Abstract
Hunter syndrome (mucopolysaccharidosis II [MPS II]), a deficiency of iduronate-2-sulfatase (IDS), causes an accumulation of glycosaminoglycans, giving rise to multiple systemic and CNS symptoms. The currently available therapies, idursulfase and idursulfase beta, are ineffective against the CNS symptoms because they cannot pass the blood-brain barrier (BBB). A novel IDS fused with anti-human transferrin receptor antibody (JR-141) has been shown to penetrate the BBB and ameliorate learning deficits in model mice. This first-in-human study evaluated the pharmacokinetics, safety, and potential efficacy of JR-141 in 14 patients with MPS II. In a dose-escalation study performed in two patients, JR-141 plasma concentrations were dose dependent and peaked at 3 hr after initiation of each infusion, and no or only mild adverse reactions were exhibited. In a subsequent 4-week evaluation at two dose levels, the plasma concentration profiles were similar between the first and final administration, indicating no drug accumulation. Levels of heparan sulfate (HS) and dermatan sulfate (DS) were suppressed in both plasma and urine and HS levels were significantly decreased in cerebrospinal fluid. Two patients experienced some amelioration of neurocognitive and motor symptoms. These results suggest that the drug successfully penetrates the BBB and could have CNS efficacy.
Keywords: mucopolysaccharidosis II, Hunter syndrome, blood brain barrier, iduronate-2-sulfatase, CNS, cognitive impairment, anti-human transferrin receptor antibody, heparan sulfate, dermatan sulfate, enzyme-replacement therapy, clinical trial
Okuyama et al. report on a first-in-human study of iduronate-2-sulfatase fused with anti-human transferrin receptor antibody (JR-141) in 14 patients with mucopolysaccharidosis II to evaluate its pharmacokinetics, safety, and potential efficacy. The results show successful penetration of the compound across the blood-brain barrier, suggestive of its effect for neurodegeneration.
Introduction
Hunter syndrome (mucopolysaccharidosis II [MPS II]) is an X-linked recessive lysosomal storage disease caused by a deficiency of iduronate-2-sulfatase (IDS).1 Since IDS is an essential enzyme for the catabolism of glycosaminoglycans (GAGs) such as heparan sulfate (HS) and dermatan sulfate (DS), mutations in the IDS gene lead to pathological accumulation of GAGs in the lysosomes in most cells throughout the body, resulting in a broad spectrum of symptoms, including coarse facies, visceromegaly (hepatosplenomegaly), umbilical hernia, joint stiffness, upper airway obstruction, and cardiac dysfunctions.1, 2, 3 In addition to these visceral and skeletal symptoms, progressive deterioration of the CNS is common in MPS II.2, 3 The estimated worldwide incidence of MPS II at birth is 0.13–1.07/100,000.4
Enzyme replacement therapy with recombinant human IDS (idursulfase, Elaprase, and idursulfase beta, Hunterase) has been used to treat MPS II and is known to improve the aforementioned somatic symptoms.5, 6, 7, 8 However, intravenously administrated idursulfase cannot cross the blood-brain barrier (BBB) in sufficient quantity to address the neurocognitive disorders caused by MPS II, which remain a debilitating issue for patients and are therefore a major focus of lysosomal disorder research.3
Attempts have been made to boost brain uptake of drugs across the BBB.9, 10, 11, 12 Various receptors (e.g., insulin13, 14 and transferrin15) have been targeted, where, by way of transcytosis, drugs were expected to be delivered to exert effects on the brain. Positive results have been reported in mice, rats, and monkeys, and recently in some patients with MPS I.14 Different administration routes (e.g., intrathecal16 and intracerebroventricular17 injections) have been attempted with the aim of directly delivering the enzymes to the brain, but practical difficulties for both patients and physicians remain significant hurdles to overcome.
A BBB-penetrating fusion protein designated JR-141, which consists of intact human IDS and anti-human transferrin receptor (hTfR) antibody, has been developed by JCR Pharmaceuticals.18 Transferrin is one of the proteins that crosses the BBB through a mechanism of receptor-mediated transcytosis, which is utilized in the delivery of JR-141 to the brain. JR-141 has been shown to be distributed in the brain parenchyma of hTfR knockin mice and monkeys after intravenous administration.18 Notably, intravenous administration of human IDS fused with anti-mouse TfR antibody has reduced GAG accumulations in both the peripheral tissues and the brains of MPS II model mice, leading to significant improvements of neurocognitive deficits.19 In addition, after administration of JR-141, the HS concentrations in the cerebrospinal fluid (CSF) decreased in parallel with those in the brain,20 indicating that GAGs in CSF can be used as an important biomarker of the pathophysiological activities of neuropathic MPS II. In this report, we present the results of a multicenter, open-label, randomized phase 1/2 clinical trial of JR-141 to evaluate its pharmacokinetics, safety, and efficacy in patients with MPS II.
Results
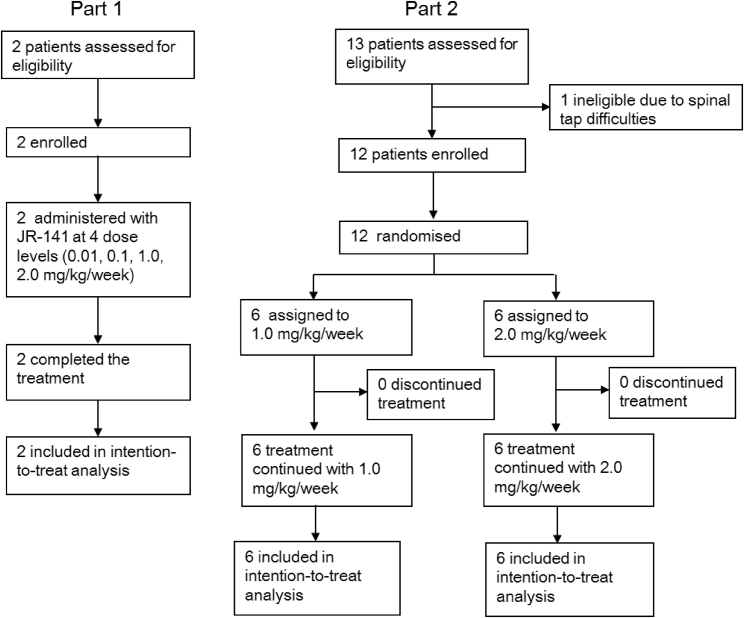
A total of 14 male patients were enrolled in the study (Figure 1), and their baseline demographics and clinical characteristics are shown in Table 1.
Figure 1.
Trial Profile
Table 1.
Demographics and Baseline Characteristics of Patients
| Subject ID | Part and Groupa | Sex and Age (Years) | Weight (kg) | MPS II-Related Intellectual Disability | Genetic Analysis Results | Anti-IDS Antibody |
|
|---|---|---|---|---|---|---|---|
| Negative/Positive | Antibody Titer | ||||||
| H1-01-01 | part 1, – | male, 33 | 47.5 | no | c.1351_1355delCCGTA | positive | 64 |
| H1-08-01 | part 1, – | male, 63 | 69.8 | no | N63D | negative | – |
| H1-02-01 | part 2, L | male, 6 | 26.7 | yes | recombination between IDS gene and IDS-2 gene | positive | 4,096 |
| H1-02-03 | part 2, L | male, 19 | 69.9 | no | – | negative | – |
| H1-03-01 | part 2, L | male, 10 | 52.0 | yes | P.Q121R | negative | – |
| H1-03-02 | part 2, H | male, 12 | 43.2 | yes | P.Q121R | positive | 1 |
| H1-04-01 | part 2, H | male, 14 | 44.2 | yes | – | negative | – |
| H1-05-01 | part 2, H | male, 10 | 29.7 | no | – | negative | – |
| H1-05-02 | part 2, L | male, 14 | 61.4 | no | – | positive | 4 |
| H1-06-01 | part 2, L | male, 7 | 21.1 | no | nonsense mutation of c.1327C > T(p.R443x) in exon9 | positive | 4,096 |
| H1-06-02 | part 2, L | male, 16 | 37.8 | yes | iduronate-2-sulfatase exon8(G365X 1092G > T) | negative | – |
| H1-07-01 | part 2, H | male, 25 | 44.7 | no | IDS C.1326C > T p.R443X | positive | 512 |
| H1-08-02 | part 2, H | male, 9 | 31.4 | yes | Ex8 TGG → TGA W337X | positive | 8,192 |
| H1-08-03 | part 2, H | male, 13 | 42.8 | yes | Ex9 CGG → CAG R468Q | negative | – |
Part 1 of the study was a dose-escalation study, and part 2 was a randomized study to evaluate the safety, pharmacokinetics, and exploratory efficacy of JR-141.
L, 1.0 mg/kg/week; H, 2.0 mg/kg/week.
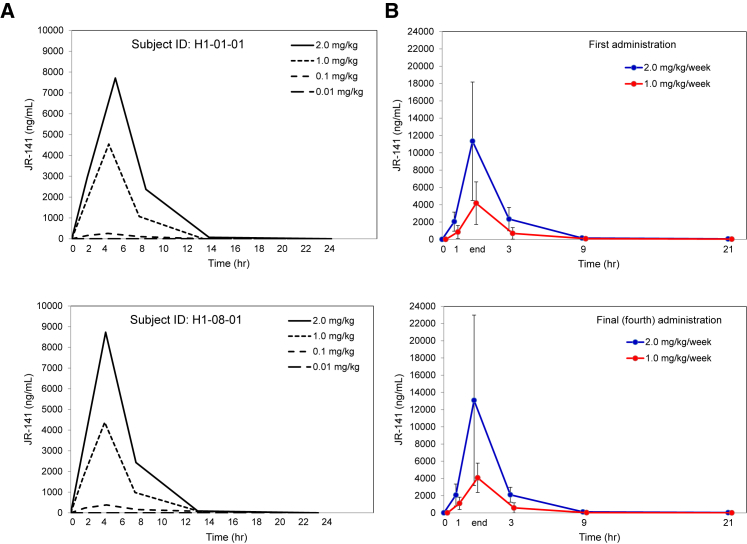
The safety of JR-141 was evaluated in two patients in a dose-escalation study (part 1). Concentrations of JR-141 in the plasma were detected in a dose-dependent manner and peaked at 3 hr after initiation of administration, because the duration of intravenous infusion was 3 hr (Figure 2A). One patient (H1-01-01) developed erythema after the first infusion (0.01 mg/kg) and during the second (0.1 mg/kg), but the erythema disappeared quickly. The same patient also experienced transient mild pyrexia (37.5°C) after the third dosing (1.0 mg/kg; Table 2). The other patient (H1-08-01) experienced no adverse reactions.
Figure 2.
Pharmacokinetics of JR-141 in Patients with MPS II
(A) Plasma concentrations of JR-141 were measured in two patients in the dose-escalation study. The indicated amount of the drug was administered intravenously once a week, and the duration of each infusion was 3 hr. JR-141 was detected in a dose-dependent manner and disappeared 24 hr after infusion. (B) JR-141 in the plasma was quantified after the first and final administrations. Data are presented as the mean results (with SD bars) for six patients receiving each dosage (1.0 or 2.0 mg/kg/week). Time 3, 9, 21: time from the end of JR-141 administration. No differences in the pharmacokinetics were observed between the first and final (fourth) administration.
Table 2.
Summary of Adverse Drug Reactions
| Part 1 | Part 2 |
Part 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group L (1.0 mg/kg/Week) | Group H (2.0 mg/kg/Week) | ||||||||
| Total n | 2 | 6 | 6 | ||||||
| Number of subjects experiencing adverse drug reactions | 1 | 3 | 3 | ||||||
| Proportion of subjects experiencing adverse drug reactions (%) | 50.0 | 50.0 | 50.0 | ||||||
| Number of adverse drug reactions | 3 | 4 | 4 | ||||||
| Adverse Events (SOC, PT) | Number of Subjects | Proportion (%) | Number of Events | Number of Subjects | Proportion (%) | Number of Events | Number of Subjects | Proportion (%) | Number of Events |
| Psychiatric Disorders | 0 | 0.0 | 0 | 1 | 16.7 | 1 | 0 | 0.0 | 0 |
| Delirium | 0 | 0.0 | 0 | 1 | 16.7 | 1 | 0 | 0.0 | 0 |
| Respiratory, Thoracic, and Mediastinal Disorders | 0 | 0.0 | 0 | 1 | 16.7 | 1 | 0 | 0.0 | 0 |
| Throat irritation | 0 | 0.0 | 0 | 1 | 16.7 | 1 | 0 | 0.0 | 0 |
| Skin and Subcutaneous Tissue Disorders | 1 | 50.0 | 2 | 1 | 16.7 | 1 | 1 | 16.7 | 2 |
| Urticaria | 0 | 0.0 | 0 | 1 | 16.7 | 1 | 1 | 16.7 | 2 |
| Erythema | 1 | 50.0 | 2 | 0 | 0.0 | 0 | 0 | 0.0 | 0 |
| General Disorders and Administration Site Conditions | 1 | 50.0 | 1 | 0 | 0.0 | 0 | 2 | 33.3 | 2 |
| Pyrexia | 1 | 50.0 | 1 | 0 | 0.0 | 0 | 2 | 33.3 | 2 |
| Investigations | 0 | 0.0 | 0 | 1 | 16.7 | 1 | 0 | 0.0 | 0 |
| Haptoglobin decreased | 0 | 0.0 | 0 | 1 | 16.7 | 1 | 0 | 0.0 | 0 |
SOC, system organ class; PT, preferred term.
In part 2 of the trial, 12 patients were allocated to receive intravenous infusions of JR-141 at 1.0 or 2.0 mg/kg/week. As in the dose escalation study, concentrations of JR-141 peaked at 3 hr after administration was initiated (Figure 2B).
JR-141 was undetectable in the plasma 24 hr after administration in all patients. The plasma concentration profiles of JR-141 did not differ between the first and final (fourth) dosing (Figures 2 and 3; Tables 3 and 4), indicating no accumulation of the drug under these conditions. JR-141 concentrations in the CSF were below the detection limit in all patients at 4–6 hr after the final dosing.
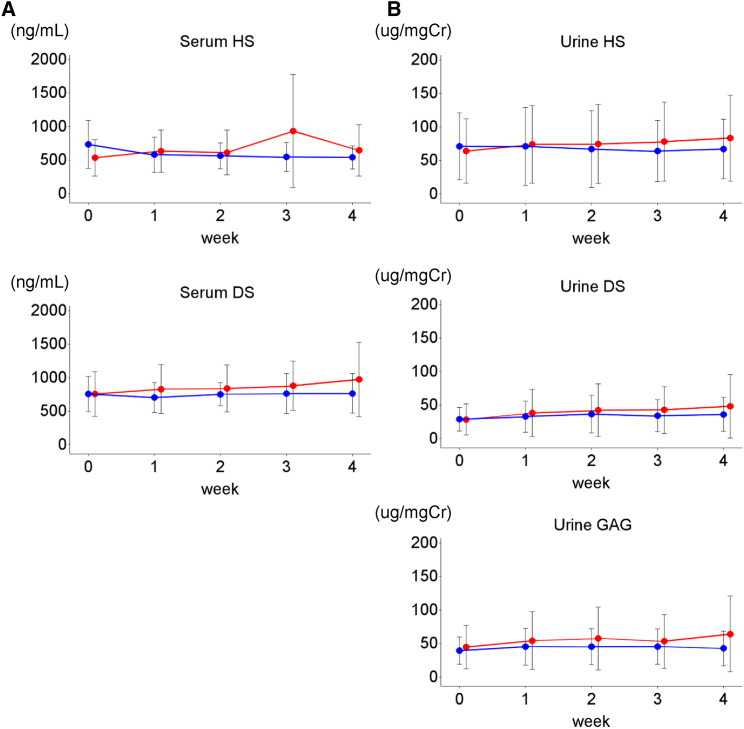
Figure 3.
HS and DS Concentrations in Serum and Urine, Part 2
Concentrations of HS and DS were determined in the serum (A) and urine (B). Data are presented as the mean results (with SD bars). Blood and urine samples were collected immediately before every administration of JR-141 (1.0 mg/kg/week [red line], n = 6; 2.0 mg/kg/week [blue line], n = 6). No significant changes in HS or DS levels were observed during the study period.
Table 3.
Pharmacokinetic Parameters of JR-141 after Initial Administration
| Parameter | Groupa | n | Mean | SD | Min | Median | Max |
|---|---|---|---|---|---|---|---|
| AUC0-t (ng·hr/mL) | L | 6 | 14,687.9 | 10,503.5 | 2,711 | 14,717.8 | 28,759 |
| H | 6 | 37,473.8 | 13,580.8 | 15,802 | 37,205.5 | 56,065 | |
| Cmax (ng/mL) | L | 6 | 4,180.0 | 2,453.6 | 1,180 | 4,010.0 | 7,900 |
| H | 6 | 11,338.3 | 6,838.9 | 6,080 | 8,635.0 | 24,700 | |
| tmax (hr) | L | 6 | 3.558 | 1.147 | 3.07 | 3.083 | 5.90 |
| H | 6 | 3.275 | 0.250 | 3.00 | 3.200 | 3.72 | |
| t1/2 (hr) | L | 2 | 4.898 | 0.944 | 4.23 | 4.898 | 5.56 |
| H | 3 | 4.099 | 0.513 | 3.72 | 3.894 | 4.68 | |
| MRT0-t (hr) | L | 6 | 2.633 | 1.101 | 1.76 | 2.098 | 4.44 |
| H | 6 | 2.852 | 0.559 | 2.11 | 2.953 | 3.46 |
AUC0-t, area under the concentration-time curve from zero to the time of the last measurable drug concentration; Cmax, maximum concentration; tmax, time to maximum concentration; t1/2, elimination half-life; MRT0-t, mean residence time.
L, 1.0 mg/kg/week; H, 2.0 mg/kg/week.
Table 4.
Pharmacokinetic Parameters of JR-141 after Final Administration
| Parameter | Groupa | n | Mean | SD | Min | Median | Max |
|---|---|---|---|---|---|---|---|
| AUC0-t (ng·hr/mL) | L | 6 | 12,857.7 | 7,681.8 | 4,914 | 12,557.9 | 24,240 |
| H | 6 | 39,186.9 | 19,294.9 | 205,77 | 33,957.1 | 74,804 | |
| Cmax (ng/mL) | L | 6 | 4,071.7 | 1,704.3 | 2,180 | 3,945.0 | 6,510 |
| H | 6 | 13,083.3 | 9,906.6 | 6,590 | 8,835.0 | 3,2800 | |
| tmax (hr) | L | 6 | 3.478 | 0.808 | 3.08 | 3.133 | 5.12 |
| H | 6 | 3.200 | 0.226 | 3.02 | 3.092 | 3.55 | |
| t1/2 (hr) | L | 0 | – | – | – | – | – |
| H | 2 | 4.482 | 1.085 | 3.71 | 4.482 | 5.25 | |
| MRT0-t (hr) | L | 6 | 1.956 | 0.675 | 1.27 | 1.796 | 3.22 |
| H | 6 | 2.558 | 0.417 | 2.16 | 2.520 | 3.24 |
AUC0-t, area under the concentration-time curve from zero to the time of the last measurable drug concentration; Cmax, maximum concentration; tmax, time to maximum concentration; t1/2, elimination half-life; MRT0-t, mean residence time.
L, 1.0 mg/kg/week; H, 2.0 mg/kg/week
Table 2 summarizes the adverse drug reactions observed during the study period. In part 2, one patient felt throat irritation and developed a transient rash during the first administration of JR-141 (1.0 mg/kg). Two patients experienced transient urticaria, one after the second administration and the other after the fourth (2.0 mg/kg). Mild to moderate pyrexia was also observed in the same two patients, but it abated quickly. One patient showed transient hypohaptoglobinemia after the final administration (1.0 mg/kg). More than 4 weeks after the completion of the study, one patient with a comorbid diagnosis of epilepsy became delirious after being prescribed a new antiepileptic; this patient was admitted to hospital for observation, hence the episode classified as a serious adverse event. Although the delirium abated after discontinuation of the new antiepileptic, we did not feel able to conclude that this episode was completely unrelated to the administration of JR-141. Overall, however, these results indicate that four administrations of JR-141 at a dosage of as high as 2.0 mg/kg/week are safe and well tolerated.
Prior to the initiation of the study, serum HS and DS levels were near normal in all patients because they were receiving idursulfase, which reduces GAG accumulation in the peripheral tissues.21 Their HS and DS levels remained substantially unchanged throughout the study period (i.e., after JR-141 administration; Figure 3A). Urinary HS and DS levels were also in the normal or near-normal range before and during the study period (Figure 3B). Although a treatment period of 4 weeks is too short to render these results conclusive, they nonetheless may suggest potential non-inferiority of JR-141 to idursulfase in reducing GAG accumulation in the peripheral tissues.
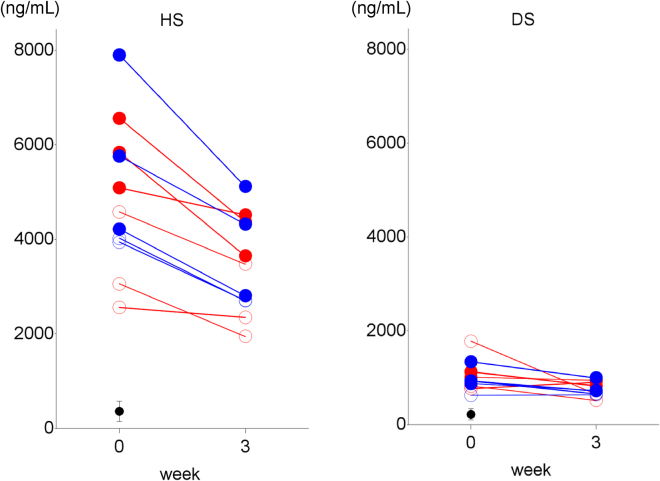
To evaluate the effects of JR-141 on neurodegeneration in MPS II, we measured the concentrations of HS and DS in CSF as biomarkers of neurodegeneration in all subjects before we initiated JR-141 administration (Figure 4). We found that the concentrations of HS were much higher than those of DS, which is consistent with the data from an MPS II study using mouse models.20 Nevertheless, both HS and DS concentrations in CSF were higher in all MPS II patients than in the nine non-MPS II subjects, indicating that the effect of idursulfase alone on the substrate accumulation in the CNS is negligible. The HS concentrations in the CSF of the patients with intellectual disability (5,895 ± 1,260 ng/mL; range, 4,220–7,900 ng/mL; n = 6) were, in alignment with a previous report,20 higher than in those without (3,632 ± 809 ng/mL; range, 2,560–4,580 ng/mL; n = 5; Figure 4), based on the reference CSF-HS value from the non-MPS II patients (363 ± 217 ng/mL). Notably, weekly intravenous administrations of JR-141 for 3 weeks reduced HS concentrations in the CSF of all patients. The values of HS in CSF before JR-141 administration were 4,615 ± 1,559 ng/mL (n = 6, mean ± SD) in the low-dose group (1.0 mg/kg/week), and 5,168 ± 1,698 ng/mL (n = 5, mean ± SD) in the high-dose group (2.0 mg/kg/week). After the final dosing of JR-141, the values had fallen to 3,387 ± 1,046 ng/mL (1.0 mg/kg/week) and 3,397 ± 1,057 ng/ml (2.0 mg/kg/week; 25.1% ± 12.9% and 31.5% ± 3.9% decrement from the initial values, respectively). The DS concentrations in CSF were also decreased significantly by 2.0 mg/kg/week of JR-141. These results suggest that intravenously administered JR-141 crosses the BBB and delivers IDS to the brain parenchyma, thereby inhibiting the pathological accumulation of GAGs.
Figure 4.
HS and DS Concentrations in the CSF, Part 2
The HS and DS concentrations in CSF before the first administration of JR-141 (1.0 mg/kg/week [red line], n = 6; 2.0 mg/kg/week [blue line], n = 5) and at week 3 are shown. The closed circles indicate the patients with intellectual disability, while the open circles suggest these without. The mean values (with SD bars) were for the subjects without MPS (n = 9).
Computed tomography revealed no marked changes in the volume of the liver or spleen of the participants during the study period. Cardiac structures and functions as evaluated by echocardiography also remained unaltered.
Before the administration of JR-141, seven patients tested positive for anti-IDS antibody, while five tested positive for anti-JR-141 antibody. Four patients were positive for both anti-IDS and anti-JR-141 antibodies. No marked increase in the titers of these antibodies was observed in any of the patients after four administrations of JR-141, regardless of dosage. In addition, no patients who were negative for either antibody at the initiation of the study developed de novo anti-drug antibodies after repeated infusions of JR-141.
Discussion
This study demonstrated the safety, tolerability, and potential efficacy of a novel compound JR-141 for patients with neuropathic MPS II. It also suggested, despite the short treatment period of 4 weeks, that JR-141 seems to be no less effective than conventional enzyme replacement therapy with idursulfase in reducing GAG concentrations in the plasma and urine, which needs to be established through a longer treatment period. Importantly, JR-141 markedly decreased HS levels in the CSF of our patients with MPS II, which remained elevated after they had been treated with idursulfase prior to this study. To the best of our knowledge, this is the first report of significant drug delivery across the BBB to address neurodegeneration in patients with MPS II.
Hendriksz et al.22 have shown a correlation between HS concentrations in CSF and intellectual disability in MPS II patients. Intellectual disability is known to develop in patients with MPS I, II, III, or VII,3, 23 all of which are associated with HS accumulation in the CNS, but not in patients with the forms of MPS that do not involve HS accumulation (MPS IV and VI). Impaired metabolism of HS leads to secondary accumulation of gangliosides that results in various CNS defects via multiple mechanisms, including tau protein hyperphosphorylation, endoplasmic reticulum stress, and increased autophagy.24, 25, 26 Therefore, HS accumulation should be regarded as a critical etiological factor in the development of CNS disorders in patients with MPS, and normalization of HS clearance in the CNS should prevent, or at least curb, the progression of neurodegeneration. In other words, HS levels in CSF are not only an informative clinical surrogate endpoint in the evaluation of efficacy of therapeutics for neurodegeneration but may also be helpful for clinical understanding of neurocognitive deficits if they correspond well to the intracellular and lysosomal HS levels, both critical for neurotoxicity.
Conventional enzyme replacement therapy with idursulfase for MPS II is ineffective against CNS disorders, because the enzyme cannot penetrate the brain through the BBB. Managing these disorders has, therefore, been a frustration in clinical practice and has left neuropathic MPS in something of a therapeutic impasse.27 Efforts have been made to improve enzyme delivery to the brain by way of intrathecal16 or intracerebroventricular17 administration, but these efforts have reportedly had very limited success in achieving sufficient brain penetration.28
In contrast to these approaches, delivery of IDS to the brain by JR-141 is achieved via the mechanism of receptor-mediated transcytosis of transferrin.18 TfRs are expressed in brain capillary endothelial cells, a component of the BBB, which enables transferrin to cross the BBB.29 We assume that JR-141 binds to TfR on the endothelial cells through the anti-TfR antibody region of the drug and is then transported into the cells and distributed in the brain parenchyma by as-yet-unidentified mechanisms, which prevent the neurotoxic accumulation of HS in the neurons. We have previously reported the penetration of human IDS fused with an engineered monoclonal antibody against murine TfRs into the brain parenchyma of MPS II model mice, which resulted in reductions in HS concentration in the brain, along with improvements in learning and memory.19 These findings were then neatly replicated with human IDS with monoclonal antibody against human TfRs administered to human TfR-knockin mice and monkeys.18 The HS concentrations in the mouse CSF correlated well with those in the mouse brain,20 which justifies the use of HS concentrations as a biomarker reflecting neuropathological disease activities in MPS II, as discussed above. These preclinical findings further reinforce the positioning of HS concentrations in CSF both as a viable efficacy endpoint in clinical trials evaluating treatment response and also as a direct biomarker of ongoing neurodegeneration. Clinical pictures of CNS disorders in MPS II are too multifaceted and varied to allow a truly viable clinical efficacy endpoint to be established in the face of diverse disease progression and severity, as well as the paucity of evaluable patient populations worldwide for participation in clinical trials.
Although this study was merely for 4 weeks and not designed to establish the efficacy of JR-141 via clinically validated measures, it may be noteworthy that two of the investigators reported some brief motor and behavioral changes in their respective patients. One 16-year-old patient in the low-dose group was reported to have learned to skip, an activity he had never attempted before, while the other, a 14-year-old in the high-dose group, showed improved gait, continence, sleep, and responsiveness toward those around him. There were notable reductions in CSF HS level from 5,840 to 3,650 ng/mL in the former, 8,940 to 2,720 ng/mL in the latter. These improvements all disappeared several weeks after administration of the test drug ceased, as documented in the patients’ medical records. Albeit merely anecdotal, these clinical observations may be useful for future clinical trials in monitoring subtle neurocognitive and behavioral changes that can be easily overlooked.
This study has several limitations. First, because MPS II is a rare disease, the number of patients available to participate was extremely limited, leading to an inevitable lack of statistical power. In addition, the marked heterogeneity of the sample population further limits the generalizability of the study results. Second, no neurocognitive or neurodevelopmental assessments were made, because we considered the limited treatment period of 4 weeks too short for any such assessments to be worthwhile. However, the aforementioned clinical observations of brief neurocognitive and motor changes even in the short period during which JR-141 was administered suggest that the drug may show salient efficacy in treating CNS disorders when administered on a long-term basis. Late-phase clinical trials are planned to investigate this. Third, the CSF levels of JR-141 were below the detection limit for pharmacokinetic measurement. This may have been because the timing of CSF sampling was too late to detect the drug before it was eliminated or because JR-141 was so swiftly and efficiently transported into the neurons after penetrating the BBB that too little was left in the CSF to measure. Nevertheless, the significant reductions in HS levels in the CSF of all patients following JR-141 administration are, we believe, robust evidence of the successful delivery of the drug to the CNS.
In conclusion, first, this phase 1/2 clinical trial of JR-141 established that JR-141 is not inferior to idursulfase in terms of its systemic effects. Second, it showed that four administrations of JR-141 at up to 2.0 mg/kg/week are safe. Third, and most notably, JR-141 significantly reduced CSF levels of GAGs, especially those of HS, which is known to be a major neurotoxin in neuropathic MPS. Further studies will be carried out to establish the efficacy of JR-141 in treating the CNS symptoms caused by neuropathic MPS II.
Materials and Methods
Study Design
We conducted a multicenter, open-label, randomized clinical trial to assess the safety, pharmacokinetics, and exploratory efficacy of intravenously administered JR-141 (NCT 03128593). The study was conducted in eight hospitals and specialized MPS centers in Japan and was in compliance with the Declaration of Helsinki. The protocol and patient informed consent were reviewed and approved by the Institutional Review Board at each participating institution.
The study consisted of two 4-week periods (Figure 1). In the first (part 1), a dose-escalation study designed to evaluate the safety of JR-141 administered to two patients by intravenous infusion at four dosage levels (0.01, 0.1, 1.0, and 2.0 mg/kg/week) was carried out. In the second 4-week period (part 2), a randomized study was done to evaluate the safety, pharmacokinetics, and exploratory efficacy of JR-141 administered by intravenous infusion at 1.0 or 2.0 mg/kg/week (six patients in each dosage group). In all patients, idursulfase was switched to JR-141 without a washout period. Appropriate amounts of JR-141 were diluted with normal saline and infused intravenously once a week. The duration of infusions was 3 hr or longer, depending on the general conditions of the patients and the investigators’ discretion. After completion of the study, all patients restarted conventional enzyme-replacement therapy with idursulfase. The study period was defined as the time from the initiation of the first infusion of JR-141 until 1 week after the last administration.
Participants and Procedures
A total of 14 male patients were enrolled in the study. Two patients (aged 33 and 63) who had no intellectual disability and were able to recognize and verbally report their subjective symptoms were selected for the dose-escalation study (part 1) and were given 0.01, 0.1, 1.0, and 2.0 mg/kg/week of JR-141 intravenously. In part 2 of the study, the other 12 patients (average age, 12.9 years; range, 6 to 25 years; SD, 5.3), seven of whom had intellectual disability, were randomly assigned to a 1.0 or 2.0 mg/kg/week of JR-141 dosing group. Key inclusion criteria were as follows: age 6 years or older (16 years or older in the initial dose-escalation study) at the time of informed consent; a diagnosis of MPS II based on the measurement of overall IDS activity in leukocytes, plasma, and cultured skin fibroblasts along with a genetic analysis; under pharmacotherapy with idursulfase (0.5 mg/kg/week) for at least 12 weeks before the initial administration of JR-141. Key exclusion criteria included a history of hematopoietic stem cell transplantation, practical inability to undergo lumbar puncture, serious drug allergy or hypersensitivity making them unsuitable for participation in the study, a history of receiving other investigational products within 4 months before the beginning of this study. All patients or legally acceptable representatives provided signed informed consent prior to enrolment. Full inclusion and exclusion criteria are listed in the appendix, and the study protocol is available online.
Randomization and Masking
As shown in Figure 1, only adult patients with mild MPS II who were able to describe their subjective symptoms were deemed appropriate for part 1 of the study, which was designed to confirm the safety of JR-141. On this basis, only two subjects were selected.
Part 2 was initiated only after safety had been confirmed in part 1. Eleven of the 12 subjects were randomized to either a 1.0 mg/kg/week or a 2.0 mg/kg/week group at an overall ratio of 1:1. However, out of safety concerns, the 6-year-old was specifically assigned to the 1.0 mg/kg/week group.
This was an open-label study with no masking.
Outcomes
Safety evaluations included continuous monitoring of adverse events as well as laboratory tests. Adverse events were recorded according to their severity (mild, moderate, severe) and their relationship to JR-141. Hematologic and serum biochemical tests and urinalyses were performed, and heart rate, body temperature, and blood pressure were also monitored. Electrocardiograms were recorded to assess general cardiac function. In addition, anti-IDS antibody and anti-JR-141 antibody levels were measured.
Pharmacokinetic evaluation was performed at all dosing points in part 1 of the study and after the first and last administrations of JR-141 in part 2. The time points for blood sampling were before dosing, 1 hr after the start of dosing, immediately after the last administration, and then 3, 9, and 21 hr afterward. Drug concentrations were measured by an electrochemiluminescence assay.
Evaluations of exploratory efficacy focused on drug delivery to the CNS as measured by reductions in the substrate concentrations in the CSF. CSF was obtained by lumbar puncture before the initial dosing and after the last dosing in part 2 of the study. HS and DS were quantified as described previously.18, 19 To evaluate systemic efficacy with regard to the somatic symptoms, HS and DS levels in the serum and urine were also measured. In addition, patients underwent computed tomography to assess hepatosplenomegaly. Cardiac structures and functions were evaluated by echocardiography.
Statistical Analysis
A non-compartment model analysis was used to calculate pharmacokinetic parameters for each subject at the time of blood collection. To analyze the pharmacokinetic endpoints, plasma drug concentrations adjusted for the plasma IDS concentration at baseline were used. Drug concentration in some subjects was 0 at 21 hr after the final administration (one of the three time points when concentration measurements were used to calculate the elimination rate constant [kel]), making it impossible to calculate not only kel, but also elimination half-life (t1/2).
Efficacy was assessed in the full analysis set, which included all patients for whom data were available on at least one exploratory endpoint after administration of JR-141. Summary statistics of efficacy endpoints were calculated.
Safety was assessed in the safety analysis set, which included all patients who received at least one dose of JR-141. The number and proportion of subjects who experienced any adverse drug reactions were recorded, along with the total number of adverse drug reactions. For all adverse events, the verbatim terms used in the case report forms were coded according to the System Organ Class and Preferred Term specified in the Medical Dictionary for Regulatory Activities version 20.1 (developed by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use).
Statistical analysis were performed using the SAS version 9.4 statistical software package (SAS Institute, Cary, NC, USA).
Author Contributions
T.Y. and K.T. conceived and designed the study, and all other authors assisted in its design. T.O., Y.E., and N.S. led the trial’s steering committee. K.M. and M.Y. wrote the first draft of the manuscript. M.Y. designed and conducted all statistical analyses. K.M., H.S., and T.H. were responsible for the pharmacokinetic assay. All authors were involved in the interpretation and critical review of the data and in drafting and revising the manuscript for important intellectual content; all approved the final version proposed by Y.S.
Conflicts of Interest
This clinical trial was funded by JCR Pharmaceuticals. The funder participated in the design of the trial, the collection, analysis and interpretation of the data, and the writing of the report; it also dealt with all the regulatory requirements for carrying out the trial. The funder is also the sole intellectual property holder as well as the manufacturer of the test drug JR-141. All authors had full access to the data used in the study, and the corresponding author had final responsibility for the completion of the manuscript and the decision to submit it for publication. T.O. reports research grants from Sanofi, JCR Pharmaceuticals, GC Pharma, Dainippon Sumitomo Pharma, Actelion, Alexion, and Anges, along with honoraria from Sanofi, Dainippon Sumitomo Pharma, Actelion, and Anges. Y.E. has conducted consultancy for JCR Pharmaceuticals, and he has been awarded grants and research support from Actelion, BioMarin Pharmaceutical Inc., and Sanofi; he has also received honoraria from Actelion, BioMarin Pharmaceutical Inc., Sanofi, Shire, and Dainippon Sumitomo Pharma. N.S. has conducted consultancy for JCR Pharmaceuticals, and he has been awarded grant/research support from Sanofi and Dainippon Sumitomo Pharma and honoraria from Actelion, BioMarin Pharmaceutical Inc., Sanofi, Shire, and Dainippon Sumitomo Pharma.
Acknowledgments
The authors are grateful to all the investigators and the patients for their contribution and commitment to the study. We thank Masafumi Kinoshita, Noboru Tanaka, and Sachiho Kida of JCR Pharmaceuticals for carrying out pharmacokinetic measurements and analyses for the entire study. Project management of the study by Sairei So of JCR Pharmaceuticals is duly appreciated. Special thanks are due to Timothy Minton of Keio University, Tokyo, for his immense editorial help.
References
- 1.Wilson P.J., Morris C.P., Anson D.S., Occhiodoro T., Bielicki J., Clements P.R., Hopwood J.J. Hunter syndrome: isolation of an iduronate-2-sulfatase cDNA clone and analysis of patient DNA. Proc. Natl. Acad. Sci. USA. 1990;87:8531–8535. doi: 10.1073/pnas.87.21.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giugliani R. The mucopolysaccharidoses. In: Mehta A., Winchester B., editors. Lysosomal Storage Disorders: A Practical Guide. First Edition. Wiley-Blackwell; 2012. pp. 94–100. [Google Scholar]
- 3.Giugliani R., Vairo F., Kubaski F., Poswar F., Riegel M., Baldo G., Saute J.A. Neurological manifestations of lysosomal disorders and emerging therapies targeting the CNS. Lancet Child Adolesc. Health. 2018;2:56–68. doi: 10.1016/S2352-4642(17)30087-1. [DOI] [PubMed] [Google Scholar]
- 4.Khan S.A., Peracha H., Ballhausen D., Wiesbauer A., Rohrbach M., Gautschi M., Mason R.W., Giugliani R., Suzuki Y., Orii K.E. Epidemiology of mucopolysaccharidoses. Mol. Genet. Metab. 2017;121:227–240. doi: 10.1016/j.ymgme.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muenzer J., Wraith J.E., Beck M., Giugliani R., Harmatz P., Eng C.M., Vellodi A., Martin R., Ramaswami U., Gucsavas-Calikoglu M. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet. Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 6.Muenzer J., Gucsavas-Calikoglu M., McCandless S.E., Schuetz T.J., Kimura A. A phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome) Mol. Genet. Metab. 2007;90:329–337. doi: 10.1016/j.ymgme.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Okuyama T., Tanaka A., Suzuki Y., Ida H., Tanaka T., Cox G.F., Eto Y., Orii T. Japan Elaprase Treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (Mucopolysaccharidosis II, MPS II) Mol. Genet. Metab. 2010;99:18–25. doi: 10.1016/j.ymgme.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Muenzer J., Beck M., Eng C.M., Giugliani R., Harmatz P., Martin R., Ramaswami U., Vellodi A., Wraith J.E., Cleary M. Long-term, open-labeled extension study of idursulfase in the treatment of Hunter syndrome. Genet. Med. 2011;13:95–101. doi: 10.1097/GIM.0b013e3181fea459. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y.J., Zhang Y., Kenrick M., Hoyte K., Luk W., Lu Y., Atwal J., Elliott J.M., Prabhu S., Watts R.J., Dennis M.S. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y.J., Atwal J.K., Zhang Y., Tong R.K., Wildsmith K.R., Tan C., Bien-Ly N., Hersom M., Maloney J.A., Meilandt W.J. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci. Transl. Med. 2014;6:261ra154. doi: 10.1126/scitranslmed.3009835. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q.H., Boado R.J., Lu J.Z., Hui E.K., Pardridge W.M. Brain-penetrating IgG-iduronate 2-sulfatase fusion protein for the mouse. Drug Metab. Dispos. 2012;40:329–335. doi: 10.1124/dmd.111.042903. [DOI] [PubMed] [Google Scholar]
- 12.Niewoehner J., Bohrmann B., Collin L., Urich E., Sade H., Maier P., Rueger P., Stracke J.O., Lau W., Tissot A.C. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 13.Boado R.J., Ka-Wai Hui E., Zhiqiang Lu J., Pardridge W.M. Insulin receptor antibody-iduronate 2-sulfatase fusion protein: pharmacokinetics, anti-drug antibody, and safety pharmacology in Rhesus monkeys. Biotechnol. Bioeng. 2014;111:2317–2325. doi: 10.1002/bit.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giugliani R., Giugliani L., de Oliveira Poswar F., Donis K.C., Corte A.D., Schmidt M., Boado R.J., Nestrasil I., Nguyen C., Chen S., Pardridge W.M. Neurocognitive and somatic stabilization in pediatric patients with severe Mucopolysaccharidosis Type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): an open label phase 1-2 trial. Orphanet J. Rare Dis. 2018;13:110. doi: 10.1186/s13023-018-0849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couch J.A., Yu Y.J., Zhang Y., Tarrant J.M., Fuji R.N., Meilandt W.J., Solanoy H., Tong R.K., Hoyte K., Luk W. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci. Transl. Med. 2013;5:1–12. doi: 10.1126/scitranslmed.3005338. 183ra57. [DOI] [PubMed] [Google Scholar]
- 16.Muenzer J., Hendriksz C.J., Fan Z., Vijayaraghavan S., Perry V., Santra S., Solanki G.A., Mascelli M.A., Pan L., Wang N. A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet. Med. 2016;18:73–81. doi: 10.1038/gim.2015.36. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka K., Tamura T., Tsuji D., Dohzono Y., Kitakaze K., Ohno K., Saito S., Sakuraba H., Itoh K. Therapeutic potential of intracerebroventricular replacement of modified human β-hexosaminidase B for GM2 gangliosidosis. Mol. Ther. 2011;19:1017–1024. doi: 10.1038/mt.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonoda H., Morimoto H., Yoden E., Koshimura Y., Kinoshita M., Golovina G., Takagi H., Yamamoto R., Minami K., Mizoguchi A. A Blood-Brain-Barrier-Penetrating Anti-human Transferrin Receptor Antibody Fusion Protein for Neuronopathic Mucopolysaccharidosis II. Mol. Ther. 2018;26:1366–1374. doi: 10.1016/j.ymthe.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonoda H., Morimoto H., Koshimura Y., Kinoshita M. Correction of central nervous system deficits in the mouse model of Hunter syndrome by recombinant iduronate 2-sulfatase crossing the blood-brain barrier. Mol. Genet. Metab. 2017;120:S125–S126. [Google Scholar]
- 20.Tanaka N., Kida S., Kinoshita M., Morimoto H., Shibasaki T., Tachibana K., Yamamoto R. Evaluation of cerebrospinal fluid heparan sulfate as a biomarker of neuropathology in a murine model of mucopolysaccharidosis type II using high-sensitivity LC/MS/MS. Mol. Genet. Metab. 2018;125:53–58. doi: 10.1016/j.ymgme.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Muenzer J., Lamsa J.C., Garcia A., Dacosta J., Garcia J., Treco D.A. Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta Paediatr. Suppl. 2002;91:98–99. doi: 10.1111/j.1651-2227.2002.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 22.Hendriksz C.J., Muenzer J., Vanderver A., Davis J.M., Burton B.K., Mendelsohn N.J., Wang N., Pan L., Pano A., Barbier A.J. Levels of glycosaminoglycans in the cerebrospinal fluid of healthy young adults, surrogate-normal children, and Hunter syndrome patients with and without cognitive impairment. Mol. Genet. Metab. Rep. 2015;5:103–106. doi: 10.1016/j.ymgmr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro E.G., Escolar M.L., Delaney K.A., Mitchell J.J. Assessments of neurocognitive and behavioral function in the mucopolysaccharidoses. Mol. Genet. Metab. 2017;122S:8–16. doi: 10.1016/j.ymgme.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Walkley S.U., Siegel D.A., Dobrenis K. GM2 ganglioside and pyramidal neuron dendritogenesis. Neurochem. Res. 1995;20:1287–1299. doi: 10.1007/BF00992503. [DOI] [PubMed] [Google Scholar]
- 25.Takamura A., Higaki K., Kajimaki K., Otsuka S., Ninomiya H., Matsuda J., Ohno K., Suzuki Y., Nanba E. Enhanced autophagy and mitochondrial aberrations in murine G(M1)-gangliosidosis. Biochem. Biophys. Res. Commun. 2008;367:616–622. doi: 10.1016/j.bbrc.2007.12.187. [DOI] [PubMed] [Google Scholar]
- 26.Ohmi K., Kudo L.C., Ryazantsev S., Zhao H.Z., Karsten S.L., Neufeld E.F. Sanfilippo syndrome type B, a lysosomal storage disease, is also a tauopathy. Proc. Natl. Acad. Sci. USA. 2009;106:8332–8337. doi: 10.1073/pnas.0903223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muenzer J., Bodamer O., Burton B., Clarke L., Frenking G.S., Giugliani R., Jones S., Rojas M.V., Scarpa M., Beck M., Harmatz P. The role of enzyme replacement therapy in severe Hunter syndrome-an expert panel consensus. Eur. J. Pediatr. 2012;171:181–188. doi: 10.1007/s00431-011-1606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardridge W.M. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS. 2011;8:7. doi: 10.1186/2045-8118-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fishman J.B., Rubin J.B., Handrahan J.V., Connor J.R., Fine R.E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res. 1987;18:299–304. doi: 10.1002/jnr.490180206. [DOI] [PubMed] [Google Scholar]