TO THE EDITOR:
Historically, metabolic studies in platelets have primarily investigated events occurring during ex vivo platelet storage, less so the consequences of metabolic alterations on platelet homeostasis and physiologic function. The importance of such studies is emphasized by the recent finding of platelet mitochondrial dysfunction in type 2 diabetes, sickle cell disease, and sepsis.1-7 These early investigations used radiometric methods with labeled glucose to study glycolysis and oxidative phosphorylation in platelets.8,9 Recently, these techniques have been supplanted by more sophisticated investigative tools that allow more rapid analysis of small volume samples, including high-resolution respirometry10,11 and Seahorse extracellular flux analysis.12-14 Glycolysis and oxidative phosphorylation, as well as glutaminolysis and fatty acid β oxidation, all function to support the metabolic demand in platelets.10,14-16 The metabolic flexibility of the platelet has been emphasized by investigations demonstrating that activated platelets exhibit a glycolytic phenotype while preserving mitochondrial function and can easily switch between glucose and fatty acid catabolism to support activation.12
The recently published study by A. K. Chauhan’s group further advances our understanding of the understudied field of platelet metabolism.13 In this paper, the authors demonstrate that dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, alters platelet metabolism and function.13 Finding salutary antiaggregatory and antithrombotic effects of dichloroacetate, the authors propose targeting of the platelet metabolic response as a novel antithrombotic approach. However, there are few points of clarification we would like to add, which we believe will be of great benefit for the platelet and mitochondria research community in their exploration of this new therapeutic avenue.13
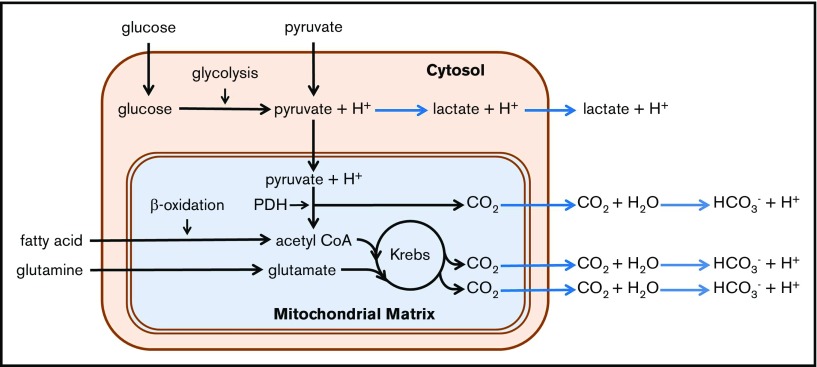
As pointed out by the investigators in this study, the extracellular acidification rate (ECAR) is a commonly used indirect measure of glycolytic rate. Key to the interpretation of this indirect measure is the test of the cell’s utilization of glucose in the presence of a series of well-defined inhibitors.12,17 Dissection of the cause of acidification is necessary, as the relationship between ECAR and glycolysis is complicated by the existence of multiple acidification mechanisms, both mitochondrial and nonmitochondrial.17 As illustrated in Figure 1, CO2 is generated within the mitochondrial matrix by the pyruvate dehydrogenase complex and through the Krebs cycle. Upon diffusion from the cell, this mitochondrially generated CO2 is rapidly hydrated to H2CO3, which dissociates to bicarbonate ion and a proton at the physiological pH of the extracellular environment. Thus, conversion of 1 molecule of glucose to lactate (so called anaerobic glycolysis) yields 2 protons, whereas a complete oxidation of glucose to CO2 by mitochondrial mechanisms yields 6 protons. In addition, platelet mitochondria can also oxidize glutamine and fatty acids to produce substrates for the Krebs cycle.9,15,16,18-24 Thus, measurement of the glycolytic rate would require subtraction of acidification by mitochondrially derived CO2.
Figure 1.
Summary of platelet catabolic pathways. Reactions leading to extracellular acidification caused by production of lactate and CO2 are shown with blue arrows. CoA, coenzyme A; Krebs, Krebs or tricarboxylic acid cycle; PDH, pyruvate dehydrogenase complex.
Another point we would like to address is the terminology used to describe cellular respiration, specifically the term aerobic glycolysis. Classically, cellular respiration is divided into 4 parts: glycolysis, pyruvate dehydrogenation, the Krebs cycle, and the electron transport chain coupled with chemiosmosis, with the last of these being the only oxygen-utilizing catabolic process. Pyruvate oxidation and the Krebs cycle are, however, dependent on oxidative phosphorylation and therefore would not occur in anaerobic conditions. Aerobic glycolysis, originally called the Warburg effect, is a phenomenon attributed mostly to cancer cells, which often rely primarily on the glycolytic part of glucose catabolism regardless of oxygenation.25-28 It is thought that the Warburg effect is a pathophysiologic adaptation of cancer cells to hypoxic conditions during early tumorigenesis. We propose that the term aerobic glycolysis be reserved for this unique setting and suggest that the term glycolysis (without the modifier) is sufficient to describe this aspect of glucose catabolism in platelets.
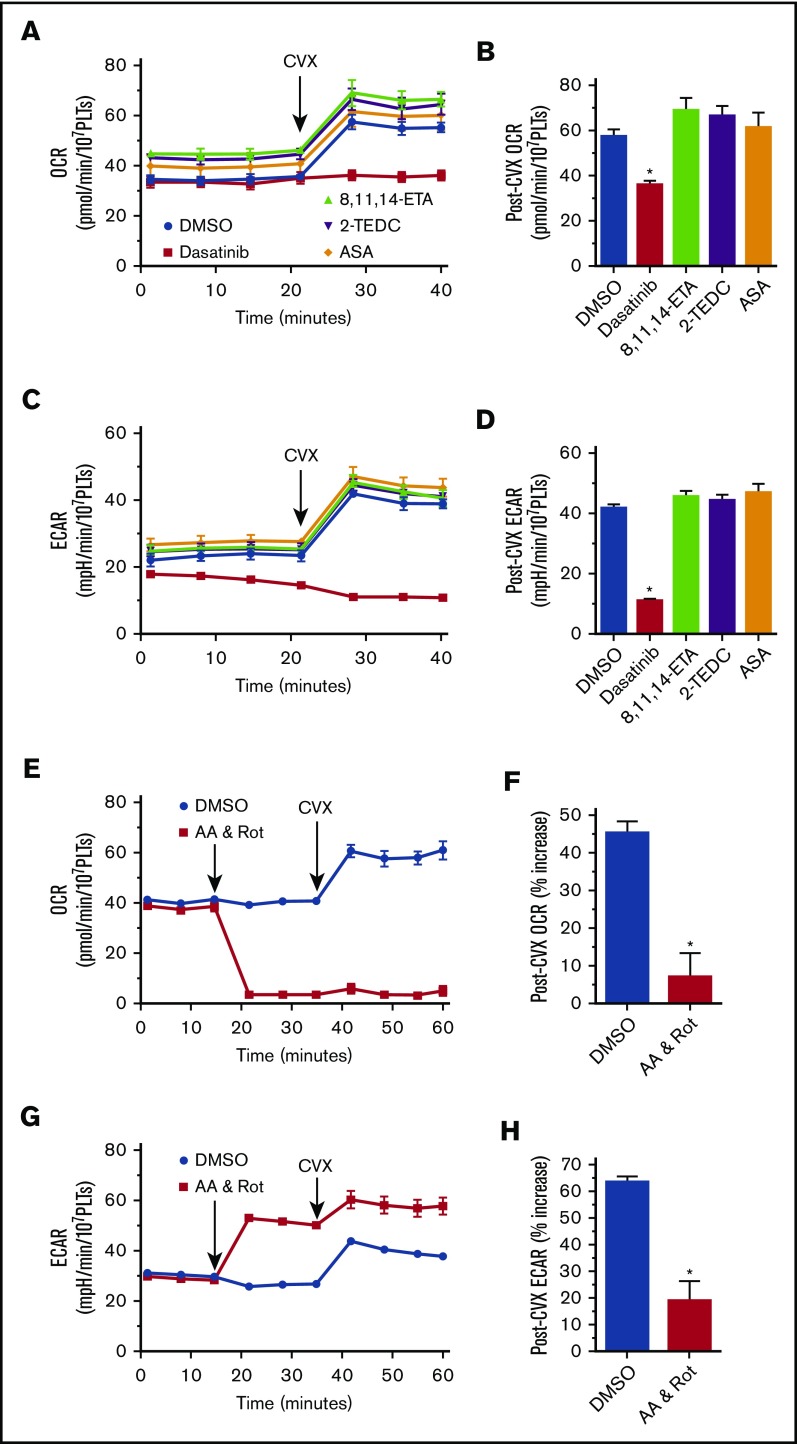
Adenosine triphosphate (ATP) plays a central role in the transfer of energy from its site of production to its site of utilization. Platelets, unlike many other cells, must abruptly transition from a “resting” state to an activated state with a similarly abrupt increase in energy consumption. The sudden increase in energy demand from the “burning” of the ATP resource during platelet activation has to be compensated if the newly activated platelet is to function within the hemostatic plug. Nayak et al have investigated a novel potential therapeutic avenue, namely preventing thrombus formation by altering the platelet’s catabolic response to activation.13 Glycolysis, although faster than oxidative phosphorylation, is not nearly as efficient in its ability to generate ATP, suggesting that an increase in oxidative phosphorylation should occur following platelet activation. Supporting this theoretical presumption, convulxin (CVX) stimulation increases both ECAR and oxygen consumption. This increase is inhibited by dasatinib, an inhibitor of the early steps of glycoprotein VI signaling.
To understand the mechanism driving this increase in oxygen utilization following CVX stimulation, we have measured the oxygen consumption rate and ECAR in the presence of various inhibitors of cyclooxygenase (acetyl salicylic acid) and lipoxygenase (2-(1-thienyl)ethyl 3,4-dihydroxybenzylidenecyanoacetate and 8,11,14-eicosatriynoic acid), none of which demonstrated an effect on metabolism in convulxin-stimulated platelets (Figure 2A-D). Preincubation of platelets with rotenone and antimycin A completely prevented this CVX-induced burst in oxygen consumption, indicating that this increased oxygen utilization is primarily of mitochondrial origin (Figure 2E-F), whereas the residual increase ECAR (Figure 2G-H) in the presence of these mitochondrial inhibitors is consistent with a compensatory increase in glycolysis (Figure 1). Distinction of the precise mechanisms of platelet metabolism will be crucial in the evaluation and development of antithrombotic therapies based on the novel therapeutic approach proposed by this study’s authors.
Figure 2.
Glycoprotein VI–stimulated platelet respiration. Oxygen consumption (A-B,E-F) and extracellular acidification (C-D,G-H) in isolated human platelets. n ≥ 4; *P < .05 vs dimethyl sulfoxide. 2-TEDC, 2-(1-thienyl)ethyl 3,4-dihydroxybenzylidenecyanoacetate; 8,11,14-ETA, 8,11,14-eicosatriynoic acid; AA, antimycin A; ASA, acetyl salicylic acid; DMSO, dimethyl sulfoxide; OCR, oxygen consumption rate; PLT, platelet; Rot, rotenone.
All studies were approved by the Institutional Review Board of Versiti Blood Research Institute.
Contribution: A.K. designed and performed the experiments and wrote the manuscript; and S.J. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andaleb Kholmukhamedov, Versiti Blood Research Institute, 8727 W Watertown Plank Rd, Milwaukee, WI 53226, e-mail: andaleb.kholmukhamedov@versiti.org.
References
- 1.Avila C, Huang RJ, Stevens MV, et al. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp Clin Endocrinol Diabetes. 2012;120(4):248-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardenes N, Corey C, Geary L, et al. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014;123(18):2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gründler K, Angstwurm M, Hilge R, et al. Platelet mitochondrial membrane depolarization reflects disease severity in patients with sepsis and correlates with clinical outcome. Crit Care. 2014;18(1):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Protti A, Fortunato F, Artoni A, et al. Platelet mitochondrial dysfunction in critically ill patients: comparison between sepsis and cardiogenic shock. Crit Care. 2015;19(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puskarich MA, Kline JA, Watts JA, Shirey K, Hosler J, Jones AE. Early alterations in platelet mitochondrial function are associated with survival and organ failure in patients with septic shock. J Crit Care. 2016;31(1):63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjövall F, Morota S, Hansson MJ, Friberg H, Gnaiger E, Elmér E. Temporal increase of platelet mitochondrial respiration is negatively associated with clinical outcome in patients with sepsis. Crit Care. 2010;14(6):R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang WH, Stitham J, Jin Y, et al. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation. 2014;129(15):1598-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen P, Wittels B. Energy substrate metabolism in fresh and stored human platelets. J Clin Invest. 1970;49(1):119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karpatkin S. Studies on human platelet glycolysis. Effect of glucose, cyanide, insulin, citrate, and agglutination and contraction on platelet glycolysis. J Clin Invest. 1967;46(3):409-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjövall F, Ehinger JK, Marelsson SE, et al. Mitochondrial respiration in human viable platelets--methodology and influence of gender, age and storage. Mitochondrion. 2013;13(1):7-14. [DOI] [PubMed] [Google Scholar]
- 11.Sowton AP, Millington-Burgess SL, Murray AJ, Harper MT. Rapid kinetics of changes in oxygen consumption rate in thrombin-stimulated platelets measured by high-resolution respirometry. Biochem Biophys Res Commun. 2018;503(4):2721-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aibibula M, Naseem KM, Sturmey RG. Glucose metabolism and metabolic flexibility in blood platelets. J Thromb Haemost. 2018;16(11):2300-2314. [DOI] [PubMed] [Google Scholar]
- 13.Nayak MK, Dhanesha N, Doddapattar P, et al. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Adv. 2018;2(15):2029-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravi S, Chacko B, Sawada H, et al. Metabolic plasticity in resting and thrombin activated platelets. PLoS One. 2015;10(4):e0123597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasta V, Meacci E, Farnararo M, Bruni P. Glutamine utilization in resting and stimulated platelets. J Biochem. 1993;114(2):163-166. [DOI] [PubMed] [Google Scholar]
- 16.Waller HD, Loehr GW, Grignani F, Gross R. Über den Energiestoffwechsel normaler menschlicher Thrombozyten [On the energy metabolism of normal human thrombocytes]. Thromb Diath Haemorrh. 1959;3:520-547. [PubMed] [Google Scholar]
- 17.Mookerjee SA, Goncalves RLS, Gerencser AA, Nicholls DG, Brand MD. The contributions of respiration and glycolysis to extracellular acid production. Biochim Biophys Acta. 2015;1847(2):171-181. [DOI] [PubMed] [Google Scholar]
- 18.Doery JC, Hirsh J, Cooper I. Energy metabolism in human platelets: interrelationship between glycolysis and oxidative metabolism. Blood. 1970;36(2):159-168. [PubMed] [Google Scholar]
- 19.Doery JC, Hirsh J, De Gruchy GC. Platelet glycolytic enzymes: effect of cellular disruption procedures on activity. Br J Haematol. 1970;19(2):145-157. [DOI] [PubMed] [Google Scholar]
- 20.Karpatkin S, Langer RM. Biochemical energetics of simulated platelet plug formation. Effect of thrombin, adenosine diphosphate, and epinephrine on intra- and extracellular adenine nucleotide kinetics. J Clin Invest. 1968;47(9):2158-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitchens CS, Newcomb TF. Human platelet respiration. J Appl Physiol. 1968;25(5):581-585. [DOI] [PubMed] [Google Scholar]
- 22.Rosenzweig A, Ways P. The oxidation of long-chain fatty acids by the formed elements of human blood. Blood. 1966;27(1):57-64. [PubMed] [Google Scholar]
- 23.Spector AA, Hoak JC, Warner ED, Fry GL. Utilization of long-chain free fatty acids by human platelets. J Clin Invest. 1970;49(8):1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warshaw AL, Laster L, Shulman NR. The stimulation by thrombin of glucose oxidation in human platelets. J Clin Invest. 1966;45(12):1923-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LePAGE GA. Glycolysis in tumor homogenates. J Biol Chem. 1948;176(3):1009-1020. [PubMed] [Google Scholar]
- 26.LePAGE GA. A comparison of tumor and normal tissues with respect to factors affecting the rate of anaerobic glycolysis. Cancer Res. 1950;10(2):77-88. [PubMed] [Google Scholar]
- 27.Potter VR. The assay of animal tissues for respiratory enzymes; further studies on oxidative phosphorylation. J Biol Chem. 1947;169(1):17-37. [PubMed] [Google Scholar]
- 28.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519-530. [DOI] [PMC free article] [PubMed] [Google Scholar]