Abstract
The cellular size and biomass of picophytoplankton were studied by flow cytometer during spring monsoon (March–May of 2015) in equatorial eastern Indian Ocean. We established an empirical relationship between forward scatter and cellular size to address the size and biomass of picophytoplankton. Results indicated that mean cell diameter of Prochlorococcus (0.60 μm) was the smallest, and then followed by Synechococcus (0.98 μm) and picoeukaryotic phytoplankton (1.05 μm). Thereafter, the biomass converted by abundance reached 0.64 μg·C·L−1 for Prochlorococcus, 0.34 μg·C·L−1 for Synechococcus, and 0.20 μg·C·L−1 for picoeukaryotic phytoplankton. Additionally, the distinct biomass contribution of picophytoplankton appeared to be affected by abundance, but not changes in cellular size. Vertically, the cellular sizes of picophytoplankton were remarkably small in upper waters, which was predominantly controlled by the nutrient availability. In contrast, they were larger in deeper waters, which was primarily attributed to the combined effects of low temperature and reduced light availability. Spatially, under the influence of high nutrient concentration induced by the different circulations and coastal upwelling, slightly high carbon biomass of picophytoplankton was observed around the coastal zones of Sri Lanka island and Sumatra, as well as the southern Bay of Bengal.
Keywords: biomass, cellular size, circulation and water mass, Indian Ocean, picophytoplankton
1. INTRODUCTION
Picophytoplankton (<2 μm) mostly composed of Prochlorococcus, Synechococcus and picoeukaryotic phytoplankton have essential roles in primary productivity in tropical and subtropical oligotrophic oceans (Stockner, 1988). Observations in the oligotrophic Pacific Ocean and Atlantic Ocean have shown that picophytoplankton accounts for approximately 60%–80% of the total primary productivity (Campbell, Liu, Nolla, & Vaulot, 1997). Since their considerably high biomass and contribution to marine primary production, picophytoplankton has been known to have large impacts on ocean ecosystem and biogeochemical cycles (Flombaum et al., 2013). The tropical Indian Ocean forms the major part of the largest warm pool on the earth, and its interaction with the monsoon plays an important role in shaping complex circulation systems on both regional and global scales (Wang, Xie, & Carton, 2004). Furthermore, the variability of nutrients, biomass, and primary production in the Indian Ocean induced by changes in physical forces have been investigated by a number of studies (McClanahan, Maina, Graham, & Jones, 2016; Roxy et al., 2016; Siswanto, 2015), which usually showed that the variability in phytoplankton standing stocks and primary production are closely related to the circulations and water masses. Although the Indian Ocean is considered as one of the largest oligotrophic areas, it has received far less attention than other oceans, particularly in terms of the size and biomass of picophytoplankton. Thus, presenting their size and biomass is critical to understand the contributions to carbon cycles of these special taxa in the Indian Ocean.
Thus far, flow cytometer (FCM) can help us to address the size and biomass of picophytoplankton at high frequency according to their cell morphological properties and fluorescence when the high‐sensitive protocol was used. For FCM, light scattering at different angles are related to the function of particle volume and secondarily shape (Latimer, 1982). However, an empirical calibration between cell diameter and side scatter (SSC) was performed to roughly estimate equivalent spherical diameter and cellular biovolume of picophytoplankton (Calvo‐Díaz & Morán, 2006; Chen et al., 2011). The range of picophytoplankton cell diameter, in general, used to establish the empirical relationship between cellular size and SSC is still critical (Gasol & Del Giorgio, 2000). Light scattering efficiency of picophytoplankton cell is a complex function of its size, structure, and refractive index, even different FCM and fixatives may yield significantly different scatter diagrams of the same sample as a function of relatively minor changes in detection geometry (Gasol & Del Giorgio, 2000). Consequently, Allman, Hann, Manchee, and Lloyd (1992) pointed out that cell diameter and light scattering should break down when comparing different species. According to the Mie theory, when particle diameter extends from 0.2 μm up to 2–3 μm or more, forward scatter (FSC) is the signal which is the most sensitive to cellular size, with a diameter d dependence of FSC in d 4‐d 6 (Morel, 1991). For example, the converting mean FSC to cell sizes for Synechococcus was done by fitting a power relationship with laboratory calibrations (FSC = a × Diameterb), and the exponent (b) was found to be 5.4, which was reasonably close to that determined by Mie light scattering theory (DuRand, Olson, & Chisholm, 2001); FSC versus biovolume data of Synechococcus reported a value of d 5.1 (Chisholm 1992); an FSC variation for Prochlorococcus during the daytime as a doubling in the average volume of the prokaryotes indicated a value of d 5.4 (Binder, Chisholm, Olson, Frankel, & Worden, 1996); the FSC related to particle sizes of reference beads for picoplankton led to the value around d 5 (Blanchot, André, Navarette, Neveux, & Radenac, 2001). Collectively, a strong correlation between FSC and cellular size has been determined by laboratory cultures of reasonably spherically shaped cells (DuRand et al., 2001; Olson, Zettler, & Anderson, 1989), despite small changes in refractive index (DuRand & Olson, 1998). Moreover, Koch, Robertson, and Button (1996) presented that FSC is chosen over SSC because of its far greater signal intensity to subcellular structure according to the theoretical basis of their approach. Actually, the relationship between FSC and picoplanktonic size has been carried out for several decades, even always involving bacterioplankton. Typically, Robertson and Button (1989) have made use of FSC to estimate bacterial size and proposed a good relationship between FSC and bacterial volume. However, some recently published papers often showed that the same large dispersion of beads and target cells in FSC limited the application of FSC and weakened its relationship with cellular size. To capture light scatter in forward angles and increase the sensitivity of this parameter, more instruments have been equipped with photomultiplier tubes (Gasol & Del Giorgio, 2000). This led Blanchot et al. (2001) to attempt a practical way for the estimation of mean cellular size what the relationship between the mean FSC and cellular diameter was determined by the power law empirically, which was assumed to stand for the mean FSC and diameters relative to those of the beads (d cell = d bead(FSC)1/5), respectively.
In this study, we established an empirical relationship between FSC and cellular size to address the cellular size and biomass of picophytoplankton, and then to understand more clearly whether and how the contrasting environmental conditions affect their variations in equatorial eastern Indian Ocean. More specifically, will the expected environmental conditions induced by the complex circulations and water masses change the cellular size and biomass of picophytoplankton?
2. MATERIALS AND METHODS
2.1. Sampling strategy
This cruise was conducted on the R/V Shiyan I during spring 2015 (March 21–May 15) in equatorial eastern Indian Ocean (EIO; 6.8°N ~5.5°S, 79.5°E ~96.1°E) as shown in Figure 1. Our study area covered the entire equatorial EIO, and 31 stations were established. In addition, four selected transects were highlighted in this study. At each station, seawater samples were collected from seven depths within the upper 200‐m water column using 12‐L Niskin bottles equipped with a Sea‐Bird CTD (Conductivity, Temperature and Depth; SBE 19 Plus) rosette sampler. Photosynthetically active radiation (PAR) was measured by an RBR sensor (XRX‐620). The euphotic depth was defined as the depth of 1% surface light penetration. Temperature and salinity were recorded at the same time.
Figure 1.
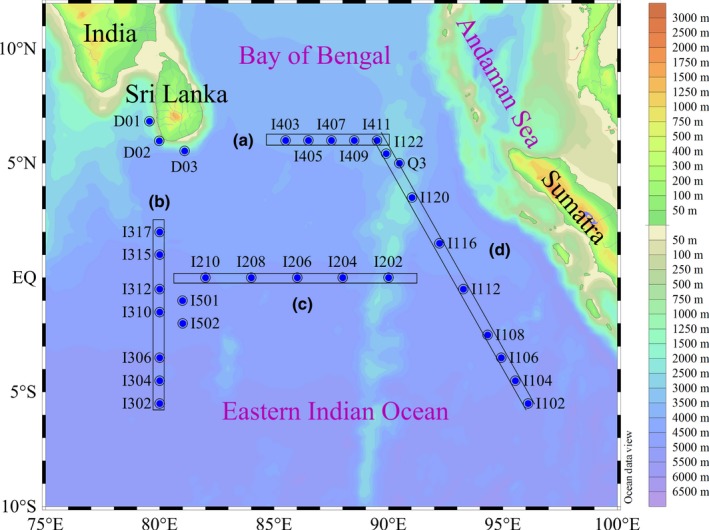
Study area and sampling stations. Four main transects (A–D) covered the entire eastern Indian Ocean were highlighted
Seawater samples for picophytoplankton analysis by FCM were preserved on board with paraformaldehyde (1% final concentration). To avoid loss of resolution and changes in cell size due to fixation or freezing, FCM samples were kept in the dark without treatment at room temperature for 10–15 min, and then quickly freeze‐trapped in liquid nitrogen until analysis in the laboratory (Den Engh et al., 2017; Sommaruga, Hofer, Alonso‐Sáez, & Gasol, 2005).
Samples for nutrient analysis were filtered using 0.45‐μm cellulose acetate membrane filters, and then immediately refrigerated at −20°C for further analysis. Nutrient concentrations including ammonium, phosphate, nitrate, nitrite, and silicate were performed by a Technicon AA3 Auto‐Analyzer (Bran + Luebbe) according to the classical colorimetric methods. Dissolved inorganic nitrogen (DIN) defined as ammonium+nitrite+nitrate was analyzed using the copper‐cadmium column reduction method. Dissolved inorganic phosphorus (DIP) and silicate (DSI) were measured using the typical spectrophotometric methods (Dai et al., 2008; Guo et al., 2014). In all ranges of tested low nutrient standards, the AA3 was more precise and more accurate and showed lower detection limit for all channels: 0.018 μmol·L−1 for DIP, 0.009 μmol·L−1 for nitrate + nitrite, and 0.012 μmol·L−1 for DSI (Dafner, 2015).
2.2. Flow cytometry analysis
Within the present study, three dominating populations, namely Synechococcus, Prochlorococcus, and picoeukaryotic phytoplankton, were manually distinguished by FCM (BD Accuri C6) according to their different amplitudes, shapes, and optical signals. The aforementioned references revealed that FSC was much more adaptable to establish the empirical relationship with cellular sizes of picophytoplankton (<2 μm). Thereafter, cellular diameters of them were enumerated by using the FCM based on their distinct FSC signatures. The histograms of example FSC frequency of three picophytoplankton groups are shown in Figure 2. We estimated the sizes of mean FSC frequency were of the order of the cell diameters of Prochlorococcus, Synechococcus, and picoeukaryotic phytoplankton, respectively. Otherwise, we presumably assumed that the optical signals of mean cell diameters of Prochlorococcus (0.6 μm) were similar to the normalized beads; subsequently, the cellular sizes with respect to biovolumes were estimated on the basis of the mean FSC frequency of Prochlorococcus, Synechococcus, and picoeukaryotic phytoplankton as measured relative to those of the mean cell diameters (Partensky, Hess, & Vaulot, 1999). The empirical relationship between the mean FSC (MFSC) and cell diameters (d cell) is shown as follows: d cell = d bead(Prochlorococcus)(MFSCcell/MFSCProchlorococcus). This hypothesis that was similar to the pattern of Blanchot et al. (2001) was an oversimplification, although the result needed to be considered cautiously and more proper calibration in further studies, the relationship was in accordance with those presently quoted.
Figure 2.
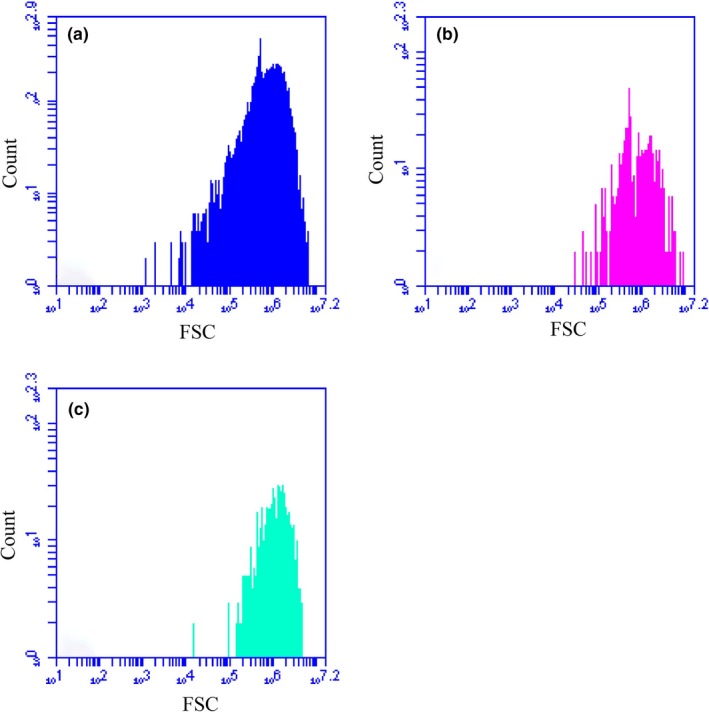
Forward scatter frequency (FSC) of (a) Prochlorococcus, (b) Synechococcus, and (c) picoeukaryotic phytoplankton in counting cells
Additionally, two different subclusters of Synechococcus were directly evidenced by two distinct peaks (bimodal distribution) in the histograms of the FSC frequency (Figure 2b), and possibly corresponding to the “SynechococcusI” and “SynechococcusII” cells (Zhao et al., 2013). Unfortunately, due to the absence of distinctive features and peak‐overlap, it was difficult to identify them by the FSC frequency. Therefore, these subclusters in the picophytoplanktonic fraction of the Indian Ocean were artificially combined and exclusively represented by Synechococcus.
3. RESULTS AND DISCUSSION
3.1. Cell size and biomass
Results from the estimated mean FSC method indicated that mean cell diameter of Prochlorococcus (0.60 ± 0.22 μm) was the smallest, followed by Synechococcus (0.98 ± 0.44 μm) and picoeukaryotic phytoplankton (1.05 ± 0.30 μm). Our estimated cellular sizes for picophytoplankton were comparable with other estimates. For example, Synechococcus cell diameters well agreed with reported sizes of 0.74–1.22 μm, for water samples collected from the Sargasso Sea (DuRand et al., 2001). Using the same method who obtained a relationship between FSC and cell size on marine picophytoplankton cultures, Shalapyonok, Olson, and Shalapyonok (2001) estimated average values of 0.91–0.95 μm and 0.98–1.14 μm for Synechococcus in the surface mixed layer and below the mixed layer, respectively. However, our estimates of cellular size of picoeukaryotic phytoplankton were slight smaller than other estimates. Estimates of picoeukaryotic phytoplankton in equivalent spherical diameter based on other methods ranged from 1 to 2 μm, for example, 1.35–2.05 μm in the central Cantabrian Sea (Calvo‐Díaz, Morán, & Suárez, 2008) and 1.93–2.07 μm in equatorial Pacific (Blanchot et al., 2001). Overall, without accompanying independent measurements to use as a comparison, we are not able to judge whether our estimates are invalid. To clearly understand the vertical pattern, all the data points of picophytoplankton cellular size against depth were analyzed to plot related fitting curves (Figure 3). The three curves in Figure 3 represented vertical variations of cell size of three picophytoplankton groups, respectively. In particular, the observed vertical trend of cell size of picoeukaryotic phytoplankton was very similar to the pattern in the Arabian Sea (Shalapyonok et al., 2001), which showed that picoeukaryotic phytoplankton in the surface were slightly larger than those in the subsurface chlorophyll maximum. Diameter minimum for Synechococcus and Prochlorococcus occurred in upper waters, whereas larger cells were recorded near the 150‐m layer of water column (Figure 3). Generally, the variations in cellular diameter were even greater with depth in equatorial EIO during spring.
Figure 3.
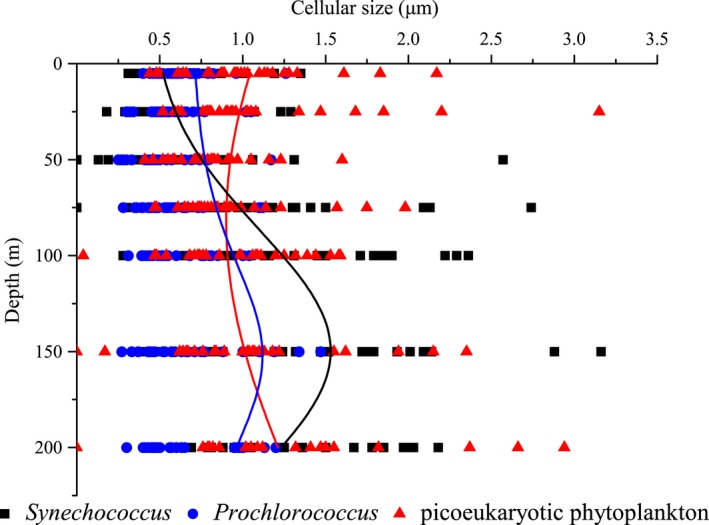
Vertical distributions of cell size in Synechococcus, Prochlorococcus, and picoeukaryotic phytoplankton
Picophytoplankton biomass could be measured directly from proximate analyses (such as chlorophyll, ATP, carbon, and nitrogen concentration) or indirectly from biovolume characteristics of the enumerated population (Frame & Hu, 1990; Hewes, Sakshaug, Reid, & olm‐Hansen, 1990; Hunter & Laws, 1981; Sun & Liu, 2003). Nevertheless, to separate Prochlorococcus, Synechococcus, and picoeukaryotic phytoplankton from other algae cells, and then to estimate the different biomass of them, respectively, using proximate analyses in situ were very difficult because of their considerably small cells. Determinations of proximate constituents such as levels of chlorophyll or detritus plus viable carbon were, therefore, inadequate measurements for use in studies on species or size‐class contributions to primary production (Sun, Liu, & Qian, 2000). For each picophytoplankton assemblage, the mean biovolume should be calculated from the mean value of these individual cell biovolumes, rather than directly from the mean cell diameter. In the equatorial EIO, the average abundances of Prochlorococcus, Synechococcus and picoeukaryotic phytoplankton were at the magnitude of 103–105 cells·ml−1 (Table 1); hence, it was a extremely complicated and heavy work to calculate the biovolume of each picophytoplanktonic cell. However, mean cell size differs greatly between species of picophytoplankton that suggests the mean cell size of species is very important with biomass (Agusti, Duarte, & Kalff, 1987; Liu, Chang, Tseng, Wen, & Liu, 2007; Marañón, 2015). Accordingly, Hillebrand, Dürselen, Kirschtel, Pollingher, and Zohary(1999) proposed that the biovolume can be calculated from the mean of measured cell diameters, not as a mean of a set of individually calculated biovolumes although there may be some errors. Indeed, the error sources within this study primarily came from the choice of mean cell diameter, in addition to the accuracy of measurement and consequent estimation of biovolume. When the two methods for mean biovolume calculation were compared, Sun and Liu (2003) found that although the latter method usually underestimated the variability, its trend had better agreement with increased measurements. Under most circumstances, the standard error was <5% of the mean biovolume after the measurement of 10 cells, we suggested that taking as many measurements as possible was better. As yet, the conversion of carbon content from biovolume based on mean cell diameter is the good way to estimate the gross biomass of picophytoplankton in the absence of direct unicellular size measurement. For example, Calvo‐Díaz and Morán (2006) obtained the mean diameters of the different groups of picophytoplankton by an empirical calibration, and thereafter assumed a spherical shape for all groups to estimate biomass by using the method of biovolume‐to‐carbon conversion in the southern Bay of Biscay; Chiang, Kuo, Chang, Wang, and Gong(2002) computed the mean cell volume based on the approximately coccoid shape of Synechococcus cells to estimate biomass in the East China Sea; Blanchot et al.(2001) attempted a very practical way for the estimation of mean cell size to calculate cellular carbon in the equatorial Pacific. Thus, the mean measured cell diameter of picophytoplankton could be used to calculate biovolume in routine analysis. The carbon biomass estimates of Synechococcus, Prochlorococcus, and picoeukaryotic phytoplankton for this study area were roughly calculated by using the mean carbon content per cell multiplied by cell abundance for each of these groups. Preferentially, we assigned the geometric shape for a picophytoplanktonic cell as a sphere (Chisholm et al., 1992). The cell diameter (d) was converted to biovolume (V) using a predictive equation (Sun et al., 2000): . Then, an average biovolume per cell was converted to the average carbon value per cell using the empirical relationship (DuRand et al., 2001; Eppley, Reid, & Strickland, 1970): logC = 0.94 × logV − 0.60. Picophytoplankton abundance (Table 1) was converted to biomass using the resulting volume to carbon conversion factors: 32 fg·C·cell−1 for Prochlorococcus, 129 fg·C·cell−1 for Synechococcus, and a carbon content of 160 fg·C·cell−1 for picoeukaryotic phytoplankton. Finally, the mean carbon concentrations reached 0.64 μg·C·L−1 for Prochlorococcus, 0.34 μg·C·L−1 for Synechococcus, and 0.20 μg·C·L−1 for picoeukaryotic phytoplankton in this study.
Table 1.
Mean values of picophytoplankton abundance (cells·ml−1) in whole equatorial EIO and four main transects (T)
| Study area/factors | Synechococcus (×103) | Prochlorococcus (×104) | Picoeukaryotic phytoplankton (×103) |
|---|---|---|---|
| Whole area | 2.65 ± 1.54 | 2.02 ± 1.07 | 1.26 ± 1.07 |
| T‐A | 2.57 ± 0.58 | 1.74 ± 1.15 | 1.65 ± 1.47 |
| T‐B | 1.18 ± 0.62 | 1.53 ± 0.79 | 0.71 ± 0.42 |
| T‐C | 1.12 ± 0.24 | 1.21 ± 0.49 | 0.66 ± 0.29 |
| T‐D | 1.66 ± 1.05 | 2.24 ± 1.28 | 1.24 ± 0.85 |
The estimated average carbon biomass of Synechococcus, Prochlorococcus, and picoeukaryotic phytoplankton tended to have different distribution patterns (Figures 4 and 5). Owing to their high abundance, the relative contribution of Prochlorococcus to the total picophytoplankton carbon biomass was higher than the other two groups, indicating that Prochlorococcus was the dominant component in terms of average carbon biomass throughout the equatorial EIO. Moreover, the average carbon biomass of three picophytoplankton groups were primarily concentrated in section A, where was profoundly influenced by surface freshwater from the Bay of Bengal runoffs (Sengupta, Bharath Raj, & Shenoi, 2006). Although Synechococcus and picoeukaryotic phytoplankton had larger cellular sizes, their abundance was approximately 1–2 orders of magnitude less abundant than Prochlorococcus. Overall, this result agreed with those reported in the regions of tropical and subtropical Pacific Ocean, Prochlorococcus was the most abundant photosynthetic organism, even accounting for 65% of the total picoplankton biomass, whereas Synechococcus and picoeukaryotic phytoplankton constituted less than 35% of the biomass (Blanchot & Rodier, 1996; Campbell, Nolla, & Vaulot, 1994; Charpy & Blanchot, 1998). Interestingly, some previous studies reported that Synechococcus could fix an order of magnitude more carbon than Prochlorococcus cells due to Synechococcus was slightly larger than Prochlorococcus, indicating that the relative biomass contributions of three picophytoplankton groups were not simply determined by abundance but also changes in cellular size (Flombaum et al., 2013). However, our finding demonstrated that the distinct biomass contributions of picophytoplankton in the equatorial EIO appeared to be affected by abundance, but not changes in cellular size. In addition to the southern Bay of Bengal, the maximal carbon biomass of Synechococcus and picoeukaryotic phytoplankton co‐occurred around the coastal zones of Sri Lanka island and Sumatra in horizontal distribution, where were potentially contributed by the freshwater discharging from coastal currents and coastal upwelling, respectively. Similarly, slightly high carbon biomass of Prochlorococcus were distributed around the coastal zones of Sri Lanka and the southern Bay of Bengal, but they, in particular, presented the highest carbon biomass in the coastal upwelling zones of Sumatra.
Figure 4.
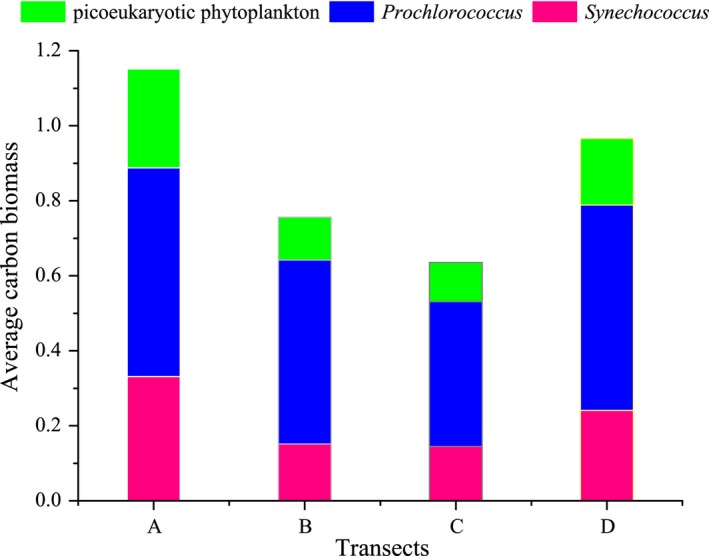
The average carbon biomass (μg·C·L−1) of Synechococcus, Prochlorococcus, and picoeukaryotic phytoplankton along transects A, B, C, and D
Figure 5.
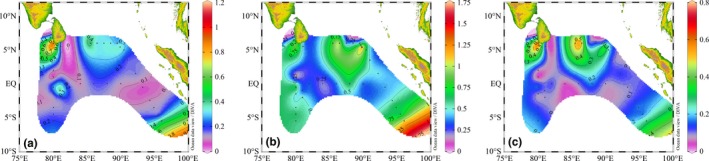
Horizontal distribution of the average carbon biomass (μg·C·L−1) for (a) Synechococcus, (b) Prochlorococcus, and (c) picoeukaryotic phytoplankton
3.2. Factors controlling on cell size and biomass
As reported previously for picophytoplankton, the differences in chlorophyll fluorescence signals (cellular sizes) observed with depth in water column were most likely due to photoacclimation (i.e., reduction of the pigment content at high light levels), cellular division, changes in quantum yield, and shifts in species composition (Campbell & Vaulot, 1993). Specifically, marine environmental variables such as temperature, light, and nutrient availability usually had apparent effects on the cellular sizes of picophytoplankton (Chen et al., 2011). Taken together, the cellular size of picophytoplankton appears to be under complex physiological and environmental control. In equatorial EIO, there were less nutrients in upper waters (5–50 m) during spring monsoon (Table 2). Figure 6 shows the Canonical Correspondence Analysis (CCA) of average carbon biomass of Prochlorococcus, Synechococcus, and picoeukaryotic phytoplankton with nutrient variables. Combined with Table 3, no significant correlations were found between Synechococcus biomass and DIP, ammonium, and nitrite (p > .05). However, Synechococcus biomass were positively correlated with nitrate and DSI (p < .01), suggesting that Synechococcus biomass including cellular sizes was favored by the optimal nitrate and DSI concentrations. Recently, Baines et al.(2012) discovered natural populations of marine cyanobacteria of the genus Synechococcus contained a significant amount of the element silicon. Demonstration of an obligate need for Si in Synechococcus would add a new dimension to their nutrient physiology and to the suite of resources influencing Synechococcus abundance in nature. Thus, the DSI might also affect Synechococcus biomass to carbon cycling as our CCA shown that could be facilitated by silicon ballasting of aggregates and fecal pellets containing Synechococcus if cells contained polymerized silica as recently reported (Brzezinski et al., 2017). In contrast with Synechococcus, Prochlorococcus, and picoeukaryotic phytoplankton biomass showed strong negative correlations with nitrate and DSI, whereas they were closely related with DIP and nitrite (p < .01), indicating that they were mostly profited from the environment in condition with high DIP and nitrite concentrations. The DIP concentration was so low in the equatorial EIO that might be a limiting factor for the growth of Prochlorococcus and picoeukaryotic phytoplankton (Table 2). Moore et al. (2002) similarly demonstrated that Prochlorococcus biomass was closely related to the nitrite concentration owing to the high‐B/A ecotypes had homologs of genes required for nitrite utilization; hence, nitrite could be available N source for such subpopulations. Conclusively, different positive correlations with each environmental variable indicated that nutrients were the crucial factors in regulating their biomass and cellular sizes. The cells of picophytoplankton in upper waters are growing slower when the nutrient levels are lower, whereas cells are on average smaller when they grow slower (DuRand et al., 2001). Results from the size fractionation method revealed that the sizes of Prochlorococcus and Synechococcus in the deep euphotic layers were significantly larger than those in the upper euphotic layers (Liu et al., 2007), and these differences in cell size were attributed to the growth rate at different nutrient concentrations. In dilution experiments, Liu et al. (1998) reported that the growth rate of Synechococcus was much higher than Prochlorococcus with nutrient availability. Furthermore, Shalapyonok et al. (2001) found that the cyanobacteria cells inhabiting the top layer were smaller than those inhabiting the deeper layer, which might be related to nutrient depletion, as well as photoacclimation. Actually, this hypothesis that lower nutrient concentration in upper waters induced smaller size was typically suitable for picophytoplankton in equatorial EIO (Figure 3). Although their cells were remarkably small in the upper waters, the smaller cells of Prochlorococcus and Synechococcus had advantages relative to larger picoeukaryotic phytoplankton cells in terms of resource acquisition and utilization in growth and reproduction, because of their very large surface area per unit volume and minimal diffusion boundary layer thickness (Raven, 1998). Collectively, the variations of cellular sizes in upper waters were closely related to the nutrient availability.
Table 2.
Average nutrient concentrations (μmol·L−1) of different water layers over 0–200 m during spring 2015. BDL: below detection limits
| Depth/Parameter | Ammonium | Phosphate | Nitrate | Nitrite | Silicate | |
|---|---|---|---|---|---|---|
| 5 m | Range | 0.14–2.69 | BDL‐0.35 | BDL‐14.50 | BDL‐1.31 | 0.29–1.47 |
| Mean | 0.63 ± 0.54 | 0.11 ± 0.07 | 0.99 ± 2.31 | 0.18 ± 0.29 | 0.86 ± 0.31 | |
| 50 m | Range | 0.01–0.71 | 0.06–0.85 | BDL‐15.38 | BDL–1.09 | 0.07–3.47 |
| Mean | 0.27 ± 0.16 | 0.23 ± 0.17 | 1.73 ± 3.02 | 0.15 ± 0.25 | 0.98 ± 0.64 | |
| 100 m | Range | 0.06–1.46 | 0.46–2.66 | 4.55–33.43 | 0.03–0.31 | 0.96–15.37 |
| Mean | 0.55 ± 0.28 | 1.23 ± 0.46 | 14.35 ± 6.62 | 0.16 ± 0.07 | 4.04 ± 2.66 | |
| 150 m | Range | 0.07–0.98 | 0.42–2.45 | 4.08–49.75 | BDL‐0.14 | 2.20–17.68 |
| Mean | 0.45 ± 0.21 | 1.12 ± 0.61 | 16.27 ± 10.93 | 0.06 ± 0.05 | 7.16 ± 3.76 | |
| 200 m | Range | 0.05–1.24 | 0.45–2.92 | 5.80–44.00 | BDL‐0.29 | 3.35–54.69 |
| Mean | 0.43 ± 0.25 | 1.20 ± 0.74 | 19.49 ± 10.27 | 0.03 ± 0.06 | 11.24 ± 11.63 | |
Figure 6.
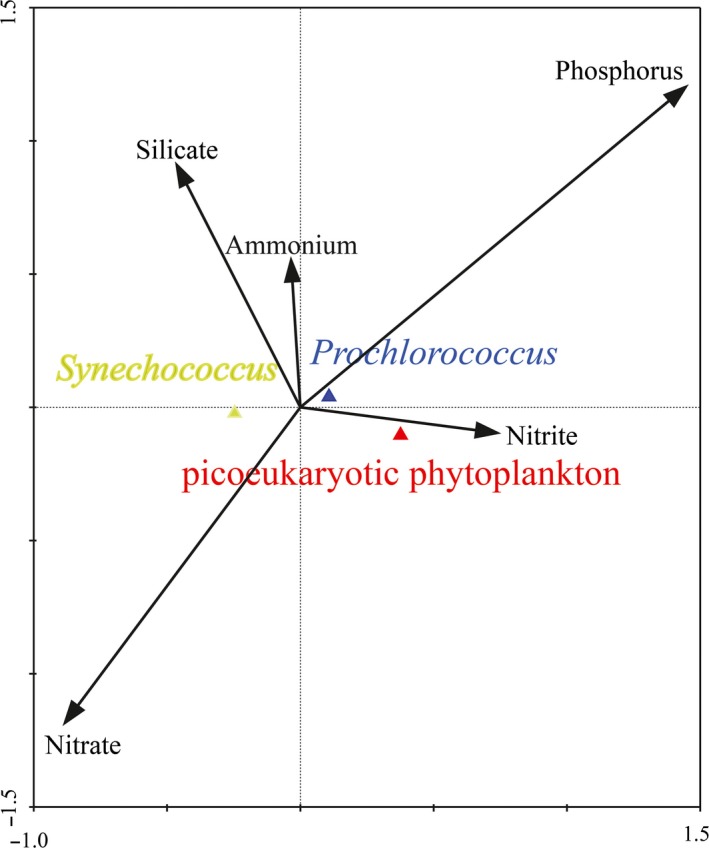
Canonical correspondence analysis of nutrient variables with the carbon biomass for three picophytoplankton groups
Table 3.
Spearman's rank correlation coefficients between environmental factors and carbon biomass of three picophytoplankton groups
| DIP | Ammonium | Nitrite | DSI | Nitrate | |
|---|---|---|---|---|---|
| Prochlorococcus | 0.139b | −0.109 | 0.301b | −0.344b | −0.321b |
| Synechococcus | −0.597 | 0.059 | −0.159 | 0.542b | 0.580b |
| Picoeukaryotic phytoplankton | −0.177 | −0.069 | 0.502b | −0.248a | −0.184 |
Correlation is significant at the .05 level (two‐tailed).
Correlation is significant at the .01 level (two‐tailed).
Due to phytoplankton that grow deeper in the water column need more pigment per cell to compensate for the decreased light levels, their cellular sizes are broadly larger under lower light intensity (Goericke & Welschmeyer, 1998). Den Engh et al. (2017) also presented that chlorophyll fluorescence, and to a lesser extent forward light scatter (an approximate proxy for cell size), increased with depth. The above‐reported increase in the relative cellular sizes of phytoplankton with decreasing light levels might be fully applicable to picophytoplankton in our dataset. Vertically, Prochlorococcus, Synechococcus, and picoeukaryotic phytoplankton inhabiting in the deeper layers were larger and had higher chlorophyll fluorescence than those inhabiting in the upper layers (Figure 3). It is well known that the PAR values vary greatly at different times of 1 day, but the abundance and cell size of picophytoplankton will not vary too much although they are potentially influenced by the light availability. Furthermore, the refraction of light is largely dependent on the particles, suspended sediments, and also color dissolved organic matter. Accordingly, it was impossible to establish the linear correlation between cell size and light level directly owing to the variability of light irradiance. According to the nonlinear fitting curve between depth and light irradiance (Figure 7), the light intensity decreased with the increasing depth in the equatorial EIO (R 2 = .65252), indicating that the decrease in light irradiance could be effectively represented by the increasing depth. Consequently, we established the linear response between cell size and the increasing depth (representing the decreasing light irradiance) to show different response relations of cell size and light intensity. Figure 8 shows the distinct response relations between cellular size and depth (light levels) and temperature for three picophytoplankton groups in the equatorial EIO. According to our analysis of response, the slopes of these lines for cell size of Prochlorococcus and Synechococcus to depth and temperature were relatively high, which represented high response levels of cell size to light levels and temperature. Thus, this observation indicated the vertical patterns of Prochlorococcus and Synechococcus in cellular sizes were the results of combined effects of photoacclimation and temperature (Figure 8). Similarly, Goericke and Welschmeyer (1998) proposed that the increase in cellular size with depth might be attributed to photoacclimation because of the need to synthesize more proteins and pigments to capture limiting photons. Thus, the organisms adjusted their chlorophyll content, size, and shape to compensate for the changes in irradiance with increasing depth. In laboratory batch cultures, Burbage and Binder (2007) reported that the cellular sizes of oceanic Prochlorococcus MIT9312 and Synechococcus WH8103 grown under high light were smaller than the sizes of cells grown under low light in semi‐continuous cultures. Otherwise, Montagnes and Franklin (2001) suggested that lower temperature could lead to larger size for Prochlorococcus and Synechococcus because of the growth dilution, with roughly 4% increase of cellular volume per centigrade decrease. Overall, the cellular sizes of Synechococcus and Prochlorococcus were larger under deep layer in the equatorial EIO, which was primarily attributed to the different responses to lower temperature and reduced light intensity. For Prochlorococcus, low‐B/A isolates are able to grow maximally at high light intensity, while high‐B/A isolates acclimate to the low light condition of the deep euphotic zone (Moore & Chisholm, 1999). Changes in the dominance of different ecotypes were consequently critical in explaining the vertical variations of their cellular sizes in addition to temperature and light availability in deeper waters. For picoeukaryotic phytoplankton, however, due to the lower capacity to photoacclimate controlled by physiological differences, their cellular sizes were larger occurring in low light layers (Campbell et al., 1997). Similarly, the pronounced increase with depth in picoeukaryotic phytoplankton size between the depth of 100 m and 200 m was the result of a dramatic change in light intensity.
Figure 7.
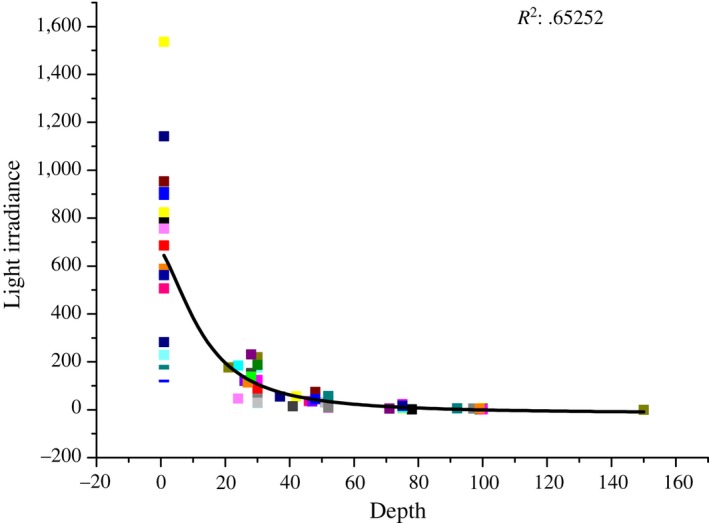
Nonlinear fitting curve between depth (m) and light irradiance (μmol photons m−2·s−1)
Figure 8.
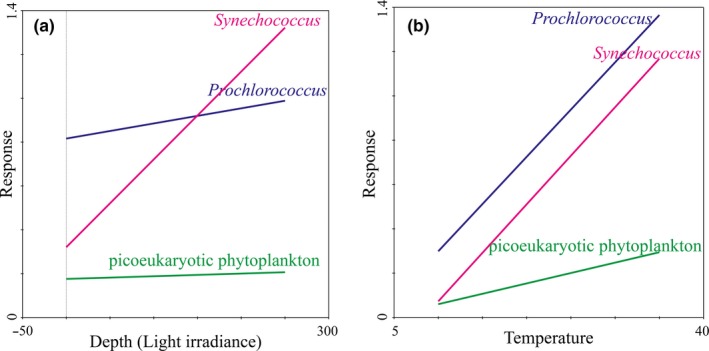
Different response relations between cellular size and light levels (depth (m)) and temperature (°C) for three picophytoplankton groups
Spatially, most of coastal waters around the Sri Lanka island are influenced by the increases of pollution and eutrophication. Simultaneously, the East India Coastal Current (EICC) along the western boundary of the Bay of Bengal flows equatorward and bifurcates east of the Sri Lanka island, but one bifurcation of its source waters characterized by nutrient enrichment continues along the coast of Sri Lanka island (Vinayachandran et al., 2005). Therefore, this studied coastal area is abundant in nutrients and suitable for picophytoplankton development. The CCA analysis revealed that significant correlations were found between the average carbon biomass of Prochlorococcus, Synechococcus, and picoeukaryotic phytoplankton and nutrient variables, reinforcing the notion that nutrient supply was the key limiting factor for the spatial distribution of average carbon biomass. Therefore, such contaminants loading from coastal currents and freshwater discharging from the EICC with high nutrients contributed to the fairly high average carbon biomass of three picophytoplankton groups in coastal waters of the Sri Lanka island. Surface temperature surrounding the Sumatra was below 29°C with a comparatively high salinity of approximately 34 during spring 2015, indicating that its hydrographic properties were prominently attributed to the coastal upwelling. The co‐occurrence of high average carbon biomass for picophytoplankton in coastal upwelling zones nearby the Sumatra was not surprisingly owing to the high nutrient availability. Waters of very low salinity and temperature (32°C and 29°C, respectively) were presented in the northern Bay of Bengal, thereby indicating that they were dramatically influenced by freshwater from the Bay of Bengal runoffs. As such, slightly high average carbon biomass of three picophytoplankton groups co‐occurred in the southern Bay of Bengal due to they could benefit from the increased nutrient availability carried by the Bay of Bengal runoffs (Mukhopadhyay, Biswas, De, & Jana, 2006). Collectively, as a result of differential responses of picophytoplankton in average carbon biomass to the changes in physical and chemical environments induced by the variable circulations and water masses, we observed very different spatial patterns of average carbon biomass for these three picophytoplankton groups.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGMENTS
The authors are grateful to D. Wang for supplying the temperature and salinity data for the cruise. We also express thanks to the crews of R/V Shiyan I. This study was supported by the National Natural Science Foundation of China (41676112 and 41276124); the National Foundation of the Indian Ocean Opening Voyage Project (41449910), and the Changjiang Scholar Program of Chinese Ministry of Education to J. Sun.
Wei Y, Sun J, Zhang X, Wang J, Huang K. Picophytoplankton size and biomass around equatorial eastern Indian Ocean. MicrobiologyOpen. 2019;8:e629 10.1002/mbo3.629
REFERENCES
- Agusti, S. , Duarte, C. M. , & Kalff, J. (1987). Algal cell size and the maximum density and biomass of phytoplankton. Limnology and Oceanography, 32(4), 983–986. 10.4319/lo.1987.32.4.0983 [DOI] [Google Scholar]
- Allman, R. , Hann, A. C. , Manchee, R. , & Lloyd, D. (1992). Characterization of bacteria by multiparameter flow cytometry. Journal of Applied Microbiology, 73(5), 438–444. 10.1111/j.1365-2672.1992.tb05001.x [DOI] [PubMed] [Google Scholar]
- Baines, S. B. , Twining, B. S. , Brzezinski, M. A. , Krause, J. W. , Vogt, S. , Assael, D. , & McDaniel, H. (2012). Significant silicon accumulation by marine picocyanobacteria. Nature Geoscience, 5(12), 886 10.1038/ngeo1641 [DOI] [Google Scholar]
- Binder, B. J. , Chisholm, S. W. , Olson, R. J. , Frankel, S. L. , & Worden, A. Z. (1996). Dynamics of picophytoplankton, ultraphytoplankton and bacteria in the central equatorial Pacific. Deep Sea Research Part II: Topical Studies in Oceanography, 43(4–6), 907–931. 10.1016/0967-0645(96)00023-9 [DOI] [Google Scholar]
- Blanchot, J. , André, J. M. , Navarette, C. , Neveux, J. , & Radenac, M. H. (2001). Picophytoplankton in the equatorial Pacific: Vertical distributions in the warm pool and in the high nutrient low chlorophyll conditions. Deep Sea Research Part I: Oceanographic Research Papers, 48(1), 297–314. 10.1016/S0967-0637(00)00063-7 [DOI] [Google Scholar]
- Blanchot, J. , & Rodier, M. (1996). Picophytoplankton abundance and biomass in the western tropical Pacific Ocean during the 1992 El Niño year: Results from flow cytometry. Deep Sea Research Part I: Oceanographic Research Papers, 43(6), 877–895. 10.1016/0967-0637(96)00026-X [DOI] [Google Scholar]
- Brzezinski, M. A. , Krause, J. W. , Baines, S. B. , Collier, J. L. , Ohnemus, D. C. , & Twining, B. S. (2017). Patterns and regulation of silicon accumulation in Synechococcus spp. Journal of phycology, 53(4), 746–761. 10.1111/jpy.12545 [DOI] [PubMed] [Google Scholar]
- Burbage, C. D. , & Binder, B. J. (2007). Relationship between cell cycle and light‐limited growth rate in oceanic Prochlorococcus (MIT9312) and Synechococcus (WH8103)(cyanobacteria). Journal of phycology, 43(2), 266–274. 10.1111/j.1529-8817.2007.00315.x [DOI] [Google Scholar]
- Calvo‐Díaz, A. , & Morán, X. A. G. (2006). Seasonal dynamics of picoplankton in shelf waters of the southern Bay of Biscay. Aquatic Microbial Ecology, 42(2), 159–174. 10.3354/ame042159 [DOI] [Google Scholar]
- Calvo‐Díaz, A. , Morán, X. A. G. , & Suárez, L. Á. (2008). Seasonality of picophytoplankton chlorophyll a and biomass in the central Cantabrian Sea, southern Bay of Biscay. Journal of marine systems, 72(1–4), 271–281. 10.1016/j.jmarsys.2007.03.008 [DOI] [Google Scholar]
- Campbell, L. , Liu, H. , Nolla, H. A. , & Vaulot, D. (1997). Annual variability of phytoplankton and bacteria in the subtropical North Pacific Ocean at Station ALOHA during the 1991–1994 ENSO event. Deep Sea Research Part I: Oceanographic Research Papers, 44(2), 167–192. 10.1016/S0967-0637(96)00102-1 [DOI] [Google Scholar]
- Campbell, L. , Nolla, H. A. , & Vaulot, D. (1994). The importance of Prochlorococcus to community structure in the central North Pacific Ocean. Limnology and Oceanography, 39(4), 954–961. 10.4319/lo.1994.39.4.0954 [DOI] [Google Scholar]
- Campbell, L. , & Vaulot, D. (1993). Photosynthetic picoplankton community structure in the subtropical North Pacific Ocean near Hawaii (station ALOHA). Deep Sea Research Part I: Oceanographic Research Papers, 40(10), 2043–2060. 10.1016/0967-0637(93)90044-4 [DOI] [Google Scholar]
- Charpy, L. , & Blanchot, J. (1998). Photosynthetic picoplankton in French Polynesian atoll lagoons: Estimation of taxa contribution to biomass and production by flow cytometry. Marine Ecology Progress Series, 57–70, 10.3354/meps162057 [DOI] [Google Scholar]
- Chen, B. , Wang, L. , Song, S. , Huang, B. , Sun, J. , & Liu, H. (2011). Comparisons of picophytoplankton abundance, size, and fluorescence between summer and winter in northern South China Sea. Continental Shelf Research, 31(14), 1527–1540. 10.1016/j.csr.2011.06.018 [DOI] [Google Scholar]
- Chiang, K. P. , Kuo, M. C. , Chang, J. , Wang, R. H. , & Gong, G. C. (2002). Spatial and temporal variation of the Synechococcus population in the East China Sea and its contribution to phytoplankton biomass. Continental Shelf Research, 22(1), 3–13. 10.1016/S0278-4343(01)00067-X [DOI] [Google Scholar]
- Chisholm, S. W. (1992). Phytoplankton size In Falkowski P. G., & Woodhead A. D. (Eds.), Primary productivity and biogeochemical cycles in the sea, Vol. 43 (pp. 213–237). Boston, MA: Springer. [Google Scholar]
- Dafner, E. V. (2015). Segmented continuous‐flow analyses of nutrient in seawater: Intralaboratory comparison of Technicon AutoAnalyzer II and Bran+Luebbe Continuous Flow AutoAnalyzer III. Limnology and Oceanography: Methods, 13(10), 511–520. 10.1002/lom3.10035 [DOI] [Google Scholar]
- Dai, M. , Wang, L. , Guo, X. , Zhai, W. , Li, Q. , He, B. , & Kao, S. J. (2008). Nitrification and inorganic nitrogen distribution in a large perturbed river/estuarine system: The Pearl River Estuary. China. Biogeosciences Discussions, 5(2), 1545–1585. [Google Scholar]
- Den Engh, V. , Doggett, J. K. , Thompson, A. W. , Doblin, M. A. , Gimpel, C. , & Karl, D. M. (2017). Dynamics of Prochlorococcus and Synechococcus at Station ALOHA revealed through flow cytometry and high‐resolution vertical sampling. Frontiers in Marine Science, 4, 359 10.3389/fmars.2017.00359 [DOI] [Google Scholar]
- DuRand, M. D. , & Olson, R. J. (1998). Diel patterns in optical properties of the chlorophyte Nannochloris sp.: Relating individual‐cell to bulk measurements. Limnology and Oceanography, 43(6), 1107–1118. 10.4319/lo.1998.43.6.1107 [DOI] [Google Scholar]
- DuRand, M. D. , Olson, R. J. , & Chisholm, S. W. (2001). Phytoplankton population dynamics at the Bermuda Atlantic Time‐series station in the Sargasso Sea. Deep Sea Research Part II: Topical Studies in Oceanography, 48(8–9), 1983–2003. 10.1016/S0967-0645(00)00166-1 [DOI] [Google Scholar]
- Eppley, R. W. , Reid, F. M. , & Strickland, J. D. H . (1970). Estimates of phytoplankton crop size, growth rate and primary production off La Jolla, CA in the period April through September 1967. In J. D. H. Strickland, (Ed.), Bulletin of the Scripps Institution of Oceanography, Vol. 17. (pp. 33‐42).
- Flombaum, P. , Gallegos, J. L. , Gordillo, R. A. , Rincón, J. , Zabala, L. L. , Jiao, N. , & Vera, C. S. (2013). Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus . Proceedings of the National Academy of Sciences, 110(24), 9824–9829. 10.1073/pnas.1307701110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame, K. K. , & Hu, W. S. (1990). The loss of antibody productivity in continuous culture of hybridoma cells. Biotechnology and bioengineering, 35(5), 469–476. 10.1002/bit.260350504 [DOI] [PubMed] [Google Scholar]
- Gasol, J. M. , & Del Giorgio, P. A. (2000). Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Scientia Marina, 64(2), 197–224. 10.3989/scimar.2000.64n2197 [DOI] [Google Scholar]
- Goericke, R. , & Welschmeyer, N. A. (1998). Response of Sargasso Sea phytoplankton biomass, growth rates and primary production to seasonally varying physical forcing. Journal of Plankton Research, 20(12), 2223–2249. 10.1093/plankt/20.12.2223 [DOI] [Google Scholar]
- Guo, S. , Feng, Y. , Wang, L. , Dai, M. , Liu, Z. , Bai, Y. , & Sun, J. (2014). Seasonal variation in the phytoplankton community of a continental‐shelf sea: The East China Sea. Marine Ecology Progress Series, 516, 103–126. 10.3354/meps10952 [DOI] [Google Scholar]
- Hewes, C. D. , Sakshaug, E. , Reid, F. M. , & Olm‐Hansen, O. (1990). Microbial autotrophic and heterotrophic eucaryotes in Antarctic waters: relationships between biomass and chlorophyll, adenosine triphosphate and particulate organic carbon. Marine Ecology Progress Series, 63, 27–35. 10.3354/meps063027 [DOI] [Google Scholar]
- Hillebrand, H. , Dürselen, C. D. , Kirschtel, D. , Pollingher, U. , & Zohary, T. (1999). Biovolume calculation for pelagic and benthic microalgae. Journal of phycology, 35(2), 403–424. 10.1046/j.1529-8817.1999.3520403.x [DOI] [Google Scholar]
- Hunter, B. L. , & Laws, E. A. (1981). ATP and chlorophyll a as estimators of phytoplankton carbon biomass. Limnology and Oceanography, 26(5), 944–956. 10.4319/lo.1981.26.5.0944 [DOI] [Google Scholar]
- Koch, A. L. , Robertson, B. R. , & Button, D. K. (1996). Deduction of the cell volume and mass from forward scatter intensity of bacteria analyzed by flow cytometry. Journal of microbiological methods, 27(1), 49–61. 10.1016/0167-7012(96)00928-1 [DOI] [Google Scholar]
- Latimer, P. (1982). Light scattering and absorption as methods of studying cell population parameters. Annual Review of Biophysics and Bioengineering, 11(1), 129–150. 10.1146/annurev.bb.11.060182.001021 [DOI] [PubMed] [Google Scholar]
- Liu, H. , Campbell, L. , Landry, M. R. , Nolla, H. A. , Brown, S. L. , & Constantinou, J. (1998). Prochlorococcus and Synechococcus growth rates and contributions to production in the Arabian Sea during the 1995 Southwest and Northeast Monsoons. Deep Sea Research Part II: Topical Studies in Oceanography, 45(10–11), 2327–2352. 10.1016/S0967-0645(98)00073-3 [DOI] [Google Scholar]
- Liu, H. , Chang, J. , Tseng, C. M. , Wen, L. S. , & Liu, K. K. (2007). Seasonal variability of picoplankton in the Northern South China Sea at the SEATS station. Deep Sea Research Part II: Topical Studies in Oceanography, 54(14–15), 1602–1616. 10.1016/j.dsr2.2007.05.004 [DOI] [Google Scholar]
- Marañón, E. (2015). Cell size as a key determinant of phytoplankton metabolism and community structure. Annual Review of Marine Science, 7, 241–264. 10.1146/annurev-marine-010814-015955 [DOI] [PubMed] [Google Scholar]
- McClanahan, T. R. , Maina, J. M. , Graham, N. A. , & Jones, K. R. (2016). Modeling reef fish biomass, recovery potential, and management priorities in the Western Indian Ocean. PLoS ONE, 11(5), e0154585 10.1371/journal.pone.0154585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnes, D. J. , & Franklin, M. (2001). Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: Reconsidering some paradigms. Limnology and Oceanography, 46(8), 2008–2018. 10.4319/lo.2001.46.8.2008 [DOI] [Google Scholar]
- Moore, L. R. , & Chisholm, S. W. (1999). Photophysiology of the marine cyanobacterium Prochlorococcus: Ecotypic differences among cultured isolates. Limnology and Oceanography, 44(3), 628–638. 10.4319/lo.1999.44.3.0628 [DOI] [Google Scholar]
- Moore, L. R. , Post, A. F. , Rocap, G. , & Chisholm, S. W. (2002). Utilization of different nitrogen sources by the marine cyanobacteria Prochlorococcus and Synechococcus . Limnology and Oceanography, 47(4), 989–996. 10.4319/lo.2002.47.4.0989 [DOI] [Google Scholar]
- Morel, A. (1991). Optics of marine particles and marine optics In Demers S. (Ed.), Particle analysis in oceanography, Vol. 27 (pp. 141–188). Berlin Heidelberg: Springer; 10.1007/978-3-642-75121-9 [DOI] [Google Scholar]
- Mukhopadhyay, S. K. , Biswas, H. D. T. K. , De, T. K. , & Jana, T. K. (2006). Fluxes of nutrients from the tropical River Hooghly at the land–ocean boundary of Sundarbans, NE Coast of Bay of Bengal, India. Journal of Marine Systems, 62(1–2), 9–21. 10.1016/j.jmarsys.2006.03.004 [DOI] [Google Scholar]
- Olson, R. J. , Zettler, E. R. , & Anderson, O. K. (1989). Discrimination of eukaryotic phytoplankton cell types from light scatter and autofluorescence properties measured by flow cytometry. Cytometry Part A, 10(5), 636–643. 10.1002/cyto.990100520 [DOI] [PubMed] [Google Scholar]
- Partensky, F. , Hess, W. R. , & Vaulot, D. (1999). Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiology and molecular biology reviews, 63(1), 106–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, J. A. (1998). The twelfth Tansley Lecture. Small is beautiful: The picophytoplankton. Functional ecology, 12(4), 503–513. 10.1046/j.1365-2435.1998.00233.x [DOI] [Google Scholar]
- Robertson, B. R. , & Button, D. K. (1989). Characterizing aquatic bacteria according to population, cell size, and apparent DNA content by flow cytometry. Cytometry Part A, 10(1), 70–76. 10.1002/cyto.990100112 [DOI] [PubMed] [Google Scholar]
- Roxy, M. K. , Modi, A. , Murtugudde, R. , Valsala, V. , Panickal, S. , Prasanna Kumar, S. , & Lévy, M. (2016). A reduction in marine primary productivity driven by rapid warming over the tropical Indian Ocean. Geophysical Research Letters, 43(2), 826–833. 10.1002/2015GL066979 [DOI] [Google Scholar]
- Sengupta, D. , Bharath Raj, G. N. , & Shenoi, S. S. C. (2006). Surface freshwater from Bay of Bengal runoff and Indonesian throughflow in the tropical Indian Ocean. Geophysical Research Letters, 33(22), 10.1029/2006GL027573 [DOI] [Google Scholar]
- Shalapyonok, A. , Olson, R. J. , & Shalapyonok, L. S. (2001). Arabian Sea phytoplankton during Southwest and Northeast Monsoons 1995: Composition, size structure and biomass from individual cell properties measured by flow cytometry. Deep Sea Research Part II: Topical Studies in Oceanography, 48(6–7), 1231–1261. 10.1016/S0967-0645(00)00137-5 [DOI] [Google Scholar]
- Siswanto, E. (2015). Atmospheric deposition—Another source of nutrients enhancing primary productivity in the eastern tropical Indian Ocean during positive Indian Ocean Dipole phases. Geophysical Research Letters, 42(13), 5378–5386. 10.1002/2015GL064188 [DOI] [Google Scholar]
- Sommaruga, R. , Hofer, J. S. , Alonso‐Sáez, L. , & Gasol, J. M. (2005). Differential sunlight sensitivity of picophytoplankton from surface Mediterranean coastal waters. Applied and environmental microbiology, 71(4), 2154–2157. 10.1128/AEM.71.4.2154-2157.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockner, J. G. (1988). Phototrophic picoplankton: An overview from marine and freshwater ecosystems. Limnology and Oceanography, 33(4part2), 765–775. 10.4319/lo.1988.33.4part2.0765 [DOI] [Google Scholar]
- Sun, J. , & Liu, D. (2003). Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of plankton research, 25(11), 1331–1346. 10.1093/plankt/fbg096 [DOI] [Google Scholar]
- Sun, J. , Liu, D. , & Qian, S. (2000). Estimating biomass of phytoplankton in the Jiaozhou bay. I. Phytoplankton biomass estimated from cell volume and plasma volume. Acta oceanologica sinica, 19(2), 97–110. [Google Scholar]
- Vinayachandran, P. N. , Kagimoto, T. , Masumoto, Y. , Chauhan, P. , Nayak, S. R. , & Yamagata, T. (2005). Bifurcation of the East India coastal current east of Sri Lanka. Geophysical Research Letters, 32(15), 10.1029/2005GL022864 [DOI] [Google Scholar]
- Wang, C. , Xie, S. P. , & Carton, J. A. (2004). A global survey of ocean–atmosphere interaction and climate variability. Earth's Climate, 1–19, 10.1029/147GM01 [DOI] [Google Scholar]
- Zhao, L. , Zhao, Y. , Zhang, W. , Zhou, F. , Zhang, C. , Ren, J. , & Xiao, T. (2013). Picoplankton distribution in different water masses of the East China Sea in autumn and winter. Chinese Journal of Oceanology and Limnology, 31(2), 247–266. 10.1007/s00343-013-2085-3 [DOI] [Google Scholar]


