Abstract
Water shortage is a major environmental stress that causes the generation of reactive oxygen species (ROS). The increase in ROS production induces molecular responses, which are key factors in determining the level of plant tolerance to stresses, including drought. The aim of this study was to determine the expression levels of genes encoding MAPKs (MAPK3 and MAPK6), antioxidant enzymes (CAT, APX and GPX) and enzymes involved in proline biosynthesis (P5CS and P5CR) in Triticum aestivum L. seedlings in response to short-term drought conditions. A series of wheat intervarietal substitution lines (ISCSLs) obtained by the substitution of single chromosomes from a drought-sensitive cultivar into the genetic background of a drought-tolerant cultivar was used. This source material allowed the chromosomal localization of the genetic elements involved in the response to the analyzed stress factor (drought). The results indicated that the initial plant response to drought stress resulted notably in changes in the expression of MAPK6 and CAT and both the P5CS and P5CR genes. Our results showed that the substitution of chromosomes 3B, 5A, 7B and 7D had the greatest impact on the expression level of all tested genes, which indicates that they contain genetic elements that have a significant function in controlling tolerance to water deficits in the wheat genome.
Introduction
Cereal crops are the basis of agricultural production in most countries. Triticum aestivum L., as one of the most commonly cultivated cereals in the world (next to rice and maize), is particularly important1. Currently, wheat cultivation covers 220 million ha2. According to the FAO, 680 million tons of wheat are produced annually3.
Water deficits represent a major global abiotic stress that limit plant productivity by inhibiting plant growth and development. Drought induces ROS overproduction and leads to the disruption of membrane integrity and osmotic balance in plant cells. The consequence of these changes is a reduction in crop quality and quantity, which causes crop yield losses4–6.
The level of ROS significantly increases under drought conditions. In plants, the enzymatic antioxidant system, which involves many enzymes, such as superoxide dismutase (SOD), catalase (CAT), and peroxidases (POX) including ascorbate peroxidase (APX), glutathione peroxidase (GP) and guaiacol peroxidase (GPX), protects cells against toxic ROS7–9. However, many studies indicate that CAT, APX and GPX play the most important roles in ROS scavenging7,8,10–14. It has been shown that the expression patterns of APX, CAT and SOD in barley under drought conditions depend on the plant development stage and genotype15. The results of previous studies indicate that genes encoding antioxidant enzymes are frequently important in developing plants with enhanced drought tolerance. Increased tolerance to drought and salt stress has been observed in Nicotiana tabacum that overexpress the APX gene16. In transgenic rice, OsMT1 gene overexpression indicates a higher level of CAT and APX activity and causes an increase in drought resistance17. Increased tolerance to abiotic stress factors (including drought) was found in an Arabidopsis thaliana GP overexpression line18.
The accumulation of osmolytes such as proline is another plant defense mechanism against stress conditions. Proline is synthesized in a two-step process catalyzed by ∆1-pyrroline-5-carboxylate synthetase (P5CS), followed by the reduction of P5C to proline by ∆1-pyrroline-5-carboxylate reductase (P5CR)19. Proline protective mechanisms during osmotic stress have been proposed to involve the stabilization of proteins and antioxidant enzymes, direct scavenging of ROS, balance of intracellular redox homeostasis, and cellular signaling promotion20. High P5CS expression was observed in rice treated with H2O221. The upregulation of both P5CS and P5CR was found in Brassica napus under salt and ABA treatment conditions22. It has also been shown that the overexpression of the P5CS gene leads to an increase in proline accumulation and enhanced stress tolerance in tobacco23,24 and wheat25.
However, ROS are also involved in signal transduction in response to stress conditions10,26,27, and MAPK cascades are major signaling pathways28. This system consists of kinases (MAPK, MAP Kinases), kinase kinases (MAPKK, MAP Kinase Kinases) and kinase kinase kinases (MAPKKK, MAP Kinase Kinase Kinases)28,29. In the Arabidopsis thaliana genome, 80 MAPKKKs, 10 MAPKKs and 20 MAPKs have been identified28,30–32. The function of wheat MAPKs is under intensive study;33–37 however, there is still little data available. MAPK3 and MAPK6 are the best-described plant MAP kinases32, and their function is well established in many species. Most data on MAP kinases are based on the analysis of transcript levels. In wheat, changes in the expression patterns of TaMAPK3 and TaMAPK6 genes were found under drought conditions and salinity stress as well as during phosphor and nitrogen deficiency35 and Mycosphaerella graminicola infection33. Studies on rice indicate that OsMAPK6 is involved in signal transduction during Magnaporthe grisea attack38, while OsMAPK3 is involved in the response to cold stress39. There are several studies in maize concerning the activation of ZmMAPK3 under cold, drought, salinity, the presence of heavy metal ions, UV and wounding40. The induction of AtMAPK3 and AtMAPK6 genes was observed in A. thaliana that was exposed to excess cadmium and copper41.
Many studies have focused on the development of varieties with increased drought resistance due to the importance of wheat in global cereal production and the worldwide problem of water deficits. The main purpose of such strategies is commonly the identification of genomic regions involved in the stress response42,43, although there is still no data concerning the initial plant reaction to water shortage. However, previous studies conducted with transcriptional or proteomic profiling indicate that the short-term plant response to stress factors can play important role in regulating gene expression and physiological responses, e.g., during hormone treatment44 or salt stress45. Yang et al.44. observed that transcriptional changes caused by short ABA treatment were much stronger than 9 days treatment and promote expression of genes encoding ethylene and JA signaling components.
The research carried out so far has shown that drought occurring during the early developmental stage determines the induction of signaling pathways as well as modification of plant growth and metabolic profile crucial for acclimation46,47. Moreover, the research carried out so far has shown that there is a correlation between traits related to drought response and increased yield for wheat48. It has been also shown, that drought stress occurs during the seedling stage can influence grain production49,50 and quality parameters51.
The main objective of this study was to examine wheat responses to short-term drought, as measured by the expression level of genes involved in signal transduction (MAPK3 and MAPK6), the activity of the antioxidant system and the proline biosynthesis in common wheat seedlings. Because of the fact that the majority of analyzed genes is encoded by more than one gene, localized on different chromosomes, we decided to focus on holistic analysis based on consensus coding sequences. Furthermore, using intervarietal substitution lines (ISCSLs), our purpose was to describe genome regions involved to the highest extent in response to drought including both: genes expression and its regulation.
Results
Expression of genes encoding enzymes involved in ROS signaling (MAPK3 and MAPK6)
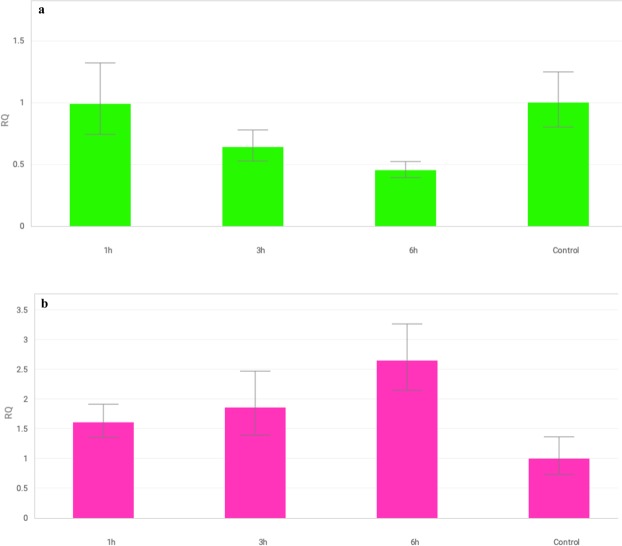
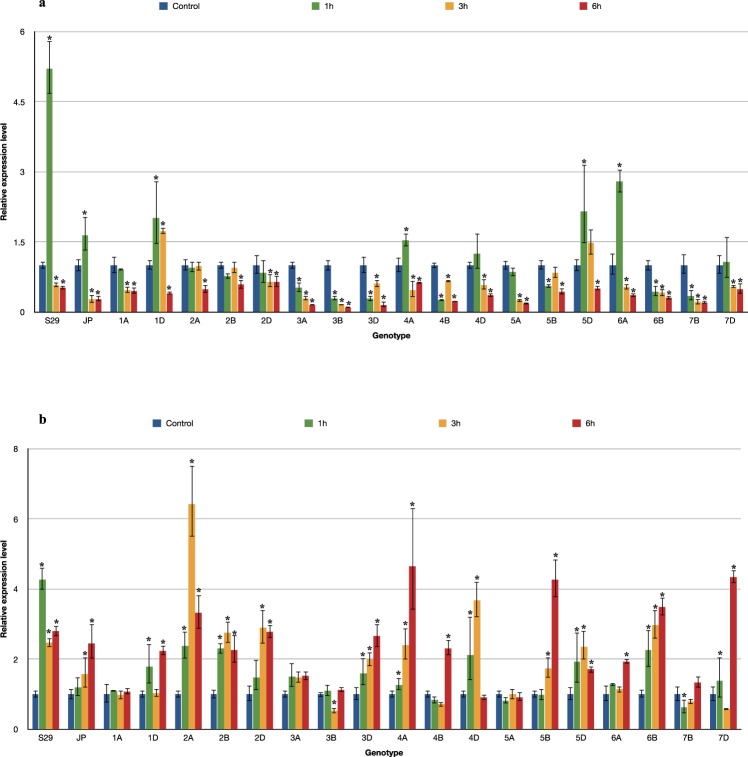
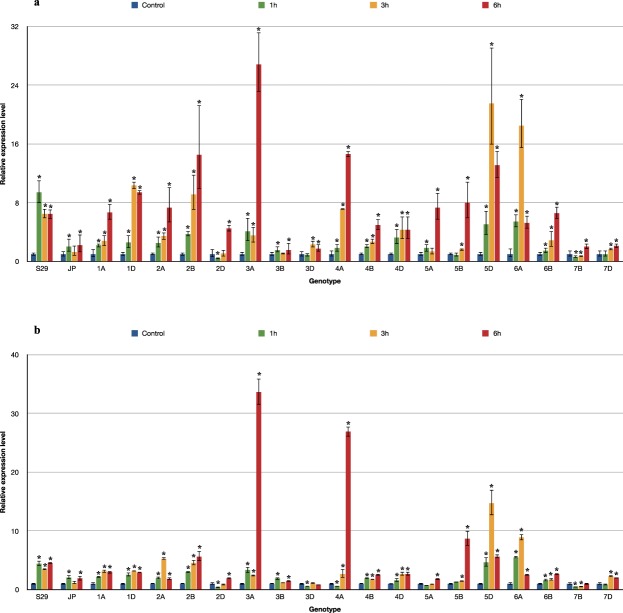
The MAPK3 and MAPK6 genes showed two different patterns of expression in the tested T. aestivum L. seedlings exposed to drought (Fig. 1a,b). TaMAPK3 transcript levels significantly decreased after 6 hours of exposure in most of the tested lines. However, different expression profiles were observed after 1 and 3 hours of stress for individual lines. Lines containing chromosomes of homoeologous groups 3 (3A, 3B, 3D), 4B, 6B and 7B showed a rapid response and immediate reduction in MAPK3 expression after 1 hour of PEG treatment. The downregulation of MAPK3 was noticed at subsequent time points (after 3 and 6 hours). Only 3D and 4B showed a transient increase in MAPK3 expression after 3 hours. A significant induction of MAPK3 was detected in the first hours of stress for the drought-tolerant cultivar S29 and lines 1D, 4A, 5D, 6A, followed by a rapid decrease in transcript levels. For the remaining forms, no MAPK3 induction was observed in the first hours of plant exposure to drought, and then a significant decrease occurred. The biggest differences in the MAPK3 expression pattern compared to the recipient were detected for lines 3A, 3B, 6B and 7B. The drought-sensitive cultivar JP showed a decrease in the transcript level after 3 hours. A similar expression pattern was recorded for 1A, 5A and 7D. MAPK3 expression in lines 4D, 5B and 5D decreased only after 6 hours of water shortage. The results of the study indicated the weakest changes in MAPK3 expression for lines containing chromosomes of homoeologous group 2 (2A, 2B, 2D) under the conditions tested (Fig. 2a).
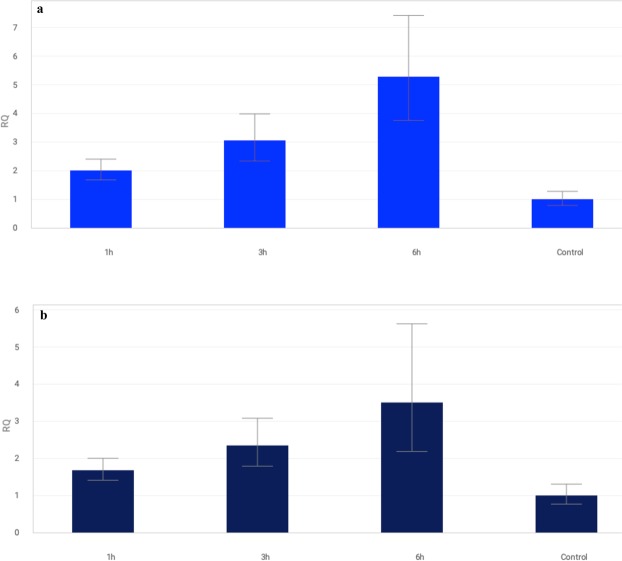
Figure 1.
The pattern of MAPK3 (a) and MAPK6 (b) genes expression alteration in S29(JP) substitution lines after 1, 3 and 6 h of 10% PEG treatment. The average values obtained for all tested genotypes in particular time point are presented in comparison to non-exposed plants (Control). Bars represent standard deviation. All samples were analyzed in three full biological and three technical replications.
Figure 2.
Changes in expression of MAPK3 (a) and MAPK6 (b) genes in S29(JP) substitution lines after 1, 3 and 6 h of 10% PEG treatment. Bars represent standard deviation. The expression level for non-exposed plants was used as calibrator (relative expression level = 1), *change significant at the 0.05 level. All samples were analyzed in three full biological and three technical replications.
Most ISCSLs showed an increase in the transcript level of MAPK6 after 6 hours. However, different expression patterns were observed during stress treatment for individual lines. Rapid MAPK6 induction already occurred after 1 hour in the drought-tolerant cultivar S29. Similar expression profiles were recorded for lines involving chromosomes of homoeologous group 2 (2A, 2B, 2D), 4D, 6B, 3D, 4A and 5D. A significant increase in MAPK6 transcript levels was detected for those lines after 3 hours of 10% PEG exposure. Lines 3A, 3B, 5A, 6A and 7B showed the most significant changes in MAPK6 expression compared to the recipient. The drought-sensitive cultivar JP exhibited a delayed response, and MAPK6 expression was elevated significantly only after 3 and 6 hours. A similar delayed induction of MAPK6 was noted for lines 4B, 5B and 7D. Most lines with the substitution of A genome chromosomes (1A, 3A, 5A) showed no significant changes in MAPK6 expression throughout the 6 hours of stress (Fig. 2b).
Expression of genes encoding antioxidant enzymes (CAT, APX and GPX)
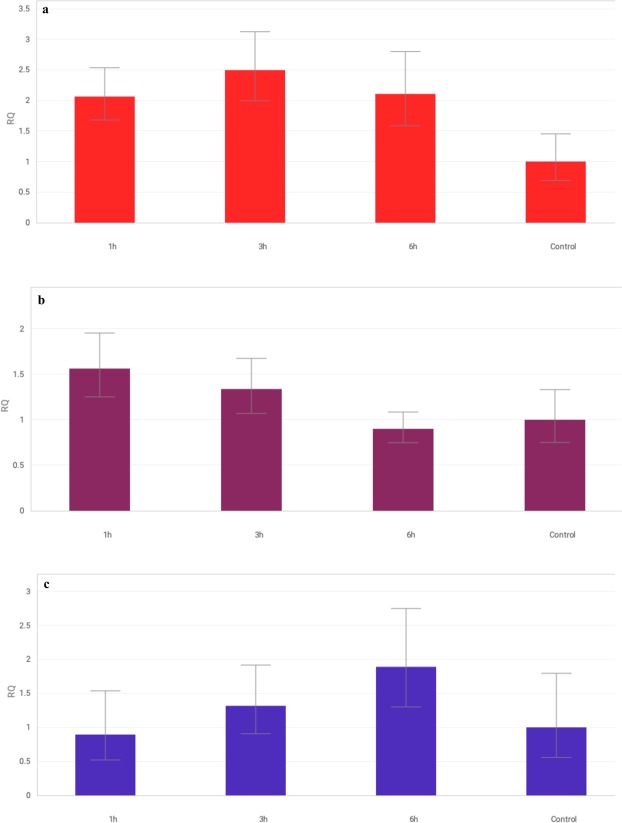
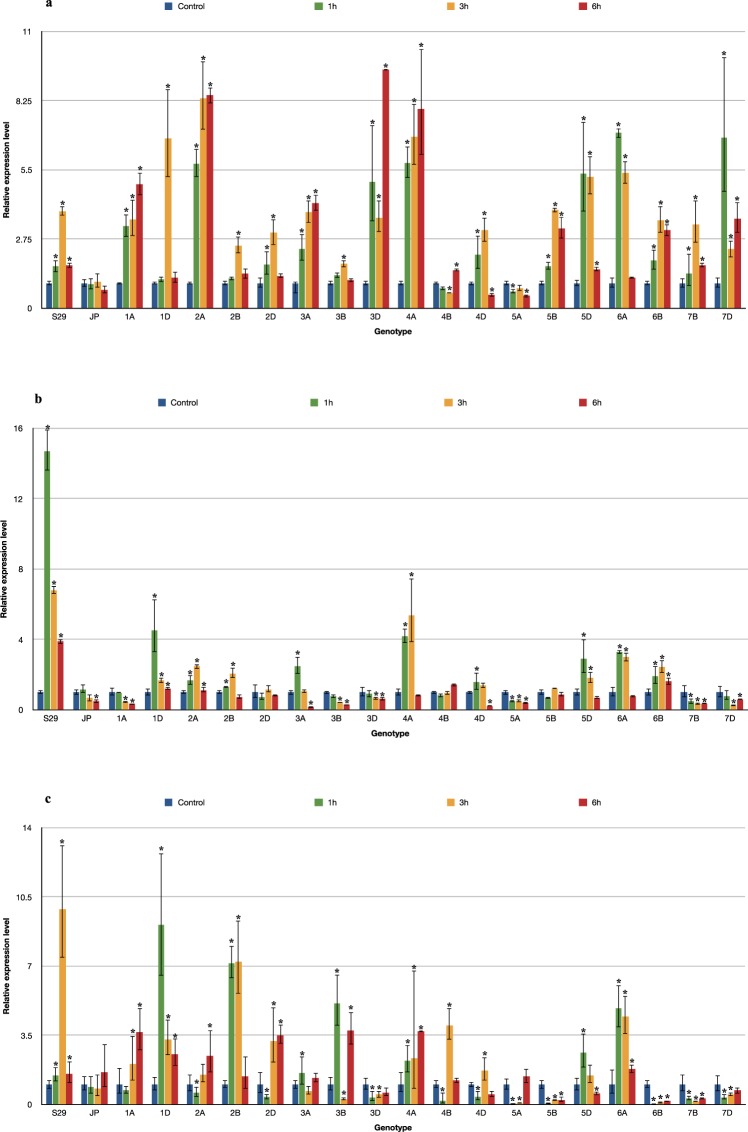
The overall pattern of CAT expression was a significant increase in transcript levels after 1 hour, which remained stable after 3 and 6 hours of stress (Fig. 3a). This expression trend was observed for lines with the substitution of A genome chromosomes (1A, 2A, 3A, 4A) and 3D, 5B and 6B forms. However, S29, ISCSLs with substitution of chromosomes from groups 2 (2B, 2D), 4 (4A, 4D), 7 (7B, 7D) and lines 1D, 5D and 6A initially showed an increase in CAT expression, followed by a decrease after 6 hours of drought. This expression pattern was characteristic for lines with the substitution of D genome chromosomes. The most significant alterations in CAT gene expression compared to the recipient (S29) were observed for lines 3B, 4B and 5A, that response was similar to the drought-sensitive cultivar JP (Fig. 4a).
Figure 3.
The pattern of CAT (a), APX (b), and GPX (c) genes expression alteration in S29(JP) substitution lines after 1, 3 and 6 h of 10% PEG treatment. The average values obtained for all tested genotypes in particular time point are presented in comparison to non-exposed plants (Control). Bars represent standard deviation. All samples were analyzed in three full biological and three technical replications.
Figure 4.
Changes in expression of CAT (a), APX (b), and GPX (c) genes in S29(JP) substitution lines after 1, 3 and 6 h of 10% PEG treatment. Bars represent standard deviation. The expression level for non-exposed plants was used as calibrator (relative expression level = 1), *change significant at the 0.05 level. All samples were analyzed in three full biological and three technical replications.
The pattern for APX gene expression was characterized by an initial increase, followed by a decrease (Fig. 3b). However, elevated transcript levels were only observed for S29, 1D, 2B, 5D, 6B and lines with the substitution of A genome chromosomes (2A, 3A, 4A, 6A). Other lines showed lower transcript levels after 1 hour (1A, 4D), 3 hours (3B, 7D) or 6 hours (JP, 3A) exposure to stress. Lines with chromosomes belonging to homoeologous groups 3 (3B, 3D), 5 (5A, 5B), 7 (7B, 7D) and lines 1A, 2D, 4B showed the greatest changes in APX expression in response to drought stress compared to the recipient (S29). No significant changes in APX expression were observed for 2D, 3D, 4B or 5B under the conditions tested (Fig. 4b).
The analysis of the GPX gene indicated different expression patterns in the tested plants (Fig. 3c). S29 showed a significant increase in expression after 3 hours, followed by a decrease during prolonged drought (6 hours). A similar trend was observed for lines 1D, 2B, 4B, 5D and 6A. The substitution of chromosomes 3A, 3D, 5A, 5B, 6B, 7B and 7D had the greatest impact on GPX expression compared to the recipient. Continuous upregulation of GPX expression was recorded in lines with the substitution of A genome chromosomes (1A, 2A, 4A). Some of the tested lines exhibited a significant decrease in GPX expression during 10% PEG treatment: lines containing chromosomes of homoeologous groups 5 (5A, 5B) and 7 (7B, 7D) and line 6B. The transient downregulation of the GPX gene occurred in the first hours, followed by an increase in the transcript level for the majority of lines with the substitution of D genome chromosomes (2D, 3D, 4D, 7D). No significant changes of GPX expression were observed for the drought-sensitive cultivar JP (Fig. 4c).
Expression of genes involved in proline biosynthesis (P5CS and P5CR)
The results of the study indicated a significant increase in the expression of both genes: P5CS and P5CR. However, elevated transcript levels of the P5CS gene were observed immediately after 1 hour of stress, while a delayed induction (after 3 hours) was noticed for P5CR (Fig. 5a,b).
Figure 5.
The pattern of P5CS (a) and P5CR (b) genes expression alteration in S29(JP) substitution lines after 1, 3 and 6 h of 10% PEG treatment. The average values obtained for all tested genotypes in particular time point are presented in comparison to non-exposed plants (Control). Bars represent standard deviation. All samples were analyzed in three full biological and three technical replications.
The exposure of plants to drought resulted in a rapid response and increase in P5CS expression in most of the tested lines. S29, JP, 1D, 2B, 4B, 4D, 5D and lines with the substitution of A genome chromosomes (1A, 2A, 3A, 6A) already showed P5CS upregulation after 1 hour of stress. Moreover, the level of transcript for the drought-tolerant cultivar was significantly higher than that for the drought-sensitive one at each time point. A delayed response was observed for lines 3D, 4A and 6B (after 3 hours) and for 2D, 5A, 5B, 7B and 7D (after 6 hours of stress). Therefore, the results of the analysis showed that lines containing chromosomes from homoeologous groups 1, 2 and 4 exhibited a fast response to drought and continuous upregulation of P5CS, while a delayed reaction to stress was observed in lines containing chromosomes from homoeologous groups 5 and 7. Line 3B did not show any changes in P5CS gene expression. The transient downregulation of the P5CS gene was found in lines 2D and 7B after 1 hour (Fig. 6a).
Figure 6.
Changes in expression of P5CS (a) and P5CR (b) genes in S29(JP) substitution lines after 1, 3 and 6 h of 10% PEG treatment. Bars represent standard deviation. The expression level for non-exposed plants was used as calibrator (relative expression level = 1), *change significant at the 0.05 level. All samples were analyzed in three full biological and three technical replications.
The results showed similar trends in P5CR and P5CS regulation for the majority of the tested lines. A significant increase in P5CR expression was detected in S29 and JP after 1 hour of stress however, the upregulation of P5CR was higher in the drought-tolerant cultivar S29 than that in drought-sensitive JP. Similar observations were made for lines 1D, 2A, 2B, 4B, 5D and 6A. A delayed response to water stress, manifested as P5CR gene upregulation, was found in lines 4A, 4D, 7D (after 3 hours) and 2D, 5B, 6B (after 6 hours). A significant decrease in the P5CR transcript level was observed for 2D, 3D, 4A and 7B after 1 hour of drought. In comparison to the recipient (S29), lines 3B, 3D, 5A, 7B and 7D showed the highest changes in the P5CS and P5CR expression profile in response to drought (Fig. 6b).
Discussion
Plant MAPK cascades have been intensively studied as a mechanism involved in the regulation of stress response. Many studies have indicated the important roles of MAPK3 and MAPK6 in plant cells33,41,52,53. However, the information available on MAPKs in T. aestivum is still limited33,35–37.
This study tested genes encoding two MAPKs (MAPK3 and MAPK6), three antioxidant enzymes (CAT, APX and GPX) and two enzymes involved in proline biosynthesis (P5CS and P5CR). According to our data, the main pattern for the MAPK6 gene was increased expression in drought-treated plants. MAPK6 gene induction during water deficiency has also been reported in wheat35 and A. thaliana32. However, some of the tested lines did not show any changes in MAPK6 expression (1A, 3A, 5A, 6A, 3B and 7B), which had also been demonstrated in wheat cultivar Chinese Spring using RNA-seq36. The downregulation of MAPK3 gene expression was observed in all tested lines. The results suggest that kinase encoded by this gene may also function in signal transduction but as negative regulator A decrease in MAPK3 expression was described for wheat under phosphorus deprivation35 and for Cucumis sativus L. under drought conditions54. However, our analysis of the MAPK3 gene indicated different expression patterns for each line during 6 hours of PEG treatment. Based on previous data, we suggest that there is no unequivocal evidence for a specific MAPK3 gene expression pattern under drought. Wen et al.35. reported no significant changes in MAPK3 expression in wheat, while Zhan et al.36. observed an increase in the TaMAPK3 transcript level. MAPK3 expression upregulation was also described in A. thaliana55,56. Wang et al.40. reported a high level of ZmMAPK3 expression after 1 hour of 10% PEG treatment and decreased expression after 6 hours. These authors suggest that there are common mechanisms underlying the abiotic stress signaling and convergent points that include the production of H2O2 generated by stress stimuli and the reliance on common signaling cascades. The versatile system also allows linking the H2O2 signal to the MAPK cascade and target genes. Identifying all cascade modules and broadening our knowledge about the regulation of all molecular mechanisms under stress conditions will provide further insight into the biological response of plants. Different profiles of MAPK3 and MAPK6 expression obtained in this study suggest that these kinases are regulated differently. Obtained results showed that MAPK6 overexpression is one of the components of plant response to drought, whereas MAPK3 do not play a role as a positive signal transduction regulator during this kind of stress. Moreover, a reverse reaction of MAPK3 versus MAPK6 expression in experimental conditions can indicate on the regulation mechanism based on feedback between these two kinases. Analysis based on ISCSLs revealed that chromosomes 3A, 3B, 6B, and 7B as well as 3A, 3B, 5A, 6A, and 7B contained genes involved in MAPK3 and MAPK6 expression regulation, respectively (Table 1). These results could provide a premise that chromosomes 3A, 3B and 7B contain genetic elements responsible for controlling of both analyzed kinases.
Table 1.
Chromosomes involved to the highest extent in regulation of analyzed genes expression according to results obtained on the basis of examined ISCSLs set.
| Gene | Chromosome |
|---|---|
| MAPK3 | 3A, 3B, 6B, 7B |
| MAPK6 | 3A, 3B, 5A, 6A, 7B |
| CAT | 3B, 4B, 5A |
| APX | 1A, 2D, 3B, 3D, 4B, 5A, 5B, 7B, 7D |
| GPX | 3A, 5A, 5B, 6B, 7B, 7D |
| P5CS | 3B, 3D, 5A, 7B, 7D |
| P5CR | 3B, 3D, 5A, 7B, 7D |
Water deficiency leads to ROS formation and the induction of genes that encode antioxidant enzymes. The involvement of those genes in promoting plant responses to unfavorable conditions has been well established in many studies57–59. It has been reported that the overexpression of genes encoding antioxidant enzymes causes higher tolerance to stress factors in A. thaliana60 and rice16,61. However, some data indicate that retaining stable gene expression can also confer drought tolerance in plants62,63.
In this study, the significant increase in CAT expression was observed in the majority of tested lines. CAT gene upregulation was also observed in wheat subjected to mild drought for 7 hours64, in Macrotyloma uniflorum after 78 hours65 and in barley after 2 days of drought66. High levels of CAT expression were observed in Cleome gynandra and Cleome spinosa during prolonged drought exposure (10 days)67. However, in this study, some of the tested lines showed a significant decrease in CAT expression after 6 hours of water stress. Previous studies have revealed variable responses to different durations and severities of drought stress in various plant species. A lower CAT transcript level was observed in wheat after 10 days68 or in barley after 9 and 16 days of stress66. Our results suggest that short-term drought increased CAT expression as a rapid plant response. However, prolonged exposure to drought could lead to the inhibition of expression and the reduction in transcript levels. PEG (10%) did not alter CAT expression in JP and lines 3B, 4B, and 5A, what can indicate the presence of encoding or regulatory elements on these chromosomes (Table 1). Similar observations were reported for Poa pratensis57,62 and Koeleria macrantha53. Moreover, in this work, we report similar expression patterns for MAPK6 and CAT genes. Xing et al.69. suggested that MAPK6, as a component of a cascade involved in signal transduction, mediates H2O2 formation and CAT expression changes. Our results can support this hypothesis and indicate, that both enzymes are involved in the complex immediate reaction to osmotic stress in wheat.
Our analysis of the genes encoding two peroxidases showed that no constitutive expression pattern can be defined for all analyzed lines (especially for GPX). One of the potential factors responsible for this situation is fact that both of these enzymes are encoded by numerous sequences localized in different parts of the wheat genome. The trend of response to examined stress revealed enhancement of both genes expression, however, the time shift between them was observed. For APX the quick response was noticed, whereas for GPX a slower building up of the response was shown.
APX gene showed its expression downregulation in lines 3B, 5A, 7B and 7D. A reduction in APX transcript level was reported previously for Arachis hypogaea L70. and in roots of P. pratensis under drought treatment conditions62. No significant changes were found in the leaves of P. pratensis, which is consistent with our results obtained for lines 2D, 3D, 4B and 5B. The upregulation of the APX gene was observed in lines with the substitution of A genome chromosomes (2A, 3A, 4A, 6A), lines 2B, 5D, 6B and the drought-tolerant cultivar S29. Many previous studies have pointed to an increase in APX expression in different plant species under drought conditions, including Pisum sativum L.71, K. macrantha63, P. pratensis57 and Hordeum vulgare L.66. We observed a higher APX expression level in S29 compared than that in JP. This outcome has also been reported in drought-tolerant and -sensitive barley under water shortage conditions66. Some data indicate that APX gene overexpression enhances tolerance to drought in transgenic rice53 and tobacco58.
Our results of GPX expression analysis are consistent with those of previous studies performed on K. macrantha63 or P. pratensis57,62. GPX upregulation was detected in some of the tested lines. The induction of the GPX gene can be the result of higher H2O2 levels in plant cells or reduced enzyme activities72. An increase in GPX expression was also recorded for K. macrantha63, a drought-tolerant cultivar of T. aestivum68, and in wheat roots72. Our study showed a significantly elevated level of GPX expression in S29. This phenomenon has been previously reported for other tolerant T. aestivym cultivars after 10 days of drought68.
The results of our research based on ISCSLs suggest that genetic elements encoding and/or regulating the genetic activity of ascorbate peroxidase and guaiacol peroxidase may be present notably on chromosomes belonging to homoeologous groups 3, 5, and 7 (Table 1).
The current results for antioxidative system have demonstrated that catalase and ascorbate peroxidase, which can efficiently scavenge H2O2 and prevent its accumulation to toxic levels, are major antioxidant enzymes. However, these enzymes have different affinities for H2O2 and play different roles in scavenging. CAT does not need a reductant to scavenge H2O2, making it reducing power-free, whereas APX needs a reductant (ascorbate)62,66,73. According to our results, the CAT expression pattern was similar to APX in most of the tested lines (S29, JP, 1D, 2A, 2B, 3A, 4A, 4B, 5A, 5D, 6A, 6B). Transcript levels significantly increased in these forms after 1, 3 and/or 6 hours of stress. The results of the analysis indicated that this expression pattern was characteristic for lines with the substitution of A genome chromosomes and lines containing chromosomes of homoeologous groups 2, 4, 5 and 6. Similar regulation of CAT and APX expression could be explained by the fact that proteins encoded by these genes are both involved in the scavenging of H2O2 produced during oxidative stress, and their gene expression is likely regulated in the same way. Bian and Jiang62 suggested that CAT and APX may facilitate efficient H2O2 scavenging in leaf cells, although CAT and APX have different affinities for H2O2. No relationship was found between the expression of these two genes in the remaining lines. Different expression patterns have been described for tobacco plants exposed to drought, where an increase in Cat3 and a decrease in strAPX expression occur74.
Proline is synthesized in a two-step process catalyzed by ∆1-pyrroline-5-carboxylate synthetase (P5CS), followed by the reduction of P5C to proline by ∆1-pyrroline-5-carboxylate reductase (P5CR)19. Both genes showed a significant increase in expression levels in the tested wheat substitution lines with 10% PEG treatment. P5CS and P5CR upregulation was also found in K. macrantha under water stress conditions63. Previous results show that increased expression of these genes may control plant responses and contribute to a higher level of RWC and proline accumulation or lead to a decrease in MDA content. The overexpression of genes involved in proline synthesis enhances drought tolerance in transgenic Glycine max75, Petunia hybrida76, Cicer arietinum77 and T. aestivum25. Our study demonstrated that the exposure of plants to 10% PEG resulted in a rapid response reflected by an immediate increase in P5CS and P5CR expression levels in the first hours of stress. This observation confirms the hypothesis, that the major mechanism underlying proline biosynthesis in response to drought is regulation at the transcriptional level. This mechanism has also been reported in other plant species under drought conditions. An increase in P5CS transcript levels was reported for Oryza sativa L. after 2 and 5 hours78, Brassica napus after 1, 2 and 6 hours22 and for A. thaliana after 5 and 12 hours of water stress79. P5CS gene upregulation has also been observed under osmotic stress conditions induced by NaCl in wheat19, moth bean (Vigna aconifolia)80, soybean81 and A. thaliana82. An increase in P5CR expression was described in P. sativum83 and A. thaliana84 after 6 and 12 hours of NaCl treatment, respectively.
Our results showed a higher level of P5CS expression in the drought-tolerant cultivar compared to that in the drought-sensitive one, which has also been found in rice78. In most of the tested lines, the P5CS gene showed an immediate induction after 1 hour of stress, while changes in P5CR were observed after 3 or 6 hours. These results suggest that the P5CS gene plays a key role in proline biosynthesis and the plant response to short-term drought. Similar expression patterns were reported for A. thaliana. Changes in AtP5CS gene expression were detected after 2 hours and in AtP5CR after 24 hours of osmotic stress82. These results can indicate that product of P5CS gene could induce P5CR expression as well. Analysis of the results obtained for each ISCSL indicates, that genes encoding enzymes associated with proline biosynthesis (P5CS and P5CR) or their regulators were located on chromosomes 3B, 3D, 5A, 7B, and 7D (Table 1). The location of these genes on 3B and 3D chromosome is supported by data from Phytozome (Table 2) however, the remaining genome regions indicated need further investigations. Moreover, lines with the substitution of the 3A and 4A chromosomes showed very strong upregulation of both proline biosynthesis genes expression after 6 hours of the experiment. This observation can indicate the presence of regulatory elements on these chromosomes.
Table 2.
Phytozome transcripts annotated to selected antioxidant and proline biosynthesis enzymes, applied for consensus coding sequences construction.
| Enzyme | EC | Phytozome transcripts |
|---|---|---|
| CAT | 1.11.1.6 | Traes_4DS_FA4454E51.1, Traes_7DL_44F6042FE.1, Traes_4BL_664A41517.1, Traes_7BL_7A3B8A199.1, Traes_4BL_825998751.1, Traes_4DL_4FC0D4B27.1, Traes_5AL_EEA9DF0FC.1,Traes_7AL_B42CCD94B.1, Traes_4BL_1852E26C9.1, Traes_6AS_7FB8F9A66.1, Traes_6DS_3522B8EF6.2, Traes_4DS_F0ABE9257.1, Traes_7AL_65B1F0872.1, Traes_7AL_F80BC8414.1 |
| APX | 1.11.1.11 | Traes_4BL_19FA6DCAD.1, Traes_6AL_80FD46553.1, Traes_4DL_8CE055F15.1, Traes_5BL_C2D4F19B1.1, Traes_4AS_9EEABCE1C.1, Traes_2DL_59D310517.2, Traes_5AS_3AFCAA6AC.1, Traes_2AL_6FA87E31C.2, Traes_5DL_690A481C7.1, Traes_5BS_DA33416FB.1, Traes_6DL_2A99B8CDC.1, Traes_4BL_FBE8A057A.1, Traes_2BL_C8F030038.2, Traes_6BL_83DE6DC09.1, Traes_2AL_0EFC246E71.1, Traes_2AL_0EFC246E7.1, Traes_5BS_C8C312966.1 |
| GPX | 1.11.1.7 | Traes_2DL_09743F9A9.1, Traes_7AS_090BA704D.1, Traes_3AS_634121F561.1, Traes_1BS_70E91236D.1, Traes_4AL_454E2A798.1, Traes_7AS_FE0E50F2E.1, Traes_1BS_C25C6D1AD.1, Traes_1BL_9D96A6922.1, Traes_7AS_0611176A1.1, Traes_7AL_AB00F8D69.1, Traes_6DS_EBBE8AE2A.2, Traes_1AL_1F3A0CD1F.1, Traes_4BL_4CDA94F8D.1, Traes_2BS_4FF4CEA9F.1, Traes_2DL_59588BD68.1, Traes_2BS_AABEC0F2F.1, Traes_2BS_19F05C27A.2, Traes_7DL_883CB0B5B.1, Traes_7DL_DB6471BF0.1, Traes_1DS_3D2F70A22.1 |
| P5CS | 2.7.2.11 | Traes_3DL_3E215D878.2, Traes_3B_C4683D0FA.2, Traes_1DL_0BB66CF71.1, Traes_1BL_31105367B.1 |
| P5CR | 1.5.1.2 | Traes_4AL_D98D91F71.1, Traes_2AL_9D35F6B8F.1, Traes_3B_1E5C683B5.1, Traes_3DL_EB6A17449.1 |
In conclusion, our study shed light on the molecular mechanisms of plant responses to short-term drought conditions. The first reaction of the tested wheat lines was characterized by changes in the transcript levels of CAT, a gene encoding an enzyme closely associated with H2O2 detoxification. Furthermore, stress induced the expression of genes involved in proline biosynthesis and the MAPK6-mediated signaling pathway. The results suggest that plant responses are controlled by differential gene expression regulation.
In the present study, we identified chromosomes associated with the initial wheat response to short-term stress using a set of S29 substitution lines (JP) with varying drought tolerance. The data indicated that the substitution of chromosomes 3B, 5A, 7B and 7D had the largest impact on the expression level of all tested genes and could play a critical function in controlling tolerance to water deficits in the wheat genome (Table 1). Moreover, we suggest that structural or regulatory genes involved in the first plant response to drought may be located on those chromosomes. Our results are consistent with previous data reported by several research groups85–88.
Further investigations should be performed, including gene expression analysis of other MAPKs and antioxidant enzymes as well as evaluation of their activity to gain new insight into drought tolerance in plants and to clarify the roles of these enzymes in the general pattern of the stress response. A better understanding of the mechanisms underlying drought tolerance will help to develop stress-tolerant genotypes. Moreover, mapping strategies should also be devised for the localization of genetic loci associated with wheat responses to drought.
Methods
Plant material and drought treatment
Wheat (Triticum aestivum L.) intervarietal substitution lines (ISCSLs), with ‘Saratovskaya 29’ (S29) as a recipient and ‘Janetzkis Probat’ (JP) as a donor, were used in the study. S29 is a drought-tolerant cultivar, and JP is a drought-sensitive one. The set of 18 ISCSLs kernels was provided by the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK, Gatersleben, Germany).
Grains were sterilized with chlorine gas for 4 hours and transferred to Petri dishes containing filter paper soaked in distilled water. For germination induction, the grains were incubated at 4 °C for 48 hours and germinated for 2 days in the dark at 24 °C. Germinating seedlings were placed in plastic pots containing MS medium and maintained in a hydroponic culture in a growth chamber for 5 days under control conditions (light/dark regime of 16/8 h at 25 ± 3 °C, relative humidity of 50 ± 10%, light intensity during the daytime was 350 μmol m−2 s−1). For stress induction polyethylene glycol (PEG) addition to the medium was used. PEG cause osmotic stress occurrence, inducing plant water deficit similar to drought condition89. The main advantage of this experimental model is fact, that hydroponic culture provides a constantly reduced water potential, which is not possible in soil models90. Drought stress was induced after 5 days of acclimatization by applying 10% PEG-6000 dissolved in MS medium solution. To avoid hypoxia occurrence, the medium was aerated consistently. Wheat seedlings were collected after 1, 3 and 6 hours of stress treatment. Plants growing in MS medium without PEG were used as a control.
Identification of analyzed genes transcripts and the detection system design
This study analyzed genes encoding antioxidant enzymes (CAT, APX, GPX), enzymes involved in proline biosynthesis (P5CS and P5CR) and two MAPKs (MAPK3 and MAPK6). For genes encoding antioxidant enzymes and enzymes involved in proline biosynthesis the Phytozome 12.1 (https://phytozome.jgi.doe.gov) database was used to identify full-length cDNA sequences within the wheat genome (Triticum aestivum v. 2.2) (Table 2)91. For identified sequences, multiple sequence alignment was performed using T-Coffee software with M-Coffee algorithm92 and the representative consensus sequence was built. For analyzed MAPKs the sufficient information about coding sequences was not present in Phytozome database. Because of that fact we decided to identify wheat ortologs of A. thaliana MAPK3 and MAPK6 genes in NCBI GenBank database. Based on the alignment results, the accessions AF079318 and AY173962 were selected for MAPK3 and MAPK6, respectively. In order to confirm the homology of these sequences with respective MAPK3 and MAPK6 sequences from other selected plant species (A. thaliana, Brachypodium distachyon, O. sativa and Zea mays) an alignment was performed using BLAST tool (https://blast.ncbi.nlm.nih.gov) (Table 3). Obtained sequences were used as templates for detection system design. Gene-specific primers and probes were designed using PrimerBLAST software (https://www.ncbi.nlm.nih.gov/tools/primer-blast/)93 (Tables 4 and 5). For qPCR dual-labeled (6-FAM as a reporter, BHQ-1 as a quencher) TaqMan probes were applied.
Table 3.
Alignment of the selected wheat MAPK3 and MAPK6 sequences with the corresponding sequences from the other species.
| Species | Accession number | E-value |
|---|---|---|
| MAPK3 | ||
| Arabidopsis thaliana | NM_114433.3 | 1e-88 |
| Brachypodium distachyon | NM_001279922.1 | 2e-39 |
| Oryza sativa | DQ826422.1 | 0.0 |
| Zea mays | EU130900.1 | 0.0 |
| MAPK6 | ||
| Arabidopsis thaliana | NM_129941.4 | 0.0 |
| Brachypodium distachyon | XM_003574199 | 4e-126 |
| Oryza sativa | EU675863.1 | 0.0 |
| Zea mays | EU965114.1 | 0.0 |
Table 4.
Sequences of primers used in qPCR.
| Primer | Primer (5′ → 3′) sequence |
|---|---|
| CAT-F | CACCTGGTGGAGAAGATCGC |
| CAT-R | TCACCTCGAAGAAGCCCTTG |
| GPX-F | GCGGTGACACCAACATCAAC |
| GPX-R | GTCCAGGTTCTCCAGGTTGG |
| APX-F | CAAGGCTCTGACCACCTCAG |
| APX-R | CATCTTCCCAGGGTGTGACC |
| P5CS-F | GATTCTCCGATGGTGCTCGT |
| P5CS-R | TTCAACACCCACAGGTCCAC |
| P5CR-F | TAAATGCCGTTGTTGCTGCC |
| P5CR-R | AGCAAAACTAACAATGGCTACCAG |
| MAPK3-F | CTTTAACCCGCTGCAGAGGA |
| MAPK3-R | GTCAAAGGAGAAGGGGTCCG |
| MAPK6-F | GAGGTCACCGCCAAGTACAA |
| MAPK6-R | CTTGTTGTCGAAGGCGTTGG |
| CJ705892-F | AACCACCGCATTTGCTGAAG |
| CJ705892-R | GACAGGGTGCCACCAACTAT |
Table 5.
Sequences of TaqMan probes used in qPCR.
| Probe | Probe (5′ → 3′) sequence |
|---|---|
| CAT | [6-FAM]ACTTCGACCGCGAGCGCATC[BHQ-1] |
| GPX | [6-FAM]AGGCCAACTGCCCCCAGTCC[BHQ-1] |
| APX | [6-FAM]GCAGGTGTTTTCCACTCAGATGGGT[BHQ-1] |
| P5CS | [6-FAM]GGACTCGGTGCTGAGGTTGGC[BHQ-1] |
| P5CR | [6-FAM]ACAACAAGATGCCGAGAGCTCTCA[BHQ-1] |
| MAPK3 | [6-FAM]AGAGGCGCTGGAGCACCCTT[BHQ-1] |
| MAPK6 | [6-FAM]CCCCATCCTCCCCATCGGCA[BHQ-1] |
| CJ705892 | [6-FAM]AGAGCCATTGTCTTGGCAGGCT[BHQ-1] |
Extraction of total RNA and qPCR (Real-Time PCR) analyses
Total RNA from wheat seedlings was extracted using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The quality and quantity of isolated RNA were evaluated by 2% agarose gel electrophoresis and spectrophotometrically using DeNovix DS-11. Total RNA (1.5 µg) was used to synthesize cDNAs using the iScript™ cDNA synthesis kit (Bio-Rad). Quantitative PCR was performed with TaqMan probes. PCRs were carried out in a total volume of 20 µl containing 80 ng of cDNA, 1× TaqMan Universal PCR Master Mix (Applied Biosystems), 800 nM of each primer and 250 nM of TaqMan probe. Standard curves were generated from five dilution points for each primer pair. In the first step, amplification was started at 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of PCRs: 95 °C for 15 s and 60 °C for 1 min. For qPCR analyses the Quant Studio3 system (ThermoFisher Scientific) was used.
The T. aestivum cDNA clone whv16n3n16 5- (GenBank accession number: CJ705892) was used as an internal standard for normalization of qPCR results, as it revealed the most stable expression in the tested wheat lines under experimental treatment conditions. In order to determine the best reference for qPCR, ten potential reference genes were selected: 5 of them were ‘typical’ reference genes (actin, tubulin, GAPDH, ubiquitin, and translation elongation factor) and the remaining 5 were selected from Genevestigator database using RefGenes tool. The validation of the reference genes was performed with 4 different algorithms: geNorm, NormFinder, BestKeeper, and RefFinder (data unpublished). Relative gene expression was calculated using the 2−∆∆Ct method94. Each sample was analyzed in three full biological and three technical replicates. For determination of the specificity of qPCR reaction NTC (No Template Control) was applied for each reaction. The results were analyzed using the dedicated relative quantification software module from ThermoFisher Cloud (ThermoFisher Scientific).
Statistical analyses
In order to determine statistical significance of the change of analyzed genes expression in comparison to respective control forms a one-way ANOVA with Dunnett’s post-hoc test was performed at 0.05 probability level based on the ∆CT values. All statistical analyses were performed using Statistica 13.1 software package (Dell Inc.).
Author Contributions
K.D., M.N., A.B. and K.K. – designed experiment A.B. – provided plant material K.D. and M.Z. – performed the experiment M.N. and K.D. – analyzed the data K.D. and H.S. – wrote the paper M.N., K.D. and A.B. – edited manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baloglu MC, Inal B, Kavas M, Unver T. Diverse expression pattern of wheat transcription factors against abiotic stresses in wheat species. Gene. 2014;550(1):117–122. doi: 10.1016/j.gene.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Shiferaw B, et al. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Security. 2013;5(3):291–317. doi: 10.1007/s12571-013-0263-y. [DOI] [Google Scholar]
- 3.FAO FAOSTAT database collection. Food and Agriculture Organization of the United Nations. Rome. http://faostat.fao.org (2017).
- 4.Zalibekov ZG. The arid regions of the world and their dynamics in conditions of modern climatic warming. Arid Ecosystems. 2011;1:1–7. doi: 10.1134/S2079096111010094. [DOI] [Google Scholar]
- 5.Maqsood M, Shehzad MA, Ahmad S, Mushtaq S. Performance of wheat (Triticum aestivum L.) genotypes associated with agronomical traits under water stress conditions. Asian Journal of Pharmaceutical and Biological Research. 2012;2:45–50. [Google Scholar]
- 6.Hossain A, et al. Evaluation of growth, yield, relative performance and heat susceptibility of eight wheat (Triticum aestivum L.) genotypes grown under heat stress. Int. J. Plant Prod. 2013;7:615–636. [Google Scholar]
- 7.Małecka A, Tomaszewska B. Reaktywne formy tlenu w komórkach roślinnych i enzymatyczne systemy obronne. Postępy Biologii Komórki. 2005;32(2):311–325. [Google Scholar]
- 8.Nowicka B, Kruk J. Reaktywne formy tlenu w roślinach — więcej niż trucizna. Kosmos – problemy Nauk Biologicznych. 2014;62(4):583–596. [Google Scholar]
- 9.Manavalan, L. P. & Nguyen, H. T. Drought tolerance in crops: physiology to genomics. Plant Stress Physiology, 2nd Edition 1–23 (2017).
- 10.Szymańska R, Strzałka K. Reaktywne formy tlenu w roślinach – powstawanie, dezaktywacja i rola w przekazywaniu sygnału. Postępy Biochemii. 2010;56(2):182–190. [PubMed] [Google Scholar]
- 11.Caverzan A, et al. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012;35:1011–1019. doi: 10.1590/S1415-47572012000600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sofo A, Scopa A, Nuzzaci M, Vitti A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015;16(6):13561–13578. doi: 10.3390/ijms160613561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becana M, et al. Reactive oxygen species and anioxidants in legume nodules. Physiol. Plantarum. 2000;109:372–381. doi: 10.1034/j.1399-3054.2000.100402.x. [DOI] [Google Scholar]
- 14.Uarrota VG, et al. The role of ascorbate peroxidase, guaiacol peroxidase, and polysaccharides in cassava (Manihot esculenta Crantz) roots under postharvest physiological deterioration. Food Chem. 2016;197:737–746. doi: 10.1016/j.foodchem.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Guan LM, Scandalios JG. Hydrogen peroxide-mediated catalase gene expression in response to wounding. Free Radical Biol. Med. 2000;28:1182–1190. doi: 10.1016/S0891-5849(00)00212-4. [DOI] [PubMed] [Google Scholar]
- 16.Badawi GH, et al. Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci. 2004;166:919–928. doi: 10.1016/j.plantsci.2003.12.007. [DOI] [Google Scholar]
- 17.Yang Z, Wu Y, Li Y, Ling HQ, Chu C. OsMT1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol. Biol. 2009;70:219–229. doi: 10.1007/s11103-009-9466-1. [DOI] [PubMed] [Google Scholar]
- 18.Gaber A, et al. Glutathione peroxidase-like protein of Synechocystis PCC 6803 confers tolerance to oxidative and environmental stresses in transgenic Arabidopsis. Physiol. Plantarum. 2006;128:251–262. doi: 10.1111/j.1399-3054.2006.00730.x. [DOI] [Google Scholar]
- 19.Tavakoli M, Poustini K, Alizadeh H. Proline accumulation and related genes in wheat leaves under salinity stress. J. Agric. Sci. Technol. 2016;18:707–716. [Google Scholar]
- 20.Liang X, Zhang L, Natarajan SK, Becker DF. Proline mechanism of stress survival. Antioxid. Redox Signal. 2013;19(9):998–1011. doi: 10.1089/ars.2012.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida A, Jagendorf AT, Hibinom T, Takabe T, Takabe T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002;163:515–523. doi: 10.1016/S0168-9452(02)00159-0. [DOI] [Google Scholar]
- 22.Xue X, Liu A, Hua X. Proline accumulation and transcriptional regulation of proline biothesynthesis and degradation in Brassica napus. BMB Rep. 2009;42:28–34. doi: 10.5483/BMBRep.2009.42.1.028. [DOI] [PubMed] [Google Scholar]
- 23.Kishor PBK, Hong Z, Miao G, Hu CAA, Verma DPS. Overexpression of D1-pyrroline-5-carboxylate synthetase increases proline overproduction and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishor PBK, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant. Cell. Environ. 2014;37(2):300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- 25.Vendruscolo ECG, et al. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. Journal of Plant Physiol. 2007;164(10):1367–1376. doi: 10.1016/j.jplph.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Mittler R, et al. ROS signaling: the new wave? Trends. Plant. Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Choudhury S, Panda P, Sahoo L, Panda SK. Reactive oxygen species signaling in plants under abiotic stress. Plant Signaling and Behavior. 2013;8(4):e23681. doi: 10.4161/psb.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha AK, Jaggi M, Raghuram B, Tuteja N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav. 2011;6(2):196–203. doi: 10.4161/psb.6.2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, et al. Genome-wide identification and analysis of MAPK and MAPKK gene families in Brachypodium distachyon. PLoS ONE. 2012;7(10):e46744. doi: 10.1371/journal.pone.0046744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem. J. 2008;4132:217–226. doi: 10.1042/BJ20080625. [DOI] [PubMed] [Google Scholar]
- 31.Hamel LP, Nicole MC, Duplessis S, Ellis BE. Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell. 2012;244:1327–1351. doi: 10.1105/tpc.112.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raja V, Majeed U, Kang H, Andrabi KI, John R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017;137:142–157. doi: 10.1016/j.envexpbot.2017.02.010. [DOI] [Google Scholar]
- 33.Rudd JJ, Keon J, Hammond-Kosack KE. The wheat mitogen-activated protein kinases TaMPK3 and TaMPK6 are differentially regulated at multiple levels during compatible disease interactions with Mycosphaerella graminicola. Plant Physiol. 2008;147(2):802–815. doi: 10.1104/pp.108.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lian W, et al. Phylogenetic Analysis and Expression Patterns of the MAPK Gene Family in Wheat (Triticum aestivum L.) J. Integr. Agr. 2012;11(8):1227–1235. doi: 10.1016/S2095-3119(12)60119-1. [DOI] [Google Scholar]
- 35.Wen Y, et al. Characterization and expression analysis of mitogen-activated protein kinase cascade genes in wheat subjected to phosphorus and nitrogen deprivation, high salinity, and drought. J. Plant Biochem. Biot. 2015;24(2):184–196. doi: 10.1007/s13562-014-0256-8. [DOI] [Google Scholar]
- 36.Zhan H, et al. Genome-wide identification and analysis of MAPK and MAPKK gene families in bread wheat (Triticum aestivum L.) Genes. 2017;8(10):E284. doi: 10.3390/genes8100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal RK, et al. Analysis of MAPK and MAPKK gene families in wheat and related Triticeae species. BMC Genomics. 2018;19(1):1–26. doi: 10.1186/s12864-018-4545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieberherr D, et al. A sphingolipid elicitor-inducible mitogen-activated protein kinase is regulated by the small GTPase OsRac1 and heterotrimeric G-protein in rice. Plant Physiol. 2005;138:1644–1652. doi: 10.1104/pp.104.057414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie G, Kato H, Imai R. Biochemical identification of the OsMKK6–OsMPK3 signaling pathway for chilling stress tolerance in rice. Biochem. J. 2012;443:95–102. doi: 10.1042/BJ20111792. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, et al. A novel mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. J. Integr. Plant Biol. 2010;52:442–452. doi: 10.1111/j.1744-7909.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- 41.Opdenakker K, Remans T, Keunen E, Vangronsveld J, Cuypers A. Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAPKinase transcript levels. Environ. Exp. Bot. 2012;83:53–61. doi: 10.1016/j.envexpbot.2012.04.003. [DOI] [Google Scholar]
- 42.Osipova SV, et al. The antioxidant enzymes activity in leaves of inter-varietal substitution lines of wheat (Triticum aestivum L.) with different tolerance to soil water deficit. Acta Physiol. Plant. 2013;35(8):2455–2465. doi: 10.1007/s11738-013-1280-3. [DOI] [Google Scholar]
- 43.Collins NC, Tardieu F, Tuberosa R. Quantitative trait loci and crop performance under abiotic stress: where do we stand? Plant Physiology. 2008;147:469–486. doi: 10.1104/pp.108.118117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, C. et al. Short-term and continuing stresses differentially interplay with multiple hormones to regulate plant survival and growth. Mol. Plant7(5) (2014). [DOI] [PubMed]
- 45.Li, W., & Li, Q. Effect of environmental salt stress on plants and the molecular mechanism of salt stress tolerance. Int. J. Environ. Sci. Nat. Res. 7(3) (2017).
- 46.Harb A, Krishnan A, Ambavaram MMR, Pereira A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010;154(3):1254–1271. doi: 10.1104/pp.110.161752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duan H, et al. Effects of drought stress on growth and development of wheat seedlings. Int. J. Agric. Biol. 2017;19(5):1119–24. [Google Scholar]
- 48.Saeedipour S. Relationship of grain yield, ABA and proline accumulation in tolerant and sensitive wheat cultivars as affected by water stress. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 2013;83:311. doi: 10.1007/s40011-012-0147-5. [DOI] [Google Scholar]
- 49.Dhanda S, Sethi G, Behl R. Inheritance of seedling traits under drought stress conditions in bread wheat. Cereal Res. Commun. 2002;30(3/4):293–300. [Google Scholar]
- 50.Debieu M, et al. Response to early drought stress and identification of QTLs controlling biomass production under drought in pearl millet. PLOS ONE. 2018;13(10):e0201635. doi: 10.1371/journal.pone.0201635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, et al. Multiple heat and drought events affect grain yield and accumulations of high molecular weight glutenin subunits and glutenin macropolymers in wheat. J. Cereal Sci. 2013;57(1):134–140. doi: 10.1016/j.jcs.2012.10.010. [DOI] [Google Scholar]
- 52.Tena G, Asai T, Chiu WL, Sheen J. Plant mitogen activated protein kinase signaling cascades. Curr. Opin. Plant Biol. 2001;4:392–400. doi: 10.1016/S1369-5266(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 53.Teige M, et al. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell. 2004;15:141–52. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, et al. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genomics. 2015;16:386. doi: 10.1186/s12864-015-1621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizoguchi T, et al. A gene encoding a mitogenactivated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold and water stress in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:765–769. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moustafa, K., Lefebvre De Vos, D., Leprince, A. S., Savouré, A. & Laurière, C. Analysis of the Arabidopsis mitogen-activated protein kinase families: organ specificity and transcriptional regulation upon water stresses. Scholarly Research Exchange 1–12, (2008).
- 57.Xu L, Han L, Huang B. Antioxidant enzyme activities and gene expression patterns in leaves of Kentucky bluegrass in response to drought and post-drought recovery. J. Am. Soc. Hortic. Sci. 2011;136:247–255. doi: 10.21273/JASHS.136.4.247. [DOI] [Google Scholar]
- 58.Singh N, Mishra A, Jha B. Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte Salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Mar. Biotechnol. 2014;16:321–332. doi: 10.1007/s10126-013-9548-6. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z, et al. Gene knockout study reveals that cytosolic ascorbate peroxidase 2 (OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS ONE. 2013;8:e57472. doi: 10.1371/journal.pone.0057472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossel JB, et al. A mutation affecting ascorbate peroxidase 2 gene expression reveals a link between responses to high light and drought tolerance. Plant, Cell Environ. 2006;29:269–281. doi: 10.1111/j.1365-3040.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 61.Prashanth SR, Sadhasivam V, Parida A. Over expression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia mayina in indica rice var. Pusa Basmati-1 confers abiotic stress tolerance. Transgenic Res. 2008;17:281–291. doi: 10.1007/s11248-007-9099-6. [DOI] [PubMed] [Google Scholar]
- 62.Bian S, Jiang Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Scientia Hortic. 2009;120(2):264–270. doi: 10.1016/j.scienta.2008.10.014. [DOI] [Google Scholar]
- 63.Jiang Y, Watkins E, Liu S, Yu X, Luo N. Antioxidative responses and candidate gene expression in prairie junegrass under drought stress. J. Am. Soc. Hortic. Sci. 2010;135(4):303–309. doi: 10.21273/JASHS.135.4.303. [DOI] [Google Scholar]
- 64.Luna CM, et al. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 2005;56:417–23. doi: 10.1093/jxb/eri039. [DOI] [PubMed] [Google Scholar]
- 65.Reddy PCO, et al. Identification of stress-induced genes from the drought tolerant semi-arid legume crop horsegram (Macrotyloma uniflorum(Lam) Verdc.) though analysis of subtracted expressed sequence tags. Plant Sci. 2008;175:372–384. doi: 10.1016/j.plantsci.2008.05.012. [DOI] [Google Scholar]
- 66.Harb A, Awad D, Samarah N. Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. J. Plant Interact. 2015;10(1):109–116. doi: 10.1080/17429145.2015.1033023. [DOI] [Google Scholar]
- 67.Uzilday B, Turkan I, Sekmen AH, Ozgur R, Karakaya HC. Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci. 2012;182:59–70. doi: 10.1016/j.plantsci.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Sheoran S, et al. Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought. Appl. Biochem. Biotech. 2015;177(6):1282–1298. doi: 10.1007/s12010-015-1813-x. [DOI] [PubMed] [Google Scholar]
- 69.Xing Y, Jia W, Zhang J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. The Plant Journal. 2008;543:440–451. doi: 10.1111/j.1365-313X.2008.03433.x. [DOI] [PubMed] [Google Scholar]
- 70.Furlan A, Bianucci E, Tordable M, Castro S, Dietz KJ. Antioxidant enzyme activities and gene expression patterns in peanut nodules during a drought and rehydration cycle. Funct. Plant Biol. 2014;41:704–713. doi: 10.1071/FP13311. [DOI] [PubMed] [Google Scholar]
- 71.Mittler R, Zilinskas BA. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. The Plant Journal. 1994;5:397–405. doi: 10.1111/j.1365-313X.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- 72.Csiszár J, et al. Different peroxidase activities and expression of abiotic stress-related peroxidases in apical root segments of wheat genotypes with different drought stress tolerance under osmotic stress. Plant Physiol. Bioch. 2011;52:119–129. doi: 10.1016/j.plaphy.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 73.de Carvalho MC. Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal. Behav. 2008;3:156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rizhsky L, Hongjian L, Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002;130:1143–1151. doi: 10.1104/pp.006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Ronde JA, Cressc WA, Krügerd GHJ, Strasserd RJ, Stadenb JV. Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. Journal of Plant Physiol. 2004;161:1211–1224. doi: 10.1016/j.jplph.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 76.Yamada M, et al. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005;56:1975–1981. doi: 10.1093/jxb/eri195. [DOI] [PubMed] [Google Scholar]
- 77.Bhatnagar-Mathur P, et al. Genetic engineering of chickpea (Cicer arietinum L.) with the P5CSF129A gene for osmoregulation with implications on drought tolerance. Mol. Breeding. 2009;23:591–606. doi: 10.1007/s11032-009-9258-y. [DOI] [Google Scholar]
- 78.Choudhary NL, Sairam RK, Tyagi A. Expression of delta1-pyrroline-5-carboxylate synthetase gene during drought in rice (Oryza sativa L.) Indian Journal of Biochemistry and Biophysics. 2005;42(6):366–370. [PubMed] [Google Scholar]
- 79.Peng LX, Gu LK, Zheng CC, Li DQ, Shu HR. Expression of MaMAPK gene in seedlings of Malus L. under water stress. Acta Biochimica et Biophysica Sinica. 2006;38(4):281–286. doi: 10.1093/abbs/38.4.281. [DOI] [PubMed] [Google Scholar]
- 80.Hu CA, Delauney AJ, Verma DP. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(19):9354–8. doi: 10.1073/pnas.89.19.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delauney A, Verma D. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol. Genet. Genomics. 1990;221(3):299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- 82.Yoshiba Y, et al. Correlation between the induction of a gene for Δ1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. The Plant Journal. 1995;7:751–760. doi: 10.1046/j.1365-313X.1995.07050751.x. [DOI] [PubMed] [Google Scholar]
- 83.Williamson, C. L. & Slocum, R. D. Molecular cloning and evidence for osmoregulation of the delta 1-pyrroline-5-carboxylate reductase (proC) gene in pea (Pisum sativum L.). Plant Physiology 100(3):1464–70. (1992). [DOI] [PMC free article] [PubMed]
- 84.Liu J, Zhu JK. Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol. 1992;114(2):591–596. doi: 10.1104/pp.114.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang DL, Jing RL, Chang XP, Li W. Identification of quantitative trait loci and environmental interactions for accumulation and remobilization of water-soluble carbohydrates in wheat (Triticum aestivum L.) stems. Genetics. 2007;176:571–584. doi: 10.1534/genetics.106.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao HX, et al. Chromosomal location of traits associated with wheat seedling water and phosphorus use efficiency under different water and phosphorus stresses. Int. J. Mol. Sci. 2009;10(9):4116–36. doi: 10.3390/ijms10094116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pinto RS, et al. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. TAG. Theor. App. Genet. 2010;121(6):1001–21. doi: 10.1007/s00122-010-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gahlaut V, et al. QTL mapping for nine drought-responsive agronomic traits in bread wheat under irrigated and rain-fed environments. PLoS ONE. 2017;12(8):e0182857. doi: 10.1371/journal.pone.0182857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lagerwerff JV, Ogata G, Eagle HE. Control of osmotic pressure of culture solutions with polyethylene glycol. Science. 1961;133:1486. doi: 10.1126/science.133.3463.1486. [DOI] [PubMed] [Google Scholar]
- 90.Frolov A, et al. A. Early responses of mature Arabidopsis thaliana plants to reduced water potential in the agar-based polyethylene glycol infusion drought model. J. Plant Physiol. 2017;208:70–83. doi: 10.1016/j.jplph.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 91.Goodstein DM, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178–86. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Notredame C, Higgins DG, Heringa J. T-Coffee: A Novel Method for Fast and Accurate Multiple Sequence Alignment. J. Mol. Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 93.Ye J, et al. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.