Abstract
Objective
This case report describes the clinical features, complications, imaging characteristics, and management of postoperative spinal adhesive arachnoiditis.
Clinical Features
A 54-year-old woman presented with right posterior thigh and leg pain after a lumbar spine fusion surgery to correct a degenerative spondylolisthesis of L3/4. Her pain was sharp and shooting and worsened with knee extension. A lumbar computed tomography myelogram demonstrated clumping and adhesion of the nerve rootlets in the cauda equina at the surgical fusion levels. Findings were consistent with spinal arachnoiditis.
Intervention and Outcome
The patient was treated with 2 sets of neural mobilization of the sciatic nerve with 15 repetitions each. Treatment was provided 2× per week for 3 weeks. The patient used the neural mobilization exercises at home and performed to tolerance. The patient’s Oswestry Questionnaire was reduced significantly by 19% with decreased pain intensity of 2 points on the verbal analogue scale.
Conclusion
Neural mobilization was used successfully in the management of a patient with postoperative spinal arachnoiditis.
Key Indexing Terms: Arachnoiditis, Failed Back Surgery Syndrome, Magnetic Resonance Imaging, Myelography, Chiropractic
Introduction
Spinal arachnoiditis (SA) is defined as inflammation and adhesions of the arachnoid membrane surrounding the spinal cord and rootlets of the cauda equine.1, 2 Low back pain and radiculopathy are the most common symptoms.1, 2 There are multiple etiologies of arachnoiditis; postoperative is the most common with a prevalence of 3% to 16%.3, 4, 5 Magnetic resonance imaging (MRI) and computed tomography (CT) myelography are effective in the diagnosis of arachnoiditis.6, 7, 8, 9, 10 There are limited treatment options for these patients. Surgical options, such as arachnoidolysis and direct spinal cord stimulation, have shown mixed results but with the risk of developing more postsurgical scarring.1, 11, 12
Pharmacologic treatment options are limited to analgesics, which carry an adverse risk profile. Corticosteroids and epidural injections are other pharmacologic options; however, they promote an increased risk of scarring and pain. Nonpharmacologic interventions, such as physiotherapy and spinal manipulation, have been limited to several case reports in failed back surgery syndrome.13, 14, 15, 16, 17, 18 However, none address the complication of arachnoiditis. The purpose of this care report is to describe neural mobilization (NM) in the management of a patient with postoperative spinal adhesive arachnoiditis.
Case Report
A 54-year-old woman presented to a chiropractic outpatient teaching clinic with the chief complaint of right-sided buttock pain and radiation to the posterior knee with occasional extension to the lateral calf. Onset was 7 months before the initial visit. She rated the pain on a verbal numeric scale as 5 of 10, with 0 being no pain and 10 being the worst. She also stated at best, the pain was a 2 of 10 and at worst, 9 of 10. The patient described the pain as a constant dull ache with occasional sharp, stabbing, and shooting pain, particularly while seated with knee extension. The patient expressed disappointment in her limited capacity to perform routine exercise. Movement, ice, stretching, and ibuprofen (400-600 mg/d) were palliative. Prolonged sitting, standing, driving, and walking uphill provoked pain and radiation into her legs. The patient denied any bladder or bowel incontinence or urgency, saddle anesthesia, or abdominal pain. She also denied any recent trauma or hospitalizations, history of cancer, unexplained weight loss, or recent constitutional symptoms.
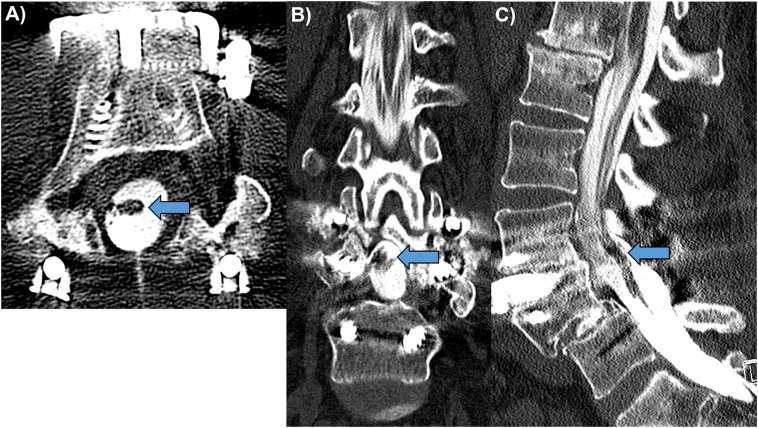
The patient had an extensive surgical history. About 20 years before the initial visit, she was in a motor vehicle accident and sustained a lumbar spine injury, leading to a posterior L4/5 pedicle screw and rod fixation with total laminectomy. This procedure eventually led to the complication of recurrent low back pain 19 years later. She was diagnosed with an unstable degenerative spondylolisthesis of L3/4. Subsequently, a consultation with another orthopedist was obtained 1 year before the chiropractic visit. That orthopedist proceeded with a posterior L3/4 pedicle screw and rod fusion and total laminectomy. This relieved the patient’s low back pain until her 6-week postoperative follow-up. At this time, she reported her low back pain was worsening. Subsequent imaging was performed (not available), and a fractured screw and failed fixation were identified. As a result, an anterior L3/4 screw and plate fixation were employed. During this postoperative period, the patient reported the onset of lumbar radicular pain. A CT myelogram was obtained and demonstrated clumping and adhesion of the cauda equina nerve rootlets. The clumped rootlets were adherent centrally within the thecal sac at the L3/4 and L4/5 surgical levels (Figs 1A-1C). The patient was given a diagnosis of postsurgical adhesive arachnoiditis.
Fig 1.
Axial (A), coronal (B), and sagittal (C) computed tomography myelogram showing type 2 clumping of the nerve rootlets of the cauda equina centrally within the thecal sac at the L3/4 and L4/5 spinal levels (arrows). There is a total laminectomy at L3 and L4 with a posterior pedicle screw and rod fixation of L3-L5, and an anterior screw and plate fixation of L3/4. There is persistence of the post-traumatic L4/5 spondylolisthesis following posterior surgical fixation.
Review of systems revealed a diagnosis of hypothyroidism 15 years before. She reported a history of hypertension that was well controlled with medication. The remaining systems were noncontributary. The patient was prescribed metoprolol (25 mg/d) for hypertension and Synthroid (50 mcg/d) for hypothyroidism. The patient’s family history was positive for stroke and colon cancer on her father’s side. Her mother had no pertinent health problems. The patient denied allergies, smoking, or consumption of alcohol.
The physical examination revealed all vitals within normal limits. Inspection of the lumbar region showed 2 scars associated with her previous surgeries. No bruising or swelling was noted. Ranges of motion of the lumbar spine were assessed and demonstrated restricted flexion (50°), extension (30°), bilateral lateral flexion (20°), and bilateral rotation (10°). Orthopedic testing demonstrated a positive right straight leg raise between 60° and 90° and a positive slump test reproducing the right leg pain. Heel-toe walk, Romberg’s sign, and femoral nerve stretch test were all negative. Tenderness was noted over L5/S1 on the right. The patient underwent a low back Oswestry questionnaire, which scored 63% disability. Based on the examination and imaging findings, a working diagnosis of SA with lumbar radiculopathy was established.
To reduce pain and improve function, a treatment plan using NM 2× per week for 3 weeks was prescribed. Because the patient’s chief complaint was right thigh and leg pain, lumbar spinal manipulation was deferred during the initial treatment plan. Neural mobilization (nerve flossing) was directed to the right extremity distally and proximally to provide sciatic nerve mobilization. The intent of this intervention was to reduce nociception, assist the desensitization of the lumbosacral nerve roots, and mobilize the periradicular adhesions.19, 20, 21, 22 This technique was designed to increase the nerve root translation within the neural foramen by applying tension to one end of the nerve root while slackening the other proximally.
For the distal application of NM, the patient was supine and the practitioner dorsiflexed the ankle and flexed the hip while maintaining knee extension on the symptomatic side. Once the barrier was approached, her radicular symptoms were provoked mildly. She then was directed to flex the cervical spine, bringing the chin to the chest, and at the same time was placed passively in plantar flexion, reducing the neural tension. Cervical flexion caused tension on the sciatic nerve while ankle plantar flexion slackened it. The patient then lowered her head to the table while the practitioner dorsiflexed the ankle. These maneuvers were performed with 10 to 15 repetitions per set, and 2 sets were used in a slow and coordinated manner. For the proximal NM, the same procedure was performed except the patient actively flexed the cervical spine to the chest, with concomitant passive extension of the hip. The patient then lowered her head to the table while the practitioner raised the lower extremity again, maintaining dorsiflexion of the ankle for continuous neural tension (Suppl. Video 1).
In addition, the patient was instructed in NM for home care. For this protocol, the patient was seated and maintained a normal lumbar curve during the maneuver. The patient flexed the cervical spine while keeping the involved knee in the flexed position. The patient then extended the cervical spine while simultaneously extending the knee and dorsiflexing the ankle (Suppl. Video 2). This protocol was repeated for 10 to 20 repetitions, in a slow controlled manner, 2× to 3× per day. The patient was highly motivated and reported compliance with the treatment plan.
Three weeks later, after completion of the treatment plan, the patient was re-evaluated. In contrast to her baseline examination, her pain level decreased 2 points on the numeric pain scale, and she was able to extend her right leg fully while in a seated position without pain. The straight leg raise test, at this point, was negative on the right. In addition, she reported improvement while exercising and was able to perform her routine with little to no pain. Her Oswestry questionnaire was graded at 44% in contrast to 63% disability at baseline. The patient was pleased with her care and reported no adverse effects during treatment. The patient provided consent for the publication of this report.
Discussion
Spinal arachnoiditis first was described in 1909 by Horsley.1, 6 The clinical presentation of SA may include resting low back pain with radiation and spasms into lower extremities. Patients may experience sensory loss, paresis, or paralysis with reduced deep tendon reflexes. Autonomic disturbances of the urinary tract or bowel also have been reported.1, 2, 3, 4, 5 The common symptoms of arachnoiditis are nonspecific and difficult to differentiate from other neural compressive disorders, such as degenerative canal stenosis and spinal tumors.1, 2, 3, 4, 5 The pathophysiology of SA involves the arachnoid membrane and 1 or more possible insults. These may include infections,1, 2, 3, 4, 5 such as tuberculosis23 or staphylococcus aureus; trauma producing hematoma or subarachnoid hemorrhage24, 25, 26; spinal tumors1, 2, 3, 4, 5; multiple spinal surgeries3, 4, 5, 27; steroid or epidural injections1, 2, 3, 4, 5; and radiopaque imaging contrast agents.7
Postoperative SA is the most common etiology.3, 4, 5 The risk of developing SA increases with the number of surgeries. Although the pathophysiology is still not well understood, a progression from spinal arachnoiditis to adhesive arachnoiditis and finally arachnoiditis ossificans has been described.1, 2, 27, 28, 29, 30 When damage occurs to the arachnoid layer, an inflammatory response triggers fibrinous exudate. The avascular nature of the arachnoid layer and the turbulence of the constant cerebrospinal fluid flow inhibit healing, resulting in adhesions of the nerve roots or thecal sac.1 Clinically significant complications have been described in association with arachnoiditis, such as syringomyelia, hydrocephalus, and cauda equina syndrome.1, 30, 31
Clinical suspicion of SA warrants imaging because the differential may include spinal tumors and cauda equina syndrome.1 Noncontrast MRI and CT myelography are the imaging modalities of choice. Magnetic resonance imaging is the preferred modality because it does not require direct intrathecal contrast injection. Its increased contrast resolution of the spinal cord and cauda equina are an additional benefit. Magnetic resonance imaging has a 92% sensitivity and a 100% specificity in the diagnosis of arachnoiditis.9 Computed tomography myelography is likely comparable. Three MRI patterns of spinal arachnoiditis have been described: (1) nerve rootlets clump together centrally in the thecal sac; (2) nerve rootlets adhere to the wall of the thecal sac, creating an “empty thecal sac”; and (3) a conglomerate mass that fills most of the thecal sac. The third type easily can be mistaken for an intradural spinal tumor.6, 7, 8 Computed tomography myelography demonstrates 2 slightly differing patterns of SA.9 Type 1 is described as the “empty thecal sac,” whereas type 2 is localized or diffuse filling defects within the thecal sac.9 Our patient demonstrated the type 2 pattern of arachnoiditis on her CT myelogram. Oil-based and iodinated water-based contrast materials were used in the past for myelographic examinations but were implicated in the development of arachnoiditis.7 Currently, the CT myelographic contrast agents of choice are water-soluble and nonionic.7 Computed tomography myelography and MRI may employ suppression algorithms to decrease artifacts from surgical hardware encountered in postoperative patients with arachnoiditis.6, 8, 9 Magnetic resonance myelography uses a specialized 3-dimensional gradient imaging technique to assess arachnoiditis without percutaneous contrast injection.10 Imaging is indicated for diagnosis and surgical planning.
Most patients with SA initially are treated with pharmacologic approaches, such as epidural or steroid injections.1, 2 Surgical options are limited for SA patients as any surgery may increase the amount of scar tissue and inflammation within the thecal sac as well as increase the risk of neurologic deficits. These surgical options include thecaloscopy, neural stimulation, arachnoidolysis, flexible endoscopy, and subarachnoid-subarachnoid shunting.11, 12, 31, 32, 33 These surgical techniques have shown short-term improvements in pain, although most patients returned to baseline or worsened in the long term.1, 12, 33 Our patient is a prime example of a failed response to multiple lumbar spine surgeries with little impact on her neurologic deficits, pain status, and quality of life.
Nonpharmacologic interventions for SA hold significant promise in the management of these patients. Postoperative patients who have been treated, for example, with spinal manipulation have shown improvement in their quality of life, although the evidence is limited to case reports.13, 14, 15, 16, 17, 18 The use of NM as an intervention for SA has not been described to date.
Neural mobilization is a manual therapy or exercise directed at restoring homeostasis by the disruption of adherent and fibrotic nerves.19, 20, 34 Animal models and some human clinical trials revealed NM reduced intraneural edema, improved intraneural fluid dispersion, reversed the increased immune response after a peripheral nerve injury or radiculopathy, and reduced thermal and mechanical hyperalgesia.19, 20, 21, 22, 34 In an anatomical study, sliding and tensioning neural mobilization techniques demonstrated lengthening of the nerve bed, which increased the nerve tension and intraneural pressure. If the nerve has maintained elevated intraneural edema, the intraneural blood flow is reduced, as seen in neuropathies. Neural mobilization reduces the intraneural edema and pressure, improving nerve function.35 A systematic and critical review on the effectiveness of NM found that patients with nerve-related low back pain and radiculopathy responded favorably to treatment.21, 22, 34, 36 The reviews also found NM did not offer any additional benefit to care in postoperative lumbar spinal pain, although there was insufficient evidence for the effectiveness of NM.34, 36 Kim et al revealed that NM and therapeutic ultrasound were beneficial in elevating the pressure pain threshold with upper-extremity delayed-onset muscle soreness compared with ultrasound alone.37
Our patient responded favorably to NM with no adverse effects. Her pain reduced 2 points on the numeric pain scale, and her lumbar Oswestry Disability Index decreased by 19%; a significant improvement is 10% according to Ostelo et al.38 Additional clinical research is necessary to elucidate the mechanisms underlying the clinical benefit of NM in postoperative patients with SA.
Limitations
Our case study has a limitation characteristic of case reports. The diagnosis and interventions cannot be generalized among the postoperative lumbar spine pain population. Assessment in short- or long-term improvements from NM in SA is also limited because this patient was lost to follow-up.
Conclusion
The complication of SA may be seen after spinal surgery. These patients commonly present with myelopathic or radiculopathic pain seen in other common diagnoses. Magnetic resonance imaging and CT myelography are sensitive and specific for demonstrating clumping of nerve roots and adhesions to the thecal sac, significantly decreasing the differential diagnosis. Neural mobilization may offer a nonpharmacologic option for SA. Albeit a short-term result, our patient had decreased pain levels and improved quality of life after NM. Therefore, neural mobilization may offer improvement in a nonsurgical setting as an intervention for SA, although randomized controlled trials would be required to determine the long-term efficacy of this treatment.
The following are the supplementary data related to this article.
Sciatic neural mobilization protocol for in-office patient care.
Sciatic neural mobilization exercise protocols for patients to do at home.
Acknowledgments
Acknowledgment
We thank Kimberly Cerf for volunteering to be our model in the NM video.
Practical Applications
-
•
Spinal arachnoiditis may be seen in a postoperative setting.
-
•
Neural mobilization has not been documented in the literature in the treatment of arachnoiditis symptoms.
-
•
Neural mobilization was used in the care of this patient.
-
•
Since there are few effective surgical and pharmacologic (opioid) options for arachnoiditis, a nonpharmacologic solution, such as NM, may be considered in the pain management of these patients.
Alt-text: Unlabelled Box
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): S.M.C., N.W.K.
Design (planned the methods to generate the results): S.M.C., N.W.K.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): N.W.K.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): E.D.J.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): E.D.J.
Literature search (performed the literature search): S.M.C.
Writing (responsible for writing a substantive part of the manuscript): S.M.C.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): S.M.C., E.D.J., N.W.K.
Other (neural mobilization videos): E.D.J.
References
- 1.Wright MH, Denney LC. A comprehensive review of spinal arachnoiditis. Ortho Nursing. 2003;22(3):215–221. doi: 10.1097/00006416-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Klein JP. Imaging of noninfectious inflammatory disorders of the spinal cord. Handbook Clin Neurol. 2016;136:733–746. doi: 10.1016/B978-0-444-53486-6.00036-3. [DOI] [PubMed] [Google Scholar]
- 3.Herrera Herrera I, Moreno de la Presa R, Gonzalez Gutierrez R, Barcena Ruiz E, Garcia Benassi JM. Evaluation of the postoperative lumbar spine. Radiologia. 2013;55(1):12–23. doi: 10.1016/j.rx.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Santos Armenia E, Prada Gonzalez R, Silva Priegue N. The postsurgical spine. Radiologia. 2016;58(S1):104–114. doi: 10.1016/j.rx.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MC, Ross JS. Postoperative spine complications. Neuroimag Clin N Am. 2014;24(2):305–326. doi: 10.1016/j.nic.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Anderson TL, Morris JM, Wald JT, Kotsenas AL. Imaging appearance of advanced chronic adhesive arachnoiditis: a retrospective review. AJR. 2017;209(3):1–8. doi: 10.2214/AJR.16.16704. [DOI] [PubMed] [Google Scholar]
- 7.Pomerantz SR. Myelography: modern technique and indications. Handb Clin Neurol. 2016;135:193–208. doi: 10.1016/B978-0-444-53485-9.00010-6. [DOI] [PubMed] [Google Scholar]
- 8.Dhagat PK, Jain M, Singh SN, Arora S, Leelakanth K. Failed back surgery syndrome: evaluation with magnetic resonance imaging. J Clin Diag Res. 2017;11(5):6–9. doi: 10.7860/JCDR/2017/24930.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro C, Reis FC. Adhesive lumbar arachnoiditis. Acta Medica Port. 1998;11(1):59–65. [PubMed] [Google Scholar]
- 10.Patel A, James SL, Davies AM, Botchu R. Spinal imaging update: an introduction to techniques for advanced MRI. Bone Joint J. 2015;97-B:1683–1692. doi: 10.1302/0301-620X.97B12.36164. [DOI] [PubMed] [Google Scholar]
- 11.Mitsuyama T, Asamoto S, Kawabata T. Novel surgical management of spinal adhesive arachnoiditis by arachnoid microdissection and ventriculo-subarachnoid shunting. J Clin Neurosci. 2011;18:1702–1704. doi: 10.1016/j.jocn.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Kashcheev AA, Arestov SO, Gushcha AO. Flexible endoscopy in surgical treatment of spinal adhesive arachnoiditis and arachnoid cysts. NN Burdenko J Neurosurg. 2013;5:41–50. [PubMed] [Google Scholar]
- 13.Coulis CM, Lisi AJ. Chiropractic management of postoperative spine pain: a report of 3 cases. J Chiropr Med. 2013;12(3):168–175. doi: 10.1016/j.jcm.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Shaughnessy J, Drolet M, Roy JF, Descarreaux M. Chiropractic management of patients post-disc arthroplasty: eight case reports. Chiropr Osteopat. 2010;18:7. doi: 10.1186/1746-1340-18-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruse RA, Cambron J. Chiropractic management of postsurgical lumbar spine pain: a retrospective study of 32 cases. J Manipulative Physiol Ther. 2011;34(6):408–412. doi: 10.1016/j.jmpt.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Morningstar MW, Strauchman MN. Manipulation under anesthesia for patients with failed back surgery: retrospective report of three cases with 1-year follow-up. J Chiropr Med. 2012;11(1):30–35. doi: 10.1016/j.jcm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gluck NI. Passive care and active rehabilitation in a patient with failed back surgery syndrome. J Manip Physiol Ther. 1996;19(1):41–47. [PubMed] [Google Scholar]
- 18.Welk AB, Werdehausen DN, Kettner NW. Conservative management of recurrent lumbar disk herniation with epidural fibrosis: a case report. J Chiropr Med. 2012;11(4):249–253. doi: 10.1016/j.jcm.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos MF, Silva TJ, Giardini CA. Neural mobilization reverses behavioral and cellular changes that characterize neuropathic pain in rats. Mol Pain. 2012;8:1–9. doi: 10.1186/1744-8069-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown LC, Gilbert KK, Brismee MJ. The effects of neurodynamic mobilization on fluid dispersion within the tibial nerve at the ankle: an unembalmed cadaveric study. J Man Manip Ther. 2011;19:26–34. doi: 10.1179/2042618610Y.0000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwornik M, Kujawa J, Bialoszewski D, Slupik A, Kiebzak W. Electromyographic and clinical evaluation of the efficacy of neuromobilization in patients with low back pain. Ortop Traumatol Rehabil. 2009;11(2):164–176. [PubMed] [Google Scholar]
- 22.Ferreira GE, Stieven FF, Araujo FX. Neurodynamic treatment for patients with nerve-related leg pain: protocol for a randomized controlled trial. J Bodyw Mov Ther. 2016;20(4):870–878. doi: 10.1016/j.jbmt.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Canova G, Boaro A, Giordan E, Longatti P. Treatment of posttubercular syringomyelia not responsive to antitubercular therapy: case report and review of literature. J Neurol Surg Rep. 2017;78:e59–e67. doi: 10.1055/s-0037-1601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caranci F, Leone G, Ugga L. Imaging of post-surgical treatment and of related complications in spinal trauma. Musculoskelet Surg. 2017;101(1):S63–S73. doi: 10.1007/s12306-017-0457-0. [DOI] [PubMed] [Google Scholar]
- 25.Basaran R, Kaksi M, Efendioglu M. Spinal arachnoid cyst associated with arachnoiditis following subarachnoid hemorrhage in adult patients: a case report and literature review. Br J Neurosurg. 2015;29(2):285–289. doi: 10.3109/02688697.2014.976175. [DOI] [PubMed] [Google Scholar]
- 26.Van Heerden J, McAuliffe W. Spinal arachnoiditis as a consequence of aneurysm-related subarachnoid hemorrhage. J Med Imaging Rad Oncol. 2013;57(1):61–64. doi: 10.1111/j.1754-9485.2012.02416.x. [DOI] [PubMed] [Google Scholar]
- 27.Kochany JZ, Tran ND, Sarria JE. Increasing back and radicular pain 2 years following intrathecal pump implantation with review of arachnoiditis. Pain Med. 2013;14(11):1658–1663. doi: 10.1111/pme.12188. [DOI] [PubMed] [Google Scholar]
- 28.Maulucci CM, Ghobrial GM, Oppenlander ME. Arachnoiditis ossificans: clinical series and review of the literature. Clin Neurol Neurosurg. 2014;124:16–20. doi: 10.1016/j.clineuro.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Kasai Y, Sudo T, Sakakibara T, Akeda K, Sudo A. A case of lumbosacral arachnoiditis ossificans. NMC Case Rep J. 2016;3(1):5–7. doi: 10.2176/nmccrj.cr.2015-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwatsuki K, Yoshimine T, Ohnishi Y. Syringomyelia associated with spinal arachnoiditis treated by partial arachnoid dissection and syrinx-far distal subarachnoid shunt. Clin Med Insights Case Rep. 2014;7:107–110. doi: 10.4137/CCRep.S14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koerts G, Rooijakkers H, Abu-Serieh B, Cosnard G, Raftopoulos C. Postoperative spinal adhesive arachnoiditis presenting with hydrocephalus and cauda equina syndrome. Clin Neurol Neurosurg. 2008;110(2):171–175. doi: 10.1016/j.clineuro.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Tachibana T, Moriyama T, Maruo K. Subarachnoid-subarachnoid bypass for spinal adhesive arachnoiditis. J Neurosurg Spine. 2014;21(5):817–820. doi: 10.3171/2014.7.SPINE131082. [DOI] [PubMed] [Google Scholar]
- 33.Di Ieva A, Barolat G, Tschabitscher M. Lumbar arachnoiditis and thecaloscopy: brief review and proposed treatment algorithm. Cent Eur Neurosurg. 2010;71(4):207–212. doi: 10.1055/s-0029-1243201. [DOI] [PubMed] [Google Scholar]
- 34.Basson A, Olivier B, Ellis R. The effectiveness of neural mobilization for neuromusculoskeletal conditions: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47(9):593–615. doi: 10.2519/jospt.2017.7117. [DOI] [PubMed] [Google Scholar]
- 35.Coppieters MW, Butler DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther. 2008;13(3):213–221. doi: 10.1016/j.math.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Efstathiou MA, Stefanakis M, Savva C, Giakas G. Effectiveness of neural mobilization in patients with spinal radiculopathy: a critical review. J Bodyw Mov Ther. 2015;19(2):205–212. doi: 10.1016/j.jbmt.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Kim MK, Cha HG, Ji SG. The initial effects of an upper extremity neural mobilization technique on muscle fatigue and pressure pain threshold of healthy adults: a randomized control trial. J Phys Ther Sci. 2016;28(3):743–746. doi: 10.1589/jpts.28.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostelo RW, Deyo RA, Stratford P. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33(1):90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sciatic neural mobilization protocol for in-office patient care.
Sciatic neural mobilization exercise protocols for patients to do at home.