Abstract
The incidence of heart valve disease increases significantly with age. Degenerative abnormalities associated with severe aortic stenosis and mitral and tricuspid regurgitation are found in not less than 10% of the population aged ≥ 75 years. Surgical treatment has been considered for years to be the treatment of choice. However, it was not uncommonly associated with high perioperative morbidity and mortality due to frequent comorbidities and overall frailty conditions of these patients. Conventional risk scores such as Society of Thoracic Surgeons and European System for Cardiac Operative Risk Evaluation may underestimate the risk of surgery in elderly patients, leading to inappropriate surgical indication. On the other hand, at least 30% of patients with severe conditions are left untreated due to prohibitive surgical risk. Interventional procedures, which are in continuous development, may be actually considered for high risk patients and, as recent results suggest, also for intermediate risk patients.
Keywords: Valve diseases, Elderly, Surgery, Interventional cardiology
Core tip: Severe heart valve diseases are not uncommon in the elderly and often treatment may be challenging due to high risks related both to relevant comorbidities and the frailty condition of elderly patients. Although surgery is still the first choice for most conditions, interventional strategies are emerging as a valid alternative both in high and intermediate risk patients. Careful evaluation is needed for each individual patient in order to establish a more appropriate strategy considering that the impact on the quality of life may be more relevant in this population than the effects on survival, which is already limited by decreased life expectancy related to ageing.
INTRODUCTION
Progressive ageing of a population is associated with an increased prevalence of chronic degenerative diseases. Among these, heart valve abnormalities represent an important public-health problem leading to high morbidity and mortality. The Euro Heart Survey on valve heart disease (VHD) published in 2003 included 5001 adults from 25 countries suffering from moderate to severe heart valve disease[1]. Native VHD was found in about 4000 patients. The remaining had had previous valve surgery. Degenerative process was the main cause of aortic involvement and mitral regurgitation (MR). Mitral stenosis (MS) was mainly due to rheumatic disease. Incidence of valvular disease increased with age. Incidence of VHD was 6% for both mitral and aortic disease in patients aged ≥ 75 years, while in younger patients (aged < 64 years), the incidence was less than 1%. Importantly, according to the Survey, more than 30% of subjects with severe, symptomatic, single VHD, usually elderly with relevant comorbidities, did not undergo surgery.
More recently, Nkomo et al[2] reported the results of echocardiographic examinations in 11911 randomly selected adults who had been prospectively assessed in three large population-based epidemiological studies[3-5]. Moreover, included in the study were 16501 adults who were assessed in community by clinically indicated echocardiography. In the first group, 615 patients (5.1%) had moderate or severe valve disease. There were no gender related differences. Prevalence of valve disease increased significantly with age from 0.7% in the group comprised of 18-year-olds to 44-year-olds to 13.3% in the group of those 75 years and older (P < 0.0001). A significant increase of VHD was reported for each increment of 10 years of ageing. This was particularly evident for aortic stenosis (AS) (hazard ratio (HR) = 2.51; 95% confidence interval (CI): 2.02 to 3.12; P < 0.0001). MR was the most frequent VHD in elderly patients (9.3%) followed by AS (2.8%), aortic regurgitation (AR) (2.0%), and finally MS (0.2%).
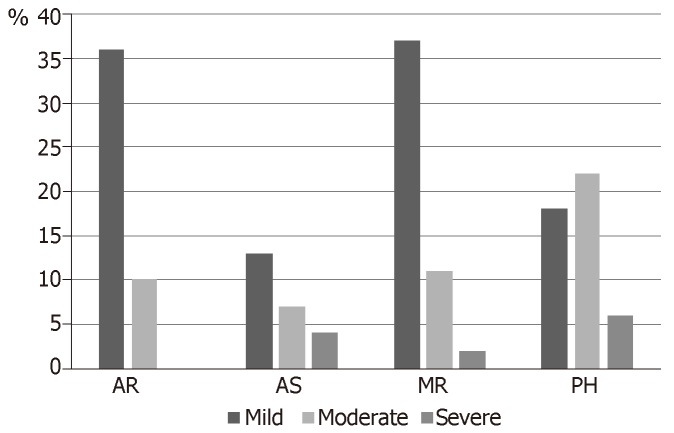
In the community group, valve disease was diagnosed in 1505 patients. Prevalence of valve diseases increased considerably with age also in this group (0.3% in 18-44 years old, 11.7% in those aged ≥ 75 years). There was a trend that showed a lower rate of diagnosis in women than in men. Both in in the population and in the community study, valve disease was associated with an increased mortality risk ratio (RR) (1.36, 95%CI: 1.15–1.62; P = 0.0005 and respectively 1.75, 95%CI: 1.61–1.90; P < 0.0001). Incidence of heart valve disease in 500 consecutive patients aged > 8 years referred to our Center for hip fracture is reported in Figure 1.
Figure 1.
Incidence of valve heart disease in 500 consecutive patients aged > 80 years referred for hip fracture.
Due to increased life expectancy in the elderly population, AS prevalence is expected to increase further. according to recent projections from The OxVALVE population cohort study in the United Kingdom, the number of elderly people with moderate or severe valvular heart disease will more than double by 2056[6].
A retrospective study from Scotland showed that among all patients hospitalized from 1 January 1997 to 31 December 2005, a final diagnosis of non-congenital aortic valve disease was made in a total of 19733 adults[7]. Discharge diagnosis was AS in 13220 (67.0%) and AR in 2807 (14.2%). Mixed aortic valve disease, or unspecified aortic valve disease, occurred respectively in 699 (3.5%) and 3007 (15.2%). Elderly patients, aged 80 and older, accounted for most of the patients included in the study. More than half had died by 31 December 2006. The risk of death (and heart failure) was 20% higher in AS in comparison to aortic insufficiency or mixed aortic valve disease. Only 19.4% of patients included in the study had aortic valve replacement during follow-up, three out four for AS. Age, female gender, and co-morbidity influenced replacement rate.
Despite the relevance of VHD as a cause of heart failure and death, the first European Heart Valve Disease survey demonstrated that the awareness and knowledge of heart valve disease in the general population was alarmingly low, and only 3.8% really knew what AS was[8]. Two years later, the second European Heart Valve Disease survey showed a mild improvement in general knowledge of heart valve disease in comparison to 2015. Despite this finding, the correct understanding of AS decreased significantly (2015: 7.2% vs 2017: 3.8%; P < 0.001)[9].
Treatment of VHD in the elderly requires careful evaluation since other than the effects on survival, already limited by decreased life expectancy related to ageing, the impact on the quality of life should be considered a relevant aspect. In elderly people, clinical outcome after surgical treatment is significantly influenced by concomitant severe comorbidities [diabetes mellitus, chronic kidney disease, cerebrovascular disease, and atrial fibrillation (AF), etc] that may impair postoperative recovery, leading to worse outcomes[10]. A multidisciplinary approach involving cardiologists, interventional cardiologists, surgeons, anesthesiologists, and geriatricians may improve the decisional process.
AORTIC VALVE
AS
Epidemiology and pathophysiology: In the elderly, degenerative AS is one of the most common types of valvular heart disease. The prevalence of AS has been reported to be between 12% and 26% depending on the diagnostic criteria employed[1,11]. In the study by Lindroos et al[12], critical AS was defined as a valve area < 0.8 cm2 or velocity ratio of < 0.35. In the 75- to 86-year-old group, the reported prevalence of disease was 2.9% (95%CI: 1.4% to 5.1%). Overall, 40% of patients with severe AS were considered to be at high surgical risk. It must be emphasized that although AS is clearly associated with adverse outcomes, even aortic sclerosis can create an increased risk of cardiovascular morbidity and mortality mainly by its being a significant risk factor for progression to AS. Degenerative calcific disease accounts for most cases of severe AS; however, a large study reported that 22% percent of octogenarians presenting for surgery for isolated AS had bicuspid valve disease[13-15].
Calcific aortic valve disease evolves over the years at a different rate in every subject. The development and progression of AS is at least in part related to active processes that have pathophysiological mechanisms in common with atherosclerotic disease[14]. First, several studies suggested that calcific degenerative AS and atherosclerosis have common risk factors such as age, smoking, hypertension, hypercholesterolemia, diabetes mellitus, and metabolic syndrome[15-18]. Since valve leaflets may have anatomic heterogeneity, different shear stresses may lead to endothelial dysfunction at the ventricular surface of the valve. Second, the loss of endothelial integrity allows lipid accumulation and cellular migration (inflammatory cells, macrophages, and T cells) in the subendothelial matrix[19] with neurohormonal activation[20]. Plaque-like subendothelial deposits may lead to downward displacement and fragmentation of the subjacent elastic lamina. The osteoblast-like activity of interstitial cells may be responsible for valvular calcification over time with a decrease in leaflet mobility[21].
AR
Epidemiology and pathophysiology: Isolated AR is significantly less common than pure AS. Degenerative and bicuspid aortic valve disease shows a different degree of both regurgitation and left ventricular obstruction; however, stenosis is usually pre-eminent. More frequently, AR is a consequence of aortic dilation and the deformation of the annulus valve. Overall prevalence of significant native AR has been reported in between 2.0% and 2.5% of patients 70 years to 83 years of age, without gender differences[22,23] although smaller studies reported a higher incidence of up to 13%. Age, aortic valve fibrocalcification, and female sex were considered independent factors related to AR, while several studies failed to find a relationship with arterial hypertension[24].
Treatment of aortic valve diseases: Surgical aortic valve replacement (SAVR) has been, for a long time, the treatment of choice for severe aortic valve disease. Improved survival and quality of life have been clearly demonstrated even in elderly patients[25-27]. Nevertheless, a non-negligible number of elderly patients are considered at very high or prohibitive risk for conventional surgical procedures, and about 30% of symptomatic subjects will never undergo surgery[1].
Non-surgical options, in particular transcatheter aortic valve replacement (TAVR), have developed as a suitable alternative to SAVR. In humans, the first transcatheter aortic stent valve was implanted in 2002, using femoral vein access and a transeptal approach[28]. In 2005, technical developments allowed for changing the approach to the transfemoral artery[29]. Transapical TAVR (TA-TAVR) has been proposed in patients with unsuitable vascular access. Several studies compared safety and efficacy between the transfemoral TAVR (TF-TAVR) and TA-TAVR. The transfemoral approach, whenever feasible, should be considered the preferable access route[30].
Initially, the indication for TAVR was limited to severely symptomatic AS with high surgical risk according to validated risk scores [Society of Thoracic Surgeons (STS) or European System for Cardiac Operative Risk Evaluation (EuroSCORE)]. At present, indications for percutaneous treatment may be extended to intermediate risk subjects. Nevertheless the use of STS-risk score (or EuroSCORE) may be misleading in very old people (aged > 80 years) since a high risk of perioperative complications may exist due to overall age and frailty per se[31-33]. Frailty, limited functional capacity according to Barthel scale, inadequate nutrition, and the need for non-cardiac surgery (most frequently oncologic or orthopedic surgery) are good indicators for TAVR, which allows a faster recovery and improved quality of life.
The randomized PARTNER 1B study first showed a decrease in death from any cause and death from cardiovascular causes in patients who underwent TAVR vs a conservative treatment[34]. The PARTNER 1A trial randomized 699 high-risk patients with severe AS to TAVR (using transfemoral or the transapical approach) or SAVR[35]. Death from any cause at 1 year was similar in the two groups, while major vascular complications (11.0% vs 3.2%, P < 0.001) and stroke (8.3% vs 4.3%, P < 0.05) were more frequent in TAVR than in SAVR. At 2 years follow-up, TAVR was associated with an increased late mortality mainly related to mechanical complications of the valve such as paravalvular leak. With first generation devices, residual AR due to para-valvular leaks was found postoperatively in about 20% of patients. Minimally invasive aortic valve replacement was proposed to manage carefully selected patients with the aim of decreasing permanent pacemaker implantation and other vascular complications that would be critical to changing patient prognosis.
In the study by Hirji et al[36], 1028 octogenarians underwent isolated aortic valve replacement between 2002 and 2015. Three hundred and six were treated by TAVR and 722 by SAVR (344 conventional and 378 minimally invasive valve replacement). Median follow-up was 35 mo. TAVR patients were relatively older (86.2 years vs 84.2 years) and in more cases had several co-morbidities. Operative mortality and mid-term survival were similar for TAVR (regardless of approach), SAVR, and minimally invasive aortic valve replacement after adjustment for confounding factors. The median in-hospital length of stay was statistically higher for the SAVR group (P < 0.05). Independent predictors of mortality were age, class III/IV New York Heart Association (NYHA), preoperative creatinine, severe chronic lung disease, and prior cardiac surgery (all P < 0.05). The authors concluded that treatment decisions should be addressed by a multi-disciplinary heart team, taking into account patient comorbidities, frailty, and quality of life.
Recently were reported the results of the FRench Aortic National CoreValve and Edwards (FRANCE-2) registry. In the study were included 2254 patients > 80 years of age who underwent TAVR. Thirty-day and 1-year mortality were not significantly different among patients aged 80 to 84 years, 85 to 89 years, and finally > 90 years (10.3% vs 9.5% vs 11.2%; P = 0.53 and respectively 19.8% vs 26.1% vs 27.7%; P = 0.16)[37].
A recent study compared carefully selected patients > 90 years old, without many comorbidities, vs younger patients who underwent TAVR. Major complications were similar, and all-cause mortality at 30 days and 1 year was not statistically different (2.9% and 12.5% in patients aged ≥ 90 vs 2.8% and 12.3% in patients aged < 90, respectively)[38].
The effects of TAVR were evaluated more recently in low-intermediate risk populations. An Italian observational, multicenter, “real-world” study included 1300 patients in a propensity-matched population. The authors did not find significant differences in mortality or major adverse cardiac and cardiovascular events between SAVR and TAVR[39].
In the PARTNER 2A randomized trial, TAVR was compared with SAVR in 2032 intermediate-risk patients. The primary endpoints were all-cause mortality or disabling stroke at 2 years. The authors did not find differences between groups. Although major vascular complications and paravalvular regurgitation were more frequent in TAVR, surgical replacement was associated with higher rates of acute kidney injury, severe bleeding, and new-onset AF[40].
The multicenter Surgical Replacement and Transcatheter Aortic Valve Implantation trial was a randomized, clinical trial that included 1746 patients at intermediate surgical risk, of whom 1660 underwent TAVR or surgical operation[41]. The primary endpoint, a composite of death from any cause or disabling stroke at 24 mo, was 12.6% in TVAR and 14% in SAVR respectively. On the basis of these results, 2017 American Heart Association/America College of Cardiology gave a IIa indication for the TAVR procedure in intermediate surgical risk[42].
Data from studies of a low-risk group for surgery, showed that SAVR is still more advantageous than TAVR. Rosato et al[43] reported that survival at 3 years was 72.0% after TAVR and 83.4% after SAVR (P = 0.0015). Further studies with new generation valve prostheses are necessary before expanding indications of TAVR in lower-risk patients.
Effects of coronary artery disease: Coronary artery disease (CAD) is frequently associated with AS, in particular in elderly patients[44]. The coexistence of CAD leads to a worse prognosis for AS of comparable severity. Surgical treatment allows correction of valve disease and at the same time coronary revascularization. Data regarding elderly subjects are limited. Less is known about the effects of CAD in elderly patients undergoing TAVR.
To evaluate the effect of age on combined AVR and concomitant coronary artery bypass graft (CABG), 452 consecutive patients (mean age 64 years) were divided into three groups: Young (n = 114), middle-aged (n = 225), and elderly (n = 113). CAD was more extensive in the elderly group. Only 62.8% of elderly patients had complete myocardial revascularization in comparison to 94.1% and 76.2%, respectively, of the other two groups (P < 0.05). In-hospital mortality was 6.4% in the elderly in comparison to 2.0% and 5.3%, respectively in the other groups. Freedom from cardiac-related death at 12 mo and 60 mo was higher in young and middle-aged patients than in elderly patients[45].
How CAD impacts patient survival following TAVR has been investigated by a recent meta-analysis. Fifteen studies including 8013 patients were examined. The median age of patients was 81.3 years, 46.6% were men, and 3899 (48.7%) had CAD. All-cause mortality at 30 days post TAVR was not significantly different between patients with and without CAD. All-cause mortality however was significantly higher at 1 year in patients with CAD in comparison with patients without CAD (OR = 1.21; 95%CI: 1.07–1.36; P = 0.002). These results suggest the need to revisit the revascularization strategies for concomitant CAD in patients with TAVR[46].
AS and MR
In the elderly, AS is frequently associated with concomitant MR (22%-48%). In severe cases affecting both valves, surgical valve replacement has usually been considered the treatment of choice. Data regarding elderly subjects is limited. In the study by Yu et al[47], 43 high-risk patients with severe AS, aged 80 ± 6 years, underwent concomitant SAVR and mitral valve (MV) surgery. Nineteen (44%) had prior cardiac surgery, and 39 (91%) were in congestive heart failure. Five patients (11.6%) died during hospitalization or at 30 days. Mortality was 25% at 6 mo, 35% at 1 year, and 45% at 2 years. Patients often needed prolonged ventilation, and 10% developed new renal failure requiring dialysis. When AS in patients at high or prohibitive surgical risk is treated by percutaneous TAVR, concomitant significant MR usually is not corrected[48,49]. Untreated MR is associated with a significant increase in mortality and morbidity[50].
The recent availability of percutaneous devices for treating MV disease may offer an alternative for the management of MR after TAVR[51]. Few limited case series reported a procedural success (decrease of degree of MR < 2+) comprised between 92% and 100% for edge to edge MV repair with MitraClipTM (Abbott Vascular, Menlo Park, CA, United States)[52]. Recurrent 3+ MR at 1 year however occurred in 21.4%. One year survival rate was 66.5%.
In conclusion, concomitant MV surgery in patients with MV disease undergoing aortic valve replacement did not give better results on long term survival than TAVR without correction of MV regurgitation. Therefore, individual assessment should guide procedural strategy in treating MR associated with severe AS.
MV
Epidemiology and pathophysiology
Several conditions may damage the MV in older patients, such as degeneration of valve leaflets, calcification of the mitral annulus commonly involving the posterior leaflet, ischemia, and rheumatic heart disease.
Anatomo-functional abnormalities of the MV apparatus may result in valve stenosis or, more frequently, regurgitation. The most common etiology of MS is rheumatic heart disease; however, it is not common that the disease remains undiagnosed up until an advanced age[53]. Degenerative MV annulus calcification is more frequent in the elderly, but it is unclear how frequent a significant hemodynamic impact might be. Functional MS related to massive annular calcification and reduced leaflet excursion has been reported in 2.5% to 18.0% of elderly patients[54]. Degenerative MS accounted for 12.5% of MS cases according to data of the Euro Heart Survey[1]. The severity of calcification has significant implications for surgery. Debridement of the posterior annulus may be challenging, and residual calcium may not allow adequate suturing of the MV prosthesis with the risk of post-operative paravalvular regurgitation due to suture dehiscence. Moreover, there is the non-negligible risk of extensive damage and posterior disruption of the left ventricle and that of death.
In industrialized countries, MR is the most frequent valvular heart disease in patients over the age of 65 years[1,2]. Elderly patients account for about 40% of all patients with MR and 4.5% are over 80 years of age. Heart failure, arrhythmia, and death may occur in patients with severe disease. Prevalence of moderate MR in the Framingham study was 11.1% in men 70 years to 83 years of age[23]. In the study, no information was reported regarding valve morphology. Secondary MR has been reported in about 25% of patients after myocardial infarction and in more than 50% in heart failure with depressed ejection fraction.
Treatment
Etiology of MV regurgitation plays a relevant role in the decision-making process, particularly in elderly patients. MV surgery is indicated only if the balance between expected clinical improvement exceeds increased operative risk related to ageing and comorbidities. Surgical treatment is clearly suggested by American guidelines for patients with primary valve disease, while no indications are provided by ESC guidelines[42,55]. A high operative mortality (15%) was reported by a recent meta-analysis including 5572 octogenarian patients[56]. Therefore, a careful multidimensional preoperative evaluation is needed for risk stratification since STS and EuroSCORE may effectively underestimate effective surgery related risks in elderly, frail patients. Left ventricular dysfunction is more frequent with concomitant CAD. Surgical revascularization increases the risk of both early and late mortality after surgery.
Secondary MR in those aged > 75 years is likely to be more frequent than primary valve disease. In this case, no clear indication for surgery exists as the clinical benefit is uncertain. When concomitant coronary artery bypass grafting is not planned, surgical intervention may be recommended only in patients with refractory symptoms after optimization of medical therapy and eventual cardiac resynchronization therapy[57,58].
MV-repair at present is the generally accepted “gold standard” treatment for degenerative MV disease. Several studies demonstrated the superiority of repair over MV replacement (MVR)[59,60]. Patients with extensive bi-leaflet or anterior leaflet prolapse and myxomatous degeneration without extensive calcification are considered good candidates for MV repair. Nevertheless, in elderly patients MV-repair as suggested by administrative American databases was performed in less than 50%. Advanced age was as an independent predictor of valve replacement[61].
The lower technical complexity of valve replacement with shorter cardio pulmonary bypass times and decreased risk of failure with need of reintervention may explain the lower rate of MV repair than expected in elderly patients. These aspects are particularly relevant due the limited life expectancy of aged patients. Nevertheless, MVR has a high short-term mortality of 25% to 30%, frequently due to congestive heart failure possibly related to alteration of the left ventricular dimensions and geometry.
Differences in long-term clinical outcomes between surgery and conservative management were evaluated by Kang et al[62] in 157 patients with severe MR aged ≥ 70 years. Median follow-up was 5.4 years. Surgery was associated with a lower mortality (HR 0.31; 95%CI: 0.13 to 0.73; P = 0.007) other than with a decrease in overall cardiac event (HR 0.26; 95%CI: 0.13 to 0.53; P < 0.001).
In a single center retrospective study in 2015, consecutive patients with moderate to severe MR were divided into two groups[63]. Patients aged > 60 years (mean age 66.98 ± 5.94 years) were considered as the elderly group (n = 680) and compared to patients < 60 years (control group, n = 1061). In total, 308/680 elderly MR were denied surgery, which was much higher than the rate of denial observed in the control group (45.29% vs 36.10%, P < 0.001). The factors associated with decreased probability of undergoing surgery were increased age, diabetes, and high risk stratification according to EuroSCORE-II. Of the 275 elderly patients with severe MR included in this study, 75 (27.27%) did not undergo surgery.
A database from the University Centre of Liepzig, Germany was examined and assessed to identify all patients aged > 70 years who underwent MV surgical procedures between 1999 and 2009. In 97% of the 2503 patients, MR was the primary indication for operation[64]. MV repair was performed in 64%. Mortality rate at 30 days was 3.1%, and survival at 5 years was 55.2%. Coronary revascularization was associated with an early and long-term poorer outcome. Several factors, such as diabetes, chronic obstructive lung disease, left ventricular function < 30%, preoperative hemodialysis, presence of endocarditis, MVR, concomitant TV procedures, urgent or emergent procedures, aortic procedures, aortic valve replacement, and CABG, were independently related younger late death[64].
A recent retrospective study by Silashi et al[65] reviewed the results after MV surgery in elderly patients treated over the past 20 years. Excluded from the study were patients with repeat cardiac surgery, endocarditis, and concomitant aortic valve replacement. Of 1776 patients with MV disease, 341 were aged ≥ 75 years. Two hundred and twenty-one underwent MV-repair and 120 MVR. One hundred thirty-five patients had concomitant coronary artery bypass grafting (39.6%). Fifty had tricuspid valve (TV) surgery (14.7%). Thirty-day mortality associated with MV repair was 5.4% vs 9.2% for MVR (P = 0.26). Concomitant CABG was more frequently performed in patients undergoing MV-repair (43.9% vs 31.7%, P = 0.03). In 27 patients, planned MV-repair was converted to MVR, mainly after invasive inspection of the MV. Moderate/severe MR was observed at follow-up in 15 cases after MV-repair (6.8%), of which four needed reintervention. After MVR, significant MR was observed in only 3 cases (2.5%). Overall 1- and 5-year survival was 90.7% and 74.2% vs 81.3% and 61.0%, respectively (P < 0.01).
In a propensity adjusted analysis of outcomes after MV surgery in patients aged > 80 years (mean age 83 years), overall operative mortality was 11% after MV-repair in comparison to 18.9% for MVR[66]. It must be underlined that this study also included patients with endocarditis (1.8% in MV-repair and 13.7% in MVR) and ischemic MV disease (32.2% in MV-repair).
Included in a meta-analysis by Shang et al[67] were seven observational clinical studies published after 2000 comparing MVP and MVR in the elderly (aged 70 years or older). Overall, 1809 patients were considered. Thirty-day mortality was significantly lower after MV repair (RR: 0.40, 95%CI: 0.25–0.64). Moreover, repair was associated with length of postoperative hospital stay and less postoperative complications in comparison to MVR. Finally long-term (1- and 5-year survival) were higher in MV-repair.
Patients at high-prohibitive risk for surgery may benefit, when technically feasible, by percutaneous interventional treatment. MitraClipTM therapy is at present the most widely used technique. The device allows for building a bridge between the anterior and posterior mitral leaflet thus mimicking the surgical technique of the Alfieri stitch. In patients treated for degenerative MR, despite good periprocedural results, the rate of recurrent severe MR after MitraClipTM therapy has been reported close to 55% at 12 mo[68]. The need for re-operations may exceed 20% at 4 years of follow-up[69].
Failure of MitraClipTM procedures may be related to the absence of concomitant annuloplasty. Failed MitraClipTM procedures may complicate eventual future MV-repair. In particular when treatment included more than one clip valves, which are often not repairable. Further techniques, such as transcatheter MV-in-ring implantation, may be considered in selected cases after failure on MV repair.
Surgery must be considered the initial “gold standard” treatment for elderly patients with degenerative MR and acceptable surgical risk should be considered. A multidisciplinary “Heart Team” should discuss the patient’s condition and various treatment opportunities. New interventional treatment options may be considered for symptomatic high risk patients.
TV
Tricuspid regurgitation (TR) is the second most common VHD after MR with an incidence of 1.2% to 1.5% in the general population[1,2]. The prevalence increases with age and in particular in females. In the group of 70 to 83 year-olds, incidence is 5.6% in women as compared to 1.5% in men[23]. Severe TR is associated with higher 1-year mortality and poorer outcomes independent of age and other comorbid conditions[70].
Primary valve disease accounts for 25% of TR. This is more common in younger patients suffering from anatomic valve abnormalities (congenital, rheumatic, neoplastic, traumatic, infective endocarditis, and endomyocardial fibrosis). Other causes of TR are lead implantation for pacing or leaflet damage due to RV biopsy[71]. In elderly subjects, functional or secondary TR due to left heart disease, often MR or AS, is by far the more common etiology of TR[70]. TR secondary to left heart valve disease is often associated with poor prognosis and difficult therapeutic choices. Pulmonary hypertension, right ventricular infarction, chronic right ventricular pacing, and history of AF are other common causes of secondary TR. The term ‘‘functional’’ may be misleading for TV disease. As with the MV, annular dilatation of the tricuspid annulus and/or dislocation of papillary muscles plays just as important of a role in causing valve malfunction[72]. Annular dilatation occurs along the anterior and posterior TV leaflet implantation; therefore, the annulus becomes more circular and planar. Geometrical abnormalities may be different between secondary TR and the so-called “idiopathic TR,” commonly attributed to ageing and AF[73,74]. In idiopathic TR, basal RV dilatation with relatively normal RV length and marked annular dilatation but with normal tenting height of leaflets is commonly observed. Where there is functional TR in patients with pulmonary hypertension, there is a spherical RV deformation, with less evident annular dilation but significantly greater tenting height. These morpho-functional differences have significant implications for treatment.
Treatment
A conservative (no touch) approach to TR was proposed in the 1960s. It was conceivable that the hemodynamic improvement related to the correction of the left-sided valve disease would result in a decrease of secondary TR. The experiences that followed, however, demonstrated that regression of TR is not the rule, and regurgitation may further increase in particular when the mitral and/or aortic valve diseases are not completely or adequately resolved during surgery. Moreover, it must be stressed that an isolated severe TR is now increasingly recognized even in patients with normal left heart valve function after either MV annuloplasty or replacement. The degree of right ventricular dysfunction indicated by annular dilatation may be related to impaired regression of further increased degree of valve regurgitation. Preoperative evaluation may give information whether TR will resolve after successful mitral surgery. Four hundred and thirteen patients with rheumatic heart disease, who did not have preoperative severe TR, underwent MVR without concomitant TV repair and were then followed for a median period of 13 years[75]. Forty-six patients (11.1%) had new severe TR. Independent predictors for new severe TR were preoperative moderate TR (HR 2.401; P = 0.008) and AF (HR 2.119; P = 0.018). Patients with new severe TR had larger right ventricles and higher pulmonary artery pressures on echocardiography.
Right ventricular failure is associated with a higher surgical mortality (from 5% to 11% and from 8% to 22% during follow-up)[76]. Preoperative right ventricular dysfunction and persisting TR are associated with a minor relief of symptoms and an impaired cardiac output response to exercise after correction of valve diseases.
Although it has been suggested that functional TR may be untreated in patients with a significant predictable decrease in the pulmonary resistance, at present we have no reliable methods to predict reversibility of the TR after correction of the left heart valve dysfunction. Moreover, methods of measuring and quantifying the degree of TR are still not reliable and repeatable. The clinical assessment may add information to echocardiography. Finally, there is no satisfactory method to assess true right ventricular function.
Often, in elderly patients with long standing disease, TR frequently poses a challenging treatment dilemma[77]. Severe TR may be tolerated for many years and sometimes managed conservatively until severe right heart failure and ascites develop. It is then often too late for correction since any therapy comes with extremely high risk, with unacceptable operative mortality. Moreover, the likelihood of functional recovery is poor. American College of Cardiology/American Heart Association guidelines do not give any Class I indications for isolated TV surgery[42]. Operative risk is high in these patients with a mortality rate of 7.9% at 30 days. Age is an independent predictor according to multivariable analysis. Reduction of right ventricle afterload after treatment of a left-sided valve lesion may lead to an improvement, even if often unpredictable, of severe TR. TV repair during left sided surgery does not appreciably increase the risks of surgery. TR repair is currently recommended in patients undergoing left-sided valve surgery. Effects of depressed right ventricular function on results of TV repair were examined by Subbotina et al[78]. Eighty-two out of 191 patients (43%) had decreased tricuspid annular plane systolic excursion (TAPSE) (13.3 ± 3.3 mm vs 20.2 ± 4.9 mm; P < 0.001). In both groups, 91% underwent ring annuloplasty. Patients with depressed right ventricular function had a higher incidence of low cardiac output syndrome after surgery (10% vs 27%, P = 0.005) and a higher early mortality. Functional improvement, expressed as change in NYHA class, was more evident in patients with preserved right ventricular function. Of 173 patients who underwent MV surgery and radiofrequency ablation of AF, only age and concomitant TV repair were independently associated with mortality according to multivariate analysis[79].
In the last years, numerous percutaneous transcatheter repair and replacement devices were developed to treat this large group of high surgical risk patients. To improve prognosis in severe TR, an earlier diagnosis and referral for treatment are essential, as are a better understanding of the different stages of disease and potential treatment options, proven safe and efficacious percutaneous options, and an evidence base for earlier surgical or percutaneous intervention of significant TR, irrespective of symptoms. The use of MitraClipTM in the tricuspid position is associated with unpredictable results. Date reported from a recent registry showed a > 50% reduction in effective regurgitant orifice area after treatment with MitraClipTM. The procedure was associated with significant clinical improvement with decrease in the NYHA functional class and longer 6 minute walking distance[80]. Other transcatheter therapies are being tested in feasibility trials. Among these the Trialign system (Mitralign, Tewksbury, Massachusetts, United States) that mimics the surgical Kay annuloplasty via a pair of pledgeted sutures delivered percutaneously through the right internal jugular vein.
ACKNOWLEDGEMENTS
The author thanks Dr. Katharine Johnson for language editing
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Manuscript source: Invited manuscript
Peer-review started: December 20, 2018
First decision: December 30, 2018
Article in press: January 26, 2019
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akin I, Chang ST, Li D S- Editor: Ji FF L- Editor: Filipodia E- Editor: Wu YXJ
References
- 1.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 3.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR, Jr, Liu K, Orden S, Pirie P. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 4.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 5.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 6.Berry C, Lloyd SM, Wang Y, Macdonald A, Ford I. The changing course of aortic valve disease in Scotland: temporal trends in hospitalizations and mortality and prognostic importance of aortic stenosis. Eur Heart J. 2013;34:1538–1547. doi: 10.1093/eurheartj/ehs339. [DOI] [PubMed] [Google Scholar]
- 7.Coffey S, d'Arcy JL, Loudon MA, Mant D, Farmer AJ, Prendergast BD OxVALVE-PCS group. The OxVALVE population cohort study (OxVALVE-PCS)-population screening for undiagnosed valvular heart disease in the elderly: study design and objectives. Open Heart. 2014;1:e000043. doi: 10.1136/openhrt-2014-000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaede L, Di Bartolomeo R, van der Kley F, Elsässer A, Iung B, Möllmann H. Aortic valve stenosis: what do people know? A heart valve disease awareness survey of over 8,800 people aged 60 or over. EuroIntervention. 2016;12:883–889. doi: 10.4244/EIJY16M06_02. [DOI] [PubMed] [Google Scholar]
- 9.Gaede L, Aarberge L, Brandon Bravo Bruinsma G, Macarthy P, Musumeci F, Zamorano P, Möllmann H. Heart Valve Disease Awareness Survey 2017: what did we achieve since 2015? Clin Res Cardiol. 2019;108:61–67. doi: 10.1007/s00392-018-1312-5. [DOI] [PubMed] [Google Scholar]
- 10.Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 12.Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 13.Roberts WC, Janning KG, Ko JM, Filardo G, Matter GJ. Frequency of congenitally bicuspid aortic valves in patients ≥80 years of age undergoing aortic valve replacement for aortic stenosis (with or without aortic regurgitation) and implications for transcatheter aortic valve implantation. Am J Cardiol. 2012;109:1632–1636. doi: 10.1016/j.amjcard.2012.01.390. [DOI] [PubMed] [Google Scholar]
- 14.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 15.Pahkala K, Hietalampi H, Laitinen TT, Viikari JS, Rönnemaa T, Niinikoski H, Lagström H, Talvia S, Jula A, Heinonen OJ, Juonala M, Simell O, Raitakari OT. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (the Special Turku Coronary Risk Factor Intervention Project for Children [STRIP] study) Circulation. 2013;127:2088–2096. doi: 10.1161/CIRCULATIONAHA.112.000761. [DOI] [PubMed] [Google Scholar]
- 16.Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2014;130:10–17. doi: 10.1161/CIRCULATIONAHA.113.005445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–1683. doi: 10.1161/CIRCULATIONAHA.114.009273. [DOI] [PubMed] [Google Scholar]
- 18.Loprinzi PD, Branscum A, Hanks J, Smit E. Healthy Lifestyle Characteristics and Their Joint Association With Cardiovascular Disease Biomarkers in US Adults. Mayo Clin Proc. 2016;91:432–442. doi: 10.1016/j.mayocp.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Bossé Y, Mathieu P, Pibarot P. Genomics: the next step to elucidate the etiology of calcific aortic valve stenosis. J Am Coll Cardiol. 2008;51:1327–1336. doi: 10.1016/j.jacc.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 20.New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res. 2011;108:1381–1391. doi: 10.1161/CIRCRESAHA.110.234146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of 'degenerative' valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 22.Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace WA, Salter DM, McKillop G, van Beek EJ, Boon NA, Rudd JH, Newby DE. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 23.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 24.Palmieri V, Bella JN, Arnett DK, Roman MJ, Oberman A, Kitzman DW, Hopkins PN, Paranicas M, Rao DC, Devereux RB. Aortic root dilatation at sinuses of valsalva and aortic regurgitation in hypertensive and normotensive subjects: The Hypertension Genetic Epidemiology Network Study. Hypertension. 2001;37:1229–1235. doi: 10.1161/01.hyp.37.5.1229. [DOI] [PubMed] [Google Scholar]
- 25.Shapira OM, Kelleher RM, Zelingher J, Whalen D, Fitzgerald C, Aldea GS, Shemin RJ. Prognosis and quality of life after valve surgery in patients older than 75 years. Chest. 1997;112:885–894. doi: 10.1378/chest.112.4.885. [DOI] [PubMed] [Google Scholar]
- 26.Brennan JM, Edwards FH, Zhao Y, O'Brien S, Booth ME, Dokholyan RS, Douglas PS, Peterson ED DEcIDE AVR (Developing Evidence to Inform Decisions about Effectiveness–Aortic Valve Replacement) Research Team. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation. 2013;127:1647–1655. doi: 10.1161/CIRCULATIONAHA.113.002003. [DOI] [PubMed] [Google Scholar]
- 27.Brennan JM, Edwards FH, Zhao Y, O'Brien SM, Douglas PS, Peterson ED Developing Evidence to Inform Decisions About Effectiveness–Aortic Valve Replacement (DEcIDE AVR) Research Team. Long-term survival after aortic valve replacement among high-risk elderly patients in the United States: insights from the Society of Thoracic Surgeons Adult Cardiac Surgery Database, 1991 to 2007. Circulation. 2012;126:1621–1629. doi: 10.1161/CIRCULATIONAHA.112.091371. [DOI] [PubMed] [Google Scholar]
- 28.Cribier AG. The Odyssey of TAVR from concept to clinical reality. Tex Heart Inst J. 2014;41:125–130. doi: 10.14503/THIJ-14-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb JG, Binder RK. Transcatheter aortic valve implantation: the evolution of prostheses, delivery systems and approaches. Arch Cardiovasc Dis. 2012;105:153–159. doi: 10.1016/j.acvd.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Biancari F, Rosato S, D'Errigo P, Ranucci M, Onorati F, Barbanti M, Santini F, Tamburino C, Santoro G, Grossi C, Covello RD, Ventura M, Fusco D, Seccareccia F OBSERVANT Research Group. Immediate and Intermediate Outcome After Transapical Versus Transfemoral Transcatheter Aortic Valve Replacement. Am J Cardiol. 2016;117:245–251. doi: 10.1016/j.amjcard.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 31.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, Harris TB, Ershler WB, Ferrucci L. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 32.Prêtre R, Turina MI. Cardiac valve surgery in the octogenarian. Heart. 2000;83:116–121. doi: 10.1136/heart.83.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheitlin MD, Gerstenblith G, Hazzard WR, Pasternak R, Fried LP, Rich MW, Krumholz HM, Peterson E, Reves JG, McKay C, Saksena S, Shen WK, Akhtar M, Brass LM, Biller J. Database Conference January 27-30, 2000, Washington D.C.--Do existing databases answer clinical questions about geriatric cardiovascular disease and stroke? Am J Geriatr Cardiol. 2001;10:207–223. doi: 10.1111/j.1076-7460.2003.00696.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 35.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 36.Hirji SA, Ramirez-Del Val F, Kolkailah AA, Ejiofor JI, McGurk S, Chowdhury R, Lee J, Shah PB, Sobieszczyk PS, Aranki SF, Pelletier MP, Shekar PS, Kaneko T. Outcomes of surgical and transcatheter aortic valve replacement in the octogenarians-surgery still the gold standard? Ann Cardiothorac Surg. 2017;6:453–462. doi: 10.21037/acs.2017.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto M, Mouillet G, Meguro K, Gilard M, Laskar M, Eltchaninoff H, Fajadet J, Iung B, Donzeau-Gouge P, Leprince P, Leuguerrier A, Prat A, Lievre M, Chevreul K, Dubois-Rande JL, Teiger E FRANCE-2 Registry Investigators. Clinical results of transcatheter aortic valve implantation in octogenarians and nonagenarians: insights from the FRANCE-2 registry. Ann Thorac Surg. 2014;97:29–36. doi: 10.1016/j.athoracsur.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 38.Tamburino C, Barbanti M, D'Errigo P, Ranucci M, Onorati F, Covello RD, Santini F, Rosato S, Santoro G, Fusco D, Grossi C, Seccareccia F OBSERVANT Research Group. 1-Year Outcomes After Transfemoral Transcatheter or Surgical Aortic Valve Replacement: Results From the Italian OBSERVANT Study. J Am Coll Cardiol. 2015;66:804–812. doi: 10.1016/j.jacc.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Abramowitz Y, Chakravarty T, Jilaihawi H, Kashif M, Zadikany R, Lee C, Matar G, Cheng W, Makkar RR. Comparison of Outcomes of Transcatheter Aortic Valve Implantation in Patients ≥90 Years Versus <90 Years. Am J Cardiol. 2015;116:1110–1115. doi: 10.1016/j.amjcard.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 40.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 41.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP SURTAVI Investigators. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 42.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM, 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 43.Rosato S, Santini F, Barbanti M, Biancari F, D'Errigo P, Onorati F, Tamburino C, Ranucci M, Covello RD, Santoro G, Grossi C, Ventura M, Fusco D, Seccareccia F OBSERVANT Research Group. Transcatheter Aortic Valve Implantation Compared With Surgical Aortic Valve Replacement in Low-Risk Patients. Circ Cardiovasc Interv. 2016;9:e003326. doi: 10.1161/CIRCINTERVENTIONS.115.003326. [DOI] [PubMed] [Google Scholar]
- 44.Enriquez-Sarano M, Klodas E, Garratt KN, Bailey KR, Tajik AJ, Holmes DR., Jr Secular trends in coronary atherosclerosis--analysis in patients with valvular regurgitation. N Engl J Med. 1996;335:316–322. doi: 10.1056/NEJM199608013350504. [DOI] [PubMed] [Google Scholar]
- 45.Perek B, Casadei V, Puślecki M, Stefaniak S, Maison D, Gwizdała A, Perek A, Szarpak Ł, Jemielity M. Clinical presentation, surgical management, and outcomes of patients treated for aortic stenosis and coronary artery disease. Does age matter? Kardiol Pol. 2018;76:655–661. doi: 10.5603/KP.2018.0005. [DOI] [PubMed] [Google Scholar]
- 46.Sankaramangalam K, Banerjee K, Kandregula K, Mohananey D, Parashar A, Jones BM, Jobanputra Y, Mick S, Krishnaswamy A, Svensson LG, Kapadia SR. Impact of Coronary Artery Disease on 30-Day and 1-Year Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis. J Am Heart Assoc. 2017;6:pii: e006092. doi: 10.1161/JAHA.117.006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu PJ, Mattia A, Cassiere HA, Esposito R, Manetta F, Kohn N, Hartman AR. Should high risk patients with concomitant severe aortic stenosis and mitral valve disease undergo double valve surgery in the TAVR era? J Cardiothorac Surg. 2017;12:123. doi: 10.1186/s13019-017-0688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nombela-Franco L, Ribeiro HB, Urena M, Allende R, Amat-Santos I, DeLarochellière R, Dumont E, Doyle D, DeLarochellière H, Laflamme J, Laflamme L, García E, Macaya C, Jiménez-Quevedo P, Côté M, Bergeron S, Beaudoin J, Pibarot P, Rodés-Cabau J. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol. 2014;63:2643–2658. doi: 10.1016/j.jacc.2014.02.573. [DOI] [PubMed] [Google Scholar]
- 49.Cortés C, Amat-Santos IJ, Nombela-Franco L, Muñoz-Garcia AJ, Gutiérrez-Ibanes E, De La Torre Hernandez JM, Córdoba-Soriano JG, Jimenez-Quevedo P, Hernández-García JM, Gonzalez-Mansilla A, Ruano J, Jimenez-Mazuecos J, Castrodeza J, Tobar J, Islas F, Revilla A, Puri R, Puerto A, Gómez I, Rodés-Cabau J, San Román JA. Mitral Regurgitation After Transcatheter Aortic Valve Replacement: Prognosis, Imaging Predictors, and Potential Management. JACC Cardiovasc Interv. 2016;9:1603–1614. doi: 10.1016/j.jcin.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 50.Khawaja MZ, Williams R, Hung J, Arri S, Asrress KN, Bolter K, Wilson K, Young CP, Bapat V, Hancock J, Thomas M, Redwood S. Impact of preprocedural mitral regurgitation upon mortality after transcatheter aortic valve implantation (TAVI) for severe aortic stenosis. Heart. 2014;100:1799–1803. doi: 10.1136/heartjnl-2014-305775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schofer J, Siminiak T, Haude M, Herrman JP, Vainer J, Wu JC, Levy WC, Mauri L, Feldman T, Kwong RY, Kaye DM, Duffy SJ, Tübler T, Degen H, Brandt MC, Van Bibber R, Goldberg S, Reuter DG, Hoppe UC. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation. 2009;120:326–333. doi: 10.1161/CIRCULATIONAHA.109.849885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.David TE, Ivanov J, Armstrong S, Christie D, Rakowski H. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg. 2005;130:1242–1249. doi: 10.1016/j.jtcvs.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 53.Kodali SK, Velagapudi P, Hahn RT, Abbott D, Leon MB. Valvular Heart Disease in Patients ≥80 Years of Age. J Am Coll Cardiol. 2018;71:2058–2072. doi: 10.1016/j.jacc.2018.03.459. [DOI] [PubMed] [Google Scholar]
- 54.Ukita Y, Yuda S, Sugio H, Yonezawa A, Takayanagi Y, Masuda-Yamamoto H, Tanaka-Saito N, Ohnishi H, Miura T. Prevalence and clinical characteristics of degenerative mitral stenosis. J Cardiol. 2016;68:248–252. doi: 10.1016/j.jjcc.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 55.Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, Ford I, Gonzalez-Juanatey JR, Gorenek B, Heyndrickx GR, Hoeft A, Huber K, Iung B, Kjeldsen KP, Longrois D, Lüscher TF, Pierard L, Pocock S, Price S, Roffi M, Sirnes PA, Sousa-Uva M, Voudris V, Funck-Brentano C Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA) Eur Heart J. 2014;35:2383–2431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 56.Biancari F, Schifano P, Pighi M, Vasques F, Juvonen T, Vinco G. Pooled estimates of immediate and late outcome of mitral valve surgery in octogenarians: a meta-analysis and meta-regression. J Cardiothorac Vasc Anesth. 2013;27:213–219. doi: 10.1053/j.jvca.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, Smith PK, Hung JW, Blackstone EH, Puskas JD, Argenziano M, Gammie JS, Mack M, Ascheim DD, Bagiella E, Moquete EG, Ferguson TB, Horvath KA, Geller NL, Miller MA, Woo YJ, D'Alessandro DA, Ailawadi G, Dagenais F, Gardner TJ, O'Gara PT, Michler RE, Kron IL CTSN. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dayan V, Soca G, Cura L, Mestres CA. Similar survival after mitral valve replacement or repair for ischemic mitral regurgitation: a meta-analysis. Ann Thorac Surg. 2014;97:758–765. doi: 10.1016/j.athoracsur.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 59.Mick SL, Keshavamurthy S, Gillinov AM. Mitral valve repair versus replacement. Ann Cardiothorac Surg. 2015;4:230–237. doi: 10.3978/j.issn.2225-319X.2015.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vassileva CM, Mishkel G, McNeely C, Boley T, Markwell S, Scaife S, Hazelrigg S. Long-term survival of patients undergoing mitral valve repair and replacement: a longitudinal analysis of Medicare fee-for-service beneficiaries. Circulation. 2013;127:1870–1876. doi: 10.1161/CIRCULATIONAHA.113.002200. [DOI] [PubMed] [Google Scholar]
- 61.Badhwar V, Peterson ED, Jacobs JP, He X, Brennan JM, O'Brien SM, Dokholyan RS, George KM, Bolling SF, Shahian DM, Grover FL, Edwards FH, Gammie JS. Longitudinal outcome of isolated mitral repair in older patients: results from 14,604 procedures performed from 1991 to 2007. Ann Thorac Surg. 2012;94:1870–7; discussion 1877-9. doi: 10.1016/j.athoracsur.2012.05.105. [DOI] [PubMed] [Google Scholar]
- 62.Kang DH, Heo R, Lee S, Baek S, Kim DH, Song JM, Song JK, Lee JW. Initial surgery versus conservative management of symptomatic severe mitral regurgitation in the elderly. Heart. 2018;104:849–854. doi: 10.1136/heartjnl-2017-311759. [DOI] [PubMed] [Google Scholar]
- 63.Zhuge RQ, Hou XP, Qi XL, Wu YJ, Zhang MZ. Clinical features and treatment options for mitral regurgitation in elderly inpatients. J Geriatr Cardiol. 2018;15:428–433. doi: 10.11909/j.issn.1671-5411.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seeburger J, Falk V, Garbade J, Noack T, Kiefer P, Vollroth M, Mohr FW, Misfeld M. Mitral valve surgical procedures in the elderly. Ann Thorac Surg. 2012;94:1999–2003. doi: 10.1016/j.athoracsur.2012.05.069. [DOI] [PubMed] [Google Scholar]
- 65.Silaschi M, Chaubey S, Aldalati O, Khan H, Uzzaman MM, Singh M, Baghai M, Deshpande R, Wendler O. Is Mitral Valve Repair Superior to Mitral Valve Replacement in Elderly Patients? Comparison of Short- and Long-Term Outcomes in a Propensity-Matched Cohort. J Am Heart Assoc. 2016;5:pii: e003605. doi: 10.1161/JAHA.116.003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chikwe J, Goldstone AB, Passage J, Anyanwu AC, Seeburger J, Castillo JG, Filsoufi F, Mohr FW, Adams DH. A propensity score-adjusted retrospective comparison of early and mid-term results of mitral valve repair versus replacement in octogenarians. Eur Heart J. 2011;32:618–626. doi: 10.1093/eurheartj/ehq331. [DOI] [PubMed] [Google Scholar]
- 67.Shang X, Lu R, Liu M, Xiao S, Dong N. Mitral valve repair versus replacement in elderly patients: a systematic review and meta-analysis. J Thorac Dis. 2017;9:3045–3051. doi: 10.21037/jtd.2017.08.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L EVEREST II Investigators. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 69.Dal-Bianco JP, Inglessis I, Melnitchouk S, Daher M, Palacios IF. Percutaneous Mitral Valve Edge-to-Edge Repair for Degenerative Mitral Regurgitation. Curr Treat Options Cardiovasc Med. 2015;17:389. doi: 10.1007/s11936-015-0389-7. [DOI] [PubMed] [Google Scholar]
- 70.Taramasso M, Vanermen H, Maisano F, Guidotti A, La Canna G, Alfieri O. The growing clinical importance of secondary tricuspid regurgitation. J Am Coll Cardiol. 2012;59:703–710. doi: 10.1016/j.jacc.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 71.Cohen SR, Sell JE, McIntosh CL, Clark RE. Tricuspid regurgitation in patients with acquired, chronic, pure mitral regurgitation. II. Nonoperative management, tricuspid valve annuloplasty, and tricuspid valve replacement. J Thorac Cardiovasc Surg. 1987;94:488–497. [PubMed] [Google Scholar]
- 72.Girard SE, Nishimura RA, Warnes CA, Dearani JA, Puga FJ. Idiopathic annular dilation: a rare cause of isolated severe tricuspid regurgitation. J Heart Valve Dis. 2000;9:283–287. [PubMed] [Google Scholar]
- 73.Topilsky Y, Khanna A, Le Tourneau T, Park S, Michelena H, Suri R, Mahoney DW, Enriquez-Sarano M. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5:314–323. doi: 10.1161/CIRCIMAGING.111.967919. [DOI] [PubMed] [Google Scholar]
- 74.Mahmood F, Kim H, Chaudary B, Bergman R, Matyal R, Gerstle J, Gorman JH, 3rd, Gorman RC, Khabbaz KR. Tricuspid annular geometry: a three-dimensional transesophageal echocardiographic study. J Cardiothorac Vasc Anesth. 2013;27:639–646. doi: 10.1053/j.jvca.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Q Tri HH, Vinh PN. Progression of Tricuspid Regurgitation after Mitral Valve Replacement for Rheumatic Heart Disease. J Heart Valve Dis. 2017;26:290–294. [PubMed] [Google Scholar]
- 76.Zack CJ, Fender EA, Chandrashekar P, Reddy YNV, Bennett CE, Stulak JM, Miller VM, Nishimura RA. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J Am Coll Cardiol. 2017;70:2953–2960. doi: 10.1016/j.jacc.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 77.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 78.Subbotina I, Girdauskas E, Bernhardt AM, Sinning C, Reichenspurner H, Sill B. Comparison of Outcomes of Tricuspid Valve Surgery in Patients with Reduced and Normal Right Ventricular Function. Thorac Cardiovasc Surg. 2017;65:617–625. doi: 10.1055/s-0037-1604450. [DOI] [PubMed] [Google Scholar]
- 79.Rostagno C, Gelsomino S, Stefàno PL, Padeletti L. Rhythmic and haemodynamic determinants of long-term survival after radiofrequency ablation of atrial fibrillation in mitral valve surgery. Eur Heart J Qual Care Clin Outcomes. 2016;2:285–290. doi: 10.1093/ehjqcco/qcw021. [DOI] [PubMed] [Google Scholar]
- 80.Nickenig G, Kowalski M, Hausleiter J, Braun D, Schofer J, Yzeiraj E, Rudolph V, Friedrichs K, Maisano F, Taramasso M, Fam N, Bianchi G, Bedogni F, Denti P, Alfieri O, Latib A, Colombo A, Hammerstingl C, Schueler R. Transcatheter Treatment of Severe Tricuspid Regurgitation With the Edge-to-Edge MitraClip Technique. Circulation. 2017;135:1802–1814. doi: 10.1161/CIRCULATIONAHA.116.024848. [DOI] [PubMed] [Google Scholar]