Abstract
The prognosis of pancreatic cancer remains dismal despite continuous and considerable efforts. Integrins (ITGs) are highly expressed in various malignant cancers. However, very few studies investigated the role of integrin α3 (ITGα3) in malignant cancers. Here, we determined the functional role of ITGα3 in pancreatic cancer. Analysis of public microarray databases and Western blot analysis indicated a unique expression of ITGα3 in human pancreatic cancer. Silencing ITGα3 expression significantly inhibited the viability and migration of human pancreatic cancer cells. Notably, ablation of ITGα3 expression resulted in a significant decrease of epidermal growth factor receptor (EGFR) expression compared with transfection of control-siRNA through an increased number of leucine-rich repeats and immunoglobulin-like domain protein 1 (LRIG1) expression. In addition, ablating ITGα3 inhibited tumour growth via blockade of EGFR signalling in vivo. Furthermore, the highly expressed ITGα3 led to a poor prognosis of pancreatic cancer patients. Our results provide novel insights into ITGα3-induced aggressive pancreatic cancer.
Introduction
Pancreatic cancer is one of the most dangerous malignancies of the digestive system characterized by rapid progression, natural invasion, and grave patient outcome1,2. Despite continuous efforts to improve its prognosis, the incidence rates of pancreatic cancer are almost equal to its death rates3. Thus, there is an urgent need to develop effective therapeutics for this deadly neoplasm.
ITGs are primary transmembrane receptors that mediate cellular interactions with extracellular matrix (ECM) and regulate tumour cell features including adhesion, migration, invasion, proliferation, and survival4–6. The integrin receptor family consists of 18 α subunits and 8 β subunits that assemble as non-covalently connected heterodimers and organized into 24 different ITGs7. Our current in silico study and another previous report suggest that ITGα3 plays a significant role in adverse prognosis of pancreatic cancer8,9. However, the underlying mechanism is poorly understood.
Human epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase (RTK) is characterized by an extracellular ligand-binding domain, a transmembrane portion, and a tyrosine kinase moiety10. Activation of EGFR signalling results in auto-phosphorylation of the tyrosine kinase domains, which amplify downstream signalling pathways such as mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway, leading to angiogenesis, growth, metastasis, and survival11,12. Due to mutations or over expression, inhibition of EGFR represents an assiduous therapeutic strategy via monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs)13. In contrast, the predominant effects of negative signalling against EGFR in mammals prevailed for a long time, mediated by inducible feedback inhibitors (IFIs) such as leucine-rich repeats and immunoglobulin-like domain protein 1 (LRIG1), receptor-associated late transducer (RALT), suppressor of cytokine signalling 4 (SOCS4), and suppressor of cytokine signalling 5 (SOCS5) that act promptly without the need for de novo protein synthesis14,15. Various reports have highlighted the effects of IFIs on mammalian EGFR and demonstrated the critical role played by these molecules in the control of homeostasis15.
In the present study, we provide robust evidences suggesting that ITGα3 is critical for pancreatic malignancy via coordination with EGFR signalling pathway involved in alteration of LRIG1 expression. In addition, ITGα3 is specifically associated with adverse prognosis in pancreatic cancer. Our results establish a rationale for ITGα3 as a promising therapeutic target in patients with pancreatic cancer.
Results
Functional expression of integrin α3 (ITGα3) in human pancreatic cancers
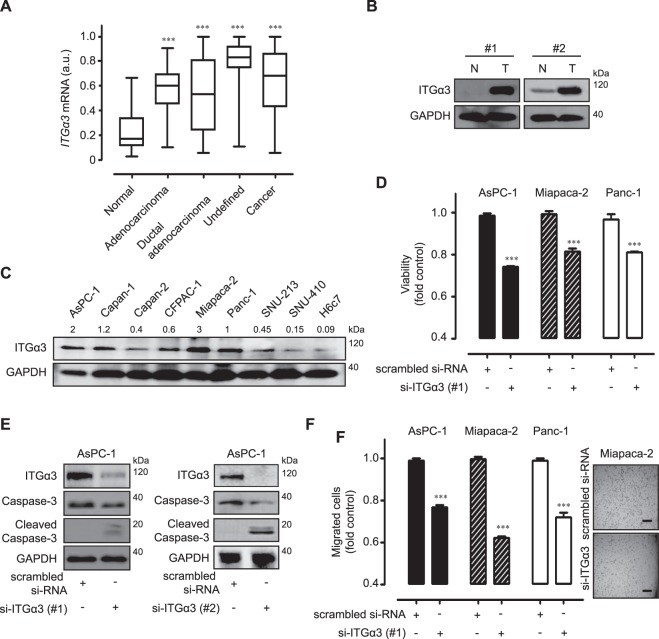
The expression profiles of ITGα3 in human pancreatic cancer samples were obtained from public microarray database Gene Expression Omnibus (GEO). Adenocarcinoma of the pancreas, ductal-adenocarcinoma samples, and undefined cancers expressed higher levels of ITGα3 than normal pancreas samples (Fig. 1A). To verify the expression patterns, we examined the ITGα3 protein levels in separate human pancreatic cancer tissues by Western blot analysis. ITGα3 was highly expressed in pancreatic cancer tissues compared with normal pancreas (Fig. 1B). In addition, ITGα3 was expressed relatively highly in eight human pancreatic cancer cells compared with human pancreatic duct epithelial H6c7 cells (Fig. 1C). To demonstrate the cellular functions of ITGα3, we inhibited ITGα3 expression by si-RNA transfection in ITGα3-expressing AsPC-1, Miapaca-2, and Panc-1 cells. Compared with AsPC-1, Miapaca-2, and Panc-1 cells transfected with control si-RNA, cells transfected with ITGα3-specific si-RNA showed significantly decreased levels of ITGα3 protein (Supplementary Fig. 1). Silencing of ITGα3 expression significantly inhibited the viability of AsPC-1, Miapaca-2, and Panc-1 cells under serum-free culture conditions (Fig. 1D). Similar result was obtained using another type of si-ITGα3 (#2) transfection (Supplementary Fig. 2A). Transfection using two different types of si-ITGα3 (#1 and #2) induced the caspase-3-mediated apoptosis (Fig. 1E). Ablation of ITGα3 expression also markedly diminished the migration of AsPC-1, Miapaca-2, and Panc-1 cells (Fig. 1F). At that time, there was no inhibition of viability between scrambled si-RNA or si-ITGα3 transfected cells (data not shown). Similar migration result was obtained using another type of si-ITGα3 (#2) transfection (Supplementary Fig. 2B). Correlations between ITGα3 expression and various anti-cancer drugs were also demonstrated using Cancer Cell Line Encyclopedia (CCLE) public database to investigate the function of ITGα3 in human pancreatic cancer drug-resistance. ITGα3 expression was negatively correlated with anti-cancer drug sensitivity in about 75% (18/24) of human pancreatic cancer cells (Table 1).
Figure 1.
Functional integrin α3 (ITGα3) expression in pancreatic cancer. (A) Transcriptional levels of ITGα3 in normal pancreas (n = 59, GSM# 388101–463724), adenocarcinoma (n = 55, GSM# 967641–1053825), ductal adenocarcinoma (n = 66, GSM# 388153–811004), and undefined samples (n = 76, GSM# 242823–414974) were analyzed using the Gene Expression Omnibus (GEO) databases (a.u. indicates arbitrary unit using the UPCs method, the P value was evaluated with Student’s t-test, ***P < 0.001, Cancer indicates the sum of adenocarcinoma, ductal adenocarcinoma, and undefined samples). (B) Protein expression of ITGα3 in pancreatic cancers and normal pancreas were analyzed using the Western blot. GAPDH was used as a control (N indicates a normal pancreas sample, T indicates a pancreatic cancer sample, #1 and #2 are separate samples, Data is representative of three individual experiments). (C) ITGα3 proteins in various human pancreatic cancer cells and H6c7 cells were detected by Western blot. GAPDH was measured as a control. Relative pixel intensities of ITGα3 were measured using ImageJ analysis software (ITGα3/GAPDH). Data is representative of three individual experiments. (D) AsPC-1, Miapaca-2, and Panc-1 cells were transfected with scrambled or ITGα3-specific siRNA for 72 h under serum-free cultured conditions. The viability was measured by WST-1 assay (n = 3; Tukey’s post-hoc test was used to detect significant differences in ANOVA, p < 0.0001; asterisks indicate a significant difference compared with 0% inhibition, ***P < 0.001). (E) AsPC-1 cells were transfected with scrambled or ITGα3-specific siRNAs (#1 and #2) for 72 h. ITGα3, caspase-3, and cleaved caspase-3 protein levels were analyzed by Western blot. GAPDH was used as a loading control. Data is representative of three individual experiments. (F) AsPC-1, Miapaca-2, and Panc-1 cells were transfected with scrambled or ITGα3-specific siRNA. After 48 h of transfection, the cells were exposed to serum-starved conditions. After 24 h of serum starvation, migrated cells were evaluated using the Transwell-migration assay (n = 3; Tukey’s post-hoc test was used to detect significant differences in ANOVA, p < 0.0001; asterisks indicate significant differences compared with 0% inhibition, ***P < 0.001, scale bar = 50 µm).
Table 1.
Drug-sensitivities correlated with integrin α3 (ITGα3) expression levels in the CCLE databases.
| Drug name | correlation | average | variation | N |
|---|---|---|---|---|
| Paclitaxel | −0.136521 | 0.7962 | 0.1585 | 28 |
| RAF-265 | −0.194086 | 0.8281 | 0.1089 | 23 |
| 17-AAG | 0.007134 | 0.7962 | 0.1585 | 28 |
| Irinotecan | −0.131134 | 0.8285 | 0.1199 | 19 |
| Lapatinib | 0.111679 | 0.7962 | 0.1585 | 28 |
| Panobinostat | −0.165702 | 0.7962 | 0.1585 | 28 |
| TKI258 | −0.400488 | 0.7962 | 0.1585 | 28 |
| Topotecan | 0.166555 | 0.7962 | 0.1585 | 28 |
| L-685458 | −0.193066 | 0.7962 | 0.1585 | 28 |
| PD-0332991 | −0.161663 | 0.8295 | 0.1112 | 22 |
| PF2341066 | −0.020896 | 0.7962 | 0.1585 | 22 |
| Erlotinib | −0.052117 | 0.7962 | 0.1585 | 28 |
| AZD0530 | −0.273753 | 0.7962 | 0.1585 | 28 |
| LBW242 | −0.049073 | 0.7962 | 0.1585 | 28 |
| TAE684 | −0.02831 | 0.7962 | 0.1585 | 28 |
| ZD-6474 | 0.353902 | 0.7899 | 0.1580 | 28 |
| PHA-665752 | −0.379134 | 0.7962 | 0.1685 | 28 |
| AZD6244 | 0.20628 | 0.7962 | 0.1585 | 28 |
| PD-0325901 | −0.070893 | 0.7962 | 0.1585 | 22 |
| Sorafenib | −0.220974 | 0.7962 | 0.1585 | 28 |
| Nilotinib | −0.07676 | 0.8290 | 0.1140 | 21 |
| Nutlin-3 | 0.109441 | 0.7962 | 0.1585 | 28 |
| PLX4720 | −0.253 | 0.7899 | 0.1585 | 27 |
| AEW541 | −0.259067 | 0.7962 | 0.1585 | 28 |
Correlation of sensitivities to various anti-cancer drugs with ITGα3 expression levels in different pancreatic cancer cells.
ƗN indicates the number of human pancreatic cancer cell lines.
These results indicate that the functional expression of ITGα3 was elevated in human pancreatic cancer.
Mechanism associated with integrin α3 (ITGα3) blockade in human pancreatic cancer cells
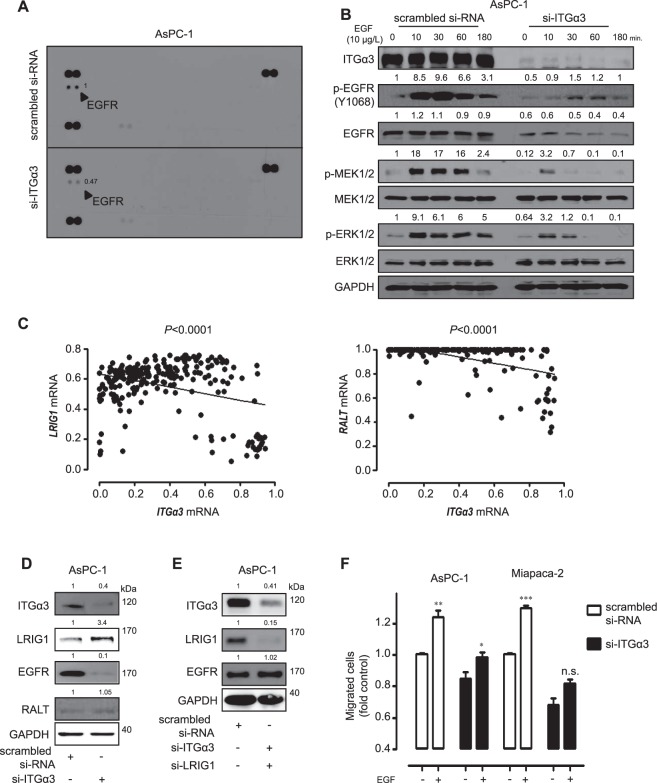
Next, we examined signal transduction pathways using phospho-RTK array to delineate the mechanism by which ablation of ITGα3 expression affected features of human pancreatic cancer cells. Transfection with si-ITGα3 significantly reduced EGFR phosphorylation in AsPC-1 cells compared with control cells (Fig. 2A). To further analyze the detailed mechanism of ITGα3 knockdown, the levels of phosphorylated EGFR were examined with varying levels of exogenous EGF (10 µg/L) treatment following si-ITGα3 transfection in AsPC-1 cells. Treatment with exogenous EGF increased phosphorylation levels of EGFR (Tyr1068), MEK1/2 (Ser217/221), and ERK1/2 (Thr202/204) in AsPC-1 cells, but not in si-ITGα3 transfected cells (Fig. 2B). Notably, silencing of ITGα3 expression decreased the EGFR expression. To verify the reduction of EGFR expression by si-ITGα3 transfection, we examined the mRNA levels of EGFR following silencing of ITGα3 expression in AsPC-1 cells. Our results revealed that suppression of ITGα3 expression had no effect on EGFR mRNA expression level (Supplementary Fig. 3). Previous studies reported that inducible feedback inhibitors (IFIs) were natural inhibitors of EGFR expression15,16. To demonstrate the involvement of IFIs expression in EGFR down-regulation by reduction of ITGα3 expression, we initially examined the correlations between IFIs and ITGα3 using the GEO public microarray database. A negative correlation was specifically found between LRIG1 or RALT and ITGα3 expression in pancreatic cancer samples (Fig. 2C). SOCS4 and SOCS5 showed a statistically non-significant or positive correlation with ITGα3 (Supplementary Fig. 4). To examine the alteration of LRIG1 or RALT expression based on ITGα3 level, we performed si-ITGα3 transfection in AsPC-1 cells. A decreased ITGα3 expression increased the level of LRIG1 expression in AsPC-1 cells, but not RALT (Fig. 2D).
Figure 2.
Associated mechanism following integrin α3 (ITGα3) blockade in human pancreatic cancer cells (A) AsPC-1 cells were transfected with scrambled or ITGα3-specific siRNA for 48 h. Human phospho-RTK array was used to determine differences in scrambled or ITGα3-specific siRNA transfection. Relative pixel intensity for p-EGFR was measured by densitometry analysis using ImageJ analysis software. Data is representative of two individual experiments. Bold arrows indicate the spot of EGFR. (B) AsPC-1 cells were transfected with scrambled or ITGα3-specific siRNA. After 48 h of transfection, the cells were exposed to serum-starved condition. After 18 h of serum starvation, AsPC-1 cells were incubated with 10 μg/L of EGF for various durations, and the cell lysates were subjected to Western blot using specific antibodies for p-EGFR (Y1068), EGFR, p-MEK1/2, MEK1/2, p-ERK1/2, ERK1/2, and GAPDH. Relative pixel intensities were measured by densitometry analysis using ImageJ analysis software. Data is representative of three individual experiments. (C) The correlation between ITGα3 and LRIG1 or RALT expression in the pancreatic cancer samples was calculated using the Gene Expression Omnibus (GEO) public microarray database (Pearson’s correlation coefficient (PCC) was used for statistical analysis). (D) AsPC-1 cells were transfected with scrambled or ITGα3-specific siRNA. After 72 h of transfection, the cell lysates were subjected to Western blot using antibodies specific for ITGα3, LRIG1, EGFR, RALT, and GAPDH. Relative pixel intensities were measured by densitometry using ImageJ analysis software. Data is representative of three individual experiments. (E) AsPC-1 cells were transfected with scrambled or combined ITGα3- and LRIG1-siRNA. After 72 h of transfection, the cell lysates were subjected to Western blot using antibodies specific for ITGα3, LRIG1, EGFR, and GAPDH. Relative pixel intensities were measured by densitometry using ImageJ analysis software. Data is representative of three individual experiments. (F) AsPC-1 and Miapaca-2 cells were transfected with scrambled or ITGα3-specific siRNA for 48 h. 10 μg/L of EGF was exogenously pretreated for 1 h. Migrated cells were evaluated using the Transwell-assay for a 6 h (n = 3; Tukey’s post-hoc test was used to detect significant difference in ANOVA, p < 0.0001; asterisks indicate a significant difference compared with 0% inhibition, *P < 0.05, **P < 0.01, ***P < 0.001, n.s. means non-significant).
To support this correlation of ITGα3, LRIG1, and EGFR expression, we performed the combined transfection with si-ITGα3 and si-LRIG1 in AsPC-1 cells. EGFR expression level was not altered following dual transfection of si-ITGα3 and si-LRIG1 (Fig. 2E). We also demonstrated the EGFR expression levels of eight human pancreatic cancer cells and H6c7 cells. Miapaca-2 had a most high EGFR expression level; in contrast, SNU-410 and H6C7 cells had relatively low EGFR expression patterns (Supplementary Fig. 5). Ablation of ITGα3 expression also significantly diminished the migration potential of AsPC-1 and Miapaca-2 cells stimulated by exogenous EGF treatment (Fig. 2F). These results clearly indicate that ITGα3 ablation decreases EGFR signalling via induction of LRIG1 expression in vitro.
In vivo effects of ITGα3 expression on human pancreatic cancer
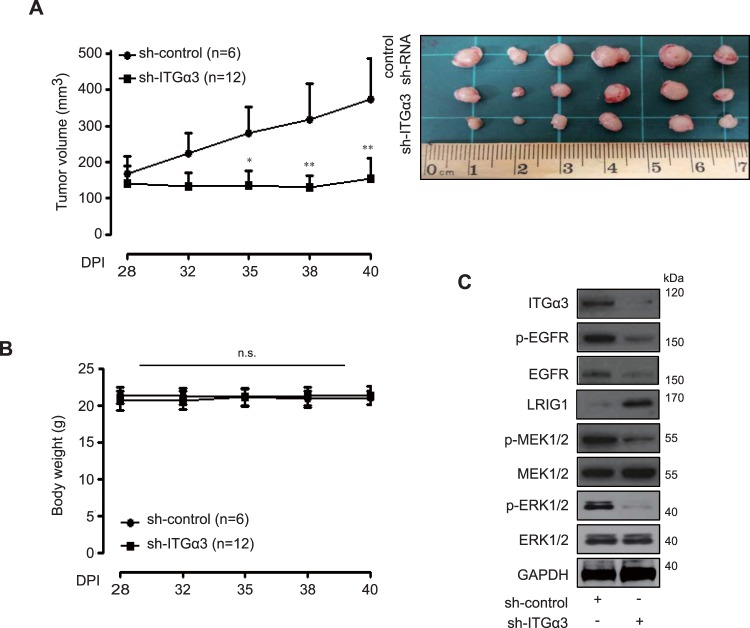
To validate the effect of ITGα3 expression in vivo, we constructed a stably reduced sh-ITGα3 AsPC-1 cell line. We first determined the expression levels and features of the established cell line in vitro. Sh-ITGα3 AsPC-1 cells showed a significantly decreased expression of ITGα3 accompanied by reduced proliferation and migration activities under serum-free conditions (Supplementary Fig. 4). The effect of decreased expression of ITGα3 was then observed in sh-ITGα3 and sh-control AsPC-1 xenograft models. Control tumours grew to a mean size of 374.19 ± 113.7 mm3 at 40 days after transplantation with sh-control AsPC-1. However, shRNA-reducible tumours showed a mean size of 153.86 ± 57.6 mm3 at 40 days after transplantation with sh-ITGα3 AsPC-1 (Fig. 3A). No weight loss was detected in the control or sh-ITGα3 group of AsPC-1 xenograft models (Fig. 3B). To investigate the in vivo roles of ITGα3, we performed Western blot analysis using sh-ITGα1 and sh-control xenograft tumour lysates. The levels of ITGα3, phospho-EGFR, EGFR, phospho-MEK1/2, and phospho-ERK1/2 were significantly decreased in sh-ITGα3 AsPC-1 xenografts compared with those in sh-control AsPC-1 xenografts. In contrast, LRIG1 was markedly increased in sh-ITGα3 AsPC-1 xenografts compared with those in sh-control AsPC-1 xenografts (Fig. 3C).
Figure 3.
In vivo effects of integrin α3 (ITGα3) expression. (A) Anti-tumour effects of sh-ITGα3 AsPC-1 xenograft models (sh-control group: n = 6, sh-ITGα3 group: n = 12) were measured for 40 days using the formula: V = 0.523 LW2 (L = length, W = width) (Tukey’s post-hoc test was used to detect significant differences in ANOVA, p < 0.0001; asterisks indicate a significant difference compared with 0% inhibition, *p < 0.05, **p < 0.01 compared with sh-control group and sh- ITGα3 group). (B) Body weight in each group was regularly measured. (C) Western blot analysis of sh-control group and sh-ITGα3 group tumour lysate was conducted with anti ITGα3, p-EGFR, EGFR, LRIG1, p-MEK1/2, MEK1/2, p-ERK1/2, and ERK1/2 antibodies. GAPDH was used for loading control. Data is representative of three individual experiments.
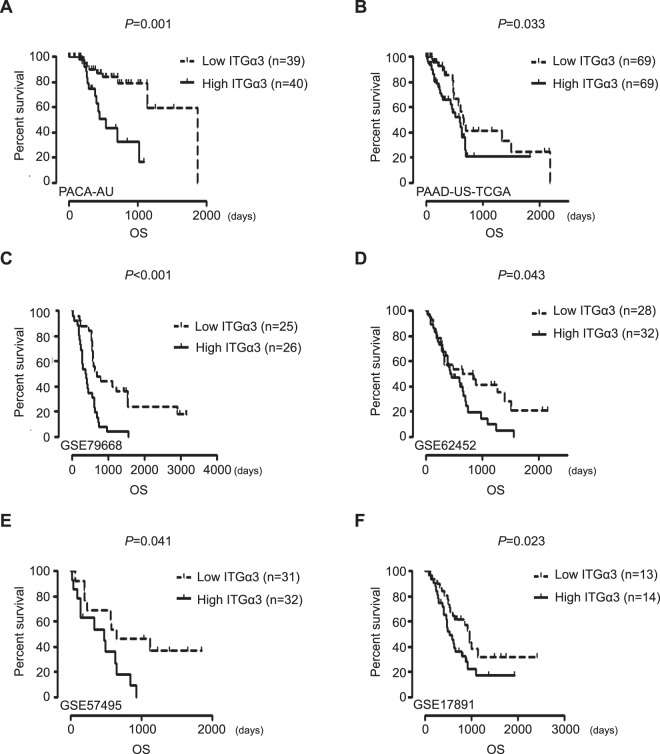
To demonstrate the role of ITGα3 in the prognosis of pancreatic cancer patients, we analyzed GEO datasets. PACA-AU, PAAD-US-TCGA, GSE79688, GSE62452, GSE57495, and GSE17891 datasets revealed that low expression of ITGα3 significantly improved median survival (MS) compared with a high expression of ITGα3 in patients with pancreatic cancer (Fig. 4A–F). To further analyze the detailed role of ITGα3 in prognosis of patients with pancreatic cancer, we subdivided PACA-AU, PAAD-US-TCGA, and GSE79668 datasets into different age groups. The highly ITGα3-expressing group at all ages showed significantly worse median survival than the poorly expressing group. Group of over 50 years had the worst median survival (MS: 393 days) in GSE79668 dataset, followed by those of over 60 years (MS: 481 days) in PAAD-US-TCGA dataset and those who were below 70 years (MS: 537 days) in PACA-AU dataset (Table 2).
Figure 4.
Integrin α3 (ITGα3) expression is associated with prognosis of pancreatic cancer patients. (A–F) Overall survival of pancreatic cancer patients was analyzed using Kaplan-Meier curves depending on the differential expression of ITGα3 in PACA-AU, PAAD-US-TCGA, GSE79688, GSE62452, GSE57495, and GSE17891 datasets (P value was calculated using Log-rank (Mantel-Cox) Test.
Table 2.
Clinical outcomes correlated with integrin α3 (ITGα3) expression levels.
| data-set-ID | stratification | P value | Low (MS) | High (MS) | Low (N) | High (N) |
|---|---|---|---|---|---|---|
| PACA-AU | ALL | 0.00163884 | 1874 | 537 | 39 | 40 |
| PACA-AU | AGE>30 | 0.00258695 | 1874 | 709 | 39 | 39 |
| PACA-AU | AGE>40 | 0.00248286 | 1874 | 709 | 38 | 39 |
| PACA-AU | AGE>50 | 0.0082042 | 1874 | 709 | 35 | 38 |
| PACA-AU | AGE>60 | 0.0238491 | 1874 | 709 | 29 | 30 |
| PACA-AU | AGE<70 | 0.00205248 | 1144 | 537 | 23 | 23 |
| PACA-AU | AGE<80 | 0.031809 | 1874 | 1021 | 35 | 38 |
| PAAD-US-TCGA | ALL | 0.0336586 | 666 | 598 | 69 | 69 |
| PAAD-US-TCGA | AGE>30 | 0.0336586 | 666 | 598 | 69 | 69 |
| PAAD-US-TCGA | AGE>40 | 0.0323066 | 652 | 511 | 68 | 69 |
| PAAD-US-TCGA | AGE>50 | 0.0113558 | 603 | 511 | 62 | 62 |
| PAAD-US-TCGA | AGE>60 | 0.00818206 | 1332 | 481 | 48 | 49 |
| PAAD-US-TCGA | AGE<70 | 0.0450421 | 1332 | 511 | 43 | 45 |
| PAAD-US-TCGA | AGE<80 | 0.0078225 | 1332 | 598 | 63 | 63 |
| GSE79668 | ALL | 0.000233238 | 702 | 382 | 25 | 26 |
| GSE79668 | AGE>30 | 0.0011241 | 647 | 371 | 25 | 25 |
| GSE79668 | AGE>40 | 0.0011241 | 647 | 371 | 25 | 25 |
| GSE79668 | AGE>50 | 0.00358437 | 601 | 341 | 22 | 22 |
| GSE79668 | AGE>60 | 0.0115516 | 611.5 | 342 | 17 | 18 |
| GSE79668 | AGE<70 | 0.00645222 | 702 | 449 | 17 | 17 |
| GSE79668 | AGE<80 | 0.000351786 | 702 | 393 | 25 | 25 |
ƗLow (MS) indicates the median survival of low expressed ITGα3 group.
ƗHigh (MS) indicates the median survival of high expressed ITGα3 group.
ƗLow (N) indicates the number of patients with low expression of ITGα3.
ƗHigh (N) indicates the number of patients with high expression of ITGα3.
The English in this document has been checked by at least two professional editors, both native speakers of English.
These results strongly indicate that low expressed ITGα3 improves the malignancy of human pancreatic cancer.
Discussion
Thorough knowledge underlying malignant pancreatic cancer features has become increasingly critical due to poor clinical outcome of patients with this disease. Evidently, survival rates of pancreatic cancer patients have not improved during the past few decades despite continuous efforts to improve their prognosis1. This grave convalescence might originate from the strong resistance of this cancer to chemotherapies, instinct metastasis to different nests, and hard to obtain early diagnosis17–19.
Results of the present study demonstrate that ITGα3 has critical functions in human pancreatic cancer features via adducing in silico-in vitro-in vivo-clinical evidences. To the best of our knowledge, this is the critical study shows that blockade of ITGα3 might be a promising strategy to inhibit malignant pancreatic cancer through ablating the strict connective signalling pathway involving epidermal growth factor receptor (EGFR).
Integrins (ITGs) play a critical role in metastasis to evade apoptosis and maintain cellular motility. It has been reported that ITGs help tumour cells acquire malignant features via interactions with their corresponding ECM components20–22. Integrin α3 (ITGα3) is a receptor for ECM molecules such as fibronectin, laminin- 5, -10, or -1123. Being a pivotal player in aggressive cancer phenotypes, α3β1 integrin plays an oncogenic role in different cancer types. It is critically involved in membrane protrusion of U251MG glioblastoma cells24 and epithelial mesenchymal transition (EMT) in breast cancer cells25. Consistent with previous reports, the current study also revealed the unique function of ITGα3 in human pancreatic cancers. All data were retrieved from the public GEO database indicated that ITGα3 was specifically over-expressed in pancreatic cancers compared with normal pancreas. The expression of ITGα3 was also confirmed in human pancreatic cancer tissues and cell lines. In contrast, it had very weak expression level in normal pancreas cells based on Western blot analysis. Of note, ITGα3 was shown to be associated with aggressive phenotypes of human pancreatic cancers. Ablation of ITGα3 expression using si-RNA transfection significantly inhibited viability and migration of human pancreatic cancer cells, indicating that silencing ITGα3 expression triggered prominent anti-cancer effects independent of its interactions with various ECM components. A recent clinical trial of gene therapy suggested remarkable therapeutic benefits and an excellent safety record26, prompting serious consideration of blockade of ITGα3 via innovative gene therapy approaches. In addition, effective small-molecule inhibitors or neutralizing antibody against ITGα3 should be developed as pancreatic cancer therapy. Based on CCLE database, there were mostly negative correlations (75%, 16/24 cases) between ITGα3 expression and various anti-cancer drugs. Interestingly, clinically approved drugs for human pancreatic cancer such as paclitaxel, irinotecan, and erlotinib27 all showed negative correlations with ITGα3 expression, indicating that ITGα3 might be potentially involved in chemo-resistance of pancreatic cancer. However, substantial correlations of ITGα3 expression and various anti-cancer drugs should be verified.
Altered levels of EGFR play an important role in the growth, invasion, adhesion, and angiogenesis of numerous cancers. It has been reported that the EGFR signalling induces tumour growth, invasion, adhesion, and angiogenesis12. Consistent with previous reports, the results of the present study suggest that EGFR signalling was regulated by ITGα3 expression in silico and in vitro for the first time. It is noteworthy that the protein level of EGFR, but not the mRNA level, is regulated by silencing ITGα3 expression, indicating that ITGα3 affects EGFR post-transcriptional processes.
Natural regulator of negative-feedback regulation in de novo EGFR expression, LRIG1 is a cell-surface transmembrane protein, which contains a leucine-rich repeat (LRR) domain and interacts with EGFR under ligand-independent conditions28,29. The natural structure of LRIG1 is sufficient for direct binding to the extracellular region of EGFR. Therefore, the intracellular domain of EGFR is dispensable for the formation of EGFR-LRIG1 complex and the LRIG1 is capable of directing ubiquitylation and degradation of EGFR28. Notably, ITGα3 silencing led to a reduction in EGFR expression levels via induction of functional LRIG1 expression. Based on our in-silico results correlating IFIs and ITGα3, the LRIG1 expression was enhanced by blockade of ITGα3 expression, implying that ITGα3 might act as “the sealing” for protection of EGFR against natural inhibitor, LRIG1. However, understanding these relationships will positively stimulate our cogitation to design an effective therapy for pancreatic cancer.
Notably, the in vivo anti-cancer effects of ITGα3 targeting were observed in the presence of an adequate number of each group (control: n = 6, sh-ITGα3: n = 12). Blockade of ITGα3 expression evoked anti-cancer effect by inhibiting EGFR signalling pathways. Selective down-regulation of EGFR expression was also demonstrated in sh-ITGα3 xenograft models compared with sh-control xenograft models. Therefore, the results of the present study showed that EGFR signalling can be controlled by ITGα3 expression through in vivo xenograft models.
A previous study reported the involvement of ITGα3 in clinical outcomes of colon and pancreatic cancers9,30. However, the clinical significance of ITGα3 in prognosis remains controversial. Miyamoto et al. suggested that poorly expressed ITGα3 is related to unfavourable prognosis31. In contrast, another study suggested that the diagnostic levels of integrin α3, β4, and β5 gene expression determine the prognosis of tongue squamous cell carcinoma22. Notably, our results strongly suggested that ITGα3 expression had clinical significance in pancreatic cancer. Low expression of ITGα3 significantly improved the prognosis of pancreatic cancer patients compared with high expression of ITGα3 according to independent PACA-AU, PAAD-US-TCGA, GSE79688, GSE62452, GSE57495, and GSE17891 datasets. Moreover, PACA-AU, PAAD-US-TCGA, and GSE79688 datasets were analyzed in further detail in different age groups. All the datasets showed that high ITGα3 expression significantly aggravated the prognosis of pancreatic cancer patients compared with low expression of ITGα3 according to age, although the significance was not considerable in older ages. To our knowledge, this is a reliable finding showing that ITGα3 expression has clinical significance in human pancreatic cancer.
Collectively, the results of the present study indicate that ITGα3 plays a pivotal role in human pancreatic malignancy based on in silico, in vitro, and in vivo-clinical evidence. Targeting ITGα3 might be a promising strategy to inhibit malignant pancreatic cancer by ablating the EGFR signalling pathway.
Material and Methods
Gene expression profile
Microarray analysis was performed from Gene Expression Omnibus (GEO) public microarray database at NCBI (https://www.ncbi.nlm.nih.gov/geo/). Histological type of samples were designated as normal pancreas or pancreatic tumour according to its annotation in GEO as described previously32. Correlations between ITGα3 mRNA expression and expression levels of EGFR were analysed in GEO public microarray database using GraphPad Prism version 5.01 for Windows (San Diego, CA, USA). Correlations between ITGα3 expression and various anti-cancer drugs were demonstrated using Cancer Cell Line Encyclopedia (CCLE, https://www.broadinstitute.org/ccle/) public database as described previously33.
Cell culture and reagents
Human pancreatic cancer cells, AsPC-1 (#21682), Capan-1 (#30079), Capan-2 (#30080), Miapaca-2 (#21420), Panc-1 (#21469), SNU-213 (#00213), and SNU-410 (#00410) were purchased at Korean Cell Line Bank (KCLB, Seoul, Korea). CFPAC-1 (CRL-1918) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). These cells were maintained as described previously32. H6c7 (ECA001) was obtained from Kerafast (Boston, MA, USA) and grown as described previously34. Monoclonal antibody for ITGα3 (sc-374242) was purchased at Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against epidermal growth factor receptor (EGFR, #4267), phospho-EGFR (Tyr1068, #3777), mitogen-activated protein kinase kinase 1/2 (MEK1/2, #4694), phospho-MEK1/2 (Ser217/221, #9154), protein kinase B (AKT, #9272), phospho-AKT (Ser473, #9271), extracellular signal-regulated kinase1/2 (ERK1/2, #9107), phospho-ERK1/2 (Thr202/204, #9106), leucine-rich repeats and immunoglobulin-like domain protein 1 (LRIG1, #12752), receptor-associated late transducer (RALT, #2440), caspase-3 (#9665), cleaved caspase-3 (#9664), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, #5174) were obtained from Cell Signaling Technology (Beverly, MA, USA). Recombinant EGF (#236-EG) was purchased at R&D Systems (Minneapolis, MN, USA).
Transfection with small interfering RNA (si-RNA)
Transfection of siRNAs was performed using Effectene reagent (Qiagen, Hilden, Germany) as described previously35. Oligonucleotides specific for ITGα3 (sc-35684 for #1 and SDH-1002 for #2) and scrambled control (sc-37007) were obtained from Santa Cruz Biotechnology and Bioneer (Daejeon, Korea), respectively. The effect of si-RNA transfection was validated by Western blotting of ITGα3 protein.
Phospho-RTK array
To demonstrate intracellular signalling by si-ITGα3 transfection in AsPC-1 cells, a phospho-RTK array kit (ARY001B, R&D Systems, Minneapolis, MN, USA) was used according to the manufacturer’s instructions.
Measurement of cell viability
To evaluate cell viability after si-ITGα3 transfection, WST-1 (#11644807001, Sigma-Aldrich, St. Louis, MO, USA) assay was performed as described previously36.
Trans-well migration assay
Migration assay was demonstrated using a Trans-well apparatus (#3422, Corning, Corning, NY, USA) as described previously33. Briefly, cells were transfected with scrambled si-RNA or si-ITGα3 for 72 h under normal cultured condition. Then, cells were applied to the upper-chamber containing RPMI without serum for 6 h, and cells that had migrated to the back side of the filter were stained. The eluted dye using 10% acetic acid was measured at 560 nm in an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Rad, Richmond, CA, USA).
Western blot analysis
To determine protein levels of ITGα3 in eight pancreatic cancers and H6c7 cells, Western blot analysis was demonstrated as described previously37. Bands were subjected to densitometry analysis using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
RNA preparation and quantitative real-time polymerase chain reaction
Total RNA extraction was performed using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and diverted to cDNA using a RNA PCR kit (Takara Bio Inc, Japan) as described previously38. Quantitative real-time PCR analysis was demonstrated using a StepOne Real-Time PCR system (Applied Biosystems) according to the manufacturers’ protocols. Primers sequences for quantitative real-time PCR for human ITGα3 (P205125V) and EGFR (P187403V) were obtained from Bioneer (Daejeon, Korea) Expression normalization was performed using GAPDH level as described previously39. Primer sequences for quantitative real-time PCR were as follows: GAPDH (forward primer, 5′-TCACTGGCATGGCCTTCCGTG-3′; reverse primer, 5′-GCCATGAGGTCCACCACCCTG-3′).
Generation of the ITGα3-specific short hairpin (sh)RNA stable AsPC-1 cell line
Plasmids specific for sh-ITGα3 and control shRNA (sc-35684-SH and sc-108060) were purchased at Santa Cruz Biotechnology to stably suppress ITGα3 expression using a sh-activated gene silencing vector system. Briefly, one day after transfection with shRNA constructs, AsPC-1 cells were grown in Dulbecco’s complete medium containing 5 µM puromycin for 3 days to select the stable transfectants.
Xenograft tumour model
Nude mice (BALB/c) were purchased at Orient (Seongnam, Korea) at 6–8 weeks of age. sh-control/AsPC-1 (1 × 107) and sh-ITGα3/AsPC-1 cells (1 × 107) were subcutaneously injected into the right flank as described previously40. Body weight was recorded about every 3 days. In vivo experiments were carried out in accordance with guidelines approved by the Animal Bioethics Committee (ABC) of Jeju National University (approval number: 2016-0049).
GSE data-set analysis
mRNA expression profiles was obtained from the Gene Expression Omnibus (GEO) public microarray data-base. We independently integrated data sets obtained from several groups using the absolute normalization method SCAN.UPC41. Then, we restricted the integration to Affymetrix Human Genome U133 Plus 2.0 Array platform (GPL570) because the normalization method is dependent on the total number of probes. All data were normalized by the default option of SCAN.UPC. Eight data sets were used: GSE9599, -15471, -16515, -17891, -32676, -39409, -42952, and -46385. Each probe was converted to EntrezID. Several probes for the same EntrezID were averaged. Quantile-quantile normalization was applied to all samples to remove batch effects. To test prognostic value of a gene, samples were divided into two groups using median gene expression level as threshold. Log-rank test was then performed using Graph Prism version 5.
Statistical analyses
All data are presented as mean ± standard deviation. Student’s t-test was used to determine significance level for comparison between two independent samples. Groups were compared by one-way analysis of variance (ANOVA) with Tukey’s post hoc test for significant main effects using SPSS 12.0 K for Windows (SPSS Inc., Chicago, IL, USA).
Supplementary information
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1A6A1A03012862) and (2017R1D1A1B03033899).
Author Contributions
J.W. Lee conceived and supervised the experiment. J.W. Lee and J. Lee designed and performed the experiments. J.W. Lee, J. Lee, C. Choi, and J.H. Kim analyzed the data. J.W. Lee wrote and proofread the manuscript.
Data Availability
Additional data are available as Supplementary information.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39628-x.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson, L. Pancreatic cancer: Promise of doublet chemotherapy. Nature reviews. Clinical oncology, 10.1038/nrclinonc.2016.138 (2016). [DOI] [PubMed]
- 3.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. The New England journal of medicine. 2014;371:2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 5.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annual review of pharmacology and toxicology. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 6.Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. The Journal of clinical investigation. 2002;109:999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grzesiak JJ, et al. Knockdown of the beta(1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis. International journal of cancer. 2011;129:2905–2915. doi: 10.1002/ijc.25942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim W, Lee J, Choi C. Robust method for identification of prognostic gene signatures from gene expression profiles. Scientific reports. 2017;7:16926. doi: 10.1038/s41598-017-17213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu GH, et al. Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma. Digestive diseases and sciences. 2011;56:1090–1098. doi: 10.1007/s10620-010-1416-x. [DOI] [PubMed] [Google Scholar]
- 10.Ray M, Salgia R, Vokes EE. The role of EGFR inhibition in the treatment of non-small cell lung cancer. The oncologist. 2009;14:1116–1130. doi: 10.1634/theoncologist.2009-0054. [DOI] [PubMed] [Google Scholar]
- 11.Cruz JJ, Ocana A, Del Barco E, Pandiella A. Targeting receptor tyrosine kinases and their signal transduction routes in head and neck cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2007;18:421–430. doi: 10.1093/annonc/mdl175. [DOI] [PubMed] [Google Scholar]
- 12.Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nature reviews. Molecular cell biology. 2011;12:104–117. doi: 10.1038/nrm3048. [DOI] [PubMed] [Google Scholar]
- 13.Killock D. Lung cancer: a new generation of EGFR inhibition. Nature reviews. Clinical oncology. 2015;12:373. doi: 10.1038/nrclinonc.2015.93. [DOI] [PubMed] [Google Scholar]
- 14.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nature reviews. Molecular cell biology. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 15.Segatto O, Anastasi S, Alema S. Regulation of epidermal growth factor receptor signalling by inducible feedback inhibitors. Journal of cell science. 2011;124:1785–1793. doi: 10.1242/jcs.083303. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson SE, et al. Suppressor of cytokine signaling (SOCS)-5 is a potential negative regulator of epidermal growth factor signaling. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2328–2333. doi: 10.1073/pnas.0409675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Molecular cancer. 2003;2:14. doi: 10.1186/1476-4598-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleeff J, et al. Pancreatic cancer. Nature reviews. Disease primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 19.Sidaway P. Pancreatic cancer: New biomarkers improve standard screening. Nature reviews. Clinical oncology. 2017;14:262. doi: 10.1038/nrclinonc.2017.35. [DOI] [PubMed] [Google Scholar]
- 20.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nature reviews. Molecular cell biology. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature reviews. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurokawa A, et al. Diagnostic value of integrinalpha3, beta4, and beta5 gene expression levels for the clinical outcome of tongue squamous cell carcinoma. Cancer. 2008;112:1272–1281. doi: 10.1002/cncr.23295. [DOI] [PubMed] [Google Scholar]
- 23.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. The Journal of biological chemistry. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 24.Makarov A, Ylivinkka I, Nyman TA, Hyytiainen M, Keski-Oja J. Ephrin-As, Eph receptors and integrin alpha3 interact and colocalise at membrane protrusions of U251MG glioblastoma cells. Cell biology international. 2013;37:1080–1088. doi: 10.1002/cbin.10134. [DOI] [PubMed] [Google Scholar]
- 25.Shirakihara T, et al. Identification of integrin alpha3 as a molecular marker of cells undergoing epithelial-mesenchymal transition and of cancer cells with aggressive phenotypes. Cancer science. 2013;104:1189–1197. doi: 10.1111/cas.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 27.Werner J, et al. Advanced-stage pancreatic cancer: therapy options. Nature reviews. Clinical oncology. 2013;10:323–333. doi: 10.1038/nrclinonc.2013.66. [DOI] [PubMed] [Google Scholar]
- 28.Gur G, et al. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. The EMBO journal. 2004;23:3270–3281. doi: 10.1038/sj.emboj.7600342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laederich MB, et al. The leucine-rich repeat protein LRIG1 is a negative regulator of ErbB family receptor tyrosine kinases. The Journal of biological chemistry. 2004;279:47050–47056. doi: 10.1074/jbc.M409703200. [DOI] [PubMed] [Google Scholar]
- 30.Hashida H, et al. Integrin alpha3 expression as a prognostic factor in colon cancer: association with MRP-1/CD9 and KAI1/CD82. International journal of cancer. 2002;97:518–525. doi: 10.1002/ijc.1625. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto S, et al. Loss of motility-related protein 1 (MRP1/CD9) and integrin alpha3 expression in endometrial cancers. Cancer. 2001;92:542–548. doi: 10.1002/1097-0142(20010801)92:3<542::AID-CNCR1353>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Lee J, Kim SJ, Kim JH. Quercetin-3-O-glucoside suppresses pancreatic cancer cell migration induced by tumor-deteriorated growth factors in vitro. Oncology reports. 2016;35:2473–2479. doi: 10.3892/or.2016.4598. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Han SI, Yun JH, Kim JH. Quercetin 3-O-glucoside suppresses epidermal growth factor-induced migration by inhibiting EGFR signaling in pancreatic cancer cells. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:9385–9393. doi: 10.1007/s13277-015-3682-x. [DOI] [PubMed] [Google Scholar]
- 34.Radulovich N, Qian JY, Tsao MS. Human pancreatic duct epithelial cell model for KRAS transformation. Methods in enzymology. 2008;439:1–13. doi: 10.1016/S0076-6879(07)00401-6. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, et al. Autocrine DUSP28 signaling mediates pancreatic cancer malignancy via regulation of PDGF-A. Scientific reports. 2017;7:12760. doi: 10.1038/s41598-017-13023-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Lee J, Kim M, Kim JH. Dietary approach to attenuate human pancreatic cancer growth and migration with innoxiousness. Journal of Functional Foods. 2017;30:303–312. doi: 10.1016/j.jff.2016.12.032. [DOI] [Google Scholar]
- 37.Lee J, Lee J, Yu H, Choi K, Choi C. Differential dependency of human cancer cells on vascular endothelial growth factor-mediated autocrine growth and survival. Cancer letters. 2011;309:145–150. doi: 10.1016/j.canlet.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Lee J, Yun JH, Jeong DG, Kim JH. DUSP28 links regulation of Mucin 5B and Mucin 16 to migration and survival of AsPC-1 human pancreatic cancer cells. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:12193–12202. doi: 10.1007/s13277-016-5079-x. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Hun Yun J, Lee J, Choi C, Hoon Kim J. Blockade of dual-specificity phosphatase 28 decreases chemo-resistance and migration in human pancreatic cancer cells. Scientific reports. 2015;5:12296. doi: 10.1038/srep12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J, et al. Blockade of VEGF-A suppresses tumor growth via inhibition of autocrine signaling through FAK and AKT. Cancer letters. 2012;318:221–225. doi: 10.1016/j.canlet.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Piccolo SR, Withers MR, Francis OE, Bild AH, Johnson WE. Multiplatform single-sample estimates of transcriptional activation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17778–17783. doi: 10.1073/pnas.1305823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data are available as Supplementary information.