Abstract
Objective
The purpose of this study was to investigate the electromyographic activity of lumbar multifidus (MF) and erector spinae (ES) muscle during forward walking (FW) and backward walking (BW) in participants with and without chronic low back pain (CLBP).
Methods
Twenty-one patients with CLBP were recruited from outpatient department of Centre for Physiotherapy and Rehabilitation Sciences, Jamia Millia Islamia. Twenty-one age-matched healthy controls without CLBP were recruited from community. Maximum voluntary isometric contraction (MVIC) was quantified for MF and ES using standard guidelines. Electromyographic activity of MF and ES was recorded using surface electrodes during FW and BW on a motorized treadmill, which was later normalized by respective MVIC’s of each muscle.
Results
Muscle activity (in percentage MVIC) was determined to be higher for both the muscles during BW. Activity of MF muscle was significantly higher in CLBP patients compared with healthy controls (P < .04). Electromyographic activity of MF and ES was significantly increased during BW (MF: P < .001; ES: P < .001) compared with FW in both healthy and CLBP groups.
Conclusion
BW leads to greater activation of the paraspinal muscles. Along with global extensor (ES), activity of core extensor (MF) is also higher during BW than FW in both healthy participants and CLBP patients. BW is a more favorable aerobic activity to enhance lumbar paraspinal recruitment. These findings may have important clinical implications in the rehabilitation of CLBP.
Key indexing terms: Low Back Pain, Muscles, Spine
Introduction
Chronic low back pain (CLBP) is a clinical entity that can be defined as back pain with duration of more than 12 weeks. Epidemiologic evidence suggests that 50% of the general population experiences CLBP and around 70% have encountered at least one occurrence of it during their lifetime.1 Chronic low back pain is associated with restriction in daily physical activities that ultimately leads to disuse atrophy of muscles.2 Moreover, weak and atrophied muscles around the lumbar spine cause immense immobility and recurring low back pain in these patients. In particular, CLBP patients present with greater atrophy of extensor muscles than of flexor muscles.3 The lumbar multifidus (MF), which is a core extensor muscle, is crucial for intersegmental stability at lumbar spine, and the superficial extensor muscles, namely longissimus thoracis and iliocostalis lumborum (also known as erector spinae [ES]), are global extensors of spine and are designed to produce gross movements and to counterbalance external loads.4 Contrary to equal distribution of MF on both sides of lumbar spine in healthy adults,5, 6 patients with CLBP present with greater atrophy of MF in lower parts of the lumbar spine.7 Furthermore, previously an inverse relationship between cross-sectional area of ES muscle and CLBP has been established.7 Hence, the size of the lumbar muscles and the extent of muscular atrophy have a relationship with occurrence and relapse of CLBP.
Rehabilitation programs for the relief of low back pain include complex exercises such as motor control exercises, muscle strengthening exercises, muscle stretching exercises, and aerobic exercises.8 Though the effect of the former three has been widely studied, the role of aerobic exercises in the rehabilitation of CLBP has been largely ignored; however, some existing reports in the literature do indicate that aerobic exercise in the form of walking is effective in reducing pain and dysfunction in LBP patients.9 Usually the term walking is considered as locomotion in forward direction; however, recent literature has given substantial importance to backward walking (BW) as well.10 BW is a method of locomotion with roots in ancient China, where it was used to attain physical fitness and well-being.11 BW has been reported to elicit better clinical outcomes than FW. It has emerged as a beneficial exercise in terms of cardiopulmonary fitness and lower limb muscle activity while simultaneously decreasing patellofemoral joint stress in runners.12 Furthermore, BW poses higher neuromuscular demands on body than FW in part because of its association with greater cadence and shorter stride length.13, 14 Previous evidence has clearly indicated that BW creates "more muscle activity in proportion to effort" compared with FW.15
The role of BW training program in preventing LBP (induced by compromised flexibility of hamstrings in LBP patients) has been proved because BW enhances hamstring and low back flexibility by virtue of the hamstring prestretch mechanism.16 A study by Masumoto et al17 elicited that BW leads to greater core muscle activity during locomotion in water compared with walking forward in the same medium. Dufek et al18 reported that BW can decrease low back pain and increase range of motion of lumbar spine specifically in the sagittal plane. However, all these previous observations were made in the absence of a control group performing FW; hence, it is difficult to conclude that the reported changes were solely a result of BW rather than walking as such. Previous observations in favor of BW16, 17, 18 have created a desire among the researchers to investigate this exercise more rigorously in order to upgrade the present conventional rehabilitation models for CLBP patients in clinical settings. Critical analysis of the literature revealed an important lacuna of not understanding the activation patterns of lumbar spine muscles during this novel task of BW compared with FW in healthy individuals and in CLBP patients. Considering these gaps in previous literature, the purpose of the present study was to investigate and compare the activation pattern of lumbar MF and ES muscles during FW vs BW in individuals with and without CLBP. The secondary objective of the study was to investigate the lumbar paraspinal muscle activation pattern in healthy individuals vs CLBP patients. We hypothesized that the activity of both core (MF) and global (ES) extensors of spine would be more pronounced during BW in both healthy and CLBP participants.
Materials and Methods
Participants
The number of participants was determined by G*Power Software version 3.1.9.2 (University of Kiel, Kiel, Germany). Using data of changes in electromyographic (EMG) activity of paraspinal muscles during FW vs BW from a previous study,17 42 participants (21 participants each in 2 groups) were determined to be necessary based on the effect size of 0.672, α level of .05, and power (1-β) of .90. Twenty-one healthy participants without any known back problems (10 men, 11 women; age 23.52 ± 2.44 years; height 161.5 ± 6.5 cm; weight 52.72 ± 8.4 kg; and body mass index [BMI] 20.1 ± 2.98 kg/m2) and 21 patients with CLBP, which was defined as low back pain for more than 3 months without any radicular symptoms and intensity of pain <6 on the visual analog scale (VAS) (12 men, 9 women; age 22.62 ± 2.7 years; height 161.1 ± 11.04 cm; weight 57.97 ± 11.16 kg; BMI 22.28 ± 3.8 kg/m2; and pain intensity on VAS 4.16 ± 1.08) were recruited by convenience sampling from the outpatient department of Centre for Physiotherapy and Rehabilitation Sciences, Jamia Millia Islamia, New Delhi, India. It was ensured by medical examination that the participants did not experience any acute or chronic cardiopulmonary and musculoskeletal problem (apart from CLBP). This study was approved by the Institutional Ethics Committee, Jamia Millia Islamia, and was conducted in accordance with the Helsinki Declaration of 1964. All participants were informed about the procedures and potential risks and were asked to sign a written consent form to participate in the study.
Experimental Procedure
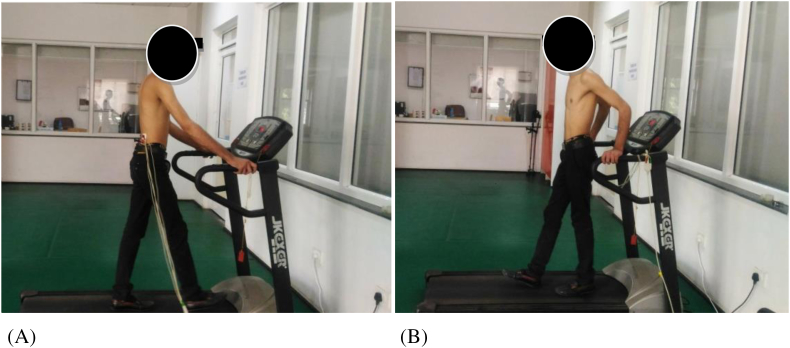
All the experimental procedures were performed at the Laboratory of Biomechanics, Centre for Physiotherapy and Rehabilitation Sciences, Jamia Millia Islamia. After recording the demographic measurements (age, height, weight, BMI, pain intensity by VAS [for CLBP patients only]), participants were subjected to exercise testing. Before commencing the exercise testing, all the participants were asked to practice BW on the same treadmill that was used for testing until they felt confident enough to proceed with the actual testing. Each test was completed by a participant in a single day. First, maximal voluntary isometric contraction (MVIC) values were obtained to normalize the EMG amplitude. Test–retest reliability of MVIC measurement for each muscle group tested in this study was determined to be good to excellent, with intraclass correlation coefficient values of 0.64 and 0.84 for MF and ES muscle, respectively. Further testing consisted of walking forward and backward on a motorized treadmill (JK EXER Treadmill, Cosco India Ltd, Gurugram, India) at self-paced comfortable speeds (Fig 1). Mean and standard deviation of walking speed in kilometers per hour for FW and BW in healthy and CLBP groups were 4.1 ± 1.09 vs 3.2 ± 1.18 (P = .91) and 2.6 ± 0.62 vs 2.3 ± 0.78 (P = .32), respectively. Each participant completed two 1-min walks in forward and backward direction at their respective comfortable speeds along with simultaneous surface EMG recording for MF and ES muscle activity. A 1-minute rest was provided between FW and BW. Verbal instructions were provided to participants to follow correct pattern of walking. Of the total 1-minute EMG record in each direction, the middle 30 seconds were used for analysis.17 Root mean square values were obtained for both the muscles during FW and BW in participants with and without CLBP. The EMG signals were filtered (band-pass filter = 50-1 000 Hz) and amplified (100 times) before data acquisition (sampling rate: 100 Hz). The analysis of raw EMG signals was performed by LabChart Software version 1.4.2 (ADInstruments, Bella Vista, Australia).
Fig 1.
Electromyographic recording during forward walking (A) and backward walking (B).
Electromyographic Recording
For evaluating muscle activity, surface EMG of lumbar MF and ES was recorded using gel-coated silver chloride surface electrodes (Medico Electrodes International Ltd, Warwick, United Kingdom). To reduce skin resistance to a minimum (≤10 k ohm), electrodes were placed on the required skin site after shaving, abrading, and cleaning the skin. Surface EMG has been reported to be a reliable method to investigate the activity of deep-seated paraspinal muscles as well,19 and a strong body of evidence exists regarding previous use of this method to evaluate the activity of MF muscle.20, 21, 22 Electromyographic signals were recorded for lumbar MF and ES muscles unilaterally on the right side. For ES muscle, the electrodes were placed 4 cm lateral to the L1 spinous process, and for lumbar MF, electrodes were placed 2 cm lateral to the midline running through the L5 spinous process22 (Fig 2). Bipolar surface electrodes were aligned parallel to the orientation of muscle fibers for both the muscles.23 A reference electrode was placed over the posterior superior iliac spine. Surface electrodes were fixed to the placement site with tape to maintain appropriate skin contact and to limit skin impedance. Electrodes were placed 2 cm apart to minimize cross-talk between the two electrodes placed on a muscle.
Fig 2.
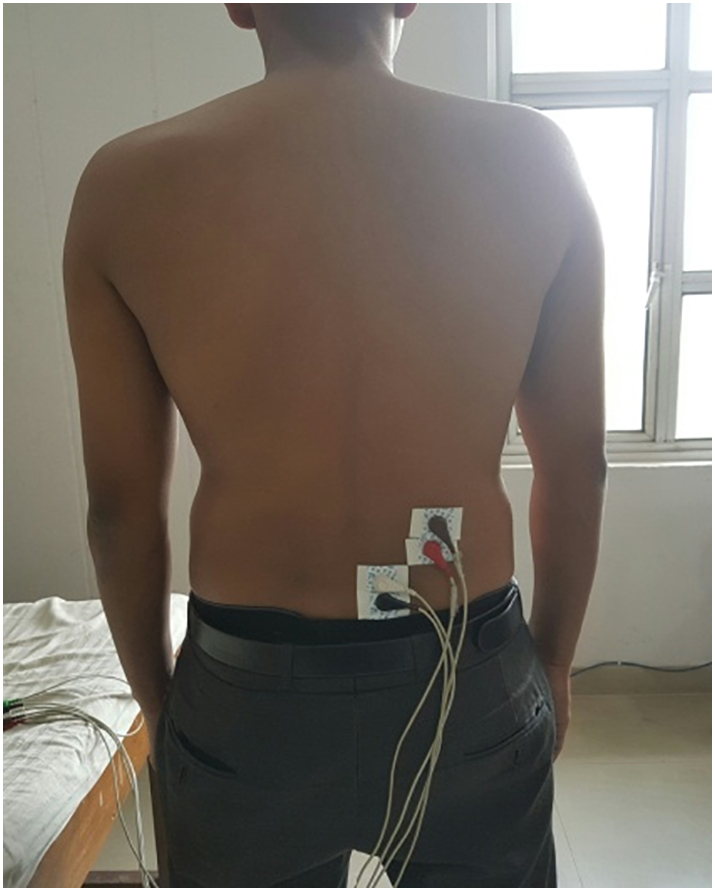
Electrode placement for recording electromyographic activity of multifidus and erector spinae muscle.
MVIC Assessment
Before testing, participants were familiarized with the assessment procedures and were trained to produce the maximal force output a day before each measurement. To obtain MVIC of lumbar MF and ES muscles, participants were made to lie in a prone position, with their hands clasped behind their buttocks and their lower extremities were stabilized firmly on the table with the help of a stabilization belt. The resistance was applied by the therapist for 5 seconds to the upper thoracic area in downward direction on completion of maximal possible lumbar extension by the participant by moving the upper body away from the table22, 24 (Fig 3). Verbal encouragement was provided by the instructors to motivate the participant in order to achieve maximal contraction levels during MVIC assessment. The participants performed this isometric contraction 3 times for each muscle with a rest interval of 1 minute between two trials. For normalization procedures, root mean square value of the EMG activity (in millivolts) during 30 seconds of treadmill walking was divided by the respective MVIC value.
Fig 3.
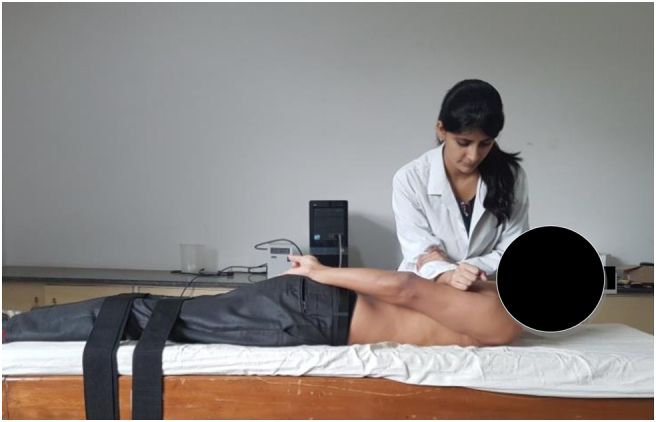
Maximum voluntary isometric contraction assessment of multifidus and erector spinae muscle.
Statistical Analysis
Data are expressed as mean ± standard deviation. All the data were analyzed using SPSS Software version 17.0 (SPSS Inc, Chicago, Illinois). The Shapiro-Wilk test was used to verify the normality of quantitative data. Demographic characteristics and criterion measures at baseline were compared between the healthy and CLBP patients by an independent t test. A 2 × 2 mixed model analysis of variance was used to analyze difference in the muscle activation between the groups (CLBP vs healthy), direction of walking (FW vs BW), and group × direction interaction effect. A paired t test was used to find differences in the activation between the two muscles during FW and BW. The significance level was set at P ≤ .05.
Results
Demographic Characteristics of Participants
Each participant completed all the testing in a single day. The demographic characteristics of participants in healthy control and CLBP group are shown in Table 1. The groups were comparable in terms of age, height, and weight (Table 1).
Table 1.
Demographic Characteristic of Participants
| Healthy n = 21 |
CLBP n = 21 |
P Value | |
|---|---|---|---|
| Variables | Mean ± SD | Mean ± SD | |
| Age (y) | 23.52 ± 2.44 | 22.62 ± 2.7 | .27 |
| Height (cm) | 161.5 ± 6.5 | 161.1 ± 11.04 | .87 |
| Weight (kg) | 52.72 ± 8.4 | 57.97 ± 11.16 | .10 |
| BMI (kg/m2) | 20.1 ± 2.98 | 22.28 ± 3.8 | .04 |
| VAS | 4.16 ± 1.08 |
BMI, body mass index; CLBP, chronic low back pain patients; SD, standard deviation; VAS, visual analog scale.
Multifidus Activity During Forward and Backward Walking
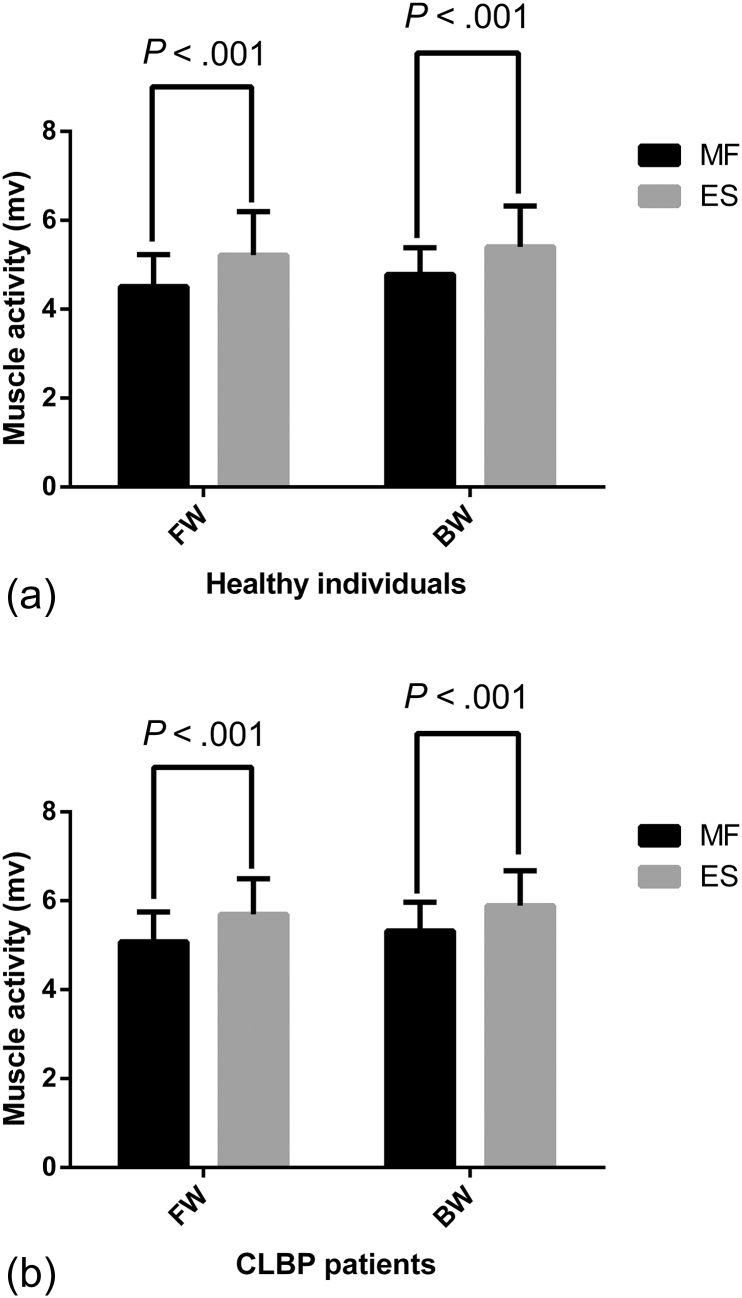
Percentage MVIC for MF during FW and BW is presented in Table 2. It was higher during BW in both groups. A 2 × 2 mixed model analysis of variance yielded a significant main effect for group, F (1, 40) = 4.365, P = .043. There was a significant main effect for direction, F (1, 40) = 44.09, P < .001, and group × direction interaction effect, F (1, 40) = 4.334, P = .04 (Table 3). There was significantly greater MF activity during BW than FW in healthy participants and CLBP patients (Fig 4).
Table 2.
Percentage MVIC During FW and BW in Healthy Participants and CLBP Patients
| Groups | FW |
BW |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Healthy | ||
| MF | 114 ± 74.2 | 140.7 ± 80.06 |
| ES | 293.2 ± 199.98 | 332.0 ± 226.33 |
| CLBP | ||
| MF | 210.4 ± 116.63 | 261.7 ± 146.01 |
| ES | 401.7 ± 318.1 | 480.7 ± 367.97 |
BW, backward walking; CLBP, chronic low back pain; ES, erector spinae; FW, forward walking; MF, multifidus; MVIC, maximal voluntary isometric contraction.
Table 3.
Summary of the Results of 2 × 2 Mixed Model Analysis of Variance for Activity of Multifidus and Erector Spinae Muscles
| Muscle Activity in Different Walking Conditions | Source | df | F | P | η2 |
|---|---|---|---|---|---|
| Multifidus | Group (main effect) | 1 | 4.365 | .043 | 0.098 |
| Direction (main effect) | 1 | 44.09 | <.001 | 0.524 | |
| Group × direction (interaction) | 1 | 4.334 | .044 | 0.0098 | |
| Erector spinae | Group (main effect) | 1 | 1.642 | .207 | 0.039 |
| Direction (main effect) | 1 | 37.85 | <.001 | 0.486 | |
| Group × direction (interaction) | 1 | 4.406 | .042 | 0.099 |
df, degrees of freedom.
Fig 4.
Comparison of muscle activity (multifidus and erector spinae) in mv during FW and BW in patients with CLBP vs healthy controls. BW, backward walking; CLBP, chronic low back pain; FW, forward walking; mv, millivolts.
Erector Spinae Activity During Forward and Backward Walking
Percentage MVIC for ES was higher during BW than FW (Table 2). Statistical analysis suggested no significant main effect for group, F (1, 40) = 1.642, P = .207. However, there was significant main effect for direction, F (1, 40) = 37.85, P < .001, and group × direction interaction effect, F (1, 40) = 4.406, P = .042 (Table 3). Activity of ES was significantly greater during BW than FW in both the groups (Fig 4).
Discussion
Purpose and Main Findings
The aim of the present study was to investigate paraspinal muscle activity (lumbar MF and ES) during FW and BW in healthy individuals and CLBP patients. Results indicated that activity of MF was greater in participants with CLBP compared with the healthy group. Primary results were in accordance to our experimental hypothesis, and the activity of both MF and ES was more pronounced during BW compared with FW.
Muscle Activation Patterns During FW and BW: Effect of Direction of Walking
Greater activation of the MF and ES in both healthy participants and CLBP patients during BW was identified in the present study. This finding can be justified by a previous study that reported that BW creates greater muscle activity in proportion to effort than FW.15 A previous study by Cole et al25 speculated that BW causes extended paraspinal muscle activity as a result of prolonged isometric contractions of paraspinals in aquatic medium. Scientific analysis of BW has illustrated greater activity in the motor cortex, thalamus, putamen, and caudate and lesser activity in the cerebellum and brainstem.26 This was suggested to indicate that BW being a novel task for an individual presents more stability challenge, leading to greater motor unit recruitment, resulting in a greater amount of skeletal muscle activation than in an equivalent familiar task.27, 28, 29
The biomechanics of BW gait are different from FW in terms of stride length and time, knee and hip range of motion, and relative stance phase. Moreover, a shorter stride length in BW is partially compensated for by increased stride frequency in maintaining the predefined walking speed,27, 29 and these changes in stride frequency consequently influence joint kinematics and kinetics.30 Changes in joint kinematics and kinetics might influence relative muscle contributions during a physical task. Apart from these kinematic differences, BW comprises lower local dynamic stability compared with walking forward.28 This lower local dynamic stability might make BW a more demanding task for the local stabilizers from the perspective of motor control.
From a biomechanical perspective, BW works on the principle of reverse control mechanism, which is opposite to the conventional heel-toe pattern of FW gait. The toe-heel pattern of BW gait places immense neuromuscular and proprioceptive demands on the body because of a lack of visual cues in the direction of movement. Linear association of neuromuscular control and proprioception with muscle strength and activity has been established by previous research,31 and longitudinal neuromuscular training has been reported to produce positive outcomes in muscle strength.32 Therefore, the neuromuscular and proprioceptive demands posed by BW may be speculated to cause enhanced muscle activity of lumbar MF and ES in the present investigation. However, these are mere speculations because we did not investigate neuromuscular control and proprioception in the present study. Furthermore, the toe-heel pattern of gait in BW might have required greater activity of both the core and global extensors of spine for accomplishing greater extension of lumbar spine in the present investigation. Greater hip extension and a concomitant extension of lumbar spine has been reported to open the disc space and decrease the compressive loads on intervertebral disc, with a concomitant reduction in pain in athletes with low back pain.18 Dufek et al18 have suggested that BW enhances lumbar muscle function by activating core stability control, which probably requires greater activation of core musculature such as MF. Moreover, there was no similar study for direct comparison of our results because no study has previously explored the activity of lumbar muscles in CLBP patients during BW apart from Masumoto et al,17 who compared lumbar muscle activity during BW on land vs water and identified greater activation of muscles in the water because of increased resistance offered by this medium. Increased demands on the neuromuscular and proprioceptive system, along with changes in biomechanical alignment of spine and lower extremity, are the probable indirect mechanisms that might lead to increased lumbar paraspinal muscle activity in CLBP patients. However, directly speculating on the biomechanical causes of the same are difficult because we did not study the kinetics and kinematics of BW gait along with lumbar paraspinal muscle activity. Still, the results are promising and are in favor of BW, which opens up opportunities to study BW in future with rigorous study designs. Based on the present findings, BW may be of importance to CLBP patients because it may help in counteracting the dysfunction of lumbar paraspinal muscles via improving the neuromuscular and proprioceptive deficits associated with CLBP.
Multifidus Muscle Activity in Healthy Controls vs CLBP Patients
The present study identified greater activation of MF muscle in CLBP patients compared with healthy controls. There might be two possible mechanisms behind this observation; first could be the Pain Adaptation Model, wherein during dynamic conditions (FW and BW) muscle pain is accompanied by hyperactivity in the back muscles33, 34, 35; the second possible mechanism is associated with the muscle spasm identified in CLBP patients.36 Paraspinal muscles as well as some of the connective tissues in the region of the spine contain sensory nerve endings sensitive to changes in length, tension, position, and movement, which may be initiated by the presence of pain and other factors. In addition, some sensory nerve endings respond to changes in position by inhibiting the muscle activity, whereas others possibly related to muscle spasm with increase in activity.37 Lumbar MF is an important stabilizer of the neutral zone of spine, which is mostly involved in CLBP, and dysfunction of MF muscle is strongly associated with CLBP.38 Persistent lumbar MF dysfunction is identified by atrophic replacement of MF muscle with fatty infiltrates. Previous investigations have identified reduced MF activity and strength in patients with CLBP compared with healthy controls.39, 40 Greater activity of lumbar MF in CLBP patients compared with healthy controls in the present study seems to be in contradiction to previous reports in which reduced MF activity was identified in CLBP patients. However, direct comparisons are difficult because MF muscle activity during BW per se was not investigated by previous studies, and this increased MF activity identified in the present study might be a compensatory strategy of the spine to provide local stabilization to the neutral zone of spine during the novel task of BW.
Erector Spinae Muscle Activity in Healthy Controls vs CLBP Patients
The result of the present study indicated that although there was no statistically significant difference between the groups for ES muscle, the percentage MVIC of ES muscle was greater compared with percentage MVIC of MF. Researchers have reported greater average ES activity during the total stride41, 42 and during periods of swing43 in CLBP patients. The absolute muscle activity level was determined to be 8% to 48% higher in patients with CLBP than in healthy controls.44 Previous studies have reported that global back extensor muscles in LBP patients exhibit higher level of activation to compensate for spinal instability.42 Present findings are in accordance with these previous observations, indicating greater activity of ES muscle for providing additional stability to spine in part to counter decreased back muscle force and concomitantly reduced capability to manage perturbations. The trunk muscles are also supplied by supraspinal and spinal centers such as the reticulospinal, and vestibulospinal neurons, which have an indirect role in postural control. Therefore, changes in the organization of these regions termed as “motor cortex reorganization” after the first incidence of back pain contribute to changes in postural control and are one of the mechanisms suggested to cause increased ES muscle activity in the present investigation.45 Also, during BW, individuals are deficient of visual cues necessary to anticipate the condition of ground; therefore there is a need to reorganize and adapt the changed information from visual, cutaneous and proprioceptive, and vestibular senses, and then enhance the movement control to maintain dynamic balance, which makes BW a more challenging task requiring greater support from the global extensors (ES) of spine.46
Strengths and limitations
The findings of the present study indicated that BW was a more demanding aerobic activity compared with conventional FW. These findings may have clinical implications in the upgradation of rehabilitation protocols for CLBP patients. BW is a more demanding leisure physical activity, which enhances recruitment of paraspinals and may provide clinicians with better outcomes in CLBP patients.
Limitations of the present study included the following. We investigated the activity of only two paraspinal muscles, which does not give a holistic outlook about lower back muscle activation patterns during BW. EMG was recorded by surface electrodes, and therefore noises from the nearby muscle might also have contributed to the observed signal. Since it was a cross-sectional study, direct implications for modifying rehabilitation protocol cannot be drawn and there is need for longitudinal clinical trials for doing the same. Our study did not investigate the lower limb muscle activity along with gait parameters; therefore it becomes difficult to elucidate the exact biomechanical mechanisms in support of the present findings. Studies in future should counter these limitations and should also include kinematic analysis along with EMG activity for a thorough analysis of BW. In addition, BW may have implications for frequent fallers because it poses greater motor control demands than FW; therefore, in future, similar studies should be conducted in frequent fallers but with extreme caution. There is need of large randomized controlled trials to understand the role of BW more precisely and to implement it in rehabilitation of CLBP.
Conclusion
In the present investigation, it was determined that paraspinal muscle (MF and ES) activity was greater during BW than FW. The CLBP group had greater activation of paraspinal muscles compared with the healthy group. There was increased ES activity compared with the MF during FW and BW in both the healthy and CLBP groups. These findings support previous statements on greater global muscle activation in CLBP patients. BW leads to greater activation of ES (global extensor) as well as of MF (core extensor) compared with traditional FW. This may have clinical implications in evolving the rehabilitation program for CLBP patients. BW may be a preferred mode of aerobic exercise in CLBP patients because it may cause greater muscle activation.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Practical Applications
-
●
This study reported that backward walking caused greater activation of lumbar extensors compared with forward walking in patients with chronic low back pain.
-
●
Backward walking may be a more favorable aerobic exercise form to combat muscle atrophy in chronic low back pain.
Alt-text: Unlabelled Box
Contributorship Information
Concept development (provided idea for the research): B.A., N.N., M.E.H.
Design (planned the methods to generate the results): B.A., N.N., P.B.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): P.B., N.N., M.E.H.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): B.A., P.B.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): B.A., P.B., D.S.
Literature search (performed the literature search): B.A., P.B.
Writing (responsible for writing a substantive part of the manuscript): B.A., P.B., D.S.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): P.B., D.S., M.E.H.
References
- 1.McGill SM. A revised anatomical model of the abdominal musculature for torso flexion efforts. J Biomech. 1996;29(7):973–977. doi: 10.1016/0021-9290(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 2.Smeets RJ, Wade D, Hidding A, Van Leeuwen PJ, Vlaeyen JW, Knottnerus JA. The association of physical deconditioning and chronic low back pain: a hypothesis-oriented systematic review. Disabil Rehabil. 2006;28(11):673–693. doi: 10.1080/09638280500264782. [DOI] [PubMed] [Google Scholar]
- 3.Beimborn DS, Morrissey MC. A review of the literature related to trunk muscle performance. Spine. 1988;13(6):655–660. [PubMed] [Google Scholar]
- 4.Panjabi M, Abumi K, Duranceau J, Oxland T. Spinal stability and intersegmental muscle forces: a biomechanical model. Spine. 1989;14(2):194–200. doi: 10.1097/00007632-198902000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Hides JA, Richardson CA, Jull GA. Magnetic resonance imaging and ultrasonography of the lumbar multifidus muscle: comparison of two different modalities. Spine. 1995;20(1):54–58. doi: 10.1097/00007632-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21(23):2763–2769. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Lee HS, Shim JS, Lee ST, Kim M, Ryu JS. Facilitating effects of fast and slope walking on paraspinal muscles. Ann Rehabil Med. 2014;38(4):514–522. doi: 10.5535/arm.2014.38.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moseley L. Combined physiotherapy and education is efficacious for chronic low back pain. Aust J Physiother. 2002;48(4):297–302. doi: 10.1016/s0004-9514(14)60169-0. [DOI] [PubMed] [Google Scholar]
- 9.Chatzitheodorou D, Kabitsis C, Malliou P. Mougios V. A pilot study of the effects of high-intensity aerobic exercise versus passive interventions on pain, disability, psychological strain, and serum cortisol concentrations in people with chronic low back pain. Phys Ther. 2007;87(3):304–312. doi: 10.2522/ptj.20060080. [DOI] [PubMed] [Google Scholar]
- 10.Laufer Y. Age and Gender related changes in the temporal spatial characteristics of forward and backward gaits. Physiother Res Int. 2003;8(3):131–142. doi: 10.1002/pri.281. [DOI] [PubMed] [Google Scholar]
- 11.Hoogkamer W, Meyns P, Duysens J. Steps forward in understanding backward gait: from basic circuits to rehabilitation. Exerc Sport Sci Rev. 2014;42(1):23–29. doi: 10.1249/JES.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 12.Flynn TW, Soutas-Little RW. Patellofemoral joint compressive forces in forward and backward running. J Orthop Sports Phys Ther. 1995;21(5):277–282. doi: 10.2519/jospt.1995.21.5.277. [DOI] [PubMed] [Google Scholar]
- 13.Vilensky JA. A kinematic comparison of backward and forward walking in humans. J Hum Mov Stud. 1987;13(1):29–50. [Google Scholar]
- 14.Flynn TW, Soutas-Little RW. Mechanical power and muscle action during forward and backward running. J Orthop Sports Phys Ther. 1993;17(2):108–112. doi: 10.2519/jospt.1993.17.2.108. [DOI] [PubMed] [Google Scholar]
- 15.Gray GW. Wynn Marketing; Adrian, MI: 1990. Chain Reaction: Successful Strategies for Closed Chain Testing and Rehabilitation. [Google Scholar]
- 16.Whitley CR, Dufek JS. Effects of backward walking on hamstring flexibility and low back range of motion. Int J Exerc Sci. 2011;4(3):192–198. [Google Scholar]
- 17.Masumoto K, Takasugi SI, Hotta N, Fujishima K, Iwamoto Y. Muscle activity and heart rate response during backward walking in water and on dry land. Eur J Appl Physiol. 2005;94(1-2):54–61. doi: 10.1007/s00421-004-1288-x. [DOI] [PubMed] [Google Scholar]
- 18.Dufek J, House A, Mangus B, Melcher G, Mercer J. Backward walking: a possible active exercise for low back pain reduction and enhanced function in athletes. J Exerc Physiol Online. 2011;14(2):17–26. [Google Scholar]
- 19.Cardozo AC, Gonçalves M. Assessment of low back muscle by surface EMG. In: Steele C, editor. Applications of EMG in Clinical and Sports Medicine. InTech; Rijeka, Croatia: 2012. pp. 151–164. [Google Scholar]
- 20.Ng JK, Richardson CA, Jull GA. Electromyographic amplitude and frequency changes in the iliocostalis lumborum and multifidus muscles during a trunk holding test. Phys Ther. 1997;77(9):954–961. doi: 10.1093/ptj/77.9.954. [DOI] [PubMed] [Google Scholar]
- 21.Menacho MO, Obara K, Conceição JS. Electromyographic effect of mat Pilates exercise on the back muscle activity of healthy adult females. J Manip Physiol Ther. 2010;33(9):672–678. doi: 10.1016/j.jmpt.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Ekstrom RA, Osborn RW, Hauer PL. Surface electromyographic analysis of the low back muscles during rehabilitation exercises. J Orthop Sports Phys Ther. 2008;38(12):736–745. doi: 10.2519/jospt.2008.2865. [DOI] [PubMed] [Google Scholar]
- 23.Kim BI, Jung JH, Shim J, Kwon HY, Kim H. An analysis of muscle activities of healthy women during Pilates exercises in a prone position. J Phys Ther Sci. 2014;26(1):77–79. doi: 10.1589/jpts.26.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendall FP, McCreary EK, Provance GP, Rodgers MM, Romani WA. 5th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. Muscles Testing and Function With Posture and Pain. [Google Scholar]
- 25.Cole AJ, Eagleston RE, Moschetti M, Sinnett E. Spine pain: aquatic rehabilitation strategies. J Back Musculoskelet Rehabil. 1994;4(4):273–286. doi: 10.3233/BMR-1994-4407. [DOI] [PubMed] [Google Scholar]
- 26.Godde B, Voelcker-Rehage C. More automation and less cognitive control of imagined walking movements in high-versus low-fit older adults. Front Aging Neurosci. 2010;2:139. doi: 10.3389/fnagi.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackney ME, Earhart GM. The effects of a secondary task on forward and backward walking in Parkinson's disease. Neurorehabil Neural Repair. 2009;24(1):97–106. doi: 10.1177/1545968309341061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogkamer W, Massaad F, Jansen K, Bruijn SM, Duysens J. Selective bilateral activation of leg muscles after cutaneous nerve stimulation during backward walking. J Neurophysiol. 2012;108(7):1933–1941. doi: 10.1152/jn.01159.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurz MJ, Wilson TW, Arpin DJ. Stride-time variability and sensorimotor cortical activation during walking. NeuroImage. 2012;59(2):1602–1607. doi: 10.1016/j.neuroimage.2011.08.084. [DOI] [PubMed] [Google Scholar]
- 30.Lacquaniti F, Ivanenko YP, Zago M. Patterned control of human locomotion. J Physiol. 2012;590(10):2189–2199. doi: 10.1113/jphysiol.2011.215137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HM, Cheng CK, Liau JJ. Correlation between proprioception, muscle strength, knee laxity, and dynamic standing balance in patients with chronic anterior cruciate ligament deficiency. Knee. 2009;16(5):387–391. doi: 10.1016/j.knee.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Holm I, Fosdahl MA, Friis A, Risberg MA, Myklebust G, Steen H. Effect of neuromuscular training on proprioception, balance, muscle strength, and lower limb function in female team handball players. Clin J Sport Med. 2004;14(2):88–94. doi: 10.1097/00042752-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Triano JJ, Schultz AB. Correlation of objective measure of trunk motion and muscle function with low-back disability ratings. Spine. 1987;12(6):561–565. doi: 10.1097/00007632-198707000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Ahern DK, Follick MJ, Council JR, Laser-Wolston N, Litchman H. Comparison of lumbar paravertebral EMG patterns in chronic low back pain patients and non-patient controls. Pain. 1988;34(2):153–160. doi: 10.1016/0304-3959(88)90160-1. [DOI] [PubMed] [Google Scholar]
- 35.Sihvonen T, Partanen J, Hänninen O, Soimakallio S. Electric behavior of low back muscles during lumbar pelvic rhythm in low back pain patients and healthy controls. Arch Phys Med Rehabil. 1991;72(13):1080–1087. [PubMed] [Google Scholar]
- 36.Sherman RA. Relationships between strength of low back muscle contraction and reported intensity of chronic low back pain. Am J Phys Med Rehabil. 1985;64(4):190–200. [PubMed] [Google Scholar]
- 37.Kumar S. Electromyography in ergonomics. In: Kumar S, Mital A, editors. Electromyography in Ergonomics. Taylor & Francis; London, UK: 1996. [Google Scholar]
- 38.Freeman MD, Woodham MA, Woodham AW. The role of the lumbar multifidus in chronic low back pain: a review. PM R. 2010;2(2):142–146. doi: 10.1016/j.pmrj.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Ahern DK, Follick MJ, Council JR, Laser-Wolston N. Reliability of lumbar paravertebral EMG assessment in chronic low back pain. Arch Phys Med Rehabil. 1986;67(10):762–765. doi: 10.1016/0003-9993(86)90014-6. [DOI] [PubMed] [Google Scholar]
- 40.Danneels L, Coorevits P, Cools A. Differences in electromyographic activity in the multifidus muscle and the iliocostalis lumborum between healthy participants and patients with sub-acute and chronic low back pain. Eur Spine J. 2002;11(1):13–19. doi: 10.1007/s005860100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallwork TL, Stanton WR, Freke M, Hides JA. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man Ther. 2009;14(5):496–500. doi: 10.1016/j.math.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Vogt L, Pfeifer K, Banzer W. Neuromuscular control of walking with chronic low-back pain. Man Ther. 2003;8(1):21–28. doi: 10.1054/math.2002.0476. [DOI] [PubMed] [Google Scholar]
- 43.Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain. 1996;64(2):231–240. doi: 10.1016/0304-3959(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 44.Tsao H, Hodges PW. Persistence of improvements in postural strategies following motor control training in people with recurrent low back pain. J Electromyogr Kinesiol. 2008;18(4):559–567. doi: 10.1016/j.jelekin.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Anders C, Wagner H, Puta C, Grassme R, Petrovitch A, Scholle HC. Trunk muscle activation patterns during walking at different speeds. J Electromyogr Kinesiol. 2007;17(2):245–252. doi: 10.1016/j.jelekin.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Nadeau S, Amblard B, Mesure S, Bourbonnais D. Head and trunk stabilization strategies during forward and backward walking in healthy adults. Gait Posture. 2003;18(3):134–142. doi: 10.1016/s0966-6362(02)00070-x. [DOI] [PubMed] [Google Scholar]