Abstract
The two ubiquitous, outside the retina, G protein-coupled receptor (GPCR) adapter proteins, β-arrestin-1 and -2 (also known as arrestin-2 and -3, respectively), have three major functions in cells: GPCR desensitization, i.e., receptor decoupling from G-proteins; GPCR internalization via clathrin-coated pits; and signal transduction independently of or in parallel to G-proteins. Both β-arrestins are expressed in the heart and regulate a large number of cardiac GPCRs. The latter constitute the single most commonly targeted receptor class by Food and Drug Administration-approved cardiovascular drugs, with about one-third of all currently used in the clinic medications affecting GPCR function. Since β-arrestin-1 and -2 play important roles in signaling and function of several GPCRs, in particular of adrenergic receptors and angiotensin II type 1 receptors, in cardiac myocytes, they have been a major focus of cardiac biology research in recent years. Perhaps the most significant realization coming out of their studies is that these two GPCR adapter proteins, initially thought of as functionally interchangeable, actually exert diametrically opposite effects in the mammalian myocardium. Specifically, the most abundant of the two β-arrestin-1 exerts overall detrimental effects on the heart, such as negative inotropy and promotion of adverse remodeling post-myocardial infarction (MI). In contrast, β-arrestin-2 is overall beneficial for the myocardium, as it has anti-apoptotic and anti-inflammatory effects that result in attenuation of post-MI adverse remodeling, while promoting cardiac contractile function. Thus, design of novel cardiac GPCR ligands that preferentially activate β-arrestin-2 over β-arrestin-1 has the potential of generating novel cardiovascular therapeutics for heart failure and other heart diseases.
Keywords: Adverse remodeling, β-arrestin, Biased signaling, Cardiac myocyte, Cardiac fibroblast, contractility, Functional divergence, G protein-coupled receptor, Heart failure, Hormone, Myocardial infarction, Signal transducer
Core tip: Presumed functionally similar for a long time, we now know that the two β-arrestins display significant functional diversity in several organs and tissues, including in the cardiovascular system. Their functional distinction also in the mammalian heart has been clearly documented over the past few years. β-arrestin-1, which is far more abundant than β-arrestin-2 in almost every tissue including the myocardium, opposes the cyclic adenosine monophosphate (cAMP)-dependent pro-contractile signaling of the β1 adrenergic receptor (β1AR), and promotes cardiac apoptosis, inflammation, and other adverse remodeling-associated processes post-myocardial infarction. Conversely, β-arrestin-2 promotes catecholamine-dependent cardiac contractility directly, via SERCA2a potentiation, and indirectly, by leaving β1AR’s cAMP-dependent pro-contractile signaling unaffected.
INTRODUCTION
Out of the four mammalian arrestins, only the two ubiquitous (outside the retina) arrestin-2 and -3, also known as β-arrestin-1 and -2 respectively, are expressed in the mammalian cardiovascular system. Like in almost every tissue, β-arrestin-1 protein is approximately 10-15-fold more abundant than β-arrestin-2 in the circulatory system, as well[1]. Both β-arrestins regulate all non-opsin G protein-coupled receptors (GPCRs), also known as seven transmembrane-spanning receptors (7TMRs), including those responsible for neurohormonal regulation of cardiovascular physiology[2,3]. For instance, cardiac function (contractility) is tightly controlled by the activity of β-adrenergic receptors (βARs) located in the membranes of cardiac myocytes[4-8]. Cardiac structure and morphology are regulated by angiotensin II (AngII) type 1 receptors (AT1Rs) present (mainly) in cardiac fibroblast and endothelial cell membranes[4,7]. Even the production and release of the regulatory hormones per se, whether it be catecholamine and corticosteroid release by the adrenal glands or activation of the renin-angiotensin-aldosterone system by the juxtagomerular apparatus of the kidneys or release of neurotransmitters by central and peripheral neurons innervating cardiovascular organs, is under tight regulation by various GPCRs[1,4,7].
Cardiovascular GPCRs can signal either through G-proteins or β-arrestins with the natural, endogenous agonist hormones activating both signal transducers at each receptor fully and equally[1,9]. Several “biased” GPCR ligands have been discovered that (relatively) selectively activate either G proteins or β-arrestins[1,9]. This “bias” in terms of the activated signal transducer is always relative but the concept of “biased” signaling and its attainability for therapeutic purposes has been challenged recently. Specifically, recent studies have shown that receptors can activate both G-proteins and β-arrestins at the same time[10] or that β-arrestins do not even need to bind the agonist-activated receptor to get (“catalytically”) activated[11]. Additionally, it was very recently clearly demonstrated that G-protein activation is absolutely necessary, at least initially upon agonist activation, for β-arrestin activation and signaling to follow[12,13]. This sequence of activation of the two signal transducers, i.e., G-proteins being activated first followed by activation of β-arrestins, is also corroborated well by the majority of structural studies on mechanisms of GPCR activation done to date. Specifically, the receptor seems to require the interaction with the heterotrimeric G-protein in order to become fully activated by the agonist. In other words, in the absence of a G-protein, agonist binding per se is simply insufficient for the receptor to break the huge energy barrier that prevents it from reaching the active state[2,14]. Taken together, G-protein activation and signaling appears to be a prerequisite for β-arrestin signaling by GPCRs and thus, discrimination between these two families of signal transducers for any given GPCR ligand, which represents the foundation of the “biased signaling” concept for GPCRs, is essentially unfeasible. However, whereas the selective stimulation of G-protein vs. β-arrestin signaling for therapeutic purposes is most likely impossible, selective stimulation of β-arrestin-1 vs β-arrestin-2 might be feasible, similarly to the selective stimulation (or inhibition) of various Gα, which is pharmacologically achievable and currently exploited therapeutically. The first hint at signaling and functional differences between the two β-arrestins came over a decade ago with the realization that β-arrestin-1, but not β-arrestin-2 which has a nuclear export signal sequence (NES), can translocate to the nucleus where it regulates gene transcription[15]. Since then, the experimental evidence supporting functional divergence between the signaling properties of the two β-arrestins both in vitro and in several tissues and organs in vivo, including in the heart, has been mounting at an accelerating pace. Thus, β-arrestin isoform-selective targeting may have a place in the design and development of novel drugs. Below, we review this evidence known so far for the cardiac β-arrestins and discuss what it could signify for heart failure drug development. Given that almost all of the in vivo studies on cardiac β-arrestins done so far are in relation to the effects of these two proteins on βAR and AT1R signaling in the heart, the evidence for cardiac β-arrestins’ functional diversity reviewed below pertains exclusively to cardiac βARs and AT1Rs.
FUNCTIONAL DIFFERENCES BETWEEN THE TWO BETA ARRESTINS IN CARDIAC BETA-AR SIGNALING
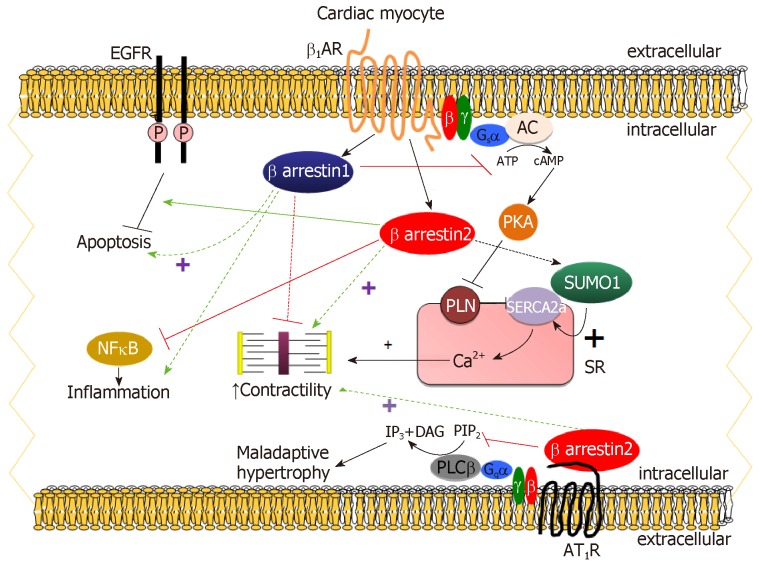
The β1AR is by far the predominant βAR subtype in human adult cardiac myocytes, representing 75%-80% of total βAR density, followed by the β2AR, which comprises about 15-18% of total cardiomyocyte βARs and the remaining 2%-3% is β3ARs[4,7,16]. β1AR stimulation by catecholamines results in the dissociation of the stimulatory G protein alpha subunit (Gαs) from Gβγ. Gαs stimulates adenylyl cyclase (AC) to produce cyclic adenosine monophosphate (cAMP), which, in turn, activates protein kinase A (PKA) and regulates different intracellular, sarcolemmal and myofibrillar substrates[4,5,7]. Thus, cAMP-dependent signaling in cardiomyocytes mediates the cellular effects of β1AR activation on stimulation of cardiac chronotropy, inotropy, dromotropy, and lusitropy (Figure 1)[4,5,7]. As co-factors of GPCR-kinases (GRKs) in βAR desensitization/downregulation, β-arrestins normally diminish the inotropic and β-adrenergic reserves of the failing heart and their inhibition should theoretically be beneficial in acute decompensated heart failure (ADHF)[4,7]. Indeed, genetic deletion of β-arrestin-1 in the heart results in several desirable therapeutic effects in heart failure, such as dramatic improvements in both cardiac β-adrenergic and inotropic reserves, amelioration of adverse remodeling and increased survival post-myocardial infarction (MI) (Figure 1)[17,18]. In contrast however, cardiac β-arrestin-2 has been shown to be cardio-protective, as it inhibits cardiac apoptosis, inflammation, and significantly attenuates overall adverse remodeling post-MI (Figure 1)[19]. One of the underlying mechanisms for the anti-inflammatory effects of cardiac β-arrestin-2 is nuclear factor-kappaB (NFĸB) inhibition in cardiac myocytes, which, again, appears to be mediated only by β-arrestin-2 and not by β-arrestin-1 in the heart (Figure 1)[19,20]. Importantly, β1AR-stimulated β-arrestin-2 was also recently documented to increase cardiac contractility both directly and indirectly (Figure 1)[20]. Directly, by interacting with Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA)-2a leading to enhanced Small Ubiquitin-related MOdifier (SUMO)-ylation of the latter[20]. This process, deficient in human heart failure, is known to directly stimulate SERCA2a activity, thereby increasing cardiac contractility[21]. β-arrestin-2 also increases cardiac function indirectly by leaving the β1AR-stimulated cAMP-dependent pro-contractile signaling intact (i.e., not desensitizing it) in cardiac myocytes in vitro and in post-MI heart failure mice in vivo (Figure 1)[20]. Importantly, these effects are not shared by the vastly more abundant in the human heart β-arrestin-1[22].
Figure 1.
The functional distinction between β-arrestin-1 and β-arrestin-2 in cardiac myocytes. ATP: Adenosine triphosphate; P: Phosphorylation; SR: Sarcoplasmic reticulum; SUMO1: Small ubiquitin-like modifier protein-1; PLN: Phospholamban; PIP2: Phosphatidyl-inositol 4,5-bisphosphate; IP3: Inositol 1,4,5-trisphosphate; DAG: 1,2-Diacylglycerol; EGFR: Epidermal growth factor receptor; AR: Adrenergic receptor.
One of the salient mechanisms for the anti-apoptotic effects of cardiac β-arrestin-2 is transactivation of the epidermal growth factor receptor (EGFR) by the cardiac β1AR (Figure 1)[18,23]. β-arrestin-1 seems again unable to stimulate this and instead, promotes cardiac apoptosis post-MI (Figure 1)[18]. Older studies had reported that mice expressing a mutant β1AR that cannot undergo GRK-dependent desensitization or activate β-arrestins in their hearts lack cardiac EGFR transactivation and suffer from massive cardiac apoptosis and left ventricular dilatation compared to wild type controls[23]. Interestingly, the β-blocker drug carvedilol, an inverse agonist towards G-protein activation[16], is a weak β-arrestin-biased agonist that activates ERKs (Extracellular signal-Regulated Kinases) via EGFR transactivation also[24,25]. It should be pointed out though that carvedilol`s “biased” β-arrestin agonism has been demonstrated only in heterologous recombinant cell systems without cardiovascular (or any other physiological) relevance (mostly, transfected HEK293 cells). However, if this holds true in actual cardiomyocytes in vivo, it might be part of the mechanism for this β-blocker`s cardio-protective effects. However, several studies do not lend support to this notion; sustained β1AR activation by catecholamines, markedly more potent activators of β-arrestin-dependent ERKs in the heart than carvedilol, increases cardiac adverse remodeling even in the absence of cardiac injury[26]. Moreover, carvedilol is also a β2- and α1AR antagonist, which may interfere with its β-arrestin-activating properties in the heart[27]. On the other hand, carvedilol`s effects in the heart are virtually exclusively mediated by the β1AR, due to its relative selectivity for the human β1AR over the other human AR subtypes (β2AR and α1AR) and to the vast preponderance of the β1AR over the rest of AR subtypes in the human adult myocardium[16]. Last but not least, recent studies have been unable to directly detect β-arrestin interactions with either β1AR or β2AR bound to carvedilol, including a study reporting the intriguing finding that carvedilol requires Gαi protein recruitment to the β1AR in order to induce EGFR transactivation via β-arrestins[28-31]. Nevertheless, carvedilol has been shown to selectively recruit β-arrestin-2 to the hyperfunctional human polymorphic Arg389 β1AR in cardiomyocytes in vitro[32]. Therefore, the more robust, compared to its Gly389 counterpart, pro-contractile signaling of this β1AR variant[33] might be, at least partly, due to its unique β-arrestin-2-interacting tropism. In this vein, very recent data from our lab indicate that carvedilol-bound β1AR uniquely stimulates β-arrestin-2-dependent SERCA2a activity and fractional shortening of cardiomyocytes in vitro[34].
FUNCTIONAL DIFFERENCES BETWEEN THE TWO BETA-ARRESTINS IN CARDIAC AT1R SIGNALING
Despite its very low abundance in adult human myocardium (density ratio of AT1R/β1AR in the non-failing human heart: approximately 1:15)[16], the AT1R plays important regulatory roles in the heart, but mainly via actions in cardiac fibroblasts and endothelial cells, rather than in cardiac myocytes[4,7,35-37]. The AngII peptide analog SII ([Sar1-Ile4-Ile8]-AngII) does not elicit Gq protein signaling when bound to the AT1R[9,38]. When it was discovered to induce β-arrestin signaling from the AT1R, the concept of biased signaling was introduced and ushered in a completely new field in cardiovascular drug development with companies designing novel “biased” AT1R ligands that only activate β-arrestins while retaining G-protein-blocking properties. SII has now been documented to not be completely β-arrestin-“biased”, as it can activate some G-protein types (e.g., Gs, Gi) from the AT1R[9]. Nevertheless, studies have shown that AT1R-elicited β-arrestin-dependent signaling in cardiac myocytes leads to cardiomyocyte proliferation without hypertrophy, which is Gq/11 protein signaling-dependent, and may even result in positive inotropy and lusitropy depending on which GRK isoform is involved (the so-called receptor “bar-coding” by GRKs). Interestingly, which β-arrestin is engaged is also crucial[39,40]. Specifically, GRK2-dependent phosphorylation followed by β-arrestin-1 binding seems to reduce, whereas GRK6-dependent phosphorylation followed by β-arrestin-2 interaction seems to promote AT1R-induced contractility in primary murine adult cardiomyocytes (Figure 1)[39]. However, AT1R-activated β-arrestins have no effect on contractility in isolated Langendorff-perfused cardiac preparations[41]. Furthermore, a recombinant AT1AR capable of only signaling through β-arrestins inhibits myocardial apoptosis and fibrosis, and enhances cardiomyocyte hypertrophy, upon transgenic overexpression in mouse hearts[42]. Interestingly, a β-arrestin-2-“biased” ligand at the AT1R is very beneficial in mice suffering from dilated cardiomyopathy as it prevents maladaptive signaling and improves myofilament Ca2+ sensitivity[43]. Thus, cardiac AT1R promotes hypertrophy and cardiomyocyte proliferation via the classic Gq protein/phospholipase C-β-signaling pathway, which is inhibited by the β-arrestins (classic AT1R desensitization), and, at the same time, β-arrestin-2 (but not β-arrestin-1) can increase cardiac function via its cardiac AT1R-dependent signaling (Figure 1).
Based on the above studies, several SII-derivative peptides have been synthesized and tested in animal models of ADHF with promising initial results[44,45]. Unfortunately, these compounds failed in large phase III clinical trials (BLAST-AHF, ClinicalTrials.gov number, NCT01966601). There are probably several reasons for this. First, findings in animal models do not always translate into humans. Second, the compounds might have not been completely β-arrestin-“biased” (i.e., maybe they had some weak, residual activity towards certain G-proteins). One intriguing possibility is that, due to the significantly lower abundance of the cardioprotective β-arrestin-2, compared to the cardio-toxic β-arrestin-1, in human cardiomyocytes[22], these β-arrestin-“biased” compounds stimulated, in reality, β-arrestin-1 instead of β-arrestin-2 in the patients` hearts and that’s why their clinical outcomes were not the desired ones. Finally, it is very likely that these compounds stimulated the AT1R only in cardiac fibroblasts, which would preclude any clinical benefit for ADHF patients. In fact, both β-arrestins have been shown to regulate human cardiac fibroblast differentiation and to mediate collagen synthesis in human failing left ventricle-derived fibroblasts, thereby promoting the adverse remodeling process of cardiac fibrosis[46].
Notably, β-arrestins have been reported to mediate signaling by the mechanical stretch-activated (unliganded) cardiac AT1R[47], which has been suggested to underlie one of the fundamental laws of cardiac physiology, the Frank-Starling mechanism of cardiac contractility[48]. Although intriguing, this finding is very difficult to interpret, given that the Frank-Starling mechanism is hemodynamically/biomechanically governed rather than dependent on hormonal receptors/effects. Besides, if it was mediated by a cardiac GPCR, then that receptor would definitely be the β1AR, the by far most abundant GPCR (and at least 15-fold more abundant than the AT1R) in cardiomyocytes[16].
Finally, β-arrestin-mediated signaling by the AT1R that can regulate cardiac function occurs in the adrenal cortex, as well. Specifically, the AT1R promotes the adrenocortical production of aldosterone, a cardio-toxic hormone elevated in chronic human heart failure, via both Gq/11-proteins and β-arrestin-1[35,49-51]. In fact, adrenal β-arrestin-1 is essential for aldosterone production, since, in mice lacking the β-arrestin-1 gene, circulating aldosterone levels do not increase even in the presence of MI[18]. Interestingly, the prototypic AT1R antagonist (ARB, angiotensin receptor blocker) losartan is a relatively G protein-“biased” antagonist, which means it cannot suppress β-arrestin-1-dependent aldosterone production[51-54]. In contrast, candesartan and valsartan are potent β-arrestin-inverse agonists at the adrenal AT1R and very effective at suppressing aldosterone in vitro and in vivo[52,53]. These differences among ARBs, which are all orthosteric antagonists, in their potency at blocking AT1R-activated β-arrestin signaling may reflect their differential abilities to suppress β-arrestin signaling by the unliganded (i.e., constitutively active) AT1R[47,55]. In other words, the ARBs seem to be inverse agonists not only for G-proteins but also for β-arrestins at the AT1R.
THERAPEUTIC IMPLICATIONS IN HEART FAILURE
It becomes clear from the above that the signaling effects of the two β-arrestins in the heart are not just different but actually diametrically opposite. This is true for several other mammalian organ systems and tissue types[56-58] and is completely corroborated by molecular, biophysical, crystallographic, and proteomic studies[59-62]. It also makes sense from the evolutionary point of view, as functional redundancy of proteins is usually not favored by natural selection. Only during embryonic development might the two β-arrestins be able to compensate for each other, since single β-arrestin-knockout mice (either β-arrestin-1- or β-arrestin-2-knockouts) are viable and breed normally but the double β-arrestin-knockout mouse is embryonic-lethal[63].
Regarding the myocardium, all the functional studies on β-arrestins done so far are in relation to either βARs or the AT1R. Studies on β-arrestin signaling from other cardiac GPCRs in cardiac cells are lacking. Based on the available data for β1AR, β2AR, or AT1R signaling through β-arrestins in cardiac cells, it can be safely concluded that β-arrestin-1 is the arrestin responsible for the classic desensitization of the cAMP-dependent pro-contractile signaling of the β1AR. This quite simply means that β-arrestin-1 exerts overall negative inotropy and is responsible (together with the elevated in human heart failure cardiac GRK2) for the diminished inotropic and adrenergic reserves of the failing human heart. In addition, β-arrestin-1 promotes cardiac apoptosis, inflammation, and accelerates cardiac adverse remodeling post-MI. In direct contrast, β-arrestin-2 promotes β1AR-mediated cardiac contractility both directly and indirectly. Directly, via augmentation of SERCA2a activity, and indirectly, by leaving the β1AR’s cAMP-dependent pro-contractile signaling intact (i.e., no desensitization). On the other hand, it inhibits apoptosis and inflammation, and overall attenuates cardiac adverse remodeling post-MI, via stimulation of a variety of molecular pathways, such as EGFR transactivation and NFκB inhibition, which β-arrestin-1 does not activate. Induction of ERK and of other mitogenic/proliferative molecular signaling pathways in cardiomyocytes play auxiliary roles in β-arrestin-2’s reverse remodeling effects, as well. Of note, the same functional distinction between the two cardiac β-arrestins (i.e., β-arrestin-1 being detrimental, β-arrestin-2 being beneficial) applies to cardiac AT1R signaling, too. Regardless of how small the contribution of this GPCR to the overall contractile function of the cardiac myocyte is, β-arrestin-2 again appears to promote contractility and cardiomyocyte survival in response to AT1R activation. Conversely, β-arrestin-1 (again in conjunction with GRK2) opposes the AT1R-dependent pro-contractile signaling in cardiac myocytes. However, β-arrestin-1 might exert an indirect beneficial effect in the hypertrophic heart by desensitizing (reducing) the pro-hypertrophic signaling of the cardiac AT1R through Gq/11-proteins. In conclusion, based on their observed effects on the signaling of both β1ARs and AT1Rs in cardiac myocytes, documented either directly (in β-arrestin-knockout mice) or indirectly (with the use of “biased” receptor ligands), cardiac β-arrestin-2 stimulation and/or β-arrestin-1 inhibition might be valid therapeutic strategies in human heart failure. By the way, it is interesting to note here that probably the exact opposite is the case in the systemic vasculature. In vascular smooth muscle cells, β-arrestin-1 appears beneficial in terms of vasodilation and attenuation of hyperplasia and β-arrestin-2 seems to promote hypertrophy/hyperplasia[58]. This should not come as a surprise at all, given the different cellular machineries and GPCR types involved in β-arrestin signaling between cardio-myocytes (mainly β1AR) and vascular smooth muscle cells (mainly AT1R and, to a lesser extent, β2AR). Besides, this is exactly what happens with the major second messenger cAMP: stimulated by the β1AR, it is pro-contractile in cardiomyocytes, but stimulated by the β2AR, it is pro-dilatory in vascular smooth muscle.
CONCLUSION
In the adult myocardium, the actions of β-arrestin-1 are detrimental, since they result in depletion of the functional and adrenergic reserves of the heart. In contrast, β-arrestin-2 is beneficial, since it can increase both of these cardiac reserves or at least preserve them in the face of a cardiac insult/damage, such as an MI. Thus, from the therapeutic standpoint, cardiac β-arrestin-1 blockade or β-arrestin-2 stimulation should be pursued for heart disease treatment. Of course, there are at least three very important questions awaiting answer in future studies in order to fully validate these strategies as therapeutic options for human heart failure. First, the effects of the two β-arrestins on the signaling of other important cardiac GPCRs in the heart, i.e., beyond the βARs and the AT1R, need to be elucidated. The second issue to resolve is what the effects of the two β-arrestins in other tissues/components of the cardiovascular system are, e.g., vasculature, platelets, adrenals, etc. This is particularly important if the pharmacological targeting of the β-arrestins with systemically administered agents is being explored. For instance, β-arrestin-2 is beneficial in the heart, in platelets, and in vascular endothelium but might be detrimental in vascular smooth muscle[58]. Its exact effects in all of these tissues need to be thoroughly investigated and determined, if a drug that stimulates this β-arrestin isoform is to be designed and developed. Finally, the third and therapeutically salient unanswered question pertains to the expression levels of the cardiac β-arrestins in the failing human heart. Although β-arrestin-2 protein is significantly under-expressed, compared to β-arrestin-1, in the non-failing human adult myocardium[22,64], which makes cardiac-specific β-arrestin-2 gene transfer an enticing approach for heart failure treatment, its protein levels (and if they change) in human heart failure remain unknown. However, given that it is significantly expressed at the mRNA level, and in fact at levels comparable to those of β-arrestin-1 mRNA, in the failing human heart[64], it is quite plausible that it might be upregulated at the protein level, similarly to GRK2, as a homeostatic mechanism of the failing human myocardium to confer cardioprotection. The only study done to date investigating β-arrestin expression in failing human hearts is quite old (was published almost 25 years ago) and could not detect any changes in the protein levels of either β-arrestin[64]. Nevertheless, although β-arrestin-1 protein probably does not change in human heart failure, because it is already highly expressed in normal, healthy human hearts, that study failed to detect any β-arrestin-2 protein at all, even in normal healthy human hearts, probably due to technical deficiencies of the antibody it used[64]. Therefore, it is rather inconclusive with regard to cardiac β-arrestin-2 protein expression in humans and whether cardiac β-arrestin-2 protein changes in human heart failure remains an open question. In fact, a much more recent study done in human cardiac fibroblasts isolated from left ventricles of heart failure patients hinted at β-arrestin-2 protein being upregulated in human failing hearts[46]. In any case, more studies are certainly warranted, especially in human cardiac specimens, to unravel the full spectrum of physiological (and pathophysiological) actions of the two cardiac β-arrestins, beginning with the answering of the three outstanding questions mentioned above. Only then will the true potential of individual cardiac β-arrestin isoform targeting for heart failure therapy be revealed, so that the pharmaceutical industry can begin its realization.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest related to this publication.
Manuscript source: Invited manuscript
Peer-review started: October 16, 2018
First decision: November 15, 2018
Article in press: January 10, 2019
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aksu T, Liberale L, Pastromas S S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
Contributor Information
Anastasios Lymperopoulos, Laboratory for the Study of Neurohormonal Control of the Circulation, Department of Pharmaceutical Sciences (Pharmacology), College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL 33328, United States. al806@nova.edu.
Shelby L Wertz, Laboratory for the Study of Neurohormonal Control of the Circulation, Department of Pharmaceutical Sciences (Pharmacology), College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL 33328, United States.
Celina M Pollard, Laboratory for the Study of Neurohormonal Control of the Circulation, Department of Pharmaceutical Sciences (Pharmacology), College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL 33328, United States.
Victoria L Desimine, Laboratory for the Study of Neurohormonal Control of the Circulation, Department of Pharmaceutical Sciences (Pharmacology), College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL 33328, United States.
Jennifer Maning, Laboratory for the Study of Neurohormonal Control of the Circulation, Department of Pharmaceutical Sciences (Pharmacology), College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL 33328, United States; Jackson Memorial Hospital, Miami, FL 33136, United States.
Katie A McCrink, Laboratory for the Study of Neurohormonal Control of the Circulation, Department of Pharmaceutical Sciences (Pharmacology), College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL 33328, United States; Massachusetts General Hospital, Boston, MA 02114, United States.
References
- 1.Desimine VL, McCrink KA, Parker BM, Wertz SL, Maning J, Lymperopoulos A. Biased Agonism/Antagonism of Cardiovascular GPCRs for Heart Failure Therapy. Int Rev Cell Mol Biol. 2018;339:41–61. doi: 10.1016/bs.ircmb.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Hilger D, Masureel M, Kobilka BK. Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol. 2018;25:4–12. doi: 10.1038/s41594-017-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson YK, Luttrell LM. The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Pharmacol Rev. 2017;69:256–297. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lymperopoulos A, Garcia D, Walklett K. Pharmacogenetics of cardiac inotropy. Pharmacogenomics. 2014;15:1807–1821. doi: 10.2217/pgs.14.120. [DOI] [PubMed] [Google Scholar]
- 6.Siryk-Bathgate A, Dabul S, Lymperopoulos A. Current and future G protein-coupled receptor signaling targets for heart failure therapy. Drug Des Devel Ther. 2013;7:1209–1222. doi: 10.2147/DDDT.S35905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capote LA, Mendez Perez R, Lymperopoulos A. GPCR signaling and cardiac function. Eur J Pharmacol. 2015;763:143–148. doi: 10.1016/j.ejphar.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Lymperopoulos A, Bathgate A. Arrestins in the cardiovascular system. Prog Mol Biol Transl Sci. 2013;118:297–334. doi: 10.1016/B978-0-12-394440-5.00012-7. [DOI] [PubMed] [Google Scholar]
- 9.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjan R, Dwivedi H, Baidya M, Kumar M, Shukla AK. Novel Structural Insights into GPCR-β-Arrestin Interaction and Signaling. Trends Cell Biol. 2017;27:851–862. doi: 10.1016/j.tcb.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Eichel K, Jullié D, Barsi-Rhyne B, Latorraca NR, Masureel M, Sibarita JB, Dror RO, von Zastrow M. Catalytic activation of β-arrestin by GPCRs. Nature. 2018;557:381–386. doi: 10.1038/s41586-018-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundmann M, Merten N, Malfacini D, Inoue A, Preis P, Simon K, Rüttiger N, Ziegler N, Benkel T, Schmitt NK, Ishida S, Müller I, Reher R, Kawakami K, Inoue A, Rick U, Kühl T, Imhof D, Aoki J, König GM, Hoffmann C, Gomeza J, Wess J, Kostenis E. Lack of beta-arrestin signaling in the absence of active G proteins. Nat Commun. 2018;9:341. doi: 10.1038/s41467-017-02661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luttrell LM, Wang J, Plouffe B, Smith JS, Yamani L, Kaur S, Jean-Charles PY, Gauthier C, Lee MH, Pani B, Kim J, Ahn S, Rajagopal S, Reiter E, Bouvier M, Shenoy SK, Laporte SA, Rockman HA, Lefkowitz RJ. Manifold roles of β-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci Signal. 2018;11:pii: eaat7650. doi: 10.1126/scisignal.aat7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weis WI, Kobilka BK. The Molecular Basis of G Protein-Coupled Receptor Activation. Annu Rev Biochem. 2018;87:897–919. doi: 10.1146/annurev-biochem-060614-033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, Pei G. Beta-arrestin signaling and regulation of transcription. J Cell Sci. 2007;120:213–218. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikawa T, Port JD, Asano K, Chidiak P, Bouvier M, Dutcher D, Roden RL, Minobe W, Tremmel KD, Bristow MR. Cardiac adrenergic receptor effects of carvedilol. Eur Heart J. 1996;17 Suppl B:8–16. doi: 10.1093/eurheartj/17.suppl_b.8. [DOI] [PubMed] [Google Scholar]
- 17.Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 18.Bathgate-Siryk A, Dabul S, Pandya K, Walklett K, Rengo G, Cannavo A, De Lucia C, Liccardo D, Gao E, Leosco D, Koch WJ, Lymperopoulos A. Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension. 2014;63:404–412. doi: 10.1161/HYPERTENSIONAHA.113.02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watari K, Nakaya M, Nishida M, Kim KM, Kurose H. β-arrestin2 in infiltrated macrophages inhibits excessive inflammation after myocardial infarction. PLoS One. 2013;8:e68351. doi: 10.1371/journal.pone.0068351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCrink KA, Maning J, Vu A, Jafferjee M, Marrero C, Brill A, Bathgate-Siryk A, Dabul S, Koch WJ, Lymperopoulos A. β-Arrestin2 Improves Post-Myocardial Infarction Heart Failure via Sarco(endo)plasmic Reticulum Ca2+-ATPase-Dependent Positive Inotropy in Cardiomyocytes. Hypertension. 2017;70:972–981. doi: 10.1161/HYPERTENSIONAHA.117.09817. [DOI] [PubMed] [Google Scholar]
- 21.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477:601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCrink KA, Maning J, Vu A, Jafferjee M, Marrero C, Brill A, Bathgate-Siryk A, Dabul S, Koch WJ, Lymperopoulos A. Cardiac βarrestin2 Improves Contractility and Adverse Remodeling in Heart Failure, But Is Underexpressed in Humans. J Am Coll Cardiol. 2017;70:2948–2949. doi: 10.1016/j.jacc.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur K, Parra S, Chen R, Charbeneau RA, Wade SM, Jay PY, Neubig RR. Gαi2 signaling: friend or foe in cardiac injury and heart failure? Naunyn Schmiedebergs Arch Pharmacol. 2012;385:443–453. doi: 10.1007/s00210-011-0705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carr R, 3rd, Schilling J, Song J, Carter RL, Du Y, Yoo SM, Traynham CJ, Koch WJ, Cheung JY, Tilley DG, Benovic JL. β-arrestin-biased signaling through the β2-adrenergic receptor promotes cardiomyocyte contraction. Proc Natl Acad Sci USA. 2016;113:E4107–E4116. doi: 10.1073/pnas.1606267113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Littmann T, Göttle M, Reinartz MT, Kälble S, Wainer IW, Ozawa T, Seifert R. Recruitment of β-arrestin 1 and 2 to the β2-adrenoceptor: analysis of 65 ligands. J Pharmacol Exp Ther. 2015;355:183–190. doi: 10.1124/jpet.115.227959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shukla AK, Westfield GH, Xiao K, Reis RI, Huang LY, Tripathi-Shukla P, Qian J, Li S, Blanc A, Oleskie AN, Dosey AM, Su M, Liang CR, Gu LL, Shan JM, Chen X, Hanna R, Choi M, Yao XJ, Klink BU, Kahsai AW, Sidhu SS, Koide S, Penczek PA, Kossiakoff AA, Woods VL, Jr, Kobilka BK, Skiniotis G, Lefkowitz RJ. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature. 2014;512:218–222. doi: 10.1038/nature13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hayre M, Eichel K, Avino S, Zhao X, Steffen DJ, Feng X, Kawakami K, Aoki J, Messer K, Sunahara R, Inoue A, von Zastrow M, Gutkind JS. Genetic evidence that β-arrestins are dispensable for the initiation of β2-adrenergic receptor signaling to ERK. Sci Signal. 2017;10:pii: eaal3395. doi: 10.1126/scisignal.aal3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Hanada K, Staus DP, Makara MA, Dahal GR, Chen Q, Ahles A, Engelhardt S, Rockman HA. Gαi is required for carvedilol-induced β1 adrenergic receptor β-arrestin biased signaling. Nat Commun. 2017;8:1706. doi: 10.1038/s41467-017-01855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCrink KA, Brill A, Jafferjee M, Valero TR, Marrero C, Rodriguez MM, Hale GM, Lymperopoulos A. β1-adrenoceptor Arg389Gly polymorphism confers differential β-arrestin-binding tropism in cardiac myocytes. Pharmacogenomics. 2016;17:1611–1620. doi: 10.2217/pgs-2016-0094. [DOI] [PubMed] [Google Scholar]
- 33.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, Weber SA, Greene SM, Hodne D, Nelson B, Morrison J, Domanski MJ, Wagoner LE, Abraham WT, Anderson JL, Carlquist JF, Krause-Steinrauf HJ, Lazzeroni LC, Port JD, Lavori PW, Bristow MR. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci USA. 2006;103:11288–11293. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lymperopoulos A, Desimine VL, McCrink KA, Maning J, Wertz SL, Markan U, Pasupuleti S, Brill A, Parker BM. Positive cardiac inotropy by carvedilol via unique beta-arrestin2-dependent SERCA2a stimulation. Eur Heart J. 2018;39:Suppl_1, 1. [Google Scholar]
- 35.Maning J, Negussie S, Clark MA, Lymperopoulos A. Biased agonism/antagonism at the AngII-AT1 receptor: Implications for adrenal aldosterone production and cardiovascular therapy. Pharmacol Res. 2017;125:14–20. doi: 10.1016/j.phrs.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Lymperopoulos A. Beta-arrestin biased agonism/antagonism at cardiovascular seven transmembrane-spanning receptors. Curr Pharm Des. 2012;18:192–198. doi: 10.2174/138161212799040475. [DOI] [PubMed] [Google Scholar]
- 37.Thomas WG, Thekkumkara TJ, Baker KM. Cardiac effects of AII. AT1A receptor signaling, desensitization, and internalization. Adv Exp Med Biol. 1996;396:59–69. [PubMed] [Google Scholar]
- 38.Saulière A, Bellot M, Paris H, Denis C, Finana F, Hansen JT, Altié MF, Seguelas MH, Pathak A, Hansen JL, Sénard JM, Galés C. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat Chem Biol. 2012;8:622–630. doi: 10.1038/nchembio.961. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA. 2006;103:16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aplin M, Christensen GL, Schneider M, Heydorn A, Gammeltoft S, Kjølbye AL, Sheikh SP, Hansen JL. Differential extracellular signal-regulated kinases 1 and 2 activation by the angiotensin type 1 receptor supports distinct phenotypes of cardiac myocytes. Basic Clin Pharmacol Toxicol. 2007;100:296–301. doi: 10.1111/j.1742-7843.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, Irie K, Holle E, Yu X, Kupershmidt S, Roden DM, Wagner T, Yatani A, Vatner DE, Vatner SF, Sadoshima J. Cardiac-specific overexpression of AT1 receptor mutant lacking G alpha q/G alpha i coupling causes hypertrophy and bradycardia in transgenic mice. J Clin Invest. 2005;115:3045–3056. doi: 10.1172/JCI25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryba DM, Li J, Cowan CL, Russell B, Wolska BM, Solaro RJ. Long-Term Biased β-Arrestin Signaling Improves Cardiac Structure and Function in Dilated Cardiomyopathy. Circulation. 2017;135:1056–1070. doi: 10.1161/CIRCULATIONAHA.116.024482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 45.Boerrigter G, Soergel DG, Violin JD, Lark MW, Burnett JC., Jr TRV120027, a novel β-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ Heart Fail. 2012;5:627–634. doi: 10.1161/CIRCHEARTFAILURE.112.969220. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Philip JL, Xu X, Theccanat T, Abdur Razzaque M, Akhter SA. β-Arrestins regulate human cardiac fibroblast transformation and collagen synthesis in adverse ventricular remodeling. J Mol Cell Cardiol. 2014;76:73–83. doi: 10.1016/j.yjmcc.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3:ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abraham DM, Davis RT, 3rd, Warren CM, Mao L, Wolska BM, Solaro RJ, Rockman HA. β-Arrestin mediates the Frank-Starling mechanism of cardiac contractility. Proc Natl Acad Sci USA. 2016;113:14426–14431. doi: 10.1073/pnas.1609308113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lymperopoulos A, Aukszi B. Angiotensin receptor blocker drugs and inhibition of adrenal beta-arrestin-1-dependent aldosterone production: Implications for heart failure therapy. World J Cardiol. 2017;9:200–206. doi: 10.4330/wjc.v9.i3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Soltys S, Koch WJ. An adrenal beta-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106:5825–5830. doi: 10.1073/pnas.0811706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Koch WJ. Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels. J Am Coll Cardiol. 2011;57:356–365. doi: 10.1016/j.jacc.2010.08.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lymperopoulos A, Sturchler E, Bathgate-Siryk A, Dabul S, Garcia D, Walklett K, Rengo G, McDonald P, Koch WJ. Different potencies of angiotensin receptor blockers at suppressing adrenal β-Arrestin1-dependent post-myocardial infarction hyperaldosteronism. J Am Coll Cardiol. 2014;64:2805–2806. doi: 10.1016/j.jacc.2014.09.070. [DOI] [PubMed] [Google Scholar]
- 53.Dabul S, Bathgate-Siryk A, Valero TR, Jafferjee M, Sturchler E, McDonald P, Koch WJ, Lymperopoulos A. Suppression of adrenal βarrestin1-dependent aldosterone production by ARBs: head-to-head comparison. Sci Rep. 2015;5:8116. doi: 10.1038/srep08116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valero TR, Sturchler E, Jafferjee M, Rengo G, Magafa V, Cordopatis P, McDonald P, Koch WJ, Lymperopoulos A. Structure-activity relationship study of angiotensin II analogs in terms of β-arrestin-dependent signaling to aldosterone production. Pharmacol Res Perspect. 2016;4:e00226. doi: 10.1002/prp2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tóth AD, Prokop S, Gyombolai P, Várnai P, Balla A, Gurevich VV, Hunyady L, Turu G. Heterologous phosphorylation-induced formation of a stability lock permits regulation of inactive receptors by β-arrestins. J Biol Chem. 2018;293:876–892. doi: 10.1074/jbc.M117.813139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srivastava A, Gupta B, Gupta C, Shukla AK. Emerging Functional Divergence of β-Arrestin Isoforms in GPCR Function. Trends Endocrinol Metab. 2015;26:628–642. doi: 10.1016/j.tem.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Lymperopoulos A, Negussie S. βArrestins in cardiac G protein-coupled receptor signaling and function: partners in crime or "good cop, bad cop"? Int J Mol Sci. 2013;14:24726–24741. doi: 10.3390/ijms141224726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lymperopoulos A. Arrestins in the Cardiovascular System: An Update Prog Mol Biol Transl Sci. 2018:In press. doi: 10.1016/bs.pmbts.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Zhan X, Gimenez LE, Gurevich VV, Spiller BW. Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual subtypes. J Mol Biol. 2011;406:467–478. doi: 10.1016/j.jmb.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhuo Y, Vishnivetskiy SA, Zhan X, Gurevich VV, Klug CS. Identification of receptor binding-induced conformational changes in non-visual arrestins. J Biol Chem. 2014;289:20991–21002. doi: 10.1074/jbc.M114.560680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurevich VV, Gurevich EV. Extensive shape shifting underlies functional versatility of arrestins. Curr Opin Cell Biol. 2014;27:1–9. doi: 10.1016/j.ceb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, 3rd, Lefkowitz RJ. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ungerer M, Parruti G, Böhm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ. Expression of beta-arrestins and beta-adrenergic receptor kinases in the failing human heart. Circ Res. 1994;74:206–213. doi: 10.1161/01.res.74.2.206. [DOI] [PubMed] [Google Scholar]