Abstract
Background:
Apremilast (Otezla®) is a phosphodiesterase 4 (PDE4) inhibitor approved for the treatment of psoriasis and psoriatic arthritis (PsA), but the reason why apremilast shows clinical effect is not fully understood. The objective of this study was to study the downstream effects of apremilast on cells of inflamed joints in immune-mediated inflammatory arthritis.
Methods:
Synovial fluid was obtained from patients with active rheumatoid arthritis (RA), PsA or peripheral spondyloarthritis (SpA; n = 18). The in vitro models consisted of synovial fluid mononuclear cells (SFMCs) or fibroblast-like synovial cells (FLSs) cultured for 48 h, SFMCs cultured for 21 days, an osteoclast pit formation assay, and a mineralization assay.
Results:
In SFMCs cultured for 48 h, apremilast decreased the production of interleukin (IL)-12/IL-23p40 (the shared subunit of IL-12 and IL-23), colony-stimulating factor 1, CD6, and CD40 and increased the production of C-X-C motif chemokine 5 dose-dependently. Apremilast had a very different response signature compared with the tumor necrosis factor alpha inhibitor adalimumab with a substantially greater inhibition of IL-12/IL-23p40. In SFMCs cultured for 21 days, apremilast increased the secretion of IL-10. In FLS cultures, apremilast decreased matrix metalloproteinase-3 production. Apremilast decreased osteoclastogenesis but did not affect mineralization by human osteoblasts.
Conclusion:
This study reveals the downstream effects of apremilast in ex vivo models of arthritis with a strong inhibition of IL-12/IL-23p40 by SFMCs. Our findings could explain some of the efficacy of apremilast seen in IL-12/IL-23-driven immune-mediated inflammatory diseases such as psoriasis and PsA.
Keywords: apremilast, cyclic adenosine monophosphate, PALACE, phosphodiesterase 4, psoriatic arthritis, rheumatoid arthritis, spondyloarthritis
Introduction
Rheumatoid arthritis (RA), psoriatic arthritis (PsA) and spondyloarthritis (SpA) are chronic immune-mediated inflammatory diseases characterized by inflammation in synovial joints. The pathogenesis involves increased levels of proinflammatory cytokines such as tumor necrosis factor alpha (TNFα), interleukin (IL)-6, IL-23 and IL-17 and inadequate anti-inflammatory responses including insufficient secretion of IL-10.1–3 In particular, in PsA and SpA, the IL-23–T helper (Th)17 axis is believed to play a significant role. This is explained by the finding of pathogenic Th17 cells in the inflammatory sites and illustrated by the clinical efficacy seen with therapeutic inhibition of the IL-12/IL-23p40 subunit (the shared subunit of IL-12 and IL-23), IL-23 and IL-17 in these diseases.4 In contrast, therapeutic inhibition of TNFα has shown clinical efficacy in both RA, PsA and SpA indicating differences in downstream effects associated with inhibition of different cytokines. Immune-mediated inflammatory arthritis is also characterized by dysregulation of bone homeostasis.1 Arresting osteoclast activity is the main way to treat systemic bone loss and the periarticular osteolysis of immune-mediated inflammatory arthritis.2
Apremilast (Otezla®) is a small molecule phosphodiesterase 4 (PDE4) inhibitor showing efficacy in the treatment of psoriasis and PsA.5 Inhibition of PDE4 in peripheral blood mononuclear cells with apremilast results in modulation of gene transcription, including decreases in genes encoding TNFα, IL-12A, and IL-23A, resulting in suppression of inflammatory responses in vitro and in vivo.6 By blocking PDE4 enzymatic activity, apremilast increases intracellular cyclic adenosine monophosphate (AMP) levels, resulting in activation of the PKA-CREB pathway and inhibition of nuclear factor kappa B (NF-κB) transcriptional activity.7 In patients with PsA, treatment with apremilast (30 mg twice daily) has been shown to decrease plasma levels of proinflammatory cytokines, including TNFα, IL-6, IL-8, MIP-1β, monocyte chemoattractant protein (MCP)-1, ferritin, IL-17 and IL-23, and increase plasma levels of the anti-inflammatory cytokines IL-10 and IL-1R antagonist. However, the time course of these pharmacodynamics shows a biphasic response, with the initial phase observed during weeks 4–16 characterized primarily by a decrease in TNFα which was significantly associated with clinical improvement [American College of Rheumatology (ACR)20 response criteria], and a second phase observed at week 40, characterized by a decrease in the Th17 pathway cytokines IL-23 and IL-17.8 The nature of this biphasic pharmacodynamic response has not been explained. Furthermore, the specific effects of apremilast in the inflamed joint have not been described, due to the lack of data from analysis of synovial biopsies.9 A number of studies have previously examined the effects of PDE4 inhibitors on osteoclasts and osteoblasts, but with inconsistent results. In a phase II clinical trial apremilast (30 mg twice daily) significantly reduced serum receptor activator of NF-κB ligand (RANKL) levels in ankylosing spondylitis patients after 12 weeks.10
The objective of this study was to analyze the downstream effects of apremilast on cells of the inflamed joint in immune-mediated inflammatory arthritis. First, we tested the effect of apremilast on the secretion of several cytokines, chemokines and growth factors by synovial fluid mononuclear cells (SFMCs) ex vivo. Then, we tested whether apremilast affect factors involved in structural changes by studying fibroblast-like synovial cells (FLSs), osteoclasts, and osteoblasts.
Methods
Study participants
SFMCs were obtained from a study population consisting of patients with chronic RA, PsA or peripheral SpA with at least one swollen joint (for obtaining synovial fluid; n = 18). The group consisted of 10 patients with RA, 3 patients with PsA, and 5 patients with human leukocyte antigen B27-associated peripheral SpA. Median C-reactive protein (CRP) was 31.5 mg/l [interquartile range (IQR) 10.0–66.5 mg/l] and median disease activity score 28 CRP (DAS28CRP) was 3.9 (IQR 3.2–4.8; Table 1). Not all patient samples were used in all experiments and the concentrations were below the detection limit for some of the analytes. The exact number of patients in each experiment has been stated in the figure legends.
Table 1.
Patient characteristics.
| Characteristic | |
|---|---|
| Diagnosis | |
| PsA patients | 3 |
| Peripheral SpA | 5 |
| RA | 10 |
| Disease activity | |
| CRP (mg/l) | 31.5 (10.0–66.5 mg/l) |
| DAS28CRP (0–10) | 3.9 (3.2–4.8) |
Data are expressed as the median with IQR.
CRP, C-reactive protein; DAS28CRP, disease activity score 28 based on CRP; IQR, interquartile range; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SpA, spondyloarthritis.
Culture conditions
Apremilast (Otezla®, Celgene Summit, NJ, USA) was used at three-fold dilutions from 1000 nM (111 nM, 333 nM, and 1000 nM) or 10-fold dilutions from 10,000 nM (100 nM, 1000 nM, and 10,000 nM). Untreated cells and cells treated with dimethyl sulfoxide (DMSO) were used as controls. Cells treated with adalimumab (Humira, Abbvie, Lake Bluff, Illinois, USA) at 5 μg/ml, the bisphosphonate alendronate (CalBiochem, Billerica, MA, USA) at 10 μM, sulfasalazine (Sigma, St. Louis, Missouri, USA) at 30 μM were used for comparison. Adalimumab is a fully humanized monoclonal antibody neutralizing TNFα. The concentration of 5 μg/ml was chosen because this concentration is the preferred steady-state trough concentration and has been used previously for in vitro experiments.11–13 Supernatants were harvested after centrifugation of the culture plates at 1200 rpm for 5 min and stored at −80°C for later analysis.
SFMC 48-hour ex vivo model
The 48-hour culture is an in vitro model of immune-mediated inflammatory arthritis dominated by lymphocytes and monocytes.11,14 SFMCs were isolated by conventional Ficoll–Paque (GE Healthcare, Chicago, Illinois, USA) density-gradient centrifugation and cryopreserved at −135°C. The cells were then thawed and seeded at a concentration of 106 cells/ml and kept in a humidified incubator at 37°C and 5% CO2 as done previously. Culture supernatants were analyzed using the Olink Proseek Multiplex interferon I panel. All samples passed the quality control. A total of 45/92 proteins were detected in >75% of the samples. Proseek Multiplex uses relative quantification. Thus, even if two different proteins have the same normalized protein expression (NPX) values, their actual concentration may differ. An increase in one NPX corresponds to a doubling of the concentration.
SFMC 21-day ex vivo model
The 21-day culture is a model of low-grade inflammatory osteoclastogenesis also containing macrophage-like synovial cells.12,15,16 SFMCs were cultured in Dulbecco’s modified Eagle’s medium, 10% fetal calf serum, penicillin, streptomycin and glutamine for 21 days. Cultures were seeded with a cell density of 106 cells/ml and kept in a humidified incubator at 37°C and 5% CO2 changing medium every 2–3 days and adding fresh compounds as previously described. Culture supernatants were analyzed for the concentration of IL-10 and MCP-1 by enzyme-linked immunosorbent assays (ELISAs; Biolegend, San Diego, California, USA) and for enzyme activity of tartrate-resistant acid phosphatase (TRAP) positive by an enzymatic assay (B-bridge International, Santa Clara, California, USA) following the instructions of the manufacturer.
Osteoclastogenesis assay
Human bone marrow mononuclear cells (Lonza, Walkersville, MA, USA) were cultured in 10% heat-inactivated fetal bovine serum (Life Technologies, Carlsbad, California, USA) in minimal essential medium-α (Life Technologies) in a final count of 8.51 × 05 cells/ml and differentiated into osteoclasts using 10 nM dexamethasone and 10 nM vitamin D for 7 days. Apremilast or other test compounds were added along with fresh medium on days 0 and 3. Cells were rinsed and fixed with 4% para-formaldehyde for 5 min. Osteoclasts were stained for TRAP using the TRAP5 staining kit (B-bridge International). ImageQuant TL (GE Healthcare, Piscataway, NJ, USA) Colony Count software was used to count total cell numbers and number of osteoclasts.
Osteoclast pit formation
RAW 264.7 mouse macrophages were plated in 24-well Osteo Surface plates (Corning, NY, USA) and incubated for 2 h to allow attachment. RANKL (50 ng/ml) was added and plates were incubated at 5% CO2, 37°C for 7 days, changing the media every 3 days and adding fresh compounds. Cells were removed with bleach and pictures of the wells were taken to assess osteoclast activity. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used for image analysis.
FLS 48-hour ex vivo model
FLSs were grown from SFMCs as previously described.17,18 Briefly, SFMCs were cultured in 25 cm2 tissue culture flasks for 2–3 weeks changing the medium every 2–3 days. FLSs were then loosened using trypsin/ethylenediaminetetraacetic acid (EDTA) and transferred to a new tissue culture flask. When the cell layer in the new tissue culture flask was 70% confluent, the FLSs were passaged by trypsin/EDTA treatment again. This was repeated and the FLSs were used for analyses at passage 4–5 as described previously.17 Culture supernatants were analyzed for the concentration of matrix metalloproteinase 3 (MMP3) by ELISA (R&D Systems, Minneapolis, Minnesota, USA) following the instructions of the manufacturer.
Osteoblast mineralization assay
Human osteoblasts were purchased, and mineralization assay was used according to the manufacturer’s instructions as previously described.19 Osteoblasts were cultured for 14 days changing the medium every 2–3 days.
Statistics
Olink Proseek Multiplex data were presented as NPX values, which is an arbitrary unit on log2 scale. First, the NPX of DMSO samples was subtracted from all other samples and then the data were analyzed using linear regression looking for dose-dependent differences and with the Student’s t-test to compare the top concentration of apremilast with DMSO and adalimumab. The p values were adjusted for multiple testing using the Bonferroni correction. All other data were transformed to ratios by dividing the value of the samples with the value of DMSO samples. Data were then analyzed with repeated measures one-way analysis of variance or the paired Student’s t-test depending on the number of groups. In all tests, the level of significance was a two-sided p value of less than 0.05. Statistical analyses were performed using GraphPad Prism for Mac (GraphPad Software, San Diego, California, USA) and Stata (StataCorp College Station, Texas, USA).
Results
Apremilast potently decreases the secretion of IL-12/IL-23p40 by SFMCs cultured for 48 h
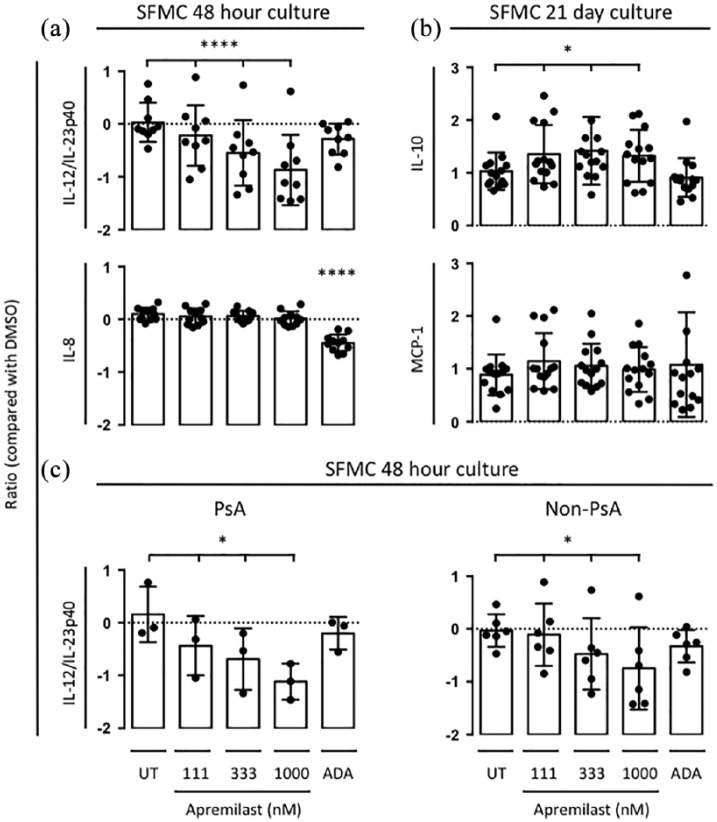
We first tested whether apremilast affect the secretion by SFMCs of a large panel of cytokines, chemokines and growth factors to evaluate the immune-modulatory effects of apremilast. SFMCs cultured for 48 h predominantly consisted of lymphocytes and monocytes. In these cultures, apremilast decreased the production of IL-12/IL-23p40 (p < 0.00001) colony-stimulating factor 1 (CSF1; also known as M-CSF; p = 0.009), CD6 (p = 0.03), CD40 (p = 0.04), and increased the production of C-X-C motif chemokine 5 (CXCL5; p = 0.003) dose-dependently (Table 2). Only the decrease in IL-12/IL-23p40 passed the Bonferroni correction (p = 0.0001). Also, the apremilast induced decrease in IL-12/IL-23p40 was significantly greater compared with adalimumab [p = 0.012; Figure 1(a)]. Many other cytokines and chemokines were decreased less potently with apremilast compared with adalimumab. We then tested whether the effect of apremilast was different in SFMCs isolated from PsA patients compared with non-PsA patients (RA and SpA patients). Apremilast at 1000 nM decreased the normalized protein expression of IL-12/IL-23p40 with a value of 1.1 [standard deviation (SD) 0.34, p < 0.03] in SFMCs isolated form PsA patients and with a value of 0.74 (SD 0.78, p = 0.03) in non-PsA patients.
Table 2.
Changes in the Olink Proseek Multiplex IFN I panel in SFMCs cultured for 48 h with DMSO control, apremilast or adalimumab.
| Protein | Apremilast dose-response |
Apremilast versus DMSO |
Apremilast versus adalimumab |
||||||
|---|---|---|---|---|---|---|---|---|---|
| p.value | Bonf.adj.p | R value | p.value | Bonf.adj.p | R value | p.value | Bonf.adj.p | R value | |
| IL-12B | <0.00001 | 0.0001 | −0.27 | 0.0067 | 0.14 | −0.71 | 0.012 | 0.039 | −0.48 |
| CXCL5 | 0.0026 | 0.06 | 0.19 | 0.028 | 0.22 | 0.51 | 0.0001 | 0.0012 | 1.19 |
| CSF-1 | 0.0088 | 0.14 | −0.12 | 0.047 | 0.22 | −0.50 | 0.0073 | 0.029 | 0.38 |
| CD6 | 0.026 | 0.32 | −0.06 | 0.35 | 0.67 | −0.08 | 0.10 | 0.17 | −0.08 |
| CD40 | 0.045 | 0.43 | −0.08 | 0.72 | 0.93 | 0.04 | 0.0087 | 0.030 | 0.22 |
| MIP-1α | 0.055 | 0.44 | −0.09 | 0.22 | 0.60 | −0.35 | 0.0039 | 0.020 | 0.54 |
| CCL19 | 0.073 | 0.44 | −0.08 | 0.014 | 0.14 | −0.32 | 0.22 | 0.32 | 0.12 |
| CCL4 | 0.073 | 0.44 | −0.08 | 0.12 | 0.44 | −0.35 | 0.0023 | 0.014 | 0.56 |
| TWEAK | 0.11 | 0.50 | −0.07 | 0.13 | 0.44 | 0.14 | 0.0084 | 0.030 | −0.27 |
| OPG | 0.12 | 0.50 | −0.07 | 0.96 | 0.98 | −0.01 | 0.036 | 0.091 | 0.22 |
| IL-18R1 | 0.13 | 0.50 | −0.05 | 0.15 | 0.45 | −0.12 | 0.12 | 0.19 | −0.11 |
| TNFSF14 | 0.13 | 0.50 | −0.05 | 0.48 | 0.75 | −0.06 | 0.58 | 0.63 | 0.04 |
| TNFB | 0.14 | 0.50 | −0.04 | 0.045 | 0.22 | −0.21 | 0.060 | 0.13 | 0.08 |
| Flt3L | 0.15 | 0.51 | −0.06 | 0.98 | 0.98 | 0.00 | 0.087 | 0.16 | −0.13 |
| MMP-1 | 0.22 | 0.69 | −0.19 | 0.50 | 0.75 | 0.35 | 0.44 | 0.52 | 0.35 |
| CXCL9 | 0.25 | 0.71 | −0.07 | 0.79 | 0.95 | −0.04 | 0.0013 | 0.012 | 0.76 |
| CD244 | 0.25 | 0.71 | −0.03 | 0.74 | 0.93 | 0.02 | 0.55 | 0.62 | −0.03 |
| IL-8 | 0.31 | 0.77 | −0.02 | 0.75 | 0.93 | 0.01 | 0.0001 | 0.0012 | 0.46 |
| OSM | 0.32 | 0.77 | −0.04 | 0.50 | 0.75 | −0.11 | 0.0015 | 0.012 | 0.38 |
| EN-RAGE | 0.36 | 0.77 | −0.05 | 0.30 | 0.61 | 0.12 | 0.42 | 0.52 | −0.06 |
| MCP-1 | 0.37 | 0.77 | −0.04 | 0.30 | 0.61 | −0.20 | 0.056 | 0.13 | 0.35 |
| IL-4 | 0.37 | 0.77 | 0.03 | 0.24 | 0.60 | 0.10 | 0.32 | 0.43 | 0.07 |
| CXCL10 | 0.38 | 0.77 | −0.06 | 0.87 | 0.98 | 0.04 | 0.0024 | 0.014 | 0.98 |
| DNER | 0.40 | 0.77 | −0.02 | 0.53 | 0.77 | 0.03 | 0.42 | 0.52 | −0.04 |
| CD5 | 0.40 | 0.77 | −0.03 | 0.38 | 0.67 | 0.10 | 0.92 | 0.92 | −0.01 |
| TGFα | 0.42 | 0.77 | −0.03 | 0.25 | 0.61 | 0.08 | 0.029 | 0.081 | 0.14 |
| CCL20 | 0.44 | 0.78 | −0.03 | 0.55 | 0.77 | −0.14 | 0.0057 | 0.025 | 0.42 |
| CCL23 | 0.48 | 0.78 | −0.02 | 0.96 | 0.98 | 0.00 | 0.21 | 0.31 | 0.10 |
| uPA | 0.50 | 0.78 | −0.03 | 0.27 | 0.61 | 0.11 | 0.13 | 0.21 | 0.10 |
| IL-6 | 0.52 | 0.78 | 0.04 | 0.88 | 0.98 | 0.04 | 0.020 | 0.059 | 0.41 |
| LAP-TGFβ1 | 0.53 | 0.78 | −0.02 | 0.47 | 0.75 | −0.06 | 0.068 | 0.14 | 0.12 |
| FGF-21 | 0.55 | 0.78 | −0.02 | 0.12 | 0.44 | 0.12 | 0.73 | 0.78 | 0.02 |
| CASP-8 | 0.55 | 0.78 | −0.02 | 0.23 | 0.60 | 0.09 | 0.50 | 0.57 | −0.05 |
| CXCL1 | 0.55 | 0.78 | 0.03 | 0.30 | 0.61 | 0.19 | 0.0008 | 0.010 | 0.56 |
| CXCL11 | 0.61 | 0.82 | −0.02 | 0.64 | 0.87 | 0.07 | 0.031 | 0.081 | 0.48 |
| HGF | 0.61 | 0.82 | −0.02 | 0.015 | 0.14 | 0.46 | 0.0008 | 0.010 | −0.83 |
| MCP-3 | 0.63 | 0.82 | −0.02 | 0.76 | 0.93 | 0.03 | 0.086 | 0.16 | 0.16 |
| IL-18 | 0.66 | 0.84 | −0.01 | 0.88 | 0.98 | −0.01 | 0.30 | 0.40 | 0.04 |
| STAMPB | 0.77 | 0.92 | 0.01 | 0.059 | 0.26 | 0.06 | 0.83 | 0.85 | −0.01 |
| CXCL6 | 0.77 | 0.92 | 0.01 | 0.010 | 0.14 | 0.11 | 0.28 | 0.39 | 0.08 |
| ADA | 0.79 | 0.92 | −0.01 | 0.045 | 0.22 | 0.18 | 0.80 | 0.83 | 0.01 |
| MCP-2 | 0.84 | 0.95 | −0.01 | 0.98 | 0.98 | 0.00 | 0.0042 | 0.020 | 0.76 |
| TNFRSF9 | 0.86 | 0.95 | 0.01 | 0.14 | 0.44 | 0.11 | 0.091 | 0.16 | 0.13 |
| TRAIL | 0.88 | 0.95 | 0.00 | 0.91 | 0.98 | 0.00 | 0.37 | 0.48 | −0.04 |
| CDCP1 | 0.89 | 0.95 | 0.00 | 0.41 | 0.70 | 0.04 | 0.45 | 0.52 | −0.04 |
| VEGF-A | 0.94 | 0.96 | 0.00 | 0.044 | 0.22 | 0.16 | 0.045 | 0.11 | 0.14 |
| 4E-BP1 | 0.94 | 0.96 | 0.00 | 0.0078 | 0.14 | 0.30 | 0.062 | 0.13 | 0.16 |
| IL-10 | 1.00 | 1.00 | 0.00 | 0.37 | 0.67 | −0.08 | 0.18 | 0.28 | 0.08 |
Bold numbers; p < 0.05.
4E-BP1, 4E-binding protein 1; ADA, adenosine deaminase; Bonf.adj.p; Bonferroni adjusted p value; CASP-8, caspase-8; CDCP1, CUB domain-containing protein 1; CSF-1, colony-stimulating factor 1; DNER, Delta and Notch-like epidermal growth factor-related receptor; EN-RAGE, extracellular newly identified receptor for advanced glycation end products (RAGE)-binding protein; FGF-21, fibroblast growth factor-21; Flt3L, FMS-like tyrosine kinase 3 ligand; HGF, hepatocyte growth factor; IL, interleukin; LAP-TGFβ1, latency-associated peptide-transforming growth factor beta; MCP, monocyte chemoattractant protein; MIP-1α, macrophage inflammatory protein 1 alpha; OPG, osteoprotegerin; OSM, oncostatin M; STAMPB, signal-transducing adaptor molecule-binding protein; TGFα, transforming growth factor alpha; TNFβ, tumor necrosis factor beta; TNFRSF9, tumor necrosis factor receptor superfamily member 9; TNFSF14, TNF superfamily member 14; TRAIL, TNF-related apoptosis-inducing ligand; TWEAK, tumor necrosis factor-like weak inducer of apoptosis; uPA, urokinase-type plasminogen activator; VEGF-A, vascular endothelial growth factor A.
Figure 1.
(a) Secretion of IL-12/IL-23p40 (n = 9) and IL-8 (n = 11) by SFMCs cultured for 48 h untreated (UT) or treated with DMSO control, apremilast or ADA. Data were presented as NPX values, which is an arbitrary unit on log2 scale. A decrease in one NPX corresponds to a two-fold decrease of the concentration. (b) Secretion of IL-10 (n = 14) and MCP-1 (n = 14) by SFMCs cultured for 21 days untreated (UT) or treated with DMSO control, apremilast or ADA. Data were normalized to untreated cultures and expressed as a ratio. (c) Secretion of IL-12/IL-23p40 by PsA (n = 3) and non-PsA (n = 6) SFMCs cultured for 48 h untreated (UT) or treated with DMSO control, apremilast or ADA. Data were presented as NPX values, which is an arbitrary unit on log2 scale. Boxes and bars indicate mean and SD. * p < 0.05. ** p < 0.01. *** p < 0.001. **** p < 0.0001.
ADA, adalimumab; DMSO, dimethyl sulfoxide; IL, interleukin; MCP-1, monocyte chemoattractant protein; NPX, normalized protein expression; PsA, psoriatic arthritis; SD, standard deviation; SFMC, synovial fluid mononuclear cell.
Apremilast increases the production of IL-10 in SFMCs cultured for 21 days
Next, the effect of apremilast on the secretion of MCP-1 and IL-10 by SFMCs cultured for 21 days was studied to evaluate the immune-modulatory capacity of apremilast in cultures with more macrophage-like synovial cells. Apremilast increased the secretion of anti-inflammatory IL-10 (p = 0.04) but did not change the secretion of MCP-1 [p = 0.5; Figure 1(b)]. This increase in IL-10 production was not seen with adalimumab treatment.
Apremilast inhibits osteoclast differentiation and pit formation
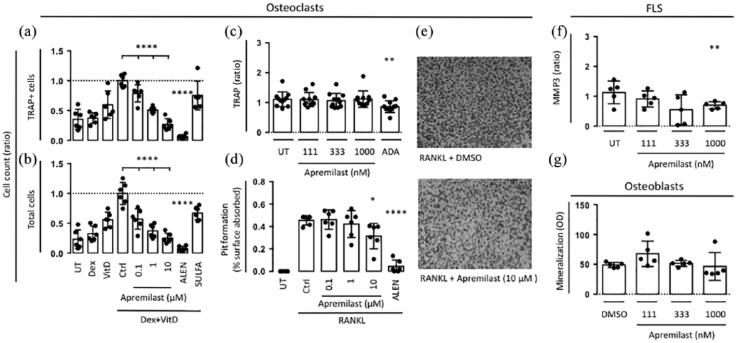
Then, to evaluate the potential role of apremilast in preventing structural damage we then studied the effect of apremilast on osteoclasts, FLSs, and osteoblasts. First, the effects of apremilast was tested on osteoclasts derived from human bone marrow mononuclear cells treated with vitamin D and dexamethasone for 7 days. The number of TRAP-positive (TRAP+) osteoclasts was reduced by apremilast at 0.1 μM, 1 μM and 10 μM by 21%, 49%, and 73%, respectively. The number of TRAP+ osteoclasts was also reduced by alendronate but not by sulfasalazine [Figure 2(a)]. Apremilast, alendronate and sulfasalazine all reduced the total cell counts [Figure 2(b)]. In contrast, apremilast did not change TRAP secretion by SFMC 21-day cultures [Figure 2(c)]. This is a model of low-grade inflammation leading to osteoclastogenesis. In order to study the effect of apremilast on osteoclast activity, a bone resorption assay was performed. The RAW264.7 mouse macrophage cell line was stimulated with RANKL for 7 days on a synthetic inorganic bone mimetic surface. Surface pitting was visually assessed and measured by image quantitation. Apremilast at 10 μM significantly inhibited osteoclast pit formation by 22% [Figure 2(d) and (e)].
Figure 2.
(a,b) Human bone marrow mononuclear cells (n = 6) were plated and incubated with vitamin D (VitD), dexamethasone (Dex) and compounds for 7 days. TRAP-positive cells and total cells were counted. (c) SFMCs were cultured for 21 days untreated (UT), or treated with DMSO, apremilast or ADA and TRAP (n = 11) secretion was measured. (d) RAW264.7 mouse macrophages were stimulated with RANKL and compounds for 7 days and surface pitting was measured. (e) Representative photographs of osteoclast pit formation. (f) Secretion of MMP3 by FLSs were cultured for 48 h untreated (UT) or treated with DMSO control or apremilast (n = 6). (g) Human osteoblasts were cultured for 14 days untreated (UT) or treated with DMSO control or apremilast and mineralization was assessed (n = 5). Boxes and bars indicate mean and SD. * p < 0.05. ** p < 0.01. **** p < 0.0001.
ADA, adalimumab; DMSO, dimethyl sulfoxide; FLS, fibroblast-like synovial cell; MMP3, matrix metalloproteinase 3; RANKL, receptor activator of nuclear factor kappa B ligand; SD, standard deviation; SFMC, synovial fluid mononuclear cell; TRAP, tartrate-resistant acid phosphatase.
Apremilast decreases the secretion of MMP3 by FLSs but shows no effect on osteoblast mineralization
Finally, the effect of apremilast on FLS and osteoblast cultures was tested. The highest concentration of apremilast decreased the secretion of MMP3 from FLSs [p = 0.005; Figure 2(f)]. Apremilast did not change osteoblast mineralization [Figure 2(g)].
Discussion
Apremilast is now a common treatment option in psoriasis and PsA and is being tested in other immune-mediated inflammatory diseases.20,21 This study reveals IL-12/IL-23p40 as a major downstream target of apremilast in the inflamed joint of immune-mediated inflammatory arthritis.
RA, PsA and SpA are all characterized by inflammation of the synovial joints. Further, several cytokines are expressed at the same level in these diseases.22 However, the underlying pathobiology of the inflamed synovial membrane is very heterogeneous both between these diseases and within each diagnostic entity. This is best illustrated by the distinct responses to different medications seen in individual patients. Most notably inhibition of IL-6 is only effective in patients with RA while inhibition of IL-23 and IL-17 seems to have stronger efficacy in psoriasis, PsA and SpA.1,4,23
The molecular target of apremilast is PDE4. Inhibition of PDE4 results in an increase in intracellular cyclic adenosine monophosphate (cAMP) dampening proinflammatory signaling.7 In cell cultures of healthy peripheral blood mononuclear cells, apremilast has shown selectivity in downstream effects with inhibition of interferon-γ-inducible protein 10, interferon-γ, monokine induced by gamma interferon, TNFα, IL-12p70, macrophage inflammatory protein 1-α, MCP-1 and granulocyte-macrophage CSF, but not IL-8, IL-1β, RANTES (regulated on activation, normal T-cell expressed and secreted), IL-10 and IL-6 production.6 In synovial membrane cell cultures, apremilast reduces TNFα.24 However, the effects of apremilast on cytokine secretion by synovial cells using multiplex systems and the specific effects of apremilast on cells involved in structural changes in arthritis had previously not been studied.
In this study, apremilast showed a robust decrease in IL-12/IL-23p40 secretion by SFMCs cultured for 48 h. Supporting this, the expression of IL-12/IL-23p40 is known to be inhibited by the cAMP analog 8-Br-cAMP.25 Further, decreased IL-12/IL-23p40 and IL-23 have been reported in lipopolysaccharide (LPS)-stimulated monocytes,26 lesional skin from patients with psoriasis27 and in plasma from patients with PsA8 treated with apremilast. This supports inhibition of IL-12/IL-23p40 as a crucial target of apremilast in immune-mediated inflammatory arthritis and this could explain some of the efficacy of apremilast seen in IL-12/IL-23-driven immune-mediated inflammatory diseases.
In SFMCs cultured for 21 days to resemble spontaneous differentiation of macrophages, apremilast increased the production of IL-10. This is in line with previous studies finding no change or an increase in IL-10 in response to apremilast treatment in vitro and in vivo.8 IL-10 is an anti-inflammatory cytokine produced by monocytes and regulatory T and B-cells with multiple effects such as suppression of cytokine secretion, antigen presentation and CD4+ T-cell activation. Therefore, increased IL-10 could also be part of the clinical efficacy seen with apremilast.
Finally, we studied the role of apremilast in models of potential structural changes in immune-mediated inflammatory arthritis. Apremilast decreased the production of MMP3 by FLSs and inhibited osteoclastogenesis at clinically relevant concentrations (0.1–1 μM). This indicates that apremilast may inhibit cartilage and bone destruction in diseases such as RA, PsA, and ankylosing spondylitis by inhibiting MMP3 production and osteoclast development. However, apremilast did not change the development of TRAP-secreting osteoclasts in SFMCs cultured for 21 days resembling low-grade spontaneous inflammatory osteoclastogenesis.15 This is in line with a number of previous studies on the effects of PDE4 inhibitors on osteoclasts showing inconsistent results. For example, the PDE4 inhibitor rolipram was reported to induce osteoclastogenesis in mouse bone marrow cells cocultured with calvarial osteoblasts, through a mechanism involving increased RANKL expression.28 In this system, PDE4 inhibition was further shown to promote parathyroid hormone-induced osteoclast formation via increased cyclooxygenase-2 expression.29 However, others have reported that PDE4 inhibition reduces osteoclastogenesis induced by prostaglandin E2 in RAW 264.7 mouse macrophages, through a mechanism involving increased cell cycle arrest.30 Taken together, this suggests that apremilast might not have a substantial direct effect on structural changes in immune-mediated inflammatory arthritis. However, prevention of structural changes with apremilast as a consequence of decreased inflammation in vivo seems plausible.
There are both limitations and strengths of this study. First, the ex vivo study design is favorable for detecting direct effects on cells activated in vivo. Further, several components of the pathobiology of immune-mediated inflammatory arthritis were assessed using SFMCs, FLSs, and osteoclastogenesis and mineralization assays. However, it is obviously never possible to directly translate in vitro findings into clinical practice. Second, the apremilast top concentrations of 1000 nM might not be sufficient to suppress all the immunological reactions in the in vitro cultures. Thus, it has previously been reported that some T-cell cytokines are suppressed with a half maximal inhibitory concentration (IC50) around 1–4 μM and that B-cell differentiation and antibody production requires substantially higher concentrations.26 However, we chose concentrations around the mean steady-state apremilast Cmax of 450 nM31 to best possible resemble in vivo conditions. Third, separation of SFMCs does not completely exclude contamination with granulocytes. Therefore, some of the results could have been influenced by leftover granulocytes in the SFMC cultures. Finally, the cohort of 18 patients was too small to justify subgroup analyses.
Conclusion
This study reveals the downstream effects of apremilast in ex vivo models of arthritis with a strong inhibition of IL-12/IL-23p40. Further, apremilast induced IL-10 production in synovial macrophages and decreased MMP3 in FLS cultures. Our findings could explain the efficacy of apremilast seen in psoriasis and PsA and holds promise for testing apremilast in other IL-12/IL-23-driven immune-mediated inflammatory diseases.
Acknowledgments
We thank Karin Skovgård Sørensen (Dept. of Biomedicine, Aarhus University) for technical assistance concerning the ELISA assays and mineralization assay. We thank everyone involved at Olink Proteomics and especially Emil Nilson for the statistical assistance regarding the Olink Multiplex data.
TWK and MA helped to design the study, collect the samples, carry out the experiments, analyzed and interpreted the data, and drafted the manuscript. SL, MAN, and LH helped to collect the samples and carry out the experiments. PS and BD helped to design the study and supervised the project. All authors helped to analyze and interpret the data, were involved in revising the manuscript, and read and approved the final manuscript.
All samples were obtained after informed written consent according to the Declaration of Helsinki and approved by the Local Ethics Committee (De Videnskabsetiske Komitéer for Region Midtjylland, project number 20121329) and the Danish Data Protection Agency.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Augustinus foundation, the Nygaard foundation, the Aage Bang foundation, and the Faculty of Health at Aarhus University.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PS is Otezla Senior Principal Investigator, Translational Development, Celgene Corporation. The study was an investigator-initiated project financed by a Sponsored Research Agreement with Celgene.
ORCID iD: Tue W. Kragstrup  https://orcid.org/0000-0002-6439-397X
https://orcid.org/0000-0002-6439-397X
Contributor Information
Tue W. Kragstrup, Department of Biomedicine, Wilhelm Meyers Allé 4, Aarhus University, DK-8000 Aarhus C, Denmark.
Mary Adams, Department of Translational Development, Celgene Corporation, Summit, NJ, USA.
Søren Lomholt, Department of Biomedicine, Aarhus University, Denmark.
Morten A. Nielsen, Department of Biomedicine, Aarhus University, Denmark
Line D. Heftdal, Department of Biomedicine, Aarhus University, Denmark
Peter Schafer, Department of Translational Development, Celgene Corporation, Summit, NJ, USA.
Bent Deleuran, Department of Biomedicine, Aarhus University, Denmark; Department of Rheumatology, Aarhus University Hospital, Denmark; Department of Clinical Medicine, Aarhus University, Denmark.
References
- 1. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017; 389: 2328–2337. [DOI] [PubMed] [Google Scholar]
- 2. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017; 376: 957–970. [DOI] [PubMed] [Google Scholar]
- 3. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016; 374: 2563–2574. [DOI] [PubMed] [Google Scholar]
- 4. Sakkas LI, Bogdanos DP. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun Rev 2017; 16: 10–15. [DOI] [PubMed] [Google Scholar]
- 5. Reed M, Crosbie D. Apremilast in the treatment of psoriatic arthritis: a perspective review. Ther Adv Musculoskelet Dis 2017; 9: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol 2010; 159: 842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez-Aso M, Montesinos MC, Mediero A, et al. Apremilast, a novel phosphodiesterase 4 (PDE4) inhibitor, regulates inflammation through multiple cAMP downstream effectors. Arthritis Res Ther 2015; 17: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schafer PH, Chen P, Fang L, et al. The pharmacodynamic impact of apremilast, an oral phosphodiesterase 4 inhibitor, on circulating levels of inflammatory biomarkers in patients with psoriatic arthritis: substudy results from a phase III, randomized, placebo-controlled trial (PALACE 1). J Immunol Res 2015; 2015: 906349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schett G, Sloan VS, Stevens RM, et al. Apremilast: a novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Ther Adv Musculoskelet Dis 2010; 2: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pathan E, Abraham S, Van Rossen E, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in ankylosing spondylitis. Ann Rheum Dis. Epub ahead of print 14 September 2012. DOI: 10.1136/annrheumdis-2012-201915. [DOI] [PubMed] [Google Scholar]
- 11. Kragstrup TW, Jalilian B, Keller KK, et al. Changes in Soluble CD18 in murine autoimmune arthritis and rheumatoid arthritis reflect disease establishment and treatment response. PLoS One 2016; 11: e0148486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lomholt S, Mellemkjaer A, Iversen MB, et al. Resveratrol displays anti-inflammatory properties in an ex vivo model of immune-mediated inflammatory arthritis. BMC Rheumatology 2018; 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. l’Ami MJ, Krieckaert CL, Nurmohamed MT, et al. Successful reduction of overexposure in patients with rheumatoid arthritis with high serum adalimumab concentrations: an open-label, non-inferiority, randomised clinical trial. Ann Rheum Diseases 2018; 77: 484–487. [DOI] [PubMed] [Google Scholar]
- 14. Kragstrup TW, Andersen T, Holm C, et al. Toll-like receptor 2 and 4 induced interleukin-19 dampens immune reactions and associates inversely with spondyloarthritis disease activity. Clin Exp Immunol 2015; 180: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greisen SR, Einarsson HB, Hvid M, et al. Spontaneous generation of functional osteoclasts from synovial fluid mononuclear cells as a model of inflammatory osteoclastogenesis. APMIS 2015; 123: 779–786. [DOI] [PubMed] [Google Scholar]
- 16. Kragstrup TW, Greisen SR, Nielsen MA, et al. The interleukin-20 receptor axis in early rheumatoid arthritis: novel links between disease-associated autoantibodies and radiographic progression. Arthritis Res Ther 2015; 18: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heftdal LD, Andersen T, Jæhger D, et al. Synovial cell production of IL-26 induces bone mineralization in spondyloarthritis. J Mol Med (Berl) 2017; 95: 779–787. [DOI] [PubMed] [Google Scholar]
- 18. Kragstrup TW, Jalilian B, Hvid M, et al. Decreased plasma levels of soluble CD18 link leukocyte infiltration with disease activity in spondyloarthritis. Arthritis Res Ther 2014; 16: R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kragstrup TW, Andersen MN, Schiøttz-Christensen B, et al. Increased interleukin (IL)-20 and IL-24 target osteoblasts and synovial monocytes in spondyloarthritis. Clin Exp Immunol 2017; 189: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braun J. New targets in psoriatic arthritis. Rheumatology (Oxford, England) 2016; 55(Suppl. 2): ii30–ii37. [DOI] [PubMed] [Google Scholar]
- 21. Kumar N, Goldminz AM, Kim N, et al. Phosphodiesterase 4-targeted treatments for autoimmune diseases. BMC Med 2013; 11: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Kuijk AWR, Reinders-Blankert P, Smeets TJM, et al. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann Rheum Dis 2006; 65: 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsai YC, Tsai TF. Anti-interleukin and interleukin therapies for psoriasis: current evidence and clinical usefulness. Ther Adv Musculoskelet Dis 2017; 9: 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCann FE, Palfreeman AC, Andrews M, et al. Apremilast, a novel PDE4 inhibitor, inhibits spontaneous production of tumour necrosis factor alpha from human rheumatoid synovial cells and ameliorates experimental arthritis. Arthritis Res Ther 2010; 12: R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng WG, Wang YB, Zhang JS, et al. cAMP elevators inhibit LPS-induced IL-12 p40 expression by interfering with phosphorylation of p38 MAPK in murine peritoneal macrophages. Cell Res 2002; 12: 331–337. [DOI] [PubMed] [Google Scholar]
- 26. Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal 2014; 26: 2016–2029. [DOI] [PubMed] [Google Scholar]
- 27. Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol 2013; 12: 888–897. [PubMed] [Google Scholar]
- 28. Takami M, Cho ES, Lee SY, et al. Phosphodiesterase inhibitors stimulate osteoclast formation via TRANCE/RANKL expression in osteoblasts: possible involvement of ERK and p38 MAPK pathways. FEBS Lett 2005; 579: 832–838. [DOI] [PubMed] [Google Scholar]
- 29. Park H, No ALSM, Lee JM, et al. PDE4 inhibitor upregulates PTH-induced osteoclast formation via CRE-mediated COX-2 expression in osteoblasts. FEBS Lett 2010; 584: 173–180. [DOI] [PubMed] [Google Scholar]
- 30. Chen L, Zheng T, Park H, et al. PDE4 inhibitor suppresses PGE2-induced osteoclast formation via COX-2-mediated p27(KIP1) expression in RAW264.7 cells. Die Pharmazie 2011; 66: 201–206. [PubMed] [Google Scholar]
- 31. Gottlieb AB, Strober B, Krueger JG, et al. An open-label, single-arm pilot study in patients with severe plaque-type psoriasis treated with an oral anti-inflammatory agent, apremilast. Curr Med Res Opin 2008; 24: 1529–1538. [DOI] [PubMed] [Google Scholar]