Abstract
There has been a revolution in nanotechnology and nanomedicine. Since 1980, there has been a remarkable increase in approved nano-based pharmaceutical products. These novel nano-based systems can either be therapeutic agents themselves, or else act as vehicles to carry different active pharmaceutical agents into specific parts of the body. Currently marketed nanostructures include nanocrystals, liposomes and lipid nanoparticles, PEGylated polymeric nanodrugs, other polymers, protein-based nanoparticles and metal-based nanoparticles. A range of issues must be addressed in the development of these nanostructures. Ethics, market size, possibility of market failure, costs and commercial development, are some topics which are on the table to be discussed. After passing all the ethical and biological assessments, and satisfying the investors as to future profitability, only a handful of these nanoformulations, successfully obtained marketing approval. We survey the range of nanomedicines that have received regulatory approval and are marketed. We discuss ethics, costs, commercial development and possible market failure. We estimate the global nanomedicine market size and future growth. Our goal is to summarize the different approved nanoformulations on the market, and briefly cover the challenges and future outlook.
Keywords: : drug delivery, nanocrystal, nanodrug, nanoliposome, nanomedicine, nanopharmaceuticals, nanopolymer, nanotechnology, market size, regulatory approval
Nanomedicine
The term ‘nanotechnology’ first came to wide public prominence in a 1986 book by K. Eric Drexler entitled ‘Engines of creation: the coming era of nanotechnology’ [1]. The concept was based on an idea first propounded by Nobel laureate Richard Feynman in a presentation he gave in 1959 entitled ‘There's Plenty of Room at the Bottom’ [2]. The first proposed applications of nanotechnology involved chemical synthesis by the application of nanoscale machines, and information storage at the atomic level [3]. Since then, nanotechnology has been applied in diverse fields such as waste water treatment [4], textile industry [5], high performance batteries [6], biology [7] and medicine [8]. In medical applications, nanotechnology has led to significant improvements in cancer therapy [9], diagnostic imaging of diseases [10], tissue engineering [11] and most importantly in drug and gene delivery systems [12].
Today, the application of nanotechnology in biomedical sciences, and healthcare as a whole, has come to be called ‘nanomedicine’ and is considered a hot growth area of nanotechnology [13,14]. Over the past few decades, the US FDA has approved commercialization of 100 nanomedicine applications and products [15]. This shows that nanotechnology is playing an immense role in today's biomedical science [16,17]. Consequently, the US federal authorities have provided more than $1.4 billion funding for the National Nanotechnology Initiative, which confirms the importance of nanotechnology [18]. Nanotechnology has attracted a huge attention around the world. According to a recent report by Forbes, nanotechnology is one of the fifth biggest growth technologies to watch over the coming decade. As a result of this growing interest, a thorough review of the currently approved nanomedicines seems to be timely [19].
There have been many different articles and review papers, covering the future landscape of nanotechnology in medical applications [20]. Although about 100 nanomedicines have been approved by the FDA [15], there has not been sufficient description of the current market trends in this field. Herein, the most recent nanomedicine agents and the market trend along with the major challenges of conventional drug-delivery systems (DDSs) are addressed. The most important advantages and disadvantages of nanotechnology in pharmaceutical applications, the common nanocarriers, which are used for medical applications, the already commercialized nanomedicines, and the global market size of nanopharmaceuticals are reviewed. Finally, the current market trends, and the challenges on the way of nanomedicines to enter to the market are briefly discussed.
Nanotechnology plays a significant role in the field of medicine and drug delivery, mainly due to the major limitations and problems that affected conventional pharmaceutical agents, and older formulations and delivery systems. One of the important problems of conventional DDSs is the difficulty in removing the residual parts of such systems, thus leaving nonbiodegradable material within the patient's body, which can cause toxicity [21]. Likewise, most of the conventional DDSs have a high initial burst of drug release immediately after drug administration, and moreover the drug solubility in conventional DDSs tends to be low [12]. In here, nanopharmaceuticals can be promising solutions for the above-mentioned problems [22]. In comparison with conventional DDSs, drug administration using nanoparticles (NPs) has several advantages, which are listed below:
NPs are much smaller than the basic material unit of conventionally formulated drugs. Attaching small-molecule therapeutic agents to these small nanocarriers will form overall nanodrugs [23].
Nanoformulation of drugs is one strategy to deliver pharmaceutical agents more precisely to the targeted tissue and reduce the overall dose and potentially toxic side effects [24].
The enhanced permeability and retention (EPR) effect can allow passive targeting and accumulation of nanosized drugs at malignant tumors and other pathological sites [23].
Nanosized formulations, in comparison with conventional microsized formulations, lead to an increased active concentration and bioavailability [25].
NPs demonstrate better safety and efficacy [26].
Nanodrugs can be far cheaper than conventional therapies [27].
Drug release can occur at a constant rate over the desired timescale [28].
On the other hand, there are various challenges on the way of using NPs in DDSs. One of the most important goals in pharmaceutical researches, is the synthesis or discovery of new chemical entities with minimum side effects and maximum clinical benefit. To improve the biological system interactions, and reducing the nonspecific toxicities (particularly against the brain), drug targeting using NPs offer several advantages [29]. Furthermore, the development of nanocarriers based on biocompatible and biodegradable polymers has led to a wider application of NPs in DDSs [30]. Another important challenge facing drug delivery using NPs is the difficulty in their synthesis using conventional physicochemical methods. Moreover, according to a number of reports, the chemical synthesis of NPs can be relatively expensive. Potentially toxic reagents might be needed for the chemical synthesis, which can have possible environmental problems and requirements for safe disposal. Furthermore, the path from laboratory to the marketplace in nanoscience has an intrinsically high level of risk, due to the fragility, instability and reactivity of nanoscale materials [31]. Additionally, nanomaterials are more complex to engineer in comparison with bulk materials, so their production and storage could be more complicated and expensive [31].
Efficiently in reaching to the target sites (target tissues which are usually far away from the administration site), controlled delivery of the drugs within a predictable and limited timescale, controlled matrix or polymer degradation, the possibility for simultaneous detection and imaging (theranostics), design of smart (stimulus responsive) drug-release systems, are additional major challenges of NPs in the drug-delivery domain [32].
According to Lipinski's rule of five, drug-like molecules tend to be water insoluble, and have a moderately lipophilic character [33]. Therefore, poor drug solubility is considered as a major challenge facing conventional DDSs. By producing particles in the nanometer scale, the surface to volume ratio is increased and consequently the dissolution rate of the drug increases, and higher bioavailability is obtained [34]. Nowadays, researchers have applied different types of nanomaterials to develop improved, efficient and biocompatible therapeutic carriers. Some common nanocarriers and their applications are summarized in Table 1.
Table 1. . Different types of nanocarriers and their main advantages and applications in biomedical fields.
| Type | Description | Advantages | Applications |
|---|---|---|---|
| Nanocrystals | Crystalline particles produced by different methods such as pearl milling, high pressure homogenization (HPH), precipitation process [38] | • Improving the dissolution rate of drugs • Improving the saturation solubility • Safe composition • Suitable for intravenous injection • High drug bioavailability |
• Cancer treatment [39] • Controlling the level of triglyceride and cholesterol [38] • Hyperthermic chemotherapy [40] |
| Polymeric NPs | Can be prepared as nanospheres or nanocapsules by different methods such as nanoprecipitation, double emulsification, polymer coating and emulsification diffusion [41] | • Can be administered by infusion, different types of injection or oral ingestion • Tunable characteristics • Able to carry multifunctional agents • Improved thermodynamic stability of cargo • Deep penetration to cells and tissues |
• Drug delivery [42] • Gene delivery [43] • Tissue engineering [44,45] |
| Liposomes | Synthetic vesicles formed from lipid bilayers, which are divided into two groups: unilamellar and multilamellar able to dissolve both water-soluble (interior) and lipid soluble drugs (bilayer) at the same time [46] | • Passive targeting of drugs • Highly efficient cargo delivery • Reducing the cargo toxicity |
• Delivery of various biomolecules such as: enzymes [47], hormones [48,49], anti-sense oligonucleotides [50], ribozymes [51], proteins/peptides [52], DNA [53] and anticancer drugs [54] |
| Magnetic NPs | These types of nanosystems can respond to external magnetic fields in a nanoscale size [55] | • Accumulation at desired sites via delivery guidance using a magnetic field • A promising choice for MRI application |
• Surface functionalization [56] • Use as a contrast agent [57] • Gene delivery [58] |
| Micelles | Spherical amphiphilic copolymer NPs formed by supramolecular assembly, having a core-shell structure with hydrophobic interior separated from the aqueous exterior [59] |
• High loading capacity • Good stability in blood • Prolonged circulation time • Low number of side effects • Protects internal drugs from degradation |
• Carrying various water insoluble drugs including: paclitaxel [60], SN-38 [61], doxorubicin [62], C6-ceramide [63] |
| Dendrimers | Synthetic tree-shaped macromolecules having a 3D monodisperse structure with branches extended from a central molecule; predictable size according to generation number [64] | • Defined MW • Uniform in shape • Host-guest entrapment properties • Extremely low polydispersity |
• Carrying various drugs including: piroxicam [65], paclitaxel [66], ketoprofen [67], methotrexate [68] |
| Mesoporous silica NPs | Mesoporous form of silica particles in nanoscale sizes with a large surface area and a solid structure [69] | • High loading capacity • Good protection ability by keeping cargo molecules inside pores • Controlled drug-release ability |
• Drug and gene delivery [70] • Bio-sensing [71] • Target specific delivery [72] • Diagnostic agent [73] • Antidote agents [74,75] |
| Carbon nanotubes | Needle-like carriers, which can easily diffuse into cell membranes by perforation. CNTs are categorized as SWCNTs which have a high near infrared optical absorbance and MWCNTs having unique physical properties such as young modulus and electrical conductivity [76] |
• Very high elastic modulus and mechanical strength • High electrical and thermal conductivity • Prolonged circulating time • Cell membrane permeability • High aspect ratio allowing high drug loading |
• Use in scaffolds for supporting bone cell growth [77] • Chemo-photothermal therapy [78] • Vaccine delivery [79] • Cancer treatment [80] • Brain glioma therapy [81] • Spinal cord injury repair [82] |
| Inorganic NPs | |||
| AuNPs | First synthesized over 150 years ago; wide use in drug delivery applications [83] | • Low cytotoxicity • Controlled size and surface • Easy synthesis • High cell permeability • Ability to bind many molecules on their surface • High drug loading capacity |
• Drug delivery [84] • Diagnosis [85] • Treatment of various diseases including: cancer [86], Alzheimer [87], diabetes [88], arthritis [89], heart failure [90] |
| QD | Semiconductor crystals with a nearly spherical structure; metalloid crystalline semiconductor that controls fluorescence emission [91] | • Small size • Good intracellular uptake and drug release • Easy surface modifications |
• Biological optical detection [92] • Cellular and intracellular targeting [93] |
AuNP: Gold NP; CNT: Carbon nanotube; MW: Molecular weight; MWCNT: Multi-walled carbon nanotube; NP: Nanoparticle; SWCNT: Single-walled carbon nanotube; QD: Quantum dot.
Recently, construction of ‘smart’ or stimulus-responsive delivery vehicles capable of responding to specific internal or external, physical or chemical stimuli was of a new domain in nanocarrier science [12,32,35]. Among possible physical stimuli, light and temperature are of great importance [26,35], while similarly, changes in pH, concentration of reducing agent or ionic strength are examples of chemical stimuli which have been applied in the design of smart DDSs [28,30]. Moreover, sophisticated passive or active targeting strategies can also be used to carry different drugs into specific targets. Different ligands or targeting agents can be attached to the nanocarrier surface, in order to direct it toward specific cells or tissues based on molecular recognition strategies [29]. The active targeting strategy has been widely applied for cancer therapy, where the targeting moieties allow the drug carrier to bind to specific biomarkers which are overexpressed on tumors. On the other hand, in passive targeting, the important parameter is the nanocarrier sizes, which leads to accumulation at tumor sites due to the characteristic leaky vasculature and absence of lymphatic drainage (EPR effect) [29].
Based on the carrier type, cargo and biological target, researchers can take the advantages of either or both of these two strategies. In fact, nowadays, drug delivery, especially delivery of chemotherapeutic drugs, is considered as the main application of nanocarriers. Smart nanocarriers that sense and respond to acidic pH and higher temperature at tumor sites, or targeted agents that recognize tumor-specific biomarkers, while at the same time effectively masking the hydrophobic drug cargos, makes them an ideal choice in tumor targeting [36,37].
Development of nanopharmaceuticals
Currently the time scale for a drug to enter the market after its initial discovery/development, may take up to two decades [94]. Among other considerations, there should be sufficient skilled scientific and medical personnel willing to spend a decade or two of their lives on one single project; the fundamental scientific premise should be novel with appropriate intellectual property protection, and the economic business plan needs to convince the investors about future profits. Market needs should be properly evaluated, and profit/risk ratios should be high enough. The distribution and shelf-life of the therapeutic agents need to be clarified as well [95]. Some key steps along the way, including intellectual property, technical issues, general costs and also the ethics and regulatory affairs of the matter are briefly discussed in the following sections. In order to decrease the risk of failure, it is important to exploit expert market evaluation to assess the market needs and opportunities [96].
From laboratory to market
During all stages of drug discovery and commercialization process, it is important to file patents to protect the intellectual properties of inventors and companies, and also save money and time from being wasted in lawsuits or even losing legal cases. Nevertheless, considering the long time it takes to gain regulatory approval of a drug, passing the required clinical trials, and finally introducing a new pharmaceutical to the market, the allowed 20-year period (depending on the country) of patent protection, diminishes the period of commercial exclusivity to 12 years or less. Therefore, the time available to make profits for the company is sometimes too short to risk the necessary resources [97]. This issue needs to be addressed by the authorities, especially taking into account the additional complications of nanopharmaceuticals. Considering ethical issues, and also the economic impact of the clinical trials on the total cost of developing nanopharmaceuticals, the issue of preclinical in vivo tests on animals and human clinical trials, the period of market exclusivity should be extended.
Techniques
Larger companies are better equipped to take a drug from lab bench to marketplace. Scientists conduct the research, engineers devise the manufacturing process, lawyers take the responsibility for filing and defending patents, and funding is provided by shareholders and existing profit streams from already marketed drugs. Furthermore, the research carried out on nanomaterials may only take a small share of the entire investments of these companies, and the risk for loss is corresponding lower. By contrast, in small start-up companies, the budget for the whole process mentioned above might be limited and the inadequate resources may prohibit these smaller companies from undertaking costly nanopharmaceutical projects, forcing them to seek other projects and opportunities. These start-up companies are often associated with academic laboratories, where the initial stages of the research are funded by government grants, so the overall costs can be lower [98]. However big-pharma companies can also take the advantage of government funding especially for ‘orphan diseases’ [99,100]. In some cases, large companies acquire these smaller companies which are developing new nanoformulations, thus allowing these scientists the funding for their next projects in exchange of making profits in a shorter time [101]. Unlike other fields, it may not be possible for the nanopharmaceutical industry to lower quality in order to save costs; substantial money and time needs to be spent to provide appropriate quality assurance in the pharmaceutical market.
Costs
The costs over the lifetime of any new drug (including nanopharmaceuticals) consist of the time and money of the original idea and preclinical research (sometimes carried out in academia and funded by government and tax payers), the industrial development, the ‘valley of death’ which includes the period of highest expenses for human clinical trials, and finally getting regulatory approval, and the phase of commercialization and marketing [99]. In a recent study carried out by Tufts Center for the study of drug development, it was reported that getting all the way to the profitable phase (excluding advertising and special marketing expenses) needs about 2870 million US$ (2013 US$) [102]. This includes $1,395 million for development of an approved product of which the clinical trials phase costs about $1012–1744 million. Taking into account the long time it takes to market a product, the relevant patents that could expire and inflation and discount rates makes it equivalent to $2558 million [102]. Post-marketing research costs to improve the drugs can be combined with the costs for the next pharmaceutical in the pipeline. It is important to remember that the high costs include those for projects that are abandoned at any stage of the process. According to The Washington Post, the group that published this report is partially funded by drug companies, therefore the estimates may be seen to be biased, and may have been multiplied many times in order to justify the elevated drug prices to the consuming public [103]. Despite the fact that 80–90% of pharmaceuticals eventually fail to obtain final approval after clinical trials (with an exact assessment of 11.8% success rate) [104,105], this rate can vary from drug to drug, and the pharmaceutical classes chosen for this study were not mentioned, therefore so the conclusion could be faulty. Tax relief, subsidies, governmental and externally-funded research funds are also omitted in these estimates. The high project failure rate is a key factor for justifying the increasing costs of developing any new pharmaceutical. This is especially relevant to the added complexity of novel nanopharmaceuticals. During the process of developing new nanopharmaceuticals, it is important to remember the legal, ethical and cost-management procedures mentioned above. Although the marketing of approved nanopharmaceuticals, considering their higher efficiency, could lead to enormous profits, however the high failure rate has deterred some large companies from investing in this area, and opportunities are still available for researchers and entrepreneurs to seize upon.
Ethics
The ethics of nanopharmaceuticals go hand-in-hand with the regulatory frameworks. The safety profile and potentially toxic effects of these materials (both to patients, manufacturing personnel and to the wider environment), which in many cases are not fully understood, making matters worse [106]. The important factor is the evaluation of the risk/benefit ratio [107]. The evaluation of this ratio itself, has its own issues, since there is no framework or clear guidelines for the assessment. Thus, decisions are necessarily based on unclear foundations and difficult-to-calculate risks for each specific case and for each type of patient [108]. Since most human clinical trials (especially for cancer) are carried out on patients with advanced disease (and usually no other alternative therapy), these trials may have no beneficial effects (and may even be harmful) to the patient [109]. Therefore it is important for the trial conductors to fully explain the procedures (informed consent) and not to raise the hopes of the patient unjustifiably [110]. Despite some stakeholders are ambivalent about mentioning the word ‘nano’, it is crucial for the sake of patients’ confidence to inform them about the presence of NPs in the treatment, even if the likelihood of signing the consent forms is put in jeopardy [111]. Nevertheless the overall ethical concepts for these nanopharmaceutical trials remain the same as any other new therapy to be clinically tested [112]. It is the obligation of the regulatory agencies to assess the benefit/risk ratio, but since the patients finally choose whether to take the risk or not [113], it is recommended that data safety monitoring committees, stakeholders and experts outside the government also advise these agencies [114]. Care must be taken not to give in to public pressure that is overcautious (and can even be positively misleading) about the possible dangers of nanopharmaceuticals, and prohibit the development of these agents altogether [115]. It not just the safety of the patients themselves that matters, but also the safety of anyone in contact with the nanomaterial such as the workers and family members should be evaluated and ethically addressed [116].
Regulations for approving nanopharmaceuticals (FDA rules & regulations)
Until recently, the FDA had not published any specific guidance document for nanopharmaceuticals (or the category of nanomaterials in general). However in August 2016, a document addressing general regulations for all nanomaterial products related to cosmetics, food ingredients and animal feedstuff was issued [117]. Since the FDA is not yet entirely convinced that nanopharmaceuticals behave very differently from other small molecule drugs, (except for some particular characteristics), definite guidance has not yet been issued [118]. On the contrary, scientists believe that nanosized materials are not only different from the same bulk material in size and surface area, but particularly in the case of therapeutics, they also differ in biodistribution, toxicity, pharmacokinetics and excretion profiles [119]. One of the problems for nanopharmaceuticals is the fact that, since such materials behave differently in various environments (especially in vivo), current testing methods are not able to give clear answers to the above questions. This makes it difficult for companies, which are required to fully test their novel pharmaceuticals before applying for approval. Each pharmaceutical agent should be evaluated for its ultimate application. For instance, an injectable drug should be tested in the bloodstream or a tablet should be evaluated in appropriate solutions to replicate the digestive tract and so forth. The FDA system for categorizing medical products is another issue. The FDA categorizes its regulations applying to drugs, medical devices, blood products, biologic agents and other groupings [94,117]. The guideline applied for categorization of combination materials relies on the ‘primary mode of action’. For instance, if a prosthetic bone cement is equipped with nanomaterials or nanotherapeutics, this material falls into the categories of both devices and drugs. Sometimes the primary mode of action is not entirely obvious and the chosen product then falls into the hands of the Office of Combination Products. Safety assessments are not only concerned with the health risk of nanopharmaceuticals, but also with the effects of nanomaterials on the environment. In one study, it was concluded that the nanomaterial interactions are the important issue, and these evaluations are not valid with the ‘pristine form’ of the nanomaterial [120]. In this regard, the US National Research Council has decided to work on a new system for environmental risk assessment, mainly based on the ‘critical elements of nanomaterial interactions’, addressing the issue of potential environmental risks and public hazards of nanomaterials [121]. As mentioned earlier, the process of marketing a new pharmaceutical takes about 10–20 years [94,99], but it should be noted that, even today, nanomaterial science is still a young field. Long term exposure effects on humans, animals and the environment have yet not been completely evaluated, and it is required that even after FDA approval, the post-market monitoring should take place [122]. Another question is concerned with ‘nonbiological complex drugs’ [123]; these are complex structures often containing nanomaterials, as well as nanosimilars [124]. It is also recommended that the FDA and similar continue to improve the regulatory frameworks for these materials [125].
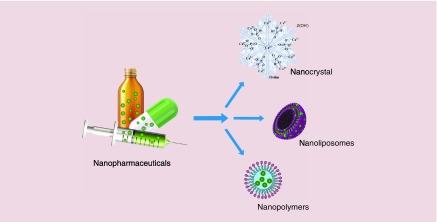
Currently a variety of nano-based pharmaceuticals have successfully entered to the market, and are utilized every day by many patients (Figure 1). These products come from various companies all around the world, and indicate the present and (likely) future success of nanomaterials as therapeutic agents. Some of the most known nanopharmaceuticals are discussed below according to the type of nanoformulation. These groups are: nanocrystals, liposomes and lipid-based, polymeric (including pegylated biologics, gels and emulsions), protein-based and metallic NPs.
Figure 1. . Schematic illustration of three types of nanopharmaceuticals available in the market.
Nanocrystals
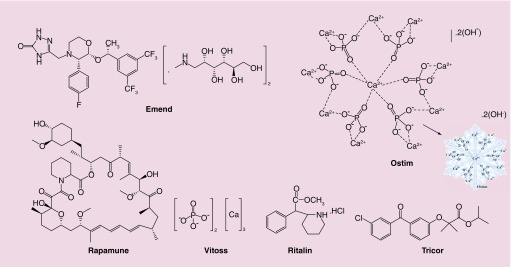
Nanocrystals are defined as crystals having at least one dimension less than 100 nm. Nanocrystalline pharmaceuticals that are currently available in the market are discussed here (Figure 2). Each product is described with its history of commercialization and mechanism of action.
Figure 2. . Chemical structures of approved nanopharmaceuticals within the nanocrystals category.
Emend®
Emend® (Merck & Co., Inc., NJ, USA) is the nanocrystalline form of the anti-emetic drug aprepitant (Figure 2) approved by FDA in 2003. It is administrated in prevention of nausea and vomiting during cancer chemotherapy, especially courses containing high dose cis-platin, known to be highly emetogenic; which means it induces vomiting and nausea in patients. Aprepitant is a specific antagonist for the human substance-P-ligand showing a high affinity to bind to neurokinin-1 (NK1) receptors in the area postrema known as the ‘vomiting center of the brain’ [126,127]. The advantage of aprepitant over other NK1 antagonists is that it has tiny or no affinity for similar receptors for serotonin (5-HT3), corticosteroids or dopamine receptors [127,128]. Thus, there will be no interference with the action of other pharmaceutical agents which target these related receptors. Moreover, the absorption of aprepitant occurs in the upper gastrointestinal (GI) tract [129], and aprepitant is not soluble in water [127]. By applying a technology called ‘Elan Drug Delivery Nanocrystals®’, these problems can be solved. Oral Emend possesses better water solubility and thus a higher possibility of absorption in the upper GI and hence higher bioavailability [129]. In 2008, the FDA approved the iv. injectable form of Emend. The active ingredient of this formula is fosaprepitant dimeglumine (Figure 2), a prodrug of aprepitant. Fosaprepitant is water-soluble and can be converted to aprepitant within 30 min after iv. administration [127,130].
Ostim®
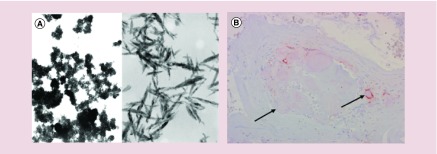
Ostim® (Osartis GmbH & Co. KG, Dieburg, Germany) is a nanocrystalline paste of calcium hydroxyapatite (HA) [Ca10(PO4)6(OH)2] (Figure 2) with crystals of 20 nm diameter approved by the FDA through the 510(k) process in 2004 (received CE approval in 2002). The structure of HA resembles natural bone minerals with the ability for osteoconduction. Osteoconduction is the ability of bone to grow on a surface, thereby providing Ostim with good biocompatibility [131]. As a bone-grafting material, Ostim can be used in orthopedic and dental surgery procedures. The process of commercially preparation includes rapid precipitations to produce a paste containing 25% water with dispersed nano-sized needle shaped HA crystals [131–133]. Figure 3, shows the formation of needle-shaped crystals.
Figure 3. . Ostim is a nanocrystalline paste of calcium hydroxyapatite.
(A) Transmission electron microscopy image of Ostim® nanocrystal (150,000×) reproduced with permission from [132] © BMC (2006) licensed with CC BY 2.0. (B) Macromorphology of Ostim, arrows showing a very little number of macrophage cells near to the particles (hydroxyapatite paste particles). Reproduced with permission from [132] © BMC (2006) licensed with CC BY 2.0.
Rapamune®
Rapamune® (Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc., PA, USA) with the generic name sirolimus (rapamycin) is known as the first nanocrystalline-type drug with FDA approval in 2010 [134]. It is used in order to prevent rejection of kidney transplants. The active ingredient of Rapamune, sirolimus, is a macrocyclic triene antibiotic derived from the bacteria Streptomyces hygroscopicus and functions as an immunosuppressant. Using the Elan Drug Delivery Nanocrystals technology involving bead/pearl milling, the release profile of the poorly soluble drug increases significantly, allowing better bioavailability [135,136]. Although, extended release is not absolutely critical for the case of Rapamune, nanoformulation leads to more convenient storage and allows oral administration [137]. Rapamune blocks T-lymphocyte proliferation induced by stimuli operating via either Ca2+-dependent or Ca2+-independent pathways [138] resulting in a weakened cellular immune system able to accept the transplanted organ. In 2015, FDA approved the use of Rapamune for the treatment of lymphangioleiomyomatosis, a rare progressive lung disease.
Vitoss®
Vitoss® (Orthovita, Inc. PA, USA), is a bestselling synthetic bone graft substitute composed of 100 nm β-tricalcium phosphate (β-TCP, Ca3[PO4]2) nanocrystals (Figure 2) approved by the FDA in 2003 through 510(k) pathway; an approval mechanism for medical devices [139]. Vitoss is able to mimic the structure of cancellous bone (also known as spongy or trabecular bone) and can fill voids or gaps in the skeletal system. Vitoss is synthesized by a high-temperature calcination process and contains porous granules of 1–4 mm diameter [133,140]. Another formulation, Vitoss Bioactive, includes conventional Vitoss combined with a bioactive agent to enhance bone remodeling speed [141]. Stryker Company (which acquired Orthovita, Inc. in 2011) also launched Vitoss BBTrauma in 2012 which consists of bioactive glass particles with a higher surface area [142].
Ritalin®
Ritalin® (Novartis, Switzerland, Basel), also known as methylphenidate (nanocrystals; Figure 2), received its FDA approval in 1955 for treatment of hyperactivity disorders in children [143,144]. The drug is mainly used to treat attention deficit hyperactivity disorder (ADHD) [145]. As a result of more widely accepted diagnoses of ADHD, the drug has been increasingly prescribed since the 1990s [146]. In 2007, general practitioners in England prescribed this drug for about 420,000 persons, and by 2012, this number had jumped to 657,000, which means a rise of 56 percent [147]. Conditions such as ADHD are supposedly linked to the dysregulation of dopamine and norepinephrine pathways in the brain, and the mechanism of action of Ritalin primarily involves inhibition of dopamine reuptake [148,149]. By preventing the reuptake of dopamine and norepinephrine their concentration increases within the synaptic cleft. Consequently, there will be an increase in cognitive and executive functions of the brain [150,151]. In addition, it can increase overall alertness of the central nervous system; resulting in short term benefits as a cost effective therapy [152,153]. As found with the majority of drugs, Ritalin also displays side effects; the most important ones are irritability, anxiety, disturbance in motor function and poor appetite [154].
TriCor®
TriCor® (Abbott Laboratories; generic name is fenofibrate, IL, USA) was approved in 2004 in order to reduce triglyceride and cholesterol levels to prevent the development of atherosclerosis; by reducing plaques on the inner wall of arteries which can lead to strokes and heart attacks. After oral administration, fenofibrate is metabolized to fenofibric acid in the intestine [155]. Fenofibric acid can lead to activation of peroxisome proliferator activated receptor α (PPAR-α). When lipoprotein lipase is activated, the production of apolipoprotein C-III (an inhibitor of lipoprotein lipase activity) decreases and lipolysis of triglyceride-rich molecules increases. This process finally reduces circulating levels of total cholesterol and triglycerides and triglyceride-rich, low-density lipoprotein, very low density lipoprotein and apolipoprotein B. It also increases high-density lipoprotein and apolipoproteins apoAI and apoAII [156]. Using Elan drug delivery nanocrystals technology, water-insoluble fenofibrate becomes more water-soluble in TriCor. Before this technology, fenofibrate required to be taken with food, and passed unchanged through the pH changes in the GI tract; because it lacks ionizable groups. The reason fenofibrate should be taken-with-food is due to the presence of surfactants and lipids in food which can emulsify it [129]. The higher water solubility of TriCor allows this drug to be taken with or without food [157].
Liposome & lipid-based nanopharmaceuticals
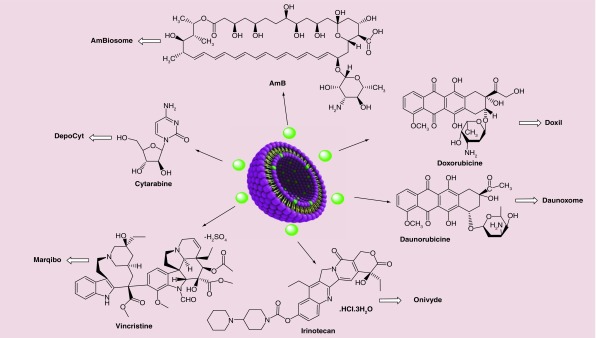
Liposomal nanoformulated drugs are recognized to be some of the most successful commercial DDSs that have been developed to overcome the side effects of many conventional drugs. For example, the liposomal formulation of doxorubicin (known by the commercial name of Doxil®) has made a great impact on the treatment of cancer, and huge benefits for pharmaceutical companies. In this section, liposome and lipid-based nanopharmaceuticals as depicted in Figure 4 are described in regard to their formula and mechanism of action.
Figure 4. . Approved nanopharmaceuticals with liposomal formulations.
Doxil® (Liposomal Doxorubicin)
Doxil (Alza, Pakistan) also known as Caelyx®, Evacet® and Lipodox®, was approved by the FDA in 1995. It is a type of nanodrug used for treatment of different cancers ranging from metastatic ovarian cancer to AIDS-related Kaposi's Sarcoma (KS) [158–160]. It is a specific formulation of doxorubicin (adriamycin) enclosed in unilamellar liposomes, the outside of which are covered with PEG (polyethylene glycol) known as ‘PEGylated liposomes’. The size of these structures varies between 80 and 90 nm. The goal of these DDS is to increase the circulation half-life leading to an enhancement in drug bioavailability [161,162]. An Indian pharmaceutical company called Sun Pharma Global FZE was the first company to manufacture the first-generation injectable Doxil from doxorubicin hydrochloride incorporated in liposomes (Figure 4) which received FDA approval in 2013 [161]. Doxil has two different mechanisms of action: intercalation into DNA molecule which causing disruption of topoisomerase and DNA repair and, intracellular production of reactive oxygen species and free radicals that damage cellular membranes by lipid peroxidation [163]. Both Doxil and Lipodox, the second-generation PEGylated liposomal doxorubicin [160], operate by a passive targeting strategy to accumulate into tumors by the EPR effect [164]. The other main advantage of liposomal doxorubicin is reducing the adverse effects of the drug, which can be toxic to several parts of the body especially the skin and the heart.
DaunoXome®
Liposomal daunorubicin (Figure 4), trade name DaunoXome® (Galen, Craigavon, UK), was approved by the FDA in 1996, and is another anthracycline anticancer drug which can be used in cancers and HIV-associated Kaposi's Sarcoma (KS) as a chemotherapy drug [159]. Moreover, there have been various clinical trials which demonstrated the applicability and efficacy of daunorubicin for different types of leukemia [165]. DaunoXome received its approval as a first-line cytotoxic therapy in advanced KS because of its effectiveness and lower side effects compared with alternative cytotoxic drugs such as adriamycin, bleomycin and vincristine [166]. The liposomes have an approximate diameter of 45 nm and consist of lipid bilayers composed of cholesterol and distearoyl phosphatidylcholine and at a 1:2 molar ratio [167]. The lipid to drug weight ratio in DaunoXome is 18.7:1 (lipid:daunorubicin). Although, the exact mechanism of DaunoXome selectivity is not clear, it is supposedly as the result of increased permeability of tumor neovasculature for particles with a similar size to DaunoXome (EPR effect). According to preclinical studies, in comparison with free daunorubicin, it was shown that DaunoXome could increase the concentration of daunorubicin in tumors while reducing drug in the brain, liver, spleen and intestine. On the other hand, myelosuppression, which can cause fever, nausea and vomiting, are the primary toxic effects of DaunoXome [168,169].
Onivyde® (irinotecan liposome injection)
Onivyde® (Merrimack Pharmaceuticals, MA, USA), also known as MM-398 or PEP02, is a liposomal nanoformulation of irinotecan (Figure 4) which was approved by the FDA in 2015 for treatment of metastatic pancreatic cancer [170,171]. It was also demonstrated that liposomal irinotecan could show synergistic effects with other anticancer agents. FOLFIRINOX is a chemotherapeutic regimen used for advanced pancreatic cancer consisting of (irinotecan, 5-fluorouracil, oxaliplatin and folinic acid), which showed a survival improvement in patients compared with gemcitabine administration [172,173]. The novel nanoliposomal formulation of irinotecan provides additional advantages, such as improvements in circulation time and drug biodistribution, passive targeting of tumors and better accumulation at tumor sites via the EPR effect, while reducing undesired side effects. However, there are still some negative effects of Onivyde including diarrhea, vomiting, abdominal pain and alopecia [173].
DepoCyt® (liposomal cytarabine)
DepoCyt® (Pacira Pharmaceuticals, NJ, USA), was approved under the accelerated approval regulations in 1999. It is a liposomal formulation of cytarabine, which is manufactured using Depofoam® technology [174]. It was also approved by the FDA in 2007 to treat a life-threatening disease called lymphomatous meningitis [161]. Liposomal cytarabine is the only liposomal drug delivered by intrathecal administration; in this case systemic exposure to cytarabine is negligible [175,176]. The liposomal formulation is composed of dipalmitoyl phosphatidyl glycerol, dioleoyl phosphatidyl choline, triolein and cholesterol. The half-life of the drug delivered by this liposomal formulation is 40 times longer than standard sample of cytarabine [177]. DepoCyt is a sustained-release formulation with cytarabine as the active agent and is designed for direct administration into cerebrospinal fluid [178]. This antineoplastic agent can affect cells during S-phase of cell division and inhibit DNA polymerase [179].
Marqibo®
Liposome vincristine sulfate, also known as Marqibo® (Talon therapeutics, CA, USA; Figure 4), was approved by the FDA in 2012. Vincristine is an anticancer alkaloid that binds to tubulin and interferes with cell division. Marqibo is vincristine encapsulated in sphingomyelin/cholesterol liposomes [180]. After administration of Marqibo (2.25 mg/m2) to adult patients with Philadelphia chromosome-negative chronic myelogenous leukemia, 35% showed a good response [181]. Liposomal vincristine shows slower clearance and higher AUC (area under the plasma drug concentration versus time curve) in comparison with conventional vincristine [182]. The liposomal formulation of vincristine offers other advantages such as: increased circulation time in blood, better release profile and better accumulation in tumors [183]. Constipation, nausea, fatigue, diarrhea and insomnia are significant side effects [184].
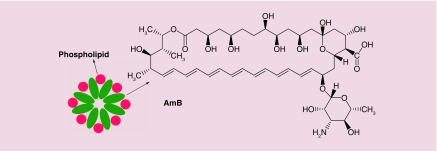
AmBisome®
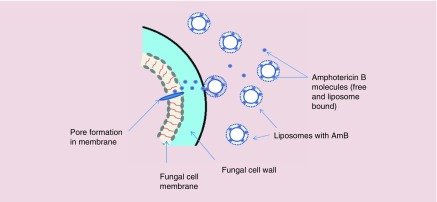
AmBisome® (NeXstar Pharmaceuticals, CA, USA), the liposomal form of Amphotericin B (AmB) or L-AmB (Figure 4), is an antifungal agent administrated for the treatment of a broad spectrum of fungal pathogens. Because it does not act via enzyme inhibition, AmB does not lead to the emergence of resistant fungal species compared with other antifungal agents [185]. The undesirable dose-limiting toxicity issues (especially nephrotoxicity) of the conventional formulation of AmB, Fungizone, which is the conventional injectable formulation of water-insoluble AmB suspended in sodium deoxycholate, led to efforts to overcome these side-effect problems [186]. Investigations were carried out on murine models focusing on particle size, higher AmB content, physicochemical stability and lower toxicity. The final optimized formulation consisted of hydrogenated soy phosphatidylcholine, cholesterol, and distearoylphosphatidylglycerol containing AmB at a molar ratio of 2:1:0.8:0.4 [185]. The advantages of liposomal AmBisome included: better pharmacokinetic properties and stability in the circulation, reduced accumulation in normal uninfected tissue, and reduced toxicity to mammalian cells compared with fungal cells making it much safer than Fungizone [185]. The mechanism of action of AmBisome is allowing the drug to transition from the liposomal membrane and then to bind to ergosterol in the fungal membrane resulting the in formation of pores, ionic leakage and eventually death of the fungal cells [187] (Figure 5).
Figure 5. . Proposed mechanism of action of AmB.
Free and liposome-bound AmB circulate in the bloodstream after injection of AmBisome. After attachment of the liposomes to the cell wall of the fungal cells, active AmB molecules are released and then bind to the cell membrane and by pore formation eventually leads to ionic leakage and cell death.
Taken with permission from [187] © Springer Nature (2016).
Figure 6. . Schematic illustration of Abelcet® lipid nanoformulation.
Vyxeos®
Daunorubicin and cytarabine encapsulated in liposomes, known as Vyxeos® (Jazz Pharmaceutics, Dublin, Republic of Ireland), received its FDA approval in 2017 for treatment of adults suffering from acute myeloid leukemia caused by previous therapy, or acute myeloid leukemia with changes related to myelodysplasia [188,189]. Cytarabine and daunorubicin are loaded into a liposomal structure at a 5:1 molar ratio [190].
By forming complexes with DNA, daunorubicin affects the synthesis of DNA and RNA controlling the expression of genes, and also generates free radicals which damage DNA [188]. By controlling DNA polymerase, cytarabine reduces the synthesis of DNA [188]. Vyxeos noticeably increases the exposure in plasma and reduces the distribution to ordinary tissues [191].
Vyxeos was prescribed to 309 patients aged 60–75 years old suffering from acute myeloid leukemia and the results showed a beneficial effect on overall survival [188]. However, it does possess some side effects; the most important ones are probably hypersensitivity reactions, cardiotoxicity and tissue necrosis [188].
Abelcet®
Another AmB lipid complex known as Abelcet (Defiante Farmaceutica, Funchal, Portugal) was approved in 1965. Like Ambisome, it is an antifungal medication which is used for treatment of serious leishmaniasis and fungal infections such as Aspergillosis, Blastomycosis, Candida, Coccidioidomycosis and Cryptococcosis [192,193]. Abelcet contains AmB and two phospholipids in a 1:1 drug to lipid molar ratio [194]. It also produces less severe side-effects compared with AmB.
Visudyne®
Visudyne® (QLT Phototherapeutics, Vancouver, Canada) is a liposomal formulation of the photosensitizer (PS), benzoporphyrin derivative mono acid ring A [195]. It received FDA approval in 2001 for treatment of choroidal neovascularization caused by wet age-related macular degeneration [196]. This growth of unwanted blood vessels in the back of the eye is one of the leading causes of blindness in adults. Visudyne is injected intravenously, followed after 10 min by shining a red laser through the pupil into the eye. The PS absorbs the light and is boosted into an excited state where it transfers its energy to ambient oxygen, producing singlet oxygen. This reactive oxygen species damages the newly formed leaky blood vessels, thus halting and even reversing the progressive loss of vision. Other than its use for age related macular degeneration, a combination of Visudyne photodynamic therapy and immunosuppression was suggested to be useful in treatment of subfoveal choroidal neovascularization which may occur as a complication of inflammatory conditions [197–200]. Side effects of Visudyne therapy were generally mild and included slight changes in vision, seeing flashes of light, dryness, redness or swelling in eyes or headache.
Polymer-based nanopharmaceuticals
Today, polymeric NPs have made great strides forward in applications for drug and gene delivery. Functional polymers can be used for encapsulation of therapeutic agents. In the area of commercial nanopharmaceuticals, pegylated drug and protein conjugates have shown major improvements over their nonpegylated counterparts [201]. ‘Pegylation’ refers to conjugation of any biomolecule to one or more chains of polyethylene glycol (PEG). In section 5 (and sub-sections) pegylated nanopharmaceuticals (Figure 7) are discussed, while in section 6 (and sub-sections) we discuss other different polymers used in nanopharmaceuticals (Figure 8).
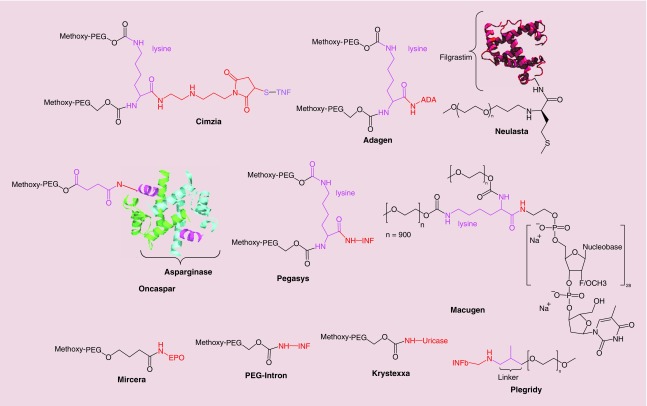
Figure 7. . Structures of PEGylated nanopharmaceuticals; linking agent is in purple and conjugation site is in red.
Figure 8. . Schematic illustration of other types of polymeric nanopharmaceuticals.
Figure 7 shows the structures of antibody, enzyme, cytokine or nucleotide conjugated to PEG through various linkers.
Cimzia®
Cimzia® (UCB, Brussels, Belgium) with the generic name ‘certolizumab pegol’ (CZP) received FDA approval in 2008, and is a PEGylated blocker of tumor necrosis factor alpha (TNF-α). Cimzia is a PEGylated Fab fragment (part of a humanized IgG antibody without the Fc region) that specifically recognizes and binds to TNF-α, thus neutralizing its activity [202]. Cimzia is used for treatment of patients with rheumatoid arthritis [203], Crohn's disease[204], psoriatic arthritis [205] and ankylosing spondylitis [206]. These diseases all are somehow related to autoimmunity; an unhealthy immune response of the patient against his/her own healthy cells. Strictly speaking, Crohn's disease is not an autoimmune disease since it seems that the content of the gut lumen triggers the response and not self-antigens [207]. TNF-α is a pluripotent pro-inflammatory cytokine and could be one of the main cytokines responsible for the autoimmune attack [202,205,206] (Figure 7).
Adagen®
Adagen® (Enzon, Inc., NJ, USA), with the generic name pegademase bovine, is PEGylated adenosine deaminase (ADA) which gained FDA approval in 1990 as the first PEGylated formulated protein on the market. Some studies [208] showed that ADA deficiency, caused by gene mutations, accounts for 14–15% of severe combined immunodeficiency disorder. A deficiency of ADA will cause accumulation of adenosine and 2′-deoxyadenosine, and this excess accumulation results in metabolic disorders which are linked to the functions of lymphocytes [209]. This disease is very rare; approval of Adagen was only based on the results with 12 patients, which gives this drug ‘orphan disease status’ [210]. Before Adagen, ADA-severe combined immunodeficiency disorder was treated with bone marrow transplants and transfusion of frozen irradiated erythrocytes [211,212], because administration of the native enzyme (ADA) was not effective. Naked ADA has a very short in vivo circulation time and also raises an immunogenic response [212]. PEGylation of ADA solved these issues. Davis et al. exhibited that PEGylated ADA had a longer circulation time also much less immunogenicity demonstrated by the absence of any detectable antibodies [212]. As shown in Figure 7, in Adagen, ADA molecules with multiple strands of attached PEG-5000, have less plasma clearance, and thus, a higher circulation time [213].
Neulasta®
Neulasta® (Amgen, Inc., CA, USA) was approved by FDA in 2002 with the generic name PEGfilgrastim, and is a PEGylated form of filgrastim. Neutropenia (low white blood cell counts) is a common adverse effect found in patients with nonmyeloid cancer who receive chemotherapy [214]. As a leukocyte growth factor, Neulasta is used for the treatment of febrile neutropenia and consequent infections arising due to lack of neutrophils. Filgrastim (the parent molecule) is recombinant methionyl human granulocyte colony-stimulating factor (r-metHuG-CSF) produced from E. coli [215]. Neulasta is synthesized by attachment of a monomethoxy-PEG aldehyde chain (20 kDa) to the N-terminal methionine residue of Filgrastim. The resulting imine is then reduced with sodium cyanoborohydride [216,217] (Figure 7). PEGylation of filgrastim results in an increased circulation time and also higher solubility of the parent molecule [217]. The half-life of the native molecule (filgrastim) is about 3.5–3.8 h, but Neulasta remains in circulation up to 42 h [216].
Oncaspar®
Oncaspar® (Enzon Pharmaceuticals Inc., NJ, USA) with the generic name pegaspargase is PEGylated-L-asparaginase (Figure 7) approved by the FDA in 1994. This agent is used to treat acute lymphoblastic leukemia, and chronic myelogenous leukemia. It is also used as an alternative in patients with these leukemias who display a hypersensitivity reaction to E. coli derived L-asparaginase [218,219]. The benefits IM/iv. administration of Oncaspar every 2 weeks compared with the three times per week needed for the native compound, L-asparaginase, are reduced hypersensitivity [220] and considerable total cost savings for patients [221,222]. The every 2 weeks requirement for the administration of Oncaspar is actually the consequence of higher half-life of the drug due to PEGylation [218].
Pegasys®
Pegasys® (previously Hoffmann-La Roche Inc., and currently Genentech USA, Inc., CA, USA) with the generic name peginterferon alfa-2a was approved by the FDA in 2002. Pegasys is recombinant human alfa-2a interferon, which is conjugated to branched PEG (40 KDa; Figure 7) [223] and is used for the treatment of hepatitis C [224] and HBeAg positive chronic hepatitis B [225]. As expected, the increased half-life of Pegasys due to PEGylation makes it possible to administer a subcutaneous injection only every 12 weeks; in comparison with the three times per week requirement for the free interferon [226]. Using Pegasys with ribavirin leads to better results for hepatitis C therapy [224]. Combining lamivudine and Pegasys also leads to higher survival in HBeAg positive chronic hepatitis B patients [225].
Somavert®
Somavert® (Pfizer Pharmaceuticals, CT, USA ) with the generic name pegvisomant (B2036-PEG) is the PEGylated analog of human growth hormone (GH) for the treatment of acromegaly, which received FDA approval in 2003. In patients with acromegaly, the pituitary gland secretes excessive amounts of growth hormone, with the consequence of abnormally enlarged forehead, jaw, hands and feet. Somavert is an antagonist of GH receptors, blocking the binding of GH, and interfering with GH signal transduction pathways, and thus reducing the serum concentration of IGF-I (which is one of the critical mediator of GH activity) [227]; by at least 50% [228]. As expected, PEGylation of the active ingredient of Somavert (B2036) will result in a reduced clearance, estimated to be 28 mL/hour for subcutaneous injection at doses of 10–20 mg, and giving an increased half-life of approximately 6 days following administration [229].
Macugen®
Pegatinib sodium is an ocular therapeutic agent discovered by EyeTech Pharmaceuticals in 2000 and received its FDA approval in 2004. Macugen® is the brand name of pegatinib sodium, which was released into the market by Pfizer Inc. This drug is administered for the wet neovascular form of age-related macular degeneration [230]. The development of Macugen was recognized as a milestone in aptamer-based nano-vectors used for treatment of human diseases [231]. The term aptamer describes an oligonucleotide sequence composed of RNA or DNA that binds to a specific target [232]. Pegatinib is an anti-VEGF aptamer with 28 nucleotides conjugated to two PEG moieties via amine groups of lysine residue with a MWt ∼50 kD (Figure 7). It functions as an anti-angiogenesis agent. Treatment with Macugen involves injection of the appropriate dosage every 6 weeks [233]. Some of the side effects of Macugen, that were observed in clinical trials, include diarrhea, eye irritation, headache and nausea.
Mircera®
Mircera® or epoetin β (EPO) conjugated to methoxy-PEG (Figure 7) is a drug formulation utilized in treatment of anemia [234,235]. Mircera received its approval in 2007 by both European commission and the FDA. EPO is a genetically recombinant form of erythropoietin, which is capable of stimulating erythropoiesis by acting on the erythropoietin receptors of bone marrow progenitor cells [236]. In Mircera, the PEG moiety (∼ 60 kD) is first linked with butanoic acid and the NHS modified structure is linked to the lysine moiety of the EPO structure via amide bonds. This formulation provides a controlled release system with a half-life of ∼ 135 h, in comparison with naked EPO with a half-life of 7–20 h [235]. The main benefit is the less frequent administration when using Mircera. Administration of Mircera is through intravenous or subcutaneous injection of 0.6 μg/kg every 2 weeks [235].
PEG-INTRON®
PEG interferon® alfa-2b is a long acting kind of interferon; its structure includes alpha interferon (INF) molecule conjugated to a mono PEG chain via succinimidyl carbonate (12 kDa; Figure 7) [237]. Its longer half-life and slower elimination leads to a less frequent administration when compared with the standard interferon molecule [237]. PEG interferon alfa-2b received its FDA approval in 2001 and is currently used as a monotherapy or along with other drugs such as ribavirin for treatment of chronic hepatitis C [238,239].
Krystexxa®
Krystexxa® (or Pegloticase, formerly Puricase, NJ, USA) manufactured by Savient Pharmaceuticals is a treatment for patients who suffer from refractory chronic gout [240,241]. Following investigations of its effectiveness in reducing uric acid levels, as well as lowering deposits of uric acid crystals in joints and soft tissues, it received FDA approval in September 2010. The EMA also approved this drug in January 2013 for treatment of tophaceous gout disorder (tophi are nodular masses of uric acid crystals) [242,243]. Pegloticase is a recombinant porcine-like uricase that can metabolize uric acid to allantoin, in a similar manner to the nonpegylated Rasburicase. Pegloticase is composed of four uniform chains of approximately 300 amino acids, in which nine of the 30 lysine residues in each chain are pegylated (PEG chains MW: 10 KDa) [243]. The improved solubility of Pegloticase removes risks of precipitates forming. Furthermore, the PEG molecules in Pegloticase result in an increased drug half-life to about 10 days (in comparison with Rasburicase which is 8 h) and a reduction in its immunogenicity [243]; Accordingly, Pegloticase is a better choice compared with other treatments especially for chronic treatment [244]. The side effects of Krystexxa, include infusion and allergic reactions which should be noted before repeating the treatment. Other minor side effects include sore throat, vomiting, nausea and chest pain [241].
Plegridy®
Plegridy® contains the active substance PEG-IFN-β-1a, which is a PEG conjugated form of glycosylated, recombinant IFN-β modified with a single, linear molecule of 20 kDa methoxy-PEG-O-2-methylpropionaldehyde (mPEG) (Figure 7) [245]. Plegridy or PEG-IFN-β-1a was approved by FDA in 2014 for treatment of relapsing remitting multiple sclerosis (RRMS) in adult patients [246]. Elevations of hepatic enzymes and liver injury have been observed with the use of plegridy. Patients should be checked for signs of hepatic damage and treatment should be stopped if icterus or other clinical symptoms of hepatic dysfunction appears [247].
Adynovate®
Adynovate®, is a recombinant pegylated anti-hemophilic factor used for treatment of hemophilia A in patients who experience repeated bleeding events [248]. Adynovate works by increasing levels of blood-clotting factor VIII in the blood in a temporary manner. Adynovate has an extended circulation time in the human body leading to reduced frequency of injection. The structure comprises coagulation factor VIII conjugated to PEG [249]. The safety and efficacy of this drug against hemophilia have been shown in clinical trials. Some common side effects include diarrhea, nausea, headache, vomiting, rash and other common allergic reactions [248].
Other types of polymer-based nanopharmaceuticals
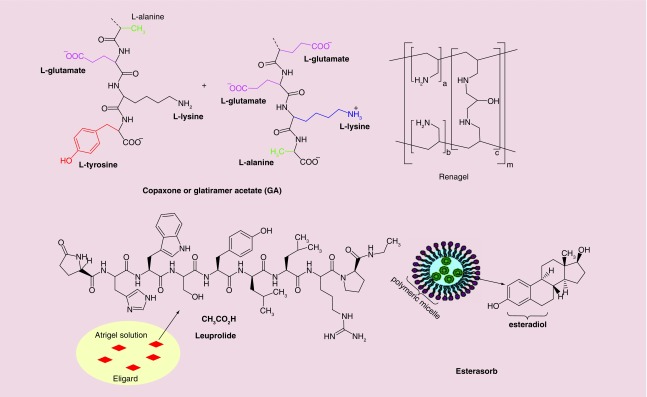
Figure 8 shows polymer-based nanopharmaceuticals (other than pegylated formulations) either themselves composed of polymer chains such as Copaxone® and Renagel, or where polymers are used to disperse drug molecules such as Eligard and Estrasorb.
Copaxone®
Copaxone or glatiramer acetate (GA otherwise known as COP-1) is a synthetic polymer consisting of L-alanine, L-glutamic acid, L-tyrosine and L-lysine (Figure 8), in a ratio of 0.141:0.338:0.427:0.095 respectively, which was approved by the FDA for treatment of multiple sclerosis (MS) [250]. Although the mechanism of action of this random copolymer is not completely understood, according to some investigations, it can suppress inflammatory responses by blocking MHC II and changing the T-cell population phenotype [251,252]. Its therapeutic benefit (especially in autoimmune disorders) can be inferred from its approval for marketing in several countries including USA, Canada and some European countries [253,254]. However, clinical trials have shown that GA caused various side effects in different organs including heart, eyes, skin, gastrointestinal and immune system [255].
Eligard®
Eligard® is a formulation of leuprolide acetate that utilizes Atrigel as a polymeric injectable nanosuspension agent (Figure 8), which received its FDA approval in 2002 as a palliative agent for treatment of prostate cancer [256]. Leuprolide acetate (known as Lupron) is a synthetic peptide-type analogue of gonadotropine-releasing hormone (GnR) which is able to interact with the GnR hormone receptor and modify gonadotropin secretion. The reduction in gonadotropin secretion reduces the level of other hormones such as luteinizing hormone (LH) and follicle stimulating hormone (FSH). The results are hypogonadism and a decrease in levels of estradiol and testosterone. Artigel is a DDS based on a polymer mixture (usually polylactic and polyglycolide) [257,258]. Eligard is an example of a nanopharmaceutical serving as a sophisticated drug-delivery system for Lupron. As an injectable liquid formulation, it solidifies in the body and slowly releases the drug over 1 month because of Atrigel biodegradation [257]. Irritation and erythema are the most common adverse effects of Eligrad after its subcutaneous injection [256].
Renagel®
Renagel®, or Sevelamer, which was approved by the FDA in 2000, is a tablet (400 or 800 mg doses) prescribed for treatment of patients with high serum levels of phosphorus [259]. Renagel is usually prescribed for cases of end-stage renal disease or in patients with hyperphosphatemia caused by chronic kidney disease [260,261]. Renagel has a polymeric network structure composed of cross-linked polyallylamine hydrochloride while the cross-linking agent is epichlorohydrin (Figure 8). The recommended starting dosage for Sevelamer is completely dependent on phosphorus serum level of the patients [262].
Estrasorb®
Estrasorb® (Novavax, Inc., MD, USA) is a topical micellar-encapsulated emulsion of estradiol (17β-estradiol) hemihydrate used to treat moderate vasomotor symptoms due to menopause. Approved in 2003, this topical lotion was, and still is, the only micellar nanopharmaceutical on the market. Vasomotor symptoms cause hot flushes, night sweats and disrupted sleep patterns by affecting the length of REM sleep, with a consequent loss of memory, fatigue and lethargy. Estradiol, the main estrogen, plays a role in reducing these symptoms [263]. In a randomized, double-blind clinical trial, volunteers who received Estrasorb, showed a reduced number of hot flushes [264]. Estrasorb is supplied in a foil pouch and is designed to be applied to the skin and penetrate the stratum corneum [265]. Estradiol binds to the nuclear estrogen receptor and alters gene transcription [266].
Zilretta®
Triamcinolone acetonide that has been embedded in a PLGA hydrogel, is known as Zilretta® (Flexion Therapeutics, MA, USA), and received its FDA approval in 2017 for treatment of knee osteoarthritis [267–269]. This formulation is delivered by intra-articular injection to reduce knee osteoarthritis pain, but its overall positive effects are still under investigation [267,270].
In spite of benefits shown in clinical trials, Zilretta gave no substantial superior benefit compared with immediate release triamcinolone in a clinical trial [268]. In clinical studies, 32 mg of Zilretta was given to 424 patients consisting of 143 patients aged 65 or more and showed that the adverse effects of Zilretta were no different between both old and young patients [268].
Protein-based nanopharmaceuticals
Abraxane®
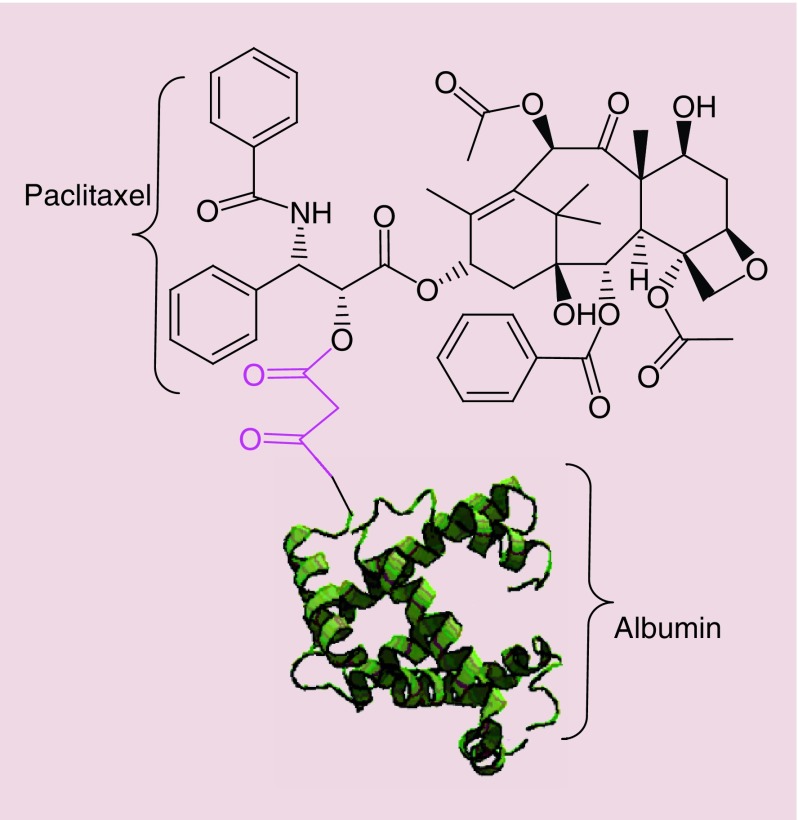
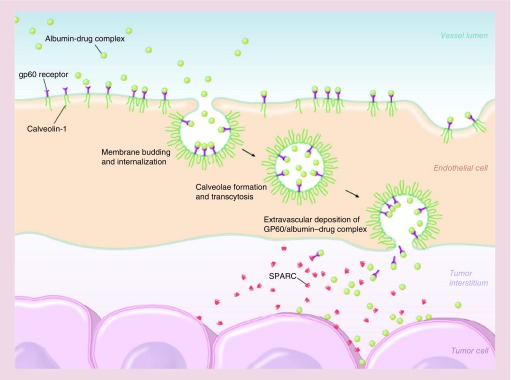
Abraxane® (Celgene Pharmaceutical Co. Ltd) or ABI-007, consists of albumin–NPs bound to paclitaxel with a particle size around 130 nm, approved by the FDA for treatment of metastatic breast cancer (2005), lung cancer (2012), and metastatic pancreatic adenocarcinoma (2013) [271]. Paclitaxel interferes with cellular mitosis by shifting the equilibrium between tubulin dimersmicrotubules in favor of microtubules, and then preventing microtubule depolymerization, hence decreasing the concentration of tubulin. Interference with microtubule dynamics by paclitaxel affects the ability of cancer cells to properly assemble a mitotic spindle, pass the metaphase/anaphase checkpoint and undergo mitosis [271,272]. Taxol® is another drug with paclitaxel as the active agent which was approved in 1998 and showed good results for cancer therapy. Paclitaxel itself is highly lipophilic and in order to make it suitable for injection, it is important to use an emulsifier/solubilizer such as Cremophor EL (polyoxyethylated castor oil) used in Taxol. High concentrations of Cremophor EL in Taxol, can induce serious toxicities and severe hypersensitivity reactions [273]. Abraxane retains the therapeutic benefits of paclitaxel while removing the toxicity of Cremophor (e.g., Taxol) [273]. After IV administration, Abraxane dissolves resulting in soluble albumin-bound paclitaxel complexes which have similar size to endogenous albumin [274]. These complexes accumulate in the tumor; partly due to the passive EPR effect in tumors; and partly due to trans endothelial transport via the albumin-binding protein (gp60) [275] (Figure 9). Studies on breast cancer have demonstrated that Abraxane has higher response rates (33 vs 19%), longer time to tumor progression (23.0 vs 16.9 weeks), less grade 4 neutropenia incidence (9 vs 22%) and more grade 3 sensory neuropathy (10 vs 2%) compared with Taxol, respectively. The higher grade 3 sensory neuropathy in Abraxane was attributed to higher doses of paclitaxel, but could be managed by standard therapy [276].
Figure 9. . Structure of albumin-bound paclitaxel known as Abraxane®.
Ontak®
Denileukin diftitox, known as Ontak® (Eisai, Japan), received its FDA approval in 1999 for treatment of T-cell lymphoma [277,278]. It consists of recombinant diphtheria toxin protein conjugated to IL-2 and was designed to bind to the IL-2 receptor. Ontak was used in leukemia and lymphoma treatment, because it could deliver diphtheria toxin selectively to target cells which expressed interleukin-2 receptors [279]. There have been some studies which showed Ontak could be used for treatment of mycosis fungoides, which is the most common form of cutaneous T-cell lymphoma [280,281]. Hypersensitivity reactions during the infusion with symptoms such as low blood pressure, back pain, fever, breath shortness, nausea and vomiting, blood test abnormalities and liver problems, weakness, rash and poor appetite are some of the side effects of using Ontak [280]. In 2006 serious problems with loss of vision emerged, and the FDA added a black box warning to the drug labeling include a description of ophthalmologic adverse events. In 2014 marketing of Ontak was discontinued in the USA [282].
Rebinyn®
The PEGylated glyco-protein drug, also known as Rebinyn® (Novo Nordisk, Bagsværd, Denmark), received its FDA approval in 2017 [283]. It is used for patients suffering from factor IX (FIX) deficiency also known as hemophilia [284]. It consists of 40 kDa PEG attached to recombinant DNA-derived coagulation FIX concentrate [285,286].
Metal-based nanopharmaceuticals
Today magnetic-based NP have vast arrays of application in drug and gene delivery [287,288] and diagnosis [289]. Feridex was a commercial product of iron oxide based NPs used as a contrast agent for MRI. In response to observed side effects its production was discontinued in 2008.
Feraheme®
Ferumoxytol, known as Feraheme® or Rienso® (AMAG Pharmaceuticals, MA, USA) was approved by the FDA in 2009, is an intravenous drug formulation with neutral pH for treatment of anemia [290,291]. It is prescribed for adult patients with evidence of iron overload [292]. Clinical studies have shown that an intravenous injection of 510 mg of ferumoxytol is well tolerated [293,294]. According to the AMAG label for Feraheme, 0.2 and 1.9% of patients who received it experienced hypersensitivity reactions and hypotension respectively. In clinical trials, some serious side effects of Feraheme were nausea, diarrhea, hypotension, constipation, dizziness and peripheral edema [295].
Market investigation
The nanopharmaceutical market can be considered to spread throughout diverse regions of the world. Therefore, assessing the market size in non-American and non-European countries with rarely supplied official statistics is not an easy task. But as with many other medical products, the USA is the largest producer and consumer of nanopharmaceuticals.
Global nanomedicine market size
Nanopharmaceuticals generally have a high added value compared with traditional pharmaceuticals. This, combined with a variable and often contradictory definition of exactly what constitutes nanotechnology and nanopharmaceuticals in different countries, and varying assessments by scientific and regulatory authorities, makes it hard to estimate accurate data on the global nanomedicine market size. In one report, the total market size for nanomedicine in 2015 was expected to be about $1 trillion [296]. With the progressive growth rate of some nanopharmaceuticals, and the ever increasing need for more efficient medicines for treating cancer, immune and nervous system diseases, as well as mitigating infectious diseases such as AIDS, the worldwide marketplace is expected to show a compound annual growth rate (CAGR) of 22% for the NPs in the life sciences as a whole [297].
It is expected that protein-based nanopharmaceuticals will have a market size of $14 ± 7 billion, and for nucleic acid-based nanopharmaceuticals it would be $7 ± 3 billion, and for small molecule-based nanopharmaceuticals, it will be $3 ± 3 billion in 2020 [298]. Another report suggested that ‘the global nanomedicine market is anticipated to reach USD 350.8 billion by 2025’, according to a new report by Grand View Research, Inc. [299].
For the market to grow even further, risk issues should be addressed, and social impacts should be evaluated. Considering the high prices of nanomaterials and nanomaterial intermediates, serious analysis of the cost-effectiveness and the required purchasing power of nations with centralized health care systems and nations with private market healthcare systems should be undertaken.
Market trends
Academic research laboratories are constantly making advances in nanomedicine [141]. The question arises to what extent these discoveries result in issued patents that can be licensed to existing or new start-up companies? The ever-increasing costs of pharmaceutical development mean that not all advances in drug delivery can necessarily be advanced to result in approved medicines. Nevertheless, it is clear that medical care represents an increasing proportion of gross domestic product in most developed countries. It is cost effective and patient friendly to diagnose and treat patients in the shortest time and in the least invasive way possible. Another major trend that has emerged in recent years has been termed ‘personalized medicine’. Discoveries in genetics and proteomics have revealed that individual patients apparently suffering from the same disease at the same stage of advancement, can in reality be very different. Nanopharmaceuticals will have a great role to play in personalized medicine. Not only will the particular drug, patient dosage and administration schedule be adjusted according to results of genetic and proteomic investigations, but the precise nanoformulation may also be tailored according to laboratory tests and biomarkers. Companies like Cerulean Pharma Inc. and Calando Pharmaceuticals have initiated this movement [300]. Combination nanomaterials, as mentioned earlier, with enhanced overall performance are the future nest-egg of the global medical market.
Nanomedicine in the face of market failure
The conventional pharmaceutical industry has experienced some major disasters over the years. Thalidomide was perhaps the first to come to public awareness. Thalidomide was first marketed in 1957 (West Germany) under the brand-name Contergan as a sedative. The disaster occurred when it was used to mitigate morning sickness in pregnant women. Shortly after the drug began to be marketed in West Germany, between 5000 and 7000 infants were born with malformation of the limbs. Throughout the world, about 10,000 cases were reported of infants with phocomelia due to thalidomide; only 50% of the 10,000 survived [301] Another major disaster was the Vioxx scandal involving non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs are divided into non-specific NSAIDs that inhibit both COX1 and COX2 (diclofenac, ibuprofen, naproxen and aspirin), while there are also COX2-specific NSAIDs (celecoxib or Celebrex, valdecoxib or Bextra, and etoricoxib or Arcoxia). There was another COX2 inhibitor on the market called rofecoxib (Vioxx), but in 2004 it was pulled from the market by Merck amid lawsuits, and blamed for causing between 88,000 and 139,000 heart attacks, 30–40% of which were fatal [302]. The total settlement costs were in excess of $5 billion. The so-called ‘Vioxx scandal’ has tainted the whole subject of NSAIDs as therapeutic agents [303].
Rapid growth in the use of nanotechnology in medicine have made great promise in treatment of several diseases such as cancer. The main class of approved nanopharmaceutical as an anticancer agents (such as doxil, abraxane) have received FDA approval due to better efficiency and less side effects in comparison with naked drug which arises from EPR effect related to nanoformulation [304]. Generally, in cancer therapy, the EPR based nanomedicine could alter the routine pathway of anti-cancer agent by delivering it directly to a designated part (i.e. tumor). According to successes and large market size of anti-cancer nanomedicine (Table 1), big and well-known pharmaceutical companies made an investment in this field. However, the threat of failure always accompanies such investments. One shocking example, have happened with BIND Therapeutics Inc. while they had declared bankruptcy in 2013. The production of BIND-014 an anticancer nanomedicine made of docetaxel coated polymer with targeted ligand, which were believed to be safer and efficient than docetaxel and had found successes in small clinical trials, failed in larger-sized trials [305]. The problem might be laid on the efficiency of nanoformulation to be better or the same as docetaxel alone. It should be considered that in development of nanomedicine, there is large distance between the products in laboratory bench to commercial products. New horizons exist in progress of new nanoformulation, however, there are several diminishing that might disappoint researchers and industries.
Challenges on the way to market
Even though there has been a huge number of reports and studies related to nanoformulation of drugs, only a handful of such nanosystems have progressed to market-related assessment and again an even smaller handful have received final approval. Based on some reports, the translation of basic science to clinical application has been less than 10% [306]. Therefore, drugs passing through what is known as the ‘valley of death’ seems not to be easy. This can lead to a time-consuming, expensive, ineffective series of investigations, which escalates health care costs as a whole [110].
The reasons for such an undesirable state of affairs probably lie in different areas and aspects of the process. One of the main challenges concerns the in vivo behavior of NPs, which is likely to be very different from their in vitro behavior. Cellular interactions, tissue transportation, diffusion and biocompatibility, are the main issues, which need to be thoroughly investigated using different animal (in vivo) models. Conducting these experiments to sufficient proof of efficacy and safety is not easy or cheap.
Another challenge, especially for tumor-targeted nanoformulations is the complexity and heterogeneous nature of tumors. Differences between different tumors, with regards to gene expression profiles, molecular patterns and degree of drug resistance, could hinder the penetration, and decrease the effectiveness of tumor-targeted NPs [307–310]. This difficulty could lead to an unsuccessful clinical trial (despite promising preclinical data in animals) and rejection of the tested nanoformulations.
Specific drug penetration into tumors, the efficiency of drug release into the target cells, and the availability of the drug loaded NPs, are other aspects which need and accurate professional experimentation [307]. Due to time and money issues, this thorough experimentation may not be available in all biomedical laboratories which itself is another problem.
The multifunctional structure and activity of some nanoformulations could be another challenge on the road to nanoformulation approval. Many investigative nanoformulations possess a hybrid structure and contain separate diagnostic and therapeutic elements. Different experiments will be needed to demonstrate the safety of such systems, and the long-term biocompatibility of these systems is still not clear [311,312]. Regarding this issue, the regulatory authorities have different restrictions, and time-consuming and expensive regulatory studies will be necessary to be sure about the long-term safety of these theranostic nanoformulations.
Most of the classical methods for the synthesis of nanoformulations are still imperfect and need further improvement and optimization. Batch-to-batch variation is another challenge that can hamper the production of sufficient stocks of nanoformulations to achieve market approval. Upscaling production methods and characterization of nanoformulations in a highly precise way are likely to be laborious, time consuming and costly [311,313,314].
In general, the challenges which limit the market entrance of nanomedicines can be categorized as financial challenges, ethical challenges and regulatory challenges [315].
With respect to financial challenges, most academic laboratories are not able to provide the required levels of financing [315]. Accordingly, most of the basic research studies will cease at the laboratory level due to financial constraint for further development and evaluation. Without financial support, not even a single basic formulation could enter into clinical trials. Recruiting specialists, obtaining bulk raw materials, scaling up of production methods, compensation for the volunteers and executing the precise measurement and evaluation required, are some of the tasks in this area, which could not take place without considerable financial investment. Based on one survey, most of basic nanomedicine researchers express a consensus opinion about the difficulties in attracting attention from commercial companies [315]. According to one researcher, companies are not eager to participate and will not risk their investment in another rival product, since they often have their own product on the way to market [315].
Ethical issues exist regarding all medicines, and nanomedicines are no exception, where issues are often based on cost/benefit evaluation. Most human clinical trials in nanomedicine are performed on people suffering from incurable diseases, or patients who have received many different treatments without any beneficial effect on their disease [316]. In spite of the valuable knowledge obtained by these clinical trials, most of the participants are unlikely to get any individual benefit by taking part in such a clinical trial. The main challenge here is in the early stage of clinical trials, where the benefit/harm ratio on the health effects caused by the nanoformulation are unknown. In this situation, many ethical issues will arise. Exaggerated caution exerted by ethical committees, the inability to provide sufficient correct information about the risks, benefits, and likely outcome of the trials, and other humanitarian issues should certainly be included. These are the main ethnical issues which can hinder the whole process of clinical trials [315–317].
The established regulations (in some cases, undesired regulations) laid down by drug authorities are another challenge on the road to new nanoformulations. The regulations of the EMA and the FDA are different from each other, are and these regulations always undergoing some periodical changes. Accordingly, such regulations can significantly affect the whole processes of clinical trials [311,315].
Once it has been decided that clinical trial needs to be planned and executed for a new nanoformulation, different drug authorities with their strict regulations will arrive on the scene. Although they can provide a source of valuable information and advice, especially in the design and development of clinical trials, their regulations usually involve differences between national and ethnic groups, and sometimes there is a request for different clinical trials on individuals from different national or ethnic groups. Delivery of precise and detailed information about the nanocharacterization and safety of the nanomedicines to the drug authorities could also be another roadblock [311,315].
All these limitations can affect the motivation of academic investigators to start or continue their basic nanodrug related experiments that will eventually reduce the overall number of nanomedicines on the market. Providing appropriate financial support, better attention to ethical issues, and establishing means for robust ethical oversight, as well as harmonizing the regulations among different countries could all facilitate and improve the bench-to-market process (Figure 10).
Figure 10. . Cellular uptake of albumin-bound nanopartcles containing paclitaxel.
Endothelial transcytosis pathways via gp60 followed by binding to that are present at the tumor site [274].
Taken with permission from [274] © Elsevier (2012).
SPARC: Cysteine-rich secreted proteins.
Conclusion
Currently marketed nanomedicines include nanocrystals, liposomes and lipid nanoparticles, PEGylated polymeric nanodrugs, other polymers, protein-based nanoparticles and metal-based nanoparticles. A range of issues must be addressed in the development of these nanostructures. Ethics, market size, possibility of market failure, costs and commercial development, are some topics which must be considered. After passing all the ethical and biological assessments, and satisfying the investors as to future profitability, only a handful of these nanoformulations are likely to become successful and profitable medicines.
Future perspective
In recent years, the rising cost of healthcare has been a worrying concern for most of the developed and even developing countries. To maximize cost efficiency, it is critical that governments acquire a deeper understanding of the cost–effectiveness of nanopharmaceuticals [304,318]. It should be noted that the first step in developing this market, is a standardized cost–effectiveness study that demonstrates whether the additional health benefits of nanopharmaceuticals compared with standard formulations could be worth the additional cost. By doing so, governments will be free to issue clear guidelines and analyze the financial rewards of developing this market [319].
The present absence of such basic information is not the only barrier hampering the efficient commercialization of nanomedicines. The progress of this market depends entirely on the assurance of patients, citizens as well as physicians about the commercial success and safety of nanomedicines. To overcome this challenge, the future market needs more public awareness of benefits, risks and safety concern regarding nanopharmaceuticals [320].
Despite all the above-mentioned challenges, the authors anticipate that the market for nanopharmaceuticals and nanomedicines will develop further over the next five years, mainly thanks to advances in bionanotechnology and nanoengineering, introduction of clear rules on new nanotechnology-based drugs, more funding from government organizations [98], more consensus on environmental issues, and formation of partnerships between nanomedicine startups or R&D departments and leading medical and pharmaceutical companies [321]. In other words, to persuade investors about the value of nanopharmaceuticals, and improve the overall health and welfare of the society, intellectual property and regulatory agencies need to change their ways meet the special needs of nanomedicines and shorten their time to regulatory approval. However, in the case of nanodrugs, it is especially important to consider the risks to health and to the environment. In some cases, it may be necessary to take a step backward and re-analyze the situation, in order to be better placed for long-term rational planning for the future, and not just think about the short-term benefits.
Another equally important factor which has attracted the attention of researchers and companies is the growing role that cancer plays in mortality and morbidity figures worldwide. For instance, in September of 2016, five out of 17 FDA-approved drugs were cancer-related. This and the high number of other cancer related pharmaceuticals approved during past years demonstrates not only the urgent requirement for better cancer treatments by the patients, but also the enormous market for cancer treatment. The combination of chemotherapy and photothermal or photodynamic therapy also holds great promise. However, these are drug device combinations and may be more difficult to advance to full approval. Diseases involving the immune system are very important to the pharmaceutical industry as well. These applications may involve stimulating the immune system to fight infections and cancer, but also down-regulating the immune system to fight against auto-immune diseases and allergies. All in all, we believe that the increasing rate of cancer-related deaths are driving forces behind the expected increase in global nanomedicine market size in coming years.
Executive summary.
‘Nanomedicine’ is an emerging field describing the production and marketing of nanoformulated drugs.
A rational pathway for the development of nanopharmaceuticals from laboratory to commercialization covers techniques, costs, ethics and US FDA approval.
Nanopharmaceuticals currently on the market are categorized into crystalline, liposome-derived, polymeric-type, protein-based and metal-based.
In each category approved nanopharmaceuticals are listed by their commercial brand name.
Their FDA approval date, chemical structure, applications and mechanisms of action are summarized.
Challenges and trends in marketing, along with the possibility of market failure are provided.
Future perspectives are given for nanopharmaceuticals and development of new nanoformulations.
Acknowledgments
F Farjadian would like to thank Shiraz University of Medical Science for a grant to support this work (1396-01-36-16248).
Footnotes
Financial & competing interests disclosure
MR Hamblin was supported by US NIH grants R01AI050875 and R21AI121700. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Eric DK. Engines of Creation. The Coming Era of Nanotechnology. Doubleday; NY, USA: 1986. [Google Scholar]
- 2.Feynman RP. There's plenty of room at the bottom. Eng. Sci. 1960;23(5):22–36. [Google Scholar]; •• The very first inkling of what would become the nanotechology revolution.
- 3.Devreese JT. Importance of nanosensors: Feynman's vision and the birth of nanotechnology. MRS Bull. 2007;32(09):718–725. [Google Scholar]
- 4.Qu X, Alvarez PJ, Li Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013;47(12):3931–3946. doi: 10.1016/j.watres.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 5.Sawhney A, Condon B, Singh K, Pang S, Li G, Hui D. Modern applications of nanotechnology in textiles. Textile Res. J. 2008;78(8):731–739. [Google Scholar]
- 6.Chan CK, Peng H, Liu G, et al. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008;3(1):31–35. doi: 10.1038/nnano.2007.411. [DOI] [PubMed] [Google Scholar]
- 7.Briggs BD, Knecht MR. Nanotechnology meets biology: peptide-based methods for the fabrication of functional materials. J. Phys. Chem. Lett. 2012;3(3):405–418. doi: 10.1021/jz2016473. [DOI] [PubMed] [Google Scholar]
- 8.Langer R, Weissleder R. Scientific discovery and the future of medicine. JAMA. 2015;313:135–136. [Google Scholar]
- 9.Coccia M, Wang L. Path-breaking directions of nanotechnology-based chemotherapy and molecular cancer therapy. Technol. Forecast. Soc. Change. 2015;94:155–169. [Google Scholar]
- 10.Karimi M, Zare H, Bakhshian NA, et al. Nanotechnology in diagnosis and treatment of coronary artery disease. Nanomedicine. 2016;11(5):513–530. doi: 10.2217/nnm.16.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parpura V. Tissue engineering: nanoelectronics for the heart. Nat. Nanotechnol. 2016;11(9):738–739. doi: 10.1038/nnano.2016.123. [DOI] [PubMed] [Google Scholar]
- 12.Karimi M, Mirshekari H, Aliakbari M, Sahandi-Zangabad P, Hamblin MR. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnol. Rev. 2016;5(2):195–207. [Google Scholar]
- 13.Farokhzad OC, Langer R. Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv. Drug Del. Rev. 2006;58(14):1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]; •• Important perspective concerning the emerging field of nanomedicine.
- 14.Sayes CM, Aquino GV, Hickey AJ. Nanomaterial drug products: manufacturing and analytical perspectives. AAPS J. 2017;19(1):18–25. doi: 10.1208/s12248-016-0008-x. [DOI] [PubMed] [Google Scholar]
- 15.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed. Nanotechnol. Biol. Med. 2013;9(1):1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dilnawaz F, Acharya S, Sahoo SK. Recent trends of nanomedicinal approaches in clinics. Int. J. Pharm. 2018;538(1–2):263–278. doi: 10.1016/j.ijpharm.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Ragelle H, Danhier F, Préat V, Langer R, Anderson DG. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opin. Drug Deliv. 2017;14(7):851–864. doi: 10.1080/17425247.2016.1244187. [DOI] [PubMed] [Google Scholar]
- 18.Iyer R, Hsia CC, Nguyen KT. Nano-therapeutics for the lung: state-of-the-art and future perspectives. Curr. Pharm. Des. 2015;21(36):5233–5244. doi: 10.2174/1381612821666150923095742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai PP, Rustomjee MT. Business potential of advanced drug delivery systems. Confocal Microscopy. 2018;23:29. [Google Scholar]
- 20.Karimi M, Zangabad PS, Ghasemi A, Hamblin MR. Smart Internal Stimulus-Responsive Nanocarriers for Drug and Gene Delivery. Morgan & Claypool Publishers; CA, USA: 2015. Future perspectives and the global drug delivery systems market. [Google Scholar]
- 21.Bhowmik D, Gopinath H, Kumar BP, Duraivel S, Kumar KS. Controlled release drug delivery systems. Pharma Innov. 2012;1(10) [Google Scholar]
- 22.Nikalje AP. Nanotechnology and its applications in medicine. Med. Chem. 2015;5:81–89. [Google Scholar]
- 23.Yih T, Al-Fandi M. Engineered nanoparticles as precise drug delivery systems. J. Cell. Biochem. 2006;97(6):1184–1190. doi: 10.1002/jcb.20796. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Ye Q, Lu M, et al. A new approach to reduce toxicities and to improve bioavailabilities of platinum-containing anti-cancer nanodrugs. Sci. Rep. 2015;5:10881. doi: 10.1038/srep10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balakumar K, Raghavan CV, Abdu S. Self nanoemulsifying drug-delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf. B. Biointerfaces. 2013;112:337–343. doi: 10.1016/j.colsurfb.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Karimi M, Sahandi Zangabad P, Baghaee-Ravari S, Ghazadeh M, Mirshekari H, Hamblin MR. Smart nanostructures for cargo delivery: uncaging and activating by light. J. Am. Chem. Soc. 2017;139(13):4584–4610. doi: 10.1021/jacs.6b08313. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review about light activated drug-delivery using nanomaterials
- 27.Bosetti R, Marneffe W, Vereeck L. Assessing the need for quality-adjusted cost–effectiveness studies of nanotechnological cancer therapies. Nanomedicine. 2013;8(3):487–497. doi: 10.2217/nnm.13.15. [DOI] [PubMed] [Google Scholar]
- 28.Karimi M, Eslami M, Sahandi-Zangabad P, et al. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8(5):696–716. doi: 10.1002/wnan.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Del. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Good review about nanomedicines and cancer concentrating on targeting strategies.
- 30.Karimi M, Moosavi Basri SM, Vossoughi M, Pakchin PS, Mirshekari H, Hamblin MR. Redox-sensitive smart nanosystems for drug and gene delivery. Curr. Org. Chem. 2016;20(28):2949–2959. [Google Scholar]
- 31.Sargent JF., Jr Nanotechnology: a policy primer. 2016.
- 32.Hosseini M, Farjadian F, Makhlouf ASH. Hosseini M, Makhlouf ASH. Industrial Applications for Intelligent Polymers and Coatings. Springer; Basel, Switzerland: 2016. Smart stimuli-responsive nano-sized hosts for drug-delivery; pp. 1–26. [Google Scholar]
- 33.Zhang M-Q, Wilkinson B. Drug discovery beyond the ‘rule-of-five’. Curr. Opin. Biotechnol. 2007;18(6):478–488. doi: 10.1016/j.copbio.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Ige PP, Baria RK, Gattani SG. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf. B. Biointerfaces. 2013;108:366–373. doi: 10.1016/j.colsurfb.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 35.Karimi M, Ghasemi A, Zangabad PS, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016;45(5):1457–1501. doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zangabad PS, Karimi M, Mehdizadeh F, et al. Nanocaged platforms: modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale. 2017;9(4):1356–1392. doi: 10.1039/c6nr07315h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farjadian F, Moghoofei M, Mirkiani S, et al. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol. Adv. 2018 doi: 10.1016/j.biotechadv.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Interesting review about using nanomaterial derived from bacterial structures as drug-targeting vehicles.
- 38.Junghanns J-UA, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008;3(3):295. doi: 10.2147/ijn.s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollis CP, Weiss HL, Leggas M, Evers BM, Gemeinhart RA, Li T. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: lessons learned of the EPR effect and image-guided drug delivery. J. Control. Release. 2013;172(1):12–21. doi: 10.1016/j.jconrel.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Z, Wu Z-K, Tang H, Tang L-J, Jiang J-H. Activity-based DNA-gold nanoparticle probe as colorimetric biosensor for DNA methyltransferase/glycosylase assay. Anal. Chem. 2013;85(9):4376–4383. doi: 10.1021/ac303575f. [DOI] [PubMed] [Google Scholar]
- 41.Crucho CIC, Barros MT. Polymeric nanoparticles: a study on the preparation variables and characterization methods. Mater. Sci. Eng. C. 2017;80(Suppl. C):771–784. doi: 10.1016/j.msec.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C. 2016;60(Suppl. C):569–578. doi: 10.1016/j.msec.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 43.Lin G, Zhang H, Huang L. Smart polymeric nanoparticles for cancer gene delivery. Mol. Pharm. 2015;12(2):314–321. doi: 10.1021/mp500656v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo B, Ma PX. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Science China Chemistry. 2014;57(4):490–500. doi: 10.1007/s11426-014-5086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith IO, Liu XH, Smith LA, Ma PX. Nano-structured polymer scaffolds for tissue engineering and regenerative medicine. Wiiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1(2):226–236. doi: 10.1002/wnan.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bang SH, Sekhon SS, Kim Y-H, Min J. Preparation of liposomes containing lysosomal enzymes for therapeutic use. Biotechnol. Bioprocess Eng. 2014;19(5):766–770. [Google Scholar]
- 48.Dubey V, Mishra D, Asthana A, Jain NK. Transdermal delivery of a pineal hormone: melatonin via elastic liposomes. Biomaterials. 2006;27(18):3491–3496. doi: 10.1016/j.biomaterials.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 49.Parmentier J, Hofhaus G, Thomas S, et al. Improved oral bioavailability of human growth hormone by a combination of liposomes containing bio-enhancers and tetraether lipids and omeprazole. J. Pharm. Sci. 2014;103(12):3985–3993. doi: 10.1002/jps.24215. [DOI] [PubMed] [Google Scholar]
- 50.Wyrozumska P, Meissner J, Toporkiewicz M, et al. Liposome-coated lipoplex-based carrier for antisense oligonucleotides. Cancer Biol. Ther. 2015;16(1):66–76. doi: 10.4161/15384047.2014.987009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mcintosh DP, Heath TD. Liposome-mediated delivery of ribosome inactivating proteins to cells in vitro . Biochim. Biophys. Acta. 1982;690(2):224–230. doi: 10.1016/0005-2736(82)90326-1. [DOI] [PubMed] [Google Scholar]
- 52.Martins S, Sarmento B, Ferreira DC, Souto EB. Lipid-based colloidal carriers for peptide and protein delivery – liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007;2(4):595–607. [PMC free article] [PubMed] [Google Scholar]
- 53.Rasoulianboroujeni M, Kupgan G, Moghadam F, et al. Development of a DNA-liposome complex for gene delivery applications. Mater. Sci. Eng. C. 2017;75:191–197. doi: 10.1016/j.msec.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Seleci M, Ag Seleci D, Scheper T, Stahl F. Theranostic liposome-nanoparticle hybrids for drug delivery and bioimaging. Int. J. Mol. Sci. 2017;18(7) doi: 10.3390/ijms18071415. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Introduces the concept of hybrid nanomaterials for theranostics, simultaneous imaging and therapy.
- 55.Frey NA, Peng S, Cheng K, Sun S. Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009;38(9):2532–2542. doi: 10.1039/b815548h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reiner AT, Ferrer N-G, Venugopalan P, Lai RC, Lim SK, Dostálek J. Magnetic nanoparticle-enhanced surface plasmon resonance biosensor for extracellular vesicle analysis. Analyst. 2017;142(20):3913–3921. doi: 10.1039/c7an00469a. [DOI] [PubMed] [Google Scholar]
- 57.Kolesnichenko V, Goloverda G, Kucheryavy P, Spinu L. Iron oxide nanoparticles with a variable size and an iron oxidation state for imaging applications. In: Eniola-Adefeso L, Decuzzi P, editors. Nanotechnology in Medicine: From Molecules to Humans. ECI Symposium Series; LA, USA: 2016. http://dc.engconfintl.org/nanotech_med/26 [Google Scholar]
- 58.Wang Y, Cui H, Li K, et al. A magnetic nanoparticle-based multiple-gene delivery system for transfection of porcine kidney cells. PLoS ONE. 2014;9(7):e102886. doi: 10.1371/journal.pone.0102886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee RW, Shenoy DB, Sheel R. Chapter 2 – Micellar nanoparticles: applications for topical and passive transdermal drug delivery A2. In: Kulkarni VS, editor. Handbook of Non-Invasive Drug Delivery Systems. William Andrew Publishing; MA, USA: 2010. pp. 37–58. [Google Scholar]
- 60.Wang J, Mongayt D, Torchilin VP. Polymeric micelles for delivery of poorly soluble drugs: preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol)-lipid conjugate and positively charged lipids. J. Drug Target. 2005;13(1):73–80. doi: 10.1080/10611860400011935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang R, Saito R, Mano Y, et al. Convection-enhanced delivery of SN-38-loaded polymeric micelles (NK012) enables consistent distribution of SN-38 and is effective against rodent intracranial brain tumor models. Drug Deliv. 2016;23(8):2780–2786. doi: 10.3109/10717544.2015.1081994. [DOI] [PubMed] [Google Scholar]
- 62.Sun L, Deng X, Yang X, et al. Co-delivery of doxorubicin and curcumin by polymeric micelles for improving antitumor efficacy on breast carcinoma. RSC Adv. 2014;4(87):46737–46750. [Google Scholar]
- 63.Wang Y, Ding Y, Liu Z, Liu X, Chen L, Yan W. Bioactive lipids-based pH-sensitive micelles for co-delivery of doxorubicin and ceramide to overcome multidrug resistance in leukemia. Pharm. Res. 2013;30(11):2902–2916. doi: 10.1007/s11095-013-1121-5. [DOI] [PubMed] [Google Scholar]
- 64.Scott RW, Wilson OM, Crooks RM. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B. 2005;109(2):692–704. doi: 10.1021/jp0469665. [DOI] [PubMed] [Google Scholar]
- 65.Prajapati RN, Tekade RK, Gupta U, Gajbhiye V, Jain NK. Dendimer-mediated solubilization, formulation development and in vitro-in-vivo assessment of piroxicam. Mol. Pharm. 2009;6(3):940–950. doi: 10.1021/mp8002489. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Z, D'Emanuele A, Attwood D. Solubility enhancement of paclitaxel using a linear-dendritic block copolymer. Int. J. Pharm. 2013;452(1–2):173–179. doi: 10.1016/j.ijpharm.2013.04.075. [DOI] [PubMed] [Google Scholar]
- 67.Manikkath J, Hegde AR, Kalthur G, Parekh HS, Mutalik S. Influence of peptide dendrimers and sonophoresis on the transdermal delivery of ketoprofen. Int. J. Pharm. 2017;521(1–2):110–119. doi: 10.1016/j.ijpharm.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Majoros IJ, Williams CR, Becker A, Baker JR., Jr Methotrexate delivery via folate targeted dendrimer-based nanotherapeutic platform. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1(5):502–510. doi: 10.1002/wnan.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug-delivery system: a review. Int. J. Pharm. Investig. 2015;5(3):124–133. doi: 10.4103/2230-973X.160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin J-T, Liu Z-K, Zhu Q-L, et al. Redox-responsive nanocarriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surf. B. Biointerfaces. 2017;155:41–50. doi: 10.1016/j.colsurfb.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Portin L. Layer by Layer Assembly of the Polyelectrolyte on Mesoporous Silicon. Biosciences, University of Eastern Finland; Joensuu, Finland: 2012. pp. 1–59. [Google Scholar]
- 72.Gary-Bobo M, Hocine O, Brevet D, et al. Cancer therapy improvement with mesoporous silica nanoparticles combining targeting, drug delivery and PDT. Int. J. Pharm. 2012;423(2):509–515. doi: 10.1016/j.ijpharm.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 73.Janib SM, Moses AS, Mackay JA. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Del. Rev. 2010;62(11):1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farjadian F, Ghasemi S, Heidari R, Mohammadi-Samani S. In vitro and in vivo assessment of EDTA-modified silica nano-spheres with supreme capacity of iron capture as a novel antidote agent. Nanomed. Nanotechnol. Biol. Med. 2017;13(2):745–753. doi: 10.1016/j.nano.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 75.Farjadian F, Ahmadpour P, Samani SM, Hosseini M. Controlled size synthesis and application of nanosphere MCM-41 as potent adsorber of drugs: a novel approach to new antidote agent for intoxication. Microporous Mesoporous Mater. 2015;213:30–39. [Google Scholar]
- 76.Karimi M, Ghasemi A, Mirkiani S, Basri SMM, Hamblin MR. Carbon Nanotubes: Properties and Classification. Morgan & Claypool Publishers; CA, USA: 2017. [Google Scholar]
- 77.Zanello LP, Zhao B, Hu H, Haddon RC. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006;6(3):562–567. doi: 10.1021/nl051861e. [DOI] [PubMed] [Google Scholar]
- 78.Yunok O, Jun OJ, Junghwan O. Photothermal-triggered control of sub-cellular drug accumulation using doxorubicin-loaded single-walled carbon nanotubes for the effective killing of human breast cancer cells. Nanotechnology. 2017;28(12):125101. doi: 10.1088/1361-6528/aa5d7d. [DOI] [PubMed] [Google Scholar]
- 79.Scheinberg DA, Mcdevitt MR, Dao T, Mulvey JJ, Feinberg E, Alidori S. Carbon nanotubes as vaccine scaffolds. Adv. Drug Del. Rev. 2013;65(15) doi: 10.1016/j.addr.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanginario A, Miccoli B, Demarchi D. Carbon nanotubes as an effective opportunity for cancer diagnosis and treatment. Biosensors (Basel) 2017;7(1) doi: 10.3390/bios7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang JTW, Rubio N, Kafa H, et al. Kinetics of functionalised carbon nanotube distribution in mouse brain after systemic injection: spatial to ultra-structural analyses. J. Control. Release. 2016;224:22–32. doi: 10.1016/j.jconrel.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roman JA, Niedzielko TL, Haddon RC, Parpura V, Floyd CL. Single-walled carbon nanotubes chemically functionalized with polyethylene glycol promote tissue repair in a rat model of spinal cord injury. J. Neurotrauma. 2011;28(11):2349–2362. doi: 10.1089/neu.2010.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain S, Hirst D, O'Sullivan J. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012;85(1010):101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gholipourmalekabadi M, Mobaraki M, Ghaffari M, et al. Targeted drug delivery based on gold nanoparticle derivatives. Curr. Pharm. Des. 2017;23(20):2918–2929. doi: 10.2174/1381612823666170419105413. [DOI] [PubMed] [Google Scholar]
- 85.Tian L, Lu L, Qiao Y, Ravi S, Salatan F, Melancon MP. Stimuli-responsive gold nanoparticles for cancer diagnosis and therapy. J. Funct. Biomater. 2016;7(3):19. doi: 10.3390/jfb7030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239(1):129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 87.Liao YH, Chang YJ, Yoshiike Y, Chang YC, Chen YR. Negatively charged gold nanoparticles inhibit Alzheimer's amyloid-β fibrillization, induce fibril dissociation, and mitigate neurotoxicity. Small. 2012;8(23):3631–3639. doi: 10.1002/smll.201201068. [DOI] [PubMed] [Google Scholar]
- 88.Barathmanikanth S, Kalishwaralal K, Sriram M, et al. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010;8(1):16. doi: 10.1186/1477-3155-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leonavičienė L, Kirdaitė G, Bradūnaitė R, et al. Effect of gold nanoparticles in the treatment of established collagen arthritis in rats. Medicina (Kaunas) 2012;48(2):91–101. [PubMed] [Google Scholar]
- 90.Spivak MY, Bubnov RV, Yemets IM, Lazarenko LM, Tymoshok NO, Ulberg ZR. Development and testing of gold nanoparticles for drug delivery and treatment of heart failure: a theranostic potential for PPP cardiology. Epma J. 2013;4(1):20. doi: 10.1186/1878-5085-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith AM, Nie S. Semiconductor nanocrystals: structure, properties, and band gap engineering. Acc. Chem. Res. 2010;43(2):190–200. doi: 10.1021/ar9001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michalet X, Pinaud F, Bentolila L, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307(5709):538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hild W, Breunig M, Göpferich A. Quantum dots–nano-sized probes for the exploration of cellular and intracellular targeting. Eur. J. Pharm. Biopharm. 2008;68(2):153–168. doi: 10.1016/j.ejpb.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 94.Bawa R, Melethil S, Simmons WJ, Harris D. Nanopharmaceuticals: patenting issues and FDA regulatory challenges. SciTech Lawyer. 2008;5(2):1–8. [Google Scholar]
- 95.Eaton MA. Improving the translation in Europe of nanomedicines (a.k.a. drug delivery) from academia to industry. J. Control. Release. 2012;164(3):370–371. doi: 10.1016/j.jconrel.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 96.Von Windheim J, Myers B. A lab-to-market roadmap for early-stage entrepreneurship. Transl. Mater. Res. 2014;1(1):16001–16001. [Google Scholar]
- 97.Bosetti R, Vereeck L. The impact of effective patents on future innovations in nanomedicine. Pharm. Patent Analyst. 2012;1(1):37–43. doi: 10.4155/ppa.11.4. [DOI] [PubMed] [Google Scholar]
- 98.Borges BJ, Carminati LS, Fernandes PM, Fernandes AaR. Regulatory framework of nanopharmaceuticals in developing countries: an analysis of the current rules in Brazil. In: Grumezescu A, editor. Inorganic Frameworks as Smart Nanomedicines. William Andrew Publishing; MA, USA: 2018. pp. 605–639. [Google Scholar]
- 99.Conde J, Artzi N. Are RNAi and miRNA therapeutics truly dead? 2015;33:141–144. doi: 10.1016/j.tibtech.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 100.Shapira P, Wang J. From lab to market? Strategies and issues in the commercialization of nanotechnology in China. Asian Bus. Manag. 2009;8(4):461–489. [Google Scholar]
- 101.Avorn J. The $2.6 billion pill – methodologic and policy considerations. N. Engl. J. Med. 2015;372(20):1877–1879. doi: 10.1056/NEJMp1500848. [DOI] [PubMed] [Google Scholar]
- 102.Dimasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 103.Milman J. Does it really cost $2.6 billion to develop a new drug? The Washington Post. 2014 www.washingtonpost.com/news/wonk/wp/2014/11/18/does-it-really-cost-2-6-billion-to-develop-a-new-drug/?utm_term=.42007dcc67d9 [Google Scholar]
- 104.Bhattacharya I, Heatherington A, Barton J. Applying the best of oncology drug development paradigms to the non-malignant space. Drug Discov. Today. 2016;21(12):1869–1872. doi: 10.1016/j.drudis.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 105.Dimasi JA, Grabowski HG, Hansen RW. The cost of drug development. N. Engl. J. Med. 2015;372(20):1972–1972. doi: 10.1056/NEJMc1504317. [DOI] [PubMed] [Google Scholar]
- 106.Erkekoglu P, Kocer-Gumusel B. Toxicity assessment of nanopharmaceuticals. In: Mihai A, editor. Inorganic Frameworks as Smart Nanomedicines. Elsevier; Oxford, UK: 2018. pp. 565–603. [Google Scholar]
- 107.Turanlı ET, Everest E. Nanomedicine. In: Ünlü H, Horing NJM, Dabrowski J, editors. Low-Dimensional and Nanostructured Materials and Devices. Springer International Publishing; NY, USA: 2016. pp. 579–587. [Google Scholar]
- 108.Tinkle S, Mcneil SE, Mühlebach S, et al. Nanomedicines: addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014;1313(1):35–56. doi: 10.1111/nyas.12403. [DOI] [PubMed] [Google Scholar]
- 109.Satalkar P, Elger BS, Hunziker P, Shaw D. Challenges of clinical translation in nanomedicine: a qualitative study. Nanomedicine. 2016;12(4):893–900. doi: 10.1016/j.nano.2015.12.376. [DOI] [PubMed] [Google Scholar]
- 110.Zamboni WC, Torchilin V, Patri AK, et al. Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin. Cancer Res. 2012;18(12):3229–3241. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Satalkar P, Elger BS, Shaw D. Naming it ‘nano’: expert views on ‘nano’ terminology in informed consent forms of first-in-human nanomedicine trials. Nanomedicine. 2016;11(8):933–940. doi: 10.2217/nnm-2015-0003. [DOI] [PubMed] [Google Scholar]
- 112.Allon I, Ben-Yehudah A, Dekel R, Solbakk J-H, Weltring K-M, Siegal G. Ethical issues in nanomedicine: tempest in a teapot? Med. Health Care Philos. 2017;20(1):3–11. doi: 10.1007/s11019-016-9720-7. [DOI] [PubMed] [Google Scholar]
- 113.Beaudry C, Allaoui S. Impact of public and private research funding on scientific production: the case of nanotechnology. Res. Policy. 2012;41(9):1589–1606. [Google Scholar]
- 114.Fatehi L, Wolf SM, Mccullough J, et al. Recommendations for nanomedicine human subjects research oversight: an evolutionary approach for an emerging field. J. Law Med. Ethics. 2012;40(4):716–750. doi: 10.1111/j.1748-720X.2012.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Graur F, Elisei R, Szasz A, et al. Ethical issues in nanomedicine. In: Vlad S, Ciupa RV, editors. International Conference on Advancements of Medicine and Health Care through Technology. Vol. 36. Springer; Berlin, Heidelberg: 2011. pp. 9–12. [Google Scholar]
- 116.Wolf SM. Introduction: the challenge of nanomedicine human subjects research: protecting participants, workers, bystanders, and the environment. J. Law Med. Ethics. 2012;40(4):712–715. doi: 10.1111/j.1748-720X.2012.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamburg MA. FDA's approach to regulation of products of nanotechnology. Science. 2012;336(6079):299–300. doi: 10.1126/science.1205441. [DOI] [PubMed] [Google Scholar]
- 118.Bawa R. FDA and Nanotech: baby steps lead to regulatory uncertainty. In: Bagchi D, Bagchi M, Moriyama H, Shahidi F, editors. Bio-Nanotechnology: A Revolution in Food, Biomedical and Health Sciences. John Wiley & Sons, Ltd; Chichester, UK: 2013. pp. 720–732. [Google Scholar]
- 119.Zolnik B, Potter TM, Stern ST. Detecting reactive oxygen species in primary hepatocytes treated with nanoparticles. Methods Mol. Biol. (Clifton, N.J.) 2011;697(3):173–179. doi: 10.1007/978-1-60327-198-1_18. [DOI] [PubMed] [Google Scholar]
- 120.Nowack B, Ranville JF, Diamond S, et al. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012;31(1):50–59. doi: 10.1002/etc.726. [DOI] [PubMed] [Google Scholar]
- 121.Louie SM, Ma R, Lowry GV, Gregory KB, Apte SC, Lead JR. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012;46(13):6893–6899. doi: 10.1021/es300839e. [DOI] [PubMed] [Google Scholar]
- 122.Bawa R. Regulating nanomedicine–can the FDA handle it? Curr. Drug Del. 2011;8(3):227–234. doi: 10.2174/156720111795256156. [DOI] [PubMed] [Google Scholar]; • Highlights the ongoing question of regulatiory authorities and nanomedicine.
- 123.Gaspar R, Aksu B, Cuine A, et al. Towards a European strategy for medicines research (2014–2020): the EUFEPS position paper on Horizon 2020. Eur. J. Pharm. Sci. 2012;47(5):979–987. doi: 10.1016/j.ejps.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 124.European Medicines A. Questions and answers on biosimilar medicines (similar biological medicinal products) 2012;44:1–1. www.medicinesforeurope.com/2012/09/27/ema-questions-and-answers-on-biosimilar-medicines-similar-biological-medicinal-products/ [Google Scholar]
- 125.Schellekens H, Stegemann S, Weinstein V, et al. How to regulate nonbiological complex drugs (NBCD) and their follow-on versions: points to consider. AAPS J. 2014;16(1):15–21. doi: 10.1208/s12248-013-9533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Andrews PLR, Kovacs M, Watson JW. The anti-emetic action of the neurokinin1 receptor antagonist CP-99,994 does not require the presence of the area postrema in the dog. Neurosci. Lett. 2001;314(1–2):102–104. doi: 10.1016/s0304-3940(01)02269-8. [DOI] [PubMed] [Google Scholar]
- 127.Merck & Co., Inc.; NJ, USA: 2015. Emend® (aprepitant) capsules, for oral use and oral suspension [Prescribing Information] [Google Scholar]
- 128.Maggi CA. The mammalian tachykinin receptors. Gen. Pharmacol. 1995;26(5):911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- 129.Junghanns JUaH, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008;3(3):295–309. doi: 10.2147/ijn.s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy-and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann. Oncol. 2010;21(Suppl. 5) doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 131.Brandt J, Henning S, Michler G, Hein W, Bernstein A, Schulz M. Nanocrystalline hydroxyapatite for bone repair: an animal study. J. Mater. Sci. Mater. Med. 2010;21(1):283–294. doi: 10.1007/s10856-009-3859-1. [DOI] [PubMed] [Google Scholar]
- 132.Huber FX, Belyaev O, Hillmeier J, et al. First histological observations on the incorporation of a novel nanocrystalline hydroxyapatite paste OSTIM® in human cancellous bone. BMC Musculoskelet. Disord. 2006;7(50) doi: 10.1186/1471-2474-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004;25(6):987–994. doi: 10.1016/s0142-9612(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 134.Narayan R, Pednekar A, Bhuyan D, Gowda C, Koteshwara K, Nayak UY. A top-down technique to improve the solubility and bioavailability of aceclofenac: in vitro and in vivo studies. Int. J. Nanomed. 2017;12:4921. doi: 10.2147/IJN.S141504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 136.Shegokar R, Müller RH. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010;399(1–2):129–139. doi: 10.1016/j.ijpharm.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 137.Kesisoglou F, Panmai S, Wu Y. Nanosizing – oral formulation development and biopharmaceutical evaluation. Adv. Drug Del. Rev. 2007;59(7):631–644. doi: 10.1016/j.addr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 138.Sehgal SN. Rapamune® (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin. Biochem. 1998;31(5):335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 139.Marya S, Ariyanayagam T, Chatterjee B, Toms AP, Crawford R. A prospective study of the efficacy of vitoss (beta tricalcium phosphate) as a bone graft substitute for instrumented posterolateral lumbar fusions. Spine J. 2017;17(3):S23. [Google Scholar]
- 140.Campion CR, Ball SL, Clarke DL, Hing KA. Microstructure and chemistry affects apatite nucleation on calcium phosphate bone graft substitutes. J. Mater. Sci. Mater. Med. 2013;24(3):597–610. doi: 10.1007/s10856-012-4833-x. [DOI] [PubMed] [Google Scholar]
- 141.Bajwa S, Munawar A, Khan W. Nanotechnology in medicine: innovation to market. Pharm. Bioprocess. 2017;5(2):11–15. [Google Scholar]
- 142.Behabtu N, Young CC, Tsentalovich DE, et al. Strong, light, multifunctional fibers of carbon nanotubes with ultrahigh conductivity. Science. 2013;339(6116):182–186. doi: 10.1126/science.1228061. [DOI] [PubMed] [Google Scholar]
- 143.Swanson JM, Wigal SB, Wigal T, et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study) Pediatrics. 2004;113(3):e206–e216. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- 144.Harris G. Warning urged on stimulants like Ritalin. New York Times. 2006:10. [Google Scholar]
- 145.Diller LH. The run on Ritalin: attention deficit disorder and stimulant treatment in the 1990s. Hastings Cent. Rep. 1996;26(2):12–18. [PubMed] [Google Scholar]
- 146.Lange KW, Reichl S, Lange KM, Tucha L, Tucha O. The history of attention deficit hyperactivity disorder. Atten. Defic. Hyperact. Disord. 2010;2(4):241–255. doi: 10.1007/s12402-010-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Doward J, Craig E. Ritalin use for ADHD children soars fourfold. Observer. 2012:6. [Google Scholar]
- 148.Hunt RD. Functional roles of norepinephrine and dopamine in ADHD. Medscape Psychiatr. 2006;11(1):2006. [Google Scholar]
- 149.Arnsten AF, Li B-M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 150.Markowitz J, Devane C, Ramamoorthy S, Zhu H-J. The psychostimulant d-threo-(R,R)-methylphenidate binds as an agonist to the 5HT1A receptor. Die Pharmazie. 2009;64(2):123–125. [PubMed] [Google Scholar]
- 151.Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J. Neurosci. 2001;21(2):RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Steele M, Weiss M, Swanson J, Wang J, Prinzo RS, Binder CE. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder. Can. J. Clin. Pharmacol. 2006;13(1):e50–e62. [PubMed] [Google Scholar]
- 153.Mott TF, Leach L. Is methylphenidate useful for treating adolescents with ADHD? J. Fam. Pract. 2004;53(9):650–663. [PubMed] [Google Scholar]
- 154.Handen BL, Feldman H, Gosling A, Breaux AM, Mcauliffe S. Adverse side effects of methylphenidate among mentally retarded children with ADHD. J. Am. Acad. Child Adolesc. Psychiatry. 1991;30(2):241–245. doi: 10.1097/00004583-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 155.Alagona P., Jr Fenofibric acid: a new fibrate approved for use in combination with statin for the treatment of mixed dyslipidemia. Vasc. Health Risk Manag. 2010;6(1):351–362. doi: 10.2147/vhrm.s6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abbott Laboratories; IL, USA: 2010. Tricor® fenofibrate tablet [Prescribing Information] [Google Scholar]
- 157.Abbott Laboratories Press Release; 2004. ‘Nanoparticle technology now allows TriCor(R) to be taken with or without food’.www.abbottinvestor.com/news-releases/news-release-details/abbott-receives-fda-approval-new-formulation-tricorr-fenofibrate [Google Scholar]
- 158.Lasic DD. Doxorubicin in sterically stabilized liposomes. Nature. 1996;380(6574):561. doi: 10.1038/380561a0. [DOI] [PubMed] [Google Scholar]
- 159.Gill PS, Wernz J, Scadden DT, et al. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. J. Clin. Oncol. 1996;14(8):2353–2364. doi: 10.1200/JCO.1996.14.8.2353. [DOI] [PubMed] [Google Scholar]
- 160.Andreopoulou E, Gaiotti D, Kim E, et al. Pegylated liposomal doxorubicin HCL (PLD; Caelyx/Doxil®): experience with long-term maintenance in responding patients with recurrent epithelial ovarian cancer. Ann. Oncol. 2007;18(4):716–721. doi: 10.1093/annonc/mdl484. [DOI] [PubMed] [Google Scholar]
- 161.Pillai G, Ceballos-Coronel ML. Science and technology of the emerging nanomedicines in cancer therapy: a primer for physicians and pharmacists. SAGE Open Med. 2013;1 doi: 10.1177/2050312113513759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4(3):95. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gordon AN, Granai C, Rose PG, et al. Phase II study of liposomal doxorubicin in platinum-and paclitaxel-refractory epithelial ovarian cancer. J. Clin. Oncol. 2000;18(17):3093–3100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- 164.Gabizon AA, Shmeeda H, Zalipsky S. Pros and cons of the liposome platform in cancer drug targeting. J. Liposome Res. 2006;16(3):175–183. doi: 10.1080/08982100600848769. [DOI] [PubMed] [Google Scholar]
- 165.Murphy T, Yee KW. Cytarabine and daunorubicin for the treatment of acute myeloid leukemia. Expert Opin. Pharmacother. 2017;18(16):1765–1780. doi: 10.1080/14656566.2017.1391216. [DOI] [PubMed] [Google Scholar]
- 166.[No authors listed] FDA approves DaunoXome as first-line therapy for Kaposi's sarcoma. Food and Drug Administration. J. Int. Assoc. Phys. AIDS Care. 1996;2(5):50–51. [PubMed] [Google Scholar]
- 167.Fassas A, Anagnostopoulos A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk. Lymphoma. 2005;46(6):795–802. doi: 10.1080/10428190500052438. [DOI] [PubMed] [Google Scholar]
- 168.Yarmolenko PS, Zhao Y, Landon C, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int. J. Hyperthermia. 2010;26(5):485–498. doi: 10.3109/02656731003789284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fosså S, Aass N, Parö G. A Phase II study of DaunoXome® in advanced urothelial transitional cell carcinoma. Eur. J. Cancer. 1998;34(7):1131–1132. doi: 10.1016/s0959-8049(97)10156-3. [DOI] [PubMed] [Google Scholar]
- 170.Passero FC, Jr, Grapsa D, Syrigos KN, Saif MW. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev. Anticancer Ther. 2016;16(7):697–703. doi: 10.1080/14737140.2016.1192471. [DOI] [PubMed] [Google Scholar]
- 171.Digiulio S. FDA Approves onivyde combo regimen for advanced pancreatic cancer. Oncology Times. 2015 [Google Scholar]
- 172.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 173.Zhang H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016;9:3001. doi: 10.2147/OTT.S105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mantripragada S. A lipid based depot (DepoFoam® technology) for sustained release drug delivery. Prog. Lipid Res. 2002;41(5):392–406. doi: 10.1016/s0163-7827(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 175.Jaeckle KA, Batchelor T, O'day SJ, et al. An open label trial of sustained-release cytarabine (DepoCyt™) for the intrathecal treatment of solid tumor neoplastic meningitis. J. Neurooncol. 2002;57(3):231–239. doi: 10.1023/a:1015752331041. [DOI] [PubMed] [Google Scholar]
- 176.Pearce H, Winter M, Beck WT. Structural characteristics of compounds that modulate P-glycoprotein-associated multidrug resistance. Adv. Enzyme Regul. 1990;30:357–373. doi: 10.1016/0065-2571(90)90026-x. [DOI] [PubMed] [Google Scholar]
- 177.Kim S, Chatelut E, Kim JC, et al. Extended CSF cytarabine exposure following intrathecal administration of DTC 101. J. Clin. Oncol. 1993;11(11):2186–2193. doi: 10.1200/JCO.1993.11.11.2186. [DOI] [PubMed] [Google Scholar]
- 178.Takayama N, Sato N, O'Brien SG, Ikeda Y, Okamoto SI. Imatinib mesylate has limited activity against the central nervous system involvement of Philadelphia chromosome-positive acute lymphoblastic leukaemia due to poor penetration into cerebrospinal fluid. Br. J. Haematol. 2002;119(1):106–108. doi: 10.1046/j.1365-2141.2002.03881.x. [DOI] [PubMed] [Google Scholar]
- 179.Galmarini CM, Thomas X, Calvo F, et al. In vivo mechanisms of resistance to cytarabine in acute myeloid leukaemia. Br. J. Haematol. 2002;117(4):860–868. doi: 10.1046/j.1365-2141.2002.03538.x. [DOI] [PubMed] [Google Scholar]
- 180.Dawidczyk CM, Kim C, Park JH, et al. State-of-the-art in design rules for drug delivery platforms: lessons learned from FDA-approved nanomedicines. J. Control. Rel. 2014;187:133–144. doi: 10.1016/j.jconrel.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Harrison TS, Lyseng-Williamson KA. Vincristine sulfate liposome injection. Biodrugs. 2013;27(1):69–74. doi: 10.1007/s40259-012-0002-5. [DOI] [PubMed] [Google Scholar]
- 182.Yang S-H, Lin C-C, Lin Z-Z, Tseng Y-L, Hong R-L. A Phase I and pharmacokinetic study of liposomal vinorelbine in patients with advanced solid tumor. Invest. New Drugs. 2012;30(1):282–289. doi: 10.1007/s10637-010-9522-3. [DOI] [PubMed] [Google Scholar]
- 183.Boman NL, Masin D, Mayer LD, Cullis PR, Bally MB. Liposomal vincristine which exhibits increased drug retention and increased circulation longevity cures mice bearing P388 tumors. Cancer Res. 1994;54(11):2830–2833. [PubMed] [Google Scholar]
- 184.Thomas DA, Sarris AH, Cortes J, et al. Phase II study of sphingosomal vincristine in patients with recurrent or refractory adult acute lymphocytic leukemia. Cancer. 2006;106(1):120–127. doi: 10.1002/cncr.21595. [DOI] [PubMed] [Google Scholar]
- 185.Takemoto K, Kanazawa K. AmBisome: relationship between the pharmacokinetic characteristics acquired by liposomal formulation and safety/efficacy. J. Liposome Res. 2016;27(3):1–9. doi: 10.1080/08982104.2016.1205087. [DOI] [PubMed] [Google Scholar]
- 186.Clemons KV, Stevens DA. Comparative efficacies of four amphotericin B formulations – Fungizone, Amphotec (Amphocil), AmBisome, and Abelcet – against systemic murine aspergillosis. Antimicrob. Agents Chemother. 2004;48(3):1047–1050. doi: 10.1128/AAC.48.3.1047-1050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stone NRH, Bicanic T, Salim R, Hope W. Liposomal Amphotericin B (AmBisome®): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs. 2016;76(4):485–500. doi: 10.1007/s40265-016-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Crain ML. Daunorubicin & CYTARABINE LIPOSome (Vyxeos™) Oncology Times. 2018;40(10):30. [Google Scholar]
- 189.Allen C. Why I'm holding onto hope for nano in oncology. Mol. Pharm. 2016;13(8):2603–2604. doi: 10.1021/acs.molpharmaceut.6b00547. [DOI] [PubMed] [Google Scholar]
- 190.Waknine Y. Medscape. FDA approves new drug for hereditary angioedema. 2011. www.medscape.com/viewarticle/748570
- 191.Mayer L, Liboiron B, Xie S, Tardi P, Paulsen K, Chiarella M. www.controlledreleasesociety.org/meetings/Documents/2016%20Abstracts/33.pdf VYXEOS™(CPX-351) Significantly improves overall survival in Phase 3 high-risk AML trial, validating the CombiPlex technology and opening opportunities for novel combinations Lawrence Mayer, Barry Liboiron, Sherwin Xie and Paul Tardi, Kim Paulsen, Michael Chiarella and Arthur Louie.
- 192.Wu TC. On the development of antifungal agents: perspective of the US Food and Drug Administration. Clin. Infect. Dis. 1994;19(Suppl. 1):S54–S58. doi: 10.1093/clinids/19.supplement_1.s54. [DOI] [PubMed] [Google Scholar]
- 193.Camarata PJ, Dunn DL, Farney AC, Parker RG, Seljeskog EL. Continual intracavitary administration of amphotericin B as an adjunct in the treatment of aspergillus brain abscess: case report and review of the literature. Neurosurgery. 1992;31(3):575–579. doi: 10.1227/00006123-199209000-00023. [DOI] [PubMed] [Google Scholar]
- 194.Veerareddy PR, Vobalaboina V. Lipid-based formulations of amphotericin B. Drugs of Today. 2004;40(2):133–146. doi: 10.1358/dot.2004.40.2.799425. [DOI] [PubMed] [Google Scholar]
- 195.Bressler NM Treatment of age-related macular degeneration with photodynamic therapy study G. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch. Ophthalmol. 2001;119(2):198–207. [PubMed] [Google Scholar]
- 196.Liu R. Methods of making liposomes containing hydro-monobenzoporphyrin photosensitizer. 1998. US Patent 5707608.
- 197.Rogers AH, Duker JS, Nichols N, Baker BJ. Photodynamic therapy of idiopathic and inflammatory choroidal neovascularization in young adults. Ophthalmology. 2003;110(7):1315–1320. doi: 10.1016/S0161-6420(03)00466-4. [DOI] [PubMed] [Google Scholar]
- 198.Parodi MB, Iacono P, Spasse S, Ravalico G. Photodynamic therapy for juxtafoveal choroidal neovascularization associated with multifocal choroiditis. Am. J. Ophthalmol. 2006;141(1):123–128. doi: 10.1016/j.ajo.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 199.Wachtlin J, Heimann H, Behme T, Foerster MH. Long-term results after photodynamic therapy with verteporfin for choroidal neovascularizations secondary to inflammatory chorioretinal diseases. Graefes Arch. Clin. Exp. Ophthalmol. 2003;241(11):899–906. doi: 10.1007/s00417-003-0734-5. [DOI] [PubMed] [Google Scholar]
- 200.Hogan A, Behan U, Kilmartin DJ. Outcomes after combination photodynamic therapy and immunosuppression for inflammatory subfoveal choroidal neovascularisation. Br. J. Ophthalmol. 2005;89(9):1109–1111. doi: 10.1136/bjo.2004.063024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016;105(2):460–475. doi: 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 202.Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): In vitro comparison with other anti-tumor necrosis factor α agents. Inflamm. Bowel Dis. 2007;13(11):1323–1332. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 203.Connock M, Tubeuf S, Malottki K, et al. Certolizumab pegol (CIMZIA®) for the treatment of rheumatoid arthritis. Health Technol. Assess. 2010;14(Suppl 2):1–10. doi: 10.3310/hta14suppl2/01. [DOI] [PubMed] [Google Scholar]
- 204.Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn's disease. N. Engl. J. Med. 2007;357(3):228–238. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 205.Mease P, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24 week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Ann. Rheum. Dis. 2013;73(1):48–55. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann. Rheum. Dis. 2013;73(1):39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Casanova JL, Abel L. Revisiting Crohn's disease as a primary immunodeficiency of macrophages. J. Exp. Med. 2009;206(9):1839–1843. doi: 10.1084/jem.20091683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Stephan J, Vlekova V, Le Deist F, et al. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J. Pediatrics. 1993;123(4):564–572. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- 209.Sigma-Tau Pharmaceuticals, Inc.; MD, USA: 2014. Adagen® (pegademase bovine) injection [Prescribing Information] [Google Scholar]
- 210.Torres C. Rare opportunities appear on the horizon to treat rare diseases. Nat. Med. 2010;16(3):241–241. doi: 10.1038/nm0310-241. [DOI] [PubMed] [Google Scholar]
- 211.Joralemon MJ, Mcrae S, Emrick T. PEGylated polymers for medicine: from conjugation to self-assembled systems. Chem. Commun. 2010;46(9):1377–1393. doi: 10.1039/b920570p. [DOI] [PubMed] [Google Scholar]
- 212.Davis S, Abuchowski A, Park YK, Davis FF. Alteration of the circulating life and antigenic properties of bovine adenosine deaminase in mice by attachment of polyethylene glycol. Clin. Exp. Immunol. 1981;46(3):649–652. [PMC free article] [PubMed] [Google Scholar]
- 213.Greenwald RB, Choe YH, Mcguire J, Conover CD. Effective drug-delivery by PEGylated drug conjugates. Adv. Drug Del. Rev. 2003;55(2):217–250. doi: 10.1016/s0169-409x(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 214.Sheridan WP, Fox RM, Begley CG, et al. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339(8794):640–644. doi: 10.1016/0140-6736(92)90795-5. [DOI] [PubMed] [Google Scholar]
- 215.Curran MP, Goa KL. Pegfilgrastim. Drugs. 2002;62(8):1207–1213. doi: 10.2165/00003495-200262080-00012. [DOI] [PubMed] [Google Scholar]
- 216.Alconcel SNS, Baas AS, Maynard HD. FDA-approved poly(ethylene glycol)-protein conjugate drugs. Polym. Chem. 2011;2(7):1442–1448. [Google Scholar]
- 217.Piedmonte DM, Treuheit MJ. Formulation of Neulasta®(pegfilgrastim) Adv. Drug Del. Rev. 2008;60(1):50–58. doi: 10.1016/j.addr.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 218.Milton HJ, Chess RB. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2(3):214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 219.Enzon Pharmaceuticals Inc.; 2006. Oncaspar® (pegaspargase) Intravenous or Intramuscular Injection [Prescribing Information] [Google Scholar]
- 220.Graham ML. Pegaspargase: a review of clinical studies. Adv. Drug Del. Rev. 2003;55(10):1293–1302. doi: 10.1016/s0169-409x(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 221.Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003;2(5):347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 222.Peters BG, Goeckner BJ, Ponzillo JJ, Velasquez WS, Wilson AL. Pegaspargase versus asparaginase in adult ALL: a pharmacoeconomic assessment. Formulary. 1995;30(7):388–393. [PubMed] [Google Scholar]
- 223.Duncan R. Polymer therapeutics: top 10 selling pharmaceuticals–what next? J. Control. Release. 2014;190:371–380. doi: 10.1016/j.jconrel.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 224.Fried MW, Shiffman ML, Rajender Reddy K, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 225.Lau GKK, Piratvisuth T, Kang XL, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2005;352(26):2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 226.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 227.Roelfsema F, Biermasz NR, Pereira AM, Romijn J. Nanomedicines in the treatment of acromegaly: Focus on pegvisomant. Int. J. Nanomed. 2006;1(4):385–398. doi: 10.2147/nano.2006.1.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Van der Lely AJ, Hutson RK, Trainer PJ, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358(9295):1754–1759. doi: 10.1016/s0140-6736(01)06844-1. [DOI] [PubMed] [Google Scholar]
- 229.Leonart LP, Tonin FS, Ferreira VL, et al. Effectiveness and safety of pegvisomant: a systematic review and meta-analysis of observational longitudinal studies. Endocrine. 2018 doi: 10.1007/s12020-018-1729-7. [DOI] [PubMed] [Google Scholar]
- 230.Gragoudas ES, Adamis AP, Cunningham ETJ, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 231.Shukla D, Namperumalsamy P, Goldbaum M, Cunningham E., Jr Pegaptanib sodium for ocular vascular disease. Indian J. Ophthalmol. 2007;55(6):427–430. doi: 10.4103/0301-4738.36476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bunka DHJ, Platonova O, Stockley PG. Development of aptamer therapeutics. Curr. Opin. Pharmacol. 2010;10(5):557–562. doi: 10.1016/j.coph.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 233.Ng EWM, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006;5(2):123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 234.Mcgahan L. Continuous erythropoietin receptor activator (Mircera) for renal anemia. Issues Emerg. Health Technol. 2008;(113):1–6. [PubMed] [Google Scholar]
- 235.Association EM. Scientific discussion: summary of product characteristics: MIRCERA (methoxy polyethyl-ene glycol-epoetin beta) 2008.
- 236.Frimat L, Mariat C, Landais P, Koné S, Commenges B, Choukroun G. Anaemia management with CERA in routine clinical practice: OCEANE (Cohorte Mircera patients non-dialyses), a national, multicenter, longitudinal, observational prospective study, in patients with chronic kidney disease not on dialysis. BMJ open. 2013;3(3):e001888. doi: 10.1136/bmjopen-2012-001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Glue P, Fang JW, Rouzier-Panis R, et al. Pegylated interferon-α2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Clin. Pharmacol. Ther. 2000;68(5):556–567. doi: 10.1067/mcp.2000.110973. [DOI] [PubMed] [Google Scholar]
- 238.Jacobson IM, Brown RS, Freilich B, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46(4):971–981. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 239.Manns M, Pockros P, Norkrans G, et al. Long-term clearance of hepatitis C virus following interferon α-2b or peginterferon α-2b, alone or in combination with ribavirin. J. Viral Hepat. 2013;20(8):524–529. doi: 10.1111/jvh.12074. [DOI] [PubMed] [Google Scholar]
- 240.Savient to Present Multiple Abstracts At the European League Against Rheumatism (EULAR) Annual Congress. 2009 [Google Scholar]
- 241.US Food and Drug Administration. Prescribing Information for KRYSTEXXA (TM): Savient Pharmaceuticals. 2009. www.accessdata.fda.gov/drugsatfda_docs/label/2010/125293s0000lbl.pdf
- 242.Food F. Drug Administration. Label and Approval History KRYSTEXXA BLA 125293 Savient Pharm. 2010.
- 243.Alconcel SN, Baas AS, Maynard HD. FDA-approved poly (ethylene glycol)–protein conjugate drugs. Polym. Chem. 2011;2(7):1442–1448. [Google Scholar]
- 244.Biggers K, Scheinfeld N. Pegloticase, a polyethylene glycol conjugate of uricase for the potential intravenous treatment of gout. Curr. Opin. Investig. Drugs. 2008;9(4):422–429. [PubMed] [Google Scholar]
- 245.Centonze D, Puma E, Saleri C, et al. Pegylation and interferons in multiple sclerosis. Farmeconomia. 2016;17(Suppl. 2):5–11. [Google Scholar]
- 246.Chaplin S, Gnanapavan S. Plegridy for the treatment of RRMS in adults. Prescriber. 2015;26(9):29–31. [Google Scholar]
- 247.Idec B. Plegridy prescribing information. 2015.
- 248.Turecek P, Romeder-Finger S, Apostol C, et al. A world-wide survey and field study in clinical haemostasis laboratories to evaluate FVIII: C activity assay variability of ADYNOVATE and OBIZUR in comparison with ADVATE. Haemophilia. 2016;22(6):957–965. doi: 10.1111/hae.13001. [DOI] [PubMed] [Google Scholar]
- 249.Konkle BA, Stasyshyn O, Chowdary P, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(9):1078–1085. doi: 10.1182/blood-2015-03-630897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Grewal IS. Emerging Protein Biotherapeutics. CRC Press; FL, USA: 2009. [Google Scholar]
- 251.Wolinsky JS, Narayana PA, O'Connor P, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann. Neurol. 2007;61(1):14–24. doi: 10.1002/ana.21079. [DOI] [PubMed] [Google Scholar]
- 252.Johnson KP. Glatiramer acetate for treatment of relapsing-remitting multiple sclerosis. Expert Rev. Neurother. 2012;12(4):371–384. doi: 10.1586/ern.12.25. [DOI] [PubMed] [Google Scholar]
- 253.Mckeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29(5):425–432. doi: 10.1007/s40263-015-0245-z. [DOI] [PubMed] [Google Scholar]
- 254.Arnon R, Aharoni R. Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications. Proc. Natl Acad. Sci. USA. 2004;101(Suppl. 2):14593–14598. doi: 10.1073/pnas.0404887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Weber MS, Hohlfeld R, Zamvil SS. Mechanism of action of glatiramer acetate in treatment of multiple sclerosis. Neurotherapeutics. 2007;4(4):647–653. doi: 10.1016/j.nurt.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Berges R. Eligard®: pharmacokinetics, effect on testosterone and psa levels and tolerability. Eur. Urol. Suppl. 2005;4(5):20–25. [Google Scholar]
- 257.Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61(2):25–31. doi: 10.1016/s0090-4295(02)02396-8. [DOI] [PubMed] [Google Scholar]
- 258.Wex J, Sidhu M, Odeyemi I, Abou-Setta AM, Retsa P, Tombal B. Leuprolide acetate 1-, 3- and 6-monthly depot formulations in androgen deprivation therapy for prostate cancer in nine European countries: evidence review and economic evaluation. Clinicoecon. Outcomes Res. 2013;5:257–269. doi: 10.2147/CEOR.S44855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Slatopolsky EA, Burke SK, Dillon MA. RenaGel, a nonabsorbed calcium- and aluminum-free phosphate binder, lowers serum phosphorus and parathyroid hormone. The RenaGel Study Group. Kidney Int. 1999;55(1):299–307. doi: 10.1046/j.1523-1755.1999.00240.x. [DOI] [PubMed] [Google Scholar]
- 260.Rosenbaum DP, Holmes-Farley SR, Mandeville WH, Pitruzzello M, Goldberg DI. Effect of RenaGel, a non-absorbable, cross-linked, polymeric phosphate binder, on urinary phosphorus excretion in rats. Nephrol. Dial. Transplant. 1997;12(5):961–964. doi: 10.1093/ndt/12.5.961. [DOI] [PubMed] [Google Scholar]
- 261.Oka Y, Miyazaki M, Takatsu S, et al. A review article: sevelamer hydrochloride and metabolic acidosis in dialysis patients. Cardiovasc. Hematol. Disord. Drug Targets. 2008;8(4):283–286. doi: 10.2174/187152908786786197. [DOI] [PubMed] [Google Scholar]
- 262.Spaia S. Phosphate binders: sevelamer in the prevention and treatment of hyperphosphataemia in chronic renal failure. Hippokratia. 2011;15(Suppl. 1):22–26. [PMC free article] [PubMed] [Google Scholar]
- 263.Buster JE. Transdermal menopausal hormone therapy: delivery through skin changes the rules. Expert Opin. Pharmacother. 2010;11(9):1489–1499. doi: 10.1517/14656561003774098. [DOI] [PubMed] [Google Scholar]
- 264.Simon JA Group FTES. Estradiol in micellar nanoparticles: the efficacy and safety of a novel transdermal drug-delivery technology in the management of moderate to severe vasomotor symptoms. Menopause. 2006;13(2):222–231. doi: 10.1097/01.gme.0000174096.56652.4f. [DOI] [PubMed] [Google Scholar]
- 265.Prausnitz MR, Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yoo JW, Lee CH. Drug delivery systems for hormone therapy. J. Control. Rel. 2006;112(1):1–14. doi: 10.1016/j.jconrel.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 267.Rai MF, Pham CT. Intra-articular drug delivery systems for joint diseases. Curr. Opin. Pharmacol. 2018;40:67–73. doi: 10.1016/j.coph.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Taylor N. Nonsurgical management of osteoarthritis knee pain in the older adult: an update. Rheum. Dis. Clin. North Am. 2018;33(4):41–51. doi: 10.1016/j.rdc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 269.Byers-Kraus V, Aazami H, Mehra P, et al. Synovial and systemic pharmacokinetics of triamcinolone acetonide following intra-articular injection of an extended release formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis. Osteoarthr. Cartil. 2017;25:S431. doi: 10.1016/j.joca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 270.Adedoyin AA. Development of injectable, stimuli-responsive biomaterials as active scaffolds for applications in advanced drug delivery and osteochondral tissue regeneration. 2018.
- 271.Celgene Pharmaceutical Co; NJ, USA: 2005. Abraxane® for Injectable Suspension [Prescribing Information] [Google Scholar]
- 272.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol [19] Nature. 1979;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 273.Sparreboom A, Scripture CD, Trieu V, et al. Comparative preclinical and clinical pharmacokinetics of a Cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in cremophor (Taxol) Clin. Cancer Res. 2005;11(11):4136–4143. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 274.Elsadek B, Kratz F. Impact of albumin on drug delivery – new applications on the horizon. J. Control. Release. 2012;157(1):4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 275.Jang K, Yoon S, Kim S-E, et al. Novel nanocrystal formulation of megestrol acetate has improved bioavailability compared with the conventional micronized formulation in the fasting state. Drug Des. Devel. Ther. 2014;8:851. doi: 10.2147/DDDT.S62176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 277.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12(10):1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 278.Manoukian G, Hagemeister F. Denileukin diftitox: a novel immunotoxin. Expert Opin. Biol. Ther. 2009;9(11):1445–1451. doi: 10.1517/14712590903348135. [DOI] [PubMed] [Google Scholar]
- 279.Kaminetzky D, Hymes KB. Denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Biologics. 2008;2(4):717–724. doi: 10.2147/btt.s3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Foss F. Clinical experience with denileukin diftitox (ONTAK) Semin. Oncol. 2006;33(1 Suppl. 3):S11–S16. doi: 10.1053/j.seminoncol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 281.Duvic M. Bexarotene and DAB389IL-2 (Denileukin Diftitox, ONTAK) in treatment of cutaneous T-cell lymphomas: algorithms. Clin. Lymphoma. 2000;1:S51–S55. doi: 10.3816/clm.2000.s.010. [DOI] [PubMed] [Google Scholar]
- 282.Schwab CL, English DP, Roque DM, Pasternak M, Santin AD. Past, present and future targets for immunotherapy in ovarian cancer. Immunotherapy. 2014;6(12):1279–1293. doi: 10.2217/imt.14.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.FDA. Rebinyn: Coagulation Factor IX (Recombinant), GlycoPEGylated. 2017 [Google Scholar]
- 284.Woods GM, Dunn MW, Dunn AL. Emergencies in hemophilia. Clin. Pediatr. Emerg. Med. 2018;19(2):110–121. [Google Scholar]
- 285.Peters R, Harris T. Advances and innovations in haemophilia treatment. Nat. Rev. Drug Discov. 2018;17(7):493–508. doi: 10.1038/nrd.2018.70. [DOI] [PubMed] [Google Scholar]
- 286.Croteau SE. Evolving complexity in hemophilia management. Pediatr. Clin. North Am. 2018;65(3):407–425. doi: 10.1016/j.pcl.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 287.Farjadian F, Ghasemi S, Mohammadi-Samani S. Hydroxyl-modified magnetite nanoparticles as novel carrier for delivery of methotrexate. Int. J. Pharm. 2016;504(1):110–116. doi: 10.1016/j.ijpharm.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 288.Majidi S, Zeinali Sehrig F, Samiei M, et al. Magnetic nanoparticles: applications in gene delivery and gene therapy. Artif. Cells Nanomed. Biotechnol. 2016;44(4):1186–1193. doi: 10.3109/21691401.2015.1014093. [DOI] [PubMed] [Google Scholar]
- 289.Farjadian F, Moradi S, Hosseini M. Thin chitosan films containing super-paramagnetic nanoparticles with contrasting capability in magnetic resonance imaging. J. Mater. Sci. Mater. Med. 2017;28(3):47. doi: 10.1007/s10856-017-5854-2. [DOI] [PubMed] [Google Scholar]
- 290.Coyne DW. Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert Opin. Pharmacother. 2009;10(15):2563–2568. doi: 10.1517/14656560903224998. [DOI] [PubMed] [Google Scholar]
- 291.Schwenk MH. Ferumoxytol: a new intravenous iron preparation for the treatment of iron deficiency anemia in patients with chronic kidney disease. Pharmacotherapy. 2010;30(1):70–79. doi: 10.1592/phco.30.1.70. [DOI] [PubMed] [Google Scholar]
- 292.Curtis BM, Barrett BJ, Djurdjev O, Singer J, Levin A, Group C-CI. Evaluation and treatment of CKD patients before and at their first nephrologist encounter in Canada. Am. J. Kidney Dis. 2007;50(5):733–742. doi: 10.1053/j.ajkd.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 293.Landry R, Jacobs PM, Davis R, Shenouda M, Bolton WK. Pharmacokinetic study of ferumoxytol: a new iron replacement therapy in normal subjects and hemodialysis patients. Am. J. Nephrol. 2005;25(4):400–410. doi: 10.1159/000087212. [DOI] [PubMed] [Google Scholar]
- 294.Spinowitz BS, Schwenk MH, Jacobs PM, et al. The safety and efficacy of ferumoxytol therapy in anemic chronic kidney disease patients. Kidney Int. 2005;68(4):1801–1807. doi: 10.1111/j.1523-1755.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 295.Mccormack PL. Ferumoxytol. Drugs. 2012;72(15):2013–2022. doi: 10.2165/11209880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 296.ETPN – Nanomedicine European Technology Platform. Src, Ncpm, Ncrd. Strategic agenda for EuroNanoMed. https://etp-nanomedicine.eu/about-nanomedicine/strategic-research-and-innovation-agenda/
- 297.BCC Research. Global markets for nanoparticle size analysis instrumentation in the life sciences. 2014. www.bccresearch.com/market-research/biotechnology/nanoparticle-size-analysis-instrumentation-life-sciences-report-bio114b.html
- 298.Moghimi SM, Peer D, Langer R. Reshaping the future of nanopharmaceuticals: adiudicium. ACS nano. 2011;5(11):8454–8458. doi: 10.1021/nn2038252. [DOI] [PubMed] [Google Scholar]
- 299.Bowman D, Marino AD, Sylvester DJ. The patent landscape of nanomedicines. Med. Res. Arch. 2017;5(9) [Google Scholar]
- 300.Business Wire. Global nanomedicine market analysis & trends report 2016 – market is poised to grow to reach approximately $1.3 trillion by 2025– research and markets. 2016. www.businesswire.com/news/home/20160804005610/en/Global-Nanomedicine-Market-Analysis-Trends-Report-2016
- 301.Ridings JE. The thalidomide disaster, lessons from the past. In: Barrow PC, editor. Teratogenicity Testing. Springer; Basel, Switzerland: 2013. pp. 575–586. [DOI] [PubMed] [Google Scholar]
- 302.Faunce T, Townsend R, Mcewan A. The Vioxx pharmaceutical scandal: Peterson v Merke Sharpe & Dohme (Aust) Pty Ltd (2010) 184 FCR 1. J. Law Med. 2010;18(1):38–49. [PubMed] [Google Scholar]
- 303.Katz JA. COX-2 inhibition: what we learned–a controversial update on safety data. Pain Med. 2013;14(Suppl. 1):S29–S34. doi: 10.1111/pme.12252. [DOI] [PubMed] [Google Scholar]
- 304.Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomed. 2014;9:4357. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ledford H. Bankruptcy of nanomedicine firm worries drug developers. Nature. 2016;533(7603):304–305. doi: 10.1038/533304a. [DOI] [PubMed] [Google Scholar]
- 306.Adams DJ. The valley of death in anticancer drug development: a reassessment. Trends Pharmacol. Sci. 2012;33(4):173–180. doi: 10.1016/j.tips.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7(9):7442–7447. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Rosenblum D, Peer D. Omics-based nanomedicine: the future of personalized oncology. Cancer Lett. 2014;352(1):126–136. doi: 10.1016/j.canlet.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 310.Roointan A, Kianpour S, Memari F, Gandomani M, Gheibi Hayat SM, Mohammadi-Samani S. Poly(lactic-co-glycolic acid): the most ardent and flexible candidate in biomedicine! Int. J. Polym. Materi. Polym. Biomater. 2018;67(17):1028–1049. [Google Scholar]
- 311.Bregoli L, Movia D, Gavigan-Imedio JD, Lysaght J, Reynolds J, Prina-Mello A. Nanomedicine applied to translational oncology: a future perspective on cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2016;12(1):81–103. doi: 10.1016/j.nano.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 312.Eetezadi S, Ekdawi SN, Allen C. The challenges facing block copolymer micelles for cancer therapy: in vivo barriers and clinical translation. Adv. Drug Del. Rev. 2015;91:7–22. doi: 10.1016/j.addr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 313.Gabizon A, Bradbury M, Prabhakar U, Zamboni W, Libutti S, Grodzinski P. Cancer nanomedicines: closing the translational gap. Lancet. 2014;384(9961):2175–2176. doi: 10.1016/S0140-6736(14)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lammers T. Smart drug delivery systems: back to the future vs. clinical reality. Int. J. Pharm. 2013;454(1):527–529. doi: 10.1016/j.ijpharm.2013.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Satalkar P, Elger BS, Hunziker P, Shaw D. Challenges of clinical translation in nanomedicine: a qualitative study. Nanomedicine. 2016;12(4):893–900. doi: 10.1016/j.nano.2015.12.376. [DOI] [PubMed] [Google Scholar]
- 316.Kimmelman J. Beyond human subjects: risk, ethics, and clinical development of nanomedicines. J. Law Med. Ethics. 2012;40(4):841–847. doi: 10.1111/j.1748-720X.2012.00712.x. [DOI] [PubMed] [Google Scholar]
- 317.Van De Poel I. How should we do nanoethics? A network approach for discerning ethical issues in nanotechnology. Nanoethics. 2008;2(1):25–38. [Google Scholar]
- 318.Weissig V, Guzman-Villanueva D. Nanopharmaceuticals (part 2): products in the pipeline. Int. J. Nanomedicine. 2015;10:1245–1257. doi: 10.2147/IJN.S65526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Liang X-J. Nanopharmaceutics: The Potential Application of Nanomaterials. World Scientific Publishing Co., Pte Ltd; Toh Tuck Link, Singapore: 2013. [Google Scholar]
- 320.Shah RB, Khan MA. Nanopharmaceuticals: challenges and regulatory perspective. In: de Villiers MM, Aramwit P, Kwon GS, editors. Nanotechnology in Drug Delivery. Springer; NY, USA: 2009. [Google Scholar]
- 321.Eaton MA. How do we develop nanopharmaceuticals under open innovation? Nanomedicine. 2011;7(4):371–375. doi: 10.1016/j.nano.2011.05.015. [DOI] [PubMed] [Google Scholar]