Autism is diagnosed via face-to-face spoken interactions. Using a novel face-to-face fMRI paradigm, Jasmin et al. report that cortical functional connectivity is heightened in social brain areas of men with autism during face-to-face conversations but not in the resting state, while subcortical-cortical connectivity is heightened in interaction and resting states.
Keywords: autism, conversation, fMRI, language, resting state
Abstract
Conversation is an important and ubiquitous social behaviour. Individuals with autism spectrum disorder (autism) without intellectual disability often have normal structural language abilities but deficits in social aspects of communication like pragmatics, prosody, and eye contact. Previous studies of resting state activity suggest that intrinsic connections among neural circuits involved with social processing are disrupted in autism, but to date no neuroimaging study has examined neural activity during the most commonplace yet challenging social task: spontaneous conversation. Here we used functional MRI to scan autistic males (n = 19) without intellectual disability and age- and IQ-matched typically developing control subjects (n = 20) while they engaged in a total of 193 face-to-face interactions. Participants completed two kinds of tasks: conversation, which had high social demand, and repetition, which had low social demand. Autistic individuals showed abnormally increased task-driven interregional temporal correlation relative to controls, especially among social processing regions and during high social demand. Furthermore, these increased correlations were associated with parent ratings of participants’ social impairments. These results were then compared with previously-acquired resting state data (56 autism, 62 control subjects). While some interregional correlation levels varied by task or rest context, others were strikingly similar across both task and rest, namely increased correlation among the thalamus, dorsal and ventral striatum, somatomotor, temporal and prefrontal cortex in the autistic individuals, relative to the control groups. These results suggest a basic distinction. Autistic cortico-cortical interactions vary by context, tending to increase relative to controls during task and decrease during test. In contrast, striato- and thalamocortical relationships with socially engaged brain regions are increased in both task and rest, and may be core to the condition of autism.
Introduction
Conversation is an important part of everyday life that provides a means to share information and express affiliation. Abnormal brain development during childhood is associated with conditions in which people struggle with conversation skills, as in the case of autism spectrum disorder (referred to hereafter as ‘autism’). This class of neurodevelopmental conditions is characterized by deficits in social functioning, communication, repetitive behaviours, and stereotyped interests (Lord et al., 2000). Structural language per se may be unimpaired except where it intersects with social processing (Mundy et al., 1990) such as in pragmatics (Baron-Cohen, 1988; Loukusa and Moilanen, 2009), and prosody (Shriberg et al., 2001; Paul et al., 2005). Non-verbal aspects of communication such as eye gaze (Loveland and Landry, 1986; Frith and Frith, 1999; Pelphrey et al., 2005) and hand gestures (Attwood et al., 1988; de Marchena and Eigsti, 2010) are also often impaired, and elicitation of deficits in these behaviours through tools like the ADOS (Autism Diagnostic Observation Schedule) (Lord et al., 2000) is standard diagnostic procedure. Indeed, one of the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) Social-Communication symptoms of autism is problems with social-emotional reciprocity as seen in normal conversational turn-taking. Severe impairments in social interaction have been linked with difficulty establishing and maintaining social relationships (Orsmond et al., 2004), thus abnormal conversation behaviour can have serious consequences for people with autism.
Despite conversation’s ubiquity and social importance, no published neuroimaging studies have measured neural activity in autistic individuals while they are engaged in face-to-face conversation with naturalistic sensory, cognitive, communicative and social demands. Instead, studies have focused on the components of conversation with which people with autism struggle. Investigations of vocal prosody have found abnormally increased brain activity both within the same network of regions active in typically developing control participants (Wang et al., 2006) and across diffuse regions outside of typical networks (Colich et al., 2012; Eigsti et al., 2012). Studies of pragmatic processing have found that integrating linguistic and real-world knowledge is associated with abnormally low levels of activity in left inferior frontal gyrus (IFG) in autistic children (Groen et al., 2010) but abnormally high levels in homologous right IFG in autistic adults (Tesink et al., 2009).
Meanwhile, resting state studies evaluating intrinsic brain organization have indicated that disordered connections between cortical regions may play a role. The bulk of the resting state literature on autism has focused on high-functioning adolescent and adult males using functional MRI (for reviews, see Picci et al., 2016; Hull et al., 2017). In seed-based connectivity studies using a small number of seeds, researchers have reported a variety of differing patterns of functional connectivity, some that show decreases in autism among cortical regions (Kennedy and Courchesne, 2008; Weng et al., 2010; Ebisch et al., 2011; Abrams et al., 2013; Alaerts et al., 2014; Jung et al., 2014; Verly et al., 2014; Fishman et al., 2015; Linke et al., 2018) and some that show increases (Redcay et al., 2013; Alaerts et al., 2014; Fishman et al., 2014, 2015; Nebel et al., 2014a, b; Chien et al., 2015). Among these, those investigating seeds used to define the so-called ‘default mode’ network (Raichle, 2015), sharing some regions with the language system (Scott et al., 2000; Turken and Dronkers, 2011; Jasmin et al., 2016), have often found decreased functional connectivity among related regions, such as the posterior cingulate, medial prefrontal cortex, and the parahippocampal gyrus (Kennedy and Courchesne, 2008; Assaf et al., 2010; see also Weng et al., 2010; Starck et al., 2013; Ypma et al., 2016). Other studies have attempted to simultaneously examine all possible combinations of regions (so-called ‘data-driven’ studies), with several of these showing decreased functional connectivity amongst cortical regions associated with social functions, such as the superior temporal sulcus (STS), medial prefrontal, temporoparietal junction, left IFG, as well as somatosensory cortex (Anderson et al., 2011; Gotts et al., 2012; Hagen von dem et al., 2013; Di Martino et al., 2014; Cerliani et al., 2015; Cheng et al., 2015), with some showing simultaneously increased functional connectivity between thalamus, striatum and some of the same cortical regions (Delmonte et al., 2013; Di Martino et al., 2014; Cheng et al., 2015; Cerliani et al., 2015; Linke et al., 2018; see also Di Martino et al., 2009; Padmanabhan et al., 2013; Nair et al., 2015; although see Nair et al., 2013). For cohorts that also include even younger participants, increased cortico-cortical functional connectivity has been observed (Supekar et al., 2013; Abbott et al., 2016; see Uddin et al., 2013 for a review; although see also Dinstein et al., 2011). There is also some question as to whether females with autism exhibit the same patterns of functional connectivity alterations as males (Alaerts et al., 2016; Ypma et al., 2016; Lai et al., 2017; Floris et al., 2018). Other studies have highlighted additional and complex aspects of the resting state functional connectivity changes (Hahamy et al., 2015).
Here we investigate the neural basis of face-to-face verbal interactions in matched samples of adolescent and adult males with and without an autism diagnosis. Participants were scanned with functional MRI while they spoke via cameras and microphones with a real interaction partner. The interactions took place under two conditions: Conversation—wherein the participant spoke spontaneously and reciprocally with their partner, and Repetition—in which the subject merely repeated their partner’s speech. These two tasks had similar sensory and motor components (both involving turn-taking), but differed crucially in their level of social demand. Conversations (but not repetitions) involved novel, spontaneous and reciprocal speech that relied on social knowledge.
We had two main objectives. First, we used a whole-brain approach to evaluate changes in between-region correlations in our two groups (Autism versus Control) and tasks (Conversation versus Repetition). Then we compared group differences in inter-region correlations at task with a larger sample of resting state data, to identify commonalities and differences between the two states.
Materials and methods
Participants
Nineteen males (aged 14.7 to 28.2 years) with autism and 20 male control participants (aged 15.1 to 32.0 years) took part in the tasks. Participants with autism were recruited from the Washington, DC metropolitan area and met DSM-5 criteria for ASD (APA, 2013) as assessed by an experienced clinician. All participants with autism received the ADOS module 4 (Lord et al., 2000). The scores from participants with autism met cut-off for the ‘broad autism spectrum disorders’ category according to criteria established by the National Institute of Child Health and Human Development/National Institute on Deafness and Other Communication Disorders Collaborative Programs for Excellence in Autism (Lainhart et al., 2006). Scores on the Social Responsiveness Scale (Constantino et al., 2003), a measure of social impairment, were obtained by parent-report. The distributions for full-scale IQ, verbal IQ, and age did not differ significantly between the autism and control groups (Supplementary Table 1).
Procedure
Each session consisted of five functional MRI runs. Runs 1, 3 and 5 were conversations and runs 2 and 4 were repetition. Prior to scanning, participants were told that they would engage in unstructured and informal conversations with the experimenter. Using a modified version of the Interest Scale questionnaire (Bodfish, 2003; Anthony et al., 2013), participants rated their level of interest in various topics such as music, games, and transportation vehicles, and indicated their top three interests, from which the experimenter selected two. The topic of the final conversation was always work or school life, depending on participant age. The topics of conversations were coded to match the categories on the Interests Scale and are listed in Supplementary Table 2. Participants chose the two nursery rhymes they would recite from a list they received before the session.
Before each run, the experimenter sat in front of a blue screen facing a camera. The run began with 16 s of rest (eight repetition times), during which the participant saw the word ‘REST’. Then, live video and audio from the experimenter were presented to the subject and the verbal interaction began (Supplementary material). The experimenter always initiated the interaction. Conversations proceeded for 6 min, and repetitions for 3 min. After each interaction, the video faded to black and a ‘STOP’ slide was displayed to the participant, followed by 30 additional seconds (15 repetition times) of rest to allow for delayed haemodynamic effects. The study was designed such that participants spent more time engaging in conversation than repetition in order to limit the duration of the entire session as a whole, and to ensure participants did not become fatigued during repetition runs.
MRI data acquisition
T2*-weighted blood oxygen level-dependent (BOLD) images were acquired on a General Electric Signa HDxt 3.0 T scanner (GE Healthcare) with an 8-channel head coil. A single-shot gradient-echo echo planar imaging sequence was used: the acceleration factor of ASSET (Array Spatial Sensitivity Encoding Technique) = 2, repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, 64 × 64 matrix, field of view = 227 mm. Forty interleaved sagittal slices with a thickness of 4 mm were used to cover whole brain (voxel size = 3.55 × 3.55 × 4 mm3). Sagittal acquisition was used to help minimize slicing artefact that can occur when using more typical axial acquisitions during overt speech (Birn et al., 2010). The conversation runs consisted of 203 repetition times and the repetition runs consisted of 113 repetition times. A high-resolution T1-weighted anatomical image (MPRAGE, magnetization-prepared rapid gradient-echo) was also obtained (124 axial slices, 1.2 mm slice thickness, field of view = 24 cm, 224 × 224 acquisition matrix).
MRI data preprocessing
Echo-planar image preprocessing was performed with AFNI (Cox, 1996). The first four repetition times of each run were removed to allow for T1 stabilization, and outlying time points in each voxel, corresponding to motion and other artefacts, were attenuated with AFNI’s 3dDespike. Volumes were slice-time corrected and co-registered to the anatomical image. Sources of motion-related and physiological noise were removed with ANATICOR (Jo et al., 2010, 2013) and aCompCorr (Behzadi et al., 2007). The regressors for these methods were created by first segmenting the anatomical scan into tissue types with Freesurfer (Fischl et al., 2002). An eroded ventricle mask was applied to the volume-registered echo-planar image data to create a nuisance time series for the ventricles. An eroded white matter mask was used to create a ‘localized’ estimate of the BOLD signal in white matter, which was averaged within a 20-mm radius sphere centred on each voxel (see Jo et al., 2010, 2013 for further discussion). For runs with physiological data, eight Retroicor (Glover et al., 2000) and five respiration volume per time (RVT, Birn et al., 2008) regressors were created from the cardiac and respiration measures, estimated at slice time 0. The multiple physiological regressors of each type represent several interpolated time points within each repetition time. Additional speech-related motion and respiration artefacts were modelled using a principal component analysis (PCA) decomposition of BOLD fluctuations in non-grey matter locations (Behzadi et al., 2007). Eroded masks of white matter and ventricles were joined into a single nuisance tissue mask and applied to each re-aligned and co-registered functional time series, which were detrended by fourth order polynomials prior to PCA in order to remove scanner drift and other very low frequency signals. The time series were then decomposed with PCA, retaining the first three principal components, as pilot analyses indicated these were the components that contained the most variance related to model-based respiration regressors (Retroicor and RVT regressors; see also Stoddard et al., 2016). The full nuisance regression model for each voxel therefore included regressors for the fourth order polynomial baseline model, one ventricle time series, one localized white matter time series, six motion parameters, five respiration volume per time regressors, eight Retroicor time series (four cardiac and four respiration regressors) and three aCompCor principal component regressors. The ‘clean’ residual time series of this model was converted into Talairach space, resampled to 3 mm3 isotropic voxels, and used in all subsequent analyses.
The magnitude of transient head motion was calculated from the six motion parameters and aggregated as a single variable using AFNI’s @1dDiffMag to calculate a Motion Index (Gotts et al., 2012; Berman et al., 2016). This measure is comparable to average Framewise Displacement over a scan (Power et al., 2012) and is in units of mm/repetition time. The grand average correlation of all voxel time series with each other, or GCOR (for global correlation level), was also calculated for each experimental run to serve as a more omnibus measure of residual global artefacts and was used as a nuisance covariate in region of interest-based, group-level analyses (Gotts et al., 2013a,b, 2017; Saad et al., 2013; Zachariou et al., 2017).
Task-related functional connectivity analyses
Our main analyses used a functional connectivity approach in which correlations among voxel- and/or region of interest-based time series were compared across conditions. In part, this choice was mandated by the lack of appropriately spaced baseline periods during naturalistic conversation, preventing the use of a more typical general linear model approach. This form of analysis was also directly comparable to that applied previously to resting state data. Our experiment used a 2 × 2 mixed factorial design with one within-subjects variable (Task) and one between-subjects variable (Group). Two types of relationships were identified: (i) inter-region correlations that differed between the autism and control groups regardless of task (i.e. main effect of Group); and (ii) inter-region correlations that differed by Group and Task (their interaction). Our functional connectivity analyses used the three steps by Gotts and colleagues (Gotts et al., 2012; Berman et al., 2016): seed definition, target region of interest selection, and region-to-region correlation analysis. We undertook all three steps for both the Group effect and the Group × Task interaction effects. For completeness, we also report the main effect of Task (Conversation > Repetition).
Seeds were identified using whole-brain ‘connectedness’ (Cole et al., 2010; Gotts et al., 2012). The average Pearson correlation of each voxel’s time series with every other voxel’s time series was calculated to create a 3D reduction of the 4D (3D + Time) dataset for each functional run (available as the AFNI function 3dTcorrMap). Since connectedness reflects the average level of correlation with the rest of the brain, it gives an indication of how involved a given brain area was with the task during the scan. This approach, akin to centrality in graph theory, has been used previously in studies of resting state (Cole et al., 2010; Gotts et al., 2012; Meoded et al., 2015; Berman et al., 2016; Stoddard et al., 2016; Watsky et al., 2018) and task-based functional connectivity (Song et al., 2015; Steel et al., 2016). Linear mixed effects models (Chen et al., 2013) were constructed whose dependent variables were the voxel-wise connectedness maps from each functional run. Group and Task and their interaction were included as fixed effects. Participant age and the motion index (computed separately for each run) were included as nuisance covariates. Participant was treated as a random intercept. Cluster correction was used to control the type I error rate. The average smoothness of the cleaned functional time series was estimated with AFNI’s 3dFWHMx, using the empirical, spatial autocorrelation function (June 2016). 3dClustSim (June 2016) was then used to run a Monte Carlo simulation with 5000 iterations within the grey matter mask in Talairach space that the analyses were performed within. Importantly, the smoothness estimates and noise simulations did not assume Gaussian distributions of activity, which has been shown to inflate the false positive rate in studies using more traditional cluster size correction (Eklund et al., 2016; Cox et al., 2017). Clusters were selected at a cluster defining threshold of P < 0.001, minimum cluster size k = 22.
The seed definition step was then followed with more typical seed-based correlation analyses. Signal within each seed region was averaged across voxels to form region of interest-averaged time series, which were correlated with the time series for every voxel in the brain, separately for each run. These correlations were Fisher z-transformed and used as dependent variables in linear mixed effects models with the same fixed and random effects as the previous step. We tested for the main effect of Group at a voxel threshold of P < 0.001, with correction by cluster size for whole-brain comparisons as well as the number of seeds tested {i.e. family-wise error (FWE) correction to P < [0.05 / (number of seeds)]}. Results of the separate seed-based tests were combined to form one composite map, first by binarizing each seed-to-whole-brain test into zeros and ones and then summing across them. This map was then thresholded at 80% of the maximum possible sum (i.e. if six seeds were tested, included voxels would need to have arisen in 5/6 tests). Clusters smaller than 20 voxels were then excluded to eliminate small singleton clusters with higher levels of voxel noise. Secondary target regions were then combined together with seed regions to arrive at a full set of regions of interest. Region-by-region matrix analyses were then conducted using the same contrasts applied to connectedness and the seed-based tests, allowing the examination of all interregional relationships. For these all-to-all matrix tests, as well as correlation with social impairment and comparison with resting state data, GCOR was included as an additional nuisance covariate (to age and motion index) in order to insure that any group effects were not due to residual global or speech-related artefacts (Gotts et al., 2013a,b, 2017; Saad et al., 2013).
Correlations with social impairment as measured with the Social Responsiveness Scale (SRS-1 Total Raw Score) (Constantino et al., 2003; Gotts et al., 2012; Ramot et al., 2017) were tested with additional models in the autism subjects, separately for the Group main effect regions of interest and the Group × Task interaction regions of interest. The analyses of Group main effect regions of interest included data from both tasks, conversation and repetition, whereas the analysis of Group × Task interaction regions of interest focused on the conversation runs, which were of primary interest.
Multiple comparisons in the region-by-region correlation tests were controlled with false discovery rate (FDR) (Genovese et al., 2002), calculated by pooling over P-values resulting from task matrix tests together (autism versus control for the Group regions of interest; interaction test in Group × Condition regions of interest; effects of SRS in both of these matrices). We report all results unthresholded in the lower triangles of the matrices, with corrected results (q < 0.05) in the upper triangles.
Comparison of task and resting state data
Several previous studies of functional connectivity at rest in male adolescents and adults with autism have observed a pattern of cortico-cortical decreases among several brain regions engaged by social tasks, such as the STS, medial prefrontal cortex, the temporoparietal junction, left IFG, and somatosensory cortex (Anderson et al., 2011; Gotts et al., 2012; Di Martino et al., 2014; Cerliani et al., 2015; Cheng et al., 2015; for reviews, see Picci et al., 2016; Hull et al., 2017). We included an explicit comparison of results in task and rest in the current study to determine which patterns of functional connectivity differences are robust across cognitive states with and without overt motor behaviours versus those that are context-specific to state. The resting state data we included have been used in previous publications (Gotts et al., 2012, 2013a, b, 2017; Plitt et al., 2015; Power et al., 2017; Ramot et al., 2017) and were collected on the same 3 T MRI scanner as the task data. The preprocessing for these data also used the same ANATICOR procedure as for the task data with the only difference being the lack of the aCompCor regressors (Behzadi et al., 2007). For rest scans, participants were instructed to lie still and relax, maintaining fixation on a central cross on the viewing screen. Participants included 56 autistic males [mean age = 19.1 years, standard deviation (SD) = 3.8 years] and 62 control males (mean age = 21.2 years, SD = 5.1 years), with the same selection/inclusion criteria as discussed above for the task data. Participant groups were matched on age, IQ, head motion, and overall measures of temporal signal to noise ratio. Informed assent and consent were obtained from all participants and/or their parent/guardian (participants younger than 18), and the experiment was approved by the NIMH Institutional Review Board (protocol 10-M-0027, clinical trials number NCT01031407).
Two sets of analyses were conducted with resting state data. In the first, the regions identified as showing a main effect of group during task were applied directly to the resting state data for quantitative comparison with task using linear mixed effect models (AFNI’s 3dLME), with Conversation and Repetition data pooled to form the condition ‘Task’. Each region-by-region combination of functional connectivity served as dependent variables, with Group and State (Task, Rest) included as fixed effects. Main effects of Group were evaluated within Task and Rest separately, along with a Group × State interaction (correction for multiple comparisons by FDR to q < 0.05). The second analysis compared whole-brain connectedness between autism and control participants in Rest, with age and motion index as nuisance covariates for comparison with the main effect of Group during Task in terms of spatial overlap (voxel-wise threshold of P < 0.001, FWE corrected using cluster size to P < 0.05; at this voxel-wise threshold, results were also corrected by FDR to q < 0.05).
Data availability
The data that support the findings of this study are openly available in XNAT https://www.xnat.org/.
Results
Verbal output
First, we examined whether participants in the autism and control groups performed the task similarly, to ensure any group differences in the neuroimaging analysis could not be explained by gross differences in task-relevant behaviour. Audio recordings were analysed with MATLAB and raw counts of words and sentences were obtained following transcription by a professional transcription service (Supplementary material). We examined SpeakingTime (defined below), total number of words uttered, number of speaking turns taken, and number of words per sentence.
For each run, SpeakingTime was calculated as the ratio of time a participant spoke during a run divided by the total time spent speaking by both the participant (PPT) and experimenter (EXP) [PPTspeech / (PPTspeech + EXPspeech)]. Ratios >0.5 indicated that the participant talked more than the experimenter. Overall there were no differences between groups or conditions for the conversation [t(37) = 0.31, P = 0.76] or repetition runs [t(37) = 0.66, P = 0.51] (Supplementary Fig. 1). The total number of words produced also did not differ between the autism and control groups [t(37) = −1.4, P = 0.17] and neither did the number of speaking turns [t(37) = 0.46, P = 0.65], nor the number of words per sentence [t(37) = 0.01, P = 0.99]. For the repetition runs, the participant repeated exactly what their partner spoke, resulting in exactly matched verbal output.
Motion
Motion index values for all runs individually (Supplementary Fig. 2) were subjected to a 2 × 2 repeated measures ANOVA with Group, Task, and Group × Task as terms. Motion did not differ by Task [F(1,74) = 0.92, P = 0.34], and Task and Group did not interact to predict motion [F(1,74) = 0.47, P = 0.50]. However, there was a statistically significant main effect of Group [F(1,74) = 9.1, P = 0.003]. To assess the overall magnitude of this group difference, we calculated the average motion index by subject and by group, and compared them. The autism group moved 57 μm per repetition time (∼ 1/17th of a mm) more than the typically developing controls (mean autism motion = 0.216 ± 0.09 mm/repetition time; mean control motion = 0.159 ± 0.07 mm/repetition time). To mitigate any remaining motion-related artefacts that were not already removed by our cleaning procedure, the motion index for each run was included as a covariate in all subsequent correlation analyses. A control analysis excluding high motion runs in the autism group and low motion runs in the control group in order to match motion levels by group also did not lead to large changes in the results reported below.
Comparison of conversation versus repetition in whole-brain connectedness
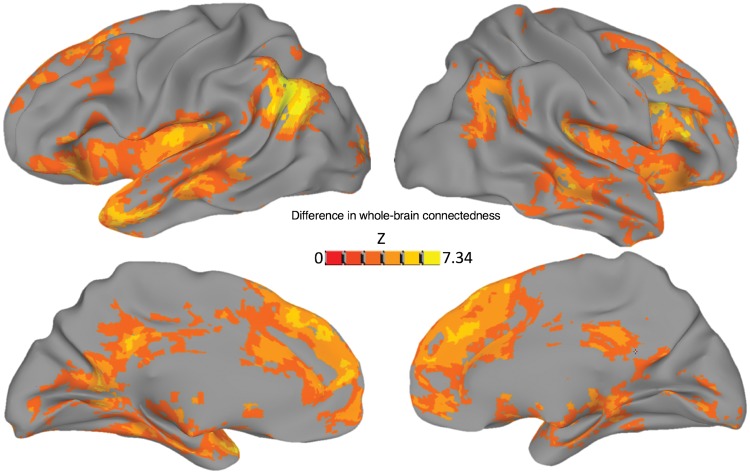
Because of the limitations of a general linear model approach during naturalistic conversation without appropriately spaced baseline periods, we instead took a basic correlational (task-driven functional connectivity) approach to the main analyses of interest (although see Supplementary Fig. 3 for a restricted general linear model analysis of local activity differences in Speaking versus Listening periods). Using this approach, we first examined the main effect of Task on whole-brain ‘connectedness’ (the correlation of each voxel with the rest of the brain) (Cole et al., 2010; Salomon et al., 2011; Gotts et al., 2012). The main effect of Task yielded highly significant results with multiple large clusters (voxel-wise threshold of P < 0.001). The strongest differences in connectedness (Conversation > Repetition) were observed in in dorsomedial prefrontal cortex, left fronto-temporal cortex with a peak in left anterior temporal lobe (prominently including the pole and STG), and left angular gyrus (Fig. 1 and Supplementary Table 3).
Figure 1.
Conversation versus Repetition. A test for the main effect of Task, across all whole-brain connectedness values from both groups, revealed a number of areas that showed greater whole-brain involvement during Conversation compared with the Repetition condition. The strongest of these results were frontal and temporal areas (especially left temporal pole), the left temporoparietal junction, and bilateral dorsomedial prefrontal cortex. Voxels significant at P < 0.001 are plotted on an inflated Freesurfer standard surface (Fischl et al., 2002).
Main effect of Group
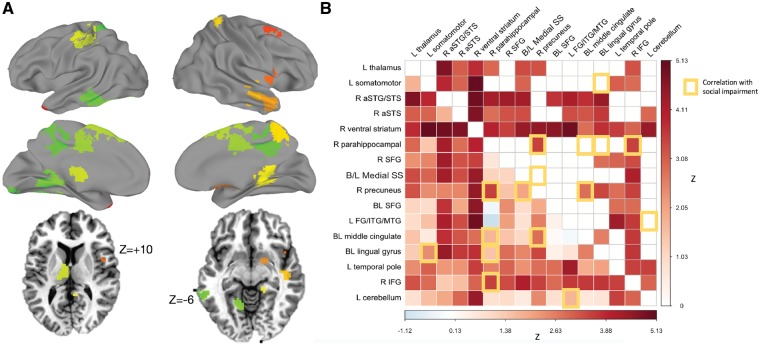
The main effect of Group on connectedness values identified six regions whose whole-brain connectedness was greater in the autism than the control group. There were no regions showing greater correlation for the control than the autism group. Increased correlation for the autism participants was found for two clusters in the right anterior temporal lobe, a cluster at the left temporal pole, the right inferior frontal gyrus and anterior insula, right ventral striatum, and somatomotor cortex (Supplementary Table 4). Using these clusters as seeds in a seed-to-whole-brain analysis (see ‘Materials and methods’ section), we identified 10 additional regions that showed greater correlation in autism than control participants (Fig. 2 and Supplementary Table 5). An analysis of the correlation values within the region × region matrix revealed a number of regional pairs whose correlations differed between the autism and control groups. This was most pronounced among right hemisphere regions such as the precuneus, anterior superior temporal gyrus and sulcus, and IFG. The most implicated region was a cluster in the right ventral striatum, which showed a significant hypercorrelation with each of the other regions, even after multiple comparisons correction (Fig. 2).
Figure 2.
Group (Autism > Control) comparison of inter-region correlations across tasks. (A) Regions of interest defined by the main effect of Group plotted on standard surfaces. Axial slices provided to show thalamus and ventral striatum regions. (B) Region-by-region matrix indicating strength of effect of autism versus control. A main effect of Group (Autism versus Control) was tested on region-by-region correlation levels. Overall, autism participants showed increased correlation between regions during the social interaction tasks, relative to controls (lower triangle unthresholded, upper triangle FDR corrected to q < 0.05). Increased functional connectivity was correlated with more extensive social impairment measured by the Social Responsiveness Scale (yellow-outlined squares).
We also identified region pairs whose correlation was predicted by the extent of social impairment (indexed by the Social Responsiveness Scale). The most strongly implicated areas were in lingual gyrus bilaterally, the middle cingulate, precuneus, and right inferior frontal gyrus (see yellow squares in Fig. 2B). All results except one were positive correlations, with one region pair showing a negative correlation (higher score → lower functional connectivity levels between the left cerebellum and the left fusiform/middle temporal gyrus). Three of these social impairment severity effects overlapped with the group effects: i.e. there were three region pairs whose correlations were both elevated in autism relative to controls, and also greater in autism participants with greater social impairment. These were the right IFG with right parahippocampal gyrus, right parahippocampal gyrus with right precuneus, and right precuneus with bilateral middle cingulate cortex.
Group × Task interactions
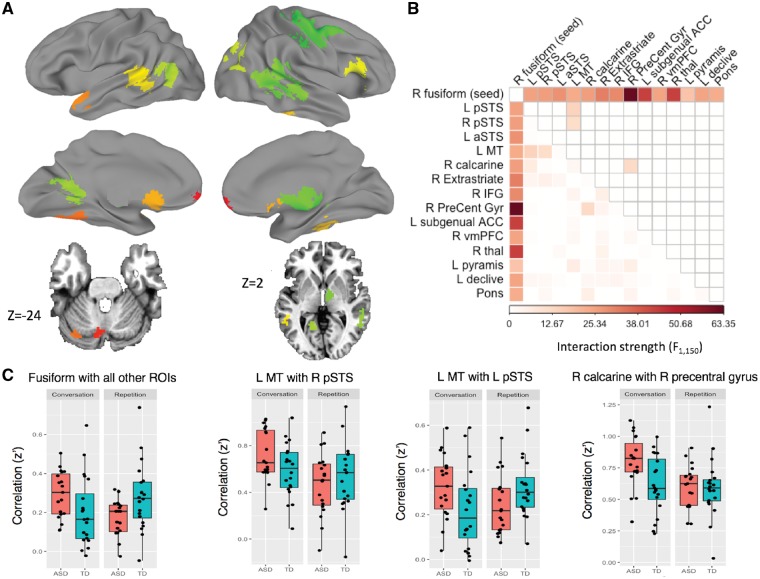
One region—in the right lateral fusiform gyrus—showed a difference in whole-brain connectedness by Group and Task (P < 0.0001, FWE corrected to P < 0.05). Using this region as a seed revealed 14 additional regions whose correlations with the seed varied by Group and Task (Fig. 3 and Supplementary Table 6). These additional regions were then aggregated with the right fusiform seed into one set of regions (15 total), and their correlations were analysed with respect to one another (which had not yet been explicitly tested). In addition to the previously determined relationships between the right fusiform seed and the other 14 regions, three more region pairs that did not include the seed also showed correlations that varied by Group and Task: left posterior STS with left middle temporal temporal cortex, left middle temporal cortex with right posterior STS, and calcarine gyrus with the right precentral gyrus. For illustration of the nature of these interaction effects, mean Fisher z-transformed correlation coefficients for each participant are plotted in Fig. 3C. Overall, autism participants showed a greater between-region increase in correlation during conversation compared to repetition than did controls. We also tested for an effect of extent of social impairment involving the Group × Task dependent regions. Only one pair survived FDR correction: functional connectivity between right extrastriate and left posterior STS showed a positive correlation with extent of social impairment (Supplementary Fig. 4).
Figure 3.
Group × Task interaction effects. (A) Group × task dependent regions of interest. (B) Region-by-region matrix. Colours indicate larger F-statistics for the interaction of Group × Task. Lower triangle = unthresholded values. Upper triangle indicates correction by FDR to q < 0.05. (C) For significant results, the raw functional connectivity values (Fisher z-transformed correlation coefficients) were extracted and plotted by Group and Task for visualization. Autistic participants showed increased correlation levels during Conversation compared to Repetition. During Conversation, autistic participants showed greater correlation levels between regions than controls.
Comparison of results during task and rest
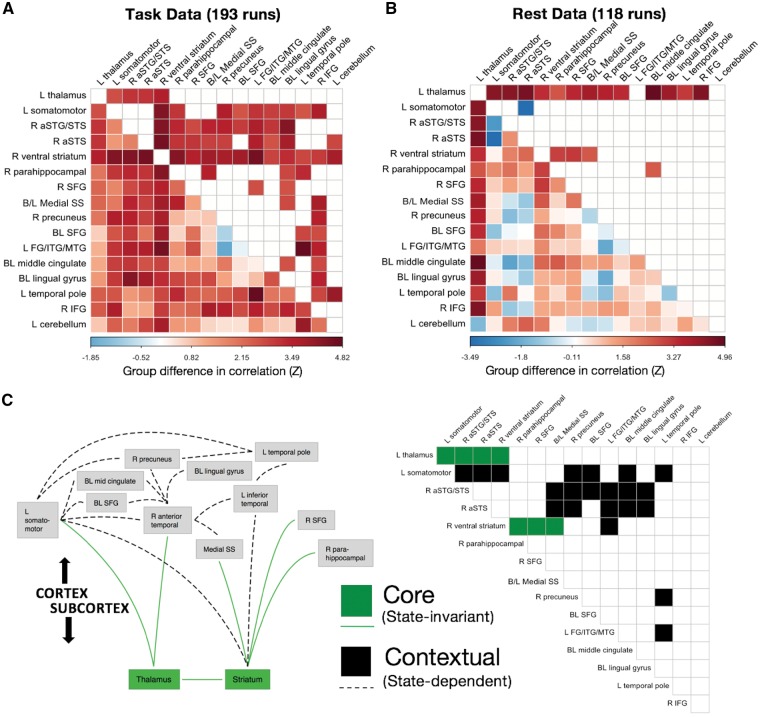
Both the main effect of Group and the interaction of Group and Task condition (Conversation versus Repetition) indicated that task-based functional connectivity in autism was greater than in controls (i.e. hypercorrelation). This pattern departs qualitatively from the results reported by some previous data-driven studies of whole-brain functional connectivity in male adolescents and adults, including those by our own laboratory (Gotts et al., 2012; Ramot et al., 2017; see also Anderson et al., 2011; Di Martino et al., 2014; Cerliani et al., 2015; Cheng et al., 2015). To examine this potential discrepancy, we therefore compared the patterns of task-based and resting state functional connectivity using the regions showing a main effect of Group in the current study. The resting state data have been used in a variety of studies published previously by our laboratory (Gotts et al., 2012, 2013a,b, 2017; Plitt et al., 2015; Power et al., 2017; Ramot et al., 2017), with groups matched on age, IQ, head motion, and measures of temporal signal to noise ratio. Region-by-region results are shown in Fig. 4, separately for Task (pooling Conversation and Repetition) and Rest. As already shown above, increases in task-based functional connectivity are observed in autism for these regions (Fig. 4A). Interestingly, increases are also observed during rest, particularly involving the left thalamus (with virtually all of the other regions) and right ventral striatum (with right parahippocampal gyrus, right superior frontal gyrus, and bilateral medial somatosensory cortex), consistent with previous reports by Di Martino et al. (2011), Cerliani et al., (2015), and Cheng et al. (2015). These common effects are highlighted as state-invariant, or ‘core’, region pairs using green squares in Fig. 4C. A number of region pairs also differ significantly by group and state (i.e. Group × State interaction; black squares in Fig. 4C), with many pairs showing increases during Task and lacking significant differences in Rest, and one region pair (left somatomotor with right anterior STS) showing a significant decrease in Rest and lacking differences in Task.
Figure 4.
Comparisons of Task and Rest. (A) Group differences in region-by-region functional connectivity during task in task-defined regions. Higher values indicate larger Autism > Control differences. (B) Group differences in region-by-region functional connectivity in resting state data, measured in the same task-defined regions. (C) Green squares and lines indicate pairs of regions with Autism > Control differences that replicate across Task and Rest. These ‘core’ effects involved the left thalamus and the right ventral striatum (corrected by FDR to q < 0.05 in both Task and Rest). Black squares and lines indicate ‘contextual’ effects occurring between cortical areas (significant Group × State interaction, corrected by FDR to q < 0.05).
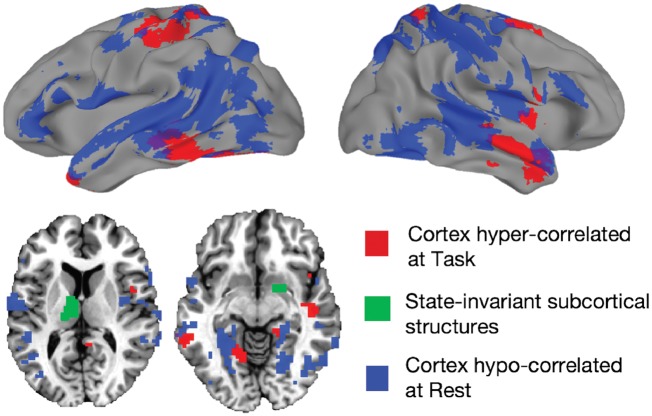
Given the relative lack of differences seen in the Rest data when using the regions detected during Task, we also compared whole-brain connectedness between autism and control at Rest. The results during Rest are shown in Fig. 5 in blue, with the regions showing a main effect of Group during Task shown in red for comparison (voxel-wise threshold of P < 0.001 for both Task and Rest, corrected to P < 0.05 by cluster size). Decreases in resting state functional connectivity in autism are indeed quite prominent, but they overlap very little with the regions showing increases during Task, explaining the lack of decreased functional connectivity seen in Fig. 4B. Despite these state-related differences, the prominent state-invariant results involving the thalamus and ventral striatum (shown in green in Figs 4C and 5) highlight notable mechanistic commonalities between Task and Rest.
Figure 5.
Regions showing the greatest hyper-correlation at task, and the greatest hypocorrelation at rest. Autism > Control cortical Task-defined regions (red) shown along with the strongest Control > Autism effects in resting state data (blue). The left thalamus and right ventral striatum regions from the present study are coloured green. These subcortical structures showed the greatest state-invariance—with the same differences in correlation with cortex during both task and rest.
Discussion
The purpose of this study was to characterize neural activity during spontaneous conversation in autism. Participants engaged in face-to-face spoken interactions while being scanned with functional MRI. Our design used two groups (people with autism versus controls) who performed two tasks (Conversation versus Repetition). Comparing neural activity during the tasks (across groups), greater whole-brain correlation was observed during conversation than repetition in regions involved with communication and social processing. Comparing the groups (across tasks), greater task-driven functional connectivity was observed for the autism than the control group, mainly between regions involved with communication and sensorimotor processing. Furthermore, functional connectivity among many region pairs, especially those involving right parahippocampal gyrus, right precuneus, right IFG and bilateral middle cingulate, was strongest in subjects with greater parent-reported social impairments observed outside of the laboratory. Next, we examined neural activity differences between groups as a function of task. We found that, particularly among visual and social areas and under high social demand, functional connectivity in the autistic (but not control) participants increased, and increases were related to social impairment. Finally, we examined the relationship between group differences in task-driven functional connectivity and group differences in previously published resting state connectivity of autism and control participants that instead exhibited a pronounced decrease in cortico-cortical correlations in autism. We found that some region pairs that were hypercorrelated for autism in Task were also hypercorrelated at Rest, and that these pairs involved the thalamus and striatum. By contrast, many more widespread regions showed strong context-specific effects (i.e. Group × State interactions), with regions hypercorrelated during Task failing to show differences in Rest and regions hypocorrelated during Rest failing to show differences in Task (Figs 4 and 5).
Increased functional connectivity in autism
We observed widespread increased functional connectivity in autism at Task. These increases were not easily explainable by head motion or other residual global artefacts because measures of these artefacts were covaried in all analyses. This is consistent with at least one other study of functional connectivity during language processing that showed increased occipital recruitment in autism during category judgements of visually-presented nouns (Feelings versus Tools and Colors; Shen et al., 2012). It also accords with activation studies showing autistic children recruit more areas than control children when judging emotion and irony in prosody (Wang et al., 2006; Colich et al., 2012; Eigsti et al., 2012), or incorporating real-world knowledge in sentence processing (Tesink et al., 2009; Groen et al., 2010). However, other studies of functional connectivity during language processing that required making judgements about imagery (Kana, 2006) or answering questions about agent-patient relationships in visually-presented sentences (Just et al., 2004) have only shown decreased functional connectivity. It may be that tasks with greater sensory demands (e.g. those involving speech versus mere reading), tasks with social components (e.g. about emotions, sarcastic/ironic prosody, and pragmatics) or tasks requiring sustained attention are more likely to elicit hypercorrelation among cortical areas in people with autism. Moreover, not all of these studies have compared groups with matched behavioural performance (as in the present study).
Increased correlation during task versus decreases during rest
For adolescent participants with autism, several previous studies of resting state functional MRI functional connectivity have observed decreased correlations among regions engaged in aspects of social processing, such as the STS, medial prefrontal cortex, left IFG, and somatosensory cortex (Anderson et al., 2011; Gotts et al., 2012; Hagen von dem et al., 2013; Di Martino et al., 2014; Cerliani et al., 2015; Cheng et al., 2015). Some of these decreases are furthermore associated with impairments of social functioning in autistic participants as measured by the Social Responsiveness Scale, which establishes that the functional connectivity decreases are relevant to behaviour (Di Martino et al., 2009; Gotts et al., 2012; Ramot et al., 2017). However, in our current study, correlated inter-regional activity was increased in autism, and these increases were also associated with impairments of social functioning using the same behavioural measure. When we analysed our previously published resting state data using the same regions of interest, we found that rather than highlighting a discrepancy between the two datasets in terms of increased versus decreased correlations, a common set of interregional relationships held across both sets. These common relationships involved the thalamus and striatum, which were hypercorrelated in autism with each other and with cortical areas. The left thalamus and caudate nucleus exhibited greater correlation with the left somatomotor cortex, the right anterior STS, and the right ventral striatum in autistic participants. The right ventral striatum further exhibited greater correlation in autism with the bilateral medial somatosensory cortex, the right parahippocampal gyrus, and the right superior frontal gyrus. Taken together, the current findings highlight two classes of relationships among regions—‘core’ state-invariant relations and ‘contextual’ state-dependent relations.
Context-specificity of cortico-cortical correlations
As discussed, many results were state-dependent—regions that were hypercorrelated during Task appeared to function at typical levels or were even hypocorrelated during Rest. Furthermore, in our task data, participants with more extensive social impairment showed the greatest interregional functional connectivity. Interestingly, an inverse relationship between functional connectivity and social impairment (using the same measure) was found in resting state data, as reported in Gotts et al. (2012) and Ramot et al. (2017). There, participants with greater parent-reported social impairment showed more strongly decreased interregional correlation. How can this apparent discrepancy be explained? One possibility is that the increased functional connectivity at task is a compensatory neural strategy (c.f.Shen et al., 2012). On this account, autistic participants with greater social impairment would require greater interregional correlation to perform social tasks similarly to controls. This compensatory neural strategy could be deployed in social situations outside the laboratory, too. Indeed, our autistic participants had normal or high IQs and language ability, and their lives seemed in many ways similar to those of controls. Like the control participants, the participants with autism talked (during the experiment) about attending schools, holding jobs, and interacting with peers. In such situations, many simple conversations are likely to occur, most of which are likely to be ‘successful’ in the sense that conversational goals—requesting or providing information, assistance, praise, guidance and so on—are accomplished. Why, then, did our autistic participants receive diagnoses if they are able to compensate and function at nearly normal levels? Even a highly successful neural strategy could result in slight but detectable behaviour differences—i.e. the compensation is not perfect, but it is ‘good enough’ to allow some people with milder autism to function (and to make autism diagnosis a non-trivial procedure requiring hours of observation by trained professionals) (Lord et al., 2000). Whether compensatory hypercorrelation observed in this study would generalize to participants with more severe forms of autism is a question that future studies should address.
Notably, the areas that were most severely hypocorrelated at Rest and are negatively correlated with degree of social impairment (Fig. 5, blue areas) do not overlap well with the areas that show the highest degree of hypercorrelation during Task (Fig. 5, red areas). This, too, may be consistent with a compensation account. If connectivity among some social brain areas is intrinsically disordered in autism (i.e. Fig. 5 blue areas), perhaps the compensation for these disordered connections takes place via other, spatially non-identical cortical areas (red areas), bringing regions that are hypocorrelated at Rest up to control levels. Novel, compensatory routing among cortical areas may take place via the thalamus, discussed below. Critically, these effects did not appear to manifest in the local activity levels as indexed by a general linear model analysis (Supplementary material and Supplementary Fig. 3), helping to establish that these effects are more selective to inter-regional interactions rather than simple activity levels in those regions.
Core features: abnormal thalamic and striatal interactions—a gating issue?
The thalamus and basal ganglia (within which the striatum resides) are strongly anatomically interconnected with each other and with the entire cerebral cortex. Through these connections, both structures gate transmission between distal cortical regions (Sherman, 2007; McNab and Klingberg, 2008). Could abnormal gating explain the abnormal cortical interactions? In our study the gates were ‘wide open’, especially the intrinsic connections measured during resting state: of the 14 task-defined cortical regions, 12 were intrinsically hyperconnected with the thalamus during rest, even after statistical correction (Fig. 4B). The fact that these regions were defined independently of the rest data is striking and suggests that intrinsic thalamocortical connectivity could partially determine which areas of cortex are likely to exhibit hypercorrelations during sustained or social tasks.
In an early study, Mizuno et al. (2006) observed increased thalamocortical correlation during a non-social visuomotor task (n = 8) and suggested that it may be ‘hyperfunctional’, helping to compensate for decreased cortico-cortical connectivity (Mizuno et al., 2006). This is unlikely (and distinct from our current proposal), given that increased thalamocortical correlation is also observed at rest (Di Martino et al., 2011, 2014; Nair et al., 2014; Cerliani et al., 2015; Cheng et al., 2015). Instead, we propose that the context-dependent increases may play this compensatory role. One previous study has examined correlation of brain activity in both task and rest states in autism, but exclusively focused on cortical results. You et al. (2013) found that overall distal connectivity was heightened in autism during a non-social sustained attention task, but decreased at rest (You et al., 2013). Furthermore, they reported that inattention problems in everyday life were associated with a greater increase in functional connectivity from rest to task in autism, from which they conclude that the increased task-driven functional connectivity is maladaptive. However, it is difficult to distinguish maladaptive from compensatory hypercorrelation in the You et al. study as the autism and control groups were not matched for behavioural performance on the in-scanner task.
Limitations and future directions
Future work should seek to characterize the differences in functional connectivity not just between task and rest states, but between different types of tasks. It is almost certain that functional connectivity patterns will vary based on task constraints, and these differences serve as the basis of results in studies that employ techniques such as Psychophysiological Interaction (PPI, Friston et al., 1997) and Dynamic Causal Modeling (DCM, Friston et al., 2003). In the present study, we observed increased functional connectivity in the autism subjects that strongly implicated right hemisphere regions known to be involved with communication and vocal processing, such as right STS and right IFG. Other tasks that require different sorts of behaviour, or with different attentional and social demands, may elicit different patterns. We hope that the comparison between a highly demanding social task and resting state, which we show in this study, can serve as a starting point for the characterization of a broader set of cognitive states.
One caveat concerning the results is that, even when wearing noise-cancelling headphones, the sensory stimulation within an MRI scanner can be intense. A growing literature suggests that autistic individuals may have abnormal sensory and motor processing (Cerliani et al., 2015; Nebel et al., 2016) and also increased anxiety (see South et al., 2017 for review). This could potentially give rise to a situation where abnormal connectivity between sensorimotor processing and emotion-related regions is observed in people with autism because of the MRI scanner environment itself. In this regard, it will be important for future studies to re-examine altered dynamics in autism using quieter neuroimaging methods such as magnetoencephalography (MEG). Along these lines, we have previously noted an encouraging correspondence between our published resting state functional MRI results and MEG phase-locking measures (Ghuman et al., 2017; see Picci et al., 2016 for review of recent MEG studies), thus suggesting that at least some of the altered dynamics we report here were not due to intense sensory stimulation.
A limiting factor in the interpretation of our results is that all participants were male and high-functioning. The reason for this was to promote consistency with the bulk of prior studies and to limit the mixture of potentially distinct patterns that may occur in females (Alaerts et al., 2016), who are rarer in frequency and more difficult to recruit in sufficient numbers to match those of males. The sample sizes were also more typical of standard functional MRI task-based studies (∼20 participants per group), which is substantially smaller than many of the more recent resting state studies. We acknowledge that these issues limit the generalizability of the current study, and we hope this may be addressed with future research with larger samples of both males and females, as well as with the inclusion of lower-functioning participants. Another issue concerns the fact that while repetition and conversation differ in social demand, they differ in other aspects as well (e.g. mnemonic or linguistic demand). Future work on the neurobiology of social aspects of conversation should seek to refine baseline and control conditions for spontaneous conversation.
To our knowledge this study is the first to characterize brain activity in autism during the context most similar to the gold standard of autism diagnosis (Lord et al., 2000)—face-to-face spoken interactions. It also emphasizes the importance of characterizing autistic brain organization in diverse task contexts and cognitive states. Further research should seek a more mechanistic understanding of whether subcortico-cortical connections may play a causal role in abnormal state-dependent cortico-cortical connections, and how disordered neural connections may be changed via interventions (Ramot et al., 2017).
Supplementary Material
Acknowledgements
We thank Bako Orionzi, Ian Eisenberg and Cynthia Peng for their assistance and our participants and their families for their participation.
Glossary
Abbreviations
- IFG
inferior frontal gyrus
- STS
superior temporal sulcus
Funding
This study was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, NIH Clinical Study Protocol 10-M-0027, Grant no.: ZIA MH002920–09, Clinical trials ID: NCT01031407, the NIMH-UCL Joint Doctoral Training Program in Neuroscience, and a Leverhulme Trust Early Career Fellowship to K.J.
Competing interests
The authors report no competing interests.
References
- Abbott AE, Nair A, Keown CL, Datko M, Jahedi A, Fishman I, et al. Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. Cerebral Cortex 2016; 26: 4034–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams DA, Lynch CJ, Cheng KM, Phillips J, Supekar K, Ryali S, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci USA 2013; 110: 12060–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts K, Swinnen SP, Wenderoth N. Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc Cogn Affect Neurosci 2016; 11: 1002–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts K, Woolley DG, Steyaert J, Di Martino A, Swinnen SP, Wenderoth N. Underconnectivity of the superior temporal sulcus predicts emotion recognition deficits in autism. Soc Cogn Affect Neurosci 2014; 9: 1589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013. [Google Scholar]
- Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, et al. Functional connectivity magnetic resonance imaging classification of autism. Brain 2011; 134: 3742–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony LG, Kenworthy L, Yerys BE, Jankowski KF, James JD, Harms MB, et al. Interests in high-functioning autism are more intense, interfering, and idiosyncratic than those in neurotypical development. Dev Psychopathol 2013; 25: 643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage 2010; 53: 247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood A, Frith U, Hermelin B. The understanding and use of interpersonal gestures by autistic and Down’s syndrome children. J Autism Dev Disord 1988; 18: 241–57. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Social and pragmatic deficits in autism: cognitive or affective? J Autism Dev Disord 1988; 18: 379–402. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007; 37: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RA, Gotts SJ, McAdams HM, Greenstein D, Lalonde F, Clasen L, et al. Disrupted sensorimotor and social–cognitive networks underlie symptoms in childhood-onset schizophrenia. Brain 2016; 139: 276–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Kenworthy L, Case L, Caravella R, Jones TB, Bandettini PA, et al. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage 2010; 49: 1099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage 2008; 40: 644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW. Interests scale. Chapel Hill, NC; 2003. [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psychiatry 2015; 72: 767–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage 2013; 73: 176–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Gu H, Zhang J, Feng J. Autism: reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain 2015; 138: 1382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien H-Y, Lin H-Y, Lai M-C, Gau SS-F, Tseng W-YI. Hyperconnectivity of the right posterior temporo-parietal junction predicts social difficulties in boys with autism spectrum disorder. Autism Res 2015; 8: 427–41. [DOI] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage 2010; 49: 3132–48. [DOI] [PubMed] [Google Scholar]
- Colich NL, Wang A-T, Rudie JD, Hernandez LM, Bookheimer SY, Dapretto M. Atypical neural processing of ironic and sincere remarks in children and adolescents with autism spectrum disorders. Metaphor Symb 2012; 27: 70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord 2003; 33: 427–33. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29: 162–73. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. fMRI clustering and false-positive rates. Proc Natl Acad Sci USA 2017; 114: E3370–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marchena A, Eigsti I-M. Conversational gestures in autism spectrum disorders: asynchrony but not decreased frequency. Autism Res 2010; 3: 311–22. [DOI] [PubMed] [Google Scholar]
- Delmonte S, Gallagher L, O’Hanlon E, McGrath J, Balsters JH. Functional and structural connectivity of frontostriatal circuitry in autism spectrum disorder. Front Hum Neurosci 2013; 7: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry 2011; 69: 847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry 2009; 65: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry 2014; 19: 659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, et al. Disrupted Neural Synchronization in Toddlers with Autism. Neuron 2011; 70: 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJH, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp 2011; 32: 1013–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti I -M, Schuh J, Mencl E, Schultz RT, Paul R. The neural underpinnings of prosody in autism. Child Neuropsychol 2012; 18: 600–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016; 113: 7900–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Neuron MD. 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–55. [DOI] [PubMed] [Google Scholar]
- Fishman I, Datko M, Cabrera Y, Carper RA, Müller R-A. Reduced integration and differentiation of the imitation network in autism: a combined functional connectivity magnetic resonance imaging and diffusion-weighted imaging study. Ann Neurol 2015; 78: 958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I, Keown CL, Lincoln AJ, Pineda JA, Müller R-A. Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry 2014; 71: 751–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris DL, Lai MC, Nath T, Milham MP, Di Martino A. Network-specific sex differentiation of intrinsic brain function in males with autism. Mol Autism. 2018; 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997; 6: 218–29. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003; 19: 1273–302. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds–a biological basis. Science 1999; 286: 1692–95. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002; 15: 870–8. [DOI] [PubMed] [Google Scholar]
- Ghuman AS, van den Honert RN, Huppert TJ, Wallace GL, Martin A. Aberrant oscillatory synchrony is biased toward specific frequencies and processing domains in the autistic brain. Biol Psychiatry Cogn Neurosci Neuroimaging 2017; 2: 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 2000; 44: 162–7. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Gilmore AW, Martin A. Brain networks, dimensionality, and global signal averaging in resting-state fMRI: hierarchical network structure results in low-dimensional spatiotemporal dynamics. bioRxiv 2017; 229567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Jo HJ, Wallace GL, Saad ZS, Cox RW, Martin A. Two distinct forms of functional lateralization in the human brain. Proc Natl Acad Sci USA 2013a; 110: E3435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. The perils of global signal regression for group comparisons: a case study of autism spectrum disorders. Front Hum Neurosci 2013b; 7: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain 2012; 135: 2711–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen WB, Tesink C, Petersson KM, van Berkum J, van der Gaag RJ, Hagoort P, et al. Semantic, factual, and social language comprehension in adolescents with autism: an FMRI study. Cereb Cortex 2010; 20: 1937–45. [DOI] [PubMed] [Google Scholar]
- Hagen von dem EAH, Stoyanova RS, Rowe JB, Baron-Cohen S, Calder AJ. Direct gaze elicits atypical activation of the theory-of-mind network in autism spectrum conditions. Cereb Cortex 2013; 24: 1485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A, Behrmann M, Malach R. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat Publ Group 2015; 18: 302–9. [DOI] [PubMed] [Google Scholar]
- Hull L, Mandy W, Petrides KV. Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism 2017; 21: 706–27. [DOI] [PubMed] [Google Scholar]
- Jasmin KM, McGettigan C, Agnew ZK, Lavan N, Josephs O, Cummins F, et al. Cohesion and joint speech: right hemisphere contributions to synchronized vocal production. J Neurosci 2016; 36: 4669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, et al. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. J Appl Math 2013; 2013: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 2010; 52: 571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Kosaka H, Saito DN, Ishitobi M, Morita T, Inohara K, et al. Default mode network in young male adults with autism spectrum disorder: relationship with autism spectrum traits. Mol Autism 2014; 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Minshew N. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 2004; 127: 1811–21. [DOI] [PubMed] [Google Scholar]
- Kana RK. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain 2006; 129: 2484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci 2008; 3: 177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Lerch JP, Floris DL, Ruigrok ANV, Pohl A, Lombardo MV, et al. Imaging sex/gender and autism in the brain: etiological implications. J Neurosci Res 2017; 95: 380–97. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: a study by the collaborative program of excellence in autism. Am J Med Genet A 2006; 140A: 2257–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke AC, Keehn RJ, Pueschel EB, Fishman I, Müller RA. Children with ASD show links between aberrant sound processing, social symptoms, and atypical auditory interhemispheric and thalamocortical functional connectivity. Dev Cogn Neurosci. 2018; 29: 117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule—generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30: 205–23. [PubMed] [Google Scholar]
- Loukusa S, Moilanen I. Pragmatic inference abilities in individuals with Asperger syndrome or high-functioning autism. A review. Res Autism Spectr Disord 2009; 3: 890–904. [Google Scholar]
- Loveland KA, Landry SH. Joint attention and language in autism and developmental language delay. J Autism Dev Disord 1986; 16: 335–49. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Publ Group 2008; 11: 103–7. [DOI] [PubMed] [Google Scholar]
- Meoded A, Morrissette AE, Katipally R, Schanz O, Gotts SJ, Floeter MK. Cerebro-cerebellar connectivity is increased in primary lateral sclerosis. Neuroimage Clin 2015; 7: 288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller R-A. Partially enhanced thalamocortical functional connectivity in autism. Brain Res 2006; 1104: 160–74. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. J Autism Dev Disord 1990; 20: 115–28. [DOI] [PubMed] [Google Scholar]
- Nair A, Carper RA, Abbott AE, Chen CP, Solders S, Nakutin S, et al. Regional specificity of aberrant thalamocortical connectivity in autism. Hum Brain Mapp 2015; 36: 4497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Keown CL, Datko M, Shih P, Keehn B, Müller R-A. Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Hum Brain Mapp 2014; 35: 4035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain 2013; 136: 1942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Barber AD, Mostofsky SH. Precentral gyrus functional connectivity signatures of autism. Front Syst Neurosci 2014a; 8: 1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, et al. Intrinsic visual-motor synchrony correlates with social deficits in autism. Biol Psychiatry 2016; 79: 633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Joel SE, Muschelli J, Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapp 2014b; 35: 567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsmond GI, Krauss MW, Seltzer MM. Peer relationships and social and recreational activities among adolescents and adults with autism. J Autism Dev Disord 2004; 34: 245–56. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Lynn A, Foran W, Luna B, O’Hearn K. Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci 2013; 7: 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Shriberg LD, McSweeny J, Cicchetti D, Klin A, Volkmar F. Brief Report: relations between prosodic performance and communication and socialization ratings in high functioning speakers with autism spectrum disorders. J Autism Dev Disord 2005; 35: 861–69. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain 2005; 128: 1038–48. [DOI] [PubMed] [Google Scholar]
- Picci G, Gotts SJ, Scherf KS. A theoretical rut: revisiting and critically evaluating the generalized under/over‐connectivity hypothesis of autism. Dev Sci 2016; 19: 524–9. [DOI] [PubMed] [Google Scholar]
- Plitt M, Barnes KA, Wallace GL, Kenworthy L, Martin A. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc Natl Acad Sci USA 2015; 112: E6699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59: 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Plitt M, Laumann TO, Martin A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage 2017; 146: 609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The brain’s default mode network. Annu Rev Neurosci 2015; 38: 433–47. [DOI] [PubMed] [Google Scholar]
- Ramot M, Kimmich S, Gonzalez-Castillo J, Roopchansingh V, Popal H, White E, et al. Direct modulation of aberrant brain network connectivity through real-time NeuroFeedback. Elife 2017; 6: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Moran JM, Mavros PL, Tager Flusberg H, Gabrieli JDE, Whitfield-Gabrieli S. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front Hum Neurosci 2013; 7: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, Jo HJ, Gotts SJ, Chen G, Martin A, et al. Correcting brain-wide correlation differences in resting-state FMRI. Brain Connect 2013; 3: 339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Bleich-Cohen M, Hahamy-Dubossarsky A, Dinstien I, Weizman R, Poyurovsky M, et al. Global functional connectivity deficits in schizophrenia depend on behavioral state. J Neurosci 2011; 31: 12972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain 2000; 123: 2400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Shih P, Öttl B, Keehn B, Leyden KM, Gaffrey MS, et al. Atypical lexicosemantic function of extrastriate cortex in autism spectrum disorder: evidence from functional and effective connectivity. Neuroimage 2012; 62: 1780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol 2007; 17: 417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Paul R, McSweeny JL, Klin A, Cohen DJ, Volkmar FR. Speech and prosody characteristics of adolescents and adults with high-functioning autism and asperger syndrome. J Speech Lang Hear Res 2001; 44: 1097–115. [DOI] [PubMed] [Google Scholar]
- Song S, Gotts SJ, Dayan E, Cohen LG. Practice structure improves unconscious transitional memories by increasing synchrony in a premotor network. J Cogn Neurosci 2015; 27: 1503–12. [DOI] [PubMed] [Google Scholar]
- South M, Rodgers J, Van Hecke A. Anxiety and ASD: Current progress and ongoing challenges. J Autism Dev Disord 2017; 47: 3679–81. [DOI] [PubMed] [Google Scholar]
- Starck T, Nikkinen J, Rahko J, Remes J, Hurtig T, Haapsamo H, et al. Resting state fMRI reveals a default mode dissociation between retrosplenial and medial prefrontal subnetworks in ASD despite motion scrubbing. Front Hum Neurosci 2013; 7: 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel A, Song S, Bageac D, Knutson KM, Keisler A, Saad ZS, et al. Shifts in connectivity during procedural learning after motor cortex stimulation: A combined transcranial magnetic stimulation/functional magnetic resonance imaging study. Cortex 2016; 74: 134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Gotts SJ, Brotman MA, Lever S, Hsu D, Zarate C, et al. Aberrant intrinsic functional connectivity within and between corticostriatal and temporal–parietal networks in adults and youth with bipolar disorder. Psychol Med 2016; 46: 1509–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep 2013; 5: 738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesink CMJY, Buitelaar JK, Petersson KM, van der Gaag RJ, Kan CC, Tendolkar I, et al. Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain 2009; 132: 1941–52. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci 2011; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, et al. Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. Neuroimage Clin 2014; 4: 374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain 2006; 129: 932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watsky RE, Gotts SJ, Berman RA, McAdams HM, Zhou X, Greenstein D, et al. Attenuated resting-state functional connectivity in patients with childhood- and adult-onset schizophrenia. Schizophr Res 2018; 197: 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S-J, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res 2010; 1313: 202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X, Norr M, Murphy E, Kuschner E, Bal E, Gaillard W, et al. Atypical modulation of distant functional connectivity by cognitive state in children with autism spectrum disorders. Front Hum Neurosci 2013; 7: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma RJF, Moseley RL, Holt RJ, Rughooputh N, Floris DL, Chura LR, et al. Default mode hypoconnectivity underlies a sex-related autism spectrum. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1: 364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci 2013; 7: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Nikas CV, Safiullah ZN, Gotts SJ, Ungerleider LG. Spatial mechanisms within the dorsal visual pathway contribute to the configural processing of faces. Cereb Cortex 2017; 27: 4124–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in XNAT https://www.xnat.org/.