Abstract
Background:
Mitochondrial DNA (mtDNA) copy number varies by cell type and energy demands. Blood mtDNA copy number has been associated with neurocognitive function in persons without HIV. Low mtDNA copy number may indicate disordered mtDNA replication; high copy number may reflect a response to mitochondrial dysfunction. We hypothesized that blood mtDNA copy number estimated from genome-wide genotyping data is related to neurocognitive impairment (NCI) in persons with HIV.
Methods:
In the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study, peripheral blood mtDNA copy number was obtained from genome-wide genotyping data as a ratio of mtDNA SNP probe intensities relative to nuclear DNA SNPs. In a multivariable regression model, associations between mtDNA copy number and demographics, blood cell counts, and HIV disease and treatment characteristics were tested. Associations of mtDNA copy number with the global deficit score (GDS), GDS-defined NCI (GDS≥0.5), and HIV-associated neurocognitive disorder (HAND) diagnosis were tested by logistic regression, adjusting for potential confounders.
Results:
Among 1,010 CHARTER participants, lower mtDNA copy number was associated with longer antiretroviral therapy duration (p<0.001), but not with d-drug exposure (p=0.85). mtDNA copy number was also associated with GDS (p=0.007), GDS-defined NCI (p<0.001), and HAND (p=0.002). In all analyses, higher mtDNA copy number was associated with poorer cognitive performance.
Conclusions:
Higher mtDNA copy number estimated from peripheral blood genotyping was associated with worse neurocognitive performance in adults with HIV. These results suggest a connection between peripheral blood mtDNA and NCI, and may represent increased mtDNA replication in response to mitochondrial dysfunction.
Keywords: DNA, Mitochondrial, HIV, Neurocognitive Disorders
INTRODUCTION
Effective antiretroviral therapy (ART) in persons with chronic HIV infection has greatly decreased the incidence of the most severe neurocognitive complication, HIV-Associated Dementia (HAD)1. Despite this improvement, milder forms of neurocognitive impairment (NCI) remain common in this population. The overall prevalence of HIV-Associated Neurocognitive Disorder (HAND) remains 30–50%2, and some studies suggest that asymptomatic forms of NCI confer risk of progression to symptomatic HAND3. The prevalence of NCI in persons with HIV may increase as the average age of the population continues to increase4, 5.
A major determinant of neuronal function is energy production from mitochondria6, 7. Mitochondria contain their own genome, with many copies of mitochondrial DNA (mtDNA) per cell. In general, the amount of mtDNA per cell closely relates to cellular energy demands, so cells with higher energy requirements (e.g. neurons and muscle cells) have more mtDNA copies8. Very high or very low mtDNA copy numbers may indicate mitochondrial dysfunction9–11. High mtDNA copy numbers are observed in patients with mitochondrial diseases who have impaired oxidative phosphorylation9, 10. This phenomenon is generally attributed to a cellular compensatory mechanism, raising mitochondrial biogenesis in response to impaired mitochondrial function12. Conversely, impairment of the mitochondrial biogenesis process itself, including mtDNA replication, causes low mtDNA levels11.
Different methods are used to quantify mtDNA copy number in a blood or tissue sample. Most include quantitative polymerase chain reaction (PCR) and involve a comparison of the amount of mtDNA to the amount of nuclear DNA. In general, these methods do not give absolute counts of mtDNA molecules per cell, but are instead relative measures with higher values indicating higher mtDNA copy number per cell13. The mtDNA copy number in peripheral blood, normalized by a nuclear DNA copy number measure, has often been used as a general proxy for the mitochondrial function in an individual. Cross-sectional population studies of blood mtDNA copy number show that the relationship to age is complex, with lower blood mtDNA copy number found in either the young or the old compared to middle-aged persons14–17. Peripheral blood mtDNA copy number has been linked to several neurological and neurocognitive phenotypes in adults without HIV18–25.
Studies of peripheral blood mtDNA copy number in persons with HIV have predominantly focused on the relationship between mtDNA copy number and HIV treatment effects26–30. No previous studies have compared peripheral blood mtDNA levels (either directly measured or estimated) and neurocognitive function in a population with HIV. We used a new method to estimate peripheral blood mtDNA copy number using the fluorescence intensity of mtDNA single-nucleotide polymorphism (SNP) probes on a genome-wide genotyping platform14. This method could allow for estimation of relative mtDNA copy number in larger populations than traditional quantitation methods. Our objective was to determine factors associated with relative mtDNA copy number, and our hypothesis was that since the central nervous system (CNS) is particularly vulnerable to mitochondrial damage, mtDNA copy number in peripheral blood would be associated with NCI in adults with HIV.
METHODS
Participants
The CNS HIV Antiretroviral Therapy Effects Research (CHARTER) is a prospective cohort study of neurologic complications of HIV infection and treatment conducted at six U.S. locations: San Diego, California; Baltimore, Maryland; Galveston, Texas; New York, New York; Seattle, Washington; and St Louis, Missouri. Institutional review boards at each site approved the study, and each participant provided written informed consent. All data utilized for these analyses were de-identified. Data were collected between 2003 and 2007 according to a protocol of comprehensive neurological, behavioral, and laboratory assessments that were standardized across sites2.
Assessment of Neurocognitive Impairment
Participants were English-speaking and underwent a comprehensive test battery that included seven neurocognitive domains2. Composite Global Deficit Score (GDS) values were derived from standardized T-scores using best available normative standards to correct for practice effects31, as well as age, education, sex, and ethnicity, as appropriate. For persons of self-reported Hispanic ethnicity, three of 15 measures were corrected for English-speaking Hispanic normative standards; the remainder were adjusted for non-Hispanic European normative standards32. The GDS as a continuous variable reflects the number and severity of neurocognitive deficits across the battery; it is the average of the deficit scores on each test, where T≥40=0 (no deficit), 35–39=1 (mild deficit), 30–34=2 (mild to moderate deficit), 25–29=3 (moderate deficit), 20–24=4 (moderate to severe deficit), and <20=5 (severe deficit). An established cutoff of GDS≥0.50 defines NCI33.
To further classify presence and severity of HAND, a published algorithm that has excellent inter-rater reliability for the presence of NCI was used34. This algorithm conforms to the Frascati criteria for diagnosing HAND35, requiring at least mild impairment in at least two of seven domains, and includes functional assessment by self-report, performance-based criteria, or both, as well as exclusions based on comorbidities (non-HIV-related risks for NCI). The assessment of HAND status in CHARTER participants required a determination that any NCI was likely due to HIV-related effects on the brain rather than comorbid conditions2. Detailed review by two senior CHARTER investigators using published guidelines35 provided categorization of neuropsychiatric comorbid conditions for all CHARTER participants as either incidental (minimal), contributing (mild-moderate), or confounding (severe) with respect to neurocognitive performance. Participants with confounding neurocognitive comorbidities, which precluded an assessment of the contribution of HIV infection to their neurocognitive performance (e.g., brain trauma, seizures, or CNS opportunistic infections), were not eligible for a diagnosis of HAND according to Frascati criteria2, 35 and were excluded from CHARTER genetic studies. Categories of HAND include asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HAD. Standardized assessments were performed by trained study personnel certified by the CHARTER coordinating center. For this analysis, we combined these three groups (ANI, MND and HAD) into a single “HAND” group, and also considered ANI separately from MND and HAD.
Genetics
Whole blood samples were collected in PAXgene tubes, and isolation of genomic nuclear DNA was performed using Puregene (Gentra Systems Inc., Minneapolis, MN, USA). Study participants had genome-wide DNA genotyping available using the Affymetrix Genome-Wide Human SNP Array 6.0 (Affymetrix, Inc., Santa Clara, CA, USA). Ancestry-informative markers were extracted from the autosomal DNA genotypes and were analyzed using EIGENSTRAT software36 to generate principal components (PC). In addition to genome-wide nuclear genotyping, full mtDNA sequencing was also performed on study participants using the GeneChip Human Mitochondrial Resequencing Array v2.0 (Affymetrix, Inc., Santa Clara, CA, USA). These data were processed using the MitoChip Filtering Protocol (MFP)37, variants were called relative to the Revised Cambridge Reference Sequence (rCRS)38, and mtDNA haplogroups were assigned using the HaploGrep program (http://haplogrep.uibk.ac.at/)39. Genome-wide genotyping data was used for the mtDNA copy number assessment as described below. Data from the mtDNA-specific genotyping chip was only used for assessing mtDNA haplogroups, not for the mtDNA copy number assessment.
MtDNA copy number measurement
Relative mtDNA copy number can be estimated from the genotyping SNP probe intensity data of genome-wide genotyping arrays14. The Affymetrix Genome-Wide Human SNP Array data were analyzed using the Affymetrix Genotyping Console. Only samples with call rates >95% were selected for further processing. For each homozygous SNP determination in an individual, the fluorescence intensity in the SNP was used, minus the fluorescence intensity in the uncalled allele as a measure of the background fluorescence “noise” level. Then, the median of this value for the mtDNA SNP probes and the median for the autosomal SNP probes were calculated. The ratio of these medians was used as an estimate of the relative copy number of the mtDNA compared to the nuclear DNA. A variant of this mtDNA copy number estimate method based on 25 genotyped mtDNA SNPs was tested against the standard quantitative PCR method40. That study concluded that the microarray-based method for mtDNA copy number was valid, with a Spearman r of 0.51 compared with PCR.
Since mtDNA copy number per cell varies greatly depending on cell type, the distribution of cell types in the peripheral blood can strongly affect the mtDNA copy number in peripheral blood. Since platelets contain mtDNA but no nuclear DNA, higher platelet count has been shown consistently to increase mtDNA copy number in peripheral blood16, 41, 42. Conversely, higher white blood cell (WBC) counts correlate with relatively lower peripheral blood mtDNA copy number per cell16, because WBCs also contain nuclear DNA. We included the platelet/WBC ratio as a covariate in the statistical models to correct for both, as described previously43.
Clinical covariates
For the analysis of the relationship between mtDNA copy number in peripheral blood and HAND, several clinical covariates were included. To represent the course of the disease treatment, we included nadir CD4+ T-cell count, estimated duration of HIV infection, duration of ART exposure, and plasma HIV RNA. We also adjusted for duration of exposure to dideoxynucleoside ART drugs (stavudine [d4T], didanosine [ddI], or zalcitabine [ddC]), collectively referred to as d-drugs, since this class has been particularly associated with mitochondrial toxicity, at least in part through disruption of mtDNA replication44–47. All covariates were collected at the same CHARTER visit as the DNA collection.
Statistical analyses
Univariate comparisons were performed by t-test for continuous variables and chi-squared test for dichotomous variables. Associations were also tested using multivariable logistic regression for dichotomous outcomes (GDS-defined NCI and HAND) and by linear regression for continuous outcomes (continuous GDS and mtDNA copy number). HAND outcomes were dichotomized as neurocognitively normal, or as impairment of any severity (ANI, MND, or HAD). Considering the importance of comorbidity to NCI, we performed stratified analyses by comorbidity severity (incidental vs. contributing; participants with severe comorbidity were excluded). We also tested for an association of mtDNA copy number with mtDNA haplogroups, with participants stratified into European, African, and Hispanic ancestry based on nuclear DNA-derived PC, as previously described48. All analyses were performed using the latest version of R.
RESULTS
Of 1,010 CHARTER participants with genome-wide genotyping and full clinical covariates available, most (78%) were men, with a median age of 44 years (Table 1). Median estimated duration of HIV infection was just over 10 years, and the median nadir and current CD4+ T-cell counts were 173 and 437 cells/mm3, respectively. Among the 748 (74%) on ART, the median ART duration was 5.6 years and 451 (60%) had plasma HIV RNA <50 copies/mL. Approximately 45% of participants had at least past exposure to d-drugs, with 122 (12% of the total; 16% of those on ART) receiving a d-drug at the study visit. At the time of the mtDNA copy number estimation, 35% of the participants had GDS values in the impaired range, and 45% of the participants were classified as having HAND.
Table 1.
Study demographics, clinical variables, and neurocognitive outcomes.
| Variable | |
|---|---|
| Total N | 1,010 |
| Female, N (%) | 227 (23) |
| Age in years, median (IQR) | 44 (39–49) |
| Non-Hispanic Black, N (%) | 469 (46) |
| Non-Hispanic White, N (%) | 429 (43) |
| Hispanic, N (%) | 94 (9) |
| Other race/ethnicity, N (%) | 18 (2) |
| Estimated duration of HIV in months, median (IQR) | 127 (60–191) |
| Currently on ART, N (%) | 748 (74) |
| Estimated total duration of ART in months, median (IQR)* | 68 (28–103) |
| Ever d-drug exposure, N (%) | 458 (45) |
| Duration d-drug exposure in those exposed in months, median (IQR) | 36 (17–66) |
| Current d-drug use, N (%)* | 122 (16) |
| Current CD4+ T-cell count, median (IQR) cells/mm3 | 437 (283–610) |
| Nadir CD4+ T-cell count, median (IQR) cells/mm3 | 173 (50–297) |
| Plasma HIV RNA <50 copies/mL, N (%)* | 451 (60) |
| Contributing comorbidities, N (%) | 359 (36) |
| GDS, median (IQR) | 0.33 [0.11–0.67] |
| GDS impairment (GDS ≥ 0.5), N (%) | 352 (35) |
| HAND, N (%) | 457 (45) |
Among those participants on ART at the study visit. IQR=interquartile range; GDS=global deficit score; HAND=HIV-associated neurocognitive disorder
The relative mtDNA copy number estimate from whole blood genotyping in the population was normally distributed (p=0.19 by Shapiro-Wilk test for normality). Consistent with previous reports in populations without HIV14–16, relative mtDNA copy number values differed significantly by sex (p<0.001), with females having higher values (Supplementary Figure). Multivariable linear regression modeling of the mtDNA copy number was adjusted for platelet/WBC ratio, age, sex, genetic PC 1–3, nadir CD4+ T-cell count, estimated duration of HIV, and duration of ART and d-drug exposure (Table 2). Sex remained highly significantly associated with mtDNA copy number (p=0.001). The most significant association with relative mtDNA copy number was with platelet/WBC ratio; higher copy number was associated with higher platelet/WBC ratio, as expected16. Participant age and the first three ancestry PC (representing the racial and ethnic diversity of the cohort) were not significantly associated with mtDNA copy number. Relative mtDNA copy number was also not significantly associated with mtDNA haplogroups (data not shown).
Table 2.
Adjusted associations between demographic and clinical variables and relative mtDNA copy number.
| Variable | Coefficient (±2 SE) | P value |
|---|---|---|
| Platelet / WBC ratio | 0.01 ± 0.001 | <0.001 |
| Age (per 10 years) | −0.03 ± 0.05 | 0.18 |
| Female sex | 0.16 ± 0.09 | 0.001 |
| Genetic PC1 | 1.40 ± 1.55 | 0.07 |
| Genetic PC2 | −0.96 ± 1.51 | 0.20 |
| Genetic PC3 | −1.01 ± 1.47 | 0.17 |
| Nadir CD4+ T-cell count (per 100 cells) | 0.008 ± 0.02 | 0.44 |
| Estimated duration of HIV (per year) | 0.0005 ± 0.01 | 0.89 |
| Estimated duration of ART (per year) | −0.02 ± 0.01 | 0.001 |
| Estimated d-drug exposure (per year) | −0.001 ± 0.01 | 0.85 |
For the HIV-specific clinical covariates, neither estimated duration of HIV infection nor duration of exposure to d-drug ART was significantly associated with mtDNA copy number (Table 2). In contrast, longer total estimated duration of ART was significantly associated with lower mtDNA copy number (p<0.001).
Higher relative mtDNA copy number was associated with GDS-defined NCI (p<0.001, Figure 1 and Table 3). In a subgroup analysis of participants on ART with plasma HIV RNA ≤ 50 copies/mL, the association between relative mtDNA copy number and impaired GDS persisted (p=0.006; data not shown). As expected, nadir CD4+ T-cell count (p=0.02) was associated with higher GDS (poorer performance), as was the Hispanic ancestry PC (p<0.0001), a finding previously observed in CHARTER and other populations with HIV48–51. The association of NCI with Hispanic ancestry was independent of relative mtDNA copy number and was consistent across all adjusted models of the NCI outcomes. We also carried out a secondary analysis using the continuous GDS value instead of the dichotomized GDS impairment measure (Supplementary Table 1). In this analysis, whole blood mtDNA copy number was also significantly associated with continuous GDS values (p=0.007), and higher mtDNA copy number was associated with higher GDS scores (poorer neurocognitive performance).
Figure 1.
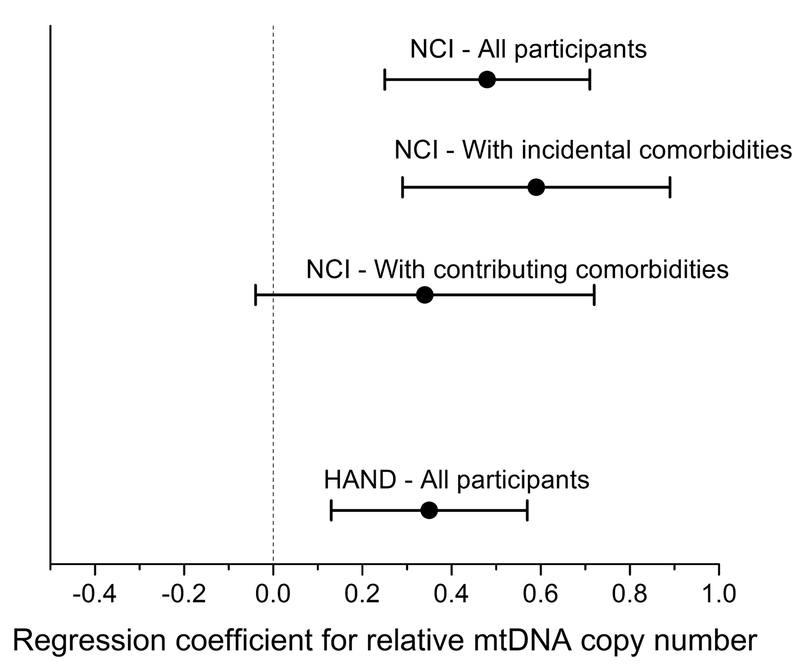
Forest plot of regression coefficients (± 2x standard error) for associations between relative mtDNA copy number and either neurocognitive impairment (NCI; GDS≥0.5) or HAND in models adjusting for nadir CD4+ T-cell count, estimated duration of HIV infection, duration of ART and d-drug exposure, and plasma HIV RNA level. Plots for associations between mtDNA copy number and NCI for subgroups stratified by baseline comorbidity status are also shown.
Table 3.
Adjusted associations between demographic and clinical variables, relative mtDNA copy number, and neurocognitive impairment by global deficit score (GDS ≥0.5).
| Variable | Coefficient (±2x SE) | P value |
|---|---|---|
| Peripheral blood mtDNA copy number | 0.48 ± 0.23 | <0.0001 |
| Platelet / WBC ratio | −0.01 ± 0.01 | 0.02 |
| Age (per 10 years) | 0.04 ± .09 | 0.68 |
| Female sex | 0.23 ± 0.34 | 0.18 |
| Genetic PC1 | −1.23 ± 5.46 | 0.65 |
| Genetic PC2 | 10.63 ± 5.40 | <0.0001 |
| Genetic PC3 | 1.39 ± 5.25 | 0.60 |
| Estimated duration of HIV (per year) | 0.01 ± 0.02 | 0.56 |
| Contributing comorbidity | 0.67 ± 0.28 | <0.0001 |
| Nadir CD4+ T-cell count (per 100 cells) | 0.10 ± 0.08 | 0.02 |
| Plasma HIV RNA ≤50 copies/mL | 0.09 ± 0.28 | 0.52 |
Higher whole blood mtDNA copy number was also significantly associated with a greater likelihood of HAND (p=0.002; Table 4). Median whole blood mtDNA copy number was significantly greater in persons with ANI (N=283) vs. those without HAND (N=553; Wilcoxon p=0.0002), but not in those with MND or HAD (N=174 [141 with MND; 33 with HAD]; p=0.35). In fully-adjusted logistic regression models, higher mtDNA copy number was associated with greater likelihood of ANI (p<0.001) but not MND/HAD (p=0.68).
Table 4.
Adjusted associations between demographic and clinical variables, relative mtDNA copy number, and any HAND.
| Variable | Coefficient (±2x SE) | P value |
|---|---|---|
| Peripheral blood mtDNA copy number | 0.35 ± 0.22 | 0.002 |
| Platelet / WBC ratio | −0.01 ± 0.01 | 0.001 |
| Age (per 10 years) | 0.01 ± 0.16 | 0.86 |
| Female sex | 0.11 ± 0.32 | 0.50 |
| Genetic PC1 | 3.04 ± 5.51 | 0.27 |
| Genetic PC2 | 9.71 ± 5.34 | 0.0003 |
| Genetic PC3 | 0.25 ± 5.22 | 0.92 |
| Estimated duration of HIV (per year) | 0.01 ± 0.02 | 0.28 |
| Contributing comorbidity | 0.77 ± 0.27 | <0.0001 |
| Nadir CD4+ T-cell count (per 100 cells) | 0.06 ± 0.04 | 0.10 |
| Plasma HIV RNA ≤50 copies/mL | 0.03 ± 0.14 | 0.83 |
Considering the importance of comorbidities in assessing HIV-associated NCI, we performed a stratified analysis by comorbidity status. In adjusted analysis of participants with incidental comorbidities (N=651), those with GDS-defined NCI (N=191) had higher median relative mtDNA copy number than those with an unimpaired GDS (p<0.001; Figure 1 and Supplementary Table 2). In participants with comorbidities that were considered potentially contributing to NCI (N=359), the difference in mtDNA copy number by GDS impairment was of a lower magnitude and not statistically significant (p=0.08; Figure 1 and Supplementary Table 3).
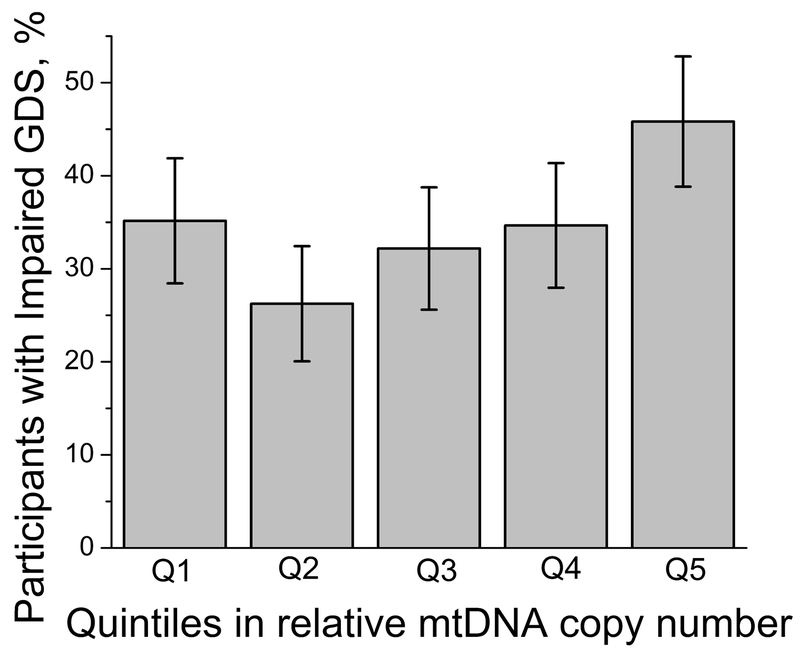
The analyses reported above tested for linear associations of mtDNA copy number with NCI measures. To test for a possible non-linear relationship, we carried out a quintile analysis, separating participants into five equally sized groups based on their mtDNA copy number. The proportion of participants with GDS impairment across mtDNA copy number quintiles was significantly non-uniform (p=0.001 by chi-square test; Figure 2), with a greater proportion of participants with GDS impairment seen in the higher quintiles of relative mtDNA copy number. Participants with the lowest mtDNA copy numbers (quintile 1) also showed an increased proportion with GDS impairment, consistent with the hypothesis that both extremes of relative mtDNA copy number may be associated with NCI.
Figure 2.
Percentage of CHARTER participants with impaired GDS within each quintile of the blood mtDNA copy number. Q1 is the lowest mtDNA copy number group and Q5 is the highest. Error bars for proportion p were calculated as 2 x SQRT(p(1-p)/N), where N is the sample size. P value=0.001 by chi-squared test.
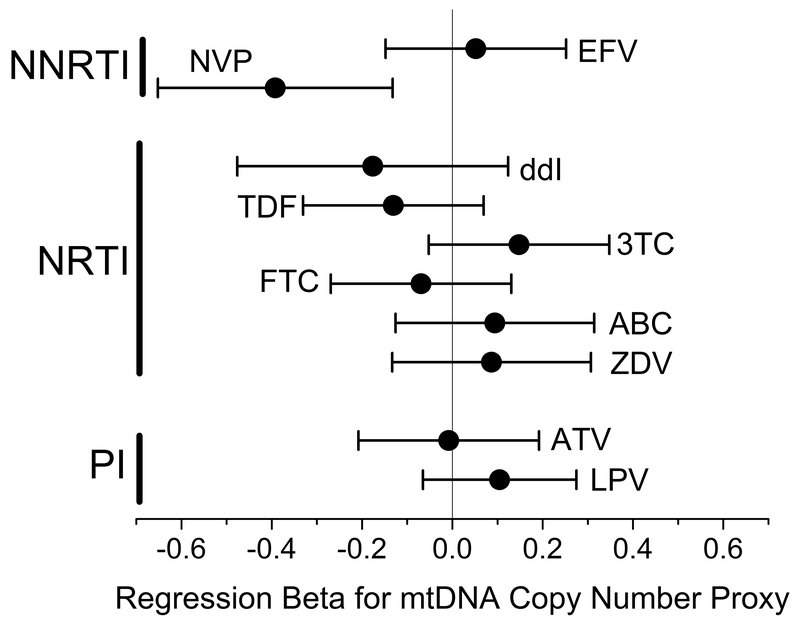
To further investigate the effect of ART on mtDNA copy number, we tested for an association between mtDNA copy number and the ART regimen at the time of sample collection in the subgroup of study participants on ART with controlled HIV infection, defined by plasma HIV RNA ≤50 copies/mL (N = 449). Drugs for which >5% of the participants were treated were analyzed, for a total of 10 different medications. Each drug was tested in a multivariable linear regression model, using 1 or 0 for current exposure to the drug or not, adjustting for the platelet/WBC ratio, age, and sex (Figure 3). The only significant association with mtDNA copy number was nevirapine (NVP) exposure, which was associated with lower mtDNA copy number (p=0.003). This association was not changed when all of the ART drugs were included simultaneously in a single model (NVP coefficient ± 2x standard error = −0.46 ± 0.32, p=0.004).
Figure 3.
Regression coefficients (± 2x standard error) for associations between duration of exposure to specific ART on the relative mtDNA copy number in peripheral blood. All ART drugs were tested in a single multivariable linear regression model, adjusting for the platelet to WBC ratio, age at measurement, and sex. (Abbreviations: NNRTI=Non-Nucleoside Reverse Transcriptase Inhibitors; NRTI=Nucleoside Reverse Transcriptase Inhibitors; PI=Protease Inhibitors; EFV=efavirenz; NVP=nevirapine; ddI=didanosine; TDF=tenofovir disoproxil fumarate; 3TC=lamivudine; FTC=emtricitabine; ABC=abacavir; ZDV=zidovudine; ATV=atazanavir; LPV=lopinavir/ritonavir.)
DISCUSSION
In this large analysis of a well-characterized cohort, higher relative mtDNA copy number in blood was associated with a greater probability of NCI in CHARTER participants. The association was not explained by potentially relevant covariates such as age, prior use of d-drug ART, or comorbidities that might have contributed to NCI, and was robust in analyses of multiple measures of NCI. Interestingly, in analyses of secondary HAND outcomes, the association between higher blood mtDNA copy number and HAND was predominantly seen in persons with ANI, not in those with more functionally significant forms of NCI, MND or HAD. These findings may suggest a biologic correlate of asymptomatic NCI that is either lost with more severe forms of NCI, or that we were underpowered to detect in these subgroups. While these cross-sectional data cannot assess temporal relationships, future studies will need to address the relationship between mtDNA copy number and transition from ANI to MND or HAD over time.
Significant associations between peripheral blood mtDNA copy number and neurocognitive phenotypes have been reported in several studies. Lower mtDNA copy number in blood compared to controls has been reported for Huntington’s disease25 and Parkinson’s disease18. While not identical to neurocognitive impairment or neurodegenerative diseases, data in neuropsychological phenotypes are mixed: significantly lower blood mtDNA levels have been reported in elderly women with depression19, but not in young adults20. A study on bipolar disorders in young adults found only a borderline significant association of lower blood mtDNA levels with more severe forms of bipolar disorder, and no association with milder bipolar disorder21. Related studies in healthy, elderly Korean women reported that lower blood mtDNA levels were significantly associated with poorer performance on the Mini-Mental State Examination22 and the Geriatric Depression Scale23. In contrast to all of these studies, which showed worse phenotypes with lower blood mtDNA level, higher blood mtDNA copy number has been significantly associated with an increased likelihood of childhood autism24.
In this analysis, we used a novel method to estimate relative peripheral blood mtDNA copy number from available genome-wide genotype data. This method has recently been used to demonstrate associations between lower mtDNA copy number and increased risk of sudden cardiac death52, incident cardiovascular disease and stroke53, and chronic kidney disease40 in populations without HIV. While studies of direct peripheral blood mtDNA quantitation have been performed in persons with HIV for many years, this is the first study to assess this particular measure in a population with HIV, and the first to assess relationships of any mtDNA copy number estimate with carefully characterized neurocognitive performance. The presence of expected relationships between this measure and sex, platelet count, and WBC provided a degree of validation, although the expected association with age was absent. While multiple studies have shown decreasing blood mtDNA copy number with age in the elderly14, 15, studies over a broader range of ages show a more complex relationship, with blood mtDNA either stable or rising with age in the young and middle-aged, then dropping in the elderly16, 17. The age distribution of our study population is in the range that has little age-related variation in peripheral mtDNA copy number, consistent with our results16.
We found an unexpected association between NVP and lower relative mtDNA copy number. ART has generally been associated with lower mtDNA copy number in peripheral blood when measured by PCR29, 54. A small study of 32 patients showed that treatments containing either NVP or 3TC were associated with significantly higher peripheral blood mononuclear cell (PBMC) mtDNA copy number compared to regimens containing ddI28. Another study of 47 participants examined changes in PBMC mtDNA copy number after switching to a nucleoside reverse transcriptase inhibitor (NRTI)-sparing ART regimen, and found that the group switched from NRTIs (d-drug and/or zidovudine) to NVP plus a protease inhibitor (lopinavir/ritonavir) had an increase in PBMC mtDNA over 48 weeks55. A recent analysis of pregnant women/infant pairs with or without HIV found lower blood mtDNA copy number in HIV-exposed infants than unexposed56. While unadjusted mtDNA copy number was lower in NVP-exposed than unexposed infants, after multivariable adjustment the difference was statistically significant only in infants exposed to the thymidine analog NRTI zidovudine, but not those exposed to NVP. NVP has also been implicated in increasing mitochondrial depolarization to levels sufficient to induce apoptosis57. We found no statistically significant associations between mtDNA copy number and NRTIs zidovudine or d4T, each of which are associated with mitochondrial toxicity in patients58–61, although our probe-intensity method of estimating relative mtDNA copy number could partially explain differences across studies. However, recent data have found good correlation between probe-intensity measurements from exome sequencing data and direct RT-PCR quantitation of mtDNA copy number in the same cell lines62.
Limitations of our study include those inherent to cross-sectional, observational analyses, including that they are more prone to bias than longitudinal studies. We did not have simultaneous measures of relative or directly measured mtDNA copy number from CSF, or CSF biomarker data from a large enough sample to include in these analyses. Thus, mechanistic insights are necessarily limited and will need to be addressed in future studies. The CHARTER study population includes a large proportion of individuals treated with earlier-generation ART drugs and so our findings may not generalize well to younger populations who have only been exposed to newer drugs. We included data from 94 persons (9% of the total population) of self-reported Hispanic ethnicity. At the time of data collection, normative population standards for correction were limited, thus NCI could be overestimated in this population. There was no control population without HIV for comparison, so we cannot draw conclusions regarding the contribution of HIV infection itself to mtDNA copy number. We did not adjust for all factors potentially related to mtDNA biogenesis and NCI, including substance use or concomitant non-ART medications. Finally, due to the exploratory nature of analyses with this novel measure, we did not adjust for multiple comparisons, but many p-values for adjusted NCI associations were robust to levels well below the p=0.05 threshold.
In summary, we present the first analysis of relative mtDNA copy number derived from genome-wide genotyping data in adults living with HIV. This is also the first analysis linking peripheral blood mtDNA copy number to HIV-associated NCI. We found a consistent association between higher relative mtDNA copy number in blood and poorer neurocognitive performance, defined by either the GDS or Frascati criteria. These results can inform longitudinal studies of NCI risk and progression in HIV by providing a novel biomarker that may already be available in population-level genotyping data. They also provide mechanistic insights, affirming a possible role for mitochondria in the pathogenesis of HIV-associated NCI. More studies are needed to assess longitudinal changes in mtDNA copy number, correlate peripheral blood and CSF or tissue-level measures of mtDNA, and explore mechanisms underlying associations between mtDNA and HIV-associated NCI. Studies like these could inform future clinical studies of NCI prediction or prognosis and mitochondria-targeted interventions.
Supplementary Material
Acknowledgements:
The authors are indebted to all study participants who made this study possible.
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; Washington University, St. Louis; and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: Scott L. Letendre, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D.; Laboratory and Virology Component: Scott Letendre, M.D. (Co-P.I.), Davey M. Smith, M.D. (Co-P.I.).; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Matthew Dawson; Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Michael J Taylor, Ph.D., Rebecca Theilmann, Ph.D.; Data Management Component: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Component: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D.; Johns Hopkins University Site: Ned Sacktor (P.I.), Vincent Rogalski; Icahn School of Medicine at Mount Sinai Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.); University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Sher Storey, PA-C.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
Funding Sources and Potential Conflicts of Interest:
Funding for this study was provided by National Institutes of Health (NIH) R01 MH095621, R01 MH107345 and K24 MH097673. CHARTER was also supported by awards N01 MH22005, HHSN271201000036C and HHSN271201000030C from the NIH. The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Footnotes
The authors report no potential conflicts of interest relevant to the work.
REFERENCES
- 1.Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment (vol 12, pg 234, 2016). Nature Reviews Neurology. May 2016;12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. December 2010;75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant I, Franklin DR Jr., Deutsch R, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. June 10 2014;82(23):2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cody SL, Vance DE. The neurobiology of HIV and its impact on cognitive reserve: A review of cognitive interventions for an aging population. Neurobiology of Disease. August 2016;92:144–156. [DOI] [PubMed] [Google Scholar]
- 5.Levine AJ, Quach A, Moore DJ, et al. Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. Journal of Neurovirology. June 2016;22(3):366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiological Reviews. January 2000;80(1):315–360. [DOI] [PubMed] [Google Scholar]
- 7.Xavier JM, Rodrigues CMP, Sola S. Mitochondria: Major Regulators of Neural Development. Neuroscientist. August 2016;22(4):346–358. [DOI] [PubMed] [Google Scholar]
- 8.Wachsmuth M, Huebner A, Li M, Madea B, Stoneking M. Age-Related and Heteroplasmy-Related Variation in Human mtDNA Copy Number. Plos Genetics. March 2016;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durham SE, Samuels DC, Cree LM, Chinnery PF. Normal levels of wild-type mitochondrial DNA maintain cytochrome c oxidase activity for two pathogenic mitochondrial DNA mutations but not for m.3243A → G. American Journal of Human Genetics. July 2007;81(1):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sitarz KS, Yu-Wai-Man P, Pyle A, et al. MFN2 mutations cause compensatory mitochondrial DNA proliferation. Brain. August 2012;135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart JD, Schoeler S, Sitarz KS, et al. POLG mutations cause decreased mitochondrial DNA repopulation rates following induced depletion in human fibroblasts. Biochimica Et Biophysica Acta-Molecular Basis of Disease. March 2011;1812(3):321–325. [DOI] [PubMed] [Google Scholar]
- 12.Chinnery PF, Samuels DC. Relaxed replication of mtDNA: A model with implications for the expression of disease. American Journal of Human Genetics. April 1999;64(4):1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cote HCF, Gerschenson M, Walker UA, et al. Quality assessment of human mitochondrial DNA quantification: MITONAUTS, an international multicentre survey. Mitochondrion. May 2011;11(3):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashar FN, Moes A, Moore AZ, et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. Journal of Molecular Medicine-Jmm. February 2015;93(2):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J, Sidore C, Butler TJ, et al. Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of similar to 2,000 Sardinians Using Tailored Sequencing Analysis Tools. Plos Genetics. July 2015;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knez J, Winckelmans E, Plusquin M, et al. Correlates of Peripheral Blood Mitochondrial DNA Content in a General Population. American Journal of Epidemiology. January 15 2016;183(2):138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mengel-From J, Thinggaard M, Dalgard C, Kyvik KO, Christensen K, Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Human Genetics. September 2014;133(9):1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyle A, Anugrha H, Kurzawa-Akanbi M, Yarnall A, Burn D, Hudson G. Reduced mitochondrial DNA copy number is a biomarker of Parkinson’s disease. Neurobiology of Aging. February 2016;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim M-Y, Lee J-W, Kang H-C, Kim E, Lee D-C. Leukocyte mitochondrial DNA (mtDNA) content is associated with depression in old women. Archives of Gerontology and Geriatrics. Sep-Oct 2011;53(2):E218–E221. [DOI] [PubMed] [Google Scholar]
- 20.He Y, Tang JS, Li ZC, et al. Leukocyte Mitochondrial DNA Copy Number in Blood Is Not Associated with Major Depressive Disorder in Young Adults. Plos One. May 2014;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Sousa RT, Uno M, Zanetti MV, et al. Leukocyte mitochondrial DNA copy number in bipolar disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. January 3 2014;48:32–35. [DOI] [PubMed] [Google Scholar]
- 22.Lee J-W, Park KD, Im J-A, Kim MY, Lee D-C. Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clinica Chimica Acta. April 2 2010;411(7–8):592–596. [DOI] [PubMed] [Google Scholar]
- 23.Kim J-H, Kim HK, Ko J-H, Bang H, Lee D-C. The Relationship between Leukocyte Mitochondrial DNA Copy Number and Telomere Length in Community-Dwelling Elderly Women. Plos One. June 13 2013;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Li Z, He Y, et al. Elevated mitochondrial DNA copy number in peripheral blood cells is associated with childhood autism. Bmc Psychiatry. March 17 2015;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen MH, Budtz-Jorgensen E, Sorensen SA, et al. Reduction in mitochondrial DNA copy number in peripheral leukocytes after onset of Huntington’s disease. Mitochondrion. July 2014;17:14–21. [DOI] [PubMed] [Google Scholar]
- 26.Cherry CL, Nolan D, James IR, et al. Tissue-specific associations between mitochondrial DNA levels and current treatment status in HIV-infected individuals. Jaids-Journal of Acquired Immune Deficiency Syndromes. August 1 2006;42(4):435–440. [DOI] [PubMed] [Google Scholar]
- 27.Cossarizza A, Pinti M, Moretti L, et al. Mitochondrial functionality and mitochondrial DNA content in lymphocytes of vertically infected human immunodeficiency virus-positive children with highly active antiretroviral therapy-related lipodystrophy. Journal of Infectious Diseases. February 1 2002;185(3):299–305. [DOI] [PubMed] [Google Scholar]
- 28.Cote HCF, Yip B, Asselin JJ, et al. Mitochondrial : nuclear DNA ratios in peripheral blood cells from human immunodeficiency virus (HIV)-infected patients who received selected HIV antiretroviral drug regimens. Journal of Infectious Diseases. June 15 2003;187(12):1972–1976. [DOI] [PubMed] [Google Scholar]
- 29.Lopez S, Miro O, Martinez E, et al. Mitochondrial effects of antiretroviral therapies in asymptomatic patients. Antiviral Therapy. February 2004;9(1):47–55. [PubMed] [Google Scholar]
- 30.McComsey G, Tan DJ, Lederman M, Wilson E, Wong LJ. Analysis of the mitochondrial DNA genome in the peripheral blood leukocytes of HIV-infected patients with or without lipoatrophy. Aids. March 8 2002;16(4):513–518. [DOI] [PubMed] [Google Scholar]
- 31.Cysique LA, Franklin D Jr., Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. June 2011;33(5):505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. Journal of Clinical and Experimental Neuropsychology. 2011 2011;33(7):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackstone K, Moore DJ, Franklin DR, et al. Defining Neurocognitive Impairment in HIV: Deficit Scores Versus Clinical Ratings. Clinical Neuropsychologist. 2012 2012;26(6):894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. September 2004;26(6):759–778. [DOI] [PubMed] [Google Scholar]
- 35.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. October 30 2007;69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. August 2006;38(8):904–909. [DOI] [PubMed] [Google Scholar]
- 37.Xie HBM, Perin JC, Schurr TG, et al. Mitochondrial genome sequence analysis: A custom bioinformatics pipeline substantially improves Affymetrix MitoChip v2.0 call rate and accuracy. BMC Bioinformatics. October 2011;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nature Genetics. October 1999;23(2):147–147. [DOI] [PubMed] [Google Scholar]
- 39.Kloss-Brandstatter A, Pacher D, Schonherr S, et al. HaploGrep: A Fast and Reliable Algorithm for Automatic Classification of Mitochondrial DNA Haplogroups. Human Mutation. January 2011;32(1):25–32. [DOI] [PubMed] [Google Scholar]
- 40.Tin A, Grams ME, Ashar FN, et al. Association between Mitochondrial DNA Copy Number in Peripheral Blood and Incident CKD in the Atherosclerosis Risk in Communities Study. Journal of the American Society of Nephrology. August 2016;27(8):2467–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banas B, Kost BP, Hillebrand S, Goebel FD. Platelets, a typical source of error in real-time PCR quantification of mitochondrial DNA content in human peripheral blood cells. European Journal of Medical Research. August 31 2004;9(8):371–377. [PubMed] [Google Scholar]
- 42.Urata M, Koga-Wada Y, Kayamori Y, Kang D. Platelet contamination causes large variation as well as overestimation of mitochondrial DNA content of peripheral blood mononuclear cells. Annals of Clinical Biochemistry. September 2008;45:513–514. [DOI] [PubMed] [Google Scholar]
- 43.Hurtado-Roca Y, Ledesma M, Gonzalez-Lazaro M, et al. Adjusting MtDNA Quantification in Whole Blood for Peripheral Blood Platelet and Leukocyte Counts. Plos One. October 13 2016;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wendelsdorf KV, Song Z, Cao Y, Samuels DC. An Analysis of Enzyme Kinetics Data for Mitochondrial DNA Strand Termination by Nucleoside Reverse Transcription Inhibitors. Plos Computational Biology. January 2009;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benbrik E, Chariot P, Bonavaud S, et al. Cellular and mitochondrial toxicity of zidovudine (AZT), didanosine (ddI) and zalcitabine (ddC) on cultured human muscle cells. Journal of the Neurological Sciences. July 1997;149(1):19–25. [DOI] [PubMed] [Google Scholar]
- 46.Cherry CL, Gahan ME, McArthur JC, Lewin SR, Hoy JF, Wesselingh SL. Exposure to dideoxynucleosides is reflected in lowered mitochondrial DNA in subcutaneous fat. Journal of Acquired Immune Deficiency Syndromes. July 1 2002;30(3):271–277. [DOI] [PubMed] [Google Scholar]
- 47.Kampira E, Dzobo K, Kumwenda J, van Oosterhout JJ, Parker MI, Dandara C. Peripheral Blood Mitochondrial DNA/Nuclear DNA (mtDNA/nDNA) Ratio as a Marker of Mitochondrial Toxicities of Stavudine Containing Antiretroviral Therapy in HIV-Infected Malawian Patients. Omics. July 2014;18(7):438–445. [DOI] [PubMed] [Google Scholar]
- 48.Hulgan T, Samuels DC, Bush W, et al. Mitochondrial DNA Haplogroups and Neurocognitive Impairment During HIV Infection. Clinical Infectious Diseases. November 1 2015;61(9):1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marquine MJ, Sakamoto M, Dufour C, et al. The impact of ethnicity/race on the association between the Veterans Aging Cohort Study (VACS) Index and neurocognitive function among HIV-infected persons. J Neurovirol. August 2016;22(4):442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marquine MJ, Heaton A, Johnson N, et al. Differences in Neurocognitive Impairment Among HIV-Infected Latinos in the United States. J Int Neuropsychol Soc. February 2018;24(2):163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mindt MR, Miranda C, Arentoft A, et al. Aging and HIV/AIDS: neurocognitive implications for older HIV-positive Latina/o adults. Behav Med. 2014;40(3):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang YY, Guallar E, Ashar FN, et al. Association between mitochondrial DNA copy number and sudden cardiac death: findings from the Atherosclerosis Risk in Communities study (ARIC). European Heart Journal. December 2017;38(46):3443–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashar FN, Zhang YY, Longchamps RJ, et al. Association of Mitochondrial DNA Copy Number With Cardiovascular Disease. Jama Cardiology. November 2017;2(11):1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montaner JSG, Cote HCF, Harris M, et al. Mitochondrial toxicity in the era of HAART: Evaluating venous lactate and peripheral blood mitochondrial DNA in HIV-infected patients taking antiretroviral therapy. Jaids-Journal of Acquired Immune Deficiency Syndromes. September 2003;34:S85–S90. [DOI] [PubMed] [Google Scholar]
- 55.Negredo E, Miro O, Rodriguez-Santiago B, et al. Improvement of Mitochondrial Toxicity in Patients Receiving a Nucleoside Reverse-Transcriptase Inhibitor-Sparing Strategy: Results from the Multicenter Study with Nevirapine and Kaletra (MULTINEKA). Clinical Infectious Diseases. September 15 2009;49(6):892–900. [DOI] [PubMed] [Google Scholar]
- 56.Jao J, Powis KM, Kirmse B, et al. Lower mitochondrial DNA and altered mitochondrial fuel metabolism in HIV-exposed uninfected infants in Cameroon. Aids. November 28 2017;31(18):2475–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karamchand L, Dawood H, Chuturgoon AA. Lymphocyte mitochondrial depolarization and apoptosis in HIV-1-infected HAART patients. Jaids-Journal of Acquired Immune Deficiency Syndromes. August 1 2008;48(4):381–388. [DOI] [PubMed] [Google Scholar]
- 58.Casula M, Weverling GJ, Wit FW, et al. Mitochondrial DNA and RNA increase in peripheral blood mononuclear cells from HIV-1-infected patients randomized to receive stavudine-containing or stavudine-sparing combination therapy. Journal of Infectious Diseases. November 15 2005;192(10):1794–1800. [DOI] [PubMed] [Google Scholar]
- 59.Fleischman A, Johnsen S, Systrom DM, et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. American Journal of Physiology-Endocrinology and Metabolism. June 2007;292(6):E1666–E1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ribera E, Carlos Paradineiro J, Curran A, et al. Improvements in Subcutaneous Fat, Lipid Profile, and Parameters of Mitochondrial Toxicity in Patients with Peripheral Lipoatrophy When Stavudine is Switched to Tenofovir (LIPOTEST Study). Hiv Clinical Trials. Nov-Dec 2008;9(6):407–417. [DOI] [PubMed] [Google Scholar]
- 61.van der Valk M, Casula M, Weverling GJ, et al. Prevalence of lipoatrophy and mitochondrial DNA content of blood and subcutaneous fat in HIV-1-infected patients randomly allocated to zidovudine- or stavudine-based therapy. Antiviral Therapy. June 2004;9(3):385–393. [PubMed] [Google Scholar]
- 62.Zhang P, Lehmann BD, Samuels DC, et al. Estimating relative mitochondrial DNA copy number using high throughput sequencing data. Genomics. October 2017;109(5–6):457–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.