Abstract
Aim:
To evaluate the persistence of biological (TNF inhibitor [anti-TNF]) and synthetic (conventional synthetic disease-modifying antirheumatic drugs [csDMARDs]) antirheumatic agents for psoriatic arthritis and their associated factors.
Methods:
A historical cohort was developed. Persistence and associated factors were evaluated at 6 and 12 months.
Results:
A total of 161 patients were included. The anti-TNF treatment presented higher persistence as compared with csDMARDs at 6 (83.4 vs 50.8%; p < 0.05) and 12 months (66.4 vs 35.6%; p < 0.05). From anti-TNFs, adalimumab and etanercept presented similar persistence, along with leflunomide and methotrexate among the csDMARDs. The factors associated with non-persistence with regard to anti-TNF agents were female sex and use of infliximab.
Conclusion:
Anti-TNF agents are important therapeutic alternatives and present lower rates of discontinuation as compared with csDMARDs.
Keywords: : antirheumatic agents, medication persistence, psoriatic arthritis
Lay abstract
Conventional synthetic disease-modifying antirheumatic drugs (csDMARD) and anti-TNF agents were included in the Brazilian National Health System in 2009, yet were not previously the target of evaluations in a Brazilian context. Persistence, that is the time between starting and discontinuing the drug, is a proxy of effectiveness and safety for antirheumatic agents. This study evaluated medication persistence and relevant factors associated with it. Patients using infliximab showed a lower medication persistence when compared with adalimumab and etanercept. The medication persistence with anti-TNF agents was higher when compared with csDMARDs. Female sex and infliximab were predictors of medication non-persistence.
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis that affects axial and peripheral joints, entheses, tendons and fascias; and is associated with cutaneous psoriasis [1,2]. It was described for the first time in 1956 by Wright and it is considered the most common extracutaneous manifestation of psoriasis [1,3].
PsA causes a decrease in occupational function and psychosocial morbidity. When compared with the general population, PsA patients present a decrease in quality of life (QoL), a functional impairment, psychosocial inability and a significant increase in their mortality index [4,5]. This impact on QoL is related to pain, skin problems, functional disability and fatigue, as well as emotional and social aspects [6]. Besides this, PsA has been associated with an increase in the risk of cardiovascular, endocrine/metabolic, gastrointestinal, respiratory and neuropsychiatric disorders, and uveitis [1,4].
Adequate PsA treatment is essential for the control of disease progression and its impact on the health of affected patients. Treatment comprises nonpharmacological and pharmacological measures. The so-called disease-modifying antirheumatic drugs (DMARDs) are commonly characterized by their capacity to decrease or revert signals and symptoms, functional impairment, QoL impairment, occupational disability and joint damage progression and, therefore, could interfere with the entire disease process. They are divided into three different classes: conventional synthetic DMARDs (csDMARDs), biological DMARDs (bDMARDs) and target-specific synthetic DMARDs [7].
After incorporation of DMARDs into the National Health System (Sistema Único de Saúde - SUS) of Brazil in 2009, few studies have evaluated the profile of request and use of these medications [8]. Considering this fact, the performance evaluation of technologies applied to health, which consists of the continued evaluation of incorporated technologies and the analysis of results obtained in the context of a health system, become an important tool, particularly in a scenery of successive increases in health costs, limitation of public health expenditures and significant technological advancement achieved in the last few years, which points to the need for postmarketing and/or postincorporation studies [9].
Besides this, the literature indicates that biological therapies, generally, lose their effectiveness over time [10,11]. There are few data from clinical trials regarding the comparative efficacy/effectiveness of medications for PsA treatment [10]. Finally, the long-term safety and the real purpose profile of medications could not be adequately evaluated in clinical trials, due to the restriction of such studies thanks to, in particular, inclusion and size criteria being driven by primary efficacy outcomes that result in low external validity for the real-world population [12].
In this context, the medication persistence has been interpreted as a composite measure when considering effectiveness, safety and utility in real life for rheumatic diseases. The more common reasons for the interruption of tumor necrosis factor inhibitors (anti-TNFs) were reported as effectiveness lack or loss and adverse events. However, the persistence in the use of medications could also be influenced by other factors, such as the available number of alternative treatment options and the characteristics of the population of treated patients [10,13].
Thus, the purpose of this present study is to describe, in the ambit of SUS, the profile of a sample of PsA patients in Brazil, evaluating their medication persistence, as a ‘proxy’ of both effectiveness and safety related to treatment.
Materials & methods
This is a historical cohort of patients requiring PsA treatment in the period between 4 November 2014 and 31 December 2016 in the SUS. These patients were seen in the Belo Horizonte Health Region, which comprises a population of over five million inhabitants distributed between 39 municipalities.
The patients who requested csDMARDs or anti-TNF drugs for the treatment of PsA were identified in the Pharmaceutical Services Management System (SiGAF), where the data of requesting and authorization for use of such medications were available.
The variables analyzed at baseline were the information contained in the documentation (administrative process) extracted from the SiGAF for the request of DMARDs, namely: sociodemographic profile; patients’ clinical profile; requested medications; examinations defined in the documents listing and those necessary for opening the treatment process for PsA.
The patients who started the use of csDMARDs or anti-TNFs and had not utilized previous therapy with medication of this same class were considered eligible. The therapy administered at study start was considered: anti-TNF (adalimumab, etanercept or infliximab) and csDMARD (methotrexate or leflunomide). Patients who started treatment with sulfasalazine or cyclosporine were excluded due to the small number of requests for these medications. The date of entrance in the cohort was defined as the first date of the dispensation of the medication in the Integrated System of Hospital Management (SIGH). All the patients were followed up to the date of the final follow-up (December/2017) or death.
The outcome evaluated was persistence, defined as the time duration between the medication's first dispensation and discontinuation. Therapy discontinuation was defined as the absence of medication dispensation for more than 90 days, verified in the Integrated System of Hospital Management (SIGH). Three comparison groups were built: anti-TNF versus csDMARD (between the classes); anti-TNF (adalimumab versus etanercept and infliximab) and csDMARD (leflunomide versus methotrexate). The proportion of persistent individuals was evaluated at 6 and 12 months of follow-up and the factors associated with persistence were verified.
The independent variables considered in the analysis of factors associated with persistence were sex, age range, body mass index (BMI), C-reactive protein examination and erythrocyte sedimentation rate, medications in use, axial impairment, peripheral impairment, nail dystrophy, bone neoformation and dactylitis.
The continuous variables of interest were graphically evaluated using the Shapiro Wilk test for verification of data normality and for the definition of the measures of central tendency and dispersion. The categorical variables were described by means of distribution of frequencies and the continuous variables by means of median (interquartile range). The proportions of persistence between the groups and between the medications were compared by means of the Pearson's χ2 test. The average time of persistence between the groups and between the medications were compared by means of T-test (two comparators) or variance analysis (ANOVA) with adjustment by the method of Bonferroni (three comparators). Kaplan–Meier survival curves were elaborated to verify the time up to treatment discontinuation, that is, the loss of treatment persistence with the medications after 6 and 12 months. The log-rank test was utilized to compare the medication persistence between the study groups. The regression by the model of proportional risks of bivariate and multivariable COX was utilized to verify the variables independently associated with time up to treatment discontinuation. A significance level of 20% has been adopted for the bivariate analysis and of 5% for the multivariable analysis. The statistical analyses were conducted utilizing the software STATA version 15.1.
This study was approved by the Institutional Review Board/Independent Ethics Committee of the Federal University of Minas Gerais (UFMG), appraisals ETIC 0069.0.203.000-1 and CAAE 44121315.2.0000.5149.
Results
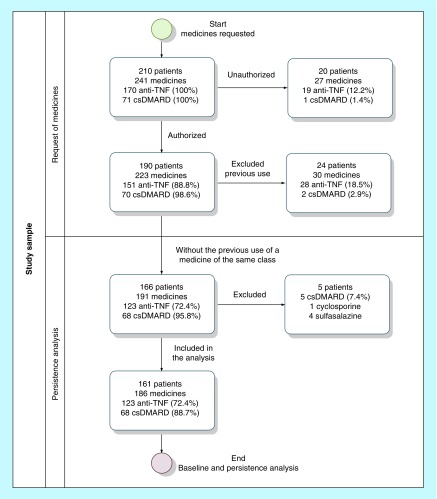
A total of 241 medications were requested by 210 patients, out of which 161 patients were included with the request of 186 medications (123 anti-TNF and 63 csDMARDs; Figure 1). Adalimumab (61%) was the most used anti-TNF, followed by etanercept (27.6%) and infliximab (11.4%). Among the csDMARDs, 54% of patients started methotrexate and 46% leflunomide (Table 1).
Figure 1. . Flowchart with the selection of patients eligible for the study.
Table 1. . Profiles of patients who started the use of medications and were included in the persistence analysis.
| Variables | Total (161) | % | Anti-TNF (123) | % | csDMARD (63) | % | p-value |
|---|---|---|---|---|---|---|---|
| Sex | 0.106 | ||||||
| Female | 91 | 56.5 | 64 | 52 | 40 | 64.5 | |
| Male | 70 | 43.5 | 59 | 48 | 22 | 35.5 | |
| Age in years | 51.6 (42.5–59.5) | 51.6 (41.4–59.4) | 50.2 (43.7–58.0) | 0.966 | |||
| Race | 0.597 | ||||||
| White | 72 | 52.5 | 56 | 53.8 | 25 | 47.2 | |
| Brown | 61 | 44.5 | 44 | 42.3 | 27 | 51.0 | |
| Black | 4 | 3 | 4 | 3.9 | 1 | 1.9 | |
| BMI in kilograms | 26.4 (23.9–29.9) | 26.9 (23.6–29.9) | 27.8 (25.4–30.9) | 0.045 | |||
| Normal | 56 | 36.6 | 48 | 41.4 | 14 | 23.3 | 0.053 |
| Overweight | 59 | 38.6 | 40 | 34.5 | 29 | 48.3 | |
| Obesity | 38 | 24.8 | 28 | 24.1 | 17 | 28.4 | |
| Drugs in use | |||||||
| Anti-TNF | 123 | 66.1 | 123 | 100.0 | – | – | |
| Adalimumab | 75 | 40.3 | 75 | 61.0 | – | – | |
| Etanercept | 34 | 18.3 | 34 | 27.6 | – | – | |
| Infliximab | 14 | 7.5 | 14 | 11.4 | – | – | |
| csDMARD | 63 | 33.9 | – | – | 63 | 100.0 | |
| Methotrexate | 34 | 15.6 | – | – | 34 | 54.0 | |
| Leflunomide | 29 | 18.3 | – | – | 29 | 46.0 | |
| Medical specialty | <0.001 | ||||||
| Reumatology | 124 | 77.0 | 92 | 74.8 | 52 | 83.9 | |
| Dermatology | 26 | 16.2 | 25 | 20.3 | 1 | 1.6 | |
| Medical clinics | 10 | 6.2 | 5 | 4.1 | 8 | 12.9 | |
| Without specialty | 1 | 0.6 | 1 | 0.8 | 1 | 1.6 | |
| CASPAR | |||||||
| Personal psoriasis | 139 | 95.9 | 110 | 96.5 | 51 | 94.4 | 0.518 |
| Family psoriasis | 9 | 6.2 | 5 | 4.4 | 5 | 9.3 | 0.212 |
| Nail dystrophy | 63 | 46 | 48 | 44.9 | 28 | 53.8 | 0.287 |
| Negative rheumatoid factor | 107 | 73.8 | 84 | 73.7 | 43 | 79.6 | 0.402 |
| Dactylitis | 79 | 54.5 | 60 | 52.6 | 30 | 55.6 | 0.723 |
| Bone neoformation | 44 | 30.3 | 33 | 29.0 | 21 | 38.9 | 0.198 |
| Impairment | |||||||
| Axial | 83 | 60.6 | 70 | 64.8 | 22 | 44.0 | 0.014 |
| Peripheral | 132 | 94.3 | 111 | 94.6 | 46 | 92.0 | 0.528 |
| Enthesitis | 46 | 50.0 | 41 | 52.3 | 10 | 37.0 | 0.147 |
| CRP mg/l | 6.0 (1.8–14.8) | 5.0 (1.5–13.5) | 7.7 (2.4–25.4) | 0.007 | |||
| >5 mg/l | 83 | 55.7 | 59 | 51.3 | 38 | 66.7 | 0.056 |
| ESR in 60 min | 14.0 (6.0–28.0) | 13.0 (5.0–25.0) | 20.0 (9.5–38.0) | 0.015 | |||
| >8/10 mg (woman/man) | 96 | 67.1 | 76 | 66.1 | 34 | 75.6 | 0.315 |
| Rheumatoid factor | 10.0 (7.7–10.0) | 10.0 (7.6–10.0) | 10.0 (8.0–11.0) | 0.277 | |||
| ALT | 20.0 (17.0–27.0) | 20.0 (17.0–27.0) | 21.0 (18.0–28.0) | 0.236 | |||
| AST | 21.0 (15.0–32.0) | 21.5 (15.0–32.0) | 22.5 (13.0–35.0) | 0.532 | |||
Italic = Median and interquartile range.
CASPAR: Criterion of Classification of Psoriatic Arthritis; csDMARD: Conventional synthetic disease-modifying antirheumatic drug; ESR: Erythrocyte sedimentation rate.
According to the Criterion of Classification of Psoriatic Arthritis (CASPAR), 95.9% of patients presented personal psoriasis, 6.2% presented familial psoriasis, 46.0% had nail dystrophy, 73.8% had negative rheumatoid factor, 54.5% has dactylitis and 30.3% had bone neoformation. In addition, 94.3% of patients have been described as presenting peripheral impairment and 60.6% presented axial impairment. Enthesitis was observed in 50.0% of patients (enthesitis data were available only in 57.1% of patients; Table 1).
It was observed that patients using anti-TNF agents presented a higher index of axial impairment than those using csDMARDs. The higher portion of medication requests came from rheumatologists’ physicians. The baseline characteristics of patients starting use of csDMARDs and anti-TNF agents are presented in Table 1. The main differences between the patients from the two groups were in the BMI value, the specialty of the physician responsible for the prescription and in the median values of the examinations of inflammatory activity (C-reactive protein and erythrocyte sedimentation rate).
At 6 and 12 months, individuals using anti-TNF agents presented higher persistence as compared with individuals under csDMARD therapy at 6 and 12 months (χ2: < 0.001; T-test: p < 0.001). Adalimumab and etanercept presented similar persistent patients’ proportions and persistence average times. Infliximab presented lower persistent patients’ proportion and persistence average time, but without statistical significance, as compared with adalimumab and etanercept (χ2: > 0.05, Bonferroni: > 0.05). Leflunomide and methotrexate had close proportions and persistence average time, without any statistical difference in the class of csDMARDs (χ2: p > 0.05; T-test: p > 0.05; Table 2).
Table 2. . Persistence in the use of anti-TNF and conventional synthetic disease-modifying antirheumatic drug medications in individuals with psoriatic arthritis.
| Medicine | % persistent 6 months | % persistent 12 months | Average in days 6 months (95% CI) | Average in days 12 months (95% CI) |
|---|---|---|---|---|
| Anti-TNF | 83.4 | 66.4 | 166.5 (159.6–173.3) | 297.3 (278.0–316.5) |
| Adalimumab | 86.5 | 69.7 | 170.4 (162.9–178.0) | 306.1 (282.4–329.9) |
| Etanercept | 84.4 | 69.0 | 168.5 (157.2–179.8) | 306.9 (274.6–339.1) |
| Infliximab | 64.7 | 43.8 | 140.4 (106.4–174.3) | 229.9 (156.9–303.0) |
| csDMARD | 50.8 | 35.6 | 135.5 (120.3–150.7) | 207.2 (171.7–242.7) |
| Leflunomide | 51.7 | 37.0 | 139.1 (116.5–161.7) | 209.9 (157.8–262.0) |
| Methotrexate | 50.0 | 34.4 | 132.4 (111.6–153.2) | 204.9 (155.6–254.2) |
csDMARD: Conventional synthetic disease-modifying antirheumatic drug.
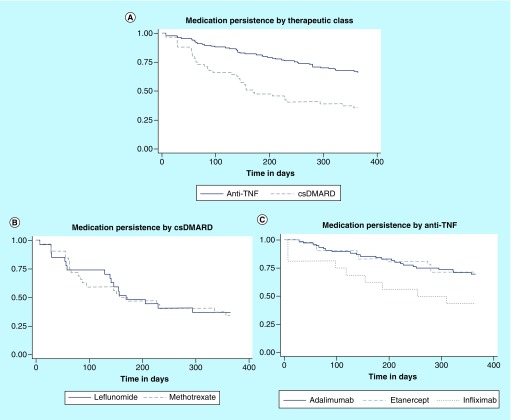
Another observation was that individuals making use of infliximab presented lower persistence as compared with those using adalimumab and etanercept after 6 months (log-rank: p = 0.054) and 12 months (log-rank: p = 0.056). However, this observed difference restricted itself to the limit of statistical significance. In the class of csDMARDs, statistically significant difference has also not been observed in the persistence values of both leflunomide and methotrexate after 6 months (log-rank: p = 0.882) and 12 months (log-rank: p = 0.841). Individuals using anti-TNF agents presented higher persistence to treatment when compared with individuals using csDMARD (log-rank: p < 0.001; Figure 2).
Figure 2. . Kaplan–Meier curves with the persistence in the treatment using anti-TNF and conventional synthetic disease-modifying antirheumatic drug.
(A) Medication persistence per therapeutic class. (B) Medication persistence per csDMARD used. (C) Medication persistence per anti-TNF used.
csDMARD: Conventional synthetic disease-modifying antirheumatic drug.
In the adjusted Cox model, the factors associated with medication non-persistence with anti-TNF agents after 6 and 12 months were the female sex and use of infliximab (Table 3). Factors associated with medication non-persistence have not been found with csDMARD medications after 6 and 12 months.
Table 3. . Persistence predictors in the use of anti-TNF agents after 6 and 12 months.
| Variables | Anti-TNF – 6 months | |||
|---|---|---|---|---|
| HR crude | p-value | HR adjusted | p-value | |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 2.08 (0.90–4.83) | 0.087 | 2.42 (1.03–5.69) | 0.043 |
| Anti-TNF | ||||
| Adalimumab | 1 | 1 | ||
| Etanercept | 1.16 (0.46–2.96) | 0.749 | 1.20 (0.48–3.07) | 0.749 |
| Infliximab | 3.08 (1.15–8.20) | 0.025 | 3.73 (1.38–10.11) | 0.010 |
| Age | ||||
| Until 50 years | 1 | – | – | |
| >50 years | 0.47 (0.22–1.10) | 0.086 | – | – |
| Variables | Anti-TNF – 12 months | |||
| HR crude | p-value | HR adjusted | p-value | |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 2.23 (1.20–4.15) | 0.011 | 2.65 (1.40–5.02) | 0.003 |
| Anti-TNF | ||||
| Adalimumab | 1 | 1 | ||
| Etanercept | 1.01 (0.52–2.01) | 0.96 | 1.07 (0.54–2.12) | 0.749 |
| Infliximab | 2.41 (1.11–5.21) | 0.025 | 3.25 (1.47–7.21) | 0.004 |
| CRP | ||||
| Until 5 mg/l | 1 | – | – | |
| >5 mg/l | 0.66 (0.36–1.21) | 0.177 | – | – |
| Rheumatoid factor | ||||
| Negative | 1 | – | – | |
| Positive | 2.64 (1.03–6.77) | 0.042 | – | – |
Only the variables with a p-value < 0.20 in the bivariate analysis were shown.
1 (reference category).
Bold: variables with significant results in the final model - multivariable analysis.
HR: Hazard ratio.
Discussion
This is a new study conducted in Brazil, whose purpose is to evaluate PsA patients treated with anti-TNF and csDMARD medications offered by means of the SUS in a real-life scenario. The respective results have demonstrated that medication persistence was higher for patients making use of anti-TNF agents as compared with those using csDMARDs, which could be partially explained by the fact that anti-TNF agents, currently, represent the last line of treatment for PsA at SUS [14]. Other different hypotheses point to the lower acquisition price of csDMARDs, which allows their purchase by the patients themselves by means of direct disbursement and, finally, the delay in disease diagnosis due to difficult access to a rheumatologist. In the latter case, treatment starts with the disease already in a more advanced stage, resulting in less satisfactory results in terms of effective disease control [15].
Both the use of infliximab and female sex were identified as factors associated with nonpersistence to treatment with anti-TNF agents and corroborate the results from other studies [16–19]. The lower persistence for women with PsA could be explained by the higher duration of disease symptoms, higher BMI, higher count of swollen and tender joints, higher disease activity score, higher polyarticular involvement, higher alteration in the inflammatory activity test examinations, higher fatigue intensity and higher functional impairment as compared with men [20,21].
An observational study of the British Society for Rheumatology has demonstrated that the main reasons for the higher discontinuation in the use of infliximab are the lower effectiveness and higher quantity of adverse events occurring with the use of this anti-TNF agent when compared with etanercept and adalimumab [18]. In this sense, the use of infliximab presents the higher potential of immunogenicity, with the development of anti-drug antibodies (ADA), as compared with the remaining anti-TNF agents. The anti-drug antibodies (ADA) are associated with decrease of clinical response and increasing incidence of reactions to infusion as well as to reactions at the application site [22,23]. Besides this, lower adhesion to treatment with infliximab has been demonstrated in other immunomediated diseases when compared with other anti-TNF agents [24], which could be explained by its higher potential for adverse reactions, mainly infusional ones and in the application site, and by its administration path [18,22–24]; patients with rheumatic diseases might present preference for subcutaneous administration instead of intravenously and for home administration instead of hospital [24]. However, this result should be interpreted with caution as infliximab has shown better persistence in particular subsets, such as in oligoarticular-PsA [26].
Differences in the persistence to treatment have not been observed between the csDMARD medications. A study has observed a persistence of 87.7% with leflunomide after 6 months in individuals with PsA who presented the main reasons for discontinuation to be the adverse events (51%) and effectiveness loss (33%) [27]. Asiri et al. have found a persistence of 64.7% after 1 year of follow-up of individuals with PsA using leflunomide [28]. Curtis et al., in a systematic review, observed a persistence of 50–94% for methotrexate in patients with rheumatoid arthritis [29]. Such results are higher to those observed in the PsA patients participating in this present study. It should be reinforced that few studies have been addressed to the action of csDMARDs in the PsA population.
Concerning anti-TNF agents, different studies have demonstrated that persistence could vary from 61 to 79% in the first year of follow-up in different countries [17,18,30,31]. Aaltonen et al. observed a persistence of 90% at 6 months and 80% at 12 months of follow-up using anti-TNF agents, without differences in the persistence between the first, second and third lines of treatment [16]. The persistence after 12 months, with the use of anti-TNF agents in these studies, was somewhat higher or similar to that verified in Brazilian individuals using anti-TNF agents and participating in the present study.
There was a mild predominance of individuals of female sex and who were overweight, which was also observed in a Canadian study [31]. The mean age was similar to that of other studies [16,32].
Adalimumab was the most requested anti-TNF agent, followed by etanercept. This anti-TNF utilization profile is consistent with the literature, where adalimumab has been the medication most commonly utilized in the treatment of PsA and other rheumatic diseases in Brazil [33–35]. This could be partially explained as adalimumab presents additional treatment indications, such as uveitis, and this is a characteristic that is not shared with etanercept. Besides this, in the SUS, there are no centers of assisted therapy for the infusion of medications, which explains the lower prescription of Infliximab.
Among the csDMARDs, the medications methotrexate and leflunomide were the most requested ones. According to the European League against Rheumatism (EULAR), methotrexate should be the csDMARD of choice in patients with PsA presenting relevant cutaneous involvement [7]. Additionally, methotrexate has been the csDMARD most utilized in association with anti-TNF agents in the treatment of PsA [17,35]. Leflunomide has also been demonstrated to be an effective alternative for the peripheral and cutaneous impairment at PsA [14]. The csDMARDs were less requested when compared with anti-TNF agents. This could be explained in part by the fact that csDMARDs are preferentially utilized in patients with peripheral impairment, due to low proven efficacy in the axial impairment [7,14].
With respect to disease classification by the Criterion of Classification of Psoriatic Arthritis (CASPAR), the occurrence was verified of a predominance of peripheral involvement, with personal psoriasis in the large majority of cases and predominance of negative rheumatoid factor, which corresponds to the findings of Taylor et al. [36].
The higher frequency of requests from rheumatologist physicians is in concordance with the PsA treatment protocol in Brazil. This protocol recommends PsA individuals are met by a team, in a specialized service, counting on a rheumatologist for the appropriate diagnosis, inclusion in the treatment and follow-up [14]. The prescription by different medical specialties (rheumatology, dermatology and general practice) could be explained by the fact that psoriasis diagnosis precedes the one of arthritis in 75% of cases [37]. Furthermore, the general practice is a medical residency that precedes the formation in rheumatology in Brazil.
Recommendations and guidelines highlight that a delayed diagnosis is a great challenge that needs to be overcome, as it negatively impacts the treatment result, and strategies promoting the early forwarding of patients and decreasing the delay in diagnosis and treatment of inflammatory arthritis have been proposed [38]. This is a problem that has been confronted in Brazil and the challenges faced are in the concentration of rheumatologist physicians in the larger cities. There are difficulties in the early forwarding of patients to a rheumatologist by the primary care physicians, mainly for patients from the countryside and small cities, as well as in the follow-up maintenance and treatment management of patients after that forwarding [39].
Another alternative to improve health outcomes is the implementation of pharmaceutical care in the SUS. Recent results demonstrate that this alternative is cost-effective and improves the quality of healthcare. [40,41]. Thus, SUS would be better able to provide an integral care, which is one of its basic premises. In this sense, complementary measures could be useful for the maximization of results and to qualify the use of medications; such measures, for instance, could include the improvement of access to rheumatologist physicians, the implementation of more efficient treatment management tools and the integration of a multidisciplinary team to follow-up PsA patients.
The use of administrative data possesses important limitations. One of them is that the objective of the records is for authorization/reimbursement/dispensation of medications from the health system and not for data collection for clinical–epidemiological studies. Therefore, this study does not reflect direct clinical effectiveness but provides a better understanding of the occurrence of nonpersistence in the PsA patients served at SUS. Another important limitation is the lack of data concerning the administrative processes, such as clinical measures, time of disease duration and mandatory examinations, which could give more information about the patients seen. The nonavailability of csDMARDs for dispensation in given periods could have negatively influenced the persistence of such medications.
Despite these limitations, the study presents potentialities. The csDMARD and anti-TNF agents were included at SUS in 2009 and were not the target of previous evaluations in the Brazilian context. Our results demonstrate the persistence in the use of these medications in a real life scenario, making it the first postincorporation study addressed to PsA in Brazil with this focus. In addition, the study has identified relevant aspects that effectively influence the treatment of PsA at SUS. The knowledge about the utilization of these medications in the Brazilian context is relevant, as the majority of publications reflect the reality of populations of other different countries. In this sense, it is observed the lower persistence to biological treatment by women, which requires a closer follow-up and an approach of the specific needs in this group of patients to improve the response to treatment and, consequently, the results in health. Finally, few studies have proposed to evaluate the use of csDMARDs within a real-life scenario. Thus, it is necessary to investigate if the persistence in the use of these medications has decreased over time, mainly in a context of innovation of medications and consequent increase in the number of alternative therapies for PsA.
Conclusion
The results demonstrate that anti-TNF agents are important therapeutic alternatives and present lower discontinuation as compared with csDMARDs after 6 and 12 months of follow-up. Adalimumab and etanercept presented similar persistence results in anti-TNF group and leflunomide and methotrexate in the csDMARDs group. Medication persistence was lower in Brazil than in other countries. Additional measures are important to improve outcomes in medication persistence such as increasing access to rheumatologists and implementing a pharmaceutical care strategy.
Future perspective
In Brazil, the Pharmaceutical Services is the main tool to get access to medicine in the SUS. However, anti-TNF and csDMARD drugs have not been previously evaluated for their efficacy, safety and cost-effectiveness for PsA in Brazil. In a time of economic recession, we need to establish a useful tool for decision-making and to identify policy options for containment and reordering of costs. In addition, as the volume, complexity and cost of new medical technologies increase, the need to assess benefits, risks and costs become increasingly important, both for new technologies and those already incorporated into the health system but not previously evaluated. In this context, the analysis of health performance is useful to subsidize the renegotiation of health prices and the optimization of the application of financial resources, with the purpose of contributing to the efficiency of public management and, consequently, to the pharmaceutical services and SUS.
Summary points.
The conventional synthetic disease-modifying antirheumatic drug (csDMARD) and TNF inhibitor (anti-TNF) agents were included at National Health System (SUS) in 2009 and were not subject to previous evaluations in the Brazilian context.
The medication persistence for psoriatic arthritis varies among different countries and data for developing countries were not found.
A total of 161 psoriatic arthritis patients were included in the present study and 186 treatments were evaluated.
The patients under anti-TNF treatment presented higher persistence as compared with patients under csDMARDs treatments at 6 and 12 months.
Adalimumab and etanercept presented similar persistence results in the anti-TNF group.
Leflunomide and methotrexate presented similar persistence results in the group of csDMARDs.
The factors associated with nonpersistence with anti-TNF agents were female sex and use of infliximab.
Factors associated with nonpersistence with csDMARDs were not found.
These results are very important because the the majority of publications reflect the reality of populations in other different countries.
Acknowledgments
The authors are grateful for the institutional support of the Research Group on Pharmacoepidemiology of UFMG, of the Department of Health of Minas Gerais State and the Ministry of Health, by means of Institutional Program of Development of the SUS (PROADI-SUS).
Footnotes
Financial & competing interests disclosure
This work was supported by National Council for Scientific and Technological Development (CNPq), Brazil (grant number 471819/2013-1) and FAPEMIG, the Minas Gerais State Research Foundation, Brazil (grant number PPM-0015-15 and 03799-16). MRRS and JBRS have received Ph.D scholarships from Coordination for the Improvement of Higher Education Personnel (CAPES), a foundation of the Ministry of Education in Brazil. AMK has received personal or institutional support from Abbvie, Janssen, Pfizer, Roche and UCB; has delivered speeches at scientific events sponsored by Janssen, Eli Lilly, Pfizer and UCB. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Author contributions
MRR da Silva collected data, analyzed the data and wrote the manuscript. JBR dos Santos collected data, analyzed the data, contributed with the discussion and reviewed the manuscript. AM Almeida, A Itria, JA Teodoro and F de Assis Acurcio designed the study, contributed with the discussion and reviewed the manuscript. AM Kakehasi contributed with the discussion and reviewed the manuscript. All authors approved the final version of the manuscript.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Carneiro S, Azevedo VF, Bonfiglioli R, et al. Comissão de Espondiloartrites da Sociedade Brasileira de Reumatologia. Recommendations for the management and treatment of psoriatic arthritis. Rev. Bras. Reumatol. 2013;53(3):227–241. [PubMed] [Google Scholar]
- 2.http://formsus.datasus.gov.br/novoimgarq/24326/4057388_345331.pdf Consenso Brasileiro De Psoríase 2012 - Guias de avaliação e tratamento Sociedade Brasileira de Dermatologia. – 2 ed. Rio de Janeiro: Sociedade Brasileira de Dermatologia, 2009. 172 p.; 1 ed.; 24cm ISBN 978-85-89240-04-8. Available in.
- 3.Barnas JL, Ritchlin CT. Etiology and pathogenesis of psoriatic arthritis. Rheum. Dis. Clin. North Am. 2015;41(4):643–663. doi: 10.1016/j.rdc.2015.07.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Boehncke WH, Qureshi A, Merola JF, et al. Diagnosing and treating psoriatic arthritis: an update. Br. J. Dermatol. 2014;170(4):772–786. doi: 10.1111/bjd.12748. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Olivieri I, D'Angelo S, Palazzi C, et al. Pharmacoeconomic issues in psoriatic arthritis. J. Rheumatol. Suppl. 2012;89:103–105. doi: 10.3899/jrheum.120258. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Gossec L, De Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann. Rheum. Dis. 2014;73:1012–1019. doi: 10.1136/annrheumdis-2014-205207. [DOI] [PubMed] [Google Scholar]
- 7.Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann. Rheum. Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; • The European League against Rheumatism (EULAR) recommendations for pharmacological treatment of psoriatic arthritis (PsA), including conventional synthetic disease-modifying antirheumatic drug (csDMARD) and TNF inhibitor (anti-TNF) drugs use.
- 8.da Silva MRR, Dos Santos JBR, Almeida AM, et al. Effectiveness and safety of anti-TNF in psoriatic arthritis patients in Brazil: a post-incorporation analysis. J. Comp. Eff. Res. 2018;7(10):989–1000. doi: 10.2217/cer-2018-0017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; •• The first paper with clinical results for anti-TNF agents in a Brazilian real-world perspective.
- 9.Brasil. 2018. http://conitec.gov.br/images/Artigos_Publicacoes/Diretrizes/DIRETRIZ_AdTS_final_ISBN.pdf Ministério da Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Departamento de Gestão e Incorporação de Tecnologias em Saúde. Comissão Nacional de Incorporação de Tecnologias no SUS – CONITEC. Diretrizes Metodológicas avaliação de desempenho de tecnologias em saúde - Desinvestimento e Reinvestimento. Ministério da Saúde, 2016. 23p.
- 10.Warren RB, Smith CH, Yiu ZZN, et al. Differential Drug Survival of Biologic Therapies for the Treatment of Psoriasis: a prospective observational cohort study from the british association of dermatologists biologic interventions register (BADBIR) J. Invest. Dermatol. 2015;135(11):2632–2640. doi: 10.1038/jid.2015.208. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Carrascosa JM, van Doorn MB, Lahfa M, et al. Clinical relevance of immunogenicity of biologics in psoriasis: implications for treatment strategies. J. Eur. Acad. Dermatol. Venereol. 2014;28(11):1424–1430. doi: 10.1111/jdv.12549. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Doval I, Carretero G, Vanaclocha F, et al. Risk of serious adverse events associated with biologic and nonbiologic psoriasis systemic therapy: patients ineligible vs eligible for randomized controlled trials. Arch. Dermatol. 2012;148(4):463–470. doi: 10.1001/archdermatol.2011.2768. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Neovius M, Arkema EV, Olsson H, et al. ARTIS Study Group. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann. Rheum. Dis. 2015;74(2):354–360. doi: 10.1136/annrheumdis-2013-204128. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brasil. 2018. http://conitec.gov.br/images/Relatorios/2017/Relatório_PCDT_Artrite_Psoríaca_Secretário_n°277.pdf Ministério da Saúde. Secretaria de Atenção a Saúde. Portaria Conjunta n° 6/2017 - Publicada em 19/07/2017. Aprova o Protocolo Clínico e Diretrizes Terapêuticas da Artrite Psoríaca.
- 15.Tillett W, Jadon D, Shaddick G, Cavill C, et al. Smoking and delay to diagnosis are associated with poorer functional outcome in psoriatic arthritis. Ann. Rheum. Dis. 2013;72(8):1358–1361. doi: 10.1136/annrheumdis-2012-202608. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Aaltonen K, Heinonen A, Joensuu J, et al. Effectiveness and drug survival of TNF-inhibitors in the treatment of psoriatic arthritis: a prospective cohort study. Semin. Arthritis Rheum. 2017;46(6):732–739. doi: 10.1016/j.semarthrit.2016.09.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; • A good paper that evaluated the effectiveness and drug survival of anti-TNF use in PsA patients in the Finland perspective.
- 17.Glintborg B, Østergaard M, Dreyer L, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum. 2011;63(2):382–390. doi: 10.1002/art.30117. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Saad AA, Ashcroft DM, Watson KD, et al. British Society for Rheumatology Biologics Register. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res. Ther. 2009;11(2):R52. doi: 10.1186/ar2670. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagerli KM, Kearsley-Fleet L, Watson KD, et al. Long-term persistence of TNF-inhibitor treatment in patients with psoriatic arthritis. Data from the British Society for Rheumatology Biologics Register. RMD Open. 2018;4(1):e000596. doi: 10.1136/rmdopen-2017-000596. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nas K, Capkin E, Dagli AZ, et al. Anatolian Group for the Assessment in Rheumatic Diseases (ANGARD) Gender specific differences in patients with psoriatic arthritis. Mod. Rheumatol. 2017;27(2):345–349. doi: 10.1080/14397595.2016.1193105. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Queiro R, Tejón P, Coto P, et al. Clinical differences between men and women with psoriatic arthritis: relevance of the analysis of genes and polymorphisms in the major histocompatibility complex region and of the age at onset of psoriasis. Clin. Dev. Immunol. 2013;2013(482691) doi: 10.1155/2013/482691. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martín-López M, Carmona L, Balsa A, et al. Serum drug levels of biologic agents in the management of rheumatoid arthritis and spondyloarthritis: a systematic review. Rheumatol. Int. 2018;38(6):975–983. doi: 10.1007/s00296-018-4022-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Thomas SS, Borazan N, Barroso N, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. BioDrugs. 2015;29(4):241–258. doi: 10.1007/s40259-015-0134-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; •• This review presents the impact of immunogenicity in the clinical response with anti-TNF agents and the difference of immunogenicity among ones.
- 24.Fidder HH, Singendonk MM, van der Have M, Oldenburg B, van Oijen MG. Low rates of adherence for tumor necrosis factor-α inhibitors in Crohn's disease and rheumatoid arthritis: results of a systematic review. World J. Gastroenterol. 2013;19(27):4344–4350. doi: 10.3748/wjg.v19.i27.4344. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important and helpful paper about the view and preferences of patients about treatment with biological agents.
- 25.Malaviya AP, Ostör AJ. Drug adherence to biologic DMARDS with a special emphasis on the benefits of subcutaneous abatacept. Patient Prefer. Adherence. 2012;6:589–596. doi: 10.2147/PPA.S23786. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iannone F, Lopriore S, Bucci R, et al. Two-year survival rates of anti-TNF-α therapy in psoriatic arthritis (PsA) patients with either polyarticular or oligoarticular PsA. Scand. J. Rheumatol. 2015;44(3):192–199. doi: 10.3109/03009742.2014.962081. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Behrens F, Finkenwirth C, Pavelka K, et al. Leflunomide in psoriatic arthritis: results from a large European prospective observational study. Arthritis Care Res. 2013;65(3):464–470. doi: 10.1002/acr.21848. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Asiri A, Thavaneswaran A, Kalman-Lamb G, Chandran V, Gladman DD. The effectiveness of leflunomide in psoriatic arthritis. Clin. Exp. Rheumatol. 2014;32(5):728–731. [PubMed] [Google Scholar]
- 29.Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J. Rheumatol. 2016;43(11):1997–2009. doi: 10.3899/jrheum.151212. [DOI] [PubMed] [Google Scholar]
- 30.Stober C, Ye W, Guruparan T, et al. Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology. 2017 doi: 10.1093/rheumatology/kex387. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Anghel LA, Farcaş AM, Oprean RN. Medication adherence and persistence in patients with autoimmune rheumatic diseases: a narrative review. Patient Prefer. Adherence. 2018;12:1151–1166. doi: 10.2147/PPA.S165101. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mease PJ, Collier DH, Saunders KC, et al. Comparative effectiveness of biologic monotherapy versus combination therapy for patients with psoriatic arthritis: results from the Corrona registry. RMD Open. 2015;1(1):e000181. doi: 10.1136/rmdopen-2015-000181. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acurcio FA, Machado MA, Moura CS, et al. Medication persistence of disease-modifying antirheumatic drugs and anti-tumor necrosis factor agents in a cohort of patients with rheumatoid arthritis in Brazil. Arthritis Care Res. 2016;68(10):1489–1496. doi: 10.1002/acr.22840. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Dos Santos JB, Almeida AM, Acurcio FA, et al. Comparative effectiveness of adalimumab and etanercept for rheumatoid arthritis in the Brazilian Public Health System. J. Comp. Eff. Res. 2016;5(6):539–549. doi: 10.2217/cer-2016-0027. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Oliveira Junior HA, dos Santos JB, Acurcio FA, et al. Poorer functionality is related to better quality of life response following the use of biological drugs: 6-month outcomes in a prospective cohort from the Public Health System (Sistema Único de Saúde), Minas Gerais, Brazil. Expert Rev. Pharmacoecon. Outcomes Res. 2015;15(3):403–412. doi: 10.1586/14737167.2015.1003367. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Taylor W, Gladman D, Helliwell P, et al. CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi: 10.1002/art.21972. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Antoni CE. Psoriatic arthritis: etiology and pathogenesis. In: Hochber MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology (4th Edition) Elsevier; Philadelphia, PA, USA: 2008. [Google Scholar]
- 38.Sørensen J, Hetland ML. All departments of rheumatology in Denmark. Diagnostic delay in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Ann. Rheum. Dis. 2015;74(3):e12. doi: 10.1136/annrheumdis-2013-204867. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva CR, Carvalho BG, Cordoni L, Júnior, Nunes EFPA. Difficulties in accessing services that are of medium complexity in small municipalities: a case study. Cien Saude Colet. 2017;22(4):1109–1120. doi: 10.1590/1413-81232017224.27002016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; • This paper shows the difficulties and particularities of health access in Brazil.
- 40.Babar ZU, Kousar R, Murtaza G, et al. Randomized controlled trials covering pharmaceutical care and medicines management: a systematic review of literature. Res. Social Adm. Pharm. 2018;14(6):521–539. doi: 10.1016/j.sapharm.2017.06.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Al-Quteimat OM, Amer AM. Evidence-based pharmaceutical care: the next chapter in pharmacy practice. Saudi Pharm. J. 2016;24(4):447–451. doi: 10.1016/j.jsps.2014.07.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]