Abstract
Background and Purpose—
Brain derived neurotrophic factor (BDNF) plays an important role in neuroplasticity and neurogenesis following ischemic and non-ischemic brain injury. The predictive value of BDNF for short- term outcome after stroke is controversial. The objective of this study was to investigate the relationship among serum BDNF level, fractional anisotropy (FA), and functional outcome during post-acute stroke rehabilitation.
Methods—
Serum BDNF levels were measured on admission to an acute inpatient rehabilitation hospital. The primary functional outcome was functional independent measures (FIM) motor subscore at discharge. The secondary outcome measures were FIM total score at discharge, FIM motor subscore on admission, length of stay in the hospital, and discharge destination. We investigated the relationship among the level of serum BDNF and FA as well as functional outcome measures.
Results—
Three hundred forty-eight consecutive stroke subjects were included in the analysis. Serum BDNF levels on admission were statistically but not clinically correlated with FIM motor subscore at discharge (r=0.173, P=0.001) and FIM total score at discharge (r=0.155, P=0.004). Receiver operating characteristics (ROC) analysis of BDNF as a predictor for FIM motor subscore improvement showed low accuracy of prediction with an area under the curve (AUC) of 0.581 (P=0.026). Serum BDNF significantly correlated with FA in the high FIM motor group (n=10, r=0.609, P=0.031) but not in the low FIM motor group (n=11, r=−0.132, P=0.349).
Conclusions—
The serum BDNF level alone offers minimum predictive value for recovery of motor function during post-acute rehabilitation. Our findings suggest that serum BDNF level may be correlated with FA.
Keywords: stroke, BDNF (brain-derived neurotrophic factor), FIM (functional independence measure), Fractional Anisotropy (FA), correlation
Introduction
Post-acute stroke rehabilitation is critical to stroke recovery. Understanding the individual biological recovery process may help to design personalized treatment and to improve outcomes. Serum biomarkers have been used widely across medical disciplines to provide guidance in rapid diagnosis, choice of treatment, and prognosis assessment. However, researchers have not yet found a serum biomarker of neurobiological recovery to guide treatment plans during the post-acute phase after stroke.
Neuroplasticity, which refers to the ability of the nervous system to respond and adapt to internal and external stimuli, is closely related to neurobiological recovery after stroke and especially to the recovery of motor function [2]. Brain-derived neurotrophic factor (BDNF) plays an important role in increasing neuroplasticity after stroke [3, 4]. BDNF regulates dendritic plasticity, increasing dendrite branching and interdendritic connections, and promoting the development and maturation of the nervous system [5]. The interaction between BDNF and tyrosine kinase receptor B (Trk B) upregulates genes essential for neuronal survival and differentiation, and thereby contributes to promoting synaptic plasticity in learning and memory [6, 7]. Besides, BDNF Val66Met polymorphism was found to affect the FA values in healthy subjects and motor function in patients after stroke. Results from Kim et al. suggest that patients with the Val allele (Val/Val) showed better motor outcomes at 1 month and 3 months compared to carriers of the Met allele [8].
Several studies have investigated the potential for serum BDNF to serve as a biomarker for a variety of disorders, including traumatic brain injury [9] and dementia [10]. Increasing interest has grown to examine the association of BDNF for functional outcome after stroke. Low serum BDNF levels were found to be associated with a poor long-term functional outcome at 2 years and 7 years after ischemic stroke [11]. However, the predictive value of BDNF for short-term outcome after stroke is controversial. In Stanne’s study, serum BDNF was not associated with short-term outcome at 3 months; on the contrary, Wang et al. [12] reports that low serum BDNF was significantly associated with poor functional outcome at 3 months post-stroke. Furthermore, no studies have investigated the association of serum BDNF level with functional recovery during the post-acute rehabilitation phase, which is a very important phase of recovery. The aim of this study was to investigate the relationship among serum BDNF, FA, and motor function in stroke patients after post-acute rehabilitation.
Methods
Study participants
The study was approved by the Institutional Review Board (IRB). Patients admitted to an acute inpatient rehabilitation facility from March 2014 to June 2015 were screened using the following inclusion and exclusion criteria: (1) older than 18 years of age, (2) stroke confirmed by computed tomography (CT) of head or/and brain magnetic resonance imaging (MRI) scan, (3) length of hospital stay greater than one week, and 4) peripheral blood serum sample collected and stored on admission. Three hundred and forty-eight subjects were included in the analysis. The following demographic and clinical data were extracted from medical records: age, sex, marital status, body mass index (BMI), blood urea nitrogen (BUN) and creatinine, hematocrit, stroke type and side, length of hospital stay, stroke risk factors (hypertension, atrial fibrillation, coronary artery disease, diabetes mellitus, current smoker), admission and discharge Functional Independence Measure (FIM), and discharge destination.
Outcome measures
The primary outcome measure of this study is the FIM motor subscore at discharge. The secondary outcome measures include FIM total score at discharge, FIM motor subscore on admission, length of hospital stay, and discharge destination. The FIM is a widely used scale that measures the functional abilities of people undergoing rehabilitation. The FIM scale is an 18-item ordinal scale, and each item’s score ranges from 1 to 7, with 1 (total assistance) being the lowest possible score and 7 (complete independence) being the highest possible score. There are 13 items in the FIM motor subscale: eating, grooming, bathing, dressing – upper body, dressing – lower body, toileting, bladder management, bowel management, bed/chair/wheelchair transfer, toilet transfer, tub/shower transfer, walk/wheelchair, and stairs. There are 5 items in the FIM cognitive subscale: comprehension, expression, social interaction, problem solving, and memory. Participants were divided into two groups of high and low FIM scores using the median as the cutoff.
Serum BDNF level measurement
Serum was obtained as part of clinical care, including tests for basic metabolic profiles. The excessive serum was stored at −80°C within 4 hours of blood drawn. All subjects had serum obtained on admission, and 79 subjects had serum obtained on week 3 after admission. Serum levels of BDNF were measured by enzyme linked immunosorbent assay (ELISA) (R&D Systems, Inc. USA). Coefficients of variation (CV) of intra- and inter-assay precision of BDNF were calculated for quality control purposes (92% and 95%, respectively).
MRI data acquisition
MRI imaging data was retrospectively collected using the Research Patient Data Registry as described in our previous publication [13]. Imaging data was processed using the Research Patient Data Registry online query tool and mi2b2 Workbench software (mi2b2 Client for RPDR; Partners HealthCare.) [14]. To reduce variation caused by imaging protocols, MRI images from a single Skyra 3T scanner from Massachusetts General Hospital were selected. The diffusion tensor imaging (DTI) data acquisition parameters were as following: repetition time = 5000 milliseconds, echo time = 96 milliseconds, inversion time = −1 milliseconds, flip angle = 90°, field of view = 220, slice thickness = 5mm, and the matrix dimension size = 160×160×28. Data was collected in 28 nonlinear diffusion directions with b = 1000s/mm2 and an additional three volumes of b = 0s/mm2.
Neuroimaging analysis
DTI data processing and region of interest (ROI) analysis were made according to the literature [15–19]. Details of the neuroimaging analyses have been described in our previous publication [13]. Briefly, FA maps were created using the Functional MRI of the Brain (FMRIB) Software Library (V5.0.6, Oxford, UK). The white matter skeleton was generated by feeding all individuals’ FA maps into tract-based spatial statistics analysis. The mean values of FA were calculated within the area of interest (ROI) placed on the bilateral corticospinal tract (CST) based on the John Hopkins University white-matter tractography atlas.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 21.0 (International Business Machines Corp., New York). Student t-tests were used to compare the mean between the low and high FIM motor scores on admission. A Chi-square test was used to assess the clinical categorical measures of gender, ethnicity, race, marital status, hypertension, atrial fibrillation, coronary artery disease, diabetes mellitus, current smoker, stroke type, stroke side, stroke site, and discharge destination. Pearson correlation was used to study the relationship between BDNF levels on admission and continuous variables including age, length of hospital stay, body mass index (BMI), prior stroke history, interval between serum BDNF measurement from onset of stroke, BUN, CREAT, HCT, FIM motor subscore at admission, FIM cognitive subscore at admission, and total FIM score at admission. Values of continuous variables were expressed as mean ± standard deviations (SD). Nonparametric correlations such as spearman rho were used to analyze those with abnormal distribution. The sensitivity and specificity of serum BDNF levels on admission to predict motor recovery were calculated with receiver operating characteristic (ROC) analysis. The gain of motor function was assessed according to the Montebello Rehabilitation Factor Score (MRFS), which was calculated using the following formula: MRFSMotor = ΔMotor FIM/(maximum Motor FIM − Motor FIMAdmission).
Results
Clinical characteristics
During March 2014 to June 2015, consecutive stroke subjects were screened, and a total of 348 were included in the analysis (mean age 67.7 ± 15.2 years, 43.7% women). The study design is outlined in Figure 1. Subjects were divided into two groups: low and high FIM motor subscores on admission using a median of FIM motor subscore on admission as the cutoff (hereafter referred to as low and high FIM motor groups). Demographic and clinical characteristics of subjects are reported in Table 1. There were significant differences in length of hospital stay and discharge destination between the low and high FIM motor groups. Fifty-eight percent of patients with low FIM motor scores on admission were discharged to a skilled nursing facility (SNF), whereas 83% patients with high FIM motor scores on admission were discharged to home (Table 1).
Fig.1.
Study flow diagram.
Table 1.
Demographical and clinical characteristics
| Characteristics | Patients with low FIM motor scores on admission (n=174) | Patients with high FIM motor scores on admission (n=174) | P value |
|---|---|---|---|
| BDNF (ng/ml) | 20.59±6.481 | 22.97±7.336 | 0.001* |
| Age (years) | 68.9±13.13 | 66.5±16.99 | 0.138 |
| Gender (n) | Male (98, 56.3%) | Male (98, 56.3%) | 1.000 |
| Female (76, 43.7%) | Female (76, 43.7%) | ||
| Body mass index (BMI, kg/m2) | 27.62±6.524 | 28.34±7.793 | 0.349 |
| Ethnicity (n) | Hispanic or latino (7, 4.0%) | Hispanic or latino (5, 2.9%) | 0.557 |
| Not Hispanic or latino (167, 96.0%) | Not Hispanic or latino (169, 97.1%) | ||
| Risk factors (n) | Hypertension (144, 82.8%) | Hypertension (136, 78.2%) | 0.279 |
| Atrial fibrillation (35, 20.1%) | Atrial fibrillation (27, 15.5%) | 0.262 | |
| Coronary artery disease (35, 20.1%) | Coronary artery disease (35, 20.1%) | 1.000 | |
| Diabetes mellitus (65, 37.4%) | Diabetes mellitus (51, 29.3%) | 0.111 | |
| Current smoker (4, 2.3%) | Current smoker (2, 1.1%) | 0.410 | |
| Prior stroke history (frequency of stroke, n) | 0.39±0.923 | 0.24±0.477 | 0.050 |
| Stroke type (n) | Ischemia (134, 77.0%) | Ischemia (144, 82.8%) | |
| Hemorrhage (36, 20.7%) | Hemorrhage (26, 14.9%) | 0.373 | |
| Both (4, 2.3%) | Both (4, 2.3%) | ||
| Right side (88, 50.6%) | Right side (70, 40.2%) | ||
| Stroke side (n) | Left side (74, 42.5%) | Left side (90, 51.7%) | 0.152 |
| Both sides (12, 6.9%) | Both sides (14, 8.0%) | ||
| Supratentorial (140, 80.5%) | Supratentorial (129, 74.1%) | ||
| Stroke site (n) | Infratentorial (29, 16.7%) | Infratentorial (42, 24.1%) | 0.189 |
| Both (5, 2.9%) | Both (3, 1.7%) | ||
| Time window of available serum BDNF on admission from onset(days) | 16.87±11.688 | 13.61±9.559 | 0.005* |
| BUN (mg/dl) | 21.14±14.135 | 21.22±11.659 | 0.957 |
| CREAT (mg/dl) | 1.00±0.586 | 1.05±0.521 | 0.388 |
| HCT (%) | 36.76±4.727 | 37.44±5.753 | 0.230 |
| Length of stay (days) | 28.3±13.64 | 17.3±7.984 | 0.000* |
| Discharge destination (n) | Home (64, 36.8%) | Home (144, 82.8%) | |
| Skilled nursing facility(SNF) (102, 58.6%) | Skilled nursing facility(SNF) (26, 14.9%) | 0.000* | |
| Acute hospital (8, 4.6%) | Acute hospital (4, 2.3%) |
Values are represented as mean ± standard deviations or sample number and percentage.FIM, functional independence measure. BDNF, brain-derived neurotrophic factor.
, P <0.050.
Serum BDNF level and functional outcomes
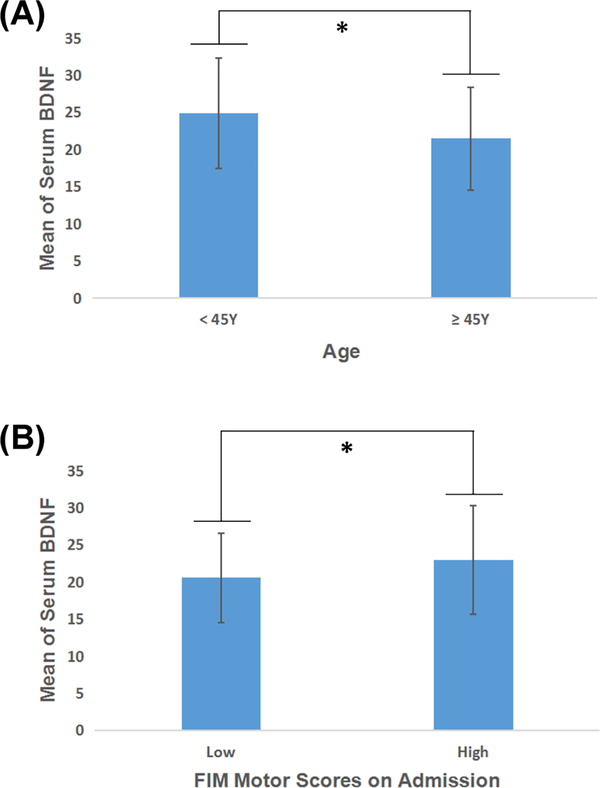
It has been shown that the rate of hospitalization after ischemic stroke increased in young people (aged less than 45 years) [20]. Studies reported that serum BDNF level negatively correlated with age [21]. In this study, serum BDNF levels of subjects who were less than 45 years old were 15% higher than those who were more than 45 years old (p<0.05, 95%CI of difference is 0.786 – 6.014, Figure 2A).
Fig.2.
Comparison of serum BDNF levels in patients with (A) different ages and (B) different FIM motor scores on admission. The BDNF level in younger stroke survivors was significantly higher than the older stroke survivors. High FIM motor group had statistically higher BDNF level than low FIM motor group with small effect size (12%). (*, P<0.050).
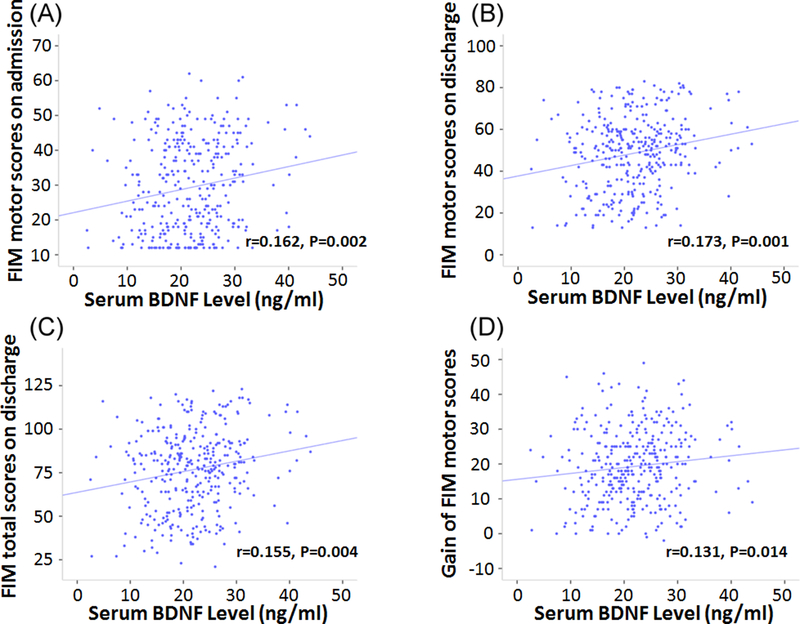
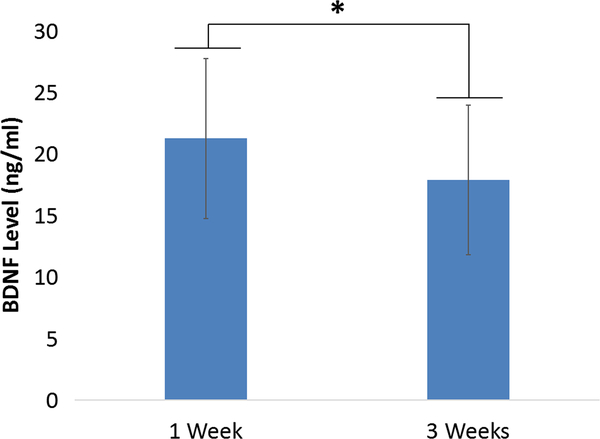
There was no significant difference between the interval of serum BDNF from admission between the two groups (low vs. high FIM motor groups) (P=0.076). Serum BDNF level was statistically significantly different between the low and high FIM motor groups (P=0.001) with low effect size. Serum BDNF level in the high FIM motor group was 12% higher than that in the low FIM motor group (Figure 2B). Serum BDNF level was statistically but not clinically correlated with FIM motor subscore on admission (r=0.173, P=0.001), FIM total score on discharge (r=0.155, P=0.004), gain of FIM motor subscore (r=0.131, P=0.014), and FIM motor score on admission (r=0.162, P=0.002) (Table 2, Figure 3). Subjects who were discharged home had significantly higher serum BDNF levels (22.5±6.90) on admission than those discharged to a SNF (20.7±7.13) (P=0.027, 95%CI of difference is 0.202 – 3.288). In addition, serum BDNF level significantly decreased (P=0.000, n=79, 95%CI of difference is 1.682 – 3.903) from week 1 (21.15±6.43) to week 3 (18.36±6.74) (Figure 4).
Table 2.
Correlation between serum BDNF level on admission and functional independence measure (FIM) outcomes in all samples.
| Variable | FIM motor on admission | FIM motor on discharge | FIM total on discharge | Gain of FIM motor scores | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| BDNF | 0.162 | 0.002* | 0.173 | 0.001* | 0.155 | 0.004* | 0.131 | 0.014* |
FIM, functional independence measure; BDNF, brain-derived neurotrophic factor.
, P <0.05.
Fig.3.
Correlation between serum BDNF level on admission and FIM outcomes in all samples. Serum BDNF level was statistically but not clinically correlated with FIM motor on admission, discharge, FIM total on discharge, gain of FIM motor scores and FIM total scores.
Fig.4.
Comparison of serum BDNF levels at week 1 and week 3 (*, P<0.050). Seven-nine subjects had serum available for BDNF measurement from both week1 and week 3 after admission to rehabilitation hospital. Pared t-test was utilized to compare the difference of BDNF at the two time-points.
Using logistical regression, high serum BDNF was associated with the high FIM motor group on admission (odds ratio 1.391, 95% CI 1.112 – 1.741, P=0,004) and on discharge (odds ratio 1.256, 95% CI 1.008 – 1.565, P=0.042) after controlling for age and marital status. Each one standard-deviation increment in BDNF was associated with a 0.203 standard-deviation increment in FIM motor score on discharge (t=3.859, P=0.000).
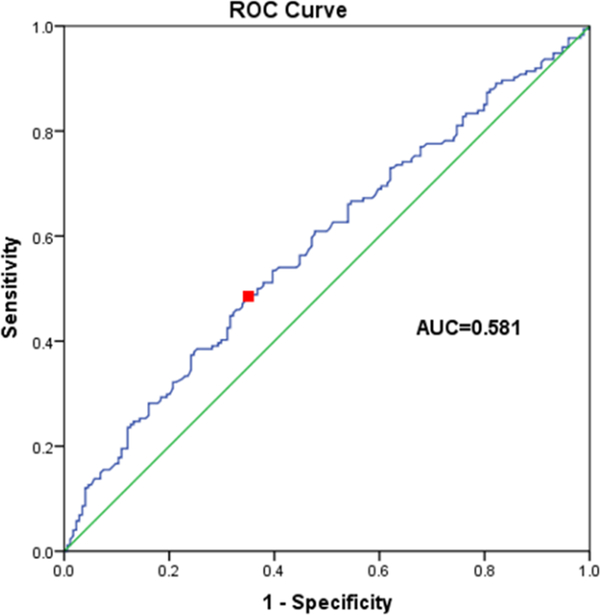
The ROC curve of serum BDNF level for predicting motor recovery is shown in Figure 5. The AUC was 0.581 (standard error = 0.063, 95% CI = 0.509 to 0.629). Derived from this curve, the best cut-off point was found to be 20.7 ng/ml. At this cut-off point, the sensitivity is 51.7%, and the specificity is 62.3%.
Fig.5.
Receiver operating characteristics (ROC) analysis of BDNF as a predictor for FIM motor subscore improvement. Area under the curve (AUC) is 0.581 (p=0.026), suggesting low accuracy of prediction.
Correlation between serum BDNF and FA in CST
In this cohort, we selected subjects with DTI available based on the imaging selection criteria (described in the methods section). Twenty-one subjects were included, with 10 in the high FIM motor group and 11 in the low FIM motor group. Serum BDNF correlated with FA of left side CST in the high FIM motor group (r=0.609, p=0.031), but not in the low FIM motor group (r=−0.132, p=0.349) (Table 3).
Table 3.
Correlation between serum BDNF level on admission and FA value in CST of patients with low and high FIM motor scores on admission.
| Variables | Cases | L_CST | R_CST | ||
|---|---|---|---|---|---|
| r | P | r | P | ||
| BDNF of patients with low FIM motor scores | 11 | −0.132 | 0.349 | −0.154 | 0.326 |
| BDNF of patients with high FIM motor scores | 10 | 0.609 | 0.031* | 0.259 | 0.235 |
BDNF, brain-derived neurotrophic factor; CST, corticospinal tract.
, P <0.050.
Discussion
The results of our study suggest that serum BDNF alone provides the minimum predictive level for motor functional outcome during post-acute rehabilitation. Interestingly, serum BDNF might correlate with FA in stroke patients.
BDNF as biomarker for stroke recovery
There are more than 30 clinical measurements to evaluate motor recovery after stroke. However, these clinical measures assess mostly functional outcomes including those gained through compensatory strategies, but may not reflect neurobiological processes. In order to achieve targeted treatment in terms of precision medicine, identification of stroke-related biomarkers plays an important role in investigating the pathological mechanism and determining the treatment method for individual stroke patients. Compared to computed tomography (CT) and magnetic resonance imaging (MRI) scans, serum biomarkers may provide an easy, low-cost approach to assess the mechanism of recovery and responsiveness to treatment. To date, however, researchers have not yet found reliable serum biomarkers for prognosis assessment and informing patient management in the post-acute stroke phase.
The correlation of BDNF and cerebral plasticity has become an interesting research area in stroke recovery [22–24]. BDNF could promote differentiation, growth, and proliferation of neural cells. The increase of BDNF expression after stroke may prevent neuronal death and reduce infarct volume. BDNF could also increase the function of microglia to reduce stroke-induced excitotoxicity [25, 26]. In addition, BDNF enhances the local anti-inflammatory effect by up-regulating the expression of IL-10 and down-regulating TNF-α [27]. Therefore, understanding the relationship between serum BDNF and neuroplasticity after stroke is important in formulating individualized treatment or functional rehabilitation strategies for stroke patients who would be responsive to BDNF modulation.
Low serum BDNF was found to be associated with poor long-term outcome after stroke [11]. However, the relationship of serum BDNF with short- term outcome is controversial; one study reported a correlation of low BDNF with poor outcome at 3 months [12], whereas another study found no correlation [11]. With a large sample size, results from our study suggest that BDNF is statistically but not clinically significantly associated with motor functional outcome during post-acute rehabilitation. The discrepancy of the results may come from possible different genetic backgrounds and different overall standard treatment that subjects received.
Blood BDNF level and brain BDNF level
Many studies suggest that serum BDNF levels reflect the BDNF level in the brain. BDNF levels in serum and brain were similar during postnatal development, and there was a positive correlation between BDNF levels in serum and the frontal cortex of rats [28]. Circulating BDNF by intravenous injection crossed the blood-brain barrier (BBB) of healthy mice in a large capacity via the saturable transport system [29]. A study reported a positive correlation between the BDNF concentration of whole blood and the hippocampus of rats, and between the BDNF concentration of plasma and the hippocampus in pigs [30]. However, there are a few studies that suggest that serum BDNF may not be associated with brain BDNF level. One study reported that plasma BDNF did not increase after stroke in rodents, suggesting that circulating BDNF levels may not mirror brain BDNF levels in this rodent stroke model [31]. Nevertheless, serum BDNF level has become an interesting target for diagnosis, prognosis, and treatment evaluation of various diseases of the central nervous system (CNS) [32, 33].
Serum BDNF stability
Several studies have investigated the stability of serum BDNF at different storage conditions and over a period of time. One study showed that the level of serum BDNF stored at 40C or 250C for 0, 1, 2, 4, 6, 24, or 48 hours after blood draw remained the same [34]. In addition, serum BDNF stored at −800C degree was stable for at least 6 months according to one study [35] and for one year by another study [36]. Several large cohort studies used serum stored at −800C degree for many years to investigate the association between serum BDNF with long-term outcome [37, 38]. In this study, we did not have serum samples from healthy subjects as a control to show the stability of BDNF over time. However, based on the well documented stability of serum BDNF stored at −800C, it is reasonable to speculate that variation in the storage duration of samples would not likely generate a significant variation in the measured level of BDNF.
Serum BDNF level and FA of CST
DTI, transcranial magnetic stimulation (TMS), functional magnetic resonance imaging (fMRI), and conventional structural MRI (sMRI) have been investigated as neurological biomarkers for post-stroke recovery [39]. FA value is widely used as a DTI metric to measure white matter integrity [40]. FA value is associated with myelin integrity, nerve fibers’ compactness, and alignment consistency of white matter [41]. A higher FA value is associated with better nerve-conductive ability [42].
The severity of white matter injury is an important factor for a patient’s outcome. One of the interesting areas of white matter injury is the neuroplasticity of the corticospinal tract (CST). Many findings suggest that CST might be involved in the functional reorganization of cortical and subcortical lesions that are important to patients’ motor functional recovery [43–45]. BDNF has been suggested to play an important role in white matter neuroplasticity [3,4]. The relationship between BDNF and FA was suggested by finding that BDNF Val66Met polymorphism affects FA values in healthy subjects [8]. Dalby et al. [46] reported that BDNF level was associated with FA in prefrontal normal-appearing white matter in a small group of patients (n=22) with late-onset depression. However, to the best of our knowledge, no previous study has explored the relationship between serum BDNF and FA in patients with stroke. The results from this study, limited by sample, suggest that serum BDNF might be correlated with FA value in CST in the high motor function group but not in the low motor function group. Our finding is consistent with previous publications that BDNF might be correlated with FA. However, the clinical significance still needs to be further explored with larger sample size and longitudinal studies.
Furthermore, in this study, DTI was collected within 5 days of onset of stroke. The predictive value of FA at acute phase for functional outcome is still controversial. Several studies suggest that FA at subacute [47] or chronic phase [48] has good predictive value for chronic functional outcome. Puig et al. [48] found that FA at 30 days, but not <12 hour or 3 days post stroke, was an independent predictor of motor outcome. Doughty et al. reported subtle reduction of FA of CST near the lesion within 80 hours after stroke, but without significant predictive value to motor outcome. Spampinato et al. [49] reported that MRI including diffusional kurtosis imaging within 4 days after stroke onset may have potential value in prediction of motor impairment. Our previous publication suggests that FA within 5 days of stroke on-set may correlate with motor function at discharge after post-acute rehabilitation [13]. In this study, the observed positive correlation between BDFN and FA in the good motor group suggests that a positive feedback loop between BDNF and FA may favor stroke recovery; however, the mechanism is not clear.
Strengths and limitations
This study explored the association between serum BDNF and functional outcomes after post-acute rehabilitation. One of the strengths of this study is the large sample size. The results show a statistically but not clinically significant correlation between BDNF and functional outcome during the early rehabilitation phase. Further studies are warranted to identify the subgroup of subjects who may have a strong correlation between BDNF and functional outcome. This study has several limitations. In this study, serum was collected after admission to inpatient rehabilitation hospital. It would be meaningful to investigate the longitudinal changes of BDNF from acute phase to chronic phase and the correlations with functional outcomes. In addition, this dataset does not have BDNF polymorphism data. The correlation between BDNF polymorphism and serum BDNF level as well as functional outcome over time is important to evaluate the utilization of BDNF polymorphism and serum BDNF in the prediction of stroke recovery. In addition, this study is the first to investigate the relationship between serum BDNF and FA in post-acute stroke patients. However, the imaging data have the limitation of a small sample size. The results from imaging data would need to be confirmed with a larger study. In this study, DTI was collected in clinical scanners as part of initial acute stroke management. To reduce the variation caused by imaging protocols, we used stringent inclusion criteria such as the same scanner from the same hospital and the same acquisition parameters (as described in the methods section). However, further study using high- quality imaging data would be warranted to confirm the findings.
In summary, results from this study suggest that serum BDNF alone has minimally predictive value for functional outcomes during post-acute stroke rehabilitation, and serum BDNF might be correlated with FA in CST in the high motor recovery group.
Acknowledgments
Funding
This study was supported by the Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health award no. UL1 TR001102); Harvard University and its affiliated academic health care centers; and Fundamental Research Funds for the Central Universities (grant no. 12ykpy39). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers or the National Institutes of Health.
This study was also supported by Shenzhen “Sanming project” (SZSM201610039).
Footnotes
Disclosures
None.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study protocol was approved by the Institutional Review Board of Spaulding Rehabilitation Hospital, the teaching affiliate of Harvard Medical School.
References
- [1].Jickling GC, Sharp FR. Blood biomarkers of ischemic stroke. Neurotherapeutics, 2011;8(3):349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7(2):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603–14. [DOI] [PubMed] [Google Scholar]
- [4].Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke, 2015;46(3):915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-dawley rats in vivo. Neuroscience. 2004;124:71–9. [DOI] [PubMed] [Google Scholar]
- [6].Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125 [DOI] [PubMed] [Google Scholar]
- [7].Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. [DOI] [PubMed] [Google Scholar]
- [8].Kim EJ, Park CH, Chang WH, Lee A, Kim ST, Shin YI, Kim YH. The brain‐derived neurotrophic factor Val66Met polymorphism and degeneration of the corticospinal tract after stroke: a diffusion tensor imaging study. Eur J Neurol. 2016;23(1):76–84. [DOI] [PubMed] [Google Scholar]
- [9].Korley FK, Kelen GD, Jones CM, Diaz-Arrastia R . Emergency Department Evaluation of Traumatic Brain Injury in the United States, 2009–2010. J Head Trauma Rehabil. 2016; 31(6):379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, Au R, Pikula A, Wolf PA, DeStefano AL, Vasan RS, Seshadri S. Serum Brain-Derived Neurotrophic Factor and the Risk for Dementia: The Framingham Heart Study. JAMA Neurol. 2014;71(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stanne TM, Åberg ND, Nilsson S, Jood K, Blomstrand C, Andreasson U, Blennow K, Zetterberg H, Isgaard J, Svensson J, Jern C. Low Circulating Acute Brain-Derived Neurotrophic Factor Levels Are Associated With Poor Long-Term Functional Outcome After Ischemic Stroke. Stroke, 2016;47(7):1943–5. [DOI] [PubMed] [Google Scholar]
- [12].Wang J, Gao L, Yang YL, Li YQ, Chang T, Man MH, Zhang XY, Guo SC, Li LH. Low Serum Levels of Brain-Derived Neurotrophic Factor Were Associated with Poor Short-Term Functional Outcome and Mortality in Acute Ischemic Stroke. Mol Neurobiol, 2017;54(9):7335–42. [DOI] [PubMed] [Google Scholar]
- [13].Wen H, Alshikho MJ, Wang Y, Luo X, Zafonte R, Herbert MR, Wang QM. Correlation of Fractional Anisotropy With Motor Recovery in Patients With Stroke After Postacute Rehabilitation. Arch Phys Med Rehabil. 2016;97(9):1487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc. 2006;1044. [PMC free article] [PubMed] [Google Scholar]
- [15].Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17(3):143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6(7):750–7. [DOI] [PubMed] [Google Scholar]
- [17].Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003;50(5):1077–88. [DOI] [PubMed] [Google Scholar]
- [18].Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A 2004;101:13335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007;34:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26(1): 115–23. [DOI] [PubMed] [Google Scholar]
- [22].Yang L, Zhang Z, Sun D, Xu Z, Yuan Y, Zhang X, Li L. Low serum BDNF may indicate the development of PSD in patients with acute ischemic stroke. Int J Geriatr Psychiatry. 2011;26(5):495–502. [DOI] [PubMed] [Google Scholar]
- [23].Pearson-Fuhrhop KM, Cramer SC. Genetic influences on neural plasticity. PMR. 2010;2(12):S227–S240. [DOI] [PubMed] [Google Scholar]
- [24].Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting Neuroplasticity for Motor Rehabilitation After Stroke: Considering the Effects of Aerobic Exercise and Genetic Variation on Brain-Derived Neurotrophic Factor. Phys Ther. 2013;93(12):1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;14(100): 8514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Elkabes S, DiCicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci. 1996;8(16):2508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jiang Y, Wei N, Zhu J, Lu T, Chen Z, Xu G, Liu X. Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediators Inflamm. 2010;372423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328(3):261–4. [DOI] [PubMed] [Google Scholar]
- [29].Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–61. [DOI] [PubMed] [Google Scholar]
- [30].Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14(3):347–53. [DOI] [PubMed] [Google Scholar]
- [31].Béjot Y, Mossiat C, Giroud M, Prigent-Tessier A, Marie C. Christine Marie. Circulating and Brain BDNF Levels in Stroke Rats. Relevance to Clinical Studies. PLoS One. 2011;6 (12):e29405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Munkholm K, Pedersen BK, Kessing LV, Vinberg M. Elevated levels of plasma brain derived neurotrophic factor in rapid cycling bipolar disorder patients. Psychoneuroendocrinology. 2014;47:199–211. [DOI] [PubMed] [Google Scholar]
- [33].Dwivedi Y Involvement of brain-derived neurotrophic factor in late-life depression. Am J Geriatr Psychiatry. 2013;21:433–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tsuchimine S, Sugawara N, Ishioka M, Yasui-Furukori N. Preanalysis storage conditions influence the measurement of brain-derived neurotrophic factor levels in peripheral blood. Neuropsychobiology. 2014;69(2):83–8. [DOI] [PubMed] [Google Scholar]
- [35].Polyakova M, Schlögl H, Sacher J, Schmidt-Kassow M, Kaiser J, Stumvoll M, Kratzsch J, Schroeter ML. Stability of BDNF in Human Samples Stored Up to 6 Months and Correlations of Serum and EDTA-Plasma Concentrations. Int J Mol Sci. 2017;18(6):1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Naegelin Y, Dingsdale H, Säuberli K, Schädelin S, Kappos L, Barde YA. Measuring and Validating the Levels of Brain-Derived Neurotrophic Factor in Human Serum. eNeuro, doi: 10.1523/ENEURO.0419-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Weistein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, Au R, Pikula A, Wolf PA, DeStefano AL, Vasan RS, Seshadri S. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol. 2014; 71(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kaess BM, Preis SR, Lieb W, Beiser AS, Yang Q, Chen TC, Hengstenberg C, Erdmann J, Schunkert H, Seshadri S, Vasan RS, CARDIoGRAM, Assimes TL, Deloukas P, Holm H, Kathiresan S, König IR, McPherson R, Reilly MP, Roberts R, Samani NJ, Stewart AF. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. J Am Heart Assoc. 2015;4(3):e001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim B, Winstein C. Can Neurological Biomarkers of Brain Impairment Be Used to Predict Poststroke Motor Recovery? A Systematic Review. Neurorehabil Neural Repair. 2017;31(1):3–24 [DOI] [PubMed] [Google Scholar]
- [40].Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Resonanace Imaging. 2001;13:534–46. [DOI] [PubMed] [Google Scholar]
- [41].Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–19. [DOI] [PubMed] [Google Scholar]
- [42].Simon NG, Narvid J, Cage T. Visualizing axon regeneration after peripheral nerve injury with magnetic resonance tractography. Neurology. 2014;83(15):1382–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jang SH, Cho SH, Kim YH, Kwon YH, Byun WM, Lee SJ, Park SM, Chang CH. Cortical activation changes associated with motor recovery in patients with precentral knob infarct. Neuroreport. 2004;15(3):395–9. [DOI] [PubMed] [Google Scholar]
- [44].Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ahn YH, Ahn SH, Kim H, Hong JH, Jang SH. Can stroke patients walk after complete lateral corticospinal tract injury of the affected hemisphere? Neuroreport, 2006;17(10):987–90. [DOI] [PubMed] [Google Scholar]
- [46].Dalby RB, Elfving B, Poulsen PH, Foldager L, Frandsen J, Videbech P, Rosenberg R. Plasma brain-derived neurotrophic factor and prefrontal white matter integrity in late-onset depression and normal aging. Acta Psychiatr Scand. 2013;128(5):387–96. [DOI] [PubMed] [Google Scholar]
- [47].Jin J, Guo Z, Zhang Y, Chen Y. Prediction of motor recovery after ischemic stroke using diffusion tensor imaging: A meta-analysis. World J Emerg Med. 2017; 8(2): 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Puig J, Blasco G, Daunis-I-Estadella J, Thomalla G, Castellanos M, Figueras J, Remollo S, van Eendenburg C, Sánchez-González J, Serena J, Pedraza S. Decreased corticospinal tract fractional anisotropy predicts long-term motor outcome after stroke. Stroke. 2013; 44: 2016–2018. [DOI] [PubMed] [Google Scholar]
- [49].Spampinato MV, Chan C, Jensen JH, Helpern JA, Bonilha L, Kautz SA, Nietert PJ, Feng W. Diffusional Kurtosis Imaging and Motor Outcome in Acute Ischemic Stroke. AJNR Am J Neuroradiol. 2017; 38(7):1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]