA
multiple system atrophy
- Myd-88
Myeloid Differentiation primary response gene-88
- NSAIDs
non-steroidal anti-inflammatory drugs
- PD
Parkinson’s disease
- PRRs
pattern recognition-receptors
- PBR/TSPO
peripheral benzodiazepine receptor
- PET
Positron emission tomography
- RBD
REM sleep behavioral disorders
- SNPs
single nucleotide polymorphisms
- SN
substantia nigra
- TLR
Toll like receptor
REFERENCES
- Allen Reish HE & Standaert DG (2015) Role of alpha-synuclein in inducing innate and adaptive immunity in Parkinson disease. J. Parkinsons Dis, 5, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin SA, Floden AM, Murphy EJ & Combs CK (2006) Alpha-synuclein expression modulates microglial activation phenotype. J. Neurosci, 26, 10558–10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK & Yamada T (2005) Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat. Disord, 11, 493–498. [DOI] [PubMed] [Google Scholar]
- Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, Desplats P, Masliah E & Lee SJ (2012) Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J. Neurosci, 32, 13454–13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkholt P, Sanchez-Guajardo V, Kirik D & Romero-Ramos M (2012) Long-term polarization of microglia upon alpha-synuclein overexpression in nonhuman primates. Neuroscience. [DOI] [PubMed] [Google Scholar]
- Bas J, Calopa M, Mestre M, Mollevi DG, Cutillas B, Ambrosio S & Buendia E (2001) Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. J. Neuroimmunol, 113, 146–152. [DOI] [PubMed] [Google Scholar]
- Beatman EL, Massey A, Shives KD, Burrack KS, Chamanian M, Morrison TE & Beckham JD (2015) Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain. J. Virol, 90, 2767–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey MJ, Sellnow RC, Sandoval IM, Sortwell CE, Lipton JW & Manfredsson FP (2018) Silencing Alpha Synuclein in Mature Nigral Neurons Results in Rapid Neuroinflammation and Subsequent Toxicity. Front. Mol. Neurosci, 11, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besong-Agbo D, Wolf E, Jessen F, Oechsner M, Hametner E, Poewe W, Reindl M, Oertel WH, Noelker C, Bacher M & Dodel R (2013) Naturally occurring alpha-synuclein autoantibody levels are lower in patients with Parkinson disease. Neurology, 80, 169–175. [DOI] [PubMed] [Google Scholar]
- Bessler H, Djaldetti R, Salman H, Bergman M & Djaldetti M (1999) IL-1 beta, IL-2, IL–6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson’s disease. Biomed. Pharmacother, 53, 141–145. [DOI] [PubMed] [Google Scholar]
- Bliederhaeuser C, Grozdanov V, Speidel A, Zondler L, Ruf WP, Bayer H, Kiechle M, Feiler MS, Freischmidt A, Brenner D, Witting A, Hengerer B, Fandrich M, Ludolph AC, Weishaupt JH, Gillardon F & Danzer KM (2016) Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol, 131, 379–391. [DOI] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y & Hirsch EC (1994) Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett, 172, 151–154. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN & Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging, 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC & Hunot S (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest, 119, 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann K, Schulte C, Schneiderhan-Marra N, Apel A, Pont-Sunyer C, Vilas D, Ruiz-Martinez J, Langkamp M, Corvol JC, Cormier F, Knorpp T, Joos TO, Bernard A, Gasser T, Marras C, Schule B, Aasly JO, Foroud T, Marti-Masso JF, Brice A, Tolosa E, Berg D & Maetzler W (2017) Inflammatory profile discriminates clinical subtypes in LRRK2-associated Parkinson’s disease. Eur. J. Neurol, 24, 427–e426. [DOI] [PubMed] [Google Scholar]
- Brudek T, Winge K, Folke J, Christensen S, Fog K, Pakkenberg B & Pedersen LO (2017) Autoimmune antibody decline in Parkinson’s disease and Multiple System Atrophy; a step towards immunotherapeutic strategies. Mol. Neurodegener, 12, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brys M, Ellenbogen A, Fanning L, Penner N, Yang M, Welch M, Koenig E, David E, Fox T, Makh S, Aldred J, Goodman I, Graham D, Weihofen A & Cedarbaum J (2018) Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose Study of Anti-Alpha-Synuclein Antibody BIIB054 in Patients with Parkinson’s Disease (S26.001). Neurology, 90. [Google Scholar]
- Caggiu E, Paulus K, Arru G, Piredda R, Sechi GP & Sechi LA (2016) Humoral cross reactivity between alpha-synuclein and herpes simplex-1 epitope in Parkinson’s disease, a triggering role in the disease? J. Neuroimmunol, 291, 110–114. [DOI] [PubMed] [Google Scholar]
- Caggiu E, Paulus K, Galleri G, Arru G, Manetti R, Sechi GP & Sechi LA (2017) Homologous HSV1 and alpha-synuclein peptides stimulate a T cell response in Parkinson’s disease. J. Neuroimmunol, 310, 26–31. [DOI] [PubMed] [Google Scholar]
- Cao S, Standaert DG & Harms AS (2012) The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J. Neuroinflammation, 9, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR & Ransohoff RM (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci, 9, 917–924. [DOI] [PubMed] [Google Scholar]
- Cebrian C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, Kanter E, Budhu S, Mandelbaum J, Vonsattel JP, Zecca L, Loike JD & Sulzer D (2014) MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun, 5, 3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine LM, Qiang J, Ashbridge E, Minger J, Yearout D, Horn S, Colcher A, Hurtig HI, Lee VM, Van Deerlin VM, Leverenz JB, Siderowf AD, Trojanowski JQ, Zabetian CP & Chen-Plotkin A (2013) Clinical and biochemical differences in patients having Parkinson disease with vs without GBA mutations. JAMA Neurol, 70, 852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi DS, Gong L, Daigneault EA & Kostrzewa RM (1992) Effects of MPTP and vitamin E treatments on immune function in mice. Int. J. Immunopharmacol, 14, 739–746. [DOI] [PubMed] [Google Scholar]
- Choi YR, Cha SH, Kang SJ, Kim JB, Jou I & Park SM (2018) Prion-like Propagation of alpha-Synuclein Is Regulated by the FcgammaRIIB-SHP-1/2 Signaling Pathway in Neurons. Cell Rep, 22, 136–148. [DOI] [PubMed] [Google Scholar]
- Choi YR, Kang SJ, Kim JM, Lee SJ, Jou I, Joe EH & Park SM (2015) FcgammaRIIB mediates the inhibitory effect of aggregated alpha-synuclein on microglial phagocytosis. Neurobiol. Dis, 83, 90–99. [DOI] [PubMed] [Google Scholar]
- Christiansen JR, Olesen MN, Otzen DE, Romero-Ramos M & Sanchez-Guajardo V (2016) alpha-Synuclein vaccination modulates regulatory T cell activation and microglia in the absence of brain pathology. J. Neuroinflammation, 13, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Zhou X & Luo BY (2012) Cytokine gene polymorphisms and Parkinson’s disease: a meta-analysis. Can. J. Neurol. Sci, 39, 58–64. [DOI] [PubMed] [Google Scholar]
- Cook DA, Kannarkat GT, Cintron AF, Butkovich LM, Fraser KB, Chang J, Grigoryan N, Factor SA, West AB, Boss JM & Tansey MG (2017) LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinsons Dis, 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DA & Tansey MG (2017) LRRK2. Springer. [Google Scholar]
- Couch Y, Alvarez-Erviti L, Sibson NR, Wood MJ & Anthony DC (2011) The acute inflammatory response to intranigral alpha-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J. Neuroinflammation, 8, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK & Graeber MB (2005) Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J. Neuroinflammation, 2, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele SG, Beraud D, Davenport C, Cheng K, Yin H & Maguire-Zeiss KA (2015) Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci Signal, 8, ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardiotis E, Tsouris Z, Mentis A-FA, Siokas V, Michalopoulou A, Sokratous M, Dastamani M, Bogdanos DP, Deretzi G & Kountouras J (2018) H. pylori and Parkinson’s disease: Meta-analyses including clinical severity. Clin. Neurol. Neurosurg, 175, 16–24. [DOI] [PubMed] [Google Scholar]
- Deleidi M & Isacson O (2012) Viral and inflammatory triggers of neurodegenerative diseases. Sci. Transl. Med, 4, 121ps123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Alvarado M, Gago B, Gorostidi A, Jimenez-Urbieta H, Dacosta-Aguayo R, Navalpotro-Gomez I, Ruiz-Martinez J, Bergareche A, Marti-Masso JF, Martinez-Lage P, Izagirre A & Rodriguez-Oroz MC (2017) Tau/alpha-synuclein ratio and inflammatory proteins in Parkinson’s disease: An exploratory study. Mov. Disord, 32, 1066–1073. [DOI] [PubMed] [Google Scholar]
- Doorn KJ, Moors T, Drukarch B, van de Berg W, Lucassen PJ & van Dam AM (2014) Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun, 2, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin-Ouellet J, St-Amour I, Saint-Pierre M, Lamontagne-Proulx J, Kriz J, Barker RA & Cicchetti F (2015) Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson’s disease. Int. J. Neuropsychopharmacol, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, Geczy CL & Halliday GM (2015) Inflammation is genetically implicated in Parkinson’s disease. Neuroscience, 302, 89–102. [DOI] [PubMed] [Google Scholar]
- Dzamko N, Gysbers A, Perera G, Bahar A, Shankar A, Gao J, Fu Y & Halliday GM (2017) Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol, 133, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison P, Ahmed I, Fan Z, Hinz R, Gelosa G, Ray Chaudhuri K, Walker Z, Turkheimer FE & Brooks DJ (2013) Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology, 38, 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, Kannarkat GT, Barnum CJ, Chang J, Chung J, Caspell-Garcia C, Taylor P, Mollenhauer B, Schlossmacher MG, Ereshefsky L, Yen M, Kopil C, Frasier M, Marek K, Hertzberg VS & Tansey MG (2017) Candidate inflammatory biomarkers display unique relationships with alpha-synuclein and correlate with measures of disease severity in subjects with Parkinson’s disease. J. Neuroinflammation, 14, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L & Vekrellis K (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci, 30, 6838–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer KL, Waxman EA, Covy JP & Giasson BI (2011) E46K human alpha-synuclein transgenic mice develop Lewy-like and tau pathology associated with age-dependent, detrimental motor impairment. J. Biol. Chem, 286, 35104–35118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Aloisi F & Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol, 28, 138–145. [DOI] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK & Stefanova N (2013) Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia, 61, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SA & Romero-Ramos M (2018) Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front. Cell. Neurosci, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszer U, Mix E, Fredrikson S, Kostulas V & Link H (1994) Parkinson’s disease and immunological abnormalities: increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol. Scand, 90, 160–166. [DOI] [PubMed] [Google Scholar]
- Foix C & Nicolesco J (1925) Anatomie cérébrale. Les noyaux gris centraux et la región Mésencéphalo-sous-optique., Suivi d’un apéndice sur l’anatomie pathologique de la maladie de Parkinson. Masson et Cie, Paris. [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A & Sudhof TC (2005) Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc. Natl. Acad. Sci. U. S. A, 102, 3413–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O’Brien DE, Casey B, Goldberg MS & Tansey MG (2008) Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci, 28, 10825–10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller JP, Stavenhagen JB & Teeling JL (2014) New roles for Fc receptors in neurodegeneration-the impact on Immunotherapy for Alzheimer’s Disease. Front. Neurosci, 8, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk N, Wieghofer P, Grimm S, Schaefer R, Buhring HJ, Gasser T & Biskup S (2013) Characterization of peripheral hematopoietic stem cells and monocytes in Parkinson’s disease. Mov. Disord, 28, 392–395. [DOI] [PubMed] [Google Scholar]
- Gagne JJ & Power MC (2010) Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology, 74, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Valera E, Spencer B, Rockenstein E, Mante M, Adame A, Patrick C, Ubhi K, Nuber S, Sacayon P, Zago W, Seubert P, Barbour R, Schenk D & Masliah E (2014) Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J. Neurosci, 34, 9441–9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, Kam W, Wilson B & Hong JS (2011a) Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect, 119, 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen H, Schwarzschild MA & Ascherio A (2011b) Use of ibuprofen and risk of Parkinson disease. Neurology, 76, 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai SJ, Mao W, Schule B, Babcock M, Schoebel S, Lorenzana C, Alexander J, Kim S, Glick H, Hilton K, Fitzgerald JK, Buttini M, Chiou SS, McConlogue L, Anderson JP, Schenk DB, Bard F, Langston JW, Yednock T & Johnston JA (2013) Elevated alpha-synuclein impairs innate immune cell function and provides a potential peripheral biomarker for Parkinson’s disease. PLoS One, 8, e71634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB & Brooks DJ (2006) In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis, 21, 404–412. [DOI] [PubMed] [Google Scholar]
- Gliem M, Schwaninger M & Jander S (2016) Protective features of peripheral monocytes/macrophages in stroke. Biochim. Biophys. Acta, 1862, 329–338. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Irizarry MC, Mariash A, Cheung B, Soto O, Schrump S, Sondel J, Kotilinek L, Day J, Schwarzschild MA, Cha JH, Newell K, Miller DW, Ueda K, Young AB, Hyman BT & Ashe KH (2003) Motor dysfunction and gliosis with preserved dopaminergic markers in human alpha-synuclein A30P transgenic mice. Neurobiol. Aging, 24, 245–258. [DOI] [PubMed] [Google Scholar]
- Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A, Hengerer B, Kassubek J, Ludolph AC, Weishaupt JH & Danzer KM (2014) Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol, 128, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden MA, Sewell RD, Yanamandra K, Davidova TV, Kucheryanu VG, Bocharov EV, Bocharova OR, Polyschuk VV, Sherstnev VV & Morozova-Roche LA (2011) Immunoprotection against toxic biomarkers is retained during Parkinson’s disease progression. J. Neuroimmunol, 233, 221–227. [DOI] [PubMed] [Google Scholar]
- Grunewald A, Kumar KR & Sue CM (2018) New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol [DOI] [PubMed] [Google Scholar]
- Guilliams M, Bruhns P, Saeys Y, Hammad H & Lambrecht BN (2014) The function of Fcgamma receptors in dendritic cells and macrophages. Nat. Rev. Immunol, 14, 94–108. [DOI] [PubMed] [Google Scholar]
- Gustafsson G, Eriksson F, Moller C, da Fonseca TL, Outeiro TF, Lannfelt L, Bergstrom J & Ingelsson M (2017) Cellular Uptake of alpha-Synuclein Oligomer-Selective Antibodies is Enhanced by the Extracellular Presence of alpha-Synuclein and Mediated via Fcgamma Receptors. Cell. Mol. Neurobiol, 37, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi M, Selvanantham T, Swinton E, Padmore RF, Tong Y, Kabbach G, Venderova K, Girardin SE, Bulman DE, Scherzer CR, LaVoie MJ, Gris D, Park DS, Angel JB, Shen J, Philpott DJ & Schlossmacher MG (2011) Parkinson’s disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J Neural Transm (Vienna), 118, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM & Stevens CH (2011) Glia: initiators and progressors of pathology in Parkinson’s disease. Mov. Disord, 26, 6–17. [DOI] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA & Payami H (2010) Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet, 42, 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C & Standaert DG (2013) MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci, 33, 9592–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Delic V, Thome AD, Bryant N, Liu Z, Chandra S, Jurkuvenaite A & West AB (2017) alpha-Synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathol Commun, 5, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Thome AD, Yan Z, Schonhoff AM, Williams GP, Li X, Liu Y, Qin H, Benveniste EN & Standaert DG (2018) Peripheral monocyte entry is required for alpha-Synuclein induced inflammation and Neurodegeneration in a model of Parkinson disease. Exp. Neurol, 300, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MA, Tsui JK, Marion SA, Shen H & Teschke K (2012) Association of Parkinson’s disease with infections and occupational exposure to possible vectors. Mov. Disord, 27, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A, Ohno K & Hirayama M (2015) Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS One, 10, e0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Inagaki T, Sawada M & Suzumura A (2000) Impaired cytokine production by peripheral blood mononuclear cells and monocytes/macrophages in Parkinson’s disease. Acta Neurol. Scand, 101, 159–164. [DOI] [PubMed] [Google Scholar]
- Hernandez DG, Reed X & Singleton AB (2016) Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J. Neurochem [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R & Payami H (2017) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord, 32, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga K, Asagi M, Itoyama Y & Iwasaki Y (2001) Increase in peripheral CD4 bright+ CD8 dull+ T cells in Parkinson disease. Arch. Neurol, 58, 1580–1583. [DOI] [PubMed] [Google Scholar]
- Hoban DB, Connaughton E, Connaughton C, Hogan G, Thornton C, Mulcahy P, Moloney TC & Dowd E (2013) Further characterisation of the LPS model of Parkinson’s disease: a comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain. Behav. Immun, 27, 91–100. [DOI] [PubMed] [Google Scholar]
- Hoenen C, Gustin A, Birck C, Kirchmeyer M, Beaume N, Felten P, Grandbarbe L, Heuschling P & Heurtaux T (2016) Alpha-Synuclein Proteins Promote Pro-Inflammatory Cascades in Microglia: Stronger Effects of the A53T Mutant. PLoS One, 11, e0162717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Ettle B, Bruno A, Kulinich A, Hoffmann AC, von Wittgenstein J, Winkler J, Xiang W & Schlachetzki JC (2016) Alpha-synuclein activates BV2 microglia dependent on its aggregation state. Biochem. Biophys. Res. Commun, 479, 881–886. [DOI] [PubMed] [Google Scholar]
- Hofmann KW, Schuh AF, Saute J, Townsend R, Fricke D, Leke R, Souza DO, Portela LV, Chaves ML & Rieder CR (2009) Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochem. Res, 34, 1401–1404. [DOI] [PubMed] [Google Scholar]
- Hou L, Bao X, Zang C, Yang H, Sun F, Che Y, Wu X, Li S, Zhang D & Wang Q (2018) Integrin CD11b mediates alpha-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol, 14, 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser MC, Chang J, Factor SA, Molho ES, Zabetian CP, Hill-Burns EM, Payami H, Hertzberg VS & Tansey MG (2018) Stool Immune Profiles Evince Gastrointestinal Inflammation in Parkinson’s Disease. Mov. Disord, 33, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser MC & Tansey MG (2017) The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis, 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KY, Fernandez-Hernandez H, Hu J, Schaffner A, Pankratz N, Hsu NY, Chuang LS, Carmi S, Villaverde N, Li X, Rivas M, Levine AP, Bao X, Labrias PR, Haritunians T, Ruane D, Gettler K, Chen E, Li D, Schiff ER, Pontikos N, Barzilai N, Brant SR, Bressman S, Cheifetz AS, Clark LN, Daly MJ, Desnick RJ, Duerr RH, Katz S, Lencz T, Myers RH, Ostrer H, Ozelius L, Payami H, Peter Y, Rioux JD, Segal AW, Scott WK, Silverberg MS, Vance JM, Ubarretxena-Belandia I, Foroud T, Atzmon G, Pe’er I, Ioannou Y, McGovern DPB, Yue Z, Schadt EE, Cho JH & Peter I (2018) Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y & Hirsch EC (1996) Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience, 72, 355–363. [DOI] [PubMed] [Google Scholar]
- Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, Gelsomino G, Moresco RM & Perani D (2013) In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat. Disord, 19, 47–52. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M & Hashizume Y (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol, 106, 518–526. [DOI] [PubMed] [Google Scholar]
- Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R & Smeyne RJ (2009) Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl. Acad. Sci. U. S. A, 106, 14063–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, Goodman I, Safirstein B, Marmon TK, Schenk DB, Koller M, Zago W, Ness DK, Griffith SG, Grundman M, Soto J, Ostrowitzki S, Boess FG, Martin-Facklam M, Quinn JF, Isaacson SH, Omidvar O, Ellenbogen A & Kinney GG (2018) Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-alpha-Synuclein Monoclonal Antibody, in Patients With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol, 75, 1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Shie FS, Liu J, Wang Y, Davis J, Schantz AM, Montine KS, Montine TJ & Zhang J (2007) Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J. Neuroinflammation, 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkonde YV, Morgan WW, Sigala J, Maffi SK, Condello C, Kuziel W, Ahuja SS & Ahuja SK (2007) Chemokines in the MPTP model of Parkinson’s disease: absence of CCL2 and its receptor CCR2 does not protect against striatal neurodegeneration. Brain Res, 1128, 1–11. [DOI] [PubMed] [Google Scholar]
- Kannarkat GT, Boss JM & Tansey MG (2013) The role of innate and adaptive immunity in Parkinson’s disease. J. Parkinsons Dis, 3, 493–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannarkat GT, Cook DA, Lee JK, Chang J, Chung J, Sandy E, Paul KC, Ritz B, Bronstein J, Factor SA, Boss JM & Tansey MG (2015) Common Genetic Variant Association with Altered HLA Expression, Synergy with Pyrethroid Exposure, and Risk for Parkinson’s Disease: An Observational and Case-Control Study. NPJ Parkinsons Dis, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsarou Z, Bostantjopoulou S, Hatzizisi O, Giza E, Soler-Cardona A & Kyriazis G (2007) [Immune factors or depression? Fatigue correlates in Parkinson’s disease]. Rev. Neurol, 45, 725–728. [PubMed] [Google Scholar]
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E & Shannon KM (2015) Colonic bacterial composition in Parkinson’s disease. Mov. Disord, 30, 1351–1360. [DOI] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ & Lee SJ (2013) Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun, 4, 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Marcogliese PC, Yang J, Callaghan SM, Resende V, Abdel-Messih E, Marras C, Visanji NP, Huang J, Schlossmacher MG, Trinkle-Mulcahy L, Slack RS, Lang AE, Canadian Lrrk2 in Inflammation, T. & Park DS (2018) Regulation of myeloid cell phagocytosis by LRRK2 via WAVE2 complex stabilization is altered in Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A, 115, E5164–E5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Cho SH, Kim KY, Shin KY, Kim HS, Park CH, Chang KA, Lee SH, Cho D & Suh YH (2009) Alpha-synuclein induces migration of BV-2 microglial cells by up-regulation of CD44 and MT1-MMP. J. Neurochem, 109, 1483–1496. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG & McGeer PL (2008) Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol. Aging, 29, 739–752. [DOI] [PubMed] [Google Scholar]
- Knott C, Stern G & Wilkin GP (2000) Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and −2. Mol. Cell. Neurosci, 16, 724–739. [DOI] [PubMed] [Google Scholar]
- Kohler O, Krogh J, Mors O & Benros ME (2016) Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol, 14, 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozina E, Sadasivan S, Jiao Y, Dou Y, Ma Z, Tan H, Kodali K, Shaw T, Peng J & Smeyne RJ (2018) Mutant LRRK2 mediates peripheral and central immune responses leading to neurodegeneration in vivo. Brain, 141, 1753–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustrimovic N, Comi C, Magistrelli L, Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F, Minafra B, Riboldazzi G, Sturchio A, Mauri M, Bono G, Marino F & Cosentino M (2018) Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J. Neuroinflammation, 15, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrador-Garrido A, Cejudo-Guillen M, Daturpalli S, Leal MM, Klippstein R, De Genst EJ, Villadiego J, Toledo-Aral JJ, Dobson CM, Jackson SE, Pozo D & Roodveldt C (2016) Chaperome screening leads to identification of Grp94/Gp96 and FKBP4/52 as modulators of the alpha-synuclein-elicited immune response. FASEB J, 30, 564–577. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, Kim WK, Junn E & Kim HS (2010) Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J. Immunol, 185, 615–623. [DOI] [PubMed] [Google Scholar]
- Lee H, James WS & Cowley SA (2017) LRRK2 in peripheral and central nervous system innate immunity: its link to Parkinson’s disease. Biochem. Soc. Trans, 45, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ & Lee SJ (2008) Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem. Biophys. Res. Commun, 372, 423–428. [DOI] [PubMed] [Google Scholar]
- Lee SB, Park SM, Ahn KJ, Chung KC, Paik SR & Kim J (2009) Identification of the amino acid sequence motif of alpha-synuclein responsible for macrophage activation. Biochem. Biophys. Res. Commun, 381, 39–43. [DOI] [PubMed] [Google Scholar]
- Li Q & Barres BA (2018) Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol, 18, 225–242. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y & Hansson O (2012) Non-motor symptoms in patients with Parkinson’s disease - correlations with inflammatory cytokines in serum. PLoS One, 7, e47387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Zhang JJ, Zhang CD, Liu R, Zheng L, Wang XJ, Chen SD & Ding JQ (2010) Altered regulation of CD200 receptor in monocyte-derived macrophages from individuals with Parkinson’s disease. Neurochem. Res, 35, 540–547. [DOI] [PubMed] [Google Scholar]
- Machado A, Herrera AJ, Venero JL, Santiago M, de Pablos RM, Villaran RF, Espinosa-Oliva AM, Arguelles S, Sarmiento M, Delgado-Cortes MJ, Maurino R & Cano J (2011) Inflammatory Animal Model for Parkinson’s Disease: The Intranigral Injection of LPS Induced the Inflammatory Process along with the Selective Degeneration of Nigrostriatal Dopaminergic Neurons. ISRN Neurol, 2011, 476158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler M, Valera E, Rockenstein E, Mante M, Weninger H, Patrick C, Adame A, Schmidhuber S, Santic R, Schneeberger A, Schmidt W, Mattner F & Masliah E (2015) Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol. Neurodegener, 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, Kang HC, Zhang J, Xu J, Chen R, Park H, Andrabi SA, Kang SU, Goncalves RA, Liang Y, Zhang S, Qi C, Lam S, Keiler JA, Tyson J, Kim D, Panicker N, Yun SP, Workman CJ, Vignali DA, Dawson VL, Ko HS & Dawson TM (2016) Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marttila RJ, Eskola J, Soppi E & Rinne UK (1985) Immune functions in Parkinson’s disease lymphocyte subsets, concanavalin A-induced suppressor cell activity and in vitro immunoglobulin production. J. Neurol. Sci, 69, 121–131. [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada Gonzalez F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, Elinav E, Sieweke MH, Schwartz M & Amit I (2016) Microglia development follows a stepwise program to regulate brain homeostasis. Science, 353, aad8670. [DOI] [PubMed] [Google Scholar]
- Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, Fazel A, Bergeron JJ, Trudeau LE, Burelle Y, Gagnon E, McBride HM & Desjardins M (2016) Parkinson’s Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell, 166, 314–327. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE & McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology, 38, 1285–1291. [DOI] [PubMed] [Google Scholar]
- Menza M, Dobkin RD, Marin H, Mark MH, Gara M, Bienfait K, Dicke A & Kusnekov A (2010) The role of inflammatory cytokines in cognition and other non-motor symptoms of Parkinson’s disease. Psychosomatics, 51, 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG & McGeer PL (2006) Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp. Neurol, 197, 275–283. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM & Cardona AE (2012) The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J. Immunol, 188, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Narabayashi H, Riederer P & Nagatsu T (1995) Transforming growth factor-beta 1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson’s disease. Neurosci. Lett, 193, 129–132. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M & Nagatsu T (1994a) Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett, 180, 147–150. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K & Nagatsu T (1994b) Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett, 165, 208–210. [DOI] [PubMed] [Google Scholar]
- Morganti JM, Nash KR, Grimmig BA, Ranjit S, Small B, Bickford PC & Gemma C (2012) The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. J. Neurosci, 32, 14592–14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley RL & Gendelman HE (2017) T cells and Parkinson’s disease. Lancet Neurol, 16, 769–771. [DOI] [PubMed] [Google Scholar]
- Muller T, Blum-Degen D, Przuntek H & Kuhn W (1998) Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson’s disease. Acta Neurol. Scand, 98, 142–144. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H & Togari A (2000) Cytokines in Parkinson’s disease. J. Neural Transm. Suppl, 143–151. [PubMed] [Google Scholar]
- Nash KR, Moran P, Finneran DJ, Hudson C, Robinson J, Morgan D & Bickford PC (2015) Fractalkine over expression suppresses alpha-synuclein-mediated neurodegeneration. Mol. Ther, 23, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata A, Tashiro K, Nukuzuma S, Nagashima K & Hall WW (1997) A rat model of Parkinson’s disease induced by Japanese encephalitis virus. J. Neurovirol, 3, 141–147. [DOI] [PubMed] [Google Scholar]
- Olesen MN, Christiansen JR, Petersen SV, Jensen PH, Paslawski W, Romero-Ramos M & Sanchez-Guajardo V (2018) CD4 T cells react to local increase of alpha-synuclein in a pathology-associated variant-dependent manner and modify brain microglia in absence of brain pathology. Heliyon, 4, e00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen Laura K., Dowd E & McKernan Declan P. (2018) A role for viral infections in Parkinson’s etiology? Neuronal Signaling, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Mizuno Y, Mori H & Halliday GM (2005) A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain, 128, 2665–2674. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yagi S, Yokokura M & Sakamoto M (2009) Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat. Disord, 15 Suppl 3, S200–204. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T & Torizuka T (2005) Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol, 57, 168–175. [DOI] [PubMed] [Google Scholar]
- Pabon MM, Bachstetter AD, Hudson CE, Gemma C & Bickford PC (2011) CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson’s disease. J. Neuroinflammation, 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parillaud VR, Lornet G, Monnet Y, Privat AL, Haddad AT, Brochard V, Bekaert A, de Chanville CB, Hirsch EC, Combadiere C, Hunot S & Lobsiger CS (2017) Analysis of monocyte infiltration in MPTP mice reveals that microglial CX3CR1 protects against neurotoxic over-induction of monocyte-attracting CCL2 by astrocytes. J. Neuroinflammation, 14, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee JW, Cooper SC, Broxmeyer HE, Cannon JR & Kim CH (2017) Parkinson disease-associated LRRK2 G2019S transgene disrupts marrow myelopoiesis and peripheral Th17 response. J. Leukoc. Biol, 102, 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Paik SR, Jou I & Park SM (2008) Microglial phagocytosis is enhanced by monomeric alpha-synuclein, not aggregated alpha-synuclein: implications for Parkinson’s disease. Glia, 56, 1215–1223. [DOI] [PubMed] [Google Scholar]
- Pessoa Rocha N, Reis HJ, Vanden Berghe P & Cirillo C (2014) Depression and cognitive impairment in Parkinson’s disease: a role for inflammation and immunomodulation? Neuroimmunomodulation, 21, 88–94. [DOI] [PubMed] [Google Scholar]
- Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB, Tyakht AV, Kovarsky BA, Alekseev DG, Kostryukova ES, Mironova YS, Izhboldina OP, Nikitina MA, Perevozchikova TV, Fait EA, Babenko VV, Vakhitova MT, Govorun VM & Sazonov AE (2017) Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med, 162, 734–737. [DOI] [PubMed] [Google Scholar]
- Pey P, Pearce RK, Kalaitzakis ME, Griffin WS & Gentleman SM (2014) Phenotypic profile of alternative activation marker CD163 is different in Alzheimer’s and Parkinson’s disease. Acta Neuropathol Commun, 2, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan JA, Stokholm K, Zareba-Paslawska J, Jakobsen S, Vang K, Gjedde A, Landau AM & Romero-Ramos M (2017) Early synaptic dysfunction induced by alpha-synuclein in a rat model of Parkinson’s disease. Sci. Rep, 7, 6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polfliet MM, Fabriek BO, Daniels WP, Dijkstra CD & van den Berg TK (2006) The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology, 211, 419–425. [DOI] [PubMed] [Google Scholar]
- Qin XY, Zhang SP, Cao C, Loh YP & Cheng Y (2016) Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol, 73, 1316–1324. [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M & Onofrj M (2009) Peripheral cytokines profile in Parkinson’s disease. Brain. Behav. Immun, 23, 55–63. [DOI] [PubMed] [Google Scholar]
- Reimer L, Vesterager LB, Betzer C, Zheng J, Nielsen LD, Kofoed RH, Lassen LB, Bolcho U, Paludan SR, Fog K & Jensen PH (2018) Inflammation kinase PKR phosphorylates alpha-synuclein and causes alpha-synuclein-dependent cell death. Neurobiol. Dis, 115, 17–28. [DOI] [PubMed] [Google Scholar]
- Ren Y, Ye M, Chen S & Ding J (2016) CD200 Inhibits Inflammatory Response by Promoting KATP Channel Opening in Microglia Cells in Parkinson’s Disease. Med. Sci. Monit, 22, 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE & Mosley RL (2007) Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J. Leukoc. Biol, 82, 1083–1094. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Kadiu I, Garg SK, Glanzer JG, Nordgren T, Ciborowski P, Banerjee R & Gendelman HE (2008) Nitrated alpha-synuclein and microglial neuroregulatory activities. J. Neuroimmune Pharmacol, 3, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL & Gendelman HE (2010) Regulatory T Cells Attenuate Th17 Cell-Mediated Nigrostriatal Dopaminergic Neurodegeneration in a Model of Parkinson’s Disease. J. Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Ostroff G, Dikengil F, Rus F, Mante M, Florio J, Adame A, Trinh I, Kim C, Overk C, Masliah E & Rissman RA (2018) Combined Active Humoral and Cellular Immunization Approaches for the Treatment of Synucleinopathies. J. Neurosci, 38, 1000–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodveldt C, Labrador-Garrido A, Gonzalez-Rey E, Fernandez-Montesinos R, Caro M, Lachaud CC, Waudby CA, Delgado M, Dobson CM & Pozo D (2010) Glial innate immunity generated by non-aggregated alpha-synuclein in mouse: differences between wild-type and Parkinson’s disease-linked mutants. PLoS One, 5, e13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R & Mazmanian SK (2016) Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell, 167, 1469–1480 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Annibali A, Jensen PH & Romero-Ramos M (2013a) alpha-Synuclein vaccination prevents the accumulation of parkinson disease-like pathologic inclusions in striatum in association with regulatory T cell recruitment in a rat model. J. Neuropathol. Exp. Neurol, 72, 624–645. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Barnum CJ, Tansey MG & Romero-Ramos M (2013b) Neuroimmunological processes in Parkinson’s disease and their relation to alpha-synuclein: microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro, 5, 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D & Romero-Ramos M (2010) Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One, 5, e8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Estes KA, Kosloski LM, Allen HE, Dempsey KM, Torres-Russotto DR, Meza JL, Santamaria PM, Bertoni JM, Murman DL, Ali HH, Standaert DG, Mosley RL & Gendelman HE (2012) CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J. Neuroimmune Pharmacol, 7, 927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K & Auvinen P (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord, 30, 350–358. [DOI] [PubMed] [Google Scholar]
- Selikhova MV, Kushlinskii NE, Lyubimova NV & Gusev EI (2002) Impaired production of plasma interleukin-6 in patients with Parkinson’s disease. Bull. Exp. Biol. Med, 133, 81–83. [DOI] [PubMed] [Google Scholar]
- Shahaduzzaman M, Nash K, Hudson C, Sharif M, Grimmig B, Lin X, Bai G, Liu H, Ugen KE, Cao C & Bickford PC (2015) Anti-human alpha-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-alpha-synuclein rat model of Parkinson’s disease. PLoS One, 10, e0116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shameli A, Xiao W, Zheng Y, Shyu S, Sumodi J, Meyerson HJ, Harding CV & Maitta RW (2016) A critical role for alpha-synuclein in development and function of T lymphocytes. Immunobiology, 221, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavali S, Combs CK & Ebadi M (2006) Reactive macrophages increase oxidative stress and alpha-synuclein nitration during death of dopaminergic neuronal cells in co-culture: relevance to Parkinson’s disease. Neurochem. Res, 31, 85–94. [DOI] [PubMed] [Google Scholar]
- Shin EC, Cho SE, Lee DK, Hur MW, Paik SR, Park JH & Kim J (2000) Expression patterns of alpha-synuclein in human hematopoietic cells and in Drosophila at different developmental stages. Mol. Cells, 10, 65–70. [DOI] [PubMed] [Google Scholar]
- Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, Cai H, Borsche M, Klein C & Youle RJ (2018) Parkin and PINK1 mitigate STING-induced inflammation. Nature, 561, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Fadler T, Dorfmeister E, Hoffmann AC, Xiang W, Winner B & Prots I (2016) Infiltrating T lymphocytes reduce myeloid phagocytosis activity in synucleinopathy model. J. Neuroinflammation, 13, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Valera E, Rockenstein E, Overk C, Mante M, Adame A, Zago W, Seubert P, Barbour R, Schenk D, Games D, Rissman RA & Masliah E (2017) Anti-alpha-synuclein immunotherapy reduces alpha-synuclein propagation in the axon and degeneration in a combined viral vector and transgenic model of synucleinopathy. Acta Neuropathol Commun, 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Williams S, Rockenstein E, Valera E, Xin W, Mante M, Florio J, Adame A, Masliah E & Sierks MR (2016) alpha-synuclein conformational antibodies fused to penetratin are effective in models of Lewy body disease. Ann Clin Transl Neurol, 3, 588–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W & Wenning GK (2011) Toll-Like Receptor 4 Promotes alpha-Synuclein Clearance and Survival of Nigral Dopaminergic Neurons. Am. J. Pathol, 179, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CH, Rowe D, Morel-Kopp MC, Orr C, Russell T, Ranola M, Ward C & Halliday GM (2012) Reduced T helper and B lymphocytes in Parkinson’s disease. J. Neuroimmunol, 252, 95–99. [DOI] [PubMed] [Google Scholar]
- Stokholm MG, Iranzo A, Ostergaard K, Serradell M, Otto M, Svendsen KB, Garrido A, Vilas D, Borghammer P, Santamaria J, Moller A, Gaig C, Brooks DJ, Tolosa E & Pavese N (2017) Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. [DOI] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K & Federoff HJ (2008) Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol. Aging, 29, 1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS & Sette A (2017) T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature, 546, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Yamada T, Nakajima S, Nakajima K, Yamamoto T & Okada H (1995) The substantia nigra is a major target for neurovirulent influenza A virus. J. Exp. Med, 181, 2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tentillier N, Etzerodt A, Olesen MN, Rizalar FS, Jacobsen J, Bender D, Moestrup SK & Romero-Ramos M (2016) Anti-Inflammatory Modulation of Microglia via CD163-Targeted Glucocorticoids Protects Dopaminergic Neurons in the 6-OHDA Parkinson’s Disease Model. J. Neurosci, 36, 9375–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T, Yokokura M, Yoshikawa E, Futatsubashi M, Kono S, Konishi T, Miyajima H, Hashizume T & Ouchi Y (2016) Extrastriatal spreading of microglial activation in Parkinson’s disease: a positron emission tomography study. Ann. Nucl. Med, 30, 579–587. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cao S, McLean PJ & Standaert DG (2008) Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol, 67, 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome AD, Standaert DG & Harms AS (2015) Fractalkine Signaling Regulates the Inflammatory Response in an alpha-Synuclein Model of Parkinson Disease. PLoS One, 10, e0140566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M & Spillantini MG (2006) Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): implications for Lewy body disorders. J. Neurosci, 26, 3942–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TA, Nguyen AD, Chang J, Goldberg MS, Lee JK & Tansey MG (2011) Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor-kappa B. PLoS One, 6, e23660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J, Fassbender K, Schwiertz A & Schafer KH (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord, 32, 66–72. [DOI] [PubMed] [Google Scholar]
- Venezia S, Refolo V, Polissidis A, Stefanis L, Wenning GK & Stefanova N (2017) Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal alpha-synucleinopathy. Mol. Neurodegener, 12, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadiego J, Labrador-Garrido A, Franco JM, Leal-Lasarte M, De Genst EJ, Dobson CM, Pozo D, Toledo-Aral JJ & Roodveldt C (2018) Immunization with alpha-synuclein/Grp94 reshapes peripheral immunity and suppresses microgliosis in a chronic Parkinsonism model. Glia, 66, 191–205. [DOI] [PubMed] [Google Scholar]
- Vlajinac H, Dzoljic E, Maksimovic J, Marinkovic J, Sipetic S & Kostic V (2013) Infections as a risk factor for Parkinson’s disease: a case-control study. Int. J. Neurosci, 123, 329–332. [DOI] [PubMed] [Google Scholar]
- Walker DG, Lue LF, Tang TM, Adler CH, Caviness JN, Sabbagh MN, Serrano GE, Sue LI & Beach TG (2017) Changes in CD200 and intercellular adhesion molecule-1 (ICAM-1) levels in brains of Lewy body disorder cases are associated with amounts of Alzheimer’s pathology not alpha-synuclein pathology. Neurobiol. Aging, 54, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chu CH, Stewart T, Ginghina C, Wang Y, Nie H, Guo M, Wilson B, Hong JS & Zhang J (2015) alpha-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc. Natl. Acad. Sci. U. S. A, 112, E1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Zhang S, Yan ZQ, Zhao YX, Zhou HY, Wang Y, Lu GQ & Zhang JD (2011) Impaired CD200-CD200R-mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free Radic. Biol. Med, 50, 1094–1106. [DOI] [PubMed] [Google Scholar]
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB & Chesselet MF (2012) Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp. Neurol, 237, 318–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y & Gross CT (2018) Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature communications, 9, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms H, Rosenstiel P, Romero-Ramos M, Arlt A, Schafer H, Seegert D, Kahle PJ, Odoy S, Claasen JH, Holzknecht C, Brandenburg LO, Deuschl G, Schreiber S, Kirik D & Lucius R (2009) Suppression of MAP kinases inhibits microglial activation and attenuates neuronal cell death induced by alpha-synuclein protofibrils. Int. J. Immunopathol. Pharmacol, 22, 897–909. [DOI] [PubMed] [Google Scholar]
- Witoelar A, Jansen IE, Wang Y, Desikan RS, Gibbs JR, Blauwendraat C, Thompson WK, Hernandez DG, Djurovic S, Schork AJ, Bettella F, Ellinghaus D, Franke A, Lie BA, McEvoy LK, Karlsen TH, Lesage S, Morris HR, Brice A, Wood NW, Heutink P, Hardy J, Singleton AB, Dale AM, Gasser T, Andreassen OA, Sharma M, International Parkinson’s Disease Genomics Consortium, N.A.B.E.C. & United Kingdom Brain Expression Consortium, I. (2017) Genome-wide Pleiotropy Between Parkinson Disease and Autoimmune Diseases. JAMA Neurol, 74, 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu MR, Brown MH, Sedgwick JD, Phillips JH & Barclay AN (2003) Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol, 171, 3034–3046. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH & Barclay AN (2000) Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity, 13, 233–242. [DOI] [PubMed] [Google Scholar]
- Xiao W, Shameli A, Harding CV, Meyerson HJ & Maitta RW (2014) Late stages of hematopoiesis and B cell lymphopoiesis are regulated by alpha-synuclein, a key player in Parkinson’s disease. Immunobiology, 219, 836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K, Gruden MA, Casaite V, Meskys R, Forsgren L & Morozova-Roche LA (2011) alpha-synuclein reactive antibodies as diagnostic biomarkers in blood sera of Parkinson’s disease patients. PLoS One, 6, e18513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York EM, Bernier LP & MacVicar BA (2017) Microglial modulation of neuronal activity in the healthy brain. Dev. Neurobiol [DOI] [PubMed] [Google Scholar]
- Zhang W, Dallas S, Zhang D, Guo JP, Pang H, Wilson B, Miller DS, Chen B, Zhang W, McGeer PL, Hong JS & Zhang J (2007) Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia, 55, 1178–1188. [DOI] [PubMed] [Google Scholar]
- Zhang W, Gao JH, Yan ZF, Huang XY, Guo P, Sun L, Liu Z, Hu Y, Zuo LJ, Yu SY, Cao CJ, Wang XM & Hong JS (2018) Minimally Toxic Dose of Lipopolysaccharide and alpha-Synuclein Oligomer Elicit Synergistic Dopaminergic Neurodegeneration: Role and Mechanism of Microglial NOX2 Activation. Mol. Neurobiol, 55, 619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS & Zhang J (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J, 19, 533–542. [DOI] [PubMed] [Google Scholar]