Abstract
Context:
Quadriceps function is a significant contributor to knee joint health that is influenced by central and peripheral factors, especially after anterior cruciate ligament reconstruction (ACLR).
Objective:
To assess differences of unilateral quadriceps isometric strength and activation between the involved limb and contralateral limb of individuals with ACLR and healthy controls.
Data Sources:
Web of Science, SportDISCUS, PubMed, CINAHL, and the Cochrane Database were all used during the search.
Study Selection:
A total of 2024 studies were reviewed. Twenty-eight studies including individuals with a unilateral history of ACLR, isometric knee extension strength normalized to body mass, and quadriceps activation measured by central activation ratios (CARs) through a superimposed burst technique were identified for meta-analysis. The methodological quality of relevant articles was assessed using a modified Downs and Black scale. Results of methodological quality assessment ranged from low to high quality (low, n = 10; moderate, n = 8; high, n = 10).
Study Design:
Meta-analysis.
Level of Evidence:
Level 2.
Data Extraction:
Means, standard deviations, and sample sizes were extracted from articles, and magnitude of between-limb and between-group differences were evaluated using a random-effects model meta-analysis approach to calculate combined pooled effect sizes (ESs) and 95% CIs. ESs were classified as weak (d < 0.19), small (d = 0.20-0.49), moderate (d = 0.50-0.79), or large (d > 0.80).
Results:
The involved limb of individuals with ACLR displayed lower knee extension strength compared with the contralateral limb (ES, –0.78; lower bound [LB], –0.99; upper bound [UB], –0.58) and healthy controls (ES, –0.76; LB, –0.98; UB, –0.53). The involved limb displayed a lower CAR compared with healthy controls (ES, –0.84; LB, –1.18; UB, –0.50) but not compared with the contralateral limb (ES, –0.15; LB, –0.37; UB, 0.07). The ACLR contralateral limb displayed a lower CAR (ES, –0.73; LB, –1.39; UB, –0.07) compared with healthy control limbs but similar knee extension strength (ES, –0.24; LB, –0.68; UB, –0.19).
Conclusion:
Individuals with ACLR have bilateral CAR deficits and involved limb strength deficits that persist years after surgery. Deficits in quadriceps function may have meaningful implications for patient-reported and objective outcomes, risk of reinjury, and long-term joint health after ACLR.
Keywords: quadriceps function, central activation ratio, isometric knee extension strength, ACLR
After anterior cruciate ligament (ACL) injury, physically active individuals most commonly seek ACL reconstruction (ACLR) with the goal of restoring knee joint stability, improving lower extremity function, and facilitating a return to a physically active lifestyle.24 However, recuperation from ACLR requires extensive rehabilitation and results in significant time loss from activity.24 Quadriceps function is consistently identified as an important aspect of recovery for individuals after ACLR because of its primary role in knee stability and association with many short-term,15,28,29,36 patient-oriented outcomes, including functional performance,4,36,42,43,54 self-reported function,27,45 rates of return to play,2 and secondary ACL injury risk.15 Despite the clear importance of recovering quadriceps function during rehabilitation, regaining function after treatment may not always be possible, and inability to address these limitations may predispose individuals to long-term functional limitations,49 declines in physical activity,3 and an elevated risk of developing knee osteoarthritis.38
Among the various methods available for assessment of quadriceps function, isometric quadriceps strength evaluation has emerged as a clinically meaningful outcome linked to specific important mid- and long-term outcomes. Isometric quadriceps strength assessment is a clinically feasible measure of quadriceps function that is highly reliable.26 Current guidelines14,15,45 recommend unilateral and limb symmetry outcome comparisons to track rehabilitation progress27,45,47 during treatment and to guide return-to-play decisions in individuals with a history of ACLR. A previous systematic review34 reported asymmetrical quadriceps strength indicating quadriceps strength deficits in the involved limb compared with the contralateral limb up to 2 years after ACLR. However, limb symmetry may overestimate quadriceps strength,53 as it does not take into account the potential for ACLR to influence contralateral neuromuscular control, resulting in bilateral quadriceps weakness.46 A gap exists in synthesizing the literature assessing unilateral strength outcomes in both limbs, which may help provide a more complete picture of quadriceps function, including problematic quadriceps strength deficits that may also result in the contralateral limb. The use of unilateral outcomes eliminates the potential crossover effect commonly seen in individuals with bilateral quadriceps functional impairments after ACLR7,21,56 and allows for clinicians to compare strength performance of injured individuals to normative outcomes for guidance in the recovery process. Furthermore, involved limb isometric quadriceps strength is a significantly better predictor of self-reported knee function45 and psychological readiness to return to sport33 when compared with limb symmetry strength and is a primary focus of this meta-analysis.
Both central and peripheral adaptations significantly contribute to persistent alterations in quadriceps function after ACLR. Arthrogenic muscle inhibition and cortical sources of reduced quadriceps activation have been implicated as a primary source of persistent weakness.17,30,32 These responses to local joint trauma diminish an individual’s ability to voluntarily contract the quadriceps muscle via spinal and cortical pathways depending on the injury characteristics and time since surgery.20,32 A common approach to quantifying the magnitude of reduction in quadriceps activation after ACLR is through the measurement of central activation ratios (CARs) using a superimposed burst technique. A previous systematic review identified CAR deficits in both the involved and contralateral limbs of individuals after ACLR compared with controls.20 This systematic review only included 4 articles assessing patients after ACLR, and these articles included heterogeneous methodology in the assessment of quadriceps strength and CAR. Many studies further exploring the underlying mechanisms contributing to quadriceps activation have since been published. Therefore, an update on the state of quadriceps activation after ACLR is necessary to reevaluate the current state of research in the topic area. The purpose of this systematic review was to compare involved limb isometric knee extension strength and quadriceps CAR with the contralateral uninvolved limb and healthy control limbs via a comprehensive analysis of the available literature. The secondary purpose of this meta-analysis was to assess the effect of participant sex and graft source on involved limb isometric knee extension strength and quadriceps CAR.
Methods
Search Strategy
An online search was performed on July 13, 2017, using Web of Science, SportDISCUS, PubMed (Medline), CINAHL (EBSCO), and the Cochrane Library and was limited to articles published after January 1, 2017. The following search terms were used: TOPIC: ((ACLR OR ACL Reconstruction) AND (quadriceps strength OR quadriceps activation OR knee extension strength or knee extension torque)). Additionally, the reference sections of relevant articles were searched and added for review if determined appropriate. After articles were retrieved from the online search and article references, a single investigator removed duplicate articles and evaluated all the article titles and abstracts deemed appropriate for inclusion.
Eligibility Criteria
Articles were included for review if they evaluated isometric knee extension strength on a machine-based dynamometer and CARs using a superimposed burst technique in individuals with a history of unilateral ACLR. Reasons for exclusion of articles included nonhuman subjects, cadavers, ACL-deficient or ACLR revision patients only, allograft tendon patients only, articles in other languages besides English, abstracts only, group treatment comparisons, contralateral surgical graft patients, use of handheld dynamometers, and nonnormalized or limb-deficit outcomes.
Reporting Quality and Publication Bias
Two investigators independently assessed the methodological quality of all articles included for the systematic review and meta-analysis through the modified Downs and Black checklist.19 The modified Downs and Black checklist consists of 15 items and is a valid methodological assessment tool for both randomized and nonrandomized studies.10,19 The highest potential score was 15, with greater scores indicating higher methodological quality. Based on the summation of the scores, articles are categorized as high (≥12), moderate (10-11), and low (≤9) methodological quality.19 Scores between the investigators were compared for each item of every article. If there was disagreement between scores of the 2 investigators, a third investigator made the final scoring decision.
Funnel plots were created to assess the effects of publication bias11 on quadriceps function outcomes. The effect size (ES) comparisons of the ACLR limb and healthy control limb of relevant articles assessing isometric quadriceps strength and CARs were plotted against the total sample size. Results that were not affected by publication bias would have symmetrical plots around the pooled ES.
Data Extraction
Descriptive statistics for studies, including participant population, time since surgery, age, and joint angle during testing, were extracted and recorded in Tables 1 and 2. Means and SDs for maximal voluntary isometric contraction (MVIC) and CAR data were extracted from each study by 2 investigators and separated by the ACLR limb (the limb that underwent ACLR), the contralateral limb (uninjured limb of ACLR participants), and limbs of healthy controls. Many of the included studies only reported 1 healthy matched limb; however, 8 articles18,27,28,30-32,41,52 reported bilateral data on healthy controls. These studies reported no statistical differences between limbs in the healthy group; therefore, pooled means and SDs were calculated between limbs to obtain a single representative healthy limb comparison for the outcomes of interest. Data pertaining to graft type and patient sex, when reported, were also extracted and analyzed for MVIC and CAR outcomes.
Table 1.
Descriptive information of articles included in MVIC ACLR and healthy group comparisons
| Study | Participant Population | Modified Downs and Black Score | Angle of Testing, deg | Injured Group | Healthy Control Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Sex | Age, y | Time Since Surgery | MVIC, N·m/kg, Mean ± SD | n | Sex | Age, y | MVIC, N·m/kg, Mean ± SD | ||||
| Kuenze et al (2015a)27 | Recreationally active | 11 | 90 | 22 | 12M, 10F | 22.5 | 31.5 mo | INV = 2.46 ± 0.83 | 24 | 12M, 12F | 21.7 | 2.72 ± 0.49 |
| Holsgaard-Larsen et al (2104)22 | Orthopaedics department patient | 10 | 90 | 23 | 23M | 27.2 | 26.5 mo | INV = 2.54 ± 0.65 CON = 2.76 ± 0.56 |
25 | 25M | 27.2 | 2.9 ± 0.54 |
| Lepley et al (2014)31 | University community | 12 | 90 | 29 | 9M, 20F | 21.2 | 48.2 mo | INV = 2.67 ± 0.76 | 29 | 9M, 20F | 21.5 | 3.13 ± 1.06 |
| Kuenze et al (2015b)28 | Recreationally active | 9 | 90 | 22 | 12M, 10F | 22.5 | 31.5 mo | INV = 2.5 ± 0.84 CON = 2.92 ± 0.65 |
24 | 12M, 12F | 21.7 | 2.835 ± 0.54 |
| Thomas et al (2015)50 | Recreationally active | 12 | 90 | 17 | 10M, 7F | 21.41 | 7-10 mo | INV = 2.03 ± 0.57 CON = 2.88 ± 0.73 |
16 | 5M, 11F | 23.38 | 2.63 ± 0.92 |
| Krishnan and Williams (2011)25 | Recreationally active | 9 | 90 | 15 | 15F | 24.73 | 2-14 y | INV = 3.67 ± 0.66 CON = 3.94 ± 0.73 |
15 | 15F | 24.73 | 4.09 ± 1.02 |
| Goetschius et al (2015)13 | Recreationally active | 9 | 60 | 32 | 18M, 14F | 24.1 | 45.1 mo | INV = 2 ± 0.6 | 32 | 15M, 17F | 24.3 | 2.6 ± 0.8 |
| Pietrosimone et al (2015)46 | University community | 11 | 90 | 28 | 9M, 19F | 21.28 | 48.1 mo | INV = 2.68 ± 0.78 | 29 | 9M, 20F | 21.55 | 3.13 ± 1.07 |
| Thomas et al (2016)52 | — | 9 | 90 | 20 | 7M, 13F | 20.65 | 212.89 d | INV = 2.03 ± 0.51 CON = 2.89 ± 0.81 |
— | — | — | — |
| Kuenze et al (2015c)30 | University, recreationally active | 14 | 90 | 22 | 12M, 10F | 22.5 | 31.5 mo | INV = 3.07 ± 1.03 CON = 3.59 ± 0.8 |
24 | 12M, 12F | 21.7 | 3.48 ± 0.67 |
| Pamukoff et al (2017)44 | Recreationally active | 11 | 90 | 20 | 6M, 14F | 21.1 | 50.7 mo | INV = 1.86 ± 0.74 | 20 | 6M, 14F | 21.2 | 2.56 ± 0.37 |
| Lepley et al (2015)32 | Healthy university/high school | 14 | 90 | 20 | 9M, 11F | 20.9 | 6 mo | INV = 2.58 ± 0.69 CON = 2.79 ± 0.82 |
20 | 9M, 11F | 21.7 | 3.53 ± 0.93 |
| Chang et al (2014)5 | Physically active (150 min of moderate exercise or 60 min of vigorous exercise per week) | 9 | 90 | 8 | 7M, 1F | 24.8 | — | INV = 2.85 ± 0.33 CON = 3.051 ± 0.477 |
10 | 8M, 2F | 26.9 | 4.18 ± 0.32 |
| Zwolski et al (2016)56 | Females with 50 h of pivoting/cutting sports per week | 9 | 60 | 15 | 15F | 18 | 9.2 mo | INV = 1.55 ± 0.5 CON = 1.88 ± 0.27 |
15 | 15F | 17.9 | 1.88 ± 0.46 |
| Jordan et al (2015)23 | Elite athletes | 12 | 70 | 8 | 3M, 5M | — | 25 mo | INV = 3.44 ± 0.63 CON = 4.43 ± 0.98 |
21 | 13M, 8F | — | 4.09 ± 0.52 |
| Goetschius and Hart (2016)12 | Recreationally active | 12 | 90 | 53 | 27M, 26F | 23.4 | 44.1 mo | INV = 2.23 ± 0.76 | 50 | 28M, 22F | 23.3 | 2.57 ± 0.76 |
| Davis et al (2017)9 | Recreationally active | 12 | 90 | 39 | 12M, 27F | 21.84 | 49.43 mo | INV = 2.83 ± 0.61 CON = 3.05 ± 0.62 |
— | — | — | — |
| Blackburn et al (2016)4 | Physically active 30 min 3 times per week | 10 | 90 | 39 | 11M, 28F | 22 | 49 mo | INV = 2.72 ± 0.62 CON = 2.94 ± 0.59 |
— | — | — | — |
| Oberlander et al (2013)41 | Participated in sports with high level of joint loading | 9 | 65 | 10 | 28 | 12 mo | INV = 2.32 ± 0.56 CON = 2.75 ± 0.57 |
— | — | — | — | |
| Zwolski et al (2015)55 | Returning to preinjury participation in pivoting or cutting | 9 | 60 | 139 | 49M, 90F | 16.7 | 8.2 mo | INV = 2.3 ± 0.5 CON = 2.6 ± 0.5 |
— | — | — | — |
| Norte et al (2018)40 | Healthy | 9 | 90 | 4 | 2M, 2F | 27.4 | 7.4 mo | INV = 1.95 ± 0.43 CON = 2.92 ± 0.8 |
— | — | — | — |
| Lepley and Palmieri-Smith(2016)35 | Orthopaedic clinic | 13 | 90 | 54 | 23M, 31F | 19.9 | 7.24 mo | INV = 2.2 ± 0.6 CON = 3.2 ± 5.8 |
— | — | — | — |
| Harput et al (2015)18 | General population, HT reconstruction | 9 | 60 | 24 | 24M | 28.1 | 1-3 mo | Month 1: INV = 1.47 ± 0.62 CON = 2.6 ± 0.63; Month 2: INV = 2.18 ± 0.65 CON = 2.88 ± 0.73; Month 3: INV = 2.61 ± 0.67 CON = 3.14 ± 0.64 |
— | — | — | — |
ACLR, anterior cruciate ligament reconstruction; CON, contralateral ACLR limb; F, female; HT, hamstring tendon; INV, involved ACLR limb; M, male; MVIC, maximal voluntary isometric contraction.
Table 2.
Descriptive information of articles included in CAR ACLR and healthy group comparisons
| Study | Participant Population | Modified Downs and Black Score | Injured Group | Healthy Control Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Sex | Age, y | Time Since Surgery | CAR, %, Mean ± SD | n | Sex | Age, y | CAR, %, Mean ± SD | |||
| Kuenze et al (2015a)27 | Recreationally active | 11 | 22 | 12M, 10F | 22.5 | 31.5 mo | INV = 84.6 ± 10.2 | 24 | 12M, 12F | 21.7 | 91.3 ± 7.3 |
| Lepley et al (2014)31 | University community | 12 | 29 | 9M, 20F | 21.2 | 48.2 mo | INV = 88.1 ± 12.0 | 29 | 9M, 20F | 21.5 | 95.9 ± 3.4 |
| Thomas et al (2015)50 | Recreationally active | 12 | 17 | 10M, 7F | 21.4 | 7-10 mo | INV = 82.0 ± 11.0 | 16 | 5M, 11F | 23.38 | 89.0 ± 10.0 |
| Otzel et al (2015)42 | Recreationally active university students | 10 | 24 | 11M, 13F | 20.2 | 39.6 mo | INV = 91.0 ± 7.0 CON = 93.0 ± 5.9 |
24 | 12M, 11F | 21.8 | 92.0 ± 4.1 |
| Pietrosimone et al (2015)46 | University community | 11 | 28 | 9M, 19F | 21.3 | 48.1 mo | INV = 88.0 ± 12.0 CON = 88.0 ± 12.0 |
28 | 9M, 20F | 21.55 | 95.5 ± 4.0 |
| Harkey et al (2016)17 | Physically active (10 min for 3 d per week) | 12 | 73 | 24M, 49F | 21.4 | 39.6 mo | INV = 90.0 ± 9.0 CON = 91.0 ± 9.0 |
74 | 24M, 50F | 21.4 | 95.0 ± 4.5 |
| Kuenze et al (2015c)30 | Recreationally active university community | 14 | 22 | 12M, 10F | 22.5 | 31.5 mo | INV = 84.6 ± 10.3 CON = 89.9 ± 9.2 |
22 | 12M, 12F | 21.7 | 91.2 ± 7.0 |
| Pamukoff et al (2017)44 | Recreationally active | 11 | 20 | 6M, 14F | 21.1 | 50.7 mo | INV = 83.3 ± 11.1 | 20 | 6M, 14F | 21.2 | 93.7 ± 3.2 |
| Lepley et al (2015)32 | Healthy university and high school populations | 14 | 20 | 9M, 11F | 20.9 | 6 mo | INV = 91.2 ± 6.2 CON = 93.1 ± 5.7 |
20 | 9M, 11F | 21.7 | 97.4 ± 1.8 |
| Goetchius and Hart (2016)12 | Recreationally active | 12 | 53 | 27M, 26F | 23.4 | 29.9 mo | INV = 84.4 ± 11.9 | 50 | 28M, 22F | 23.3 | 91.0 ± 8.2 |
| Chang et al (2014)5 | Physically active (150 min of moderate exercise or 60 min of vigorous exercise per week) | 9 | 8 | 7M, 1F | 24.8 | — | INV = 76.0 ± 3.0 CON = 77 ± 3.0 |
10 | 8M, 2F | 26.9 | 93.5 ± 2.5 |
| Luc-Harkey et al (2017)37 | — | 12 | 27 | 7M, 20F | 21.8 | 44.47 mo | INV = 88.6 ± 7.67 CON = 89.4 ± 9.10 |
— | — | — | — |
| Norte et al (2018)40 | Orthopaedic clinic | 9 | 4 | 2M, 2F | 27.4 | 7.4 mo | INV = 94.6 ± 2.7 CON = 81.4 ± 5.16 |
— | — | — | — |
| Thomas et al (2016)52 | — | 9 | 20 | 7M, 13F | 20.7 | 212.89 d | INV = 87.0 ± 12.0 CON = 85.0 ± 57.0 |
— | — | — | — |
| Lepley and Palmieri-Smith (2016)35 | Orthopaedic clinic | 13 | 54 | 23M, 31F | 19.9 | 7.24 mo | INV = 88.8 ± 9.1 CON = 92.7 ± 7.3 |
— | — | — | — |
| Blackburn et al (2016)4 | Physically active participants (30 min 3 times per week) | 10 | 39 | 11M, 28F | 22 | 49 mo | INV = 89.0 ± 9.0 CON = 89.0 ± 9.0 |
— | — | — | — |
ACLR, anterior cruciate ligament reconstruction; CAR, central activation ratio; CON, contralateral ACLR limb; F, female; INV, involved ACLR limb; M, male.
Data Analysis
Data were used to calculate weighted means, SDs, and 95% CIs accounting for study sample size for MVIC (N·m/kg) and CAR (%) outcomes of each limb. Standardized Cohen d ESs with associated 95% CIs, visually depicted via forest plots, were also calculated. Forest plots were organized by time from surgery in chronological order from top to bottom of the plot (see Figures A1-A6 in the Appendix, available in the online version of this article). ESs were classified as weak (d < 0.19), small (d = 0.20-0.49), moderate (d = 0.50-0.79), or large (d > 0.80).8 For comparisons between the ACLR limb and healthy control limb, a positive ES indicates greater quadriceps function in the healthy control limb and a negative ES indicates poorer quadriceps function in the ACLR limb. For comparisons between the ACLR limb and the contralateral limb, a positive ES indicates greater quadriceps function in the contralateral limb and a negative ES indicates poorer quadriceps function in the ACLR limb. For comparisons between the contralateral limb and healthy control limb, a positive ES indicates greater quadriceps function in the healthy control limb and a negative ES indicates poorer quadriceps function in the contralateral limb. These results were considered conclusive if the 95% CI crossed the y-axis. In addition to calculating individual ESs, a random-effects model meta-analysis approach was used to calculate combined pooled ESs and 95% CIs for each grouping of variables (represented by diamonds on the forest plots).
Based on the available data for patient sex, ES comparisons were made between female ACLR limbs and male ACLR limbs for MVIC and CAR. For available data on graft type, ES comparisons were made between the ACLR and contralateral limbs for hamstring tendon autografts and patellar tendon autografts. A random-effects model meta-analysis approach was used to calculate combined pooled ESs and 95% CIs when possible.
Results
Literature Search
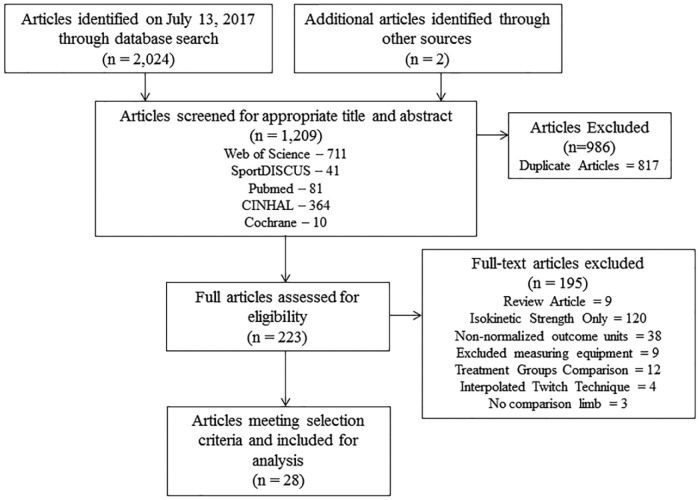
Article search results and the process of article removal are presented in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram (Figure 1).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of article selection for meta-analysis.
The initial search obtained 2024 articles. A total of 1209 articles remained once duplicates were removed. The remaining abstracts and titles of articles were evaluated to further reduce the search to 223 articles. From these articles, 9 articles were removed because they were systematic reviews. All these systematic reviews did not include unilateral quadriceps strength outcomes in their analysis, with the exception of 1 that evaluated CAR. The reference section of the systematic review20 that evaluated CARs in ACLR individuals was evaluated for article inclusion. A total of 120 articles were removed because they only provided isokinetic strength assessment. Nine articles were removed because they used equipment to measure isometric strength besides a machine-based dynamometer, such as a handheld dynamometer. Twelve articles were removed because they involved the comparison of outcomes of more than 1 ACLR group. Four articles were removed because they used the interpolated twitch technique to calculate CAR and not the superimposed burst technique. Three articles were removed because contralateral or healthy control comparison limb groups were not included. After full-text review, a total of 28 articles remained for evaluation.
Assessment of Methodological Quality
Based on the modified version of the Downs and Black checklist, 10 studies5,13,18,25,28,40,41,52,55,56 of the 28 were classified as low quality, 8 studies4,22,27,29,36,42,44,46 were classified as moderate quality, and 10 studies9,12,17,23,30-32,35,37,50 were classified as high quality. Of the articles assessed, 12% were not representative of overall clinical populations, 40% had selection bias, 36% did not identify a comparison group, 100% did not blind the assessors, 61% did not describe potential confounding variables, 9% did not adjust for potential confounders, and 73% did not report the appropriate sample size to power the study. The methodological quality for all articles included in the meta-analysis is reported in Tables 1 through 4.
Table 4.
Descriptive information of articles included in quadriceps function patellar tendon and hamstring tendon group ACLR comparisons
| Study | Participant Population | Modified Downs and Black Score | Patellar Tendon Group | Hamstring Tendon Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Sex | Age, y | Time Since Surgery | MVIC, N·m/kg, Mean ± SD | CAR, %, Mean ± SD | n | Sex | Age, y | Time Since Surgery | MVIC, N·m/kg, Mean ± SD | CAR, %, Mean ± SD | |||
| Norte et al (2018)40 | Orthopaedic clinic | 9 | — | — | — | — | — | — | 4 | 2M, 2F | 27.4 | 7.4 mo | INV = 1.95 ± 0.43 CON = 2.92 ± 0.8 |
INV = 94.66 ± 2.66 CON = 81.37 ± 5.16 |
| Harput et al (2015)18 | General population | 9 | — | — | — | — | — | — | 24 | 24M | 28.1 | 1 mo | Month 1: INV = 1.47 ± 0.62 CON = 2.6 ± 0.63 Month 2: INV = 2.18 ± 0.65 CON = 2.88 ± 0.73 Month 3:INV = 2.61 ± 0.67 CON = 3.14 ± 0.64 |
— |
| Thomas et al (2016)52 | — | 9 | 20 | 7M, 13F | 20.7 | 212.9 d | INV = 2.03 ± 0.51 CON = 2.89 ± 0.81 |
INV = 87 ± 12 CON = 85 ± 57 |
— | — | — | — | — | — |
| Lepley and Palmieri-Smith(2016)35 | Orthopaedic clinic | 13 | 54 | 23M, 31F | 19.9 | 7.2 mo | INV = 2.2 ± 0.6 CON = 3.2 ± 5.8 |
INV = 88.8 ± 9.1 CON = 92.7 ± 7.3 |
— | — | — | — | — | — |
ACLR, anterior cruciate ligament reconstruction; CAR, central activation ratio; CON, contralateral ACLR limb; F, female; INV, involved ACLR limb; M, male; MVIC, maximal voluntary isometric contraction.
Knee Extension MVIC Torque
The healthy control limb had the largest weighted mean MVIC (2.93 ± 0.72 N·m/kg; 95% CI, 2.39-2.56), followed by the ACLR contralateral limb (2.90 ± 0.63 N·m/kg; 95% CI, 2.61-3.18) and ACLR limb (2.38 ± 0.63 N·m/kg; 95% CI, 2.13-2.63). When comparing the ACLR limb with the healthy control limb, the combined meta-analysis effect was considered moderate with narrow CIs that did not cross the y-axis. When evaluating the individual studies, 5 studies5,13,23,32,44 demonstrated a strong effect, 3 studies22,50,56 demonstrated a moderate effect, and 7 studies12,25,27,28,30,31,46 demonstrated a small effect. Notably, all included data points reported a conclusive negative ES, meaning that each study observed MVIC values that were lower in the ACLR limb compared with the healthy limb (Table 5).
Table 5.
Effect sizes and 95% CIs for articles including MVIC ACLR and healthy groups
| Study | ACLR Limb vs Healthy Control Limb | ACLR Limb vs Contralateral Limb | Contralateral Limb vs Healthy Control Limb |
|---|---|---|---|
| Harput et al (2015)18 (1 mo) | — | −1.77 [−2.45, −1.11] | — |
| Harput et al (2015)18 (2 mo) | — | −1.00 [−1.60, −0.40] | — |
| Harput et al (2015)18 (3 mo) | — | −0.80 [−1.38, −0.21] | — |
| Lepley et al (2015)32 | −1.14 [−1.18, −0.47] | −0.27 [−0.89, 0.35] | −0.83 [−1.47, −0.18] |
| Lepley and Palmieri-Smith (2016)35 | — | −1.40 [−1.83, −0.98] | — |
| Norte et al (2018)40 | — | −1.31 [−2.84, 0.22] | — |
| Zwolski et al (2015)55 | — | −0.60 [−0.84, −0.36] | — |
| Zwolski et al (2016)56 | −0.68 [−1.33, −0.02] | −0.80 [−1.54, −0.05] | 0.00 [−0.64, 0.64] |
| Oberlander et al (2013)41 | — | −0.73, [−1.63, 0.18] | — |
| Jordan et al (2015)23 | −1.06 [−2.11, −0.02] | −1.14 [−2.19, −0.08] | 0.41 [−0.58, 1.40] |
| Holsgaard-Larsen et al (2014)22 | −0.56 [−1.17, −0.02] | −0.36 [−0.94, 0.23] | −0.25 [−0.82, 0.32] |
| Kuenze et al (2015a)27 | −0.38 [−0.96, 0.20] | — | −0.14 [−0.44, 0.72] |
| Kuenze et al (2015b)28 | −0.47 [−1.06, 0.12] | −0.55 [−1.16, 0.05] | −0.15 [−0.43, 0.73] |
| Kuenze et al (2015c)30 | −0.47 [−1.05, 0.12] | −0.55 [−1.15, 0.05] | — |
| Blackburn et al (2016)4 | — | −0.36 [−0.81, 0.09] | — |
| Davis et al (2017)9 | — | −0.35 [−0.80, 0.09] | — |
| Goetschius and Hart (2016)12 | −0.44 [−0.84, 0.05] | — | — |
| Goetschius et al (2015)13 | −0.84 [−1.35, −0.33] | — | — |
| Pietrosimone et al (2015)46 | −0.47 [−1.00, 0.05] | — | — |
| Lepley et al (2014)31 | −0.49 [−1.01, 0.03] | — | — |
| Pamukoff et al (2017)44 | −1.17 [−1.84, −0.50] | — | — |
| Krishnan and Williams (2011)25 | −0.48 [−1.20, 0.25] | −0.38 [−1.10, 0.34] | −0.16 [−0.88, 0.55] |
| Thomas et al (2013)51 | — | −1.25 [−1.92, −0.56] | — |
| Thomas et al (2015)50 | −0.77 [−1.48, −0.06] | −1.27 [−2.00, −0.53] | 0.29 [−0.39, 0.98] |
| Chang et al (2014)5 | −3.90 [−5.48, −2.35] | −0.81 [−1.83, 0.21] | −3.01 [−4.36, −1.65] |
| Pooled effect size | −0.76 [−0.98, −0.53] | −0.78 [−0.99, −0.58] | −0.24 [−0.68, 0.19] |
ACLR, anterior cruciate ligament reconstruction; MVIC, maximal voluntary isometric contraction.
Similar findings were observed when the ACLR limb was compared with the contralateral limb with narrow CIs that did not cross the y-axis. The combined meta-analysis effect was considered strong. Eight5,18,23,35,40,50,51,56 of the included studies demonstrated a strong effect, 4 studies28,30,41,55 had a moderate effect, and 5 studies4,9,22,25,32 had a small effect. All included data points reported a conclusive negative ES, meaning that each included study observed MVIC values that were lower in the ACLR limb compared with the contralateral limb (Table 6).
Table 6.
Effect sizes and 95% CIs for articles including CAR ACLR and healthy groups
| Study | ACLR Limb vs Healthy Control Limb | ACLR Limb vs Contralateral Limb | Contralateral Limb vs Healthy Control Limb |
|---|---|---|---|
| Lepley et al (2015)32 | −1.32 [−2.01, −0.64] | −0.31 [−0.94, 0.31] | −0.99 [−1.64, −0.33] |
| Lepley and Palmieri-Smith (2016)35 | — | −0.47 [−0.85, −0.09] | — |
| Norte et al (2018)40 | — | 2.81 [0.86, 4.77] | — |
| Kuenze et al (2015a)27 | −0.74 [−1.35, −0.13] | — | — |
| Kuenze et al (2015c)30 | −0.75 [−1.35, −0.15] | −0.54 [−1.14, 0.06] | −0.15 [−0.74, 0.44] |
| Harkey et al (2016)17 | −0.70 [−1.03, −0.36] | −0.11 [−0.44, 0.21] | −0.56 [−0.89, 0.23] |
| Goetschius and Hart (2016)12 | −0.64 [−1.03, −0.24] | — | — |
| Luc-Harkey et al (2017)37 | — | −0.09 [−0.63, 0.44] | — |
| Pietrosimone et al (2015)46 | −0.23 [−0.75, 0.30] | 0.00 [−0.53, 0.53] | −0.23 [−0.75, 0.30] |
| Lepley et al (2014)31 | −0.87 [−1.41, −0.33] | — | — |
| Blackburn et al (2016)4 | — | 0.00 [−0.44, 0.44] | — |
| Pamukoff et al (2017)44 | −1.25 [−1.93, −0.57] | — | — |
| Thomas et al (2015)50 | −0.65 [−1.35, 0.05] | −0.15 [−0.47, 0.77] | — |
| Otzel et al (2015)42 | −0.17 [−0.74, 0.40] | −0.30 [−0.87, 0.27] | 0.19 [−0.37, 0.76] |
| Chang et al (2014)5 | −6.10 [−8.30, −3.90] | −0.32 [−1.30, 0.67] | −5.76 [−7.85, −3.66] |
| Pooled effect size | −0.84 [−1.18, −0.50] | −0.15 [−0.37, 0.07] | −0.73 [−1.39, −0.07] |
ACLR, anterior cruciate ligament reconstruction; CAR, central activation ratio.
When comparing the contralateral limb to the healthy control limb, the combined meta-analysis effect was considered small with narrow CIs that crossed the y-axis. Two5,32 of the included studies demonstrated strong negative effects, 3 studies22,23,50 demonstrated small effects, and 4 studies25,27,28,56 were classified as weak. Data were heterogeneous and inconsistent, as individual studies demonstrated both negative (contralateral limb MVIC smaller than healthy limb) and positive (contralateral limb MVIC greater than healthy limb) effects (Table 5).
Quadriceps Central Activation Ratio
CAR weighted mean results were similar to the MVIC weighted means where the healthy control limb had the largest weighted mean CAR (93.44% ± 5.24%; 95% CI, 90.34%-96.54%) followed by the contralateral limb (90.00% ± 8.04%; 95% CI, 85.24%-94.75%) and ACLR limb (87.46% ± 9.66%; 95% CI, 82.73%-92.20%). When comparing the ACLR limb to the healthy control limb, the combined meta-analysis effect was considered strong with CIs that did not cross the y-axis. When evaluating the individual studies, 4 studies5,31,32,44 demonstrated a strong effect, 5 studies12,17,27,30,50 demonstrated a moderate effect, 1 study46 demonstrated a small effect, and 1 study42 demonstrated a weak effect. Notably, all included data points reported a conclusive negative ES, meaning that each study observed CAR values that were lower in the ACLR limb compared with the healthy limb (Table 6).
For the comparison of the ACLR to contralateral limb, the combined meta-analysis effect was considered weak, with CIs that crossed the y-axis. One study40 demonstrated a strong positive effect, 1 study30 had a moderate effect, 4 studies5,32,35,42 had a small effect, and 5 studies4,17,37,46,51 had a weak effect. Data were heterogeneous and inconsistent, as individual studies demonstrated negative (ACLR limb CAR smaller than contralateral limb), positive (ACLR limb MVIC greater than contralateral limb), and zero (ACLR limb MVIC is equal to contralateral limb) effects (Table 5).
When comparing the contralateral limb to the healthy control limb, the combined meta-analysis effect was considered moderate, with CIs that did not cross the y-axis. When evaluating the individual studies, 2 studies5,32 demonstrated a strong effect, 1 study17 demonstrated a moderate effect, 1 study46 had a small effect, and 2 studies30,42 demonstrated a weak effect. All but 1 study reported a negative ES, meaning that these studies observed CAR values that were lower in the contralateral limb compared with the healthy limb (Table 6).
Effects of Sex and Graft Type
Only 1 study29 reported a sex-based comparison for MVIC of the ACLR limbs between female and male participants, yielding a weak effect with wide CIs. Two studies29,42 reported a sex-based comparison for CAR of the ACLR limbs between female and male participants. Data were heterogeneous, as 1 study42 demonstrated a weak positive effect while the other29 yielded a small negative effect. The combined meta-analysis effect for these 2 studies was considered weak, with CIs that crossed the y-axis (see Table 3).
Table 3.
Descriptive information of articles included in quadriceps function male and female ACLR comparisons
| Study | Participant Population | Modified Downs and Black Score | Male Group | Female Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age, y | Time Since Surgery | MVIC, N·m/kg, Mean ± SD | CAR, %, Mean ± SD | n | Age, y | Time Since Surgery | MVIC, N·m/kg, Mean ± SD | CAR, %, Mean ± SD | |||
| Otzel et al (2015)42 | Recreationally active university students | 10 | 24 | 21.3 | 39.6 mo | — | INV = 90 ± 7.8 CON = 92 ± 7.6 |
13 | 20.2 | 30.0 mo | — | INV = 92 ± 6.5 CON = 95 ± 3.9 |
| Kuenze et al (2014)29 | Active volunteers from university community (able to complete 30 min of aerobic exercise) | 10 | 13 | 24.1 | 43.5 mo | INV = 2.63 ± 0.68 | INV = 75.4 ± 10.2 | 13 | 24.2 | 45.8 mo | INV = 2.56 ± 0.7 | INV = 75 ± 16.4 |
ACLR, anterior cruciate ligament reconstruction; CAR, central activation ratio; CON, contralateral ACLR limb; F, female; INV, involved ACLR limb; M, male; MVIC, maximal voluntary isometric contraction.
No study reported a direct comparison between graft types; therefore, comparisons were made between the ACLR and contralateral limbs for the individual graft types. Two studies18,40 reported ACLR and contralateral limb MVIC for hamstring tendon autografts. All ESs were negative and ranged from moderate to strong. The combined meta-analysis effect for these 4 studies was considered strong, with CIs that did not cross the y-axis, indicating that the ACLR limb was weaker than the contralateral limb in patients with hamstring tendon grafts. Only 1 study40 reported ACLR and contralateral limb CAR data for hamstring tendon autografts, yielding a strong positive effect with CIs that did not cross the y-axis. Two studies35,52 reported ACLR and contralateral limb MVIC for patellar tendon autografts. Both ESs were negative and classified as small or strong. The combined meta-analysis effect for these 2 studies was considered moderate, with CIs that crossed the y-axis. Finally, 2 studies35,52 reported ACLR and contralateral limb CAR data for patellar tendon autografts. ESs were heterogeneous, with 1 demonstrating a weak positive effect and the other classified as a small negative effect. The combined meta-analysis effect for these 2 studies was considered small, with CIs that crossed the y-axis (see Table 4).
Discussion
This analysis provides insight into the magnitude and consistency of persistent quadriceps weakness and decreased activation among individuals with a history of ACLR. The primary result of this review was that participants with ACLR consistently experience weakness and activation deficits in the involved limb compared with the control group. Quadriceps weakness was present in the involved limb compared with the contralateral limb in ACLR individuals. Although not homogeneous among all included studies, the contralateral limbs of the ACLR participants also demonstrated lesser CAR, but not strength, compared with healthy controls. Overall, these results suggest that individuals returning to ACLR continue to experience involved limb strength deficits and bilateral CAR deficits despite completion of rehabilitation and clearance from health care professionals to return to physical activity.
When comparing limb outcomes within ACLR participants and outcomes of ACLR participants compared with healthy controls, conclusive moderate ESs indicate lower knee extension MVIC in the involved limb of ACLR patients. These deficits persist across time18,25,32 and may contribute to alter movement patterns for years after surgery, leading to a greater risk of developing osteoarthritis.4 While the magnitude of this ES is only moderate, 100% of the studies pooled for analysis indicated decreased knee extension strength in the involved limb of ACLR patients compared with their contralateral limb and with healthy controls. Decreases in quadriceps muscle size39,40,52 and alterations in morphology39 appear soon after initial injury and may continue to persist for up to 6 months when many individuals return to play. These factors are hypothesized to contribute to deficits in involved limb strength. The homogeneous results of previous studies included in this meta-analysis support the need for continued focus on improving involved limb knee extension strength after ACLR.
A recent review of the literature32 reported a 14% limb symmetry deficit between involved and contralateral limbs for up to 12 months after ACLR surgery, further supporting the results of this analysis. A limitation of limb symmetry assessment is the lack of information provided about the state of quadriceps strength in the contralateral limb. The previous limb symmetry strength review32 provides important information about a commonly used strength outcome measure, but our analysis differs because it addresses the magnitude of differences in both limbs, especially in the contralateral limb. This analysis demonstrates heterogeneous results and low, inconclusive ESs regarding MVIC differences between the contralateral and healthy limbs. This inconclusive finding supports the drawbacks of only using symmetry-based assessment and further supports the additional use of unilateral strength-based assessments to help provide a more complete picture of quadriceps strength. While inconclusive, these results demonstrate that contralateral deficits may continue to impair some but not all individuals after ACLR. Clinicians should be aware of these potential changes and should consistently reevaluate and incorporate interventions that focus on bilateral quadriceps strength.
Individual perceptions of knee function are affected by quadriceps strength after ACLR.27,45 Unilateral strength is a strong predictor of self-reported knee function, and individuals with ACLR who achieve 3.0 N·m/kg of knee extension strength also report better knee-related function.27,45 Based on the individual studies included in this analysis, individuals with ACLR do not appear to consistently achieve satisfactory levels of quadriceps strength (3.0 N·m/kg) regardless of time since surgery (see Figure A7 in the Appendix, available online). This finding was not homogeneous, as 2 of the studies23,25 reported means and CIs that included the cutoff value for optimal involved limb quadriceps strength (3.0 N·m/kg). However, it should be noted that these results may differ because 1 of the studies23 included elite athletes who consistently train at higher rates compared with the general healthy population while the other study25 included participants who reported minimal knee-related symptoms or functional limitations after surgery, which may not be consistent with the general population. These findings indicate not only that individuals with ACLR experience decrements in involved limb quadriceps strength but that the magnitude of this decrement may be sufficient to result in persistent reductions in patient-reported knee-related function.
CAR is also altered in individuals with a history of ACLR. Our results demonstrate a moderate effect for differences in CAR between both the ACLR-involved limb and contralateral limb compared with healthy controls. This result was homogeneous for the involved limb across all studies, while all but 1 study30 indicated a decrement in the contralateral limb compared with healthy controls. The results of this analysis are similar to a previous systematic review20 that reported CAR deficits in the involved and contralateral limb. Bilateral deficits in quadriceps activation after ACLR are common and should be addressed during rehabilitation by creating an effective treatment targeting central activation deficits.16 Targeting neural mechanisms through disinhibitory therapeutic modalities and exercise interventions in individuals with decreased CAR has been shown to help improve strength in individuals after surgery.16
We attempted to evaluate the effects of patient sex and graft type on knee extension MVIC strength and CAR after ACLR. Females reported decreased subjective knee function, rates of return to sport, and engagement in physical activity compared with males.48 Only 1 study29 reported isometric knee extension strength comparisons, and 2 studies29,42 reported CAR comparisons between males and females (see Table 3). Both studies29,42 yielded heterogeneous results that were weak and inconclusive. Unlike participant sex, graft type is a modifiable surgical factor that has been shown to have effects on functional performance and patient-reported outcomes after ACLR.1,6 Unfortunately, none of the included studies directly compared knee extension MVIC strength or quadriceps CAR between graft types (see Table 4). The lack of studies evaluating patient sex and graft differences reveals a significant limitation in this analysis and a gap in the literature on isometric knee extension strength and CAR assessment. Sex and graft differences in quadriceps function may contribute to negative outcomes after ACLR, but this is only speculation, and future research should assess unilateral quadriceps strength and activation in these groups.
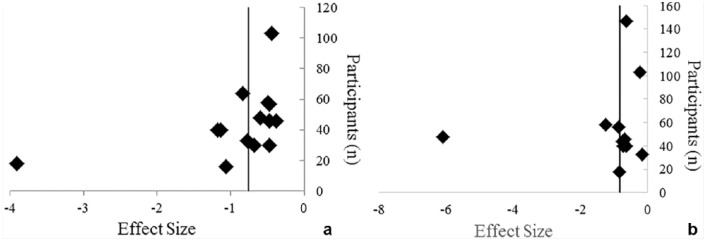
Based on the methodological heterogeneity of the studies, it is difficult to assess the role that surgical factors, including time since surgery, pain at time of testing, and meniscal outcomes, contribute to changes in quadriceps function. Many studies did not report these surgical factors and they could not be included in this analysis. The effect of surgical factors should be taken into consideration when designing studies assessing quadriceps function moving forward. Additionally, it should be noted that a small number of laboratories were responsible for many of the included studies and that some participants may have overlapped within studies conducted in the same laboratories. If this occurred, overlapping data extracted from these studies may not be independent of one another, which should be considered when interpreting study results. Publication bias was apparent in the analysis, as seen by the increased number of studies to the right of the pooled ES line compared with the left in Figures 2a and 2b. This asymmetrical shift indicates a trend toward a smaller ES where involved limb quadriceps function may not be different compared with the contralateral or healthy limb. One article5 with a small sample size demonstrated that a larger ES indicated poorer involved limb quadriceps function compared with healthy or control limbs. The random-effects model meta-analysis approach was chosen to help account for factors that may contribute to heterogeneity between studies such as sample size. Replication of this work in a great number of settings would likely reduce bias and improve the generalizability of these results and testing methodology.
Figure 2.
Publication bias assessment of (a) isometric quadriceps strength5,11,12,22,23,25,27,28,30,31,32,43,45,49,51,54 and (b) central activation ratio5,12,17,28,30-32,41,43,45,49 effect size comparisons of the anterior cruciate ligament–reconstructed limb and healthy control limb.
Regaining quadriceps function after ACLR is imperative to improve a number of physical and psychological outcomes when returning to daily life and knee function years beyond the cessation of treatment. Unfortunately, individuals with a history of ACLR demonstrate persistent decreases in involved limb quadriceps strength and activation compared with healthy controls. Quadriceps activation also remains decreased in the contralateral limb compared with controls. Factors that contribute to these changes in function are multifaceted and should be continuously assessed and addressed with evidence-based treatment approaches. A gap exists in the literature evaluating sex-based differences in quadriceps function.
Clinical Recommendations
Involved limb quadriceps function is persistently affected after ACLR. The source and functional manifestations of these impairments is multifactorial and may differ during assessment depending on the stage of rehabilitation. Unilateral and symmetry-based assessment may provide valuable evidence about the source and magnitude of quadriceps dysfunction affecting individuals with ACLR, which may help guide evidence-based rehabilitation strategies.
Supplemental Material
Supplemental material, Appendix for Quadriceps Strength and Volitional Activation After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis by Caroline Lisee, Adam S. Lepley, Thomas Birchmeier, Kaitlin O’Hagan and Christopher Kuenze in Sports Health: A Multidisciplinary Approach
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Abrams GD, Harris JD, Gupta AK, et al. Functional performance testing after anterior cruciate ligament reconstruction: a systematic review. Orthop J Sports Med. 2014;2:2325967113518305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. Am J Sports Med. 2011;39:538-543. [DOI] [PubMed] [Google Scholar]
- 3. Bell DR, Pfeiffer KA, Cadmus-Bertram LA, et al. Objectively measured physical activity in patients after anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45:1893-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blackburn JT, Pietrosimone B, Harkey MS, Luc BA, Pamukoff DN. Quadriceps function and gait kinetics after anterior cruciate ligament reconstruction. Med Sci Sport Exerc. 2016;48:1664-1670. [DOI] [PubMed] [Google Scholar]
- 5. Chang E, Kim KM, Hertel J, Hart JM. Repeated bouts of exercise in patients with anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2014;46:769-775. [DOI] [PubMed] [Google Scholar]
- 6. Chee MY, Chen Y, Pearce CJ, et al. Outcome of patellar tendon versus 4-strand hamstring tendon autografts for anterior cruciate ligament reconstruction: a systematic review and meta-analysis of prospective randomized trials. Arthroscopy. 2017;33:450-463. [DOI] [PubMed] [Google Scholar]
- 7. Chung KS, Ha JK, Yeom CH, et al. Are muscle strength and function of the uninjured lower limb weakened after anterior cruciate ligament injury? Two-year follow-up after reconstruction. Am J Sports Med. 2015;43:3013-3021. [DOI] [PubMed] [Google Scholar]
- 8. Cohen J. Statistical Power Analysis for Behavioral Sciences. New York, NY: Academic Press; 1977. [Google Scholar]
- 9. Davis HC, Troy Blackburn J, Ryan ED, et al. Quadriceps rate of torque development and disability in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2017;46:52-56. [DOI] [PubMed] [Google Scholar]
- 10. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goetschius J, Hart JM. Knee-extension torque variability and subjective knee function in patients with a history of anterior cruciate ligament reconstruction. J Athl Train. 2016;51:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goetschius J, Kuenze CM, Hart JM. Knee extension torque variability after exercise in ACL reconstructed knees. J Orthop Res. 2015;33:1165-1170. [DOI] [PubMed] [Google Scholar]
- 14. Gokeler A, Welling W, Zaffagnini S, Seil R, Padua D. Development of a test battery to enhance safe return to sports after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50:804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harkey MS, Gribble PA, Pietrosimone BG. Disinhibitory interventions and voluntary quadriceps activation: a systematic review. J Athl Train. 2014;49:411-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harkey MS, Luc-Harkey BA, Lepley AS, et al. Persistent muscle inhibition after anterior cruciate ligament reconstruction: role of reflex excitability. Med Sci Sports Exerc. 2016;48:2370-2377. [DOI] [PubMed] [Google Scholar]
- 18. Harput G, Kilinc HE, Ozer H, Baltaci G, Mattacola CG. Quadriceps and hamstring strength recovery during early neuromuscular rehabilitation after ACL hamstring-tendon autograft reconstruction. J Sport Rehabil. 2015;24:398-404. [DOI] [PubMed] [Google Scholar]
- 19. Hart HF, Culvenor AG, Collins NJ, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2016;50:597-612. [DOI] [PubMed] [Google Scholar]
- 20. Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45:87-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiemstra LA, Webber S, MacDonald PB, Kriellaars DJ. Contralateral limb strength deficits after anterior cruciate ligament reconstruction using a hamstring tendon graft. Clin Biomech (Bristol, Avon). 2007;22:543-550. [DOI] [PubMed] [Google Scholar]
- 22. Holsgaard-Larsen A, Jensen C, Mortensen NH, Aagaard P. Concurrent assessments of lower limb loading patterns, mechanical muscle strength and functional performance in ACL-patients—a cross-sectional study. Knee. 2014;21:66-73. [DOI] [PubMed] [Google Scholar]
- 23. Jordan MJ, Aagaard P, Herzog W. Rapid hamstrings/quadriceps strength in ACL-reconstructed elite alpine ski racers. Med Sci Sports Exerc. 2015;47:109-119. [DOI] [PubMed] [Google Scholar]
- 24. Kaeding CC, Léger-St-Jean B, Magnussen RA. Epidemiology and diagnosis of anterior cruciate ligament injuries. Clin Sports Med. 2017;36:1-8. [DOI] [PubMed] [Google Scholar]
- 25. Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuenze C, Eltouhky M, Thomas A, Sutherlin M, Hart J. Validity of torque-data collection at multiple sites: a framework for collaboration on clinical-outcomes research in sports medicine. J Sport Rehabil. 2016;25:173-180. [DOI] [PubMed] [Google Scholar]
- 27. Kuenze C, Hertel J, Saliba S, Diduch DR, Weltman A, Hart JM. Clinical thresholds for quadriceps assessment after anterior cruciate ligament reconstruction. J Sport Rehabil. 2015;24:36-46. [DOI] [PubMed] [Google Scholar]
- 28. Kuenze CM, Foot N, Saliba SA, Hart JM. Drop-landing performance and knee-extension strength after anterior cruciate ligament reconstruction. J Athl Train. 2015;50:596-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuenze CM, Hertel J, Hart JM. Quadriceps muscle function after exercise in men and women with a history of anterior cruciate ligament reconstruction. J Athl Train. 2014;49:740-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuenze CM, Hertel J, Weltman A, Diduch D, Saliba SA, Hart JM. Persistent neuromuscular and corticomotor quadriceps asymmetry after anterior cruciate ligament reconstruction. J Athl Train. 2015;50:303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lepley AS, Ericksen HM, Sohn DH, Pietrosimone BG. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. Knee. 2014;21:736-742. [DOI] [PubMed] [Google Scholar]
- 32. Lepley AS, Gribble PA, Thomas AC, Tevald MA, Sohn DH, Pietrosimone BG. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25:828-839. [DOI] [PubMed] [Google Scholar]
- 33. Lepley AS, Pietrosimone B, Cormier ML. Quadriceps function, knee pain, and self-reported outcomes in patients with anterior cruciate ligament reconstruction. J Athl Train. 2018;53:337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lepley LK. Deficits in quadriceps strength and patient-oriented outcomes at return to activity after ACL reconstruction: a review of the current literature. Sports Health. 2015;7:231-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lepley LK, Palmieri-Smith RM. Pre-operative quadriceps activation is related to post-operative activation, not strength, in patients post-ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016;24:236-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lepley LK, Palmieri-Smith RM. Quadriceps strength, muscle activation failure, and patient-reported function at the time of return to activity in patients following anterior cruciate ligament reconstruction: a cross-sectional study.J Orthop Sports Phys Ther. 2015;45:1017-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luc-Harkey BA, Harkey MS, Pamukoff DN, et al. Greater intracortical inhibition associates with lower quadriceps voluntary activation in individuals with ACL reconstruction. Exp Brain Res. 2017;235:1129-1137. [DOI] [PubMed] [Google Scholar]
- 38. Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49:806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noehren B, Andersen A, Hardy P, et al. Cellular and morphological alterations in the vastus lateralis muscle as the result of ACL injury and reconstruction. J Bone Joint Surg Am. 2016;98:1541-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Norte GE, Knaus KR, Kuenze C, et al. MRI-based assessment of lower extremity muscle volumes in patients before and after ACL reconstruction. J Sport Rehabil. 2018;27:201-212. [DOI] [PubMed] [Google Scholar]
- 41. Oberlander KD, Bruggemann GP, Hoher J, Karamanidis K. Altered landing mechanics in ACL-reconstructed patients. Med Sci Sports Exerc. 2013;45:506-513. [DOI] [PubMed] [Google Scholar]
- 42. Otzel DM, Chow JW, Tillman MD. Long-term deficits in quadriceps strength and activation following anterior cruciate ligament reconstruction. Phys Ther Sport. 2015;16:22-28. [DOI] [PubMed] [Google Scholar]
- 43. Palmieri-Smith RM, Lepley LK. Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med. 2015;43:1662-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pamukoff DN, Pietrosimone BG, Ryan ED, Lee DR, Blackburn JT. Quadriceps function and hamstrings co-activation after anterior cruciate ligament reconstruction. J Athl Train. 2017;52:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pietrosimone B, Lepley AS, Harkey MS, et al. Quadriceps strength predicts self-reported function post-ACL reconstruction. Med Sci Sports Exerc. 2016;48:1671-1677. [DOI] [PubMed] [Google Scholar]
- 46. Pietrosimone BG, Lepley AS, Ericksen HM, Clements A, Sohn DH, Gribble PA. Neural excitability alterations after anterior cruciate ligament reconstruction. J Athl Train. 2015;50:665-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rohman E, Steubs JT, Tompkins M. Changes in involved and uninvolved limb function during rehabilitation after anterior cruciate ligament reconstruction: implications for Limb Symmetry Index measures. Am J Sports Med. 2015;43:1391-1398. [DOI] [PubMed] [Google Scholar]
- 48. Tan SHS, Lau BPH, Khin LW, Lingaraj K. The importance of patient sex in the outcomes of anterior cruciate ligament reconstructions. Am J Sports Med. 2015;44:242-254. [DOI] [PubMed] [Google Scholar]
- 49. Tengman E, Brax Olofsson L, Stensdotter AK, Nilsson KG, Hager CK. Anterior cruciate ligament injury after more than 20 years. II. Concentric and eccentric knee muscle strength. Scand J Med Sci Sports. 2014;24:e501-e509. [DOI] [PubMed] [Google Scholar]
- 50. Thomas AC, Lepley LK, Wojtys EM, McLean SG, Palmieri-Smith RM. Effects of neuromuscular fatigue on quadriceps strength and activation and knee biomechanics in individuals post-anterior cruciate ligament reconstruction and healthy adults. J Orthop Sports Phys Ther. 2015;45:1042-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomas AC, Villwock M, Wojtys EM, Palmieri-Smith RM. Lower extremity muscle strength after anterior cruciate ligament injury and reconstruction. J Athl Train. 2013;48:610-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thomas AC, Wojtys EM, Brandon C, Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport. 2016;19:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wellsandt E, Failla MJ, Snyder-Mackler L. Limb symmetry indexes can overestimate knee function after anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2017;47:334-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xergia SA, Pappas E, Zampeli F, Georgiou S, Georgoulis AD. Asymmetries in functional hop tests, lower extremity kinematics, and isokinetic strength persist 6 to 9 months following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2013;43:154-162. [DOI] [PubMed] [Google Scholar]
- 55. Zwolski C, Schmitt LC, Quatman-Yates C, Thomas S, Hewett TE, Paterno MV. The influence of quadriceps strength asymmetry on patient-reported function at time of return to sport after anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43:2242-2249. [DOI] [PubMed] [Google Scholar]
- 56. Zwolski C, Schmitt LC, Thomas S, Hewett TE, Paterno MV. The utility of limb symmetry indices in return-to-sport assessment in patients with bilateral anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44:2030-2038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix for Quadriceps Strength and Volitional Activation After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis by Caroline Lisee, Adam S. Lepley, Thomas Birchmeier, Kaitlin O’Hagan and Christopher Kuenze in Sports Health: A Multidisciplinary Approach